Abstract
The treatment efficiency and predictors of atezolizumab plus bevacizumab therapy for unresectable hepatocellular carcinoma in real-world practice have not been established. This study aimed to assess the efficacy and safety of atezolizumab plus bevacizumab and to investigate predictors of progression-free survival and overall survival. Patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab therapy in 19 hospitals were enrolled before treatment and observed prospectively. The outcomes of 222 patients in this cohort were analyzed. The objective response rate and disease control rate were 22.0% and 70.6%, respectively, whereas the median progression-free survival was 5.7 months. Independent risk factors for shortened progression-free survival were younger age (<75 years; 3.9 months vs. 8.6 months), higher number of intrahepatic tumors (≥5; 4.0 months vs. 7.9 months), macrovascular invasion (2.3 months vs. 6.7 months), and higher neutrophil-to-lymphocyte ratio (≥3.03; 3.0 months vs. 7.8 months). The median overall survival was not reached; however, independent risk factors for shortened overall survival were absence of hyperlipidemia, higher number of intrahepatic tumors (≥5), macrovascular invasion, higher α-fetoprotein level (≥400 ng/mL), worse Child–Pugh score (≥6), and higher neutrophil-to-lymphocyte ratio (≥3.03). Severe adverse events (grade ≥3) were observed in 96 patients (36.0%), with proteinuria being the most frequent. In conclusion, patients with older age, lower number of intrahepatic tumors, absent macrovascular invasion, and lower neutrophil-to-lymphocyte ratio are expected to have better progression-free survival with atezolizumab plus bevacizumab therapy for unresectable hepatocellular carcinoma.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, and its systemic treatment has rapidly changed. The IMbrave150 trial indicated that the combination of atezolizumab and bevacizumab, an immune-checkpoint inhibitor (ICI) and an anti-vascular endothelial growth factor monoclonal antibody, was superior to sorafenib with respect to overall survival (OS) and progression-free survival (PFS) [1, 2]. Based on the results of the trial, atezolizumab plus bevacizumab therapy has been recommended as the primary systemic treatment for unresectable HCC (uHCC) [3–5].
Recent studies reported the early efficacy and safety of atezolizumab plus bevacizumab in real-world practice and investigated the predictive factors associated with therapeutic outcomes in early experiences [6–9]. Some retrospective studies showed that the CRAFITY score, composed of C-reactive protein and α-fetoprotein (AFP) levels, was useful as an early-time prognostic indicator in patients treated with atezolizumab plus bevacizumab for uHCC [10, 11]. Other studies also demonstrated the utility of neutrophil-to-lymphocyte ratio (NLR) as a clinical index of systemic inflammation associated with early therapeutic response to atezolizumab plus bevacizumab therapy [7, 9]. While these simple clinical indicators are useful, they have only been studied retrospectively in early clinical practice, and clinical indicators associated with the therapeutic efficacy of atezolizumab plus bevacizumab have not been studied prospectively.
This study aimed to assess the efficacy and safety of atezolizumab plus bevacizumab therapy in the real world using a prospective cohort and to investigate the predictors of PFS and OS in patients receiving atezolizumab plus bevacizumab therapy for uHCC.
Materials and methods
Patients
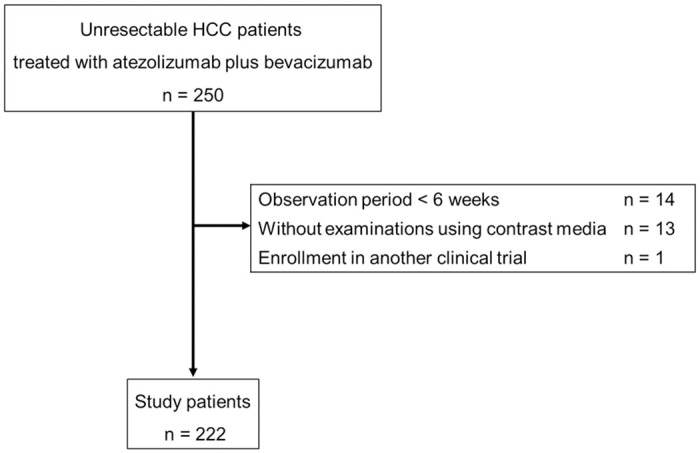
In total, 250 patients with uHCC treated with atezolizumab plus bevacizumab from November 2020 to August 2022 at Osaka University Hospital and 18 affiliated hospitals in the Osaka Liver Forum were enrolled before commencing treatment and were prospectively observed. At hospitals in the Osaka Liver Forum, patients with uHCC deemed unsuitable for surgical resection, liver transplantation, radiofrequency ablation, or transarterial chemoembolization were considered to be eligible for atezolizumab plus bevacizumab therapy. In some cases, patients with Barcelona Clinic Liver Cancer stage A were also considered to be eligible for treatment. In principle, patients with Child–Pugh class A were treated; nevertheless, depending on each attending physicians’ judgement, some patients with Child–Pugh class B were also treated. The eligibility criteria for this prospective cohort were as follows: (1) patients treated with atezolizumab plus bevacizumab therapy at hospitals in the Osaka Liver Forum; (2) patients with Eastern Cooperative Oncology Group performance status of 0 or 1; and (3) patients who consented to this study. The exclusion criteria were as follows: (1) patients with an observation period of <6 weeks; (2) patients who did not undergo examinations using contrast media; and (3) patients who were enrolled in another clinical trial (Fig 1). Among the 250 patients, 222 patients were included in the analysis.
