ABSTRACT
Historically, all efforts against tuberculosis were focused on rapid diagnosis and effective treatment to break the chain of transmission of Mycobacterium tuberculosis. However, in the last few years, more and more evidence has been found on the dramatic consequences of the condition defined as post-tuberculosis lung disease (PTLD). Approximately one third of patients surviving pulmonary tuberculosis face considerable ongoing morbidities, including respiratory impairment, psychosocial challenges, and reduced health-related quality of life after treatment completion. Given the important global and local burden of tuberculosis, as well as the estimated burden of PTLD, the development of a consensus document by a Brazilian scientific society-Sociedade Brasileira de Pneumologia e Tisiologia (SBPT)-was considered urgent for the prevention and management of this condition in order to allocate resources to and within tuberculosis services appropriately and serve as a guide for health care professionals. A team of eleven pulmonologists and one methodologist was created by the SBPT to review the current evidence on PTLD and develop recommendations adapted to the Brazilian context. The expert panel selected the topics on the basis of current evidence and international guidelines. During the first phase, three panel members drafted the recommendations, which were divided into three sections: definition and prevalence of PTLD, assessment of PTLD, and management of PTLD. In the second phase, all panel members reviewed, discussed, and revised the recommendations until a consensus was reached. The document was formally approved by the SBPT in a special session organized during the 2023 SBPT Annual Conference.
Keywords: Tuberculosis, Post-infectious disorders, Disease management
RESUMO
Historicamente, todos os esforços contra a tuberculose concentraram-se no diagnóstico rápido e no tratamento efetivo para quebrar a cadeia de transmissão do Mycobacterium tuberculosis. No entanto, nos últimos anos, têm sido encontradas mais e mais evidências sobre as dramáticas consequências da condição definida como doença pulmonar pós-tuberculose (DPPT). Aproximadamente um terço dos pacientes que sobrevivem à tuberculose pulmonar enfrenta morbidades consideráveis e persistentes, incluindo comprometimento respiratório, desafios psicossociais e redução da qualidade de vida relacionada à saúde após o término do tratamento. Diante da importante carga global e local da tuberculose, bem como da carga estimada da DPPT, considerou-se urgente o desenvolvimento de um documento de consenso por uma sociedade científica brasileira - a Sociedade Brasileira de Pneumologia e Tisiologia (SBPT) - para a prevenção e manejo dessa condição, a fim de alocar recursos de forma adequada para e nos serviços de tuberculose e servir de guia para os profissionais de saúde. Uma equipe de onze pneumologistas e um metodologista foi criada pela SBPT para revisar as evidências atuais sobre a DPPT e desenvolver recomendações adaptadas ao contexto brasileiro. O painel de especialistas selecionou os temas com base nas evidências atuais e diretrizes internacionais. Durante a primeira fase, três membros do painel redigiram as recomendações, que foram divididas em três seções: definição e prevalência de DPPT, avaliação da DPPT e manejo da DPPT. Na segunda fase, todos os membros do painel analisaram, discutiram e revisaram as recomendações até chegar a um consenso. O documento foi aprovado formalmente pela SBPT em sessão especial organizada durante o Congresso Anual da SBPT de 2023.
Descritores: Tuberculose, Transtornos pós-infecções, Gerenciamento clínico
INTRODUCTION
Tuberculosis, in addition to the physical and psychological problems that are associated with the disease, the disease-related stigma, and considerable financial costs, also entails a condition defined as post-tuberculosis lung disease (PTLD), which has so far been little studied, but which has recently received the spotlight. 1
Historically, all efforts against tuberculosis were focused on rapid diagnosis and effective treatment, trying to break the chain of transmission of Mycobacterium tuberculosis. However, in the last few years, the impact of damaging long-term sequelae due to pulmonary tuberculosis has duly been valued for individual patients, their households, their communities, and health systems. 2
Approximately one third of patients who survive pulmonary tuberculosis face, after treatment completion, a considerable and often underrecognized burden of ongoing morbidity, including respiratory impairment, psychosocial challenges, and reduced health-related quality of life. 3 , 4 Even mortality is higher in PTLD patients, with a risk of death up to six times higher than in the general population. 5 , 6
Despite the recent increase in PTLD-related publications, epidemiological data of the global burden and morbidity associated with PTLD are still limited because of lack of priority by national tuberculosis programs and the relative complexity of the diagnostic approach, which includes, among others, clinical, radiological, and lung function assessment. 7 , 8
It is evident that PTLD sequelae contribute to excess mortality and morbidity, 9 causing radiological and functional disabilities, favoring other complications (e.g., other infections, hemoptysis, etc.), impairing quality of life, and, therefore, boosting hospitalization and costs to health care systems. Early assessment and management of PTLD-related morbidity guided by solid recommendations by a scientific society are of paramount importance to ensure appropriate allocation of resources to and within tuberculosis services in order to provide quality-assured PTLD prevention, diagnosis, and treatment. 3
The present Sociedade Brasileira de Pneumologia e Tisiologia (SBPT, Brazilian Thoracic Association) recommendations apply to patients with PTLD in the Brazilian context. A panel of 11 pulmonologists and one methodologist, including two international experts, were invited by the SBPT to review the current knowledge on the topic and develop Brazilian-specific recommendations. An expert panel selected the topics based on current evidence and international guidelines available as of July of 2023. During the first phase, three panel members drafted the recommendations, which were divided into three sections: PTLD description and prevalence; PTLD assessment; and PTLD management. In the second phase, all panel members reviewed, discussed, and revised the recommendations until a consensus was reached. The document was formally approved by the SBPT in a special session organized during the 2023 SBPT Annual Conference, held in the city of Curitiba, Brazil.
