Invasive pathogenic bacteria overcome the defense mechanisms of their animal host and proliferate at its expense. They all have their own lifestyle and target organs, leading to a variety of symptoms and diseases, which could suggest the existence of great diversity among the bacterial virulence strategies. However, there are only a very restricted number of basic virulence mechanisms while genetic reshuffling introduces endless modulations and combinations. One of these basic mechanisms was unravelled only in the last few years. By this mechanism, sometimes referred to as “type III,” extracellularly located bacteria that are in close contact with a eukaryotic cell deliver toxic bacterial proteins into the cytosol of this cell. The animal pathogens sharing this type of system are Yersinia spp., Salmonella spp., Shigella spp., enteropathogenic and enterohemorrhagic Escherichia coli, Pseudomonas aeruginosa, Chlamydia psittaci (43), and Bordetella spp. (123). This mechanism is also found in the plant pathogens that elicit the so-called “hypersensitive response,” such as Erwinia amylovora, Pseudomonas syringae, Xanthomonas campestris, and Ralstonia solanacearum (for reviews, see references 1, 112, and 113). Finally, a related system is encountered in Rhizobium spp., where it serves not pathogenic but symbiotic purposes (115).
In this minireview, I will describe the Yersinia “Yop virulon,” which represents a paradigm for these type III systems. I will first give an overview of the system, and then I will describe the complex secretion-translocation apparatus made up of the Syc cytosolic chaperones, the Ysc secretion channel, the Yop translocators, and some proteins involved in the control of Yop release. I will finally deal with the “effector” Yops, the reason for the whole system. The aim is not to be exhaustive but rather to present our current view of this fast-evolving topic and to discuss ideas in a lively, stimulating, and perhaps also provocative way. The emphasis will be on bacteriology rather than on eukaryotic cell biology. I apologize for any involuntary bias the reader may discover in favor of the species Yersinia enterocolitica, with which I am working. More information on Yersinia virulence in general is available in exhaustive reviews (17, 20, 76), and a complete review of type III systems appeared recently (45).
AN OVERVIEW OF THE SYSTEM
The Yersinia lifestyle.
The Yop virulon, a weapon common to Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica, endows these three pathogens with the capacity to resist the nonspecific immune response. In particular, it protects them from the macrophage by destroying its phagocytic and signalling capacities and, finally, inducing its apoptosis. In agreement with these in vitro observations, pathological examinations of experimentally infected animals indicate that yersiniae are largely extracellular (100).
Unravelling the basic model.
When placed at 37°C in a medium deprived of Ca2+ ions, Yersinia spp. cease growing and, instead, secrete a set of proteins called Yops. This unusual capacity, strictly correlated with virulence, is encoded by a very conserved 70-kb plasmid that has been completely sequenced in Y. enterocolitica (49) and Y. pestis (44, 78).
Purified secreted Yops have no cytotoxic effect on cultured cells, although live extracellular yersiniae have such an activity (91). Cytotoxicity was nevertheless found to depend on the capacity of the bacterium to secrete YopE and YopD. However, YopE alone was found to be cytotoxic when microinjected into the cells. This led to the hypothesis that YopE is a cytotoxin that needs to be injected into the eukaryotic cell’s cytosol by a mechanism involving YopD in order to exert its effect (92). In 1994, this hypothesis was demonstrated by two different approaches. The first one was based on immunofluorescence and confocal laser scanning microscopy examinations (93). The second approach was based on a reporter enzyme strategy introduced by Sory and Cornelis (104). The reporter system consisted of the calmodulin-activated adenylate cyclase domain (called Cya) of the Bordetella pertussis cyclolysin (35). Infection of a monolayer of eukaryotic cells by a recombinant Y. enterocolitica producing a Yop-Cya hybrid enzyme led to accumulation of cyclic AMP in the cells. Since the enzyme is not functional in the bacterial cell and in the culture medium because of a lack of calmodulin, this accumulation of cyclic AMP signified the internalization of YopE-Cya into the cytosol of eukaryotic cells (104). Thus, extracellular yersiniae inject YopE into the cytosol of eukaryotic cells by a mechanism that involves at least one other Yop protein, YopD. YopH was later demonstrated to be also injected into the target cell cytosol (80, 103), and YopB was shown to be required for delivery of YopE and YopH, like YopD. These observations led to the present concept that Yops are a collection of intracellular effectors (including YopE and YopH) and proteins required for translocation of these effectors across the plasma membrane of eukaryotic cells (including YopB and YopD) (Fig. 1).Delivery into eukaryotic cells of less abundant Yop proteins such as YopO/YpkA, YopP/YopJ, and YopT turned out to be more difficult to demonstrate because of the limited sensitivity of the methods used. However, the difficulty could be circumvented by the use of mutant strains of yersiniae in which most of the genes encoding effectors have been knocked out, facilitating the traffic of the remaining Yops (10, 38). Most of the Yop proteins have been sorted out by the two different methods. Six Yops, YopE, YopH, YpkA/YopO, YopM, YopP/YopJ, and YopT, appeared to be intracellular effectors (11, 38, 47, 50, 80, 93, 103–105). The role of LcrV, a Yop with a different name (see below), is not so clear. One recent report indicates that it is required for the extrusion of YopB and YopD from the bacterial cell (97), while another concludes that it is necessary for the deployment of YopB (70). Whatever the exact role, both reports conclude that LcrV is indirectly necessary for the delivery of the effectors. YopB, YopD, and LcrV will thus be considered translocator Yops. Not surprisingly, they are encoded by three neighboring genes arranged as an operon. Another Yop, YopN, behaves as a plug closing the secretion channel (see below). Although it was first believed that YopN is not translocated (11), more recent data indicate that this Yop also ends up in the cytosol of the target cell (57).
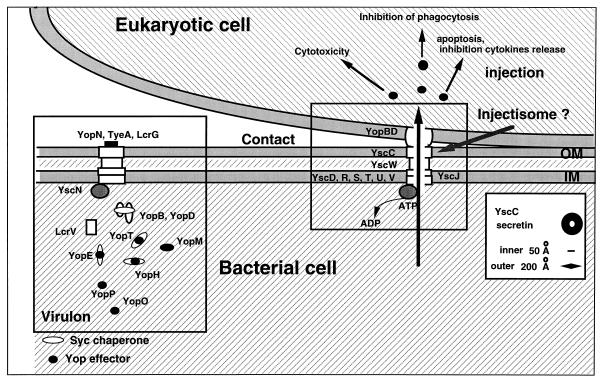
FIG. 1.
The basic model. When yersiniae are placed at 37°C in a rich environment, the Ysc secretion apparatus is installed and a stock of Yop proteins is synthesized. As long as there is no contact with a eukaryotic cell, a stop valve, possibly made of YopN, TyeA, and LcrG, blocks the Ysc secretion channel. Upon contact with the eukaryotic target cell, a sensor interacts with a receptor on the cell surface, which results in the opening of the secretion channel at the zone of contact. The Yops are then transported through the secretion channel, and the Yop effectors are translocated across the plasma membrane guided by YopB and -D. During their intrabacterial stage, Yops are capped with their specific chaperones, presumably to prevent premature associations. The rectangle on the left encloses the virulon, while that on the right encloses the injectisome.