Fig 1. Flowchart of study enrollment.
The Ethics Committee of Osaka University Hospital (UMIN-000034611) and each participating institution approved the study protocol. Written informed consent was obtained from all participants at each institute.
Treatment with atezolizumab plus bevacizumab and assessment of adverse events (AEs)
All 222 patients intravenously received atezolizumab 1200 mg plus bevacizumab 15 mg/kg once every 3 weeks. If any unacceptable or serious AE related to the drug occurred, the administration was interrupted until symptoms diminished to grade 1 or 2. The patients continued treatment until the treatment failed or an unacceptable AE occurred. The relative intensity of bevacizumab was defined as the ratio of the amount of the actual dose to the standard dose until the treatment was discontinued. Data on AEs were collected during treatment, and AEs were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Assessment of hepatic function
The prothrombin time in patients who took oral warfarin was assigned a score of 1 point to calculate the Child–Pugh score [12, 13]. The albumin-bilirubin (ALBI) score was calculated using the following formula; (log10 (total bilirubin [mg/dL] ×17.1) ×0.66) + (albumin [g/dL] × 10 × -0.085) [14]. In addition, the modified ALBI (mALBI) grade was used to categorize liver function [15].
Assessment of therapeutic efficacy
Imaging studies, such as contrast-enhanced computed tomography and magnetic resonance imaging, were performed every 6–8 weeks. The therapeutic response was assessed on the Response Evaluation Criteria in Solid Tumor version 1.1 (RECIST version 1.1) [16] and modified RECIST (mRECIST) in each institution [17]. The objective response rate (ORR) was defined as the sum of the percentage of complete response and partial response. The disease control rate (DCR) was defined as the sum of the percentage of complete response, partial response, and stable disease. PFS was the time from the start date of treatment to the date of progressive disease or death. OS the time from the start date of treatment to the date of death or patients’ last follow-up date.
Statistical analysis
Patient characteristics were expressed as medians and interquartile ranges (IQRs) for continuous variables and as actual numbers and percentages for categorical variables. OS and PFS were calculated using the Kaplan–Meier method, and their statistical differences were evaluated using the log-rank test. Cox proportional hazard models were used to identify independent factors associated with PFS and OS.
The NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count from peripheral complete blood counts. The cutoff value of NLR’s predictive capability for radiological progressive disease was calculated using a receiver operating characteristic curve based on sensitivity and specificity using the Youden index [18]. Cutoff values for other factors such as age [19], AFP level [1], maximum tumor diameter, number of tumors [19, 20], Child–Pugh score, and mALBI grade [7, 21, 22] were based on previous studies.
Statistical significance was set at a P value of < 0.05. All analyses were performed using SPSS statistical software version 24.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Table 1 shows the baseline characteristics of the study participants, as well as the characteristics according to the treatment line. The median age of patients was 73 years, and 176 (79.3%) study patients were men. One hundred and ten (49.5%), 96 (43.2%), 15 (6.8%), and 1 (0.5%) patient had Child–Pugh scores of 5, 6, 7, and 8, respectively. One hundred and six (47.7%) patients had more than four intrahepatic tumors, 37 (16.7%) patients had macrovascular invasion, and 88 (39.6%) patients had extrahepatic metastasis. Among all patients, 23 had no data on gastroesophageal varices prior to treatment; 111 (50.0%), 64 (28.8%), and 24 (10.8%) patients had F0, F1, and F2 gastroesophageal varices, respectively. The median NLR was 2.44 (IQR: 1.78–3.55). One hundred and forty-seven (66.2%) patients received atezolizumab plus bevacizumab as their first systemic chemotherapy, whereas 75 (33.8%) received another regimen before treatment. Table 1 also shows the characteristics according to the treatment line. The median observation period was 9.9 months.
Table 1. Patient characteristics.
| Characteristic | All | First-line systemic treatment | Second- or later-line systemic treatment | |
|---|---|---|---|---|
| n = 222 | ||||
| n = 147 | n = 75 | |||
| Age, years | Median (IQR) | 73 (66–79) | 75.0 (67–80) | 71 (62–76) |
| Sex, n (%) | Male | 176 (79.3) | 116 (78.9) | 60 (80.0) |
| Female | 46 (20.7) | 31 (21.1) | 15 (20.0) | |
| Hypertension, n (%) | Absent | 67 (30.2) | 47 (32.0) | 20 (26.7) |