DEFINITION
PTLD was defined during the First International Symposium on Post-Tuberculosis disease, held in Stellenbosch, South Africa, as the “evidence of chronic respiratory abnormality, with or without symptoms, attributable at least in part to previous (pulmonary) tuberculosis.” 1 PTLD includes a wide variety of functional and structural lung sequelae, ranging from mild to severe disorders. Cavitation, bronchiectasis, pleural thickening, fibrosis, and pulmonary hypertension are some examples of tuberculosis sequelae. In addition, patients may suffer colonization and infection with Aspergillus fumigatus, nontuberculous mycobacteria, and other bacteria. 1 , 10 - 16 Lung function deficits described in patients with PTLD include obstruction, restriction, or a mixed pattern, and approximately 10% of those lose more than half of their lung function. 17 In fact, as compared to the general population, patients with PTLD are twice more likely to have spirometric abnormalities. 18 As a consequence of lung sequelae, persistent respiratory symptoms are frequent, such as dyspnea, cough, wheezing, and reduced exercise capacity. 19
EPIDEMIOLOGY
Despite the recent increase in the number of PTLD-related publications, the real epidemiological burden of PTLD is not well known. 12 It is estimated that up to 50% of tuberculosis survivors live with some kind of sequelae. 20 Also, the mortality rate in this group of patients can be up to 3-to 6-fold higher when compared with that in the general population. 5 , 6 , 21
According to previous studies, the prevalence of PTLD can vary from 18% to 87%. 22 This wide range can be attributed to the different scenarios analyzed and to the diverse parameters used to diagnose PTLD. In a recent comparison of three different cohort studies involving PTLD patients in Brazil, Italy, and Mexico, pulmonary function tests showed different results, the majority of which showed obstructive, mixed, and normal patterns, respectively. 23
Another study used radiological patterns to evaluate the prevalence of PTLD, and the results varied according to the type of examination (chest CT or X-ray) and the residual abnormality found (cavitation, bronchiectasis, fibrosis, nodules, emphysema, or consolidation). 24
The possible different ways to characterize and define PTLD hinder the generalization of several studies, and few data can correlate structural damage identified in chest radiological imaging, functional impairment, respiratory symptoms, and quality of life. In 2022, a meta-analysis conducted by Maleche-Obimbo et al. 25 identified pooled prevalence of abnormal lung function, persistent respiratory symptoms, and radiological abnormalities of 46.7%, 41.0%, and 64.6%, respectively. The magnitude of any type of PTLD varied according to HIV status, geographic settings, smoking habits, and urban/rural settings.
ASSESSMENT OF PTLD
Ideally, every patient completing tuberculosis treatment should be clinically evaluated to identify post-tuberculosis sequelae as soon as possible. 26 Essential examinations/investigations recommended to identify PTLD are described in Chart 1. It is likely that not all of those examinations/investigations can be performed in many settings. In that case, some examinations/investigations should be prioritized, especially in those patients with persistent symptoms. 1 , 4 , 10 , 12 , 22 , 26 - 32
Chart 1. Recommended examinations to be conducted at the end of tuberculosis treatment.
| Parameter | Measurement tool |
|---|---|
| Clinical history and examination | Symptoms: cough, dyspnea, wheezinga
Environmental exposuresa Comorbiditiesa Physical examination: respiratory rate, heart rate, and BMIa |
| Chest imaging | Chest X-raya
Chest CT scanning |
| Pulmonary function testing | Spirometry, including pre- and post-bronchodilator testa
Plethysmography DLco Six-minute walk testa |
| Blood gas analysis | Arterial blood gas analysis Pulse oximetrya |
| Cardiopulmonary evaluation | Cardiopulmonary exercise testing |
| Subjective evaluation | Symptom score and quality-of-life instrumenta |
Examinations/investigations that should be prioritized when all cannot be carried out.
Clinical history and examination
The clinical patterns of PTLD include a wide spectrum of signs and symptoms, varying from asymptomatic to severe disability. Tuberculosis survivors usually present with a high prevalence of respiratory manifestations such as chronic cough and dyspnea. 33 The clinical examination must focus on respiratory rate, heart rate, and BMI on a routine basis. 1
Patients with residual structural abnormalities can have infectious exacerbations and are even at a higher risk of having active tuberculosis again. 34 Bacterial, viral, fungal, and nontuberculous mycobacterial diseases, which might complicate due to subsequent hemoptysis, can be severe and potentially life-threatening. 7 Patients also experience high rates of hospitalization and respiratory-related mortality. 33
It is noteworthy that factors other than tuberculosis sequelae can influence PTLD and its outcomes, such as environmental exposure to smoking, substance abuse, biomass smoke and occupational exposures. In addition, cardiopulmonary comorbidities and those caused by other diseases can also worsen PTLD outcomes. 1 , 7 , 22
Chest imaging
Although chest imaging is an important tool for evaluating PTLD patients, it should not be used alone to define post-tuberculosis lung damage, as patients can be asymptomatic or have no functional impairment, and have abnormal imaging indicating post-tuberculosis cure. 35
Both chest X-rays and chest CTs are useful in the evaluation of lung structural damage of tuberculosis survivors. 24 Although CT imaging showed to be more sensitive in the identification of a diverse range of residual pathologies, 24 CT availability, costs, and radiation impact must be taken into consideration to decide which option best fits each specific case and scenario.
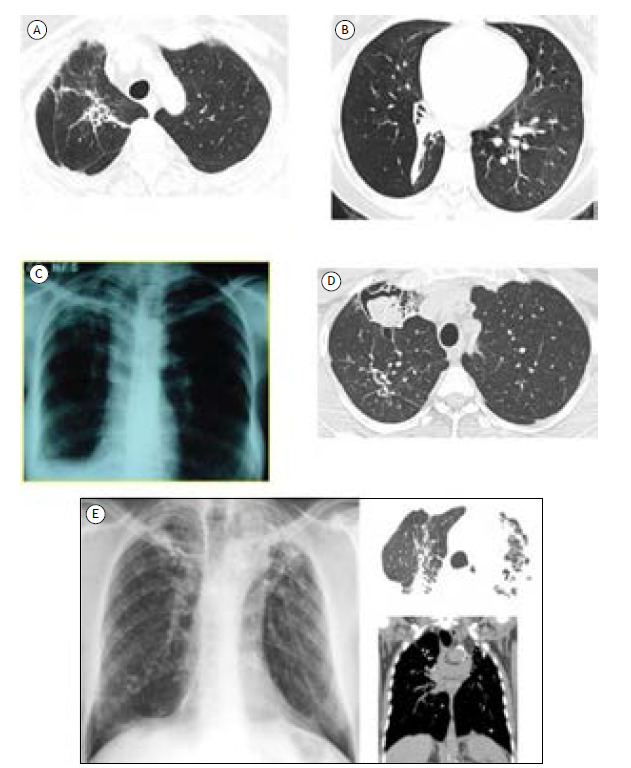
The most common radiological patterns of PTLD reported in a systematic review were cavitation, bronchiectasis, and fibrosis. In addition, nodules, consolidation, emphysema, pleural thickening, and mosaic patterns can also be noticed. 35 Figure 1 shows some radiological patterns of PTLD.