This model of intracellular delivery of Yop effectors by extracellular adhering bacteria is largely supported by a number of other results. Among others, it is supported by immunological observations. While antigens processed in phagocytic vacuoles of phagocytes are cleaved and presented by major histocompatibility complex class II molecules, epitope 249 to 257 of YopH produced by Y. enterocolitica during a mouse infection is presented by major histocompatibility complex class I molecules, like cytosolic proteins (108).
The yersinia virulence plasmid thus encodes a specialized secretion-translocation system and Yop effectors. About 50 genes are involved, and they occupy three quarters of the plasmid. Thirty-five genes encoding the secretion and translocation machineries form a continuous block flanked on both sides by more dispersed genes encoding effectors and their chaperones.
Conditions of operation.
Yop delivery requires living bacteria adhering to their target cell. In Y. pseudotuberculosis, invasin (Inv) is the main adhesin promoting Yop translocation (80). In Y. enterocolitica, the adhesin YadA is more important than Inv (104), but either YadA or Inv will suffice to initiate the contact between Y. enterocolitica and epithelial cells to allow subsequent translocation (16). The situation is not clear for Y. pestis, which lacks these two adhesins.
Delivery of effector Yops into eukaryotic cells appears to be a directional phenomenon in the sense that the majority of the Yop effector molecules produced are directed into the cytosol of the eukaryotic cell and not to the outside environment (93). There is some disagreement about the degree of “directionality.” By using various Cya reporters, Boland et al. (11) determined that roughly only half of the amount of Yops secreted by Y. enterocolitica is directed into cultured macrophages. In contrast, Persson et al. (80) reported that Y. pseudotuberculosis delivers more than 99% of the YopH-associated protein tyrosine phosphatase activity into HeLa cells. Whether the discrepancy originates from the bacterium or from the system used for the study is not known. Whatever the degree, both groups observed that more Yops are delivered into eukaryotic cells than into the culture supernatant, which implies that contact with eukaryotic cells triggers the release.
Finally, translocation occurs not from intravesicular yersiniae but exclusively from extracellular yersiniae. Indeed, selective killing of extracellular bacteria by gentamicin inhibits cytotoxicity (80, 91) and translocation of YopH. Moreover, the use of cytochalasin D, which inhibits the entry of bacteria into cells, does not greatly affect the amount of YopE that is internalized (104). Translocation of Yops is thus the feat of extracellular yersiniae.
THE SYC CYTOSOLIC CHAPERONES
In the first stages of the process, Yops appear to be cytosolic, and some of them are associated with specific chaperones called Sycs (specific Yop chaperones) (117).
Syc cytosolic chaperones SycD, SycE, SycH, SycT, and SycN.
Normal secretion of YopE, YopH, YopN, and YopT by a wild-type yersinia requires the presence of SycE, SycH, SycN, and SycT, respectively (47, 48, 116, 117). All of these chaperones are small (14 to 15 kDa), acidic (pI 4.4 to 5.2) proteins with a putative C-terminal amphiphilic α-helix, and each binds only to its partner Yop. SycE and SycH possess a conserved leucine repeat motif in this α-helix structure, where most of the hydrophobic residues, essentially leucines, are present on the same side of the helix. A consensus sequence was derived by Wattiau et al. (118) from the alignment of this conserved leucine repeat of SycE, SycH, and their homologs (LLWxRxPLxxxxxxxLxxxLExLVxxAExL). In Y. enterocolitica, each of these chaperones is encoded by a gene located close to the gene encoding the corresponding Yop (49). SycD is slightly different from this group of chaperones, and it will be treated separately. No chaperone has been found for YopO/YpkA, YopP/YopJ, YopK/YopQ, YopR, LcrV, or YopM. Although the sequence of pYV227 reveals the existence of an additional putative orphan chaperone (ORF155) (49), it is likely that not every Yop has a chaperone.
Role of SycE and SycH.
Research on Syc chaperones focused first on SycE and SycH. Each of these two chaperones binds to its partner Yop at a unique site spanning roughly residues 20 to 70 (103) (Fig. 2). Surprisingly, when this site is removed, the Yop is still secreted—though maybe in reduced amounts—and the chaperone becomes dispensable for secretion (121). This suggests that it is the binding site itself that creates the need for the chaperone and, thus, that the chaperone somehow protects this site from premature associations which lead to degradation. In agreement with this hypothesis, SycE has an antidegradation role, since the half-life of YopE is longer in wild-type bacteria than in sycE mutant bacteria (19, 32). The antidegradation role of SycH is not as clear as that of SycE, because YopH can be detected in the cytosol of sycH mutant bacteria (116). In addition to this putative bodyguard role, SycE also acts as a secretion pilot, leading the YopE protein to the secretion locus (5). Finally, both SycE and SycH are also required for efficient translocation of their partner Yops into eukaryotic cells (57, 103, 121) (see below).
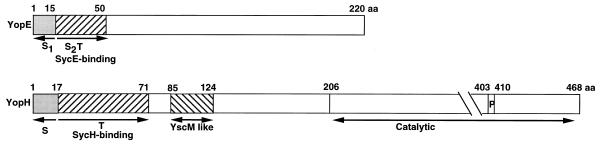
FIG. 2.
Schematic representation of YopE and YopH. S1, first secretion domain; S2/T, second secretion domain and translocation domain; P, catalytic P-loop site. Residues 85 to 124 of YopH present a significant but unexplained similarity to the hypothetical regulator LcrQ/YscM. aa, amino acids.
SycD, the chaperone of YopB and YopD.
SycD (called LcrH in Y. pseudotuberculosis and Y. pestis) (7, 86) is a chaperone serving both YopB and YopD (69, 116). In the absence of SycD, YopD and YopB are less detectable inside the bacterial cell (69, 116). SycD appears to be somewhat different from SycE and SycH in that it binds to several domains of YopB (69). This evokes SecB, a molecular chaperone in E. coli which is dedicated to the export of newly synthesized proteins (54) and also has multiple binding sites on its targets (51).
SycD resembles the chaperone IpgC from Shigella flexneri, which has been shown to prevent the association between IpaB and IpaC (60). The similarity between IpgC and SycD suggested that SycD could play a similar role and would thus prevent the intrabacterial association of YopB and YopD. Surprisingly, intrabacterial YopB and YopD seem to be associated in the bacterium, even in the presence of SycD (69). Since YopB and YopD also have the capacity to bind to LcrV (97), one could speculate that SycD prevents premature association of YopB, YopD, and LcrV (69).
RECRUITMENT FOR EXPORT
To be secreted, Yops need to be specifically recognized by the secretion apparatus. Two secretion signals have been identified.
A first secretion signal on Yops.
When Yop secretion was established, it appeared that Yops are recognized by their N terminus but that no classical signal sequence is cleaved off during Yop secretion (64). The minimal region shown to be sufficient for secretion was gradually reduced to 17 residues of YopH (103), to 15 residues of YopE (103) (Fig. 2), and to 15 residues of YopN (5).
