ABSTRACT
COVID-19 vaccines have played an important role in reducing the impact of the current pandemic. Previously, we developed NARUVAX-C19 vaccine based on a recombinant Wuhan spike protein extracellular domain expressed in insect cells and formulated with a squalene emulsion adjuvant (Sepivac SWE™). The current study assessed the immunogenicity, efficacy, and safety of NARUVAX-C19 vaccine in rhesus macaques and hamsters. Macaques immunized intramuscularly with two doses of NARUVAX-C19 vaccine showed no adverse effects and demonstrated cellular immunity as assessed by T cell IFN-γ responses against spike protein, in addition to inducing a humoral response. Serum from immunized animals neutralized the homologous wild-type SARS-CoV-2 virus as well as the Alpha and Delta variants. In hamsters, immunization with NARUVAX-C19 vaccine protected against a heterologous challenge with the Delta virus, as reflected by reduced lung and nasal viral loads and lung pathology in immunized animals. Nevertheless, some NARUVAX-C19 vaccinated animals were still shown to transmit infection to naïve sentinel animals. Overall, NARUVAX-C19 vaccine induced broadly cross-neutralizing antibody and T cell IFN-γ responses in rhesus macaques and provided heterologous protection of hamsters against infection by the Delta virus variant. This data supports the utility of squalene emulsion-based adjuvanted recombinant vaccine in protection against SARS-CoV-2 and supports their continued clinical development.
KEYWORDS: COVID-19, SARS-CoV-2, vaccine, adjuvant, immunogenicity, safety, viral transmission, hamsters, non-human primates
Introduction
Since the first identification of human infections caused by the SARS-CoV-2 virus in Wuhan, China in late 2019, there have been over 750 million cases and 6.8 million deaths registered worldwide.1 SARS-CoV-2 infection can cause severe clinical manifestations, particularly in elderly individuals with multiple medical comorbidities.2 Fortunately, vaccines help reduce the risk of severe disease consequent upon COVID-19.3 Vaccines have proven less effective at providing durable protection against SARS-CoV-2 infection and transmission, due to relatively rapidly waning of vaccine protection. The continued evolution of the SARS-CoV-2 virus supports the need for ongoing COVID-19 vaccine development efforts and research.
The four main COVID-19 vaccine approaches include nucleic acid, adenoviral vector, inactivated virus, and protein subunit vaccines. Notable examples of these different vaccine types are the mRNA vaccines developed by Moderna and BioNTech-Pfizer,4 the adenovirus vector vaccine developed by AstraZeneca,5 the inactivated whole virus vaccines (BBIBP-CorV) developed by Sinopharm,6 and the recombinant spike protein subunit vaccines developed by Vaxine/Cinnagen (SpikoGen®)7 and Novavax.8 Subunit protein approaches have extensive past experience, being used to produce a wide range of licensed vaccines, including for influenza, hepatitis B and human papilloma virus.9,10
We developed a subunit vaccine called NARUVAX-C19 based on a recombinant spike protein extracellular domain (ECD) expressed in insect cells that was formulated with Sepivac SWE™ (SWE), a nano-emulsion squalene oil adjuvant. SWE is included as an adjuvant in COVAC-2, a subunit COVID-19 vaccine candidate developed by the University of Saskatchewan which is in Phase II human trials.11 Results of a previous study of NARUVAX-C19 showed that it induced both Th1 and Th2-mediated cellular responses in mice and protected against a challenge with homologous SARS-CoV-2 wild-type (WT) virus strain (Wuhan-Hu-1) in Syrian hamsters.12 The present study presents data completing the preclinical regulatory package on NARUVAX-C19, reporting on its safety and immunogenicity in nonhuman primates (rhesus macaques) and its efficacy in protecting against a heterologous Delta variant challenge in hamsters.
Methods
Recombinant spike protein and vaccine formulation
The detailed method for obtaining spike protein ECD was described previously.13 Briefly, the spike protein was identified from the genome sequence of the wild-type SARS-CoV-2 Wuhan-Hu-1 strain in NCBI (accession number: NC_045512).14 Recombinant baculovirus containing the codon optimized spike protein ECD with various modifications was propagated in Sf9 cells and the expanded virus then used to infect Tni cells for protein expression. The purified spike protein was mixed with SWE adjuvant in a 1:1 ratio by volume. The hamster studies also included an adjuvant comparator group where the spike protein ECD was mixed with an aluminum hydroxide adjuvant (Alhydrogel® adjuvant 2%, InvivoGen, CA, USA) at a final concentration of 0.25 mg/mL (0.05 mg Aluminum per hamster dose). Injection of phosphate-buffered saline (PBS) was used as a negative control. All vaccine formulations were sterile and contained less than 2.0 EU of endotoxin per dose. Once the vaccines were prepared, they were stored at 2–8°C and used to vaccinate animals the following day.
NARUVAX-C19 safety and immunogenicity studies in rhesus macaques
Nine clinically healthy male rhesus macaques (Macaca mulatta) were used, with three animals randomly assigned to each group based on body weight, ensuring that the average weight in each group did not differ by more than 10%. Prior to inclusion in the study, the macaques underwent a mandatory quarantine period of 21 days during which they were acclimated to the animal housing conditions and personnel. The quarantine measures included deworming according to the following regimen: Metronid 50 intramuscularly twice with a 48-hours interval, at a dose of 0.2 mL/kg of body weight. Ivermek was administered 7 days after the Metronid injection, at a dose of 0.05 mL/kg, via intramuscular injection. The animals were housed in individual cages, each with a unique identification number. The cages were equipped with a centralized water supply system. The ambient air temperature was maintained at 21–28°C, with a relative humidity of 40–70%, and natural daylight duration. Blood samples were collected at predetermined time intervals for hematological and biochemical safety analyses and immunogenicity assessment including sera and collection of peripheral blood mononuclear cells (PBMC) samples to assess cellular immunity. Regular measurements were taken of the animals’ weight and temperature. The control group consisted of three animals injected with PBS, the second group were injected with NARUVAX-C19 0.5 mL containing 5 µg of spike protein, and the third group with NARUVAX-C19 0.5 mL containing 25 µg of spike protein. Vaccine was administered intramuscularly into the thigh muscle on two occasions, 14 days apart with the first injection into the left, and the second injection into the right, leg. The duration of the study was 28 days.
