ABSTRACT
Healthcare workers (HCWs) are the target population for vaccination against coronavirus disease (COVID-19) as they are at a high risk of exposure and transmission of pathogens to patients. Neutralizing antibodies developed after COVID-19 vaccination decline within few months of vaccination. Several factors, including age and sex, can affect the intensity, efficacy, and duration of immune response to vaccines. However, sex-specific analyses of humoral responses to COVID-19 vaccines are lacking. This study aimed to evaluate sex-based differences in anti-S/RBD (Receptor Binding Domain) responses at three different time points after the second dose of mRNA COVID-19 vaccine in HCWs in relation to age, and to investigate the role of sex hormones as potential markers of response. Anti-S/RBD levels after two doses of the mRNA vaccine were collected from 521 HCWs naïve to COVID-19, working at two Italian Clinical Centers. Multiple regression analysis was applied to evaluate the association between anti-S levels and sex, age, and plasma levels of sex hormones. Significantly higher anti-S/RBD response to the COVID-19 vaccination was found in female HCWs, and a significant and more abrupt decline in response with time was observed in women than that in men. A novel, positive association of testosterone plasma levels and higher anti-S levels in male HCWs was found, suggesting its potential role as sex specific marker in males. In conclusion, understanding the sex-based differences in humoral immune responses to vaccines may potentially improve vaccination strategies and optimize surveillance programs for HCWs.
KEYWORDS: COVID-19, vaccine, sex difference, anti-S/RBD, estrogen, testosterone, progesterone, healthcare workers
Introduction
Healthcare workers (HCWs) are among the groups at the highest risk of exposure to pathogens since they are in direct contact of patients or handle potentially infected material. Before the availability of an efficient vaccine, coronavirus disease (COVID-19) fatally affected 80,000–180,000 HCWs from January 2020 to May 2021.1
Hence, HCWs should be appropriately vaccinated to reduce the chance of contracting or spreading vaccine-preventable diseases by protecting themselves, the patients, and their family members. Recently, the COVID-19 pandemic has generated significant interest in vaccine development and effectiveness, as well as in public health policies related to the use of vaccines. The World Health Organization has reported data from 119 countries by September 2021, stating that on an average two out of five HCWs are fully vaccinated.2 The availability of safe and effective vaccines has been crucial to contain the infection and to limit the social and economic consequences of the pandemic for public and occupational health.3,4 HCWs were the first to be vaccinated in several countries, such as Italy, receiving the mRNA vaccine BNT162b2 (Pfizer). In this context, knowledge of the intensity and duration of antibody responses, which may be correlated with protection, both in convalescent and vaccinated individuals, is presumably one of the most important issues to be addressed.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection of target cells is mediated by the receptor‐binding domain (RBD) in the structure of the S‐protein.5 Neutralizing antibodies are directed to the RBD of the Spike (S) protein, which has been identified as immunogenic.6 Since the antibody response to the S‐protein correlates with neutralizing antibodies,7 anti-S antibodies are usually associated with protection from COVID-19 development.7–12
The neutralizing antibody levels decline 1–4 months after the onset of infection symptoms13 and post-vaccination.14 Moreover, the vaccine antibody response in HCWs has been analyzed using different types of antibodies [total anti-S immunoglobulin (Ig)G, anti-S/RBD, AU (antibody units) and/or BAU (binding antibody units), and neutralizing antibodies]. Consequently, the direct comparison of findings from different studies is not always possible, partly due to the use of different immunoassay(s).
Women are more immunoreactive than men in response to infections and antiviral vaccines, as females usually produce higher antibody levels than those by males on infection or vaccination.15–17 However, it is still unclear if the more robust antibody response translates to increased vaccine efficacy in females. Few studies on COVID-19-vaccinated HCWs or individuals have shown different antibody responses between male and female vaccine recipients.18–21 However, targeted sex-disaggregated analyses of serologic responses to anti-SARS-CoV-2 vaccines are rarely performed, and controversial results have been reported.
Hence, this study aimed to evaluate sex-based differences in anti-S/RBD antibody levels at different time points after the second dose of the mRNA COVID-19 vaccine in HCWs, considering age at vaccination. In addition, to elucidate the mechanisms underlying the different immunological responses to COVID-19 vaccination, between male and female HCWs, and to identify potential sex-specific biomarkers, we analyzed the possible association between the levels of anti-S/RBD antibodies and sex hormones, such as estrogen, progesterone, and testosterone, which are characteristic markers of sex specific immune responses.15,16
Materials and methods
Study design and study population
We conducted a follow-up study among vaccinated HCWs, working at the Spallanzani Italian hospital for infectious diseases and Bambino Gesù Children’s Hospital in Rome. HCWs were asked to visit the laboratory for testing after the second dose of mRNA COVID-19 vaccination, concomitantly with periodic health surveillance. This study included 521 HCWs with no history of SARS-CoV-2 infection, as demonstrated using molecular (reverse transcriptase-polymerase chain reaction) and antibody assays (Elecsys®Anti-N, Roche), and those receiving two primary doses of BNT162b2 vaccine (30 μg), 21 days apart, according to the immunization schedule.