| Present | 155 (69.8) | 100 (68.0) | 55 (73.3) | |
| Diabetes mellitus, n (%) | Absent | 135 (60.8) | 82 (55.8) | 53 (70.7) |
| Present | 87 (39.2) | 65 (44.2) | 22 (29.3) | |
| Hyperlipidemia, n (%) | Absent | 182 (82.0) | 120 (81.6) | 62 (82.7) |
| Present | 40 (18.0) | 27 (18.4) | 13 (17.3) | |
| ECOG PS, n (%) | 0 | 206 (92.8) | 138 (93.9) | 68 (90.7) |
| 1 | 16 (7.2) | 9 (6.1) | 7 (9.3) | |
| Prior systemic therapy, n (%) | Absent | 147 (66.2) | - | - |
| Present | 75 (33.8) | |||
| Etiology, n (%) | Viral | 120 (54.1) | 68 (46.3) | 52 (69.3) |
| Non-viral | 102 (45.9) | 79 (53.7) | 23 (30.7) | |
| Child–Pugh score classification, n (%) | 5 | 110 (49.5) | 74 (50.3) | 36 (48.0) |
| 6 or 7 or 8 | 112 (50.5) | 73 (49.7) | 39 (52.0) | |
| ALBI score | Median (IQR) | -2.35 (-2.65 to -2.01) | -2.34 (-2.61 to -2.06) | -2.35 (-2.70 to -2.02) |
| mALBI grade, n (%) | 1 or 2a | 127 (57.2) | 81 (55.1) | 46 (48.0) |
| 2b or 3 | 95 (42.8) | 66 (44.9) | 29 (39.7) | |
| Maximum intrahepatic tumor size, mm | Median (IQR) | 25.5 (16.0–46.3) | 26.0 (16.0–51.0) | 25.0 (14.0–37.0) |
| Intrahepatic tumor number, n (%) | ≤4 | 116 (52.3) | 79 (53.7) | 37 (49.3) |
| ≥ 5 | 106 (47.7) | 68 (46.3) | 38 (50.7) | |
| Macrovascular invasion, n (%) | Absent | 185 (83.3) | 122 (83.0) | 63 (84.0) |
| Present | 37 (16.7) | 25 (17.0) | 12 (16.0) | |
| Extrahepatic metastasis, n (%) | Absent | 134 (60.4) | 92 (62.6) | 42 (56.0) |
| Present | 88 (39.6) | 55 (37.4) | 33 (44.0) | |
| Gastroesophageal varices, n (%) | F0 | 111 (50.0) | 71 (48.3) | 40 (53.3) |
| F1 | 64 (28.8) | 39 (26.5) | 25 (33.3) | |
| F2 | 24 (10.8) | 17 (11.6) | 7 (9.3) | |
| no data | 23 (10.4) | 20 (13.6) | 3 (4.0) | |
| Barcelona Clinic Liver Cancer stage, n (%) | A or B | 109 (49.1) | 73 (49.7) | 36 (48.0) |
| C | 113 (50.9) | 74 (50.3) | 39 (52.0) | |
| AFP, ng/mL | Median (IQR) | 17 (4–747) | 17 (4–398) | 20 (4–1032) |
| NLR | Median (IQR) | 2.44 (1.78–3.55) | 2.55 (1.78–3.73) | 2.29 (1.78–3.37) |
ECOG PS, Eastern Cooperative Oncology Group performance status; ALBI, albumin-bilirubin; mALBI, modified albumin-bilirubin; AFP, α-fetoprotein; NLR, neutrophil-to-lymphocyte ratio.
Efficacy of atezolizumab plus bevacizumab therapy
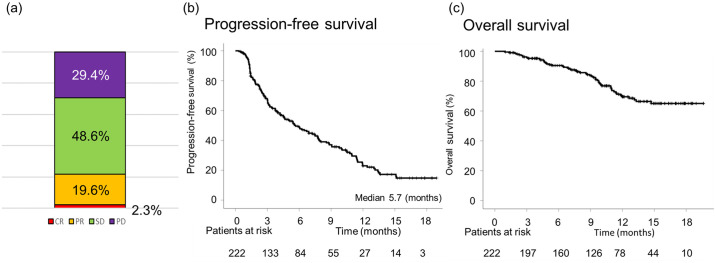
Fig 2a presents the best therapeutic responses based on RECIST version 1.1. The ORR and DCR were 22.0% and 70.6%, respectively. Eight patients were not evaluated after the treatment commenced. During the observation period, 133 patients exhibited disease progression, and 53 patients in the whole cohort died. Among these 53 patients, death was due to liver-related diseases in 48 patients, infection in two patients, and HCC rupture in one patient, immune-related AE (hepatotoxicity) in one patient, and gastrointestinal perforation in one patient. The median PFS was 5.7 months, and the median OS was not yet reached (Fig 2b and 2c). Focusing on the first-line systemic treatment group, the best therapeutic responses based on RECIST version 1.1 were similar (S1a Fig). The median PFS was 7.8 months, and the median OS had not yet reached (S1b and S1c Fig). The therapeutic efficacy in all patients and the first-line treatment group based on mRECIST was compatible with those based on RECIST version 1.1 (S2 Fig).
Fig 2.
(a) Therapeutic efficacy of atezolizumab plus bevacizumab therapy. (b) PFS and (c) OS of all patients receiving atezolizumab plus bevacizumab therapy.
In the entire cohort, 151 patients discontinued atezolizumab plus bevacizumab therapy because of progressive disease and AEs. Out of these 151 patients, 61 (40.4%), 29 (19.2%), 15 (9.9%), and 46 (30.5%) received lenvatinib, other molecular target agents, other treatments such as transarterial chemoembolization and radiation therapy, and best supportive care, respectively.
Clinical factors associated with PFS
Pretreatment clinical information was analyzed to investigate risk factors for the shortened PFS based on RECIST version 1.1. Univariate analysis identified age, maximum intrahepatic tumor size, number of intrahepatic tumors, macrovascular invasion, and NLR as significant factors associated with PFS. In contrast, hepatitis etiology or prior systemic therapy was not associated with PFS. Multivariate analysis identified younger age, a higher number of intrahepatic tumors, macrovascular invasion, and higher NLR as independent factors associated with the shorter PFS (Table 2). The median PFS was shorter in the group with younger age (<75 years; 3.9 months vs. 8.6 months), higher number of intrahepatic tumors (≥5; 4.0 months vs. 7.9 months), macrovascular invasion (2.3 months vs. 6.7 months), and higher NLR (≥3.03; 3.0 months vs. 7.8 months) than in the other groups (Fig 3).