Injection site assessment
The injection site was shaved. External assessments of the injection site for edema, erythema, or any other signs of inflammation were conducted for a duration of 7 days after each injection (days 0–7 and 14–21)
Complete blood count and biochemistry
As part of the safety assessment blood samples were taken on days 7 and 21 after vaccine administration for a complete blood count and biochemistry. An automatic hematological analyzer MEK-7300K (Nihon Kohden, Japan) was used following the manufacturer’s instructions to determine the following blood parameters: hemoglobin (Hgb), red blood cell (RBC), mean cell volume (MCV), mean cell hemoglobin (MCH), hematocrit (Hct), platelets (Plt), white blood cell (WBC), monocytes (MO), lymphocytes (LY), neutrophils (NE), eosinophils (EO), and basophils (BA). A biochemical analyzer StatFax 3300 (USA) was used to measure the following biochemistry parameters: glucose (GLU), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), total bilirubin (TB), urea nitrogen (BUN), creatinine (CRE), cholesterol (CHOL), triglycerides (TG), total protein (TP), albumin (ALB), globulin (GLB), sodium (Na), and potassium (K).
RBD-ACE2 binding inhibition antibodies
The SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Kit (GenScript, Piscataway, US) was used as per the manufacturer’s guidelines. Briefly, the samples and controls were incubated with HRP-conjugated RBD (HRP-RBD) at 37°C for 30 min. Next, the mixture was added to wells of a 96-well plate coated with hACE2 and incubated at 37°C for 15 min to allow for capture. The plates were then washed and then TMB solution added prior to incubation at room temperature for 15 min, followed by the addition of stop solution to halt the reaction. The optical density (OD) was measured at 450 nm using a spectrophotometric method. The sample inhibition percentage was calculated as (1 – mean OD of sample/mean OD of negative control) x 100%. Samples with an inhibition percentage less than 30% were categorized as “negative” for RBD-ACE2 binding inhibition antibodies, while samples with an inhibition percentage equal to or greater than 30% were considered positive for RBD-ACE2 binding inhibition antibodies. Based on the level of inhibition of RBD-ACE2 binding antibodies, the samples were further classified into three categories: low (30–59% inhibition), medium (60–89% inhibition), and high (≥90% inhibition).
Neutralizing antibodies to Wuhan, Alpha, and Delta variants
The serum samples were complement-inactivated at 56°C for 30 min. Then, they were serially two-fold diluted in medium (DMEM-2% FCS-1% Antibiotic-Antimycotic, Gibco™) to achieve final dilutions of 1:10 to 1:10,240. Subsequently, the diluted serum samples were incubated in duplicates at a 1:1 ratio with 1000 TCID50 of the SARS-CoV-2 (hCoV-19/Kazakhstan/KazNAU-NSCEDI-4635/2020, WT variant with spike protein mutations D614G and M153T; or hCoV-19/Kazakhstan/KazNAU-NSCEDI-Kaissar/2021, Alpha variant; or hCoV-19/Kazakhstan/KazNARU-NSCEDI-5526/2021, Delta variant). After 1 h of incubation at room temperature, the serum-virus mixture was transferred to a 96-well plate (#3596, Corning) that had been previously covered with a monolayer of Vero-E6 cell culture. The monolayer was obtained by seeding 5 × 104 cells per well and incubating for 24 h until the confluence reached about 95%. After an additional 1 h of incubation, the inoculums were removed, fresh medium was added, and the plates were further incubated at 37°C with 5% CO2 for 3 days. The neutralizing antibody titer was determined as the highest dilution of serum that inhibited the cytopathic effect in 100% of the wells. Visual assessment of the cytopathic effect was conducted using a MIB-R trinocular inverted biological microscope (LOMO-Microsystems, Russia) at a magnification of x10.
Spike-specific T cell IFN-γ production
Peripheral Blood Mononuclear Cells (PBMCs) were cultured in 24-well flat bottom plates (#04618024, Sigma-Aldrich, USA) at a concentration of 1 × 106 cells/well (1 mL) in RPMI-1640 + GlutaMax medium (#2242279, Gibco) supplemented with 20 mM HEPES (#15630–080, Gibco), 10% heat-inactivated FBS, and 1% Antibiotic-Antimycotic (#15240096, Gibco™). The cultures were maintained in a 5% CO2 incubator (INCO 153, Memmert, Germany) at 37°C. To stimulate the T cells, 10 µg of rSpike was added to the wells, with some wells left unstimulated to serve as negative controls. After incubation for 72 h, the supernatant was collected for the analysis of IFN-γ production using the Rhesus Macaque IFN-gamma ELISA kit (#EP8RB, ThermoFisher Scientific), following the manufacturer’s instructions. The data were presented as the difference in concentrations (pg/mL) between the stimulated and non-stimulated samples.
T cell proliferation
T cell proliferation analysis was conducted by incubating PBMCs of rhesus macaques for 5 min in the dark on ice with 5 µM CFSE (Carboxyfluorescein succinimidyl ester, eBioscience™, #2298273, Invitrogen, USA). The reaction was then quenched with 3% FBS. The splenocytes were cultured at a concentration of 1 × 106 cells/mL in 24-well plates for 5 days at 37°C in 5% CO2, either with or without 10 µg of WT spike protein ECD. Next, the cells were incubated with an antibody specific to CD3+ surface markers (CD3 Antibody, anti-non-human primate, PE, Miltenyi Biotec B.V. & Co. KG, Germany) at a concentration of 0.2 µl per 100 µl of PBMC cells for 30 min in the dark on ice. After incubation, the cells were resuspended in Flow Cytometry Staining Buffer (Invitrogen, USA) up to 0.5 mL, stained with 0.25 µg of 7-AAD solution to exclude dead cells, and incubated for 5–10 min in the dark (BioLegend). At least 5 × 105 cells were analyzed for each sample using the Attune™ NxT flow cytometer (Thermo Fisher Scientific, USA) with the Attune NxT Software program (Thermo Fisher Scientific, USA). The analysis was performed within a lymphocyte gate isolated on the FCS/SSC dot plot. The T cell proliferation index was calculated as the ratio of samples cultured with and without spike protein, relative to the total number of live proliferating CD3+CFSE+ cells.