Figure 1. Radiological patterns of post-tuberculosis lung disease. In A, a chest CT scan showing irregular dense opacity with bronchiectasis from the hilum to the right lung apex associated with apical pleural thickening, elevation of the hilum, and volumetric reduction on this side. In B, a chest CT scan showing consolidative opacity with a fibroatelectatic appearance in the right lower lobe, predominantly affecting the anterior, lateral, and posterior basal segments, determining its volumetric reduction and highlighting the associated bronchiectasis in the anterior basal segment. Cylindrical bronchiolectasis in the upper segment of the right lower lobe. In C, a chest X-ray showing fibrotic opacity in the right upper lobe with pleural thickening and volumetric reduction of the right lung, leading to elevation of the right phrenic dome. In D, a chest CT scan showing a residual excavated lesion in the anterior segment of the right upper lobe filled with mobile contents upon change of decubitus, corroborating repercussions of superimposed saprophytic fungal infectious involvement. In E, imaging scans showing confluent laminar atelectasis, volumetric loss, and architectural distortion in the upper lobes, with intervening calcifications, favoring chronic/residual changes.
Pulmonary function testing
In 2015, a study designated Burden of Obstructive Lung Disease 11 assessed the association of pulmonary function impairment with a history of tuberculosis in a large, international, population-based sample and found that previous tuberculosis was associated with both airflow obstruction and spirometric restriction, and it should be considered as a potentially important cause of obstructive disease and reduced lung function.
Until now, there has been no consensus on which disorder is the most prevalent in individuals with tuberculosis sequelae. A recent prospective cohort study conducted in Malawi showed that 34.4% of participants had abnormal spirometry by the end of antituberculosis treatment. 33 After 3 years, 27.9% still presented abnormal pulmonary function test results, mostly of obstructive nature (15.8%). 33
The healing process that the lungs undergo during and after antituberculosis treatment is likely to cause structural damage, leading to loss of parenchymal tissue and restrictive pattern on spirometry. It is less clear what mechanisms cause airflow obstruction associated with tuberculosis. The two most widely accepted hypotheses are (i) the development of airway disease (bronchiectasis and bronchial stenosis), and (ii) immunological factors that can induce bronchial hyperresponsiveness. 11
Therefore, whenever plethysmography or lung volume measurements are feasible, they should complement spirometry to confirm obstructive, restrictive, or even mixed patterns of pulmonary disease since the type of ventilatory defect can be heterogeneous and vary in different populations. 23
Decreases in DLco can occur even in patients with normal spirometry and could be a better tool for lung function evaluation in PTLD patients. 36 Furthermore, they can also correlate with cardiopulmonary exercise testing and predict oxygen consumption when that test is unavailable. 37
As well as for the evaluation of other pulmonary and cardiac diseases, the six-minute walk test (6MWT) helps evaluate PTLD patients. This test is cheap, simple, and very useful for studying functional limitations in tuberculosis survivors and for designing appropriate rehabilitation programs for PTLD patients. 38
Blood gas analysis
In patients with severe clinical, radiological, and/or functional disabilities, arterial blood gas analysis (whenever possible) or even oxygen saturation measurement using pulse oximetry can identify hypoxemic patients.
The indications for home oxygen therapy should be the same as those for patients with chronic airway diseases, that is, Pao2 < 55 mmHg, Spo2 < 88% on room air, Pao2 between 56 and 59 mmHg associated with cor pulmonale, and/or hematocrit > 55%. 39
Cardiopulmonary exercise testing
Cardiopulmonary exercise testing provides a global assessment of integrative exercise responses involving cardiovascular, respiratory, muscular, and metabolic systems during exertion, being considered the gold standard for cardiorespiratory functional assessment. 40
In 2022, Curry et al. 37 found that, although statistically significant, the correlations of any lung function patterns, if measured using spirometry, DLco, or even plethysmography, with oxygen consumption measured on cardiopulmonary exercise testing, which is considered to be the gold standard for lung capacity measurement, were weak. 1 Despite its importance, cardiopulmonary exercise testing is not always available.