Table 2. Univariate and multivariate analyses of PFS-related factors.
| Variable | Category | Univariate analysis | P-value | Multivariate analysis | P-value |
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| Age, years | <75 | 1 | 1 | ||
| ≥75 | 0.645 (0.464–0.895) | 0.009 | 0.674 (0.484–0.939) | 0.02 | |
| Sex | Male | 1 | |||
| Female | 1.036 (0.700–1.533) | 0.860 | |||
| Hypertension | Absent | 1 | |||
| Present | 0.920 (0.652–1.297) | 0.632 | |||
| Diabetes mellitus | Absent | 1 | |||
| Present | 0.946 (0.677–1.321) | 0.745 | |||
| Hyperlipidemia | Absent | 1 | |||
| Present | 1.023 (0.673–1.555) | 0.914 | |||
| Prior systemic therapy | Absent | 1 | |||
| Present | 1.360 (0.974–1.901) | 0.071 | |||
| Etiology | Viral | 1 | |||
| Non-viral | 1.006 (0.902–1.121) | 0.917 | |||
| ECOG PS | 0 | 1 | |||
| 1 | 0.981 (0.530–1.814) | 0.951 | |||
| Maximum intrahepatic tumor size, mm | <50 | 1 | 1 | ||
| ≥50 | 1.546 (1.076–2.222) | 0.018 | 1.023 (0.693–1.509) | 0.91 | |
| Intrahepatic tumor number | ≤4 | 1 | 1 | ||
| ≥5 | 1.832 (1.320–2.542) | <0.001 | 1.879 (1.339–2.637) | <0.001 | |
| Macrovascular invasion | Absent | 1 | 1 | ||
| Present | 2.226 (1.509–3.285) | <0.001 | 2.266 (1.511–3.397) | <0.001 | |
| Extrahepatic metastasis | Absent | 1 | |||
| Present | 1.115 (0.803–1.549) | 0.516 | |||
| AFP, ng/mL | <400 | 1 | |||
| ≥400 | 1.171 (0.821–1.671) | 0.383 | |||
| Child–Pugh score | 5 | 1 | |||
| 6 or 7 | 1.045 (0.757–1.443) | 0.789 | |||
| mALBI grade | 1 or 2a | 1 | |||
| 2b or 3 | 1.146 (0.828–1.586) | 0.412 | |||
| NLR | <3.03 | 1 | 1 | ||
| ≥3.03 | 2.038 (1.457–2.851) | <0.001 | 1.812 (1.273–2.577) | 0.001 |
CI, confidence interval; PFS, progression-free survival; ECOG PS, Eastern Cooperative Oncology Group performance status; ALBI, albumin-bilirubin; mALBI, modified albumin-bilirubin; AFP, α-fetoprotein; NLR, neutrophil-to-lymphocyte ratio.
Fig 3.
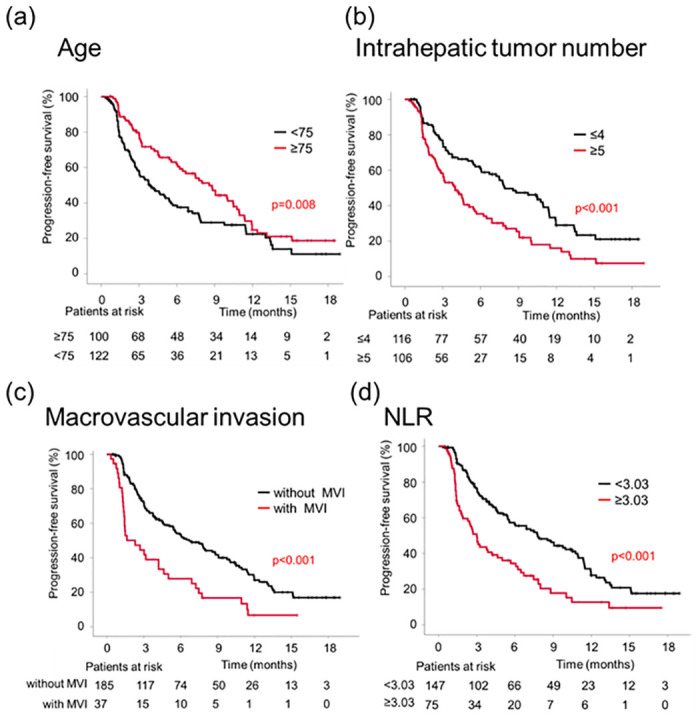
Kaplan–Meier curves for PFS according to age (a), intrahepatic tumor number (b), macrovascular invasion (c), and NLR (d).