Hamster challenge study procedures
Vaccination
Male Syrian hamsters, aged 6–8 weeks, were sourced from the laboratory animal nursery of the M. Aikimbayev National Scientific Center for Especially Dangerous Infections (NSCEDI). To ensure proper acclimatization, the animals were housed in ventilated cages with HEPA filters (Allentown, USA) for 7 days before the experiment. For immunization, the hamsters received intramuscular injections in the thigh, of 5 µg of rSpike formulated with either SWE or Alum adjuvants, while a control group received injections of PBS. The vaccination in a volume of 200 µl was administered twice, with a 21-day interval between doses.
Assessment of the vaccine protective effectiveness
On day 21 after the 2nd vaccine dose, the hamsters were challenged with 1 × 106 TCID50 of SARS-CoV-2 Delta intranasally under intraperitoneal anesthesia with ketamine (100 mg/kg) and xylazine (10 mg/kg). The challenge virus was diluted in DMEM medium and 100 µl of the diluted virus was placed into the nostrils, approximately 50 µl to each nostril, using a pipette. Subsequently, the animals were observed closely twice daily for 3 days post challenge with their body weight recorded daily. On day 3 after the challenge, the animals were sacrificed, and samples from the nasal turbinates and lungs collected. For histopathological examination, the three right lung lobes of each animal were fixed in 10% formalin. To determine the virus titer, two lobes of the left lung were homogenized in 1 ml of DMEM using a TissueLyser II (QIAGEN) device, vibrating at 300 cycles per minute for 60 s. After centrifugation (5000 g for 15 min at 4°C), the supernatant was collected and stored at −70°C for later analysis.
Virus transmission analysis
Naive sentinel hamsters were placed in the same cage as infected animals on day 2 post challenge with two sentinel hamster added to four challenged animals in cages measuring 19“x 10” x 8.” Oropharyngeal swabs were taken from all challenged hamsters on day 2 post challenge to assess viral load before the sentinels were housed together with the infected animals. The sentinels were in contact with the infected animals for one day, after which they were separated and housed individually for an additional 2 days before being sacrificed for assessment of viral load in the nasal turbinates and lungs and assessment of the lungs by histology.
Determination of the infectious virus load
Virus titers in homogenates of respiratory tract tissues were determined using 50% tissue culture infectious dose (TCID50) analysis. The tissue homogenates were diluted ten-fold in a medium (DMEM-2% FCS-1% antibiotic-antimycotic) and then transferred in quadruplicate into 96-well plates containing Vero-E6 confluent cells. Following this, the plates were incubated at 37°C with 5% CO2 for 5 days. The titration results were visually assessed by studying the cellular monolayer under a microscope, looking for specific cytopathic effects such as cell rounding and cell separation from the monolayer. The viral titer was determined using the Reed and Muench method and expressed as log10 TCID50/mL.
Histological analysis of hamster lungs
After excision, the lungs of hamsters were fixed in 10% neutral buffered formaldehyde, washed with water, and treated with four portions of 100% isopropyl alcohol, followed by two portions of xylene. The material was then soaked in four portions of paraffin, and blocks were created. Histological blocks were sectioned to a thickness of 5 µm using a microprocessor microtome MZP-01 (CB Technom, Russia). To prepare the sections for examination, the sections were dewaxed in two portions of xylene and three portions of ethyl alcohol with reduced concentrations (96%, 80%, 70%). They were then stained with hematoxylin (#05–002, BioVitrum, Russia) and eosin (#C0362, DiaPath, Italy). Subsequently, the sections were clarified in increasing concentrations of ethyl alcohol (70%, 80%, 96%) and two portions of xylene. The sections were covered with glass coverslips using the synthetic medium Bio Mount (#2813, Bio Optica, Italy).
Microscopic examination of the lungs followed classical canons adopted for parenchymal organs, and the description of pathological conditions induced by SARS-CoV and SARS-CoV-2 was used for narration. Each slide was quantitatively assessed based on the severity of histological changes, including interstitial pneumonia, alveolitis, bronchiolitis, alveolar destruction, interstitial infiltration, pulmonary hemorrhage, and peribronchiolar inflammation. For evaluation of pathological changes in peribronchiolar and perivascular infiltrates, a scoring system was used: 0 – no changes, 1 – slight changes, moderate changes, 3 – severe changes. Interstitial and intra-alveolar infiltrates, alveolar edema, and hemorrhages were assessed based on their incidence in the studied sections: 0 – no changes, 1 – ≤10%, 2 – 11–25%, 3 – 26–50%, 4 – 50–75%, 5 – ≥75%. Neutrophil infiltration was evaluated by the presence of cells in the field of vision at x400 magnification, with at least 20 fields of vision examined: 0 – no neutrophils in the field of vision, 1 – 1 neutrophil in the field of vision, 2 – 2–5 neutrophils in the field of vision, 3 - more than 5 neutrophils in the field of vision (Table 1). The slides were examined using the Mshot microscope (China), model MF52-N, with photos taken at a magnification of x40 using Mshot MS23 camera tips (China) and the MShot Image Analysis System program (China). All measurements were recorded in µm.
Table 1.
Scoring system of lung pathological changes.
| Assessable symptom | Maximum number of scores for one symptom |
|---|---|
| Perivascular infiltrates | 3 |
| Peribronchial infiltrates | 3 |
| Interstitial infiltrates | 5 |
| Intra-alveolar infiltrates | 5 |
| Neutrophil infiltration | 3 |
| Alveolar edema | 5 |
| Hemorrhages | 5 |
| Maximum score | 29 |
Biosafety and bioethics
Safety studies of the NARUVAX-C19 vaccine in rhesus macaques were carried out at the Research Institute of Medical Primatology in Sochi, Russian Federation. The study protocol, designated as No. 74 and dated June 24, 2021, received approval from the Institutional Animal Care and Use Committee of the Research Institute of Medical Primatology.