There is no similarity between the secretion domains of the Yops, which suggested recognition of a conformational motif of the nascent protein (62). To explain why proteins with no common signal could be recruited by the same secretion apparatus, Wattiau and Cornelis (117) suggested that the Syc chaperones could serve as pilots. However, this hypothesis was questioned when it appeared that YopE could be secreted even if its chaperone-binding domain had been deleted (32, 121). It was then concluded that secretion was dependent only on the short N-terminal signal, but secretion of a Yop lacking only this N-terminal signal had never been tested.
Systematic mutagenesis of the secretion signal by Anderson and Schneewind (5) led to doubts about the proteic nature of this signal. No point mutation could be identified that specifically abolished the secretion of YopE or YopN. Moreover, some frameshift mutations that completely altered the peptide sequences of the signals also failed to prevent secretion. Anderson and Schneewind (5) concluded from these observations that the signal that leads to the secretion of Yops could be in the 5′ end of the mRNA rather than in the peptide sequence.
A second secretion signal.
To determine whether this N-terminal (or 5′-terminal) signal is absolutely required for YopE secretion, Cheng et al. (19) deleted codons 2 to 15, and they observed that 10% of the hybrid proteins deprived of the N-terminal secretion signal were still secreted. They inferred that there is a second secretion signal, and they showed that this second, and weaker, secretion signal corresponds to the SycE-binding site. Not surprisingly, this secretion signal is functional only in the presence of the SycE chaperone (19), rejuvenating the pilot hypothesis (117). The Syc chaperone could ensure the stability and proper conformation of the protein and target it to the secretion channel. At the moment of secretion, the chaperone must be released from the partner Yop to allow secretion.
Thus, YopE and YopH (possibly also YopN and YopT) are likely to have two secretion signals (Fig. 2). For the other Yops, the N-terminal secretion signal would be the only one. No signal sequence is removed from YopB, YopD, and LcrV upon secretion, but their secretion signal has not been identified.
THE YSC SECRETION MACHINERY: A BIOLOGICAL SYRINGE
Global composition.
The Ysc secretion apparatus is complex and far from being completely characterized. Some elements have been localized in the inner membrane, while others are inserted in the outer membrane. It is thought to form a continuous channel across the two bacterial membranes, but this has not been formally proven. It is encoded by four contiguous loci that are called virA (yopN, tyeA, sycN, yscXYV, lcrR), virB (yscNOPQRSTU), virG, and virC (yscABCDEFGHIJKLM) in Y. enterocolitica (3, 6, 23, 27, 48, 63). The yscV gene was initially described in Y. pestis as lcrD (lcr for low calcium response) (81, 83). In total, 29 genes have been identified within these loci. Knockout mutants have been constructed for most of them, and with very few exceptions, they are deficient in Yop secretion.
We are still very far from assigning a role to each of the 29 components of the Ysc machine. However, I suspect that one of them, still unidentified, is some kind of endoglucanase. Indeed, the mesh of the peptidoglycan is presumably too small to allow installation of the Ysc apparatus and needs to be locally dismantled.
Characterized elements.
YscC (52, 63, 83) belongs to the family of secretins, a group of outer membrane proteins involved in the transport of various macromolecules and filamentous phages across the outer membrane. Like the other secretins, it exists as a very stable multimeric complex of about 600 kDa (52, 83) that forms a ring-shaped structure with an external diameter of about 200 Å and an apparent central pore of about 50 Å (52). In comparison, the pIV secretion of phage f1 has an internal diameter of about 80 Å, allowing the passage of the filamentous capsid with a diameter of 65 Å (59). Lipoprotein YscW (previously called VirG) (2) is required for efficient targeting of the YscC complex to the outer membrane (52). The Ysc apparatus also contains another lipoprotein called YscJ (Fig. 1).
Four proteins have been shown to span the inner membrane (Fig. 1): YscD (83), YscR (27), YscU (4), and YscV (formerly LcrD) (82). According to their sequences, two other proteins, YscS and YscT, are probably also anchored in the inner membrane. Finally, YscN is a 47.8-kDa protein with ATP-binding motifs (Walker boxes A and B) resembling the β catalytic subunit of FoF1 proton translocase and related ATPases (120). It probably energizes the secretion process.
Secreted Ysc proteins.
Somewhat unexpectedly, the loci encoding the secretion apparatus also encode a few proteins that are themselves secreted by the machine. These are LcrQ/YscM, YopR (the product of yscH) (3), YopN, YscO (74), and YscP (75, 106). LcrQ/YscM is thought to be a regulator (see below), and YopN is considered the plug closing down the channel and will be described below. YscO is necessary for secretion of all of the Yops (74), but this is not the case for YscP (75) or YopR (3).
Overlapping of the secretion and translocation functions.
We will see below that YopN, TyeA, and LcrG are required to keep the secretion channel closed. They must thus be somehow associated with the secretion channel. YopN appeared to be dispensable for translocation of the effectors across the eukaryotic cell membrane (11, 93). However, TyeA is necessary for translocation of YopE and YopH (50) and LcrG is required for efficient translocation of all of the known Yop effectors into macrophages (98). These observations suggest that secretion and translocation are tightly coupled operations carried out by the same complex apparatus.
A supramolecular structure resembling the flagellum: the injectisome?
The Ysc machinery, like any type III secretion machinery, contains several pieces that have homologues in the flagellar assembly apparatus, which suggests some relationship between the two structures. This assumption has been strikingly supported by the recent electron microscopic observation of the type III apparatus of Salmonella typhimurium (53). This apparatus, evoking a syringe, resembles the basal body of a flagellum extended by a straight rod. This rod extends outside the bacterial cell, which indicates that the elements that allow secretion across the two bacterial membranes are only one part of a more complex supramolecular structure. In this context, it is not surprising that some Ysc proteins appear to be “secreted.” The core of the Ysc apparatus would be the syringe, while secreted Ysc proteins or the translocator Yops would form the needle.
To the question of whether the flagellum has a type III secretion system, the answer for me is undoubtedly yes, but the flagellum is obviously much more than a secretion apparatus. The flagellum is an organelle including a type III secretion apparatus. Thus, we should no longer call the virulence organelles type III but should rather introduce a new name evoking an organelle, perhaps something like injectisome, and consider that this organelle includes a type III secretion apparatus. The injectisome itself would only be a part of the whole virulon, which also includes the effector Yops.
To the question of which one is the ancestor, my answer is the flagellum because swimming must have been the first necessity before aggressing eukaryotes. Thinking along this line, it would be interesting to look at whether or not such organelles would also be used by environmental bacteria to protect themselves against protozoa.
THE TRANSLOCATION MACHINERY: THE NEEDLE?
Translocators YopB, YopD, and LcrV.