Clinical factors associated with OS
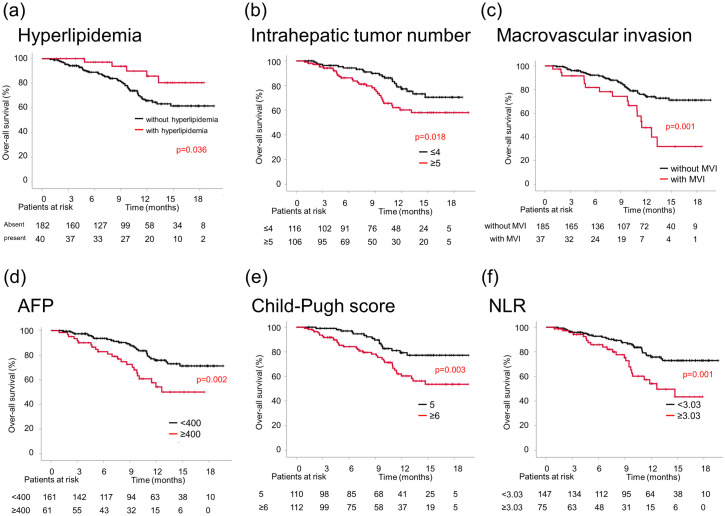
Univariate analysis for OS showed that hyperlipidemia, maximum intrahepatic tumor size, several intrahepatic tumors, macrovascular invasion, AFP level, Child–Pugh score, mALBI grade, and NLR were significant factors associated with OS (Table 3). The Child–Pugh score and mALBI grade were related; however, only the Child–Pugh score was included in the multivariate analysis. Multivariate analysis revealed that the absence of hyperlipidemia, higher number of intrahepatic tumors, macrovascular invasion, higher AFP level, worse Child–Pugh score, and higher NLR were significantly associated with poor prognoses (Table 3). The median OS was shorter in the group without hyperlipidemia (not reached vs. not reached), higher number of intrahepatic tumors (≥5; not reached vs. not reached), macrovascular invasion (11.5 months vs. not reached), higher AFP level (≥400 ng/mL; 12.6 months vs. not reached), worse Child–Pugh score (≥6; not reached vs. not reached), and higher NLR (≥3.03; 12.6 months vs. not reached) than in the other groups (Fig 4).
Table 3. Univariate and multivariate analyses of OS-related factors.
| Variable | Category | Univariate analysis | P-value | Multivariate analysis | P-value |
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | ||||
| Age, years | <75 | 1 | |||
| ≥75 | 1.077 (0.628–1.847) | 0.786 | |||
| Sex | Male | 1 | |||
| Female | 1.444 (0.784–2.661) | 0.239 | |||
| Hypertension | Absent | 1 | |||
| Present | 0.696 (0.397–1.220) | 0.206 | |||
| Diabetes mellitus | Absent | 1 | |||
| Present | 0.810 (0.458–1.430) | 0.467 | |||
| Hyperlipidemia | Absent | 1 | 1 | ||
| Present | 2.581 (1.027–6.487) | 0.044 | 2.700 (1.055–6.911) | 0.038 | |
| Prior systemic therapy | Absent | 1 | |||
| Present | 1.100 (0636–1.902) | 0.733 | |||
| Etiology | Viral | 1 | |||
| Non-viral | 0.948 (0.789–1.139) | 0.568 | |||
| ECOG PS | 0 | 1 | |||
| 1 | 2.097 (0.946–4.647) | 0.068 | |||
| Maximum intrahepatic tumor size, mm | <50 | 1 | 1 | ||
| ≥50 | 2.438 (1.387–4.285) | 0.002 | 1.479 (0.791–2.764) | 0.220 | |
| Intrahepatic tumor number | ≤4 | 1 | 1 | ||
| ≥5 | 1.950 (1.110–3.321) | 0.02 | 2.084 (1.161–3.740) | 0.014 | |
| Macrovascular invasion | Absent | 1 | 1 | ||
| Present | 2.569 (1.426–4.628) | 0.002 | 2.128 (1.146–3.951) | 0.017 | |
| Extrahepatic metastasis | Absent | 1 | |||
| Present | 1.122 (0.652–1.932) | 0.678 | |||
| AFP, ng/mL | <400 | 1 | 1 | ||
| ≥400 | 2.307 (1.337–3.982) | 0.003 | 2.422 (1.371–4.280) | 0.002 | |
| Child–Pugh score | 5 | 1 | 1 | ||
| 6 or 7 | 2.370 (1.331–4.221) | 0.003 | 2.942 (1.594–5.429) | 0.001 | |
| mALBI grade | 1 or 2a | 1 | |||
| 2b or 3 | 1.982 (1.15–13.413) | 0.014 | |||
| NLR | <3.03 | 1 | 1 | ||
| ≥3.03 | 2.361 (1.368–4.037) | 0.002 | 1.920 (1.072–3.437) | 0.028 |
CI, confidence interval; OS, overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status; ALBI, albumin-bilirubin; mALBI, modified albumin-bilirubin; AFP, α-fetoprotein; NLR, neutrophil-to-lymphocyte ratio.
Fig 4.
Kaplan–Meier curves for OS according to hyperlipidemia (a), intrahepatic tumor number (b), macrovascular invasion (c), AFP level (d), Child–Pugh score (e), and NLR (f).
Treatment-related AEs
Overall, 209 (94.1%) patients experienced AEs, with 80 (36.0%) experiencing severe AEs (grade ≥3). No significant differences in the frequency of serious AEs were observed, irrespective of the presence or absence of each comorbidity and prior systemic therapy (S1 Table). The most frequent AE was proteinuria (43.2%), followed by hypertension (31.1%), fatigue (30.2%), decreased appetite (22.1%), fever (20.7%), bleeding (20.3%), rash (18.5%), increased aspartate aminotransferase or alanine aminotransferase levels (15.8%), thyroid dysfunction (14.4%), and diarrhea (9.9%). The most frequent AE of grade ≥3 was proteinuria (12.6%) (Table 4). In total, 23 (10.4%) patients received systemic steroid treatment for immune-related AEs. The indications for systemic steroid use included interstitial pneumonia (n = 7), liver injury (n = 5), skin lesions (n = 4), colitis (n = 1), pancreatitis (n = 1), pleurisy (n = 1), persistent fever of unknown origin (n = 1), rhabdomyolysis (n = 1), Guillen-Barre syndrome (n = 1), and disseminated intravascular coagulation (n = 1). The frequency of AEs in the first-line systemic treatment group was similar to that in the later-line systemic treatment group (Table 4).