All activities involving the SARS-CoV-2 virus were conducted in the BSL-3 (Biosafety Level 3) and ABSL-3 (Animal Biosafety Level 3) laboratories of NSCEDI, adhering to the international standard ISO 35001:2019 “Biological Risk Management for Laboratories and Other Related Organizations.” The laboratory animals were housed in individually ventilated cages (Techniplast, Italy, and Allentown, USA) and were maintained under a 12/12 day and night cycle. This study was conducted in full compliance with both national and international laws and guidelines pertaining to the handling of laboratory animals. The protocol received approval from the Institutional Animal Care and Use Committee of NSCEDI, Protocol No. 4, dated September 22, 2020.
Statistical analysis
The statistical data analysis was performed using GraphPad Prism program, version 9.0.0 (San Diego, California, USA). To assess differences in antibody titers, T cell proliferation, cytokine production, weight loss, viral load, and pathological changes in the lungs between the groups of animals, the Tukey multiple comparison test was applied. For statistical analysis of IgG titers and neutralizing antibodies, data were converted to log2. The limit of detection for viral titer was 1.2 log10 TCID50/mL, while for IgG titers, it was 6.0 log2, and for neutralizing antibodies, it was 2.3 log2. Geometric mean titers (GMT) were calculated for all antibody types with a 95% confidence interval. For all comparisons, a P-value of less than 0.05 was considered statistically significant.
Results
NARUVAX-C19 vaccine immunogenicity and safety in rhesus macaques
Body weight, temperature, clinical examination, and injection site assessment
During the 28-day experiment, there were no adverse deviations in the clinical status of the macaques in any of the experimental groups that received 5 or 25 µg NARUVAX-C19 vaccine. The animals remained active with no significant differences in food consumption or body weight (Supplementary Figure S1a) compared to the control group. There were no signs of erythema or edema at the injection site at any time. All groups of macaques exhibited minimal fluctuations in average body temperature, with the pattern of temperature changes similar between the groups (Supplementary Figure S1b).
Blood biochemical and hematological analysis
Higher levels of urea and creatinine were seen at day 7 and day 21 in the groups that received 5 or 25 µg of NARUVAX-C19 vaccine when compared to the control group, although all results remained within the physiological ranges. These changes were more pronounced in animals given the 25-µg vaccine dose (Figure S2). Receipt of the vaccine did not lead to changes in any hematological parameters (Figure S3).
Vaccine-induced humoral immune responses
At 21 days after the 1st NARUVAX-C19 vaccination and day 7 after the booster, animals showed increased serum titers of anti-spike IgG binding antibodies by ELISA (Figure 1), as well as ACE2-RBD blocking antibodies, and neutralizing antibody titers against WT, Alpha, and Delta viruses. No significant differences in spike binding IgG, ACE2-RBD blocking antibody, and WT, Alpha, and Delta neutralizing antibody titers were observed between the 5 and 25 μg NARUVAX-C19 doses.
Figure 1.
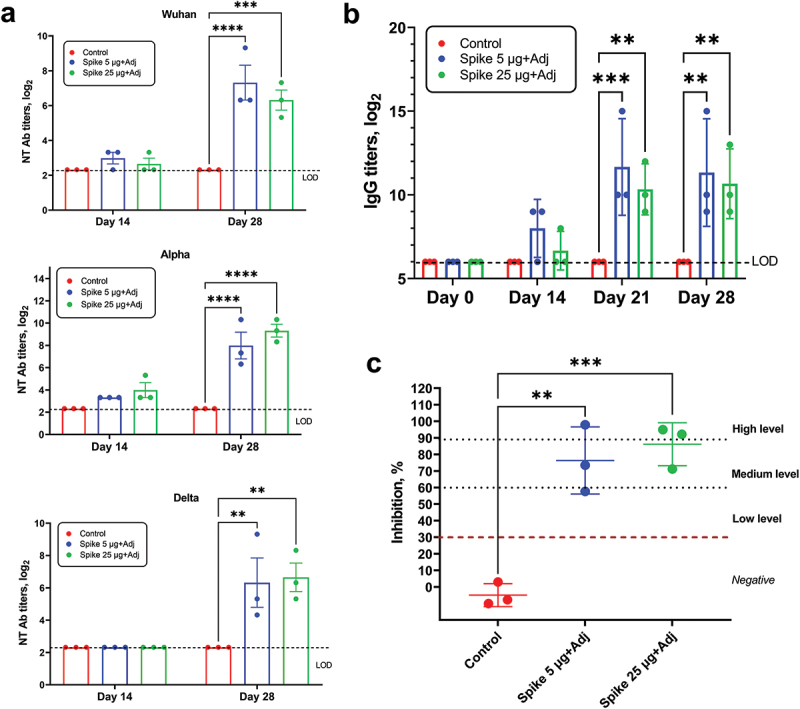
SARS-CoV-2 antibody responses in macaques after vaccination. (a) Virus neutralizing antibody titers against Wuhan, Alpha and Delta variants of SARS-CoV-2 virus in macaques on days 14 and 28 after initial immunization with 5 and 25 µg NARUVAX-C19 vaccine; (b) Anti-rSpike IgG antibody titers in macaques at days 0, 14, 21, and 28 after primary immunization (c) RBD-ACE2 blocking antibody levels in macaques at day 28 after primary immunization. RBD-ACE2 blocking antibodies were determined according to the level of inhibition: negative (<30%), low (30-59%), medium (60-89), and high (90≤). Differences in the studied variables between animal groups were assessed using Tukey’s multiple comparisons test. A P < 0.05 value was considered a significant difference. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Vaccine-induced cellular immune response
The potential for NARUVAX-C19 vaccine to induce cellular immunity in macaques was assessed by measuring T cell proliferation and IFN-γ production by PBMC after restimulation with spike protein. Spike-stimulated IFN-γ levels was significantly higher on day 28 in the groups that received either 5 or 25 μg NARUVAX-C19 vaccine when compared to controls (Figure 2). A significant increase in spike-stimulated T cell proliferation was also seen on day 28 (Figure 2). There was no significant difference in the level of T cell responses between the two vaccine doses.
