Abstract
In C. elegans, DAF-7/TGF-beta signaling regulates development, metabolism, and behavior. In addition loss of daf-7 leads to an increase of the glutamate receptor GLR-1. In daf-7(e1372) mutants, GLR-1 tagged with GFP (GLR-1::GFP) accumulates in wide puncta along the ventral nerve cord of the animal. Previous automated analyses of GLR-1::GFP accumulation relied on the proprietary software, IgorPro, for measurement of GLR-1::GFP puncta size, intensity, and density. We did a side-by-side comparison of analyses by IgorPro and an open source macro written for Fiji to analyze images from animals expressing GLR-1::GFP in wild type and daf-7(e1372) backgrounds. Analyses by the two programs were in strong agreement and are in accordance with previously published data on the effects of daf-7(e1372) on GLR-1::GFP accumulation. Based on these data, we conclude that the Fiji platform is a robust method for analyzing the accumulation of a fluorescently-tagged neurotransmitter receptor and that the Fiji puncta plugin will be applicable for image analysis for other neural markers.
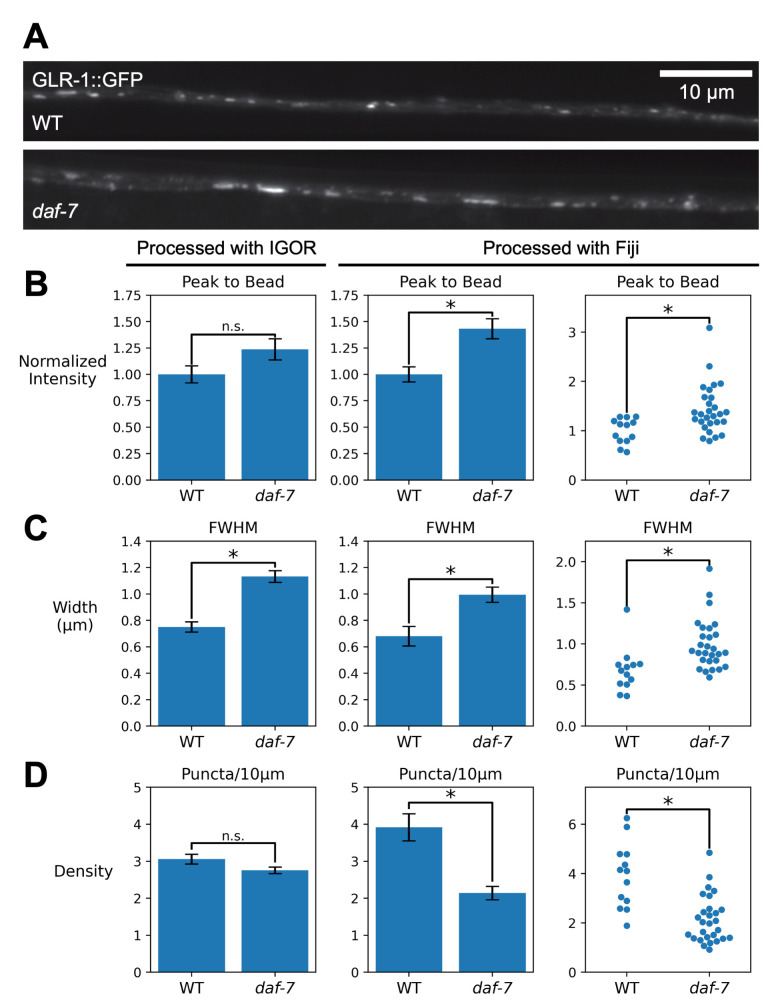
Figure 1. Comparison of quantification of GLR-1::GFP puncta using Igor and Fiji software.
A. Representative images of WT and daf-7 mutant animals expressing GLR-1::GFP under the control of the glr-1 promoter. B-D. Left, original quantification using IgorPro software; Center; analysis of the same data set using the Fiji macros. Error bars represent SEM. Right, ImageJ quantification displayed as swarm plots to show average intensity values for individual animals. B. Quantification of puncta intensity (peak-to-bead) per animal. C. Quantification of puncta width measured in microns as Full-width at Half-maximal (FWHM) fluorescence. D . Quantification of puncta density per 10 microns, by animal.
Description
The C. elegans DAF-7/TGF-beta signaling pathway relays information about an animal’s environmental state to tissues throughout the body. Signaling through this pathway regulates the developmental decision to enter dauer (Golden and Riddle 1984, Thomas et al. 1993) , and acts in later developmental stages to regulate several aspects of metabolism, reproduction and behavior (Guimenny and Savage-Dunn 2013; Meisel et al. 2014; McKnight et al. 2014; Entchev et al. 2015; Fletcher and Kim 2017; Hilbert and Kim 2017; Pekar et al. 2017; Wexler et al. 2020; Schiffer et al. 2020; Tataridas-Pallas et al. 2021; Yamamoto and Savage-Dunn 2023). Additionally, DAF-7/TGF-beta pathway has previously been shown to regulate levels of the glutamate receptor GLR-1 (McGehee et al. 2015; McGehee 2019) .