The study-protocol included the following three time points to evaluate anti-S antibody levels: after obtaining written informed consent, blood samples were collected approximately 15 days after vaccination (T1) and thereafter, approximately 2 months (T2) and 5 months after vaccination (T3), to follow up the humoral response to BNT162b2.
This study was conducted after obtaining ethical approval from ISS (AOO-ISS 09/05/2021–0017778); all participants provided written informed consent.
Serologic analysis
Serum samples from vaccinated HCWs were tested using two commercial chemiluminescence microparticle antibody assays, the SARS-CoV-2-specific anti-N and the anti-S/RBD tests (AdviseDx SARS-CoV-2 IgG II and SARS-CoV-2 IgG II Quant, respectively, ARCHITECT®, i2000sr Abbott Diagnostics), according to the manufacturers’ instructions. Arbitrary units (AU)/mL > 50 were considered positive. The anti-S serologic assay, AdviseDx SARS-CoV-2 immunoglobulin (Ig)G II assay is a chemiluminescent microparticle immunoassay for the qualitative and semi-quantitative detection of IgG antibodies to SARS-CoV-2. The assay was designed to detect serum or plasma IgG antibodies against the RBD of the S1 subunit of SARS-CoV-2 spike protein among individuals suspected or confirmed to be infected with SARS-CoV-2.
Measurement of plasma testosterone, 17β-estradiol, and progesterone levels
Plasma samples were collected from a subgroup of 112 HCWs (45 males and 67 females) at T2 post-vaccination. Sex hormones levels was quantified using the following competitive enzyme linked immunosorbent assay (ELISA) kits: Free Testosterone (KGE010 R&D Systems, Intra-Assay: CV 3.1%, inter-assay: co-efficient of variation (CV) 6.3%), 17β-estradiol (ab108667 Abcam, intra-Assay: CV < 9%, inter-assay CV < 10%), progesterone (ab108670 Abcam, intra-Assay: CV < 4%, inter-assay CV ≤ 9.3). All ELISA kits were used according to the manufacturers’ instructions.
Statistical analyses
Categorical variables were summarized using frequencies and percentages, whereas continuous variables were summarized using medians and interquartile ranges. Geometric means with their confidence intervals (95% CI) were calculated for anti-S/RBD (AU/mL) concentrations according to sex and the following three age groups: 20–44, 45–55, and 56–85 years, to evaluate the potential effect of sex hormones. When the variables of interest were not normally distributed, analyses were performed after a natural logarithmic transformation. The non-parametric Mann – Whitney test was used to assess differences between two independent groups, and the Kruskal – Wallis test was used to compare more than two groups.
A mixed linear regression model with a random intercept and beta (slope) per individual was applied to estimate the effect of post-vaccination elapsed time on the level of anti-S/RBD antibodies. Briefly, the dependent variable was the anti-S antibody level and the independent variable was the time from the second vaccine dose. To evaluate sex-based differences in the slopes of anti-S antibodies, we considered sex in the model and its interaction with time. The regression parameter beta and its relative 95% CI were estimated for both men and women. We plotted the estimated fixed effects using the models described above.
Furthermore, regression models were applied to investigate the potential effect of sex hormones on anti-S/RBD antibody levels in a subgroup of the study population. Simple and multiple linear regression models for males and females, with hormones and age groups (20–44; 45–55; and 56–85 years) as covariates, were applied.
SAS/STAT version 9.4 was used for statistical analyses.
Results
Description of the study population
The study included 521 HCWs, 137 men (26%) and 384 women (74%), who were not previously infected with SARS-CoV-2 and received two doses of the mRNA vaccine (BNT162b2), working at the Spallanzani Institute of Infectious Diseases and at the Bambino Gesù Children’s Hospital in Rome, Italy. The median age was 43 years, which was marginally higher in male workers (45 years) than those in female workers (41 years) (Table 1).
Table 1.
Demographic characteristics of the study population.
| Study population | Males | Females | |
|---|---|---|---|
| n = 521 | 137 (26.3%) | 384 (73.7%) | |
| median age | 43 | 45 | 41 |
| IQR; range | (31–52); (22–81) | (34–55); (24–66) | (30–51); (22–81) |
| age groups | |||
| 20–44 years | 282 (54.2%) | 63 (46.0%) | 219 (57.0%) |
| 45–55 years | 157 (3.1%) | 43 (31.4%) | 114 (29.7%) |
| 56–85 years | 82 (15.7%) | 31 (22.6%) | 51 (13.3%) |
IQR: interquartile range.
To evaluate the potential effect of sex hormones, we subclassified the HCWs into the following three age groups: 20–44, 45–55, and 56–85 years, including female HCWs in the pre-menopausal, menopausal, and post-menopausal age groups, respectively, and age-matched male HCWs. As expected, age-based classification of the study population revealed that majority of the HCWs belonged to the youngest age group, 20–44 age (54%) among males (46%) and females (57%).