Symptom and quality of life scores
Specific severity scores are still unavailable for PTLD patients, but there is consensus on the urgency of a scoring system evaluating mortality, health-related quality of life, rate of lung function decline, exacerbations/hospitalizations, and tuberculosis recurrence. 1
Different questionnaires of health-related quality of life are available and should be used in the follow-up of PTLD patients, such as the St George’s Respiratory Questionnaire and the Short-Form Health Survey (with 12 or 36 questions). 1 , 26
Specific considerations for children
Evaluation at the end of treatment should follow the same recommendations proposed for adults, although there is a lack of data regarding children. Chest CT is not usually indicated due to radiation exposure, but it may be considered in cases with chronic symptoms and abnormal radiological findings in order to assess the extent of disease and/or exclude other diagnoses. Pulmonary function tests should be considered in all 4-to 6-year-old children with severe lung impairment. In children ≥ 4 years of age, exercise capacity can be assessed using the 6MWT. Quality of life questionnaires such as the EQ-5D-Y and the Toddler and Infant (TANDI) instrument can be used with local adaptations for younger children. 26
MANAGEMENT OF PTLD
Currently, there are no evidence-based guidelines for PTLD management, but the increasing scientific literature on the topic has raised several issues that could help the follow-up of these patients. 2 , 26
Inhaled and oral treatment
For those whose functional obstructive disease has been established, inhaled bronchodilators may be useful to reduce symptoms of dyspnea and prevent a decline in lung function. 7 Despite the absence of evidence to recommending routine bronchodilator use in PTLD, small studies have suggested that long-acting β2 agonists and long-acting muscarinic antagonists could improve lung function and dyspnea. 7 , 24
Inhaled corticosteroids must be avoided since they can increase the frequency of exacerbations and the risk of mycobacterial diseases. 41 , 42 However, following the recommendations applied to noncystic bronchiectasis, in cases of PTLD associated with asthma, inhaled corticosteroid therapy may be justified. 24 Similarly, for chronic inflammation in patients with noncystic fibrosis bronchiectasis, the use of macrolides is recommended for a minimum period of 6-12 months in patients with bronchiectasis and at least two exacerbations per year. 39
Infectious complications
Clinical approach to exacerbations of infectious and noninfectious etiology must be the same as those applied for noncystic fibrosis bronchiectasis. 39
In addition to respiratory infections of bacterial and viral etiology, fungal complications are frequent in post-tuberculosis lung sequelae. Aspergillus sp. can present in different ways and severity levels-from only colonization in a fungus ball shaping (aspergilloma) to infiltration in the lung parenchyma and/or pleural tissue with destruction and new cavities (chronic pulmonary aspergillosis). 7 , 32 Diagnostic criteria for this last presentation include the presence of respiratory or constitutional symptoms for at least 3 months, suggestive radiological findings, and serological or microbiological evidence of Aspergillus sp. Treatment for aspergillomas in asymptomatic patients could be only “follow-up”; however, surgical management is necessary in cases with multiple episodes of hemoptysis. On the other hand, invasion and destruction of lung parenchyma will require antifungal management. 32 Prescription of long-term oral antifungal drugs, such as itraconazole at a dose of 400 mg/day or voriconazole at a dose of 400 mg/day, administered for at least 6 months is the recommended first-line therapy for chronic pulmonary aspergillosis and has been associated with improvement in quality of life, relief of symptoms, and delay in disease progression. 43
Pulmonary rehabilitation
Former tuberculosis patients with clinical, functional, or radiological findings consistent with PTLD should be evaluated for pulmonary rehabilitation (PR). 26 Chart 2 shows the indications for PR in detail, including impaired pulmonary function and/or impaired DLco 44 ; abnormal blood gas analysis results and/or nocturnal and exercise-induced desaturation 45 ; impaired exercise capacity 1 , 38 , 46 , 47 ; persistent respiratory symptoms 48 - 51 ; ineffective cough and/or difficulty to clear bronchial secretions 52 , 53 ; at least one hospitalization or two exacerbations in the last 12 months 1 , 28 , 54 , 55 ; presence of comorbid conditions, including COPD, asthma, bronchiectasis, pulmonary fibrosis, pulmonary hypertension, and/or need for surgery 11 , 18 , 56 ; and impaired quality of life. 57 - 59
Chart 2. Indications for pulmonary rehabilitation.
| • Impaired pulmonary function showing airflow obstruction or restriction (or mixed abnormalities) and bronchodilator response and/or impaired DLco |
| • Abnormal blood gas: Pao2 < 80 mmHg and/or Paco2 > 45 mmHg and/or nocturnal and exercise-induced desaturation |
| • Impaired exercise capacity |
| • Persistent respiratory symptoms (dyspnea, cough, sputum, wheezing, chest pain, and fatigue) |
| • Ineffective cough and/or difficulty to clear bronchial secretions |
| • At least one hospitalization or two exacerbations within the last 12 months |
| • Presence of comorbid conditions, including COPD, asthma, bronchiectasis, pulmonary fibrosis, pulmonary hypertension, and/or need for surgery |
| • Impaired quality of life |
The PR program should be coordinated according to the local organization of health services, taking into consideration feasibility, effectiveness, and cost-effectiveness criteria. 26 The core components of a PR program are summarized in Chart 3.
Chart 3. Core components of a rehabilitation program.
| Component | Indication | Methods | |
|---|---|---|---|
| Intervention | Adaptation to special settings and situations | ||
| Aerobic exercise: endurance training | Impaired exercise capacity, limited by dyspnea and/or other respiratory symptoms Restriction in activities of daily living 11 , 32 |
• Treadmill and/or cycle ergometer • 30 min. 2-5 times/week for 4-8 weeks • Intensity set according to maximal oxygen consumption, Luxton equation, or 80% of maximum heart rate adjusted for dyspnea • Inpatients, outpatients, or telemonitoring • Suggest maintenance program |
• Free walking • 30 min. 2-5 times/week for 4-8 weeks • Intensity set according to perceived dyspnea • Outpatients or home setting • Suggest maintenance program |
| Strength training: upper and lower extremities (limited evidence for tuberculosis) |
Reduced muscle mass and strength of peripheral muscles; lower muscle weakness with risk for falls Impaired activities of daily living involving the upper extremities (including dressing, bathing, and household tasks) 11 |
