Abstract
A low temperature hydrogen borrowing approach to generate secondary amines using benzimidazole-based N-heterocyclic carbene (BNHC) ruthenium complexes is reported. A series of the piano-stool complexes of the type [(η6-p-cymene)(BNHC)RuCl2] (1a–g) were synthesized via one-pot reaction of the NHC salt precursor, Ag2O, and [RuCl2(p-cymene)]2 and characterized using conventional spectroscopic techniques. The geometry of two precursors, [(η6-p-cymene)(Me4BnMe2BNHCCH2OxMe)RuCl2] (1f) and [(η6-p-cymene)(Me5BnMe2BNHCCH2OxMe)RuCl2] (1g), was studied by single crystal X-ray diffraction. These catalysts were found to dehydrogenate alcohols efficiently at temperatures as low as 50 °C to allow Schiff-base condensation and subsequent imine hydrogenation to afford secondary amines. Notably, this ruthenium-based procedure enables the N-alkylation of aromatic and heteroaromatic primary amines with a wide range of primary alcohols in excellent yields of up to 98%. The present methodology is green and water is liberated as the sole byproduct.
Keywords: Benzimidazol-2-ylidenes, ruthenium complexes, amine alkylation, C-N bond formation, mild conditions
1. Introduction
Amines, organic derivatives of ammonia, are extensively found in bioactive molecules and medicines [1]. Amines are the key precursor in the manufacture of a number of relevant therapeutics medicines [2–4]. Conventionally, the most common methods for producing alkylated amines involve alkyl halides [5] or stoichiometric reducing agents, which are used for reduction of imines formed between carbonyls and amines [6,7]. The toxicity of the alkylating and reducing reagents and the generation of huge volumes of undesired byproducts are all significant disadvantages of these reactions. To address these difficulties, catalytic techniques have been devised including Buchwald–Hartwig amination [8], hydroamination [9,10], and hydroaminomethylation [11] as well as hydrogen borrowing or hydrogen autotransfer (HB/HA) methodologies [12].
In the HB/HA procedure, first dehydrogenation of the alcohol produces the equivalent aldehyde, which then undergoes reductive amination to produce the required amine. Because the alcohol functions as the hydrogen donor, an additional hydrogen source is not required in this approach. Furthermore, because a variety of alcohol derivatives are easily available from renewable feedstocks, this technology is particularly well suited for the valorization of biomass or biomass-derived building blocks. The HB/HA technique is the most attractive methodology for their synthesis [13–15]. These reactions are notable for being not only ecologically friendly, but also atom efficient, with only water as a byproduct. Grigg [16] and Watanabe [17] independently described the first examples of amine alkylation with alcohols via hydrogen borrowing while employing the homogeneous ruthenium catalysts [(PPh3)4RhH] and [(PPh3)3RuCl2]. Since that time, several noble metal-based Ru [18–21], Pd [22–24], Ir [25–27], and Pt [28] complexes and nonnoble metal-based Mn [29] Co [30] Ni [31], and Fe [32] complexes have been used. Heterogeneous catalysts [33], biocatalysts [34,35], and chiral catalysts [36,37] have also been used. Importantly, many of the catalysts that have previously been described for this reaction require relatively high temperatures of 100 °C or greater and high catalytic loading [38–43], but some other complexes have comparative working conditions for this reaction [18,44].
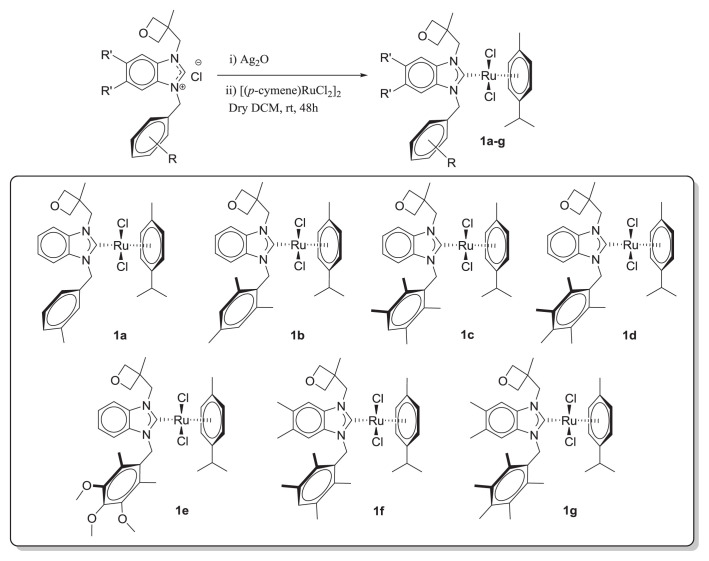
N-Heterocyclic carbene (NHC) ligands have become a common alternative to phosphine ligands in homogeneous catalysis over the last 30 years [45–48], especially in combination with ruthenium salts [44,49–52]. We recently described the synthesis of benzimidazolium salts (the precursors to benzimidazole-based NHC (BNHC) ligands) and their silver(I) complexes, which were determined to be active catalysts for carboxylation of epoxides to generate carbonates [53] and aldehyde–amine–alkyne coupling. The preparation and identification of new ruthenium(II) complexes having the general formula [(η6-p-cymene)(BNHC)RuCl2] (1a-g) are described in the present paper (Scheme 1). The hydrogen borrowing approach was used to test these complexes as catalysts for the N-alkylation of anilines and amine-substituted heterocycles with a variety of alcohols.
Scheme 1.
Synthesis of ruthenium p-cymene BNHC complexes.
2. Experimental section
2.1. Materials and methods
All metal complex preparation methods and catalytic reactions were performed using normal Schlenk procedures. Reagents were bought from commercial sources and were not purified prior to use. The melting point of the produced compounds was determined using open capillary tubes in an Electrothermal 9200 melting point device. A PerkinElmer Spectrum 100 spectrometer with a range of 4000–400 cm−1 was utilized for FT-IR analysis. NMR spectra were obtained using a Bruker Ascend 400 Avance III HD, which operated at 400 MHz (1H) and 100 MHz (13C) using tetramethyl silane as an internal reference. NMR experiments were conducted in high-quality 5-mm Young NMR tubes. Chemical shifts (δ) and coupling constants (J) are expressed in parts per million (ppm) and hertz (Hz). 13C chemical shifts are given relative to deuterated solvents (=77.16 ppm for CDCl3). 1H NMR spectra are referenced to residual protonated solvents (=7.26 ppm for CDCl3).
2.2. General synthetic methodologies used for the synthesis of benzimidazol-2-ylidene ruthenium complexes, 1a-g
Complexes [(η6-p-cymene)(BNHC)RuCl2] were synthesized in a one-step process through transmetalation. Dimeric complex of ruthenium [RuCl2(p-cymene)]2 (0.19 mmol) was added to Ag(I)–BNHC complexes (0.383 mmol) in situ without isolation and the mixture was stirred at 25 °C in dichloromethane (DCM) for 48 h. Orange–brown complexes 1a, 1b, 1c, 1d, 1e, 1f, and 1g of ruthenium carbene were isolated in good yields of 42.5%–80%. Data regarding the 1H and 13C NMR spectra are given in Tables 1 and 2.