Figure 2.
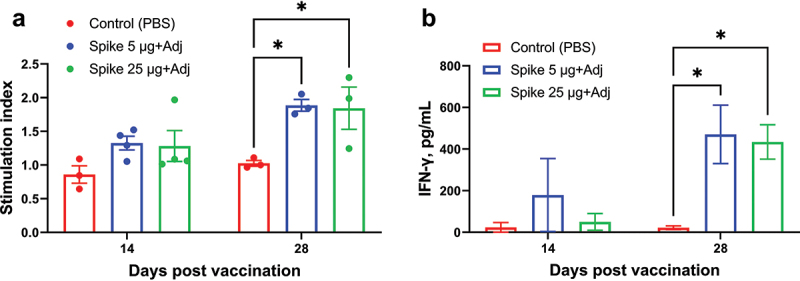
Antigen-specific T cell proliferation (a) and IFN-γ production by peripheral blood mononuclear cells (b) from vaccinated and control macaques on days 14 and 28 of the experiment. The stimulation index was calculated as the difference (Δ) in the number of proliferating (CFSE+) T cells (CD3+) between rSpike-stimulated and non-stimulated cells. IFN-γ data are presented as concentration differences (pg/mL) between samples with and without stimulation with rSpike. Differences between groups were assessed using Tukey’s multiple comparisons test. P < 0.05 was considered significant. *P < 0.05.
Vaccine efficacy against heterologous Delta virus challenge in hamsters
The efficacy of NARUVAX-C19 vaccine containing 5 µg of rSpike protein formulated with either SWE or aluminum hydroxide (alum) adjuvant was assessed in hamsters. The immunized hamsters were then given a heterologous Delta virus challenge. All challenged animals lost weight through to 2 days post challenge, with only the NARUVAX-C19 vaccinated group then showing weight gain on day 3 (Figure 3a). The viral loads in the lungs and nasal turbinates of NARUVAX-C19 vaccinated hamsters when sacrificed at Day 3 were significantly lower than in the control group (Figure 3b). Histological analysis of the lungs at termination showed that the NARUVAX-C19 immunized animals had significantly less lung pathology as compared to the control group and the alum-adjuvanted rSpike vaccine group. Notably, the severity of lung lesions was similar in the alum-adjuvanted rSpike and control groups. Features of acute respiratory distress syndrome typical of SARS-CoV-2 infection, were observed in the lungs of animals from the control and the alum-adjuvanted rSpike vaccine groups but not the NARUVAX-C19 vaccinated groups (Figure 3d).
Figure 3.
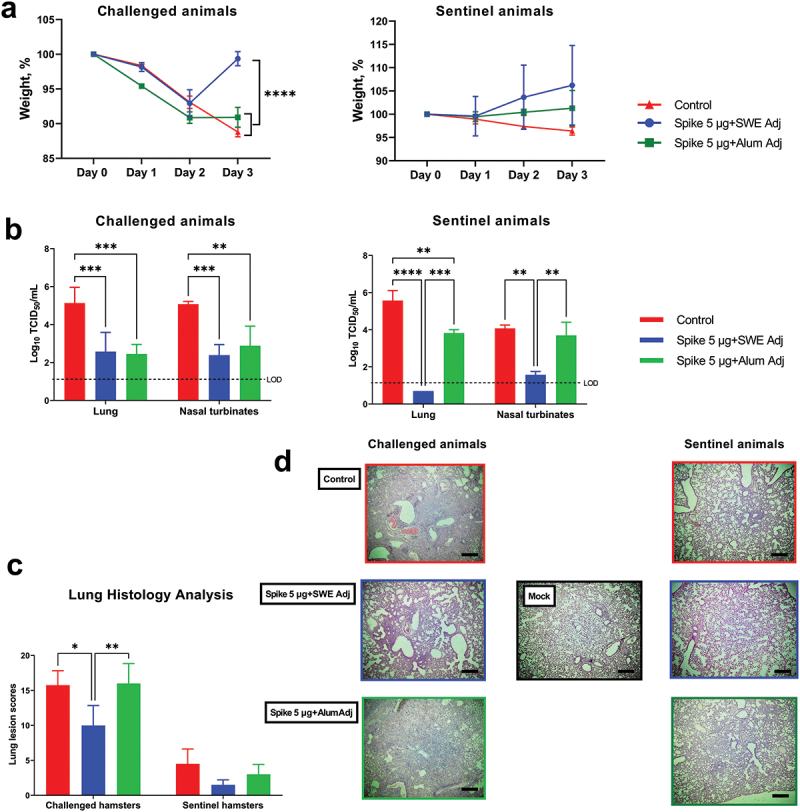
Efficacy against SARS-CoV-2 infection and viral transmission of NARUVAX-C19 vaccine in Syrian hamsters. All animals were intranasally challenged with SARS-CoV-2 Delta variant. Shown are 1) changes in body weight (a); 2) viral load in nasal turbinates and lungs (expressed as log10 TCID50/mL; (b) on day 3 after the challenge; 3) lung pathology on day 3 after challenge (c). The protective effect of vaccine due to the viral transmission was assessed by the weight dynamics of sentinel animals (a), viral load in respiratory organs of sentinel animals (added to the cages with challenged hamsters on day 1; b), lung pathology (c, d). Photographs were taken at x40 magnification. Scale bars are 500 µm. Differences between groups were assessed by Tukey’s, multiple comparison test. P < 0.05 was considered significant. *P < 0.05, **P < 0.01 and ***P < 0.001 and ****P < 0.0001.
Virus transmission studies in hamsters
Hamsters immunized with NARUVAX-C19 vaccine and challenged with Delta virus demonstrated reduced transmission of their infection to sentinel animals when compared to the control group. Interestingly, sentinel animals exposed to the challenged NARUVAX-C19 group did not lose weight during the observation period (Figure 3a) and no virus was detected in their lungs at termination (Figure 3a), although virus was detected in their nasal turbinates, albeit at significantly lower levels than the control and alum-adjuvanted rSpike vaccinated groups (Figure 3c). In contrast, the alum-adjuvanted rSpike vaccine demonstrated no ability to block transmission of infection to sentinel animals when compared to the control group.