Regulation of protein levels and localization within the nervous system of C. elegans can be studied by microscopy of fluorescently tagged proteins. The images produced require software to extract information, and return quantitative data. In previous studies, GFP-tagged GLR-1 (GLR-1::GFP) has been analyzed in the ventral nerve cord using the proprietary software, IgorPro. Those studies showed that animals harboring the daf-7(e1372) mutation have increased levels of GLR-1::GFP, which can be seen as both an increase in the fluorescence intensity of GLR-1::GFP puncta and an increase in the width of the puncta (McGehee et al. 2015, McGehee 2019) .
Here, we compared the quantification and analysis done using IgorPro with that using a new Fiji macro set (Hulsey-Vincent, et al., A 2023, Schindelin et al., 2012) . We analyzed the ventral nerve cord expression of GLR-1::GFP in daf-7 mutants (N = 28) compared to wildtype animals (N = 13). Analysis using IgorPro and Fiji showed a 23% and 43% increase in peak-to-bead intensity values, respectively, with only the Fiji analysis having statistical significance ( Figure 1B, K -S test, p=0.57, Igor Pro, and p= 0.0028, Fiji),. Both IgorPro and the Fiji program similarly described 51% and 46% increases, respectively, in the width of GLR-1::GFP puncta in daf-7 mutants compared with wild type ( Figure 1C, K -S test, p= 7.6e-6, Igor Pro, and p = 0.00076, Fiji). Analysis by IgorPro and Fiji showed a 10% and 45% decrease in puncta density ( Figure 1C, K -S test p=0.10, IgorPro, and p=0.0058, Fiji). We note that there was a difference in the quantification of peak-to-bead fluorescence and density measurements reported by the two analysis tools. For both peak-to-bead fluorescence and density, we see that the two analyses show similar trends. However, we are not able to determine the precise reason for the disparities in density measurements. In the future, it would be useful to do a careful investigation of the correlation between width and density measurements in mutants such as these. In addition, identification and quantification of puncta widths was reliable using the new Fiji protocol. This is important because “out-of-the-box” thresholding methods that are available in Fiji were not able to reliably identify all puncta in this data set.
Overall, we believe that the Fiji program provides an accessible and robust alternative to the Igor proprietary software package for quantification of GLR-1::GFP. Of major importance for this dataset was the consistency in accurately reporting the width of puncta, especially given the clear differences in the width of GLR-1::GFP puncta in daf-7 mutant animals that have been previously reported (McGehee, et al., 2015; McGehee, 2019) . While we report differences in the change in puncta intensity using the open-sources Fiji macro compared with Igor, the overall trend from the analyses of the two software platforms is the same. The analysis reported here, in combination with analyses of GFP::SNB-1 images in the dorsal nerve cord motor neurons (Hulsey-Vincent, et al., A, B, 2023 ) suggests that the open-source Fiji puncta macro is useful for analyzing other accumulation of fluorescent proteins (neurotransmitter receptors, synaptic markers, etc.) in C. elegans neurons.
Methods
Imaging was performed as previously described (McGehee et al., 2015) . Briefly, L4 worms with GLR-1:: GFP were paralyzed with 30 mg/ml 2,3-butandione monoxamine. Immobilized worms were placed onto a slide to image the anterior ventral nerve cord (VNC). Z-series stacks were collected using a Carl Zeiss Axiovert M1 microscope with GFP filter. Images of the VNC were collected with an Orca-ER CCD camera (Hamamatsu) and Metamorph (version 7.1) software (Molecular Devices). For the quantitative analyses of fluorescent puncta, the maximum intensity projections of Z-series stacks were utilized. Line scans of the ventral cord puncta were produced using the Meta-Morph (version 6.0), and analyzed with the Igor Pro (version 5) (Wavemetrics) (Burbea et al., 2002) . Statistical significance of any differences between wild type and mutant strain values was determined in Igor using a Kolmogorov–Smirnov Test. Graphs of puncta intensities show data normalized to wild type values.
Fluorescent intensity of 0.5 µm FluoSphere beads (Invitrogen) was measured to monitor the Arc lamp output.
Analysis with Fiji was performed as in Hulsey-Vincent, et al., A 2023 with the following settings: minimum puncta size = 10, radius = 2, sigma = 0.5, method = Phansalkar.
The data was tested for normality using a Shaprio-Wilks test, and was found to not be normally distributed (p < 0.05). To account for this, all data was tested for significant differences using a Kolmogorov–Smirnov Test.
Reagents
|
Strain name |
Genotype |
Available from the CGC |
|
KP1147 |
nuIs24(Pglr-1::glr-1::gfp)IV |
|
|
KP3079 |
daf-7(e1372)III; nuIs24(Pglr-1:glr-1::gfp)IV |
Acknowledgments
Acknowledgments
We would like to thank Peter Juo and Jeremy Dittman for initial help in using the Igor Customizable software, Eric Luth, Andrew Stoehr, and Dan van Hees for helpful discussions, and Jackie Rose for critical reading of the manuscript.