Humoral response to the COVID-19 vaccine by sex and age
In all vaccinated individuals, a high antibody response was achieved 16 days after vaccination (geometric mean 2,169 AU/mL, 95%CI: 2,040–2,306). As shown in Table 2, the geometric means of the antibody levels decreased to 539 (95%CI: 506–574) and 179 (95%CI: 167–191) at 2.5- and 5-months post-vaccination, respectively.
Table 2.
Geometric means and 95%CI of anti-S/RBD levels (AU/mL) at the three considered time points after second dose of vaccine; p-values refer to the comparison among groups at each measurement.
| I measurement | II measurement | III measurement | |
|---|---|---|---|
| days since second vaccination | |||
| median (IQR); (range) |
16 (15–17); (12–37) |
77 (76–78); (34–101) |
154 (151–156); (122–243) |
| Geometric mean and (95% CI) of anti-S/RBD levels (AU/mL) | |||
| all individuals | 2,169 (2,040–2,306) | 539 (506–574) | 179 (167–191) |
| males | 1,640 (1,447–1,857) | 421 (372–476) | 149 (132–170) |
| females | 2,397 (2,240–2,565) | 588 (548–632) | 191 (177–206) |
| p-values males vs females | <.001 | <.001 | <.001 |
| Age groups | |||
| 20–44 | 2,407 (2,234–2,595) | 599 (553–649) | 205 (189–223) |
| 45–55 | 2,034 (1,824–2,267) | 493 (439–552) | 159 (142–179) |
| 56–85 | 1,714 (1,403–2,094) | 444 (370–533) | 139 (114–171) |
| p-values for age groups | .002 | .002 | <.001 |
| males aged | |||
| 20–44 | 1,822 (1,528–2,171) | 495 (414–593) | 183 (152–219) |
| 45–55 | 1,635 (1,532–1,978) | 387 (317–473) | 134 (109–168) |
| 56–85 | 1,329 (944–1,869) | 340 (254–456) | 114 (85–152) |
| p-values for age groups | .166 | .015 | .008 |
| females aged | |||
| 20–44 | 2,609 (2,409–2,825) | 634 (579–691) | 212 (193–233) |
| 45–55 | 2,208 (1,939–2,515) | 539 (471–618) | 169 (148–194) |
| 56–85 | 2,001 (1,566–2,558) | 522 (415–656) | 158 (120–208) |
| p-values for age groups | .037 | .121 | .010 |
Sex-specific analysis revealed that the anti-S/RBD concentrations in females were higher than those in males; these differences were statistically significant (p < .05) at each time point considered (16, 77, and 154 days post-vaccination).
Age stratified analysis showed the highest anti-S/RBD levels in the youngest workers (20–44 years), among both females and males (Table 2, Table S1).
In Figure 1a, the statistically significant (p ≤ .001) sex-based differences in anti-S/RBD antibody levels, reported on a log scale, revealed a decrease in anti-S/RBD concentrations with time, in both males and females, which was evident in all age groups (Figure 1b-d). The waning of anti-S/RBD antibody levels after vaccination, was further evaluated using a mixed regression model (Figure 2), to determine the effect of time on antibody concentrations in male and female HCWs. The results in Figure 2a show a greater decline in anti-S/RBD levels among female HCWs than those in males, with a slope difference of 0.030 (p < .05) at all post-vaccination intervals considered. This indicates that antibody levels declined more abruptly in women than those in men. Age-stratified analysis of the antibody decrease demonstrated a statistically significant difference between male and female workers in the youngest (20–44 years) age group (Figure 2b; slope difference −0.039, p = .009).
Figure 1.
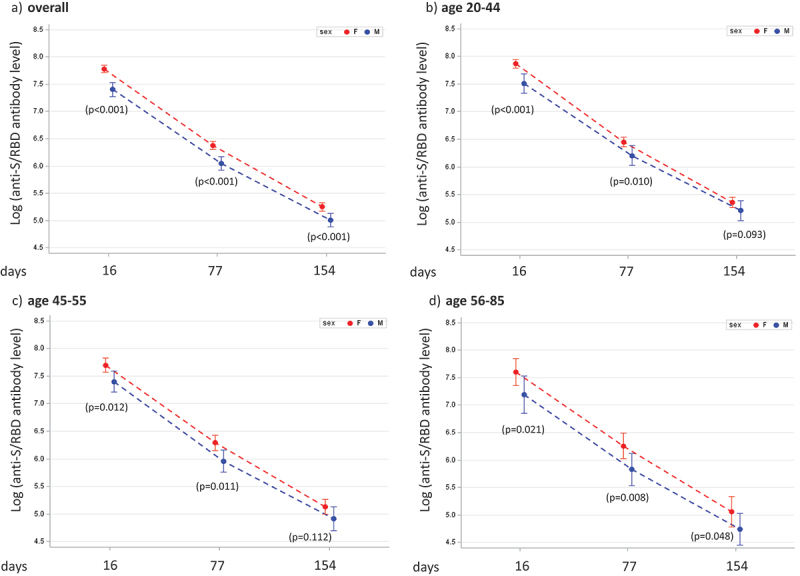
Measurements of anti-S/receptor binding domain (RBD) (by natural log transformed values and 95%CI) in the groups classified based on sex and age: (a) overall; (b) 20–44 years; (c) 45–55 years and (d) 56–85 years; p-values by Wilcoxon – Mann–Whitney test are shown for each post-vaccination time point.
