Abstract
Background & Aims
Functional cure is achieved by a limited number of patients with chronic hepatitis B (CHB) after nucleotide analogue(s) and interferon treatment. It is urgent to develop therapies that can help a larger proportion of patients achieve functional cure. The present study was designed to explore the anti–hepatitis B virus (HBV) potency of interleukin-6 family cytokines and to characterize the underlying mechanisms of the cytokine displaying the highest anti-HBV potency.
Methods
HBV-infected cells were used to screened the anti-HBV potency of interleukin-6 family cytokines. The concentration of oncostatin M (OSM) in patients with chronic HBV infection was examined by enzyme-linked immunosorbent assay. The underlying mechanism of OSM anti-HBV was explored through RNA-seq. C57BL/6 mice injected with rAAV8-1.3HBV were used to explore the suppression effect of OSM on HBV in vivo.
Results
OSM is the most effective of the interleukin-6 family cytokines for suppression of HBV replication (percentage of average inhibition: hepatitis B surface antigen, 34.44%; hepatitis B e antigen, 32.52%; HBV DNA, 61.57%). Hepatitis B e antigen–positive CHB patients with high OSM levels had lower hepatitis B surface antigen and hepatitis B e antigen than those with low levels. OSM activated JAK-STAT signaling pathway promoting the formation of STAT1-IRF9 transcription factor complex. Following this, OSM increased the expression of various genes with known functions in innate and adaptive immunity, which was higher expression in patients with CHB in immune clearance phase than in immune tolerance phase (data from GEO: GSE65359). Interferon-induced transmembrane protein 1, one of the most differentially expressed genes, was identified as an HBV restriction factor involved in OSM-mediated anti-HBV effect. In vivo, we also found OSM significantly inhibited HBV replication and induced expression of antiviral effector interferon-induced transmembrane protein 1.
Conclusions
Our study shows that OSM remodels the immune response against HBV and exerts potent anti-HBV activity, supporting its further development as a potential therapy for treating CHB.
Keywords: Interleukin-6 Family Cytokines, Oncostatin M, Hepatitis B Virus, JAK-STAT Signaling, Interferon-Induced Transmembrane Protein 1
Graphical abstract
Summary.
OSM exerts higher potency in suppression of HBV replication than other IL6 family cytokines. OSM activates JAK-STAT signaling and then promotes antiviral effectors expression to remodel antiviral response. IFITM1, identified as an HBV restriction factor, involves OSM-mediated anti-HBV effect.
Hepatitis B virus (HBV) infection, a serious public health problem, causes acute or chronic hepatitis, cirrhosis, and hepatocellular carcinoma. More than 296 million individuals are affected by chronic HBV infection worldwide.1 Complete eradication of HBV in patients with chronic hepatitis B (CHB) is rarely achieved, because of the persistence of covalently closed circular DNA (cccDNA). Functional cure characterized by hepatitis B surface antigen (HBsAg) seroconversion and sustained silencing of cccDNA has been considered as an ideal end point.2 Currently, nucleotide(s) analogues and interferon (IFN)-α are commonly used to treat chronic HBV infection; these efficiently control viral replication. However, only 1% of patients with CHB can achieve a functional cure after treatment with nucleotide(s) analogues, and only 3%–8% of patients with CHB after treatment with Peg-IFN-α.3 It is urgent to develop new drugs and evaluate their effects to cure HBV infection.
Reservoirs for HBV replication,4 high viral burden, and immune defects5,6 are obstinate barriers to HBV clearance. The International Coalition to Eliminate HBV proposes HBV cure strategies, namely curing of HBV infection without killing infected cells and inducing immune control to safely eliminate infected cells.2 Several novel antivirals therapies have been proposed and have shown promise in clinical trials. ARB-1467, a small interfering RNA, cleaves target HBV mRNAs to interfere with HBV DNA replication and production of HBV virions.7 GS9620, an innate immunity activator, enhances host antiviral immunity and leads to sustained decreases in serum HBV DNA and HBsAg.8 Moreover, cytokines with potential direct antivirus and immunomodulatory activity are expected to be candidate for the development of new therapies. For example, interleukin (IL)2 restores HBV-specific CD8+ T-cell responses in nonresponder patients after IFN-α therapy to improved clinical outcomes including hepatitis B e antigen (HBeAg) seroconversion.9
IL6 family cytokines share a common pleiotropic signaling receptor subunit gp130, which engages several signaling pathways (eg, JAK-STAT, MAPK, and PI3K-Akt). These cytokines have multiple physiological functions,10 involved in metabolism, inflammation, and liver regeneration. Mounting evidence suggests that these cytokines have protective effects against infections.11, 12, 13 In HBV-infected hepatocytes, IL6 has been shown to represses HBV replication by inhibiting taurocholic acid cotransport polypeptide (NTCP)-mediated viral entry and hepatocyte nuclear factor 4α (HNF4α)-mediated cccDNA transcription.11,14 However, the effect of other IL6 family cytokines on HBV replication has remained elusive. OSM, IL6 family cytokine, was reported to synergize with IFN-α in the inhibition of hepatitis A and C virus replication in Huh7, a hepatic carcinoma cell.15 OSM treatment upregulated the expression of multiple antiviral genes, antigen processing and presentation genes, and immunoregulatory genes and triggered expansion of the CD8+ T cell population (through IL7 and IL15Rα).16 These results question whether and how OSM could affect HBV replication.
To explore this question, we first screened the anti-HBV potency of IL6 family cytokines and found that OSM is the most effective among the examined cytokines for suppression of HBV replication. Second, we explored the underlying mechanisms of the anti-HBV effect mediated by OSM. We identified OSM-activated JAK-STAT signaling pathway and increased antiviral effectors and immunostimulatory factors expression, which make contribution to HBV immune clearance. IFN-induced transmembrane protein 1 (IFITM1) functions as an HBV restriction factor that participated in OSM anti-HBV activity. Our study identified OSM as a cytokine with promising therapeutic potential for CHB.
Results
OSM Exerts the Potent Anti-HBV Activity More Than Other Interleukin-6 Family Cytokines
The effect of IL6 on suppression HBV replication has been demonstrated,14 but the effect of other IL6 family cytokines on HBV remains unknown. Thus, we screened the anti-HBV potency of IL6 family cytokines available from biotechnology companies, including IL6, leukemia inhibitory factor (LIF), OSM, ciliary neurotrophic factor (CNTF), cardiotrophin-1, IL11, and IL31.17 In stably transformed cell lines, HepG2.2.15 and HepAD38 cells, OSM, IL6, CNTF, and IL31 significantly reduced the levels of HBeAg (Figure 1A and C). In transient transfection system, Huh7-1.3 cells, all of IL6 family cytokines significantly reduced the levels of HBV DNA (Figure 1B). Notably, in 3 types of HBV-expressed hepatoma cells, OSM exerts the potent anti-HBV activity than other IL6 family cytokines (Figure 1A-C). The percentage of average inhibition for OSM on HBsAg, HBeAg, and HBV DNA in 3 types of HBV-expressed hepatoma cells were 34.44%, 32.52%, and 61.57%, respectively.
Figure 1.