Table 1.
Selected 1H NMR data for 1.

| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | 4 | 5 | 6 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 1a | - | 7.09 (t) 7.18 (td), 7.24– 7.40 (m) |
4.50 (s) | 4.74–5.05 (m) | 1.98 (s) | 1.15 (s) | 1.25 (d) | 2.93 (hept) | 5.86–5.72 (m) | 5.41 and 5.27 (s) | 2.21 (s) | 6.98 (d), 6.80 (d) 6.39(d) |
| 1b | - | 7.19 (dd), 7.04 (d) 7.01–6.93 (m) |
5.13 (s) | 4.73 and 4.39 (d) | 1.88 (s) | 1.22 (s) | 1.32 (d) | 3.03 (hept) | 6.68 (s), 6.57 (d), 5.72 (d), 5.43–5.31 (m) | 5.52 (d) | 2.49(s), 2.25(s) 1.80 and 1.71 (s) |
6.57 (s) 6.68 (s) |
| 1c | - | 7.16 (t) 7.02 (d) 6.97 (s) 6.91 (t) |
5.17 (d) | 4.75 and 4.39 (d) | 1.79 (s) | 1.21 (s) | 1.32 (d) | 3.02 (hept) | 6.36 and 5.53 (d) 5.71 (s) |
5.44 and 5.53 (s) | 2.34, 2.06, and 1.71 (s) | 7.22 (s) |
| 1d | 7.21–7.07 (m) 6.99 (d), 6.94–6.83 (m) |
5.18 (d) | 4.76 and 4.40 (d) | 1.95 (s) | 1.19 (s) | 1.32 (d) | 3.02 (hept) | 6.35 (d), 5.70 (s) 5.44 (s), 4.44 (s) |
5.53 (d) | 2.47–1.77 (m) | – | |
| 1e | 7.32–7.23 (m), 7.22–7.15 (m), 7.06 (d) | 5.03 (d) | 4.79 and 4.44 (d) | 1.91 (s) | 1.15 (s) | 1.28 (d) | 3.02 (hept) | 6.26 (d), 5.78 (m), 4.54 (d) | 5.43 (d) | 3.80 and 3.70 (s) | 6.43 (s) | |
| 1f | 2.28 and 2.02 (s) | 7.16 and 6.98 (s) | 5.13 (d) | 4.74 and 4.37 (d) |
1.90 (s) | 1.21 (s) | 1.31 (d) | 3.02 (hept) | 6.05, 5.63 (s), 5.36 (d) | 5.51 (d) | 2.45–2.21 (m), 1.16–1.81(m) | 6.77 (s) |
| 1g | 2.27 and 2.28 (s) | 7.22–6.92 (m), 6.74 (s) | 5.14 (d) | 4.75 and 4.38 (d) | 2.00 (s) | 1.19 (s) | 1.31 (d) | 3.01 (hept) | 6.01, 5.63 (s), 5.46–5.31 (m) | 5.51 (d) | 2.44–2.29 (m) 2.16–2.01 (m) 1.92 (s) |
– |
Table 2.
Selected 13C NMR data for 1.

| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | 2 | 4 | 5,13,16 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 14 | 15 |
| 1a | 190.3 | - | 138.7, 137.2, 135.6, 135.5, 128.8, 128.3, 126.7, 122.9, 112.0, 109.3 | 52.7 | 40.3 | 98.9 | 21.5 | 18.5 | 21.7 | 30.7 | 55.5 | 20.6 |
| 1b | 187.7 | - | 137.4, 135.9, 135.4, 128.5, 111.5, 109.9, 109.6 | 50.0 | 40.8 | 98.6 | 21.3 | 18.5 | 21.6 | 30.7 | 54.2 | 20.9 |
| 1c | 187.5 | - | 135.9, 135.4, 131.9, 131.5, 122.9, 122.8, 111.8, 109.7, 109.5 | 50.8 | 40.8 | 98.5 | 21.2 | 18.6 | 23.2 | 30.7 | 54.2 | 20.9, 20.5, 16.2 |
| 1d | 187.5 | - | 135.9, 135.5, 135.2, 128.9, 122.9, 122.6, 112.0, 109.6, 109.5 | 51.4 | 40.8 | 98.5 | 21.1 | 18.6 | 21.1 | 30.8 | 54.4 | 17.2 |
| 1e | 189.7 | - | 153.4, 137.3, 135.7, 135.4, 132.4, 123.4, 112.0, 110.4, 109.9, 104.0 | 53.3 | 40.7 | 98.6 | 20.7 | 18.6 | 21.3 | 30.7 | 54.6 | 60.9, 56.1 |
| 1f | 184.9 | 20.4, 20.3 | 134.6, 134.1, 131.8, 131.7, 109.8, 109.6, 98.6 | 50.6 | 40.9 | 98.6 | 21.3 | 18.5 | 21.2 | 30.6 | 53.9 | 20.4, 20.3 |
| 1g | 184.9 | 20.4, 20.3 | 135.1, 134.6, 134.2, 131.6, 131.6, 129.0, 112.5, 109.8, 109.5 | 51.1 | 40.8 | 98.6 | 21.2 | 18.5 | 21.3 | 30.7 | 54.1 | 20.4, 20.3, 17.2 |
1a. Yield: 63%; orange–brown solid: mp 172–174 °C.
1b. Yield: 55%; orange–brown solid: mp 172–174 °C.
1c. Yield: 67%; orange–brown solid: mp 180–182 °C.
1d. Yield: 78%; orange–brown solid: mp 180–182 °C.
1e. Yield: 48%; orange–brown solid: mp 298.5–298.7 °C.
1f. Yield: 80%; light brown solid: mp 145–148 °C.
1g. Yield: 42.5%; dark brown solid: mp 242–243 °C.
2.2.1. X-ray crystallography
X-ray measurements were performed with a STOE IPDS II diffractometer at room temperature using graphite-monochromated MoKα radiation by applying the w-scan method. Data collection and cell refinement were carried out using X-AREA, while data reduction was applied using X-RED32. The structure was solved by direct methods with SIR2019 [54] and refined by means of the full-matrix least-squares calculations on F2 using SHELXL-2018 [55]. All H atoms were located in difference maps and then treated as riding atoms, fixing the bond lengths at 0.98, 0.93, 0.97, and 0.96 Å for methine CH, aromatic CH, CH2, and CH3 atoms, respectively. The displacement parameters of the H atoms were fixed at Uiso(H) = 1.2 Ueq (1.5 Ueq for CH3). Crystal data, data collection, and structure refinement details are given in Table 3. The molecular graphic was generated using OLEX2 [56].
Table 3.