Figure 2.
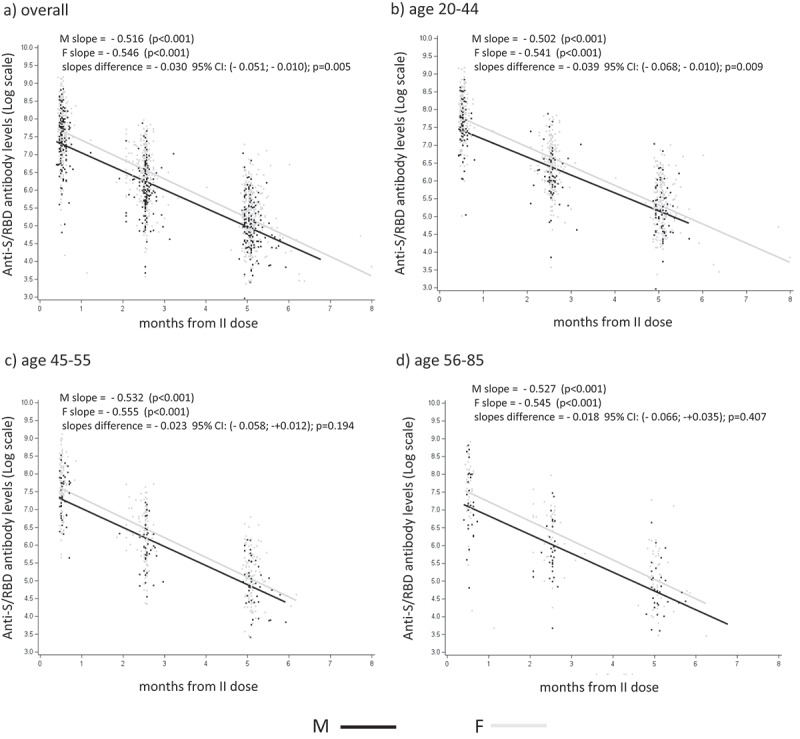
Analysis of sex differences in anti-S/RBD waning. Scatters for the log values of anti-S/RBD antibody levels with the regression lines estimated using mixed regression model for males (M) and females (F), panel (a) all age groups; panel (b) 20–44 years; panel (c) 45–55 years; panel (d) 56–85 years; p-values relative to anti-S/RBD antibody slope differences in M vs F are shown in each panel.
Furthermore, among male HCWs, anti-S/RBD decline was greater in adult age groups (>45 years) than that in the youngest (20–44 years), as indicated by the increasing values of the slopes in Figure 2b–d.
Sex hormones and anti-S/RBD antibody concentrations
Multiple regression analysis was performed to examine the possible association of estradiol, progesterone, and testosterone plasma levels with anti-S/RBD concentrations in a subgroup of 112 HCWs (45 males, 67 females). A significant positive association (beta value= +0.712; p = .014) between testosterone plasma levels and anti-S/RBD concentrations was observed only in male HCWs (Figure 3d). However, both female and male HCWs lacked a linear association between plasma progesterone and estradiol levels and anti-S/RBD concentrations (Figure 3a,b,e,f). These results were also confirmed after adjusting for age in the multiple regression models (Figure S1a – f).
Figure 3.
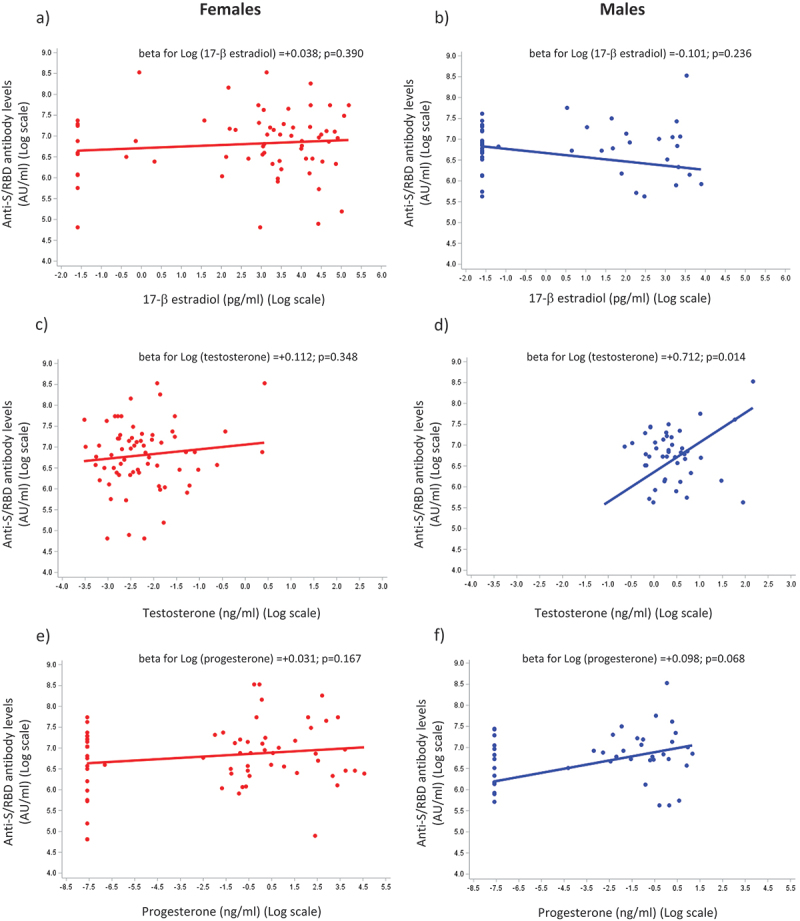
The effect of sex hormones on the anti-S/RBD response to vaccination. Simple regression models for anti-S/RBD antibody levels applied in a subgroup of healthcare workers (HCWs, n = 112) using the log of sex hormone plasma levels as a covariate; 17-β estradiol: a (females) and B (males); testosterone: C (females) and D (males); progesterone: E (females) and F (males); beta estimates (slopes) with relative p-values obtained using regression models are shown in each panel.