OSM is the most effective among the examined IL6 family cytokines for suppression of HBV replication. (A) HepG2.2.15, (B) Huh7-1.3, and (C) HepAD38 cells were treated with IL6, LIF, OSM, CNTF, cardiotrophin-1 (CT-1), IL11, or IL31 at a concentration of 20 ng/mL for 72 hours. The levels of extracellular HBsAg, HBeAg, and HBV DNA were quantitated. One-way analysis of variance. (D) Quantitation of OSM concentration by enzyme-linked immunosorbent assay in serum from health control subjects and patients with chronic HBV infection in different phases. Kruskal-Wallis test. (E) Serum OSM, HBsAg, and HBeAg levels in HBeAg-positive CHB patients. Mann-Whitney. HC, health control; I, HBeAg-positive chronic HBV infection; II, HBeAg-positive CHB; III, HBeAg-negative chronic HBV infection; IV, HBeAg-negative CHB. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. PBS, phosphate-buffered saline.
We subsequently explored and compared the serum OSM concentrations in the course of chronic HBV infection. Compared with healthy control subjects, the serum OSM concentration was significantly decreased in the HBeAg-positive CHB patients (Figure 1D). The levels of HBsAg and HBeAg in HBeAg-positive CHB patients with the high serum OSM levels were lower than those in HBeAg-positive CHB patients with the low serum OSM levels (Figure 1E). These findings reveal the potential of OSM to suppress HBV replication.
OSM Inhibits HBV Replication Without Killing Infected Cells
To investigate how OSM affects HBV replication, we first exposed HBV-expressing cell lines to different concentrations of OSM. Regardless of dose, OSM effectively suppressed HBsAg, HBeAg, and HBV DNA secretion, doing so in a noncytopathic manner (Figure 2A-C). As the dose-response curves show, OSM exhibited significant anti-HBV activity at a concentration of 20 ng/mL. cccDNA is the stable form of HBV DNA within the nucleus, and HBV pgRNA and HBcAg are identified as ideal biomarkers reflecting HBV genome transcription activity.18 We then investigated the intracellular HBV RNAs and antigens levels after OSM treatment. OSM treatment led to significantly decreased levels of HBV transcripts (pgRNA and total RNA) and of viral antigens (HBsAg and HBcAg) (Figure 2D and E). The OSM effect on inhibition HBV replication was also verified in HepG2-NTCP cells naturally infected with HBV (Figure 2F-H). These results indicated that OSM can inhibit HBV replication in a noncytopathic manner.
Figure 2.
OSM suppresses HBV replication in a noncytopathic manner. (A-C) The levels of extracellular HBsAg, HBeAg, and HBV DNA, as well as cell viability were examined in HepG2.2.15, Huh7-1.3, and HepAD38 cells treated with different doses of OSM for 3 days (0, 5, 10, 20, 50, 100 ng/mL). One-way analysis of variance. (D) RNA extracted from HepG2.2.15 and Huh7-1.3 cells treated with OSM (20 ng/mL) for 24 hours was assessed for the levels of intracellular HBV RNAs with quantitative polymerase chain reaction. Unpaired t test. (E) Protein extracted from HepG2.2.15 and Huh7-1.3 cells treated with OSM for 48 hours were subjected to immunoblotting with antibodies for the indicated intracellular viral antigens. Relative gray values of HBsAg and HBcAg were normalized by β-actin. (F) Scheme outlining the HBV infection experiments. (G) The levels of extracellular HBsAg, HBeAg, and HBV DNA, as well as cell viability were examined in HepG2-NTCP cells naturally infected with HBV and treated with different doses of OSM for 3 days (0, 5, 10, 20, 50, 100 ng/mL). One-way analysis of variance. (H) RNA and protein samples from HepG2-NTCP cells naturally infected with HBV and then treated with OSM were analyzed for the levels of intracellular HBV RNAs and viral antigens. Unpaired t test. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. PBS, phosphate-buffered saline.
OSM Remodels the Immune Response Against HBV of Hepatocytes
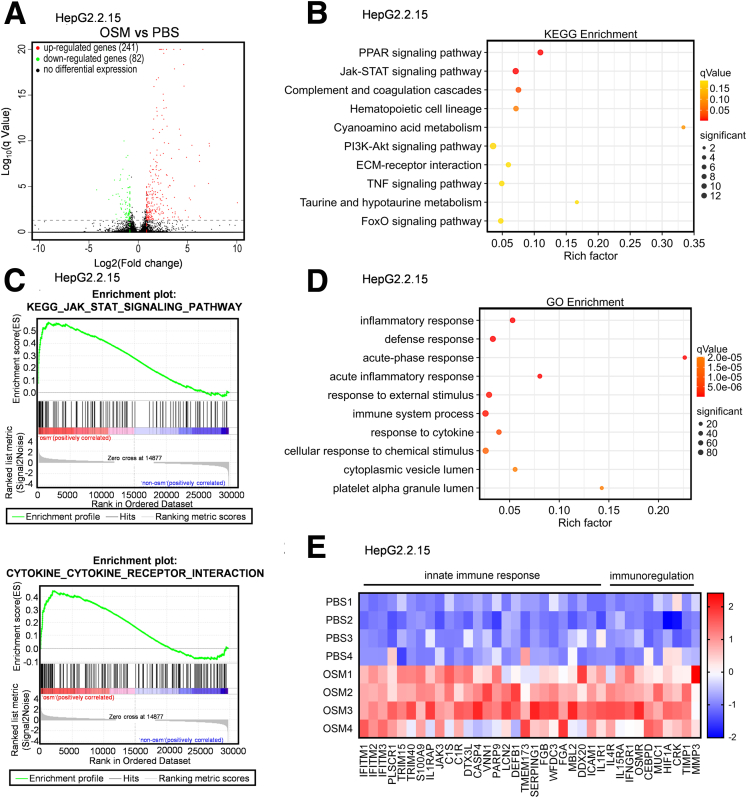
To explore the mechanisms underlying the observed OSM-mediated suppression of HBV replication, we performed RNA sequencing of HepG2.2.15 cells treated with OSM. OSM treatment resulted in upregulation of 241 genes and in downregulation of 82 genes (Figure 3A). Access to the detailed RNA-seq data is available in Supplementary Table 1. The Kyoto Encyclopedia of Genes and Genomes and Gene Set Enrichment Analysis indicated that the OSM-induced genes were enriched for the JAK-STAT signaling pathway (Figure 3B and C, Table 1), which is known to function in IFN-α activating innate immune responses against viruses.19 The Gene Ontology analysis indicated that the differentially expressed genes were enriched for functional annotations associated with terms including “inflammatory response,” “defense response,” and “immune system process” (Figure 3D). We generated a list of the significantly upregulated genes with known functions in innate and adaptive immunity (Figure 3E). To evaluate the potential anti-HBV functional roles of these differentially expressed genes, we also analyzed a published dataset from human liver biopsy (GEO: GSE65359) and found that a lot of these genes were higher expression in patients with CHB in immune clearance phase than in immune tolerance phase (Figure 4A). These results suggest that OSM is able to facilitate HBV immune clearance for HBV-infected cells.
Figure 3.
OSM elicits multiple signaling pathways and biologic process. (A) HepG2.2.15 cells were mock-treated or treated with OSM for 24 hours. Total RNA of the cells was extracted for RNA-seq analysis. The volcano plots show differentially expressed genes in the HepG2.2.15 cells treated with OSM. (qValue ≤ .05, Foldchange >1.8) (B) Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment of the differentially expressed genes was used to predicate OSM-activated signaling pathway. (C) Gene Set Enrichment Analysis of the transcription profile was used to predicate OSM-activated signaling pathway. (D) Gene Ontology (GO) enrichment of the differentially expressed genes was used to predicate OSM-activated biologic processes. (E) The heat map shows different expressed genes with known functions in innate and adaptive immunity.