Crystal data and structure refinement parameters for 1f and 1g.
| Parameters | 1f | 1g |
|---|---|---|
| CCDC depository | 2085163 | 2173756 |
| Color/shape | Dark red/prism | Light brown/prism |
| Chemical formula | [RuCl2(C10H14)(C25H32N2O)] | [RuCl2(C10H14)(C26H34N2O)] |
| Formula weight | 682.71 | 696.73 |
| Temperature (K) | 296(2) | 296(2) |
| Wavelength (Å) | 0.71073 Mo Kα | 0.71073 Mo Kα |
| Crystal system | Triclinic | Orthorhombic |
| Space group | P–1 (No. 2) | Pbca (No. 61) |
| Unit cell parameters | ||
| a, b, c (Å) | 7.1553(5), 15.4318(12), 15.5762(12) | 7.2829(2), 21.3438(5), 43.0193(13) |
| α, β, γ (°) | 86.525(6), 85.527(6), 77.055(6) | 90, 90, 90 |
| Volume (Å3) | 1669.4(2) | 6687.1(3) |
| Z | 2 | 8 |
| Dcalc. (g/cm3) | 1.358 | 1.384 |
| μ (mm−1) | 0.659 | 0.659 |
| Absorption correction | Integration | Integration |
| Tmin., Tmax. | 0.7919, 0.9579 | 0.8533, 0.9667 |
| F 000 | 712 | 2912 |
| Crystal size (mm3) | 0.48 × 0.23 × 0.09 | 0.40 × 0.09 × 0.05 |
| Diffractometer/measurement method | STOE IPDS II/ω scan | STOE IPDS II/ω scan |
| Index ranges | −9 ≤ h ≤ 9, −20 ≤ k ≤ 20, −20 ≤ l ≤ 20 | −8 ≤ h ≤ 8, −25 ≤ k ≤ 25, −52 ≤ l ≤ 52 |
| θ range for data collection (°) | 1.928 ≤ θ ≤ 27.676 | 1.894 ≤ θ ≤ 25.646 |
| Reflections collected | 26,948 | 48,873 |
| Independent/observed reflections | 7728/6314 | 6304/3620 |
| R int. | 0.0768 | 0.0927 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 7728/0/380 | 6304/0/385 |
| Goodness-of-fit on F2 | 0.999 | 0.906 |
| Final R indices [I > 2σ(I)] | R1 = 0.0362, wR2 = 0.0727 | R1 = 0.0417, wR2 = 0.0708 |
| R indices (all data) | R1 = 0.0519, wR2 = 0.0768 | R1 = 0.1006, wR2 = 0.0833 |
| Δρmax., Δρmin. (e/Å3) | 0.56, −0.34 | 0.37, −0.40 |
2.3. A general approach: N-alkylation of amines with alcohols
At room temperature, compound 1e (1 mol %), KOtBu (75 mol %), alcohols (1 mmol), and amine (1 mmol) were added to a 15-mL reaction tube in a glove box. The tube was then closed and taken out of the glove box. The reaction mixture was then heated at 120 °C for 12 h with degassed toluene (3 mL). After cooling to room temperature, the reaction mixture was diluted with ethyl acetate, filtered, and vacuum dried. The product was purified using a suitable mixture of petroleum ether and ethyl acetate in column chromatography over silica gel (300–400 mesh) (80:1).
2.4. A general approach: aniline N-methylation with methanol
In a glove box, amine (1 mmol), MeOH (2 mL), 1e (1 mol %), and KOtBu were introduced into a 15-mL sealing tube (75 mol %). The tube was then removed from the glove box and sealed with a screw cap. At 110 °C, the reaction mixture was agitated for 12 h. The liquid was diluted with ethyl acetate and filtered through a short pad of silica after cooling to room temperature (2 cm in a Pasteur pipette). Ethyl acetate was used to wash the silica. The crude residue was refined by column chromatography (SiO2, petroleum ether:ethyl acetate = 80:1) after the filtrate had evaporated.
3. Results and discussion
3.1. Preparation of ruthenium(II) complexes
Starting with previously described benzimidazolium salts [53,57], the addition of Ag2O followed by [(p-cymene)RuCl2]2 in dry dichloromethane resulted in the formation of the corresponding [(η6-p-cymene)(RBNHCCH2OxMe)RuCl2] (1a–g) compounds after 48 h at ambient temperature (Scheme 1). A large band was observed in the FT-IR spectra of the free ligands in the 1572–1556 cm−1 range, which corresponds to the vibration of the C=N bonds in ligands. In the ruthenium complex, these bands shifted to the 1461–1486 cm−1 range, which clearly indicated the shifting of double bond (C=N) character to single bond character υ(NCN). The 1H NMR spectra of these complexes revealed that the characteristic downfield NCHN signal of the salts had disappeared. The methine proton of the p-cymene group was located as a septet between 2.97 and 3.02 ppm for the respective complexes, while the methyl protons of the p-cymene appeared at 1.15–1.21 ppm. In the 13C NMR spectra of complexes 1a–g, the carbene carbon attached to ruthenium gave characteristic signals in the range of 184.9–190.3 ppm (see Tables 1 and 2 and Sup Inf).
3.2. Structural analysis
The molecular structures of 1f and 1g with complete atom numbering are displayed in the Figure, while important bond distances and angles are listed in Tables 3 and 4. Both structures consist of a BNHC ligand coordinated to a ruthenium center, which also features a p-cymene and two chloride ligands in the coordination sphere. Compound 1f crystallizes in triclinic space group P–1 with two molecules in the unit cell, while 1g crystallizes in orthorhombic space group Pbca with eight molecules in the unit cell.
Figure.
Molecular structures of 1f (a) and 1g (b) drawn at the 30% probability level. H atoms have been omitted for clarity.
Table 4.
Selected geometric parameters for 1f and 1g.
| Parameters | 1f | 1g | Parameters | 1f | 1g |
|---|---|---|---|---|---|
| Bond lengths (Å) | Bond angles (°) | ||||
| Ru1–Cg | 1.7098(11) | 1.7058(17) | Cl1–Ru1–Cl2 | 84.35(3) | 84.05(4) |
| Ru1–Cl1 | 2.4122(7) | 2.4193(11) | Cl1–Ru1–C1 | 95.53(7) | 95.76(10) |
| Ru1–Cl2 | 2.4288(7) | 2.4307(11) | Cl1–Ru1–Cg | 124.38(4) | 122.90(7) |
| Ru1–C1 | 2.074(2) | 2.096(4) | Cl1–Ru1–Carene | 88.20(7)–158.11(7) | 85.23(11)–159.05(10) |
| Ru1–Carene | 2.161(2)–2.249(2) | 2.194(4)–2.240(4) | Cl2–Ru1–C1 | 90.89(7) | 91.24(10) |
| N1–C1 | 1.361(3) | 1.364(5) | Cl2–Ru1–Cg | 127.60(4) | 126.94(7) |
| N2–C1 | 1.370(3) | 1.366(4) | Cl2–Ru1–Carene | 91.24(7)–156.33(6) | 89.88(11)–157.66(11) |
| C1–Ru1–Cg | 123.23(7) | 124.93(12) | |||
| C1–Ru1–Carene | 86.78(9)–153.24(10) | 87.70(14)–157.11(15) | |||
| N1–C1–N2 | 105.29(18) | 104.7(3) |
Note: Cg represents the centroid of the arene ring.
In the structures, the BNHC ligand is coordinated to Ru(II) in a monodentate manner via a neutral carbenic carbon, while the arene ring of p-cymene is coordinated to the metal ion in an η6-fashion. The complexes can be identified as characteristic three-legged piano stool complexes with a pseudooctahedral geometry that is common for ruthenium half-sandwich arene complexes. Furthermore, the geometry around the metal atoms may be regarded as a tetrahedron with considerable trigonal distortion, if bonding to the p-cymene centroid is considered.