Discussion
Vaccination is a crucial strategy to prevent infectious diseases and one of the most cost-effective public and occupational health measure available. The antibody response stimulated by vaccines is affected by several factors, depending on both the individual and the vaccine, which can positively or negatively affect vaccine efficacy and the duration of the antibody response.22 Sex disparity in immune response to microbial antigens and vaccines has been reported earlier, with females displaying stronger innate and adaptive immune responses than those of males.23,24
The aim of the present study was to investigate sex-specific humoral responses to COVID-19 vaccination and the waning of anti-S/RBD antibodies in HCWs. The results demonstrated notable differences in immune responses to the COVID-19 vaccine between males and females, in all age groups. Specifically, female HCWs exhibited significantly higher anti S/RBD antibody concentrations than those in males, suggesting that females generate a more robust antibody response against the spike protein of SARS-CoV-2 than that by males. This finding aligns with previous research highlighting the generally stronger immune response in females than that in males following various vaccinations,15,22,25 including that for COVID-19. In particular, Lasting Y. and coworkers reported lower anti-S measures in male HCWs than those in female HCWs after two doses of COVID-19 vaccine in a large prospective cohort study conducted in Israel.26 Anastassopoulou et al.18 and Yamamoto S. et al.27 highlighted similar sex disparity in anti-S responses among HCWs from Greece and Japan.
Age is an important parameter affecting antibody responses, since T and B cell functions decrease with aging, with consequent insufficient responses to vaccinations.15 Several studies have reported an inverse relationship between age and antibody responses post-vaccination against Hepatitis A and B Viruses, pneumococcus, Tick-Born Encephalitis, tetanus, and SARS-CoV-2.22,28–30 Consistently, our results have indicated that, considering all participants, younger HCWs (20–44 years) developed an average of 1.4 times higher anti-S levels than those among HCWs in the >56 years age group.
An important aspect of the post-vaccination response is the natural tendency of antibody levels to decrease with time. The course of the post-vaccine anti-S response is crucial in the pandemic period, particularly among HCWs, who are highly and repeatedly exposed to the risk of infection. With regard to the post-vaccine antibody concentration, Naaber et al.31 reported that the RBD antibody response decreases six weeks after vaccination compared to that observed one week after vaccination. However, to our knowledge, sex-based differences in waning of the humoral immune responses have not yet been addressed. In this study, our results indicated a significant decrease in the anti-S/RBD levels in all individuals, consistent with previous research.14,31,32 Interestingly, a more abrupt decline in anti-S antibody levels was observed in young female HCWs than those in males, confirming, in part, the observation in Japanese HCWs by Yamamoto S. et al.,27 who, however, did not analyze this effect considering age and sex simultaneously. We cannot plausibly explain the faster decrease of anti-S levels in females than those in males, after the two doses of vaccine.
Despite the waning of antibodies over time, memory B and T cell populations may last longer than those after natural infections,33,34 therefore it is not known whether the reduction in antibody levels corresponds to reduced vaccine protection.
With regard to age, sex disparity in the anti-S antibody decline was statistically significant in the youngest age group; among males HCWs, a faster decline in anti-S/RBD levels was observed in HCWs aged >45 years than those in the youngest age group (20–44 years). These findings indicate a greater decreasing rate in anti-S/RBD levels with age in males than those in females, who displayed a more constant decline in post-vaccination antibodies with age. These findings deserve further investigation to clarify sex- and age-related disparities in COVID-19 vaccine responses, which could provide useful tools for orienting public health decision makers.
In addition to the genetic and epigenetic factors contributing to sex-specific differences in vaccine responses, hormonal variations between males and females may play an important role.