• Free weights (dumbbells and ankle-braces) • 20-30 min. 2-5 times/week for 4-8 weeks • 2-3 sets of 6-12 repetitions • Intensity set to 80% of maximal voluntary contraction and/or adjusted for muscle fatigue • Inpatients, outpatients, or telemonitoring • Suggest maintenance program |
• Free weights (dumbbells and ankle-braces) • 20-30 min. 2-5 times/week for 4-8 weeks • 2-3 sets of 6-12 repetitions • Intensity set according to perceived muscle fatigue • Outpatients or home setting • Suggest maintenance program |
| Inspiratory muscle training (limited evidence for tuberculosis) |
Impaired respiratory muscle function, altered respiratory mechanics, decreased chest wall compliance, or pulmonary hyperinflation | • Load threshold devices, seated and using a nose clip • Interval training: sets of 10 exercise repetitions interspersed with 10-second breaks • 15-20 min. 2-5 times/week for 4-8 weeks • Loads from 30% to 80% of maximal inspiratory pressure |
• Not applicable |
| Airway clearance techniques | Difficult-to-remove secretions or mucous plugs; frequent bronchial exacerbations (≥ 2/year) Concomitant diagnosis of bronchiectasis |
• Choose the suitable technique for the subject among those available, based on respiratory capacity, mucus rheology, patient collaboration, and patient preferences • 15-30 min. one or more times/day • Choose the duration of treatment based on chronic (long-term) or acute (short-term) problem • Suggest maintenance program when needed |
• Choose the suitable technique for the subject among those available, based on respiratory capacity, mucus rheology, patient collaboration, and patient preferences • 15-30 min. one or more times/day • Choose the duration of treatment based on chronic (long-term) or acute (short-term) problem • Suggest maintenance program when needed |
| Long-term oxygen therapy (limited evidence for tuberculosis) |
Resting hypoxemia despite stable condition and optimal medical therapy (partial pressure of oxygen < 55 mmHg or ≤ 60 mmHg with evidence of peripheral edema, polycythemia [haematocrit ≥ 55%], or pulmonary hypertension) | • Titrate oxygen flow to maintain oxygen saturation > 92-93%. • Long-term oxygen therapy should be initiated on a flow rate of 1 L/min and titrated up in 1 L/min increments until oxygen saturation > 90% at rest has been achieved • An arterial blood gas analysis should then be performed to confirm that the target partial pressure of oxygen ≥ 60 mmHg at rest has been achieved • Ambulatory and nocturnal oximetry may be performed to allow more accurate flow rates to be prescribed for exercise and sleep, respectively • Provide formal education to patients referred home • Schedule periodic reassessment at 3 months |
• Titrate oxygen flow to maintain oxygen saturation > 92-93%. • Long-term oxygen therapy should be initiated on a flow rate of 1 L/min and titrated up in 1 L/min increments until oxygen saturation > 90% at rest has been achieved. • Non-hypercapnic patients initiated on long-term oxygen therapy should have their flow rate increased by 1 L/min during sleep in the absence of any contraindications • Ambulatory oximetry may be performed to allow more accurate flow rates to be prescribed for exercise • Provide formal education to patients referred home • Schedule periodic reassessment every 3 months |
| Long-term nocturnal noninvasive mechanical ventilation (limited evidence for tuberculosis) |
Chronic stable hypercapnia (partial pressure of carbon dioxide > 45-60 mmHg) despite optimal medical therapy Noninvasive ventilation could be applied during aerobic training in case of severe breathlessness or reduced exercise resistance |
• Not initiating long-term noninvasive ventilation during admission for acute or chronic hypercapnic respiratory failure, favoring reassessment at 2-4 weeks after resolution • Titrate noninvasive ventilation settings • Titrate mask • Plan education • Consider noninvasive ventilation during exercise • Schedule an educational meeting and verify the ability of the subject and/or a caregiver to manage noninvasive ventilation at home |
• Probably not applicable |
| Nutritional support | Malnutrition (BMI < 16 kg/m2 or < 17 kg/m2 in patients with tuberculosis-HIV coinfection, patients with MDR tuberculosis, or in those who are pregnant or are lactating mothers) | • Nutritional assessment • Tailored treatment: foods and medical supplements • Need for financial incentive and transportation access should be evaluated |
• Nutritional assessment • Tailored treatment: foods and medical supplements • Need for financial incentive and transportation access should be evaluated |
| Psychological support | Social isolation, depression, and/or anxiety Impaired health status and/or quality of life despite optimal pharmacological treatment Low adherence to medical treatment |
• Psychological assessment • Psychological support • Consider self-help group |
• Psychological assessment • Psychological support • Consider self-help group |
min.: minutes; and MDR: multidrug resistant.
Evaluation of effectiveness of PR should be carried out by comparing the core variables before and after PR, 26 as shown in Chart 4.
Chart 4. Evaluation of pulmonary rehabilitation effectiveness.
| Essential and conditional examinations/investigations | Adaptation to special settings and situations |
|---|---|
| Lung function | |
| • Spirometry (FEV1, FVC, FEV1/FVC) • Plethysmography |
• Spirometry (FEV1, FVC, FEV1/FVC) |
| Gas transfer | |
| • Pao2
• Paco2 • Pulse oximetry (SpO2, % desaturation) • DLco, Kco |
• Pulse oximetry (SpO2, % desaturation) |
| Exercise capacity | |
| • 6MWT • Vo2max • ISWT • 5STS |
• 6MWT • 5STS |
| Health-related quality of life | |
| • EUROHIS-QoL 8 • SGRQ • WHOQOL-BREF • Pediatric: EQ-5D-Y and TANDI |
• EUROHIS-QoL 8 • SGRQ • WHOQOL-BREF • Pediatric: EQ-5D-Y and TANDI |
| Self-reported symptoms | |
| • mMRC • VAS • modified Borg scale |
• mMRC • VAS • modified Borg scale |
| Acute infectious exacerbations (e.g., in bronchiectasis) requiring antibiotic and/or steroid treatment | |
| Number of episodes | Number of episodes |
| Hospitalization | |
| Number of episodes/hospital days | Number of episodes/hospital days |
| Mortality | |
| Number of deaths | Number of deaths |
Kco: carbon monoxide transfer coefficient; 6MWT: six-minute walk test; ISWT: incremental shuttle walk test; 5STS: 5 repetitions sit-to-stand test; EUROHIS-QoL: European Health Interview Survey-Quality of Life; SGRQ: St. George’s Respiratory Questionnaire; WHOQOL-BREF: World Health Organization Quality of Life Instrument, brief version; TANDI: Toddler and Infant (instrument); mMRC: modified Medical Research Council; and VAS: visual analogue scale.
Vaccination
Similarly to other chronic respiratory disorders, PTLD may cause infectious complications, and some of these can be prevented with vaccinations. Influenza, pneumococcal, and COVID-19 vaccines should be recommended for PTLD patients.