Among the 12 secreted Yops, only 2, YopB and YopD, have hydrophobic domains (37), suggesting that they could interact with membranes. YopD is a 33.3-kDa protein with a central 31-amino-acid-long hydrophobic region (37). The Eisenberg plot analysis (25) suggests that it is a transmembrane protein (37). YopB is a 41.8-kDa protein (37) with two central hydrophobic regions separated by only 15 amino acids (37). It has a moderate level of similarity to proteins of the RTX family of α-hemolysins and leukotoxins (8, 111). YopB and YopD are encoded by the large lcrGV-sycD-yopBD operon (7, 67, 86), which also encodes LcrG, LcrV, and SycD/LcrH, the chaperone of YopB and YopD.
YopB and YopD are both required for translocation of effector Yops across the eukaryotic cell membrane (11, 38, 92, 93, 104). The fact that YopB resembles proteins of the RTX toxin family suggests that the translocation apparatus could involve some kind of pore, in which YopB would be the main element. The observation of Håkansson et al. (39) that Yersinia has a YopB- and contact-dependent lytic activity on sheep erythrocytes supports this hypothesis. This YopB-dependent lytic activity is higher when the effector yop genes are deleted, suggesting that the pore is normally filled with effectors during contact (39). By analysis of the protective effect of sugars of increasing size, Håkansson et al. (39) estimated the internal diameter of the putative pore to be between 12 and 35 Å.
YopB and YopD bind to each other (69), suggesting that they interact with each other at some stage to fulfill their function. They could be associated within the putative pore, but the pore has been neither purified nor observed by electron microscopy. According to Holmström et al. (42), the size of the putative pore is controlled by the 21-kDa YopK/YopQ protein that is encoded outside the lcrGV-sycD-yopBD operon (64).
The lcrGV-sycD-yopBD operon also encodes the LcrV protein. At variance with YopB and YopD, this Yop exhibits a certain degree of polymorphism (88). LcrV has been described as a regulatory protein involved in the calcium response, since an lcrV in-frame deletion mutant was found to be Ca2+ independent and downregulated in the transcription of yop genes (7, 77, 85, 102, 110). However, other data (97) indicate that LcrV could be a functional element of the translocation apparatus, since deletion of the entire lcrV gene abolishes the secretion of LcrV, YopB, and YopD but has no effect on the secretion of the other Yops. Another recent report suggests that LcrV rather facilitates the expression (or stability) and secretion of YopB (70). In agreement with these observations, LcrV interacts with both the YopB and YopD proteins (97), as well as with LcrG (70, 71, 98). From these observations, one can envision LcrV as a third component of the delivery apparatus. One could speculate that it forms some kind of short needle underneath YopB and YopD, but as I have mentioned before, this function could also be fulfilled by the secreted Ysc proteins, such as YscO and YscP.
Is the needle installed before contact with a eukaryotic cell?
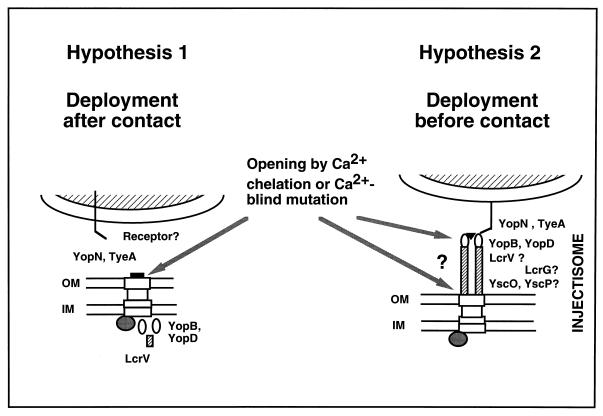
At variance with most of the Ysc proteins, the translocators, like the Yop effectors, are secreted in vitro upon Ca2+ chelation. This led to the hypothesis that the translocation elements (the needle?) are not installed in the presence of Ca2+ or prior to contact with cells (Fig. 3, hypothesis 1). However, several observations indicate that the Ysc secretion apparatus (the syringe) is installed at 37°C in the presence of Ca2+ ions (52). One could thus wonder whether or not some translocators would also be installed on top of the syringe prior to cellular contact (Fig. 3, hypothesis 2). Like the Ysc syringe, the needle would also require sensitive immunoblotting methods to be detected because this situation would be very different from the massive release observed upon Ca2+ chelation. Electron microscopy will probably be the method of choice to settle that question.
FIG. 3.
Two models for translocation. In the first hypothesis, the Ysc secretion apparatus (the syringe) is installed in the bacterial membranes but is closed by YopN and TyeA. Upon contact with a eukaryotic cell, the plug is removed and the translocation apparatus, composed of YopB, YopD, and LcrV (the needle?), grows into the eukaryotic cell. This model is supported by the observation that in vitro, the translocator Yops are secreted only upon Ca2+ chelation, like the other Yops. In this hypothesis, Ca2+ chelation would remove the YopN-TyeA plug, allowing secretion of all of the Yops. In the second hypothesis, the needle is installed before contact. This model is inspired by the electron microscopy observations of Kubori et al. (53) with S. typhimurium. In this model, Ca2+ chelation would either remove a cap at the tip of the needle or separate the needle from the basal body, leaving a large hole and inducing massive secretion of all of the Yops. LcrG could be a critical element at the base of the needle. OM, outer membrane; IM, inner membrane.
CONTROL OF YOP RELEASE
In vivo control of Yop release by cell contact.
When yersiniae are incubated at 37°C in a Ca2+-containing eukaryotic cell culture medium, they do not secrete Yops. Nevertheless, they inject Yops into the eukaryotic cells. This implies that contact with these cells somehow triggers the system and suggests that a particular bacterial ligand could be involved in this contact.
If such a ligand exists, its loss might well result in a permanently open secretion channel and in permanent secretion at 37°C. In other words, such mutants would be insensitive to Ca2+ repression and, hence, be Ca2+ blind. Such mutants have been isolated.
Ca2+-blind mutants.
The isolation of Ca2+-blind mutants, i.e., mutants that secrete Yops even in the presence of Ca2+ (122), allowed the identification of three proteins that are required to keep the channel closed in the presence of Ca2+: YopN (29), LcrG (98, 101), and TyeA (50).
YopN is a 32.6-kDa protein encoded together with ysc genes (29, 48, 50, 114). Under low-Ca2+ conditions, most of the YopN produced is released into the culture supernatant, while in the presence of Ca2+, YopN is not released but is exposed at the bacterial cell surface (29, 50).
TyeA is a 10.8-kDa protein encoded immediately downstream of yopN (29, 50, 114). Like YopN, TyeA is loosely associated with the membrane. It has the capacity to bind to YopN (50) and has been named TyeA by Iriarte et al. because it plays a role in the translocation of some Yop effectors (50). It is probably meaningful that YopN and TyeA are encoded by genes that are buried among ysc genes. It suggests that these two proteins belong to the Ysc syringe and could represent some kind of external cap.
At variance with YopN and TyeA, LcrG is encoded by the operon devoted to the translocators. It is an 11.0-kDa protein that has been shown to be primarily cytosolic (71, 101), and in spite of efforts with both Y. pestis and Y. enterocolitica, it remains uncertain whether some LcrG is surface exposed. One argument in favor of the notion that LcrG is surface exposed is based on the facts that it binds heparan sulfate proteoglycans and that heparin interferes with translocation of YopE into HeLa cells (15) (see below).