As sex hormone receptors are expressed on immune cells, sex steroid hormones (i.e., 17-β-estradiol, progesterone, and testosterone) modulate humoral and cellular immune responses.35–37 In particular, estrogens are associated with enhanced immune responses, including higher antibody production, whereas progesterone has an anti-inflammatory role.36,38,39 In addition, high levels of testosterone have an anti-inflammatory effect, suppressing the expression of the pro-inflammatory cytokines tumor necrosis factor-α, interleukin (IL)-1β and IL-6, and potentiating the expression of the anti-inflammatory IL-10.40–42 However, the effect of testosterone on acquired immunity is rather controversial, since it has been reported to have an immunosuppressive as well as immunomodulating effect. Regarding its immunosuppressive role, testosterone downregulates T and B cells immune activation.39,42 Potluri T. and coworkers39 (2019) clearly showed that antibody responses specific to influenza H1N1 vaccination inversely correlate with plasma testosterone levels in adult men. Moreover, Furman et al.43 (2018) has reported a subdued antibody response to trivalent influenza vaccination in males with high levels of plasma testosterone. However, different studies analyzing young male populations have shown a positive effect of high plasma testosterone levels on IgG production in response to seasonal trivalent influenza and Hepatitis B vaccinations.44,45
The results of our analysis of sex hormones in HCWs indicated a significant association between high plasma levels of testosterone with high anti-S/RBD plasma concentration in male HCWs, suggestive of a positive immunomodulatory effect of testosterone. Notably, it is not possible to predict how testosterone influences anti-S/RBD antibodies production, and more generally, why this hormone exerts contrasting effects on the immune system. A possible explanation could be its local conversion by immune cells to estrogenic metabolites, or a different pattern distribution of androgen receptors on immune cells.46–48 However, further studies are necessary to evaluate whether the observed association between high plasma testosterone levels and high anti-S/RBD serum concentrations in males is a direct effect of this hormone or it is an epiphenomenon due to other factors playing a role in humoral immune responses. Nevertheless, our data support the role of testosterone as a sex-specific marker to predict the response to the COVID-19 vaccine in men.
However, we did not find any significant association between plasma levels of estradiol and progesterone and anti-S concentrations, both, in male and female HCWs.
In summary, the novelty of this study lies in investigating the mechanisms underlying sex disparity in humoral response to the COVID-19 vaccine based on the hormonal milieu, highlighting the potential immunomodulatory rather than immunosuppressive function of testosterone.
This study had some limitations. In particular, the sample size for the hormonal analysis was relatively small, and the findings should be validated in larger cohorts. Additionally, other clinical variables, such as comorbidities and ongoing therapies, have not been thoroughly evaluated, which could potentially confound the observed sex-specific differences. Finally, the study population mainly consisted of HCWs who were relatively younger than the general Italian population, potentially reducing the extent of the results on a general scale.
In conclusion, disparities in the humoral immune response to COVID-19 vaccination and waning of antibody levels are, at least partly, affected by age and sex. The contribution of sex hormones to the modulation of the sex-specific response to vaccination deserves further investigation. The observed sex-specific differences in vaccine responses highlight the importance of considering sex as a biological variable in medical research.
Finally, our findings have broad implications for COVID-19 vaccination: male-female differences in vaccination responses should be taken into account to optimize and customize health surveillance programs for HCWs.
Supplementary Material
Acknowledgments
The authors thank Fabiola Diamanti and Daniela Diamanti for their technical support.
Funding Statement
The study was supported by BRIC-INAIL ID 27 “Study of organizational, immunological and gender aspects for the prevention, diagnosis, surveillance and management of occupational biological risks in healthcare personnel: an integrated and personalized approach” - FASC. AB39, to AR, VP, SZ.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors contributions
SA performed the experiments, analyzed the results, and contributed in drafting the manuscript; EI performed the experiments and analyzed the results; MD and FC conducted statistical analyses; MD drafted the statistical methods and results; RRDP and MRV provided data banks of HCWs; SZ and RC coordinated the collection of plasma samples and anamnestic data from HCWs; VP coordinated and collected plasma samples and anamnestic data from HCWs; KM, FM, FC performed anti-S/RBD tests; NV contributed to data analysis and drafting the manuscript; EO contributed to interpretation and discussion of sex hormones results; AR conceptualized the study, coordinated experimental activities, and drafted the manuscript; PT coordinated the project and contributed to the final draft. All the authors have read and agreed to the final version of the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2273697.