Influenza vaccination must be repeated annually following local vaccination campaigns. For pneumococcal prevention, the Brazilian Immunization Association recommends either the 13-valent pneumococcal conjugate vaccine or the 15-valent pneumococcal conjugate vaccine, which has stronger immunogenic-use depends on availability-and, 6 months to 1 year later, the 23-valent pneumococcal polysaccharide vaccine, which can be boosted by a second dose administered 5 years later. 24
There are some vaccines recommended for the general population (or specific age groups) of which PTLD patients are likely to benefit from, such as those against tetanus, diphtheria, pertussis, measles, and shingles. Measles vaccination is recommended if there is no evidence of immunity (e.g., being born before 1957, lack of documented proof of having received the measles-mumps-rubella vaccine, or laboratory evidence of immunity or disease) is lacking. Patients with PTLD > 50 years of age can also benefit from vaccination against shingles. The tetanus-diphtheria-pertussis vaccine is recommended for the general population and should be considered for not previously vaccinated PTLD patients; also, booster doses should be repeated every 10 years in adults. 60
Education and counseling for PTLD patients
Every patient who participates in a PR program should undergo counseling and health education. Patients should be educated on the basic principles of the disease (epidemiology, clinical aspects, transmission, diagnosis, and treatment); common symptoms that they might experience after acute disease; how to monitor and manage their symptoms at home, and when they should visit a health care facility/call a doctor; and risks of reinfection and how they can manage this risk. In addition, patients should be counseled about the benefits of a healthy lifestyle (such as physical activity, adequate nutrition, and smoking cessation). Telehealth, videos, and booklets can be used for patient education. Family member of patients should also be encouraged to participate. Counseling/health education should include maintenance of the results obtained with PR through a follow-up plan. 26 , 61
FINAL CONSIDERATIONS
As more and more evidence is available on the deleterious consequences of PTLD, the development of a consensus statement directed toward this condition has become a priority for the SBPT. Approximately one third of patients who survive pulmonary tuberculosis can face a considerable ongoing morbidity, and the present recommendations apply to them. In the present manuscript, prepared by 11 pulmonologists and one methodologist with extensive experience in this area, recommendations for prevention, diagnosis, and management have been made, and the latest international guidelines have been adapted to the Brazilian context.
ACKNOWLEDGMENTS
We would like to acknowledge the support from Lia D’Ambrosio (Public Health Consulting Group, Lugano, Switzerland) and Rosella Centis (Istituti Clinici Scientifici Maugeri, IRCCS, Tradate, Italy) for their useful comments on the manuscript.
Footnotes
Financial support: The present document received financial support from the GSK by means of an educational grant to the Brazilian Thoracic Association.
REFERENCES
- 1.Allwood BW, van der Zalm MM, Amaral AFS, Byrne A, Datta S, Egere U, et al. Post-tuberculosis lung health perspectives from the First International Symposium. Int J Tuberc Lung Dis. 2020;24(8):820–828. doi: 10.5588/ijtld.20.0067. [DOI] [PubMed] [Google Scholar]
- 2.van Kampen SC, Wanner A, Edwards M, Harries AD, Kirenga BJ, Chakaya J. International research and guidelines on post-tuberculosis chronic lung disorders a systematic scoping review. BMJ Glob Health. 2018;3(4):e000745. doi: 10.1136/bmjgh-2018-000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Kampen SC, Wanner A, Edwards M, Harries AD, Kirenga BJ, Chakaya J. International research and guidelines on post-tuberculosis chronic lung disorders a systematic scoping review. BMJ Glob Health. 2018;3(4):e000745. doi: 10.1136/bmjgh-2018-000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nightingale R, Carlin F, Meghji J, McMullen K, Evans D, van der Zalm MM, et al. Post-TB health and wellbeing. Int J Tuberc Lung Dis. 2023;27(4):248–283. doi: 10.5588/ijtld.22.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romanowski K, Baumann B, Basham CA, Ahmad Khan F, Fox GJ, Johnston JC. Long-term all-cause mortality in people treated for tuberculosis a systematic review and meta-analysis. Lancet Infect Dis. 2019;19(10):1129–1137. doi: 10.1016/S1473-3099(19)30309-3. [DOI] [PubMed] [Google Scholar]
- 6.Ranzani OT, Rodrigues LC, Bombarda S, Minto CM, Waldman EA, Carvalho CRR. Long-term survival and cause-specific mortality of patients newly diagnosed with tuberculosis in São Paulo state, Brazil, 2010-15 a population-based, longitudinal study. Lancet Infect Dis. 2020;20(1):123–132. doi: 10.1016/S1473-3099(19)30518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allwood BW, Byrne A, Meghji J, Rachow A, van der Zalm MM, Schoch OD. Post-Tuberculosis Lung Disease Clinical Review of an Under-Recognised Global Challenge. Respiration. 2021;100(8):751–763. doi: 10.1159/000512531. [DOI] [PubMed] [Google Scholar]
- 8.Visca D, D Ambrosio L, Centis R, Pontali E, Tiberi S, Migliori GB. Post-TB disease a new topic for investigation-and why it matters. Int J Tuberc Lung Dis. 2021;25(4):258–261. doi: 10.5588/ijtld.21.0040. [DOI] [PubMed] [Google Scholar]
- 9.Dodd PJ, Yuen CM, Jayasooriya SM, van der Zalm MM, Seddon JA. Quantifying the global number of tuberculosis survivors a modelling study. Lancet Infect Dis. 2021;21(7):984–992. doi: 10.1016/S1473-3099(20)30919-1. [DOI] [PubMed] [Google Scholar]