Defining Cg as the centroid of the arene ring, the Ru–Cg distance is 1.7098(11) Å in 1f and 1.7058(17) Å in 1g, while the Cl1–Ru1–Cg, Cl2–Ru1–Cg and C1–Ru1–Cg angles are 124.38(4), 127.60(4), and 123.23(7)° in 1f, and 122.90(7), 126.94(7), and 124.93(12)° in 1g, respectively. The Cl1–Ru1–Cl2, Cl1–Ru1–C1 and Cl2–Ru1–C1 angles are smaller than the ideal tetrahedral angle (109.47°), which is compensated for by extension of the Cg–Ru–L (L is Cl1, Cl2, or C1) angles. The ruthenium atom is bound to the arene ring with a mean Ru–C bond distance of 2.21 Å in both complexes. The Ru1–C1 bond distance is 2.074(2) Å in 1f and 2.096(4) Å in 1g, while the Ru–Cl bonds range from 2.4122(7) to 2.4307(11) Å. The structural data of the complexes are consistent with those of previously reported NHC-Ru(II)(p-cymene)Cl2 complexes [51,58–63].
3.3. Optimization of amine alkylation with alcohols
The ability of synthesized (BNHC)Ru complexes that might promote amine alkylation was then evaluated, as shown in Table 5. In the presence of potassium tert-butoxide, 1.0 mol % of ruthenium complex 1e, which features meta and para methoxy substitution, was fully benzylated 4-methoxy aniline (>99% conversion, entry) after 12 h at 120 °C to generate secondary amine product A. KOtBu was an efficient base for obtaining high yields. However, conversion was not possible when substituting weaker bases for KOtBu, such as K2CO3 and Na2CO3. KOtBu was required at 75 mol % to achieve satisfactory conversion. Surprisingly, the reaction still reached 98% conversion at a lower temperature of 70 °C (Table 5, entry 2). However, this trial did lead to the observation of imine product B (92:8 A:B ratio).
Table 5.
The use of benzyl alcohol to optimize the N-alkylation of 4-methoxyaniline.

| ||||||
|---|---|---|---|---|---|---|
| Entry | Cat (mol %) | Base (75 mol %) | Temp (°C) | Time (h) | Conversion (%) | A/B |
| 1 | 1e (1.0) | KOtBu | 120 | 12 | > 99 | >99/0 |
| 2 | 1e (1.0) | KOtBu | 70 | 12 | 98 | 92/8 |
| 3 | 1e (1.0) | KOtBu | 50 | 12 | 96 | 88/12 |
| 4 | 1e (0.5) | KOtBu | 70 | 12 | 96 | 65/35 |
| 5 | 1e (1.0) | KOtBu | 70 | 5 | 98 | 87/13 |
| 6 | 1f (1.0) | KOtBu | 70 | 12 | 94 | 85/15 |
| 7 | 1g (1.0) | KOtBu | 70 | 12 | 92 | 78/22 |
| 8a | 1e (1.0) | KOtBu | 70 | 12 | 26 | -b |
| 9c | 1e (1.0) | KOtBu | 70 | 12 | 5 | -b |
Reaction conditions: All reactions were conducted in 2 mL of toluene and conversion is based on 1H NMR spectroscopy.
An open-air environment.
Mixture of products.
Reaction was conducted in water.
Lowering the temperature even further to 50 °C still allowed 96% conversion with lower selectivity for product A (88:12, Table 5, entry 3). A more pronounced loss of selectivity was observed when the catalyst loading was lowered to 0.5 mol % (65:35, Table 5, entry 4). Similarly, stopping a 70 °C reaction after 5 h revealed 98% conversion, but incomplete imine hydrogenation. Compounds 1f and 1g, featuring significant methyl substitution, were slightly less effective for this reaction (Table 5, entries 6 and 7). When the reaction was carried out in an open-air environment or in water, conversion was significantly reduced (Table 5, entries 8 and 9).
Table 6.
Aniline alkylation using a variety of primary alcohols.
Table 7.
The use of benzyl alcohol to alkylate a variety of primary amines.
Table 8.
Methylation of aromatic amines.
Table 9.
Comparison of Ru–NHC catalyst (1e) with reported NHC systems.
| S/NO | Cat (mol %) | Substrate 1 Alcohol |
Substrate 2 Primary amine |
Temp (°C) | Time (h) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 1 | aniline | Substituted alcohol | 70 | 12 | 92 | This work |
| 2 | 2.5 | - | - | 120 | 24 | 97 | [38] |
| 3a | 1.0 | Substituted aniline | MeOH | 150 | 24 | 84 | [67] |
| 4 | 0.5 | - | - | 130 | 24 | 85 | [68] |
| 5 | 1.0 | - | - | 135 | 36 | 95 | [69] |
For the same reaction we use 1e (1 mol %) at 110 °C for 12 h and we get 97% selective yield.
3.4. N-Alkylation on aniline with substituted primary alcohols
Encouraged by these findings, the scope of aniline N-alkylation under mild conditions (70 °C, 12 h) using 1e was explored. Table 6 illustrates that both electron-rich and electron-deficient benzylic alcohols worked well, yielding alkylated aniline derivatives 2a–j in 55%–94% isolated yield. Catalysis was compatible with several functional groups, including methoxy groups (2c and 2f), halides (2a and 2d), and trifluoromethyl groups (2i). Debromination was observed in the case of para-bromobenzyl alcohol, but the brominated product was extracted in a reasonable yield (Table 6, 55%). At 90 °C, the sterically hindered ortho-methyl benzylic alcohol and ortho-methoxy benzylic alcohol still allow monoalkylated amine products 2e and 2f in 90% and 87% yield, respectively. Products 2g and 2h were obtained in 65% and 71% yield when heterocyclic alcohols such as 2-furylmethanol and 2-thiophenemethanol were utilized as substrates. Using the aliphatic alcohol heptanol afforded aniline derivative 2j in 75% isolated yield (Table 6). The N-alkylation of anilines with secondary alcohols like 1-phenethyl alcohol, cyclohexanol, and isopropyl alcohol, on the other hand, was ineffective, generating only trace amounts of products. This observation is consistent with a mechanism that involves alcohol dehydrogenation to generate an aldehyde intermediate.
3.5. N-Alkylation of substituted anilines and heterocyclic amines with benzyl alcohol
The scope of amines that could undergo alkylation was then explored (Table 7). The N-alkylated products 3a–i were obtained in good yield (81%–92%) from substrates containing either electron-donating or electron-withdrawing substituents. For example, 1,3-benzodioxan-5-amine was treated with benzyl alcohol to produce 3h in high yield (Table 7, 87%). Heteroaromatic amines like 2-aminopyridine, 3-aminopyridine, and 2-aminopyrimidine were successfully converted into products 3d–f in good yield (Table 7, 81%–86%). The secondary amine morpholine (3i), on the other hand, was not tolerated. The N-alkylation of p-nitroaniline with benzyl alcohol and the N-alkylation of aniline with 4-nitrobenzyl alcohol were also not successful, even with a greater catalyst loading (2 mol %) when conducted at 110 °C. These observations indicate that nitro groups are not tolerated by 1e.