The viral transmission study showed that disease severity, as characterized by weight loss (Figure 3a) and lung inflammation (Figure 3c), was significantly lower in the infected sentinel animals when compared to directly virus challenged naïve animals, even when the respiratory viral loads were equivalent in infected sentinel animals and infected directly challenged animals (Figure 3b). Furthermore, while viral loads were reduced in the turbinates and lungs of both NARUVAX-C19 and alum-adjuvanted rSpike immunized animals, a clear difference was seen in viral loads in the sentinel animals from these groups. NARUVAX-C19 immunized groups were associated with significantly reduced turbinate and lung viral loads in their co-housed sentinels when compared to alum-adjuvanted rSpike and control groups (Figure 3b).
Discussion
The rapid development of vaccines to mitigate the burden of COVID-19 is a historic achievement. However, the early promise of the inactivated virus, mRNA and viral vector vaccines, in providing high level protection against severe disease caused by SARS-CoV-2 infection was tempered by the recognition that vaccine protection against infection was of relatively short duration15 and that, although rare, potentially vaccine-associated adverse reactions could occur including severe thrombosis associated with adenoviral vector vaccines,16 and myocarditis/pericarditis associated with mRNA vaccines.17,18 Vaccines based on adjuvanted protein antigens have been successfully used for many decades with excellent safety and efficacy.19 NARUVAX-C19 is a subunit COVID-19 vaccine based on recombinant spike protein ECD combined with SWE adjuvant. NARUVAX-C19 showed strong immunogenicity in mice and protected against ancestral WT viral infection in Syrian hamsters where it also blocked virus transmission to naïve sentinels.12 Additionally, in a cat model of SARS-CoV-2 infection, NARUVAX-C19 (Pets) vaccine not only protected the lower respiratory tract against infection but also protected the cats against severe viral myocarditis.20
As part of the completion of the NARUVAX-C19 vaccine regulatory dossier, we sought to establish a NARUVAX-C19 vaccine safety and immunogenicity in non-human primates, and to conduct a study in hamsters to establish NARUVAX-C19 vaccine’s ability to protect against infection and transmission of a heterologous virus variant, namely the Delta variant. As shown, NARUVAX-C19 vaccine was immunogenic, well tolerated, and safe in macaques, inducing immune responses similar to those seen with a commercial full-length spike protein vaccine formulated with a saponin-based adjuvant, Matrix-M (NVX-CoV2373, Novavax).21 In macaques, NARUVAX-C19 vaccine induced both humoral as well as T cell proliferation and IFN-γ responses against spike protein. After receipt of two vaccine doses, serum antibodies in immunized animals were able to block ACE2-RBD binding and to neutralize WT virus as well as the Alpha and Delta variants. This is consistent with previous data on this vaccine in mice where maximal spike binding IgG and neutralizing antibody responses required two vaccine doses.12 Similarly, in olive baboons receiving the subunit vaccine NVS-Com 2373 the antibody response to a single immunization was only formed after 21 days following vaccination.21 NARUVAX-C19 also induced T cell responses against spike protein in the macaques as reflected by T cell proliferation and IFN-γ recall responses, consistent with a Th1 cellular immune response, and similar to previous T cell findings in mice.12 Interestingly, the humoral and cellular responses of the NARUVAX-C19 vaccine was similar for both the 5 and 25 µg doses, suggesting that the 5 µg dose was already sufficient to induce a maximal response. Similarly, the Novavax Phase 1 clinical trial showed minimal serum antibody differences induced by their 5 and 25 µg doses, with the 5 µg dose consequently selected as the vaccine formulation to take forward in clinical development.22 Similarly, a monkey study of another COVID-19 vaccine also demonstrated higher antigen-specific IgG, virus neutralizing antibodies and cellular immune response with a 5 µg as compared to a 25-µg dose.21
Another important question is the extent to which COVID-19 vaccines are able to provide protection against infection and transmission of heterologous SARS-CoV-2 variants.23,24 Hamsters are a convenient model for studying SARS-CoV-2 infection and transmission, although the infection even in naïve hamsters is generally extremely transient with virus being rapidly cleared and often being no longer being detectable just 3–4 days post-challenge.25 Our recent studies showed that vaccination of hamsters with two doses of NARUVAX-C19 resulted not only prevention of clinical disease and weight loss in infected hamsters but also provided 100% protection against transmission to sentinel unimmunized animals.12 The current hamster study extends these findings and shows that NARUVAX-C19 vaccine similarly provides significant protection of hamsters against clinical disease caused by the heterologous Delta variant, as reflected by reduced lung and nasal turbinate viral load and faster recovery of early weight loss post challenge. This accords well with results of other COVID-19 vaccines that have shown vaccine cross-protection against newer SARS-CoV-2 variants.26–28 NARUVAX-C19 vaccinated animals also were less likely to transmit Delta infection to sentinel animals, than control animals or those immunized with alum-adjuvanted rSpike vaccine. In addition, where some infected NARUVAX-C19 vaccinated animals still transmitted Delta infection to sentinels, what was remarkable was that these recipient animals demonstrated significantly less disease severity than animals acquiring infection from control or alum-adjuvanted rSpike immunized infected animals. Notably, those animals that acquired infection from NARUVAX-C19 vaccinated animals exhibited less weight loss, had no detectable lung virus and reduced nasal turbinates viral loads as compared to infections transmitted by control or alum-adjuvanted rSpike immunized animals. This reduced disease severity in recipient sentinels suggests that the NARUVAX-C19 vaccinated animals either were transferring a lower virus dose to their sentinels or that the virus they were shedding while still infectious, may have been modified in some way in the infected immunized host so as to reduce virus virulence in the recipient animal. Interestingly, the alum-adjuvanted rSpike immunized animals had similar virus loads in their nasal turbinates as the NARUVAX-C19 vaccinated animals, but did not show the same effect in respect of reduced clinical disease in their recipient sentinels that became infected. Alum-adjuvanted SARS CoV vaccines were shown to cause enhanced SARS-CoV lung disease, which was prevented if the same vaccines were formulated with more Th1-skewed adjuvants.29 Our study data suggests caution might similarly need to be exercised when using Th2-biased adjuvants such as alum in SARS-CoV-2 vaccines.