Funding Statement
This work was partially supported by a Western Washington University Research and Sponsored Programs grant to H. H-V.
References
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002 Jul 3;35(1):107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Patel DS, Zhan M, Steele AJ, Lu H, Ch'ng Q. A gene-expression-based neural code for food abundance that modulates lifespan. Elife. 2015 May 12;4:e06259–e06259. doi: 10.7554/eLife.06259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M, Kim DH. Age-Dependent Neuroendocrine Signaling from Sensory Neurons Modulates the Effect of Dietary Restriction on Longevity of Caenorhabditis elegans. PLoS Genet. 2017 Jan 20;13(1):e1006544–e1006544. doi: 10.1371/journal.pgen.1006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, Riddle DL. A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc Natl Acad Sci U S A. 1984 Feb 1;81(3):819–823. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer ER, Pérez CL, Van Gilst MR, Lee BH, Ashrafi K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 2008 Aug 1;8(2):118–131. doi: 10.1016/j.cmet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny TL, Savage-Dunn C. TGF-β signaling in C. elegans. WormBook. 2013 Jul 10;:1–34. doi: 10.1895/wormbook.1.22.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert ZA, Kim DH. Sexually dimorphic control of gene expression in sensory neurons regulates decision-making behavior in C. elegans. Elife. 2017 Jan 24;6 doi: 10.7554/eLife.21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey-Vincent, H., N. Alvinez, S. Witus, J.R. Kowalski, and C. Dahlberg. 2023. A Fiji process for quantifying fluorescent puncta in linear cellular structures. microPublication Biology . 10.17912/micropub.biology.001003. [DOI] [PMC free article] [PubMed]
- Hulsey-Vincent, M. McClain, M. Buckley, Kowalski, J.R., H., and C. Dahlberg. 2023. Comparison and agreement between two image analysis tools for quantifying GFP::SNB-1 puncta in an fshr-1 mutant in C. elegans . microPublication Biology . 10.17912/micropub.biology.001005. [DOI] [PMC free article] [PubMed]
- Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell. 2014 Oct 9;159(2):267–280. doi: 10.1016/j.cell.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee AM, Moss BJ, Juo P. The DAF-7/TGF-β signaling pathway regulates abundance of the Caenorhabditis elegans glutamate receptor GLR-1. Mol Cell Neurosci. 2015 Jun 5;67:66–74. doi: 10.1016/j.mcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee A. The GLR-1 phenotypes of the daf-7(e1372) allele are not temperature sensitive. MicroPubl Biol. 2019 Aug 26;2019 doi: 10.17912/micropub.biology.000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight K, Hoang HD, Prasain JK, Brown N, Vibbert J, Hollister KA, Moore R, Ragains JR, Reese J, Miller MA. Neurosensory perception of environmental cues modulates sperm motility critical for fertilization. Science. 2014 May 16;344(6185):754–757. doi: 10.1126/science.1250598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekar O, Ow MC, Hui KY, Noyes MB, Hall SE, Hubbard EJA. Linking the environment, DAF-7/TGFβ signaling and LAG-2/DSL ligand expression in the germline stem cell niche. Development. 2017 Aug 15;144(16):2896–2906. doi: 10.1242/dev.147660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998 Sep 18;94(6):751–759. doi: 10.1016/s0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]
- Schiffer JA, Servello FA, Heath WR, Amrit FRG, Stumbur SV, Eder M, Martin OM, Johnsen SB, Stanley JA, Tam H, Brennan SJ, McGowan NG, Vogelaar AL, Xu Y, Serkin WT, Ghazi A, Stroustrup N, Apfeld J. Caenorhabditis elegans processes sensory information to choose between freeloading and self-defense strategies. Elife. 2020 May 5;9 doi: 10.7554/eLife.56186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012 Jun 28;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataridas-Pallas N, Thompson MA, Howard A, Brown I, Ezcurra M, Wu Z, Silva IG, Saunter CD, Kuerten T, Weinkove D, Blackwell TK, Tullet JMA. Neuronal SKN-1B modulates nutritional signalling pathways and mitochondrial networks to control satiety. PLoS Genet. 2021 Mar 4;17(3):e1009358–e1009358. doi: 10.1371/journal.pgen.1009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993 Aug 1;134(4):1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler LR, Miller RM, Portman DS. C. elegans Males Integrate Food Signals and Biological Sex to Modulate State-Dependent Chemosensation and Behavioral Prioritization. Curr Biol. 2020 Jun 11;30(14):2695–2706.e4. doi: 10.1016/j.cub.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KK, Savage-Dunn C. TGF-β pathways in aging and immunity: lessons from Caenorhabditis elegans. Front Genet. 2023 Sep 5;14:1220068–1220068. doi: 10.3389/fgene.2023.1220068. [DOI] [PMC free article] [PubMed] [Google Scholar]