Table 1.
Gene Set Enrichment Analysis of the Transcription Profile
| Name | Size | ES | NES | NOM p-val | FDR q-val | FWER p-val |
|---|---|---|---|---|---|---|
| JAK_STAT_SIGNALING_PATHWAY | 111 | 0.5695844 | 2.202358 | 0 | 0 | 0 |
| COMPLEMENT_AND_COAGULATION_CASCADES | 61 | 0.61601466 | 2.165244 | 0 | 6.04E-04 | 0.001 |
| SYSTEMIC_LUPUS_ERYTHEMATOSUS | 35 | 0.5853503 | 1.860786 | 0.003552398 | 0.028592726 | 0.073 |
| DORSO_VENTRAL_AXIS_FORMATION | 20 | 0.66275966 | 1.81925 | 0.001901141 | 0.03405774 | 0.112 |
| CYTOKINE_CYTOKINE_RECEPTOR_INTERACTION | 172 | 0.4438064 | 1.817586 | 0 | 0.02798018 | 0.115 |
| BASAL_TRANSCRIPTION_FACTORS | 34 | 0.5685683 | 1.778937 | 0.003508772 | 0.03876292 | 0.183 |
| PROTEASOME | 44 | 0.539104 | 1.769257 | 0 | 0.036826856 | 0.197 |
| TYPE_II_DIABETES_MELLITUS | 40 | 0.5387349 | 1.761326 | 0.001834862 | 0.03544926 | 0.216 |
| N_GLYCAN_BIOSYNTHESIS | 46 | 0.51554537 | 1.728825 | 0.001798561 | 0.043714907 | 0.29 |
| INSULIN_SIGNALING_PATHWAY | 125 | 0.43912852 | 1.726543 | 0 | 0.04005217 | 0.294 |
| LEISHMANIA_INFECTION | 55 | 0.49606344 | 1.722884 | 0.003663004 | 0.037348606 | 0.299 |
| DILATED_CARDIOMYOPATHY | 71 | 0.47831494 | 1.711091 | 0.001694915 | 0.04011772 | 0.333 |
| PROTEIN_EXPORT | 24 | 0.58278537 | 1.691659 | 0.005597015 | 0.04430231 | 0.379 |
| ADIPOCYTOKINE_SIGNALING_PATHWAY | 59 | 0.4717128 | 1.688701 | 0.005102041 | 0.042596214 | 0.389 |
| O_GLYCAN_BIOSYNTHESIS | 21 | 0.5932059 | 1.687495 | 0.006734007 | 0.03983155 | 0.39 |
ES, Enrichment Score; FDR, False Discovery Rate; FWER, Family-Wise Error Rate; NES, Normalized Enrichment Score; NOM, Nominal p-value.
Figure 4.
OSM-upregulated genes involve in HBV immune clearance. (A) The transcriptomes of patients with CHB in immune-tolerant phase and in immune-clearance phase liver tissue from a published dataset (GEO: GSE65359) were analyzed. The heat map shows the landscape of OSM-upregulated genes implicated in activation of innate immunity. (B) The expression of these genes between patients with CHB in immune tolerant phase and in immune clearance. Benjamini-Hochberg. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
OSM Elicits JAK-STAT Signaling to Suppress HBV Replication
To determinate if OSM inhibits HBV replication through JAK-STAT signaling, we performed immunoblotting against STATs proteins in HBV-infected cells. As shown in Figure 5A, STAT1, STAT3, and STAT5 were significantly phosphorylated at 10 and 30 minutes after OSM treatment. Next, we used ruxolitinib to block STAT1 and STAT5 phosphorylation, and used AG490 to block STAT3 phosphorylation (Figure 5B). The inhibitory effect of OSM on HBsAg and HBV DNA secretion was almost abolished when STAT1 and STAT5 phosphorylation were abolished, whereas no attenuation was observed on inhibition of STAT3 phosphorylation (Figure 5C). Moreover, ruxolitinib diminished the effect of OSM on the inhibition of HBV RNAs and antigens (Figure 5D). We also used STAT5-IN to block the interaction of STAT5 and its target genes, then the inhibitory effect of OSM on HBV was slight attenuation (Figure 5E). These results suggested an important role of STAT1 in OSM eliciting the anti-HBV response. Finally, we found that OSM induced the expression of IRF1, IRF3, and IRF9, and immunofluorescence demonstrates the upregulated expression of IRF9 was primarily located in nucleus (Figure 5F). Co-IP assays with an anti-STAT1 antibody revealed that OSM facilitated the interaction between STAT1 and IRF9 (Figure 5G), which could be a transcription factor complex to promote the expression of cytokine-responsive genes.19 Taken together, our results demonstrate that OSM can suppress HBV replication through activating the JAK-STAT signaling pathway.
Figure 5.
OSM suppresses HBV replication through the JAK-STAT signaling pathway. (A) Extracts from HepG2.2.15 cells treated with OSM (20 ng/mL) for 0∼60 minutes were analyzed for STAT activation. (B) HepG2.2.15 cells were pretreated with ruxolitinib (1 μM) and the AG490 (10 μM) for 1 hour and then were treated with OSM (20 ng/mL) for 10 minutes. The phosphorylation levels of STAT1, STAT3, and STAT5 were examined by immunoblotting. (C, E) The supernatant HBsAg and HBV DNA levels were examined in the indicated groups. One-way analysis of variance. (D) The inhibition effects of OSM on intracellular HBV RNAs and HBV antigens were analyzed in HepG2.2.15 cells pretreated with or without ruxolitinib. Inhibition ratios of OSM for intracellular pgRNA and total RNA for the indicated cell lines were calculated as (1 - OSM-treatment/sham-treatment) ×100. Relative gray values of HBsAg and HBcAg were analyzed by Image J and normalized to β-actin. Unpaired t test. (F) The expression levels of IRFs in HepG2.2.15 cells treated with OSM were examined by quantitative polymerase chain reaction and immunoblotting. Unpaired t test. (G) STAT1-IRF9 heterodimer formation was examined by coimmunoprecipitation in Huh7-1.3 cells treated with OSM. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
IFITM1 is an HBV Restriction Factor Participating in the Anti-HBV Effect Mediated by OSM
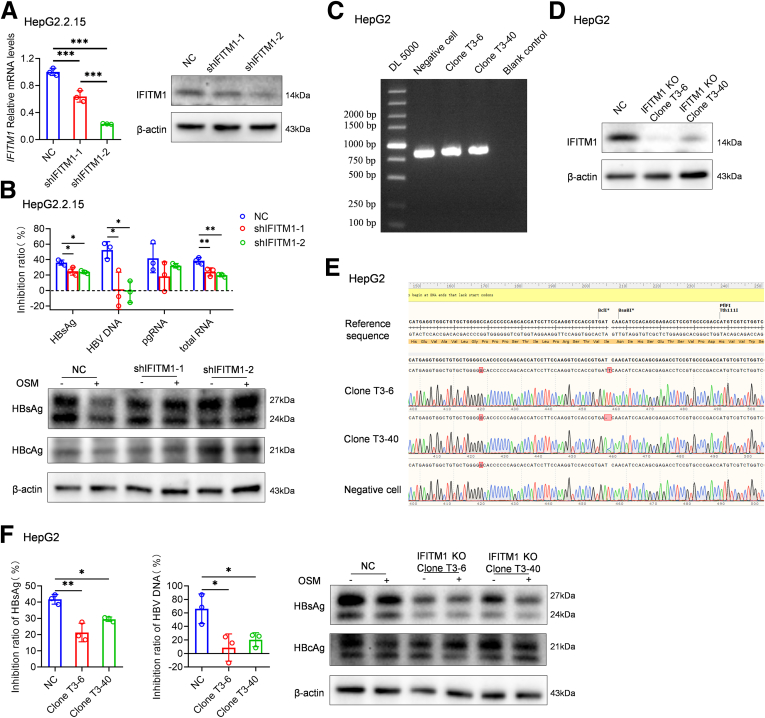
A subset of genes, including IFITMs, tripartite motif containing proteins (TRIMs), and S100 Calcium Binding Protein A9 (S100A9), were significant upregulated by OSM (Figure 3E). The expression of numbers of OSM-upregulated genes was further confirmed by quantitative polymerase chain reaction and immunoblotting (Figures 6A and 7A). In dataset GSE65359, lots of these genes were also highly expressed in patients in the immune clearance phase, such as IFITM1, TRIM15, and S100A9 (Figures 6B and 4B). Then we used ruxolitinib and AG490 to selectively block JAK-STAT signaling. Consistent with the inhibitory effect of OSM on HBV replication, the effect of OSM on the expressions of these genes was significantly reduced by ruxolitinib (Figures 6C and 7B); no impact was detected for AG490 (Figure 7C). These results further demonstrate that the anti-HBV activity mediated by OSM is dependent on these antiviral effectors.