To summarize, YopN, TyeA, and LcrG are necessary to keep the secretion channel closed or intact. They could be part of the physiological cap, but it is not necessarily so. Indeed, Ca2+-blind (yopN, tyeA, and lcrG) mutants could have an “open” apparatus, but it is equally possible that they have a “broken” apparatus instead (Fig. 3, hypothesis 2). Finally, it is reasonable to assume that YopN and TyeA are associated with each other, but there is no evidence that LcrG is associated with YopN and TyeA.
Effect of chelating Ca2+ ions.
Ca2+ chelation results in a massive release of Yops. It is likely that Ca2+ chelation specifically displaces one of the three proteins mentioned above, but again, this protein does not need to be the physiological cap. In the hypothesis that the needle is normally installed at 37°C (Fig. 3, hypothesis 2), one could envision Ca2+ chelation either uncapping the needle or breaking it at another site. In both hypotheses, Ca2+ chelation would open the channel and trigger massive Yop secretion. Now, let us come back to the basic question.
Is YopN, TyeA, or LcrG the sensor involved in eukaryotic cell recognition?
As mentioned previously, it seems reasonable to assume that a mutant lacking the sensor would be constitutively open and thus have the Ca2+-blind phenotype. In that hypothesis, one of the three proteins could be the sensor. The observation that LcrG binds to heparan sulfate proteoglycans (15) is consistent with that hypothesis, but the significance of this binding is not clear (see below). As far as YopN and TyeA are concerned, it is not known whether they interact with eukaryotic cells. Finally, one cannot exclude the possibility that a mutant lacking the sensor would be constitutively closed. In that hypothesis, the phenotype would be “nonsecreting,” a phenotype shared by most of the ysc mutants.
Is there a eukaryotic cell receptor?
The fact that LcrG binds to heparin-agarose beads and to heparan sulfate proteoglycans (15) suggests that there could be a specific cell receptor. In agreement with this observation, the addition of exogenous heparin decreases the level of YopE-Cya translocation into HeLa cells, indicating that the interaction between LcrG and proteoglycans favors translocation. However, proteoglycans cannot be the only cell receptor because heparin does not affect translocation into macrophages (16) and does not affect Yop secretion in vitro (15). The question of a specific receptor is thus still open.
ACTING IN THE EUKARYOTIC CELL: THE POISON
Disturbing the eukaryotic cell in order to avoid the reaction that would clear yersiniae is what it is all about. We have seen in the Introduction that six effectors have been identified. Taking into account the information supplied by the sequence, it seems reasonable to assume that the list is now complete. We will briefly mention the action of each of them.
YopE.
YopE (30, 64) is a 23-kDa cytotoxin that leads to disruption of the microfilament structure (92). Its exact enzyme activity and target are still unknown. Along with YopH, it blocks phagocytosis.
YopH.
YopH (14, 61) is a powerful protein tyrosine phosphatase with a molecular mass of 51.0 kDa (36). It contributes to the inhibition of bacterial uptake by dephosphorylation of p130Cas and FAK and disruption of peripheral focal complexes (9, 79, 90). YopH inhibits phagocytosis by polymorphonuclear neutrophils and macrophages, mediated by complement receptors (96) or Fc receptors, respectively (26).
YopM.
YopM (11, 24, 28, 58, 67) is a strongly acidic protein with a molecular mass of approximately 40 kDa. Unlike the other Yop effector proteins that are well conserved among different Yersinia species, YopM is somewhat heterogeneous (12). Due to the presence of leucine-rich repeats, YopM shows similarity to a great number of eukaryotic proteins, but its intracellular target and action remain unknown.
YopO/YpkA.
YpkA is a protein kinase (33) with some similarity to the COT (cancer Osaka thyroid) oncogene product, a cytosolic serine/threonine protein kinase expressed in hematopoietic cells and implicated in signal transduction by growth factors (41). YpkA catalyzes autophosphorylation of a serine residue in vitro. Infection of HeLa cells with a multiple yop mutant overproducing YpkA leads to a morphological alteration of the cells different from those mediated by YopE and YopH. The cells round up but do not detach from the extracellular matrix. Inside HeLa cells, the YpkA protein is targeted to the inner surface of the plasma membrane (38). No target protein of YpkA/YopO has been identified.
YopP/YopJ.
YopP (YopJ in Y. pestis and Y. pseudotuberculosis) is a 32.5-kDa protein encoded by the same operon as YpkA/YopO (24, 34). It induces apoptosis of murine macrophages (65, 66) but not of other cell types, such as epithelial cells or fibroblasts (65, 66).
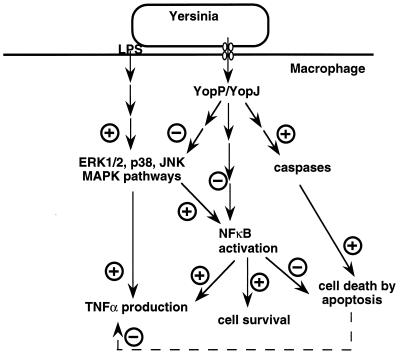
Injection of YopP/YopJ into macrophages also leads to a significant reduction in the release of tumor necrosis factor alpha (TNF-α), a proinflammatory cytokine playing a central role in the development of the immune and inflammatory responses to infection (10, 73) (Fig. 4). This reduction probably results from the inhibition of NF-κB activation by YopP/YopJ (94, 99). YopP/YopJ has also been shown to inhibit the ERK1/2, p38, and JNK mitogen-activated protein kinase (MAPK) activities (Fig. 4) (10, 73, 95). One can thus speculate that YopP would act upstream or at the junction of cascades leading to apoptosis on the one hand and to the inhibition of TNF-α on the other hand.
FIG. 4.
Model showing the effects of YopP/YopJ on the macrophage intracellular cascades. Lipopolysaccharide (LPS) activates the ERK1/2, JNK, and p38 MAPK pathways, which leads to increased TNF-α production. Activated MAPKs can lead to NFκB activation; activated NFκB can, in turn, enhance TNF-α transcription. Translocated YopP/YopJ induces macrophage apoptosis by a mechanism involving caspase activation. It also downregulates MAPKs and impairs NFκB activation, two effects that could explain the YopP/YopJ-induced reduction of TNF-α production. See the text for details and references.
Interestingly, YopP and YopJ share a high level of similarity to Xanthomonas campestris AvrRxv (119) and Rhizobium y410 (31). No function is known for AvrRxv and y410. However, AvrRxv is one of many avirulence proteins identified in plant pathogens that elicit the hypersensitive response, a process that is likely to result from the activation of a programmed cell death pathway (119). However, no cytotoxic effect has been described for AvrRxv. Animal and plant pathogens, therefore, share a type III secretion-dependent effector to elicit programmed cell death in their respective hosts.
YopT.
YopT is a 35.5-kDa protein that has been described and characterized recently (47). YopT induces a cytotoxic effect in HeLa cells and macrophages. The effect on HeLa cells consists of disruption of the actin filaments and alteration of the cell cytoskeleton.