There is an ongoing need for vaccines able to efficiently prevent SARS-CoV-2 transmission. The mechanism whereby NARUVAX-C19 vaccine was able to reduce the virulence of the transmitted virus in recipient animals is currently unknown but raises interesting questions. In particular, it would be interesting in future studies to test whether the virus shed by NARUVAX-C19 vaccinated animals is modified in some way so as to reduce its virulence in subsequent recipients.
This study has several limitations. Although results were consistent within each group, the size of the macaque groups was small due to resource limitations and challenges were not able to be performed in these animals. Similarly, it is not known how the protection against the Delta variant mediated by NARUVAX-C19 vaccine might translate to cross-protection against more recent Omicron variants.30,31 Furthermore, NARUVAX-C19 vaccine’s ability to inhibit virus transmission in small rodents may or may not translate to much larger humans. In the future, we plan to test an updated Omicron spike protein version of NARUVAX-C19 vaccine for its ability to protect against Omicron infection and transmission. Notably, populations infected with different variants during epidemics develop different immune memories, which has implications for protection against future variants of concern.32
In conclusion, the current studies showed NARUVAX-C19 vaccine to be safe and immunogenic in rhesus macaques where administration of two intramuscular doses 2 weeks apart resulted in robust broadly neutralizing humoral and T cell IFN-γ responses. NARUVAX-C19 vaccine was shown for the first time to protect Syrian hamsters against the heterologous Delta variant. Notably, it reduced Delta virus transmission to naive sentinels and even where transmission occurred seemingly reduced virus virulence in the recipient infected animals via an as-yet unknown mechanism. This data supports ongoing clinical development of NARUVAX-C19 vaccine as a highly effective and safe subunit COVID-19 vaccine. Based on these studies NARUVAX-C19 vaccine has been recommended to advance to clinical studies by the regulatory authority of the Republic of Kazakhstan.
Supplementary Material
Acknowledgments
We express our sincere gratitude to the Research Institute of Medical Primatology in Sochi, Russia, for their valuable contribution to our study. Special thanks addressed to Djina Karal-ogly and her colleagues, whose meticulous efforts and professionalism allowed us to successfully organize and conduct the safety evaluation study of the NARUVAX-C19 vaccine. The authors thank Sandybayev N.T., Strochkov V.M., and Belousov V., for sequencing the SARS-CoV-2 viruses used in this study. We also thank Turebekov N., Fomin G., for ensuring biosecurity and safety aspects of research, as well as for conducting histological studies of hamster lung tissue samples, Turegeldieva D.A., Zhambyrbayeva L.S., and Sarmantayeva K.B., for care and maintenance of laboratory animals. We would like to express our gratitude to Professor T. Yespolov, the former rector of KazNARU, and Professor T. Yerubaev, the former director of NSCEDI. We are truly thankful to them for creating conducive research conditions within these organizations.
Funding Statement
This research was supported by the Kazakh National Agrarian Research University (KazNARU) and partially by the grant [AP09259609] funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan. Development of the recombinant spike protein used in these studies was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Contracts HHS-N272201400053C and HHSN272201800024C.
Disclosure statement
KrT and KtT are affiliated with T&TvaX. NP is affiliated with Vaxine. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
Kr.T., N.P. - conceptualization; Kt.T. - data curation; Kr.T., N.P. - formal analysis; Kr.T. - funding acquisition; Kt.T., M.S. - investigation; Kr.T. - project administration; Kr.T., N.P. - resources; M.S. - software; Kr.T. - supervision; M.S. - validation; Kr.T., N.P., M.S. - visualization; Kr.T. - writing ± original draft; Kr.T., N.P. - writing ± review & editing. M.S. and Kt.T. authors have contributed equally to this work and share first authorship. All authors contributed to the article and approved the submitted version.
Data availability statement
Data are available from the corresponding author upon request.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2258571.
References
- 1.World Health Organization . Coronavirus disease (COVID-19) pandemic. 2021. [accessed 2023 June 21]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.Baj J, Karakuła-Juchnowicz H, Teresiński G, Buszewicz G, Ciesielka M, Sitarz R, Forma A, Karakuła K, Flieger W, Portincasa P, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020. Jun 5;9(6):1753. doi: 10.3390/jcm9061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, Holgersson J, Nielsen N, Bentzer P, Veroniki AA, et al. Interventions for treatment of COVID-19: second edition of a living systematic review with meta-analyses and trial sequential analyses (the LIVING project). PloS One. 2021. Mar 11;16(3):e0248132. doi: 10.1371/journal.pone.0248132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B, Wu X, Liu J, Zhao D, Li Y.. Advances in COVID-19 mRNA vaccine development. Signal Transduction Targeted Ther. 2022. Mar 23;7(1):94. doi: 10.1038/s41392-022-00950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021. Jan 9;397(10269):72–10. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, Xu W, Zhao Y, Li N, Zhang J, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020. Aug 6;182(3):713–21.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabarsi P, Anjidani N, Shahpari R, Mardani M, Sabzvari A, Yazdani B, Roshanzamir K, Bayatani B, Taheri A, Petrovsky N, et al. Safety and immunogenicity of SpikoGen®, an Advax-CpG55.2-adjuvanted SARS-CoV-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations. Clin Microbiol Infect. 2022. Sep;28(9):1263–71. doi: 10.1016/j.cmi.