Figure 6.
OSM induces the anti-HBV effector IFITM1 expression. (A) The expression of IFITM1 and IFITM2 in HepG2.2.15 cells at the indicated time points after OSM treatment (20 ng/mL) were measured by quantitative polymerase chain reaction and immunoblotting. Unpaired t test. (B) The expression of IFITM1 and IFITM2 between patients with CHB in immune tolerant phase and in immune clearance phase liver tissue from a published dataset (GEO: GSE65359) was analyzed. Benjamini-Hochberg. (C) OSM-induced IFITM1 and IFITM2 expression were analyzed in HepG2.2.15 cells pretreat with or without ruxolitinib. One-way analysis of variance. (D-F) HepG2-NTCP and HepG2.2.15 cells were transfected with LV-Control or LV-IFITM1. Supernatants were collected to measure extracellular HBsAg, HBeAg, and HBV DNA. RNA and protein samples from cells were used to measure intracellular HBV RNAs and HBV antigens. Unpaired t test. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. PBS, phosphate-buffered saline.
Figure 7.
OSM induces numerus genes with essential functions in innate immunity. (A) The mRNA levels of antiviral effectors in HepG2.2.15 cells at the indicated time points after OSM treatment (20 ng/mL) were measured by quantitative polymerase chain reaction. The protein levels of S100A9, STING, and LCN2 in HepG2.2.15 cells at 48 hours after OSM treatment (20 ng/mL) were measured by immunoblotting. Unpaired t test. (B, C) The expression of antiviral effectors was analyzed by quantitative polymerase chain reaction and immunoblotting in the indicated groups. One-way analysis of variance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
IFITMs have been identified as the important host effector molecules against viruses. IFITM1, IFITM2, and IFITM3 are known to function in a sequential and combined manner to inhibit hepatitis C virus entry.20 The HBV core protein can inhibit expression of IFITM1 rather than IFITM2 and IFITM3.21 Thus, we aimed to investigate the inhibitory effect of IFITM1 on HBV replication through its overexpression. HBV replication parameters, including HBsAg, HBeAg, HBV DNA levels in the supernatant, and intracellular HBV transcripts and viral antigens, were observed to significantly decrease in HepG2-NTCP and HepG2.2.15 cells overexpressing IFITM1 (Figure 6D-F). We also investigated the effects of IFITM1 on the anti-HBV effect of OSM by knockdown and knockout: the knockdown of IFITM1 in HepG2.2.15 cells significantly compromised the inhibitory effect of OSM on HBsAg and HBV DNA secretion, and intracellular HBsAg and HBcAg production (Figure 8A and B). The anti-HBV effect of OSM was also significantly compromised in an IFITM1 knockout HepG2 cell lines (Figure 8C-F). These findings underscore the role of IFITM1 in mediating the anti-HBV effects of OSM.
Figure 8.
IFITM1 involves in OSM-mediated anti-HBV activity. (A) HepG2.2.15 cells were transfected with LV-NC, LV-shIFITM1-1, or LV-shIFITM1-2. IFITM1 levels in the indicated groups were assessed by quantitative polymerase chain reaction and by immunoblotting. (B) The inhibitory effects of OSM on extracellular HBsAg and HBV DNA and on intracellular HBV antigens were analyzed in IFITM1 knockdown cell lines. Inhibition ratios of OSM on HBV replication parameters for the indicated cell lines were calculated as (1 - OSM-treatment/sham-treatment) ×100. (C) CRISPR gene editing technology was used to create IFITM1 knockout HepG2 cells. Genotyping of single IFITM1 knockout HepG2 cell clones. Map of electrophoresis of polymerase chain reaction products. (D) Immunoblot against IFITM1. (E) Alignment of sequencing diagram. (F) The inhibitory effects of OSM on extracellular HBsAg and HBV DNA and intracellular HBV transcripts and antigens were analyzed in IFITM1 knockout cell lines. Inhibition ratios of OSM on extracellular HBsAg and HBV DNA for indicated cell lines were calculated as (1 - OSM-treatment/sham-treatment) ×100. One-way analysis of variance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
OSM Inhibits HBV Replication In Vivo
The persistent HBV replication mouse model was used to validate the anti-HBV activity of OSM in vivo. A total of 12 persistent HBV replication mice were divided into 2 groups, which were respectively subcutaneously injected with 100 μL phosphate-buffered saline or 50 ng rmOSM in 100 μL phosphate-buffered saline daily. Blood and liver samples were collected according to the schedule presented in Figure 9A. Compared with the vehicle control group, the levels of serum HBsAg and HBV DNA were decreased in mice treated with OSM for 2 weeks (Figure 9B). Routine analyses of liver function and hepatic pathology analysis indicated that OSM can suppress HBV replication without inducing hepatotoxicity (Figure 9C and D). The mRNA levels of antiviral effectors Ifitm1 and immunoregulation factors Il-15rα were significantly increased in the livers of OSM-treated mice compared with the control group (Figure 9E). The IFITM1 protein level showed a similar pattern to mRNA expression (Figure 9F). Notably, OSM had no effect on a set of other known antiviral effectors listed in Figure 3E in vivo (Figure 9G). These results further demonstrate that OSM promotes persistent HBV replication mice immune clearance virus.
Figure 9.
Suppression of established HBV infection by OSM in vivo. (A) Schematic model of the study design. (B) The levels of serum HBsAg and HBV DNA in rAAV8-mediated HBV replication mice PBS or OSM treatment groups. (C) The levels of ALT and AST in rAAV8-mediated HBV replication mice treated with PBS or OSM for 2 weeks. (D) Hepatic pathology analysis of rAAV8-mediated HBV replication mice treated PBS or OSM. (E, F) The expression of Ifitm1 and Il-15ra were analyzed in liver of rAAV8-mediated HBV replication mice exposed to OSM. Relative gray value of IFITM1 were analyzed by Image J and normalized to β-actin. Unpaired t test. (G) The mRNA levels of the indicated antiviral effectors were analyzed in livers of rAAV8-mediated HBV replication mice exposed to OSM. Unpaired t test. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. ALT, alanine aminotransferase; AST, aspartate aminotransferase; PBS, phosphate-buffered saline.