3.6. N-Methylation of anilines
N-Methylamines are commonly employed as intermediates and building blocks in the production of bulk and fine chemicals, as well as materials [64,65]. Due to the higher activation barrier (21 kcal mol−1) of methanol dehydrogenation compared to that of higher alcohols, such as ethanol (16 kcal mol−1), methanol can be a problematic substrate for the N-alkylation of amines [66]. Therefore, the N-methylation of amines with methanol was examined to further broaden the scope of 1e promoted C–N bond formation. To our delight, we were able to successfully N-methylate anilines with methanol in the presence of 1.0 mol % of 1e at 110 °C (Table 8). As indicated in Table 8, the majority of the catalytic reactions were efficient, yielding at least 81% of the desired product (Table 8, 4a–g in 46%–97% yield). When 2-iodoaniline was used, the reaction produced 5g in moderate isolated yields (46%) and was also dehalogenated. Biologically important motifs like pyridine-2-amine and 3-trifluoromethylaniline were also successfully methylated (Table 8, 4d and 4e). Despite the use of copious MeOH and high temperatures, we did not observe dialkylation products in any case. Attempts to obtain N-methylate aliphatic amines like benzylamine and n-hexylamine, on the other hand, were ineffective, giving just traces of methylated product.
A comparison of the most active catalyst (1e) with other reported catalysts is shown in Table 9. It is the best Ru–NHC-based catalyst in terms of low catalyst loading (1 mol %) and low temperature (70 °C). Furthermore, most of the literature reports that the hydrogen transfer reaction is used to convert the alcohol into ketone or aldehyde using Ru–NHC complexes. Very few reports of such secondary amine products through the coupling of primary amine and alcohols have been reported using ruthenium–NHC complexes. These Ru–NHC complexes are also applicable for the conversion of highly complicated products like methanol and convert them into secondary amines.
3.7. Proposed mechanism
Given that catalysis requires the presence of KOtBu (75 mol % relative to 1.0 mol % of 1e), it is reasonable to propose that a salt metathesis reaction occurs to form the corresponding bis(tert-butoxide) complex, which can undergo σ-bond metathesis with incoming benzyl alcohol to generate intermediate A (Scheme 2).
Scheme 2.
A plausible mechanism for C–N bond formation catalyzed by 1e.
Alternatively, intermediate A may be generated directly from 1e if any KOBn is generated in solution. This intermediate can undergo subsequent β-hydride elimination steps to generate B and ultimately dihydride complex C. As has been observed for other hydrogen borrowing C–N bond forming reactions, the in situ generation of aldehyde results in Schiff base condensation with any primary amine that is present to generate the corresponding aldimine (this is also the reason why secondary amines do not become alkylated again to yield tertiary amines). This aldimine can insert into dihydride C to generate intermediate E. At this point, the desired secondary amine product can be liberated in one of two ways. Reductive elimination from E can occur to generate intermediate D (as shown in Scheme 2) or E can undergo σ-bond metathesis with the next equivalent of benzyl alcohol to generate intermediate B. If Ru(0) intermediate D is formed during the reaction, it quickly reacts with any alcohol or hydrogen that is present to generate the corresponding hydride complex.
4. Conclusion
We have described the synthesis and characterization of a series of ruthenium complexes with BNHC proligands that feature a variety of benzyl group substitution patterns. Through a HB/HA mechanism, these compounds were discovered to be highly effective catalysts for the selective monoalkylation of aromatic primary amines. Complex 1e was the most active of the catalysts tested, and it is one of the most active ruthenium catalysts ever reported for amine alkylation given that it operates efficiently at temperatures as low as 50 °C at low catalyst loadings (1.0 mol %). A wide range of (hetero)aromatic amines and primary alcohols were successfully converted into secondary amines in good to exceptional isolated yields, including physiologically relevant examples. The methylation of primary amines was also achieved using methanol, a transformation that is particularly difficult to demonstrate.
Supporting Information
Characterizing data of ruthenium–BNHC complex 1a
Dichloro-[1-((3-methyloxetan-3-yl)methyl)-3-(3-methylbenzyl)benzimidazole-2-ylidene](p-cymene) ruthenium(II) (1a)
1H NMR spectrum of ruthenium–BNHC complex 1a (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1a (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1a.
Characterizing data of ruthenium–BNHC complex 1b
Dichloro-[1-((3-methyloxetan-3-yl)methyl)-3-(2,4,6-trimethylbenzyl)benzimidazole-2-ylidene](p-cymene) ruthenium(II) (1b)
1H NMR spectrum of ruthenium–BNHC complex 1b (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1b (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1b.
Characterizing data of ruthenium–BNHC complex 1c
Dichloro-[1-((3-methyloxetan-3-yl)methyl)-3-(2,3,5,6-tetramethylbenzyl)benzimidazole-2-ylidene](p-cymene) ruthenium(II) (1c)
1H NMR spectrum of ruthenium–BNHC complex 1c (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1c (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1c.
Characterizing data of ruthenium–BNHC complex 1d
Dichloro-[1-((3-methyloxetan-3-yl)methyl)-3-(2,3,4,5,6-pentamethylbenzyl)benzimidazole-2-ylidene](p-cymene) ruthenium(II) (1d)
1H NMR spectrum of ruthenium–BNHC complex 1d (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1d (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1d.
Characterizing data of ruthenium–BNHC complex 1e
Dichloro-[1-((3-methyloxetan-3-yl)methyl)-3-(3,4,5-trimethoxybenzyl)benzimidazole-2-ylidene](p-cymene) ruthenium(II) (1e)
1H NMR spectrum of ruthenium–BNHC complex 1e (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1e (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1e.
Characterizing data of ruthenium–BNHC complex 1f
Dichloro-[(5,6-dimethyl-1-((3-methyloxetan-3-yl)methyl)-3-(2,3,5,6-tetramethylbenzyl)benzimidazole-2-ylidene](p-cymene) ruthenium(II) (1f)
1H NMR spectrum of ruthenium–BNHC complex 1f (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1f (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1f.
Characterizing data of ruthenium–BNHC complex 1g
Dichloro-[(5,6-dimethyl-1-((3-methyloxetan-3-yl)methyl)-3-(2,3,4,5,6-pentamethylbenzyl)benzimidazole-2-ylidene](p-cymene) ruthenium(II) (1g)
1H NMR spectrum of ruthenium–BNHC complex 1g (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1g (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1g.
Characterizing data of the tested (2a–h) substituted benzyl alcohol with aniline by the complex 1e
N-(4-chlorobenzyl)aniline (2a)
1H NMR (400 MHz, CDCl3) δ 7.32 (s, 4H), 7.20 (dd, J = 8.4, 7.5 Hz, 2H), 6.76 (t, J = 7.3 Hz, 1H), 6.66–6.57 (m, 2H), 4.32 (s, 2H), 4.05 (s, 1H).
13C NMR (101 MHz, CDCl3) δ 147.9, 138.0, 132.9, 129.3, 128.7, 117.8, 112.9, 47.6.
1H NMR and 13C NMR spectrum of 2a (in CDCl3, 25 °C, TMS, 400 MHz).