- 10.Pasipanodya JG, McNabb SJ, Hilsenrath P, Bae S, Lykens K, Vecino E. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health. 2010;10:259–259. doi: 10.1186/1471-2458-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amaral AF, Coton S, Kato B, Tan WC, Studnicka M, Janson C. Tuberculosis associates with both airflow obstruction and low lung function BOLD results. Eur Respir J. 2015;46(4):1104–1112. doi: 10.1183/13993003.02325-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allwood BW, Stolbrink M, Baines N, Louw E, Wademan DT, Lupton-Smith A. Persistent chronic respiratory symptoms despite TB cure is poorly correlated with lung function. Int J Tuberc Lung Dis. 2021;25(4):262–270. doi: 10.5588/ijtld.20.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzies NA, Quaife M, Allwood BW, Byrne AL, Coussens AK, Harries AD. Lifetime burden of disease due to incident tuberculosis a global reappraisal including post-tuberculosis sequelae [published correction appears in Lancet Glob. Health. 2022;10(3):e336. doi: 10.1016/S2214-109X(21)00367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pontali E, Silva DR, Marx FM, Caminero JA, Centis R, D'Ambrosio L, et al. Breathing Back Better A State of the Art on the Benefits of Functional Evaluation and Rehabilitation of Post-Tuberculosis and Post-COVID Lungs. Arch Bronconeumol. 2022;58(11):754–763. doi: 10.1016/j.arbres.2022.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Garcia MA, Guan WJ, de-la-Rosa D, Athanazio R, Oscullo G, Shi MX, et al. Post-TB bronchiectasis from pathogenesis to rehabilitation. Int J Tuberc Lung Dis. 2023;27(3):175–181. doi: 10.5588/ijtld.22.0566. [DOI] [PubMed] [Google Scholar]
- 16.Hsu D, Irfan M, Jabeen K, Iqbal N, Hasan R, Migliori GB, et al. Post tuberculosis treatment infectious complications. Int J Infect Dis. 2020;92S:S41–S45. doi: 10.1016/j.ijid.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, et al. Pulmonary impairment after tuberculosis. Chest. 2007;131(6):1817–1824. doi: 10.1378/chest.06-2949. [DOI] [PubMed] [Google Scholar]
- 18.Tiberi S, Torrico MM, Rahman A, Krutikov M, Visca D, Silva DR. Managing severe tuberculosis and its sequelae from intensive care to surgery and rehabilitation. J Bras Pneumol. 2019;45(2):e20180324. doi: 10.1590/1806-3713/e20180324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migliori GB, Caminero Luna J, Kurhasani X, van den Boom M, Visca D, D'Ambrosio L, et al. History of prevention, diagnosis, treatment and rehabilitation of pulmonary sequelae of tuberculosis. Presse Med. 2022;51(3):104112–104112. doi: 10.1016/j.lpm.2022.104112. [DOI] [PubMed] [Google Scholar]
- 20.Mpagama SG, Msaji KS, Kaswaga O, Zurba LJ, Mbelele PM, Allwood BW. The burden and determinants of post-TB lung disease. Int J Tuberc Lung Dis. 2021;25(10):846–853. doi: 10.5588/ijtld.21.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller TL, Wilson FA, Pang JW, Beavers S, Hoger S, Sharnprapai S. Mortality hazard and survival after tuberculosis treatment. Am J Public Health. 2015;105(5):930–937. doi: 10.2105/AJPH.2014.302431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage from epidemiology to pathophysiology. Eur Respir Rev. 2018;27(147):170077–170077. doi: 10.1183/16000617.0077-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva DR, Freitas AA, Guimarães AR, D'Ambrosio L, Centis R, Muñoz-Torrico M, et al. Post-tuberculosis lung disease a comparison of Brazilian, Italian, and Mexican cohorts. J Bras Pneumol. 2022;48(2):e20210515. doi: 10.36416/1806-3756/e20210515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meghji J, Simpson H, Squire SB, Mortimer K. A Systematic Review of the Prevalence and Pattern of Imaging Defined Post-TB Lung Disease. PLoS One. 2016;11(8):e0161176. doi: 10.1371/journal.pone.0161176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maleche-Obimbo E, Odhiambo MA, Njeri L, Mburu M, Jaoko W, Were F. Magnitude and factors associated with post-tuberculosis lung disease in low- and middle-income countries A systematic review and meta-analysis. PLOS Glob Public Health. 2022;2(12):e0000805. doi: 10.1371/journal.pgph.0000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migliori GB, Marx FM, Ambrosino N, Zampogna E, Schaaf HS, van der Zalm MM, et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis. 2021;25(10):797–813. doi: 10.5588/ijtld.21.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesov D, Butov D, Reimann M, Heyckendorf J, Myasoedov V, Butov T. Impact of lung function on treatment outcome in patients with TB. Int J Tuberc Lung Dis. 2021;25(4):277–284. doi: 10.5588/ijtld.20.0949. [DOI] [PubMed] [Google Scholar]
- 28.Muñoz-Torrico M, Rendon A, Centis R, D'Ambrosio L, Fuentes Z, Torres-Duque C, et al. Is there a rationale for pulmonary rehabilitation following successful chemotherapy for tuberculosis . J Bras. Pneumol. 2016;42(5):374–385. doi: 10.1590/S1806-37562016000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiberi S, Torrico MM, Rahman A, Krutikov M, Visca D, Silva DR. Managing severe tuberculosis and its sequelae from intensive care to surgery and rehabilitation. J Bras Pneumol. 2019;45(2):e20180324. doi: 10.1590/1806-3713/e20180324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muñoz-Torrico M, Cid-Juárez S, Gochicoa-Rangel L, Torre-Bouscolet L, Salazar-Lezama MA, Villarreal-Velarde H. Functional impact of sequelae in drug-susceptible and multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2020;24(7):700–705. doi: 10.5588/ijtld.19.0809. [DOI] [PubMed] [Google Scholar]
- 31.Ross J, Ehrlich RI, Hnizdo E, White N, Churchyard GJ. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax. 2010;65(11):1010–1015. doi: 10.1136/thx.2009.129999. [DOI] [PubMed] [Google Scholar]