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mallory RM, Formica N, Pfeiffer S, Wilkinson B, Marcheschi A, Albert G, McFall H, Robinson M, Plested JS, Zhu M, et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2022. Nov;22(11):1565–76. doi: 10.1016/S1473-3099(22)00420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Pinho Favaro MT, Atienza-Garriga J, Martínez-Torró C, Parladé E, Vázquez E, Corchero JL, Ferrer-Miralles N, Villaverde A. Recombinant vaccines in 2022: a perspective from the cell factory. Microb Cell Fact. 2022. Oct 5;21(1):203. doi: 10.1186/s12934-022-01929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayani F, Hashkavaei NS, Arjmand S, Rezaei S, Uskoković V, Alijanianzadeh M, Uversky VN, Ranaei Siadat SO, Mozaffari-Jovin S, Sefidbakht Y. An overview of the vaccine platforms to combat COVID-19 with a focus on the subunit vaccines. Prog Biophys Mol Biol. 2023. Mar;178:32–49. doi: 10.1016/j.pbiomolbio.2023.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . COVID-19 vaccine tracker and landscape. [accessed 2023 Mar 30]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 12.Tabynov K, Turebekov N, Babayeva M, Fomin G, Yerubayev T, Yespolov T, Li L, Renukaradhya GJ, Petrovsky N, Tabynov K. An adjuvanted subunit SARS-CoV-2 spike protein vaccine provides protection against Covid-19 infection and transmission. NPJ Vaccines. 2022. Feb 23;7(1):24. doi: 10.1038/s41541-022-00450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Honda-Okubo Y, Huang Y, Jang H, Carlock MA, Baldwin J, Piplani S, Bebin-Blackwell AG, Forgacs D, Sakamoto K, et al. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine. 2021. Sep 24;39(40):5940–53. doi: 10.1016/j.vaccine.2021.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–9. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nashwan A, Yassin M, Soliman A, De Sanctis V, Ibrahim M. mRNA-based COVID-19 vaccines booster dose: benefits, risks and coverage. Acta Biomed. 2022. Jul 1;93(3):e2022236. doi: 10.23750/abm.v93i3.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremer EJ. Pros and cons of adenovirus-based SARS-CoV-2 vaccines. Mol Ther. 2020. Nov 4;28(11):2303–4. doi: 10.1016/j.ymthe.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block JP, Boehmer TK, Forrest CB, Carton TW, Lee GM, Ajani UA, Christakis DA, Cowell LG, Draper C, Ghildayal N, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination - PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022. Apr 8;71(14):517–23. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt HP, Attia S. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology (Basel). 2021. Aug 5;10(8):752. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vetter V, Denizer G, Friedland LR, Krishnan J, Shapiro M. Understanding modern-day vaccines: what you need to know. Ann Med. 2018. Mar;50(2):110–20. doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 20.Tabynov K, Orynbassar M, Yelchibayeva L, Turebekov N, Yerubayev T, Matikhan N, Yespolov T, Petrovsky N, Tabynov K. A spike protein-based subunit SARS-CoV-2 vaccine for pets: safety, immunogenicity, and protective efficacy in juvenile cats. Front Vet Sci. 2022. Mar 14;9:815978. doi: 10.3389/fvets.2022.815978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian JH, Patel N, Haupt R, Zhou H, Weston S, Hammond H, Logue J, Portnoff AD, Norton J, Guebre-Xabier M, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021. Jan 14;12(1):372. doi: 10.1038/s41467-020-20653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020. Dec 10;383(24):2320–32. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove CA, Galloway J, et al. Safety and efficacy of the NVX-CoV2373 coronavirus disease 2019 vaccine at completion of the placebo-controlled phase of a randomized controlled trial. Clin Infect Dis. 2023. Feb 8;76(3):398–407. doi: 10.1093/cid/ciac803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020. Dec 31;383(27):2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braxton AM, Creisher PS, Ruiz-Bedoya CA, Mulka KR, Dhakal S, Ordonez AA, Beck SE, Jain SK, Villano JS. Hamsters as a model of severe acute respiratory syndrome Coronavirus-2. Comp Med. 2021. Oct 1;71(5):398–410. doi: 10.30802/AALAS-CM-21-000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammadi M, Shayestehpour M, Mirzaei H. The impact of spike mutated variants of SARS-CoV2 [Alpha, Beta, Gamma, Delta, and Lambda] on the efficacy of subunit recombinant vaccines. Braz J Infect Dis. 2021. Jul-Aug;25(4):101606. doi: 10.1016/j.bjid.2021.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Vercruysse T, Sanchez-Felipe L, Kerstens W, Rasulova M, Bervoets L, De Keyzer C, Abdelnabi R, Foo CS, Lemmens V, et al. Updated vaccine protects against SARS-CoV-2 variants including Omicron (B.1.1.529) and prevents transmission in hamsters. Nat Commun. 2022. Nov 4;13(1):6644. doi: 10.1038/s41467-022-34439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Doremalen N, Schulz JE, Adney DR, Saturday TA, Fischer RJ, Yinda CK, Thakur N, Newman J, Ulaszewska M, Belij-Rammerstorfer S, et al. ChAdOx1 nCoV-19 (AZD1222) or nCoV-19-Beta (AZD2816) protect Syrian hamsters against Beta Delta and Omicron variants. Nat Commun. 2022. Aug 8;13(1):4610. doi: 10.1038/s41467-022-32248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honda-Okubo Y, Barnard D, Ong CH, Peng BH, Tseng CT, Petrovsky N, Perlman S. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015. Mar;89(6):2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerardi V, Rohaim MA, Naggar RFE, Atasoy MO, Munir M. Deep structural analysis of myriads of Omicron sub-variants revealed hotspot for vaccine escape immunity. Vaccines (Basel). 2023. Mar 15;11(3):668. doi: 10.3390/vaccines11030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcantara MC, Higuchi Y, Kirita Y, Matoba S, Hoshino A. Deep mutational scanning to predict escape from bebtelovimab in SARS-CoV-2 Omicron subvariants. Vaccines (Basel). 2023. Mar 22;11(3):711. doi: 10.3390/vaccines11030711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanBlargan LA, Adams LJ, Liu Z, Chen RE, Gilchuk P, Raju S, Smith BK, Zhao H, Case JB, Winkler ES, et al. A potently neutralizing SARS-CoV-2 antibody inhibits variants of concern by utilizing unique binding residues in a highly conserved epitope. Immunity. 2021. Oct 12;54(10):2399–416.e6. doi: 10.1016/j.immuni.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author upon request.