N-(4-methylbenzyl)aniline (2b)
1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 7.4 Hz, 2H), 7.68 (d, J = 11.1 Hz, 4H), 7.21 (t, J = 7.2 Hz, 1H), 7.13 (d, J = 7.7 Hz, 2H), 4.77 (s, 2H), 4.31 (s, 1H), 2.85 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 148.7, 137.3, 136.8, 129.7, 128.0, 117.9, 113.3, 48.5, 21.6.
1H NMR and 13C NMR spectrum of 2b (in CDCl3, 25 °C, TMS, 400 MHz).
N-(4-methoxybenzyl)aniline (2c)
1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 8.6 Hz, 2H), 7.26–7.17 (m, 2H), 6.93 (dd, J = 9.2, 2.4 Hz, 2H), 6.76 (t, J = 7.3 Hz, 1H), 6.67 (d, J = 7.7 Hz, 2H), 4.28 (s, 2H), 3.98 (s, 1H), 3.83 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.9, 148.3, 131.5, 129.3, 128.8, 117.5, 114.1, 112.9, 55.3, 47.8.
1H NMR and 13C NMR spectrum of 2c (in CDCl3, 25 °C, TMS, 400 MHz).
N-(4-bromobenzyl)aniline (2d)
1H NMR (400 MHz, CDCl3) δ 7.43 (dd, J = 6.8, 1.5 Hz, 2H), 7.21 (d, J = 7.3 Hz, 2H), 7.15 (tt, J = 7.2, 1.7 Hz, 2H), 6.74–6.68 (m, 1H), 6.62–6.53 (m, 2H), 4.25 (s, 2H), 3.92 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 145.9, 136.0, 130.9, 127.3, 126.7, 115.8, 110.9, 45.6.
1H NMR and 13C NMR spectrum of 2d (in CDCl3, 25 °C, TMS, 400 MHz).
N-(2-methylbenzyl)aniline (2e)
1H NMR (400 MHz, CDCl3) δ 7.25 (dt, J = 14.9, 7.9 Hz, 5H), 7.13 (d, J = 7.3 Hz, 1H), 6.76 (t, J = 7.3 Hz, 1H), 6.68 (d, J = 7.8 Hz, 2H), 4.32 (s, 2H), 4.02 (s, 1H), 2.39 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 148.3, 139.4, 138.3, 129.3, 128.6, 128.3, 128.0, 124.6, 117.5, 112.9, 48.4, 21.5.
1H NMR and 13C NMR spectrum of 2e (in CDCl3, 25 °C, TMS, 400 MHz).
N-(2-methoxybenzyl)aniline (2f)
1H NMR (400 MHz, CDCl3) δ 7.26 (ddd, J = 14.8, 11.4, 4.3 Hz, 3H), 7.00 (d, J = 13.3 Hz, 2H), 6.87 (d, J = 8.1 Hz, 1H), 6.77 (dd, J = 10.4, 4.2 Hz, 1H), 6.68 (d, J = 7.5 Hz, 2H), 4.34 (s, 2H), 3.98 (s, 1H), 3.83 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 160.0, 148.2, 141.3, 129.7, 129.3, 119.8, 117.6, 113.0, 112.7, 55.2, 48.3.
1H NMR and 13C NMR spectrum of 2f (in CDCl3, 25 °C, TMS, 400 MHz).
N-(furan-2-ylmethyl)aniline (2g)
1H NMR (400 MHz, CDCl3) δ 7.40–7.33 (m, 1H), 7.22–7.15 (m, 2H), 6.77–6.72 (m, 1H), 6.68 (ddd, J = 4.6, 2.1, 1.1 Hz, 2H), 6.36–6.29 (m, 1H), 6.23 (dd, J = 3.2, 0.8 Hz, 1H), 4.32 (s, 2H), 3.95 (s, 1H). 13C NMR (101 MHz, CDC3) δ 152.7, 147.6, 141.9, 129.2, 118.0, 113.1, 110.3, 106.9, 41.4.
1H NMR and 13C NMR spectrum of 2g (in CDCl3, 25 °C, TMS, 400 MHz).
N-(3,5-bis(trifluoromethyl)benzyl)aniline (2h)
1H NMR (400 MHz, CDCl3) δ 7.84 (s, 2H), 7.79 (s, 1H), 7.18 (td, J = 7.8, 0.7 Hz, 2H), 6.77 (td, J = 7.4, 0.8 Hz, 1H), 6.60 (dd, J = 7.7, 0.8 Hz, 2H), 4.47 (s, 2H), 4.18 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 147.2, 142.5, 129.4, 118.5, 113.0, 47.7.
1H NMR and 13C NMR spectrum of 2h (in CDCl3, 25 °C, TMS, 400 MHz).
Characterizing data of the tested (3a–h) substituted aniline with benzyl alcohol by the complex 1e
N-benzyl-4-chloroaniline (3a)
1H NMR (400 MHz, CDCl3) δ 7.34–7.30 (m, 4H), 7.26 (dd, J = 8.8, 4.6 Hz, 1H), 7.10–7.03 (m, 2H), 4.26 (d, J = 5.5 Hz, 2H), 4.02 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 147.9, 138.0, 132.9, 129.3, 128.7, 117.8, 112.9, 47.6.
1H NMR and 13C NMR spectrum of 3a (in CDCl3, 25 °C, TMS, 400 MHz).
N-benzyl-4-methylaniline (3b)
1H NMR (400 MHz, CDCl3) δ 7.41–7.19 (m, 5H), 6.96 (d, J = 8.3 Hz, 2H), 6.53 (d, J = 8.4 Hz, 2H), 4.27 (s, 2H), 3.86 (s, 1H), 2.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 146.0, 139.7, 129.8, 128.6, 127.5, 127.2, 113.0, 48.7, 20.4.
1H NMR and 13C NMR spectrum of 3b (in CDCl3, 25 °C, TMS, 400 MHz).
N-benzyl-4-methoxyaniline (3c)
1H NMR (400 MHz, CDCl3) δ 7.37 (dt, J = 20.8, 10.2 Hz, 4H), 7.31–7.24 (m, 1H), 6.81 (d, J = 8.8 Hz, 2H), 6.63 (d, J = 8.8 Hz, 2H), 4.30 (s, 2H), 3.76 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 152.2, 142.4, 139.7, 128.6, 127.6, 114.9, 114.2, 55.8, 49.3.
1H NMR and 13C NMR spectrum of 3c (in CDCl3, 25 °C, TMS, 400 MHz).
N-benzylpyridin-2-amine (3d)
1H NMR (400 MHz, CDCl3) δ 8.10–8.00 (m, 1H), 7.46–7.28 (m, 5H), 7.27–7.21 (m, 1H), 6.55 (ddd, J = 7.1, 5.0, 0.9 Hz, 1H), 6.33 (d, J = 8.4 Hz, 1H), 5.16 (s, 1H), 4.47 (d, J = 5.8 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 158.7, 148.2, 139.2, 128.6, 127.3, 113.1, 106.7, 46.3.
1H NMR and 13C NMR spectrum of 3d (in CDCl3, 25 °C, TMS, 400 MHz).