Table 4. Treatment-related adverse events.
| Treatment-related adverse events | All n = 222 | First-line systemic treatment n = 147 | Second- or later-line systemic treatment n = 75 |
|---|---|---|---|
| Any adverse event | |||
| Any grade, n (%) | 209 (94.1) | 139 (94.6) | 70 (93.3) |
| Grade ≥3, n (%) | 80 (36.0) | 46 (31.3) | 34 (43.0) |
| Proteinuria | |||
| Any grade, n (%) | 96 (43.2) | 60 (40.8) | 36 (48.0) |
| Grade ≥3, n (%) | 28 (12.6) | 10 (6.8) | 18 (22.8) |
| Hypertension | |||
| Any grade, n (%) | 69 (31.1) | 54 (36.7) | 15 (20.0) |
| Grade ≥3, n (%) | 13 (5.9) | 11 (7.5) | 2 (2.5) |
| Fatigue | |||
| Any grade, n (%) | 67 (30.2) | 46 (31.3) | 21 (28.0) |
| Grade ≥3, n (%) | 6 (2.7) | 5 (3.4) | 1 (1.3) |
| Decreased appetite | |||
| Any grade, n (%) | 49 (22.1) | 37 (25.2) | 12 (16.0) |
| Grade ≥3, n (%) | 7 (3.2) | 6 (4.1) | 1(1.3) |
| Fever | |||
| Any grade, n (%) | 46 (20.7) | 26 (17.7) | 20 (26.7) |
| Grade ≥3, n (%) | 1 (0.5) | 0 (0) | 1 (1.3) |
| Bleeding | |||
| Any grade, n (%) | 45 (20.3) | 29 (19.7) | 16 (21.3) |
| Grade ≥3, n (%) | 15 (6.8) | 7 (4.8) | 8 (10.1) |
| Rash | |||
| Any grade, n (%) | 41 (18.5) | 26 (17.7) | 15 (20.0) |
| Grade ≥3, n (%) | 3 (1.4) | 3 (2.0) | 0 (0) |
| Increased AST or ALT | |||
| Any grade, n (%) | 35 (15.8) | 22 (15.0) | 13 (17.3) |
| Grade ≥3, n (%) | 8 (3.6) | 5 (3.4) | 3 (3.8) |
| Hypothyroidism | |||
| Any grade, n (%) | 32 (14.4) | 19 (12.9) | 13 (17.3) |
| Grade ≥3, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | |||
| Any grade, n (%) | 22 (9.9) | 14 (9.5) | 8 (10.7) |
| Grade ≥3, n (%) | 3 (0) | 1 (0.7) | 2 (2.5) |
| Hoarse voice | |||
| Any grade, n (%) | 19 (8.6) | 16 (10.9) | 3 (4.0) |
| Grade ≥3, n (%) | 0 (0) | 0 (0) | 0 (0) |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
In the entire cohort, the median relative intensity of bevacizumab during the initial 6 months was 100% (IQR: 87.5–100%). The median relative intensity did not differ between patients with and without hypertension (100% vs. 100%, P = 0.102), diabetes (100% vs. 100%, P = 0.418), and elderly age (100% vs. 100%, P = 0.149).
Discussion
This study investigated the efficacy and safety of atezolizumab plus bevacizumab therapy for uHCC in real-world practice. The ORR and DCR were 22.0% and 70.6%, respectively, and the median PFS was 5.7 months in the whole cohort. Clinical factors associated with worse PFS were younger age, a higher number of intrahepatic tumors, macrovascular invasion, and higher NLR. This study revealed the therapeutic outcomes and predictive factors for atezolizumab plus bevacizumab therapy in the prospective cohort registered before treatment.
Previous small-group retrospective studies on atezolizumab plus bevacizumab therapy reported an ORR and DCR for the first systemic treatment of 24.0–50.0% and 57.7–66.6%, respectively [6, 7, 9, 11, 23–27]. In addition, Japanese studies reported a median PFS of approximately 8.0–9.0 months for the first systemic treatment [27, 28]. The ORR, DCR, and PFS in these previous retrospective studies were similar to those of the IMbrave 150 trial, which reported an ORR and DCR based on RECIST version 1.1 of 27.3% and 73.6%, respectively, and a median PFS of 6.9 months [1]. The present study revealed that the therapeutic results in real-world practice. The efficacy was comparable to the results of the IMbrave 150 trial although the present study included patients who received atezolizumab plus bevacizumab therapy not only as first systemic treatment but also second-line or later systemic treatment.