Discussion
In this study, we found that IL6 family cytokines have different efficacies in inhibiting HBV replication, and noted that OSM exerts especially high potency as an anti-HBV cytokine. OSM inhibits HBV replication without any hepatotoxicity, both in vitro and in vivo. Moreover, JAK-STAT signaling activity was elicited by OSM. STAT1, STAT3, and STAT5 were activated, and STAT1 combined with IRF9 to form a transcription factor complex. Subsequently, OSM stimulates the expression of relevant components of innate immunity, such as IFITM1, TRIM15, and S100A9, and the expression of the immunomodulatory factors IL15Rα. These relevant components of innate immunity were highly expressed in patients in the immune clearance phase. IL15Rα was reported to restore HBV-specific immune cells activity.16 Taken together, OSM treatment is satisfied to the therapeutic strategies of functional HBV cure to suppress HBV DNA replication, inhibit HBsAg production, and restore host innate and adaptive immune response.22
OSM is a cytokine belonging to the IL6 superfamily, released by activated antigen-presenting cells.15 OSM signals via a heterodimeric receptor composed of the gp130 subunit and either LIF receptor or OSMRβ subunit, which is abundant in cells of hepatocellular lineage.12 OSM is a multifunctional cytokine with known functions in liver regeneration and differentiation, acute-phase response, and lipid metabolism.23, 24, 25 Studies have shown that OSM synergizes with IFN-α in the inhibition of hepatitis A and C virus replication.12,15 Our study first demonstrates the anti-HBV activity of OSM. Compared with other IL6 family cytokines, OSM can suppress HBV replication in transient transfection systems and stably transformed cell lines, especially in inhibitory of HBsAg secretion. LIF and OSM share a common receptor gp130/LIF receptor complex,26 whereas the inhibition of LIF on HBV replication was slight. A key observation in this paper was the inhibitory of OSM on HBV replication in rAAV-8-mediated HBV replication mouse. In the murine system, OSM was reported to bind with high affinity only to the gp130/OSMRβ comple.27 It is thus reasonable to think that OSM exerts anti-HBV effect mainly though gp130/OSMRβ complex.
OSMRβ contains the conserved proline-rich box1 region in the intracellular membrane-proximal region, which can bind with JAK1 and JAK2.28 JAK-STAT signaling is a critical signaling pathway in IFN-mediated inhibition of HBV replication.29 IFN-α signaling uses a complex comprising STAT1, STAT2, and IRF9 to activate the transcription of hundreds of IFN-stimulated genes.30 In this study, we found that OSM triggers the JAK-STAT signaling components STAT1, STAT3, and STAT5. IRF9 is an immunomodulatory transcription factor that mediates the response to IFN-α downstream of the JAK-STAT signaling pathway.31 The increased levels of nuclear IRF9 promoted the transcription of genes under the control of ISRE motifs. Our study reveals that OSM upregulates the expression of IRF9 in the nucleus and promotes interaction of STAT1 and IRF9. STAT1 activation is associated with enhanced expression of several antiviral genes. Moreover, OSMRβ can recruit Shc and activate MAP kinase cascades (ERK, p38, JNK).28 Hosel et al11 reported that MAKP signaling directly downregulates the expression of HNF4α, which is a transcription factor known for regulating HBV transcription. In the present study, we found that OSM decreases the HNF4α expression to suppress HBV replication through the MAPK signaling pathway (Figure 10). It is possible that OSM elicits crosstalk between JAK-STAT and MAPK signaling to suppress HBV replication.
Figure 11.
OSM promotes the expression of genes with known functions in histone modifications. (A) The expression of genes associated with histone modification in HepG2.2.15 treated with OSM. Benjamini-Hochberg. (B) The RNA level of MUC1 in HepG2.2.15 treated with OSM. Unpaired t test. ∗∗P < .01. FDR, false discovery rate; PBS, phosphate-buffered saline.
Figure 10.
OSM activates MAKP signaling and suppresses HNF4α expression. (A) The mRNA levels of the indicated transcription factors in HepG2.2.15 cells at 24 hours after OSM treatment (20 ng/mL) were measured by quantitative polymerase chain reaction. The HNF4α and HBx protein levels in HepG2.2.15 cells (48 hours after OSM treatment [20 ng/mL]) were measured by immunoblotting. Unpaired t test. (B) Extracts from HepG2.2.15 cells treated with OSM for 0∼60 minutes were analyzed for ERK and p38 activation by immunoblot. (C, D) HepG2.2.15 cells were pretreated with PD98059 (ERK inhibitor) for 1 hour and were then treated with OSM. The HBsAg and HBV DNA levels in supernatants were examined for the indicated groups. The protein levels of HNF4α and IFITM1, and phosphorylation levels of ERK and were examined by immunoblotting. One-way analysis of variance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
IFITMs are antiviral effector proteins of the innate immune system with functions in host-pathogen interactions.32 IFITM1-3 are capable of blocking the early stages of viral replication and of restricting entry for a broad range of enveloped viruses. IFITM1 is localized to both the plasma membrane and to early endosomes, whereas IFITM2 and IFITM3 predominantly reside in later endosomes and lysosomes.20 HBV infection could downregulate the expression of IFITM1 through HBV core protein interactions with BAF200 even under IFN-α treatment.21 Our study found that IFITM1 makes contributions to OSM inhibitory activities for HBV replication, especially at the viral antigen level. IFITM1 have been known to exert antiviral activities by restriction of virus entry.33 Besides, functional protein association network obtained from the String database indicates that IFITM1 is involved in biologic processes including 2'-5'-oligoadenylate synthetase (OAS) antiviral response and ISG15 antiviral mechanism. These findings inspired us that IFITM1 might also restrict HBV entry into hepatocyte and promote ISG15 and OAS anti-HBV activity.
However, there are several limitations to this study that should be considered in future research seeking to assess the roles of OSM toward functional cure for HBV. Further studies are needed to illuminate the mechanism through which OSM affects (or not) cccDNA stability and/or transcription: MUC1, a significant upregulated factor in HepG2.2.15 cells treated with OSM (Figure 11), has been demonstrated to trigger promoter-specific H3K27 trimethylation through activating EZH2 expression and interacting with EZH2.34 Additionally, Guerrieri’s35 study reported that HBx and DLEU2 corecruitment on cccDNA displaces EZH2 from viral chromatin, thus boosting transcription and viral replication. In the present study, we found that OSM reduced HBx expression (Figure 10A). Therefore, we speculate that OSM may promote EZH2-mediated methylation of cccDNA H3K27 by promoting MUC1 expression and reducing HBx. Further studies are also needed to illuminate the immunoregulatory activity of OSM on HBV-specific or nonspecific immunocytes: given that IL15Rα has been known to be important for expansion of the CD8+ T-cell population,16 these findings have revealed a potential role of OSM in host immune defense against viral infection by promoting the expression of IL15Rα in hepatocytes.
Collectively, our data demonstrate OSM could remodel the innated immune response against HBV of hepatocytes. Besides, OSM may behave as a trigger of adaptive immune responses through stimulating immunoregulator. These findings shed a new light on developing a new therapy for helping more patients with CHB to achieve functional cure.