N-benzylpyridin-3-amine (3e)
1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 2.9 Hz, 1H), 7.94 (dd, J = 4.7, 1.2 Hz, 1H), 7.34 (d, J = 4.4 Hz, 4H), 7.30–7.24 (m, 1H), 7.04 (dd, J = 8.3, 4.7 Hz, 1H), 6.85 (ddd, J = 8.3, 2.8, 1.2 Hz, 1H), 4.32 (s, 2H), 4.25 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 144.0, 138.8, 138.5, 136.1, 128.7, 127.4, 123.7, 118.5, 47.8.
1H NMR and 13C NMR spectrum of 3e (in CDCl3, 25 °C, TMS, 400 MHz).
N-benzylpyrimidin-2-amine (3f)
1H NMR (400 MHz, CDCl3) δ 8.19 (d, J = 3.3 Hz, 2H), 7.33 (q, J = 7.9 Hz, 4H), 7.28–7.23 (m, 1H), 6.50 (t, J = 4.8 Hz, 1H), 5.96 (s, 1H), 4.62 (d, J = 5.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 158.0, 139.1, 128.6, 127.5, 127.2, 110.7, 45.4.
1H NMR and 13C NMR spectrum of 3f (in CDCl3, 25 °C, TMS, 400 MHz).
N-benzylbenzo[1,3]dioxol-5-amine (3g)
1H NMR (400 MHz, CDCl3) δ 7.40–7.33 (m, 4H), 7.30–7.25 (m, 1H), 6.66 (d, J = 8.2 Hz, 1H), 6.27 (d, J = 2.3 Hz, 1H), 6.07 (dd, J = 8.3, 2.3 Hz, 1H), 5.84 (d, J = 0.5 Hz, 2H), 4.26 (s, 2H), 3.67 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 148.3, 143.9, 139.7, 139.4, 128.6, 127.5, 127.2, 108.6, 104.4, 100.6, 96.0, 49.2.
1H NMR and 13C NMR spectrum of 3g (in CDCl3, 25 °C, TMS, 400 MHz).
N-benzyl-3,5-bis(trifluoromethyl)aniline (3h)
1H NMR (400 MHz, CDCl3) δ 7.43–7.31 (m, 5H), 7.17 (s, 1H), 6.97 (s, 2H), 4.45 (s, 1H), 4.36 (d, J = 5.4 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 148.6, 137.6, 128.9, 127.8, 127.5, 111.9, 111.0, 48.0.
1H NMR and 13C NMR spectrum of 3h (in CDCl3, 25 °C, TMS, 400 MHz).
Characterizing data of the tested (4a–e) substituted aniline with methanol by the complex 1e
N-methylaniline (4a)
1H NMR (400 MHz, CDCl3) δ 6.72 (t, J = 7.8 Hz, 2H), 6.24 (t, J = 7.3 Hz, 1H), 6.15 (d, J = 7.8 Hz, 2H), 3.21 (s, 1H), 2.36 (s, 3H).
1H NMR and 13C NMR spectrum of 4a (in CDCl3, 25 °C, TMS, 400 MHz).
N,4-dimethylaniline (4b)
1H NMR (400 MHz, CDCl3) δ 7.02 (d, J = 8.5 Hz, 2H), 6.56 (d, J = 8.3 Hz, 2H), 3.44 (s, 1H), 2.82 (s, 3H), 2.26 (s, 3H).
1H NMR spectrum of 4b (in CDCl3, 25 °C, TMS, 400 MHz).
4-Methoxy-N-methylaniline (4c)
1H NMR (400 MHz, CDCl3) δ 6.82–6.74 (m, 2H), 6.63–6.55 (m, 2H), 3.74 (s, 3H), 2.79 (s, 3H), 0.82 (s, 1H).
1H NMR spectrum of 4c (in CDCl3, 25 °C, TMS, 400 MHz).
Chloro-N-methylaniline (4d)
1H NMR (400 MHz, CDCl3) δ 6.11 (dd, J = 4.5, 3.8 Hz, 2H), 5.50 (d, J = 8.1 Hz, 2H), 2.62 (s, 1H), 1.78 (s, 3H).
1H NMR spectrum of 4d (in CDCl3, 25 °C, TMS, 400 MHz).
N-methylpyridin-2-amine (4e)
1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 2.8 Hz, 1H), 7.91 (dd, J = 4.7, 1.1 Hz, 1H), 7.05 (dd, J = 8.3, 4.7 Hz, 1H), 6.82 (ddd, J = 8.3, 2.8, 1.3 Hz, 1H), 3.80 (dd, J = 14.9, 10.1 Hz, 1H), 2.80 (s, 3H).
1H NMR spectrum of 4e (in CDCl3, 25 °C, TMS, 400 MHz).
N-methyl-3,5-bis(trifluoromethyl)aniline (4f)
1H NMR (400 MHz, CDCl3) δ 7.13 (s, 1H), 6.91 (s, 2H), 4.16 (s, 1H), 2.89 (d, J = 5.0 Hz, 3H).
1H NMR spectrum of 4f (in CDCl3, 25 °C, TMS, 400 MHz).
Acknowledgments
Z.N. gratefully acknowledges the Higher Education Commission of Pakistan (HEC) for research funding as an IRSIP fellow at Arizona State University (USA). The authors express their gratitude to TÜBİTAK for financing PhD research (2216 Research Fellowship Program) and BÇ thanks TÜBA for financial support. This study was supported by the Scientific Research Projects Unit of Ondokuz Mayıs University (Project No: PYO.FEN.1906.19.001).
Appendix A. Supplementary data
CCDC 2085163 and 2173756 contain the supplementary crystallographic data for the compounds reported in this article. These data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK.
Funding Statement
Z.N. gratefully acknowledges the Higher Education Commission of Pakistan (HEC) for research funding as an IRSIP fellow at Arizona State University (USA).