The present prospective study revealed that age ≥75 years was associated with prolonged PFS in patients treated with atezolizumab plus bevacizumab therapy. In contrast, previous studies with shorter observation periods investigating the tolerability and efficacy of atezolizumab plus bevacizumab therapy in elderly patients observed no significant difference in PFS between younger and older patients [2, 29]. However, other previous studies reported better rates of response to ICIs in older patients with melanoma and showed that younger patients had a higher expression of regulatory T cells than older patients [30, 31]. Furthermore, CD8+ effector T cells were reduced in younger patients. This is responsible for the difference in the therapeutic responses between younger and older patients [31]. Such immune change in the tumor microenvironment also reduces the therapeutic benefit of atezolizumab plus bevacizumab therapy for HCC [32]. Additionally, age-related changes in the tumor microenvironment may affect the therapeutic response of HCC. To our knowledge, the present study is the first to report on the association between older age and better therapeutic efficacy of ICIs for uHCC. The results of this prospective study suggest that atezolizumab plus bevacizumab therapy may be preferred when selecting the systemic treatment regimen for elderly patients with uHCC. In contrast, this study observed no difference in OS between the older and younger patients. Ando et al. reported that subsequent systemic treatment was associated with the prognosis after first-line systemic treatment in patients with HCC [21]. After discontinuing atezolizumab plus bevacizumab therapy for HCC, some multikinase inhibitors, such as sorafenib and lenvatinib, could be used as subsequent treatment. Regarding the association between intolerance for multikinase inhibitors and age, Kinoshita et al. reported that a lower dose intensity causing poor therapeutic efficacy was observed in patients aged ≥80 years treated with lenvatinib for HCC [33]. The intolerance to subsequent multikinase inhibitors might contribute to the unimproved prognosis in elderly patients in this study. However, the median OS was not reached, and further long-term observation on OS is required.
Of the other clinical indicators associated with therapeutic efficacy in this study, NLR was a valid inflammatory parameter induced by tumor growth or microenvironment [34]. Recently, NLR has been identified as a factor that can predict therapeutic outcomes for various solid tumors, including HCC [35–37]. In addition, some studies reported that NLR was a prognostic factor in HCC treated with ICIs, such as atezolizumab plus bevacizumab [7, 19, 23, 38] and nivolumab [39]. The present prospective study confirmed that NLR was certainly associated with PFS.
This study has some limitations. First, the observation period was insufficient to analyze OS. Only approximately a quarter of the study patients died, and the median OS had not been reached. Second, only indicators used in routine clinical practice were assessed in this study without considering factors such as biological markers and genetic predisposition.
In conclusion, this multicenter prospective observational study suggested that younger age under 75 years, more than four intrahepatic tumors, macrovascular invasion, and elevated NLR of more than 3.03 would be risk factors for the shortened PFS in atezolizumab plus bevacizumab therapy for uHCC.
Supporting information
Therapeutic efficacy of atezolizumab plus bevacizumab therapy (a) and PFS (b) and OS (c) of patients in the first-line systemic treatment group.
(TIF)
Therapeutic efficacy (a) and PFS (b) based on mRECIST in all patients. Therapeutic efficacy (c) and PFS (d) based on mRECIST in the first-line systemic treatment group.
(TIF)
(DOCX)
(XLSX)
Acknowledgments
The authors thank Saiseikai Senri Hospital, Nishinomiya Municipal Central Hospital, and National Hospital Organization Osaka Minami Medical Center.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 2.Vithayathil M, D’Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, et al. Impact of older age in patients receiving atezolizumab and bevacizumab for hepatocellular carcinoma. Liver Int. 2022;42(11):2538–2547. doi: 10.1111/liv.15405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10(3):181–223. doi: 10.1159/000514174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llovet JM, Villanueva A, Marrero JA, Schwartz M, Meyer T, Galle PR, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD Consensus Conference. Hepatology. 2021;73 Suppl 1: 158–191. doi: 10.1002/hep.31327 [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75(4):960–974. doi: 10.1016/j.jhep.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa Y, Tsuchiya K, Kurosaki M, Yasui Y, Kaneko S, Tanaka Y, et al. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs. 2022;40(2):392–402. doi: 10.1007/s10637-021-01185-4 [DOI] [PubMed] [Google Scholar]
- 7.Eso Y, Takeda H, Taura K, Takai A, Takahashi K, Seno H. Pretreatment neutrophil-to-lymphocyte ratio as a predictive marker of response to atezolizumab plus bevacizumab for hepatocellular carcinoma. Curr Oncol. 2021;28(5):4157–4166. doi: 10.3390/curroncol28050352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka T, Hiraoka A, Tada T, Hirooka M, Kariyama K, Tani J, et al. Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice. Hepatol Res. 2022;52(9):773–783. doi: 10.1111/hepr.13797 [DOI] [PubMed] [Google Scholar]
- 9.Chon YE, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Predictive biomarkers of survival in patients with advanced hepatocellular carcinoma receiving atezolizumab plus bevacizumab treatment. Cancer Med. 2023;12(3):2731–2738. doi: 10.1002/cam4.5161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatanaka T, Kakizaki S, Hiraoka A, Tada T, Hirooka M, Kariyama K, et al. Prognostic impact of C-reactive protein and alpha-fetoprotein in immunotherapy score in hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Hepatol Int. 2022;16(5):1150–1160. doi: 10.1007/s12072-022-10358-z [DOI] [PubMed] [Google Scholar]
- 11.Teng W, Lin CC, Su CW, Lin PT, Hsieh YC, Chen WT, et al. Combination of CRAFITY score with alpha-fetoprotein response predicts a favorable outcome of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Am J Cancer Res. 2022;12(4):1899–1911. [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56(7):593–619. doi: 10.1007/s00535-021-01788-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. Hepatol Res. 2021;51(7):725–749. doi: 10.1111/hepr.13678 [DOI] [PubMed] [Google Scholar]