Materials and Methods
Patients and Healthy Participants
This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Approval No. MRCTA, ECFAH of FMU [2019]052). Written consent was obtained from each participant included in this study. Two hundred and two untreated patients with chronic HBV infection and sixty-four healthy control subjects were recruited from the First Affiliated Hospital of Fujian Medical University (Fujian, China). Patients were diagnosed with chronic HBV infection and classified into 4 phases36: (1) HBeAg-positive chronic HBV infection, (2) HBeAg-positive CHB, (3) HBeAg-negative chronic HBV infection, and (4) HBeAg-negative CHB. The concentration of serum OSM was quantified by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Cell Culture
HepG2.2.15, HepAD38, Huh7, and HepG2 cells were cultured in Dulbecco’s modified Eagle medium (Meilunbio, Liaoning, China) containing 10% fetal bovine serum (Gibco, CA), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Beyotime, Shanghai, China) and placed at 37°C in 5% CO2 humidified incubators. HepG2.2.15 and HepAD38 stably expresses HBV genome. Huh7 cells were transfected with HBV 1.3-mer wild-type replicon (QCHENG BIO, Shanghai, China) using transfection reagent FuGENE HD (Promega, Fitchburg, WI) to generate HBV transfection–based cell culture Huh7-1.3. HBV particles harvested from the culture supernatant of HepAD38 cells were used to infect HepG2-NTCP cells. HBLV-h-IFITM1 shRNA-ZsGreen-PURO (Table 2; Hanbio, Shanghai, China) and LV-IFITM1 (Genechem, Shanghai, China) were used to establish IFITM1 knockdown or overexpression cell lines.
Table 2.
siRNA Sequence for IFITM1
| Gene | Sequence | |
|---|---|---|
| IFITM1 | sh1 | CATCCTGTTACTGGTATTCGGCTCT |
| sh2 | CCTGTTACTGGTATTCGGCTCTGTG |
CRISPR gene editing technology was used to create IFITM1 knockout HepG2 cells. The endogenous IFITM1 gene was targeted and mutated using a transient transfection of RNP containing the gRNA sequence: CTCGCTGTGGATGTTGATCA CGG. Following transfection, the cells were seeded into 96-well plates using limit dilution, resulting in the generation of isogenic single clones. These clones were subsequently selected from the wells and subjected to DNA sequencing for screening, ultimately leading to identification of the isogenic knockout clones. Clone T3-6 exhibited an insertion mutation, whereas Clone T3-40 had a deletion mutation, both of which were chosen for further analysis.
Investigational Drugs
The recombinant human cytokines IL6, IL11, IL31, IL35, LIF, OSM, CNTF, and cardiotrophin-1 were purchased from R&D Systems and Novoprotein (Shanghai, China). AG490 (STAT3 phosphorylation inhibitor), ruxolitinib (JAK1/2 phosphorylation inhibitor), and STAT5-IN (STAT5-DNA binding inhibitor) were manufactured by MedChemExpress (Shanghai, China).
A Recombinant Adeno-Associated Virus Type 8–Mediated HBV Replication Mouse Model
To establish an rAAV8-mediated HBV replication mouse model, recombinant adeno-associated virus type 8 carrying the 1.3-mer wild-type HBV genome (rAAV8-1.3HBV, FivePlus Molecular Medicine Institute, Beijing, China) was intravenous injected into C57BL/6 mice (Weitong Lihua Laboratory Animal Technology, Zhejiang, China) as previously described.37 According to serum HBsAg and HBV DNA levels, HBV replication mice were randomized into different treatment groups and subcutaneously injected with 200 μL phosphate-buffered saline or 250 ng/mL recombinant mouse OSM (rmOSM-CF, R&D Systems) daily for 2 weeks. The mice were bled to monitor the HBsAg, HBeAg, and HBV DNA levels every week. Following this, the mice were sacrificed.
Real-Time Polymerase Chain Reaction and RNA Sequencing
Total RNA was isolated from cells and mice livers by the standard TRIzol method. Real-time reverse transcription polymerase chain reaction was performed as previously described.38 The primers used in this study are shown in Table 3. GAPDH served as the endogenous control in cells samples, whereas β-actin served as the endogenous control in mice samples. The ΔΔCt method was used to analyze quantitative polymerase chain reaction results.
Table 3.
Primer Sequences for Quantitative Polymerase Chain Reaction
| Gene | Primer | |
|---|---|---|
| GAPDH | F | CACATGGCCTCCAAGGAGTAAG |
| R | TGAGGGTCTCTCTCTTCCTCTTGT | |
| HBV pgRNA | F | CTCAATCTCGGGAACCTCAATGT |
| R | TGGATAAAACCTAGCAGGCATAAT | |
| HBV total RNA | F | ATGGCTGCTAGGCTGTGCTGC |
| R | ACGGTGGTCTCCATGCGACG | |
| IRF1 | F | CTGTGCGAGTGTACCGGATG |
| R | ATCCCCACATGACTTCCTCTT | |
| IRF2 | F | CATGCGGCTAGACATGGGTG |
| R | GCTTTCCTGTATGGATTGCCC | |
| IRF3 | F | AGAGGCTCGTGATGGTCAAG |
| R | AGGTCCACAGTATTCTCCAGG | |
| IRF4 | F | GCTGATCGACCAGATCGACAG |
| R | CGGTTGTAGTCCTGCTTGC | |
| IRF5 | F | GGGCTTCAATGGGTCAACG |
| R | GCCTTCGGTGTATTTCCCTG | |
| IRF6 | F | CCCCAGGCACCTATACAGC |
| R | TCCTTCCCACGGTACTGAAAC | |
| IRF7 | F | CCCACGCTATACCATCTACCT |
| R | GATGTCGTCATAGAGGCTGTTG | |
| IRF8 | F | ATGTGTGACCGGAATGGTGG |
| R | AGTCCTGGATACATGCTACTGTC | |
| IRF9 | F | GCCCTACAAGGTGTATCAGTTG |
| R | TGCTGTCGCTTTGATGGTACT | |
| IFITM1 | F | CTTCATAGCATTCGCCTACTCC |
| R | TGTCACAGAGCCGAATACCAG | |
| IFITM2 | F | GCCCTTGACCTGTATTCCACT |
| R | TTTGAAGTCACAGCAGCACCA | |
| TRIM15 | F | TCCCTGAAGGTGGTCCATGAG |
| R | CAGGATCTTGCCCGAGGATT | |
| TRIM40 | F | CATCTCCAGGGCAGTAACACA |
| R | TAATTCCTTGGCCGTCCTT | |
| S100A9 | F | GGAACGCAACATAGAGACCAT |
| R | CAGATCTTTTCGCACCAGCTC | |
| DEFB1 | F | TGATCATTACAATTGCGTCAG |
| R | GAATAGAGACATTGCCCTCC | |
| VNN1 | F | AAGGAATTTAAAGGCACTGTC |
| R | GAGCGTACACTTCATTTGGT | |
| CASP4 | F | TGGGCAAAGATTTCCTCACT |
| R | CATTTGTCCTGCCATACGTTG | |
| DTX3L | F | GCTGCATTTCTCCTTCTGAACC |
| R | CTAGCAAAAGACTCACGAGCTG | |
| PARP9 | F | TGCCAAAGACCATGTAAAACACC |
| R | CCTCATACATCTCTTCCACGTT | |
| DDX20 | F | GCTGCGGGCTCGATTTAATTG |
| R | GTCCAAAGCTATGGTGGAGAAC | |
| LCN2 | F | AGCACCAACTACAACCAGCAT |
| R | CAGCTCCTTGGTTCTCCCGTA | |
| PLSCR1 | F | ATGCTTCTCACCCGGAAACAA |
| R | GGTAGCCACTATATCCTGGAGG | |
| STING | F | CCAGAGCACACTCTCCGGTA |
| R | CGCATTTGGGAGGGAGTAGTA | |
| LIL1R1 | F | GAAATCTCCTAATGGTGCTA |
| R | AATATTAATGCCAAAAGCAAC | |
| MBL2 | F | CTTATGCAGAACTGGCAAC |
| R | ATTCTAGTTACATGAAGCGAT | |
| Mouse β-actin | F | TGTCCACCTTCCAGCAGATGT |
| R | AGCTCAGTAACAGTCCGCCTAG | |
| Mouse Ifitm1 | F | TAGCCTATGCCTACTCCGTGA |
| R | CATCTAATGGCACAGACAACGA | |
| Mouse Il-15rα | F | AATCAGATACCGCAATGACCA |
| R | GCCACTTTCGTCATTTTGGA |
RNA sequencing and data processing were performed by the Shanghai Sangon Biotech Corporation (Shanghai, China) using Illumina Hiseq. The criteria to identify differentially expressed genes were qValue < .05 and FoldChange >1.8. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses were conducted for the differentially expressed genes. Gene Set Enrichment Analysis was performed for the transcription profile using Gene Set Enrichment Analysis 3.0.