REGULATION OF TRANSCRIPTION OF THE VIRULON GENES
As we have seen, in vitro, Yop secretion occurs only at 37°C in the absence of Ca2+. Transcription of the ysc genes and the yop genes is strongly thermoinduced. This thermoregulation results from the interplay between the transcriptional activator VirF (LcrF in Y. pestis and Y. pseudotuberculosis) and chromatin structure. Transcription of the yop genes is repressed by Ca2+ or by mutations in the secretion apparatus. This second regulation is a feedback inhibition mechanism exerted by the closed secretion apparatus on transcription of the genes encoding the proteins to be secreted.
The positive loop.
VirF/LcrF is a 30.9-kDa protein that belongs to the AraC family of regulators (22). In Y. enterocolitica, the virF gene itself is strongly thermoregulated and VirF is active only at 37°C. Indeed, when virF is transcribed at low temperature from a tac promoter, the yop genes are only poorly transcribed. By contrast, at 37°C, the response to isopropyl-β-d-thiogalactopyranoside (IPTG) mimics the normal response to thermal induction (56). Thus, the expression of the yop regulon is first controlled by temperature, and the expression of some of its genes is reinforced by the action of VirF, the synthesis of which is also temperature controlled. The most likely hypothesis is that temperature could somehow modify the structure of the chromatin, making the promoters more accessible to VirF. Rohde et al. (89) confirmed that temperature alters DNA supercoiling and DNA bending by VirF in Y. enterocolitica, and they hypothesized that temperature dislodges a repressor, perhaps histone-like protein YmoA (21), bound on promoter regions of VirF-sensitive genes and of some other thermoregulated genes.
Feedback control of Yop synthesis by the secretion apparatus: LcrQ/YscM.
By analogy with the secreted antisigma factor involved in the regulation of flagellum synthesis (46, 55), Rimpiläinen et al. (87) suggested that feedback inhibition could be mediated by a repressor that is normally expelled via the Yop secretion machinery. In Y. pseudotuberculosis, they suggested that LcrQ, a 12.4-kDa secreted protein encoded by the last gene of the virC locus, could be this hypothetical regulator because overproduction of this protein abolishes Yop production. In Y. enterocolitica, there are two homologues of LcrQ: YscM1 and YscM2 (3, 107). How LcrQ or YscM1 and YscM2 work is still unknown, but their mode of action is probably indirect.
CONCLUSIONS AND FUTURE PERSPECTIVES
Although we have come close to a global picture, it is important to stress that many basic questions remain. Indeed, the system may now become a victim of its popularity, and after a period of excessive suspicion, we might fall into a period of oversimplification. I will thus end with a few questions that will allow us to remember the complexity of the system and the main present issues.
What is a Yop?
The term Yop was coined by Wolf-Watz and colleagues (13) to qualify the Yersinia outer membrane proteins initially described by Portnoy et al. (84) and Straley and Brubaker (109) and shown by Heesemann and colleagues (40) to be “released” from the bacterium. Later, Michiels, other coworkers, and I showed that these proteins are true extracellular proteins secreted by a new system, called Ysc (for Yop secretion), and encoded by the same plasmid (62–64). Yops can thus be defined as yersinia proteins secreted by the Ysc apparatus under low-Ca2+ conditions. By this definition, LcrV, the protective antigen of plague known since the mid-1950s (18), is clearly a Yop. Moreover, it is encoded by the same operon as the translocator Yops and is involved in translocation. LcrV is not the only Yop with a different name; four other proteins (YopR, YscO, YscP, and LcrQ/YscM) that are secreted under the same conditions as Yops appear to be encoded by operons devoted to secretion. Since this is also the case for YopN, there is no reason to exclude these four proteins from the Yop family. Thus, Yop proteins form a family of secreted proteins that includes intracellular effectors and several components of the secretion-translocation apparatus that is released from the bacterium upon Ca2+ chelation. In spite of the constant care of yersiniologists, nomenclature remains somewhat unfriendly. For the sake of clarity, new names could be introduced when the functions of all of these proteins become clear.
All of the Yops are thus elements of the complex apparatus described here. However, it must be stressed that this does not exclude the possibility that some Yops have another role, on their own, independent of the system, simply as proteins released in the organism. Indeed, Nakajima et al. provided evidence for a direct immunosuppressive effect of purified LcrV injected into mice (68).
What does the yersinia organelle look like?
By analogy with S. typhimurium, it is very likely that the Ysc proteins and the translocators encoded by 35 contiguous genes form a unique organelle. In this minireview, it was assumed that it resembles the salmonella organelle. However, it must be clearly stated that this is no more than an assumption. What are the elements of the Ysc syringe, what are the components of the needle, and what is the role of LcrV and LcrG in the organelle seem to be the most haunting questions in that respect. Electron microscopy techniques such as those that provided wonderful images of the assembling flagellum (72) or the S. typhimurium type III apparatus (53) will certainly help answer many of these questions.
Is a pore formed in the eukaryotic cell?
Although not firmly established, the existence of a pore is very likely, as suggested by the contact hemolysis shown by Håkansson et al. (39), but this pore needs to be characterized. Does it consist of YopB alone or of YopB associated with YopD? What is the role of LcrV in the assembly of this hypothetical pore? Could it form some kind of needle underneath YopB and YopD? What is the function of LcrG in translocation? Do the translocators interact with the effector Yops during translocation?
What does contact control mean?
Contact control is one of the most appealing aspects of the system, but as we have seen, several major questions remain. (i) Is the translocation apparatus deployed prior to contact? (ii) Does Ca2+ chelation really mimic contact, or does it cause another event leading to massive artifactual leakage? (iii) What is the degree of “directionality”? (iv) Last but not least, how does contact lead to the opening of the channel. Generally, contact means an interaction between a receptor and a ligand. However, in this case, no specific receptor at the cell surface has been identified. Recognition of heparan sulfate proteoglycans by LcrG is a first clue but certainly not the definite answer. To identify a receptor, we first have to guess the identity of the bacterial ligand. Although several pieces of the system have been localized at the bacterial surface, the actual bacterial ligand has not been identified. Is it YopN, YopN-associated TyeA, or LcrG, or could it be a protein that is not even involved in the control of secretion by Ca2+ ions?
What is the recruitment signal, and what is the function of the Syc chaperones?
The unravelling of the Ysc secretion pathway will also require long collaborative efforts. There are still more than 25 components to localize in the bacterium, and we feel in the situation of the do-it-yourself enthusiast who thinks that there must be extra pieces in his assembly kit! However, in this area, the hottest question is perhaps that of the secretion signal. Does the Ysc apparatus recognize mRNA, and if so, how? What is the exact role of the Syc chaperones? Are they bodyguards, pilots, or both?
Tools and toys for the cell biologist.
Finally, cell biology has a lot of answers to give. Why do yersiniae deliver six effectors to the same target cell? Are the six effectors needed to neutralize the same phagocyte? Would the action of some effectors be very rapid (YopH?) but reversible while the action of others would be slower but irreversible (YopP/YopJ)? Or, alternatively, would some effectors be specifically designed for some cell types and others for other cell types?