- 14.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6(4):325–336. doi: 10.1159/000479984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 17.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 18.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: [DOI] [PubMed] [Google Scholar]
- 19.Maesaka K, Sakamori R, Yamada R, Tahata Y, Imai Y, Ohkawa K, et al. Hyperprogressive disease in patients with unresectable hepatocellular carcinoma receiving atezolizumab plus bevacizumab therapy. Hepatol Res. 2022;52(3):298–307. doi: 10.1111/hepr.13741 [DOI] [PubMed] [Google Scholar]
- 20.Shomura M, Okabe H, Sato E, Fukai K, Shiraishi K, Hirose S, et al. Hypothyroidism is a predictive factor for better clinical outcomes in patients with advanced hepatocellular carcinoma undergoing lenvatinib therapy. Cancers (Basel). 2020;12(11):3078. doi: 10.3390/cancers12113078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando Y, Kawaoka T, Suehiro Y, Yamaoka K, Kosaka Y, Uchikawa S, et al. Analysis of post-progression survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Oncology. 2020;98(11):787–797. doi: 10.1159/000509387 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Takata K, Yokoyama K, Fukuda H, Yamauchi R, Fukunaga A, et al. Pretreatment modified albumin-bilirubin grade is an important predictive factor associated with the therapeutic response and the continuation of atezolizumab plus bevacizumab combination therapy for patients with unresectable hepatocellular carcinoma. Curr Oncol. 2022;29(7):4799–4810. doi: 10.3390/curroncol29070381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JH, Chen YY, Kee KM, Wang CC, Tsai MC, Kuo YH, et al. The prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma receiving atezolizumab plus bevacizumab. Cancers (Basel). 2022;14(2):343. doi: 10.3390/cancers14020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuma M, Uojima H, Hattori N, Arase Y, Fukushima T, Hirose S, et al. Safety and efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma in early clinical practice: A multicenter analysis. Hepatol Res. 2022;52(3):269–280. doi: 10.1111/hepr.13732 [DOI] [PubMed] [Google Scholar]
- 25.Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers (Basel). 2022;14(7):1747. doi: 10.3390/cancers14071747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheon J, Yoo C, Hong JY, Kim HS, Lee DW, Lee MA, et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022;42(3):674–681. doi: 10.1111/liv.15102 [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa M, Inoue M, Ogasawara S, Maruta S, Okubo T, Itokawa N, et al. Clinical effects and emerging issues of atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma from Japanese real-world practice. Cancer. 2023;129(4):590–599. doi: 10.1002/cncr.34559 [DOI] [PubMed] [Google Scholar]
- 28.Maesaka K, Sakamori R, Yamada R, Doi A, Tahata Y, Miyazaki M, et al. Comparison of atezolizumab plus bevacizumab and lenvatinib in terms of efficacy and safety as primary systemic chemotherapy for hepatocellular carcinoma. Hepatol Res. 2022;52(7):630–640. doi: 10.1111/hepr.13771 [DOI] [PubMed] [Google Scholar]
- 29.Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Safety and efficacy of atezolizumab plus bevacizumab in elderly patients with hepatocellular carcinoma: A multicenter analysis. Cancer Med. 2022;11(20):3796–3808. doi: 10.1002/cam4.4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosrati A, Tsai KK, Goldinger SM, Tumeh P, Grimes B, Loo K, et al. Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 2017;116(9):1141–1147. doi: 10.1038/bjc.2017.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kugel CH, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 2018;24(21):5347–5356. doi: 10.1158/1078-0432.CCR-18-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28(8):1599–1611. doi: 10.1038/s41591-022-01868-2 [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita A, Hagiwara N, Osawa A, Akasu T, Matsumoto Y, Ueda K, et al. Poor tolerability of lenvatinib in elderly patients ≥80 years old with hepatocellular carcinoma: A multicenter observational study. J Oncol Pharm Pract. 2023;29(3):626–636. doi: 10.1177/10781552221077039 [DOI] [PubMed] [Google Scholar]
- 34.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–488. doi: 10.4149/BLL_2021_078 [DOI] [PubMed] [Google Scholar]
- 35.da Fonseca LG, Barroso-Sousa R, Bento Ada S, Blanco BP, Valente GL, Pfiffer TE, et al. Pre-treatment neutrophil-to-lymphocyte ratio affects survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Med Oncol. 2014;31(11):264. doi: 10.1007/s12032-014-0264-5 [DOI] [PubMed] [Google Scholar]
- 36.Personeni N, Giordano L, Abbadessa G, Porta C, Borbath I, Daniele B, et al. Prognostic value of the neutrophil-to-lymphocyte ratio in the ARQ 197–215 second-line study for advanced hepatocellular carcinoma. Oncotarget. 2017;8(9):14408–14415. doi: 10.18632/oncotarget.14797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Zhang W, Niu R, Li Y, Zhou X, Han X. A combination of the preoperative neutrophil-to-lymphocyte and lymphocyte-to-monocyte ratios as a useful predictor of survival outcomes following the transarterial chemoembolization of huge hepatocellular carcinoma. Saudi Med J. 2020;41(4):376–382. doi: 10.15537/smj.2020.4.24911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, et al. Neutrophil-lymphocyte ratio predicts early outcomes in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: a multicenter analysis. Eur J Gastroenterol Hepatol. 2022;34(6):698–706. doi: 10.1097/MEG.0000000000002356 [DOI] [PubMed] [Google Scholar]
- 39.Dharmapuri S, Özbek U, Lin JY, Sung M, Schwartz M, Branch AD, et al. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020;9(14):4962–4970. doi: 10.1002/cam4.3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Therapeutic efficacy of atezolizumab plus bevacizumab therapy (a) and PFS (b) and OS (c) of patients in the first-line systemic treatment group.
(TIF)
Therapeutic efficacy (a) and PFS (b) based on mRECIST in all patients. Therapeutic efficacy (c) and PFS (d) based on mRECIST in the first-line systemic treatment group.
(TIF)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.