References
- 1.World Health Organization . Health and care worker deaths during COVID-19. Geneva (CH); 2021 Oct 20 [accessed May 11 2023]. https://www.who.int/news/item/20-10-2021-health-and-care-worker-deaths-during-covid-19. [Google Scholar]
- 2.World Health Organization . WHO and partners call for action to better protect health and care workers from COVID-19. Geneva (CH); 2021 Oct 21 [accessed May 11 2023]. https://www.who.int/news/item/21-10-2021-who-and-partners-call-for-action-to-better-protect-health-and-care-workers-from-covid-19. [Google Scholar]
- 3.Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, Meyers LA, Neuzil KM, Langley JM, Fitzpatrick MC, et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021;73(12):2257–9. doi: 10.1093/cid/ciab079. PMID: 33515252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC.. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–302. doi: 10.1016/S1473-3099(22)00320-6. PMID: 35753318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat Rev Microbiol. 2021;19(11):685–700. doi: 10.1038/s41579-021-00630-8. PMID: 34535791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min L, Sun Q. Antibodies and vaccines target RBD of SARS-CoV-2. Front Mol Biosci. 2021;8:671633. doi: 10.3389/fmolb.2021.671633. PMID: 33968996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27(7):1147–8. doi: 10.1038/s41591-021-01432-4. PMID: 34239135. [DOI] [PubMed] [Google Scholar]
- 8.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi: 10.1038/s41591-021-01377-8. PMID: 34002089. [DOI] [PubMed] [Google Scholar]
- 9.Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, Indenbaum V, Mandelboim M, Doolman R, Amit S, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. PMID: 34224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A covid-19 milestone attained - a correlate of protection for vaccines. N Engl J Med. 2022;387(24):2203–6. doi: 10.1056/NEJMp2211314. PMID: 36507702. [DOI] [PubMed] [Google Scholar]
- 11.Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, Dull P, Plotkin SA. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–8. doi: 10.1016/j.vaccine.2021.05.063. PMID: 34210573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo Sasso B, Agnello L, Giglio RV, Gambino CM, Ciaccio AM, Vidali M, Ciaccio M. Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT162b2 vaccine. Sci Rep. 2022;12(1):8679. doi: 10.1038/s41598-022-12750-z. PMID: 35606426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford KHD, Dingens AS, Eguia R, Wolf CR, Wilcox N, Logue JK, Shuey K, Casto AM, Fiala B, Wrenn S, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;223(2):197–205. doi: 10.1093/infdis/jiaa618. PMID: 33535236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jo DH, Minn D, Lim J, Lee KD, Kang YM, Choe KW, Kim KN. Rapidly declining SARS-CoV-2 antibody titers within 4 months after BNT162b2 vaccination. Vaccines (Basel). 2021;9(10):1145. doi: 10.3390/vaccines9101145. PMID: 34696253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and Gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33(1):577–99. doi: 10.1146/annurev-cellbio-100616-060718. PMID: 28992436. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen H, Klein SL. Sex differences in immunity to viral infections. Front Immunol. 2021;12:720952. doi: 10.3389/fimmu.2021.720952. PMID: 34531867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciarambino T, Barbagelata E, Corbi G, Ambrosino I, Politi C, Lavalle F, Ruggieri A, Moretti A. Gender differences in vaccine therapy: where are we in COVID-19 pandemic? Monaldi Arch Chest Dis. 2021;91(4). doi: 10.4081/monaldi.2021.1669. PMID: 33840183. [DOI] [PubMed] [Google Scholar]
- 18.Anastassopoulou C, Antoni D, Manoussopoulos Y, Stefanou P, Argyropoulou S, Vrioni G, Tsakris A, Fast PE. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: a mixed effects model across two vaccination periods. PloS One. 2022;17(4):e0266958. doi: 10.1371/journal.pone.0266958. PMID: 35486622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward H, Whitaker M, Flower B, Tang SN, Atchison C, Darzi A, Donnelly CA, Cann A, Diggle PJ, Ashby D, et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat Commun. 2022;13(1):907. doi: 10.1038/s41467-022-28527-x. PMID: 35173150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen A, Stromme M, Moyassari S, Chadha AS, Tartaglia MC, Szoeke C, Ferretti MT. COVID-19 vaccines: considering sex differences in efficacy and safety. Contemp Clin Trials. 2022;115:106700. doi: 10.1016/j.cct.2022.106700. PMID: 35149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapira G, Abu Hamad R, Weiner C, Rainy N, Sorek-Abramovich R, Benveniste-Levkovitz P, Rock R, Avnat E, Levtzion-Korach O, Bar Chaim A, et al. Population differences in antibody response to SARS-CoV-2 infection and BNT162b2 vaccination. FASEB J. 2022;36(4):e22223. doi: 10.1096/fj.202101492R. PMID: 35239233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2):e00084–18. doi: 10.1128/CMR.00084-18. PMID: 30867162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fathi A, Addo MM, Dahlke C. Sex differences in immunity: implications for the development of novel vaccines against emerging pathogens. Front Immunol. 2021;11:601170. doi: 10.3389/fimmu.2020.601170. PMID: 33488596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41(2):239–49. doi: 10.1007/s00281-018-0726-5. PMID: 30547182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchevsky NG, Li G, Aley P, Costa Clemens SA, Barrett JR, Belij-Rammerstorfer S, Bibi S, Clutterbuck E, Dold C, Felle S, et al. An exploratory analysis of the response to ChAdOx1 nCoV-19 (AZD1222) vaccine in males and females. EBioMedicine. 