- 32.Bongomin F. Post-tuberculosis chronic pulmonary aspergillosis An emerging public health concern. PLoS Pathog. 2020;16(8):e1008742. doi: 10.1371/journal.ppat.1008742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nightingale R, Chinoko B, Lesosky M, Rylance SJ, Mnesa B, Banda NPK. Respiratory symptoms and lung function in patients treated for pulmonary tuberculosis in Malawi a prospective cohort study. Thorax. 2022;77(11):1131–1139. doi: 10.1136/thoraxjnl-2021-217190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marx FM, Floyd S, Ayles H, Godfrey-Faussett P, Beyers N, Cohen T. High burden of prevalent tuberculosis among previously treated people in Southern Africa suggests potential for targeted control interventions. Eur Respir J. 2016;48(4):1227–1230. doi: 10.1183/13993003.00716-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh S, Allwood BW, Chiyaka TL, Kleyhans L, Naidoo CC, Moodley S. Immunologic and imaging signatures in post tuberculosis lung disease. Tuberculosis (Edinb) 2022;136:102244–102244. doi: 10.1016/j.tube.2022.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta MB, Bagri S, Garg A, Singh DK, Choudhary P, Sahni S. Pulmonary function in cured pulmonary tuberculosis cases. Indian J Tuberc. 2022;69(4):535–538. doi: 10.1016/j.ijtb.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Curry BD, van T Wout E, Maasdorp E, Nortje A, Irusen EM, Maree D, et al. Correlation between lung function tests and peak oxygen consumption in post-TB lung disease. Int J Tuberc Lung Dis. 2022;26(3):259–267. doi: 10.5588/ijtld.21.0504. [DOI] [PubMed] [Google Scholar]
- 38.Sivaranjini S, Vanamail P, Eason J. Six minute walk test in people with tuberculosis sequelae. Cardiopulm Phys Ther J. 2010;21(3):5–10. doi: 10.1097/01823246-201021030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira MC, Athanazio RA, Dalcin PTR, Figueiredo MRF, Gomes M, Freitas CG. Brazilian consensus on non-cystic fibrosis bronchiectasis. J Bras Pneumol. 2019;45(4):e20190122. doi: 10.1590/1806-3713/e20190122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Thoracic SocietyAmerican College of Chest Physicians ATS/ACCP Statement on cardiopulmonary exercise testing [published correction appears in Am J Respir Crit Care Med. 2003 May 15;1451-2] Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 41.Venkitakrishnan R, Ramachandran D, Augustine J, Cleetus M. Inhaled corticosteroids and risk of tuberculosis-How bad is the risk . Indian. J Tuberc. 2022;69(2):128–130. doi: 10.1016/j.ijtb.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-García MÁ, Oscullo G, García-Ortega A, Matera MG, Rogliani P, Cazzola M. Inhaled Corticosteroids in Adults with Non-cystic Fibrosis Bronchiectasis From Bench to Bedside. A Narrative Review. Drugs. 2022;82(14):1453–1468. doi: 10.1007/s40265-022-01785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bongomin F, Harris C, Hayes G, Kosmidis C, Denning DW. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. PLoS One. 2018;13(4):e0193732. doi: 10.1371/journal.pone.0193732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 45.Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Pt 1Am J Respir Crit Care Med. 1999;160(5):1525–1531. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- 46.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D. An official European Respiratory Society/American Thoracic Society technical standard field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 47.Jones SE, Kon SS, Canavan JL, Patel MS, Clark AL, Nolan CM. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax. 2013;68(11):1015–1020. doi: 10.1136/thoraxjnl-2013-203576. [DOI] [PubMed] [Google Scholar]
- 48.Grønseth R, Vollmer WM, Hardie JA, Ólafsdóttir IS, Lamprecht B, Buist AS. Predictors of dyspnoea prevalence results from the BOLD study. Eur Respir J. 2014;43(6):1610–1620. doi: 10.1183/09031936.00036813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325. doi: 10.1002/j.2048-7940.1989.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 52.Sancho J, Servera E, Díaz J, Marín J. Comparison of peak cough flows measured by pneumotachograph and a portable peak flow meter. Am J Phys Med Rehabil. 2004;83(8):608–612. doi: 10.1097/01.PHM.0000133431.70907.A2. [DOI] [PubMed] [Google Scholar]
- 53.American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 54.Kim SJ, Lee J, Park YS, Lee CH, Lee SM, Yim JJ. Effect of airflow limitation on acute exacerbations in patients with destroyed lungs by tuberculosis. J Korean Med Sci. 2015;30(6):737–742. doi: 10.3346/jkms.2015.30.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Global Initiative for Chronic Obstructive Lung Disease (GOLD) [homepage on the Internet] 2020 Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. Bethesda: GOLD; https://goldcopd.org/2023-gold-report-2 [Google Scholar]
- 56.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 57.Datta S, Gilman RH, Montoya R, Quevedo Cruz L, Valencia T, Huff D. Quality of life, tuberculosis and treatment outcome; a case-control and nested cohort study. Eur Respir J. 2020;56(2):1900495–1900495. doi: 10.1183/13993003.00495-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silva PA, Soares SM, Santos JF, Silva LB. Cut-off point for WHOQOL-bref as a measure of quality of life of older adults. Rev Saude Publica. 2014;48(3):390–397. doi: 10.1590/S0034-8910.2014048004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jo YS, Park S, Kim DK, Yoo CG, Lee CH. The cutoff point of clinical chronic obstructive pulmonary disease questionnaire for more symptomatic patients. BMC Pulm Med. 2018;18(1):38–38. doi: 10.1186/s12890-018-0601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasiri MJ, Silva DR, Rommasi F, Zahmatkesh MM, Tajabadi Z, Khelghati F, et al. Vaccination in post-tuberculosis lung disease management: A review of the evidence [published online ahead of print, 2023 Sep 5] Pulmonology. 2023 doi: 10.1016/j.pulmoe.2023.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Siddiq MAB, Rathore FA, Clegg D, Rasker JJ. Pulmonary Rehabilitation in COVID-19 patients A scoping review of current practice and its application during the pandemic. Turk J Phys Med Rehabil. 2020;66(4):480–494. doi: 10.5606/tftrd.2020.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]