Cytotoxicity Test
Hepatoma cells cultured in 96-well plates were treated with different concentrations of rhOSM for 3 days. After treatment, any cytotoxicity effect of rhOSM on hepatoma cells was assessed by enhanced cell counting kit-8 (Beyotime) following the instruction manual.
Immunoblotting and Coimmunoprecipitation
Proteins were extracted from cells using cell lysis buffer with protease and phosphatase inhibitor cocktail (Beyotime), separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and then subjected to immunoblotting with the antibodies shown in Table 4. Relative gray values of target protein were analyzed by Image J and normalized by β-actin or GAPDH. Co-IP was performed using a Pierce Co-Immunoprecipitation Kit (Thermo Scientific, Waltham, MA) following the instruction manual.
Table 4.
Antibodies Used for Western Blot and Immunostaining
| Antibody | Company | Cat# | Host | Dilution |
|---|---|---|---|---|
| STAT1 | Cell Signaling Technology | #14994 | Rabbit | 1:1000 5 μL for IP |
| phospho-STAT1 | Cell Signaling Technology | #9167 | Rabbit | 1:1000 |
| STAT2 | Cell Signaling Technology | #72604 | Rabbit | 1:1000 |
| phospho-STAT2 | Cell Signaling Technology | #88410 | Rabbit | 1:1000 |
| STAT3 | Cell Signaling Technology | #12640 | Rabbit | 1:1000 |
| phospho-STAT3 | Cell Signaling Technology | #9145 | Rabbit | 1:1000 |
| STAT4 | Affinity | AF6441 | Rabbit | 1:1000 |
| phospho-STAT4 | Beyotime | AF3441 | Rabbit | 1:1000 |
| STAT5 | Affinity | AF6305 | Rabbit | 1:1000 |
| phospho-STAT5 | Beyotime | AF2005 | Rabbit | 1:1000 |
| STAT6 | Beyotime | AF1534 | Rabbit | 1:1000 |
| phospho-STAT6 | Beyotime | AF5950 | Rabbit | 1:1000 |
| IRF1 | Cell Signaling Technology | #8478 | Rabbit | 1:1000 |
| IRF3 | Cell Signaling Technology | #11904 | Rabbit | 1:1000 |
| IRF9 | Cell Signaling Technology | #76684 | Rabbit | 1:1000 |
| IFITM1 | Thermo Fisher Scientific | PA5-27495 | Rabbit | 1:1000 |
| β-actin | Cell Signaling Technology | #8457 | Rabbit | 1:1000 |
| GAPDH | Cell Signaling Technology | #5174 | Rabbit | 1:1000 |
| S100A9 | Affinity | DF7596 | Rabbit | 1:1000 |
| IFITM2 | Affinity | DF8964 | Rabbit | 1:1000 |
| LCN2 | Affinity | AF7362 | Rabbit | 1:1000 |
| STING | Beyotime | AF8073 | Rabbit | 1:1000 |
| HBsAg | Novus Biologicals | NB100-62652 | Rabbit | 1:1000 |
| HBcAg | Abcam | ab8637 | Mouse | 1:1000 |
| p38 | Beyotime | AF1111 | Rabbit | 1:1000 |
| ERK1/2 | Beyotime | AF1051 | Rabbit | 1:1000 |
| phospho-p38 | Cell Signaling Technology | #4511 | Rabbit | 1:1000 |
| phospho-ERK1/2 | Cell Signaling Technology | #4370 | Rabbit | 1:1000 |
| HNF4α | Abcam | ab92378 | Rabbit | 1:1000 |
| HBx | Abcam | ab2741 | Mouse | 1:1000 |
Measurement of HBsAg, HBeAg, and HBV DNA
HBsAg and HBeAg were quantitated using a commercially available kit with a microparticle chemiluminescence immunoassay analyzer Architect I2000 (Abbott Laboratories, Woodmere, NY). HBV DNA quantitation was performed using a commercially available real-time fluorescence HBV DNA quantitation kit (Shengxiang Biotech Company, Hunan Province, China).
Statistical Analysis
Statistical analysis was performed in GraphPad Prism version 8.0 (GraphPad Software, Boston, MA). Data in all the graphs present the mean ± standard error of the mean of at least 3 biologic replicates. Statistical significance was determined using Student unpaired 2-tailed t test, 1-way analysis of variance, Mann-Whitney test, or Kruskal-Wallis test. P < 0.05 was considered statistically significant.
Acknowledgements
The authors thank all patients and healthy volunteers for their participation, and the staffs from their hospital and School of Medical Technology and Engineering, Fujian Medical University for their collaboration. Yuchen Ye, Ya Fu, and Caorui Lin contributed equally to this research.
CRediT Authorship Contributions
Yuchen Ye (Conceptualization: Equal; Investigation: Lead; Writing – original draft: Lead)
Ya Fu (Data curation: Lead)
Caorui Lin (Methodology: Lead)
Ye Shen (Formal analysis: Equal; Investigation: Equal)
Qingqing Yu (Formal analysis: Equal; Investigation: Equal)
Xiaobao Yao (Formal analysis: Equal; Investigation: Equal)
Qunfang Huang (Data curation: Equal)
Can Liu (Methodology: Equal)
Yongbin Zeng (Data curation: Equal; Methodology: Equal)
Tianbin Chen (Methodology: Equal)
Songhang Wu (Data curation: Equal)
Zhen Xun (Conceptualization: Equal; Formal analysis: Equal; Writing – review & editing: Equal)
Qishui Ou (Conceptualization: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the National Natural Science Foundation of China (grant numbers 81971996, 82030063, 82102467) and the Joint Fund for Medical and Health guiding project of Xiamen (grant number 3502Z20224ZD1219).
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.jcmgh.2023.10.003.
Contributor Information
Zhen Xun, Email: xunzhen@fjmu.edu.cn.
Qishui Ou, Email: ouqishui@fjmu.edu.cn.
Supplementary Material
References
- 1.Collaborators. PO. HBV progress towards coverage targets. 2021. 10-04/2022-02-06 http://cdafound.org/polaris-countries-dashboard/. Accessed 2019.