In conclusion, research on yersiniae has been extremely fruitful in terms of concepts. The Yop virulon being at the leading edge of type III secretion, it probably represents the most suitable system for investigation of its most basic aspects, such as control, secretion, and translocation. Once again, fundamental research on a system with very little, if any, commercial or health interest has led to new ideas, which we believe will pay off sooner or later.
ACKNOWLEDGMENTS
I thank Robert Macnab, Sophie Bleves, Aoife Boyd, Maite Iriarte, and Cécile Neyt for critical reading and discussions.
The yersinia project is supported by the Belgian Fonds National de la Recherche Scientifique Médicale (Convention 3.4595.97), the Direction générale de la Recherche Scientifique-Communauté Française de Belgique (Action de Recherche Concertée 94/99-172), and the Interuniversity Poles of Attraction Program–Belgian State, Prime Minister’s Office, Federal Office for Scientific, Technical and Cultural affairs (PAI 4/03).
REFERENCES
- 1.Alfano J R, Collmer A. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol. 1997;179:5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui A, Scheen R, Lambert de Rouvroit C L, Cornelis G R. VirG, a Yersinia enterocolitica lipoprotein involved in Ca2+ dependency, is related to ExsB of Pseudomonas aeruginosa. J Bacteriol. 1995;177:4230–4237. doi: 10.1128/jb.177.15.4230-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. doi: 10.1111/j.1365-2958.1995.mmi_18020343.x. [DOI] [PubMed] [Google Scholar]
- 4.Allaoui A, Woestyn S, Sluiters C, Cornelis G R. YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J Bacteriol. 1994;176:4534–4542. doi: 10.1128/jb.176.15.4534-4542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 6.Bergman T, Erickson K, Galyov E E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman T, Håkansson S, Forsberg Å, Norlander L, Macellaro A, Backman A, Bolin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhakdi S, Mackman N, Nicaud J M, Holland I B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black D S, Bliska J B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland A, Cornelis G R. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland A, Sory M P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 12.Boland, A., et al. Unpublished data.
- 13.Bolin I, Portnoy D A, Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985;48:234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolin I, Wolf-Watz H. The plasmid encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988;2:237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 15.Boyd A P, Sory M P, Iriarte M, Cornelis G R. Heparin interferes with translocation of Yop proteins into HeLa cells and binds to LcrG, a regulatory component of the Yersinia Yop apparatus. Mol Microbiol. 1998;27:425–436. doi: 10.1046/j.1365-2958.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyd, A. P. 1998. Unpublished data.
- 17.Brubaker R R. Factors promoting acute and chronic diseases caused by yersiniae. Clin Microbiol Rev. 1991;4:309–324. doi: 10.1128/cmr.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burrows T W, Bacon G A. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956;37:481–493. [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 20.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M.-P. Sory, and I. Stainier. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev., in press. [DOI] [PMC free article] [PubMed]
- 21.Cornelis G R, Sluiters C, Delor I, Geib D, Kaniga K, Lambert de Rouvroit C L, Sory M P, Vanooteghem J C, Michiels T. ymoA, a Yersinia enterocolitica chromosomal gene modulating the expression of virulence functions. Mol Microbiol. 1991;5:1023–1034. doi: 10.1111/j.1365-2958.1991.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 22.Cornelis G R, Sluiters C, Lambert de Rouvroit C L, Michiels T. Homology between virF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J Bacteriol. 1989;171:254–262. doi: 10.1128/jb.171.1.254-262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelis G R, Sory M P, Laroche Y, Derclaye I. Genetic analysis of the plasmid region controlling virulence in Yersinia enterocolitica O:9 by Mini-Mu insertions and lac gene fusions. Microb Pathog. 1986;1:349–359. doi: 10.1016/0882-4010(86)90067-7. [DOI] [PubMed] [Google Scholar]
- 24.Cornelis G R, Vanooteghem J-C, Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987;2:367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 26.Fallman M, Andersson K, Håkansson S, Magnusson K E, Stendahl O, Wolf-Watz H. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect Immun. 1995;63:3117–3124. doi: 10.1128/iai.63.8.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields K A, Plano G V, Straley S C. A low-Ca2+ response (LCR) secretion (ysc) locus lies within the lcrB region of the LCR plasmid in Yersinia pestis. J Bacteriol. 1994;176:569–579. doi: 10.1128/jb.176.3.569-579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsberg A, Bolin I, Norlander L, Wolf-Watz H. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb Pathog. 1987;2:123–137. doi: 10.1016/0882-4010(87)90104-5. [DOI] [PubMed] [Google Scholar]
- 29.Forsberg A, Viitanen A M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 30.Forsberg A, Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988;2:121–133. doi: 10.1111/j.1365-2958.1988.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 31.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 32.Frithz-Lindsten E, Rosqvist R, Johansson L, Forsberg Å. The chaperone-like protein YerA of Yersinia pseudotuberculosis stabilizes YopE in the cytoplasm but is dispensable for targeting to the secretion loci. Mol Microbiol. 1995;16:635–647. doi: 10.1111/j.1365-2958.1995.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 33.Galyov E E, Hakansson S, Forsberg Å, Wolf-Watz H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature. 1993;361:730–732. doi: 10.1038/361730a0. [DOI] [PubMed] [Google Scholar]
- 34.Galyov E E, Hakansson S, Wolf-Watz H. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J Bacteriol. 1994;176:4543–4548. doi: 10.1128/jb.176.15.4543-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guan K L, Dixon J E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990;249:553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- 37.Håkansson S, Bergman T, Vanooteghem J-C, Cornelis G, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Håkansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 39.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 40.Heesemann J, Algermissen B, Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984;46:105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higashi T, Sasai H, Suzuki F, Miyoshi J, Ohuchi T, Taikai S, Mori T, Kakunaga T. Hamster cell line suitable for transfection assay of transforming genes. Proc Natl Acad Sci USA. 1990;87:2409–2412. doi: 10.1073/pnas.87.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fallman M, Magnusson K E, Wolf-Watz H, Forsberg Å. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 43.Hsia R, Pannekoek Y, Ingerowski E, Bavoil P M. Type III secretion genes identify a putative virulence locus of Chlamydia. Mol Microbiol. 1997;25:351–359. doi: 10.1046/j.1365-2958.1997.4701834.x. [DOI] [PubMed] [Google Scholar]
- 44.Hu, P., J. Elliot, P. McCready, E. Skowronski, J. Garnes, A. Kobayashi, A. V. Carrano, R. Brubaker, and E. Garcia. 1998. Yersinia pestis plasmid pCD1, complete plasmid sequence. Genbank accession no. AF053946. [DOI] [PMC free article] [PubMed]
- 45.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 47.Iriarte M, Cornelis G R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol Microbiol. 1998;29:913–929. doi: 10.1046/j.1365-2958.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 48.Iriarte, M., and G. R. Cornelis. Assignment of SycN, YscX, and YscY, three new elements of the Yersinia Yop virulon. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 49.Iriarte, M., I. Lambermont, C. Kerbourch, and G. R. Cornelis. The complete sequence of the Yersinia enterocolitica pYVe227 virulence plasmid. Submitted for publication.