2022;81:104128. doi: 10.1016/j.ebiom.2022.104128. PMID: 35779491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lustig Y, Gonen T, Meltzer L, Gilboa M, Indenbaum V, Cohen C, Amit S, Jaber H, Doolman R, Asraf K, et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat Immunol. 2022;23(6):940–6. doi: 10.1038/s41590-022-01212-3. PMID: 35534723. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Oshiro Y, Inamura N, Nemoto T, Horii K, Okudera K, Konishi M, Ozeki M, Mizoue T, Sugiyama H, et al. Durability and determinants of anti-SARS-CoV-2 spike antibodies following the second and third doses of mRNA COVID-19 vaccine. Clin Microbiol Infect. 2023;29(9):e1201.1–e15. doi: 10.1016/j.cmi.2023.05.020. PMID: 37236545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73(11):2065–72. doi: 10.1093/cid/ciab381. PMID: 33906236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman J, Thakur N, Peacock TP, Bialy D, Elrefaey AME, Bogaardt C, Horton DL, Ho S, Kankeyan T, Carr C, et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nature Microbiology. 2022;7(8):1180–8. doi: 10.1038/s41564-022-01163-3. PMID: 35836002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hägg S, Religa D. COVID vaccination in older adults. Nature Microbiology. 2022;7(8):1106–7. doi: 10.1038/s41564-022-01166-0. PMID: 35836001. [DOI] [PubMed] [Google Scholar]
- 31.Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, Haljasmägi L, Rumm AP, Maruste R, Kärner J, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. PMID: 34514454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. PMID: 34614326; PMCID: PMC8522797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan HX, Lee WS, Wragg KM, Kelly HG, Esterbauer R, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12(1):1162. doi: 10.1038/s41467-021-21444-5. PMID: 33608522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terreri S, Piano Mortari E, Vinci MR, Russo C, Alteri C, Albano C, Colavita F, Gramigna G, Agrati C, Linardos G, et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host & Microbe. 2022;30(3):400–8.e4. doi: 10.1016/j.chom.2022.01.003. PMID: 35134333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi T, Iwasaki A. Sex differences in immune responses. Sci. 2021;371(6527):347–8. doi: 10.1126/science.abe7199. PMID: 33479140. [DOI] [PubMed] [Google Scholar]
- 36.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. doi: 10.1038/nri.2016.90. PMID: 27546235. [DOI] [PubMed] [Google Scholar]
- 37.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–21. doi: 10.1111/acel.12326. PMID: 25720438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harding AT, Heaton NS. The impact of estrogens and their receptors on immunity and inflammation during infection. Cancers Basel. 2022;14(4):909. doi: 10.3390/cancers14040909. PMID: 35205657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potluri T, Fink AL, Sylvia KE, Dhakal S, Vermillion MS, Vom Steeg L, Deshpande S, Narasimhan H, Klein SL. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. 2019;4(1):29. doi: 10.1038/s41541-019-0124-6. PMID: 31312529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupuis ML, Maselli A, Pagano MT, Pierdominici M, Ortona E. Immune response and autoimmune diseases: a matter of sex. Ital J Gender-Specific Med. 2019;5(1):11–20. doi: 10.1723/3148.31294. [DOI] [Google Scholar]
- 41.Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129–40. doi: 10.1080/13685538.2018.1482487. PMID: 29925283. [DOI] [PubMed] [Google Scholar]
- 42.Gubbels Bupp MR, Jorgensen TN. Androgen-Induced Immunosuppression. Front Immunol. 2018;9:794. doi: 10.3389/fimmu.2018.00794. PMID: 29755457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111(2):869–74. doi: 10.1073/pnas.1321060111. PMID: 24367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowak J, Pawłowski B, Borkowska B, Augustyniak D, Drulis-Kawa Z. No evidence for the immunocompetence handicap hypothesis in male humans. Sci Rep. 2018;8(1):7392. doi: 10.1038/s41598-018-25694-0. PMID: 29743556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rantala MJ, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nat Commun. 2012;3(1):694. doi: 10.1038/ncomms1696. PMID: 22353724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vottero A, Rochira V, Capelletti M, Viani I, Zirilli L, Neri TM, Carani C, Bernasconi S, Ghizzoni L. Aromatase is differentially expressed in peripheral blood leukocytes from children, and adult female and male subjects. Eur J Endocrinol. 2006;154(3):425–31. doi: 10.1530/eje.1.02102. PMID: 16498056. [DOI] [PubMed] [Google Scholar]
- 47.Surman SL, Jones BG, Penkert RR, Sealy RE, Marion T, Thomas PG, Neale G, Xu B, Hurwitz JL. How estrogen, testosterone, and sex differences influence serum immunoglobulin isotype patterns in mice and humans. Viruses. 2023;15(2):482. doi: 10.3390/v15020482. PMID: 36851695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butts CL, Jones YL, Lim JK, Salter CE, Belyavskaya E, Sternberg EM. Tissue expression of steroid hormone receptors is associated with differential immune responsiveness. Brain Behav Immun. 2011;25(5):1000–7. doi: 10.1016/j.bbi.2010.11.003. PMID: 21074604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