- 2.Revill P.A., Chisari F.V., Block J.M., et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;4:545–558. doi: 10.1016/S2468-1253(19)30119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung S., Choi H.S.J., Gehring A., et al. Getting to HBV cure: the promising paths forward. Hepatology. 2022;76:233–250. doi: 10.1002/hep.32314. [DOI] [PubMed] [Google Scholar]
- 4.Martinez M.G., Boyd A., Combe E., et al. Covalently closed circular DNA: the ultimate therapeutic target for curing HBV infections. J Hepatol. 2021;75:706–717. doi: 10.1016/j.jhep.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Schuch A., Salimi Alizei E., Heim K., et al. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut. 2019;68:905–915. doi: 10.1136/gutjnl-2018-316641. [DOI] [PubMed] [Google Scholar]
- 6.Lebosse F., Testoni B., Fresquet J., et al. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol. 2017;66:897–909. doi: 10.1016/j.jhep.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Soriano V. Hepatitis B gene therapy coming to age. AIDS Rev. 2018;20:125–127. [PubMed] [Google Scholar]
- 8.Niu C., Li L., Daffis S., et al. Toll-like receptor 7 agonist GS-9620 induces prolonged inhibition of HBV via a type I interferon-dependent mechanism. J Hepatol. 2018;68:922–931. doi: 10.1016/j.jhep.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Wang D., Fu B., Shen X., et al. Restoration of HBV-specific CD8(+) T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-alpha therapy. Signal Transduct Target Ther. 2021;6:376. doi: 10.1038/s41392-021-00776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb Perspect Biol. 2018;10:a028415. doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosel M., Quasdorff M., Wiegmann K., et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 12.Larrea E., Echeverria I., Riezu-Boj J.I., et al. Characterization of the CD40L/oncostatin M/oncostatin M receptor axis as an antiviral and immunostimulatory system disrupted in chronic HCV infection. J Hepatol. 2014;60:482–489. doi: 10.1016/j.jhep.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Carneros D., Santamaría E.M., Larequi E., et al. Cardiotrophin-1 is an anti-inflammatory cytokine and promotes IL-4-induced M2 macrophage polarization. FASEB J. 2019;33:7578–7587. doi: 10.1096/fj.201801563R. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo G.A., Scisciani C., Pediconi N., et al. IL6 inhibits HBV transcription by targeting the epigenetic control of the nuclear cccDNA minichromosome. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larrea E., Aldabe R., Gonzalez I., et al. Oncostatin M enhances the antiviral effects of type I interferon and activates immunostimulatory functions in liver epithelial cells. J Virol. 2009;83:3298–3311. doi: 10.1128/JVI.02167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larrea E., Riezu-Boj J.I., Aldabe R., et al. Dysregulation of interferon regulatory factors impairs the expression of immunostimulatory molecules in hepatitis C virus genotype 1-infected hepatocytes. Gut. 2014;63:665–673. doi: 10.1136/gutjnl-2012-304377. [DOI] [PubMed] [Google Scholar]
- 17.Giraldez M.D., Carneros D., Garbers C., et al. New insights into IL-6 family cytokines in metabolism, hepatology and gastroenterology. Nat Rev Gastroenterol Hepatol. 2021;18:787–803. doi: 10.1038/s41575-021-00473-x. [DOI] [PubMed] [Google Scholar]
- 18.Tseng C.W., Liu W.C., Ko P.H., et al. The predictive role of hepatitis B biomarkers on HBV reactivation following direct-acting antiviral therapy in HBV/HCV coinfected patients. Viruses. 2022;14:1812. doi: 10.3390/v14081812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan G., Song H., Xu F., et al. When hepatitis B virus meets interferons. Front Microbiol. 2018;9:1611. doi: 10.3389/fmicb.2018.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayana S.K., Helbig K.J., McCartney E.M., et al. The interferon-induced transmembrane proteins, IFITM1, IFITM2, and IFITM3 inhibit hepatitis C virus entry. J Biol Chem. 2015;290:25946–25959. doi: 10.1074/jbc.M115.657346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T., Ke Z., Liu W., et al. Human hepatitis B virus core protein inhibits IFNalpha-induced IFITM1 expression by interacting with BAF200. Viruses. 2019;11:427. doi: 10.3390/v11050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong G.L.H., Gane E., Lok A.S.F. How to achieve functional cure of HBV: stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76:1249–1262. doi: 10.1016/j.jhep.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Luo P., Wang P.X., Li Z.Z., et al. Hepatic oncostatin M receptor beta regulates obesity-induced steatosis and insulin resistance. Am J Pathol. 2016;186:1278–1292. doi: 10.1016/j.ajpath.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K., Nonaka H., Saito H., et al. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635–644. doi: 10.1002/hep.20086. [DOI] [PubMed] [Google Scholar]
- 25.Kurash J.K., Shen C.N., Tosh D. Induction and regulation of acute phase proteins in transdifferentiated hepatocytes. Exp Cell Res. 2004;292:342–358. doi: 10.1016/j.yexcr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Guerriero M.L., Dudka A., Underhill-Day N., et al. Narrative-based computational modelling of the Gp130/JAK/STAT signalling pathway. BMC Syst Biol. 2009;3:40. doi: 10.1186/1752-0509-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Souza P.P.C., Henning P., Lerner U.H. Stimulation of osteoclast formation by oncostatin M and the role of WNT16 as a negative feedback regulator. Int J Mol Sci. 2022;23:3287. doi: 10.3390/ijms23063287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermanns H.M. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015;26:545–558. doi: 10.1016/j.cytogfr.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 29.You H., Qin S., Zhang F., et al. Regulation of pattern-recognition receptor signaling by HBX during hepatitis B virus infection. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.829923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesev E.V., LeDesma R.A., Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4:914–924. doi: 10.1038/s41564-019-0421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuno T., Mejido J., Zhao T., et al. IRF9 is a key factor for eliciting the antiproliferative activity of IFN-alpha. J Immunother. 2009;32:803–816. doi: 10.1097/CJI.0b013e3181ad4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler M., Wrensch F., Bosch P., et al. Analysis of IFITM-IFITM interactions by a flow cytometry-based FRET assay. Int J Mol Sci. 2019;20:3859. doi: 10.3390/ijms20163859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song L., Chen J., Hao P., et al. Differential transcriptomics analysis of IPEC-J2 cells single or coinfected with porcine epidemic diarrhea virus and transmissible gastroenteritis virus. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.844657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajabi H., Hiraki M., Kufe D. MUC1-C activates polycomb repressive complexes and downregulates tumor suppressor genes in human cancer cells. Oncogene. 2018;37:2079–2088. doi: 10.1038/s41388-017-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salerno D., Chiodo L., Alfano V., et al. Hepatitis B protein HBx binds the DLEU2 lncRNA to sustain cccDNA and host cancer-related gene transcription. Gut. 2020;69:2016–2024. doi: 10.1136/gutjnl-2019-319637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinese Society of Infectious Diseases CMA, Chinese Society of Hepatology CMA The guideline of prevention and treatment for chronic hepatitis B: a 2022 update. Chinese Society of Hepatology. 2022;24:1335–1356. [Google Scholar]
- 37.Xun Z., Lin J., Yu Q., et al. Taurocholic acid inhibits the response to interferon-alpha therapy in patients with HBeAg-positive chronic hepatitis B by impairing CD8(+) T and NK cell function. Cell Mol Immunol. 2021;18:461–471. doi: 10.1038/s41423-020-00601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J., He Y., Chen J., et al. A critical role of transcription factor YY1 in rheumatoid arthritis by regulation of interleukin-6. J Autoimmun. 2017;77:67–75. doi: 10.1016/j.jaut.2016.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.