- 50.Iriarte M, Sory M P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khisty V J, Munske G R, Randall L L. Mapping of the binding frame for the chaperone SecB within a natural ligand, galactose-binding protein. J Biol Chem. 1995;270:25920–25927. doi: 10.1074/jbc.270.43.25920. [DOI] [PubMed] [Google Scholar]
- 52.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–798. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 53.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galan J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 54.Kumamoto C A, Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985;163:267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kutsukake K. Excretion of the anti-ς factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243:605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- 56.Lambert de Rouvroit C L, Sluiters C, Cornelis G R. Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol Microbiol. 1992;6:395–409. [PubMed] [Google Scholar]
- 57.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 58.Leung K Y, Straley S C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPlb α. J Bacteriol. 1989;171:4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Linderoth N A, Simon M N, Russel M. The filamentous phage pIV multimer visualized by scanning transmission electron microscopy. Science. 1997;278:1635–1638. doi: 10.1126/science.278.5343.1635. [DOI] [PubMed] [Google Scholar]
- 60.Menard R, Sansonetti P J, Parsot C, Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 61.Michiels T, Cornelis G. Nucleotide sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb Pathog. 1988;5:449–459. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- 62.Michiels T, Cornelis G R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991;173:1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michiels T, Vanooteghem J-C, Lambert de Rouvroit C L, China B, Gustin A, Boudry P, Cornelis G R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mills S D, Boland A, Sory M P, Van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mulder B, Michiels T, Simonet M, Sory M P, Cornelis G. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect Immun. 1989;57:2534–2541. doi: 10.1128/iai.57.8.2534-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neyt, C., and G. R. Cornelis. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol. Microbiol., in press. [DOI] [PubMed]
- 70.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol. 1998;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohnishi K, Ohto Y, Aizawa S I, Macnab R M, Iino T. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium. J Bacteriol. 1994;176:2272–2281. doi: 10.1128/jb.176.8.2272-2281.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmer L E, Hobbie S, Galan J E, Bliska J B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNFa production and downregulation of the MAP kinases p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 74.Payne, P. L., and S. C. Straley. YscO of Yersinia pestis is a mobile core component of the Yop secretion system. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 75.Payne, P. L., and S. C. Straley. YscP of Yersinia pestis is a secreted component of the Yop secretion system. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 76.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perry R D, Harmon P A, Bowmer W S, Straley S C. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986;54:428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perry R D, Straley S C, Fetherston J D, Rose D J, Gregor J, Blattner F R. DNA sequencing and analysis of the low-Ca2+ response plasmid pCD1 of Yersinia pestis KIM5. Infect Immun. 1998;66:4611–4623. doi: 10.1128/iai.66.10.4611-4623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Persson C, Carballeira N, Wolf-Watz H, Fällman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 81.Plano G V, Barve S S, Straley S C. LcrD, a membrane-bound regulator of the Yersinia pestis low-calcium response. J Bacteriol. 1991;173:7293–7303. doi: 10.1128/jb.173.22.7293-7303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Portnoy D A, Moseley S L, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Price S B, Cowan C, Perry R D, Straley S C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rimpiläinen M, Forsberg Å, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roggenkamp A, Geiger A M, Leitritz L, Kessler A, Heesemann J. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect Immun. 1997;65:446–451. doi: 10.1128/iai.65.2.446-451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rohde J R, Fox J M, Minnich S A. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol Microbiol. 1994;12:187–199. doi: 10.1111/j.1365-2958.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 90.Rosqvist R, Bolin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosqvist R, Forsberg Å, Rimpiläinen M, Bergman T, Wolf-Watz H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol Microbiol. 1990;4:657–667. doi: 10.1111/j.1365-2958.1990.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 92.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage TNF-a production. EMBO J., in press. [DOI] [PMC free article] [PubMed]
- 95.Ruckdeschel K, Machold J, Roggenkamps A, Schubert S, Pierre J, Zumbihl R, Liautard J P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 96.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun. 1996;64:724–733. doi: 10.1128/iai.64.3.724-733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarker M R, Sory M P, Boyd A P, Iriarte M, Cornelis G R. LcrG is required for efficient internalization of Yersinia Yop effector proteins into eukaryotic cells. Infect Immun. 1998;66:2976–2979. doi: 10.1128/iai.66.6.2976-2979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schesser K, Spiik A-K, Dukuzumuremyi J-M, Neurath M F, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 100.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sory M P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sory M P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 105.Sory, M. P., C. Kerbourch, and G. R. Cornelis. Unpublished data.
- 106.Stainier, I., and G. R. Cornelis. Unpublished data.
- 107.Stainier I, Iriarte M, Cornelis G R. YscM1 and YscM2, two Yersinia enterocolitica proteins causing down regulation of yop transcription. Mol Microbiol. 1997;26:833–843. doi: 10.1046/j.1365-2958.1997.6281995.x. [DOI] [PubMed] [Google Scholar]
- 108.Starnbach M N, Bevan M J. Cells infected with Yersinia present an epitope to class I MHC-restricted CTL. J Immunol. 1994;153:1603–1612. [PMC free article] [PubMed] [Google Scholar]
- 109.Straley S C, Brubaker R R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci USA. 1981;78:1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 111.Strathdee C A, Lo R Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989;171:916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van den Ackerveken G, Bonas U. Bacterial avirulence proteins as triggers of plant disease restistance. Trends Microbiol. 1997;5:394–398. doi: 10.1016/S0966-842X(97)01124-4. [DOI] [PubMed] [Google Scholar]
- 113.Van Gijsegem F, Gough C L, Zischek C, Niqueux E, Arlat M, Genin S, Barberis P, German S, Castello P, Boucher C. The hrp gene locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol Microbiol. 1995;15:1095–1114. doi: 10.1111/j.1365-2958.1995.tb02284.x. [DOI] [PubMed] [Google Scholar]
- 114.Viitanen A M, Toivanen P, Skurnik M. The lcrE gene is part of an operon in the lcr region of Yersinia enterocolitica O:3. J Bacteriol. 1990;172:3152–3162. doi: 10.1128/jb.172.6.3152-3162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Viprey V, Del Greco A, Golinowski W, Broughton W J, Perret X. Symbiotic implications of type III protein secretion machinery in Rhizobium. Mol Microbiol. 1998;28:1381–1389. doi: 10.1046/j.1365-2958.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 116.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 118.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 119.Whalen M C, Wang J F, Carland F M, Heiskell M E, Dahlbeck D, Minsavage G V, Jones J B, Scott J W, Stall R E, Staskawicz B J. Avirulence gene avrRxv from Xanthomonas campestris pv. vesicatoria specifies resistance on tomato line Hawaii 7998. Mol Plant-Microbe Interact. 1993;6:616–627. doi: 10.1094/mpmi-6-616. [DOI] [PubMed] [Google Scholar]
- 120.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woestyn S, Sory M P, Boland A, Lequenne O, Cornelis G R. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol Microbiol. 1996;20:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 122.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuk M H, Harvill E T, Miller J F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]