References
- 1.Brown BR. The Organic Chemistry of Aliphatic Nitrogen Compounds. Oxford, UK: Oxford University Press; 1994. [Google Scholar]
- 2.Hu S, Tat D, Martinez CA, Yazbeck DR, Tao J. An efficient and practical chemoenzymatic preparation of optically active secondary amines. Organic Letters. 2005;7:4329–4331. doi: 10.1021/ol051392n. [DOI] [PubMed] [Google Scholar]
- 3.Zhu A, Zhan W, Liang Z, Yoon Y, Yang H, et al. Dipyrimidine amines: a novel class of chemokine receptor type 4 antagonists with high specificity. Journal of Medicinal Chemistry. 2010;53(24):8556–8568. doi: 10.1021/jm100786g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Zhou H, Aguilar A, Liu L, Bai L, et al. Structure-based discovery of BM-957 as a potent small-molecule inhibitor of Bcl-2 and Bcl-xL capable of achieving complete tumor regression. Journal of Medicinal Chemistry. 2012;55(19):8502–8514. doi: 10.1021/jm3010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvatore RN, Yoon CH, Jung KW. Synthesis of secondary amines. Tetrahedron. 2001;57(37):7785–7812. doi: 10.1016/S0040-4020(01)00722-0. [DOI] [Google Scholar]
- 6.Boros EE, Thompson JB, Katamreddy SR, Carpenter AJ. Facile reductive amination of aldehydes with electron-deficient anilines by acyloxyborohydrides in TFA: application to a diazaindoline scale-up. The Journal of Organic Chemistry. 2009;74(9):3587–3590. doi: 10.1021/jo900157z. [DOI] [PubMed] [Google Scholar]
- 7.Alonso F, Riente P, Yus M. Nickel nanoparticles in hydrogen transfer reactions. Accounts of Chemical Research. 2011;44(5):379–391. doi: 10.1021/ar1001582. [DOI] [PubMed] [Google Scholar]
- 8.Yang BH, Buchwald SL. Palladium-catalyzed amination of aryl halides and sulfonates. Journal of Organometallic Chemistry. 1999;576(1–2):125–146. doi: 10.1016/S0022-328X(98)01054-7. [DOI] [Google Scholar]
- 9.Beller M, Seayad J, Tillack A, Jiao H. Catalytic Markovnikov and anti-Markovnikov functionalization of alkenes and alkynes: recent developments and trends. Angewandte Chemie International Edition. 2004;43(26):3368–3398. doi: 10.1002/anie.200300616. [DOI] [PubMed] [Google Scholar]
- 10.Hannedouche J, Schulz E. Asymmetric hydroamination: a survey of the most recent developments. Chemistry A European Journal. 2013;19(16):4972–4985. doi: 10.1002/chem.201203956. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed M, Seayad AM, Jackstell R, Beller M. Amines made easily: a highly selective hydroaminomethylation of olefins. Journal of the American Chemical Society. 2003;125(34):10311–10318. doi: 10.1021/ja030143w. [DOI] [PubMed] [Google Scholar]
- 12.Guillena G, Ramon DJ, Yus M. Hydrogen autotransfer in the N-alkylation of amines and related compounds using alcohols and amines as electrophiles. Chemical Reviews. 2010;110(3):1611–1641. doi: 10.1021/cr9002159. [DOI] [PubMed] [Google Scholar]
- 13.Watson AJ, Williams JM. The give and take of alcohol activation. Science. 2010;329(5992):635–636. doi: 10.1126/science.1191843. [DOI] [PubMed] [Google Scholar]
- 14.Nandakumar A, Midya SP, Landge VG, Balaraman E. Transition-metal-catalyzed hydrogen-transfer annulations: access to heterocyclic scaffolds. Angewandte Chemie International Edition. 2015;54(3):11022–11034. doi: 10.1002/anie.201503247. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Su C, Xu Q. N-Alkylation by hydrogen autotransfer reactions. In: Guillena G, Ramón D, editors. Hydrogen Transfer Reactions Topics in Current Chemistry Collections. Springer; Cham: 2016. pp. 291–364. [Google Scholar]
- 16.Grigg R, Mitchell T, Sutthivaiyakit S, Tongpenyai N. Transition metal-catalysed N-alkylation of amines by alcohols. Journal of the Chemical Society, Chemical Communications. 1981:611–612. doi: 10.1039/C39810000611. [DOI] [Google Scholar]
- 17.Watanabe Y, Tsuji Y, Ohsugi Y. The ruthenium catalyzed N-alkylation and N-heterocyclization of aniline using alcohols and aldehydes. Tetrahedron Letters. 1981;22(28):2667–2670. doi: 10.1016/S0040-4039(01)92965-X. [DOI] [Google Scholar]
- 18.Huang M, Li Y, Lan XB, Liu J, Zhao C, et al. Ruthenium (II) complexes with N-heterocyclic carbene–phosphine ligands for the N-alkylation of amines with alcohols. Organic & Biomolecular Chemistry. 2021;19:3451–3461. doi: 10.1039/D1OB00362C. [DOI] [PubMed] [Google Scholar]
- 19.Das K, Nandi PG, Islam K, Srivastava HK, Kumar A. N–alkylation of amines catalyzed by a ruthenium–pincer complex in the presence of in situ generated sodium alkoxide. European Journal of Organic Chemistry. 2019;2019(40):6855–6866. doi: 10.1002/ejoc.201901310. [DOI] [Google Scholar]
- 20.Celaje JJA, Zhang X, Zhang F, Kam L, Herron JR, et al. A base and solvent-free ruthenium-catalyzed alkylation of amines. ACS Catalysis. 2017;7(2):1136–1142. doi: 10.1021/acscatal.6b03088. [DOI] [Google Scholar]
- 21.Imm S, Baehn S, Neubert L, Neumann H, Beller M. An efficient and general synthesis of primary amines by ruthenium-catalyzed amination of secondary alcohols with ammonia. Angewandte Chemie International Edition. 2010;49(44):8126–8129. doi: 10.1002/anie.201002576. [DOI] [PubMed] [Google Scholar]
- 22.Mamidala R, Mukundam V, Dhanunjayarao K, Venkatasubbaiah K. Cyclometalated palladium pre-catalyst for N-alkylation of amines using alcohols and regioselective alkylation of sulfanilamide using aryl alcohols. Tetrahedron. 2017;73(16):2225–223. doi: 10.1016/j.tet.2017.03.001. [DOI] [Google Scholar]
- 23.Dang TT, Ramalingam B, Shan SP, Seayad AM. An efficient palladium-catalyzed N-alkylation of amines using primary and secondary alcohols. ACS Catalysis. 2013;3(11):2536–2540. doi: 10.1021/cs400799n. [DOI] [Google Scholar]
- 24.Martinez-Asencio A, Yus M, Ramon DJ. Palladium (II) acetate as catalyst for the N-alkylation of aromatic amines, sulfonamides, and related nitrogenated compounds with alcohols by a hydrogen autotransfer process. Synthesis. 2011;22:3730–3740. doi: 10.1055/s-0030-1260238. [DOI] [Google Scholar]
- 25.Guérin V, Legault CY. Synthesis of NHC-iridium (III) complexes based on N-iminoimidazolium ylides and their use for the amine alkylation by borrowing hydrogen catalysis. Organometallics. 2021;40(3):408–417. doi: 10.1021/acs.organomet.0c00726. [DOI] [Google Scholar]
- 26.Luo N, Zhong Y, Wen H, Luo R. Cyclometalated iridium complex-catalyzed N-alkylation of amines with alcohols via borrowing hydrogen in aqueous media. ACS Omega. 2020;5(42):27723–27732. doi: 10.1021/acsomega.0c04192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita KI, Furukawa S, Morishima N, Shimizu M, Yamaguchi R. N-alkylation of aqueous ammonia with alcohols leading to primary amines catalyzed by water-soluble N-heterocyclic carbene complexes of iridium. ChemCatChem. 2018;10(9):1993–1997. doi: 10.1002/cctc.201702037. [DOI] [Google Scholar]
- 28.Tsuji Y, Takeuchi R, Ogawa H, Watanabe Y. Platinum complex catalyzed transformation of amine. N-alkylation and N-allylation using primary alcohols. Chemistry Letters. 1986;15(3):293–294. doi: 10.1246/cl.1986.293. [DOI] [Google Scholar]
- 29.Fertig R, Irrgang T, Freitag F, Zander J, Kempe R. Manganese-catalyzed and base-switchable synthesis of amines or imines via borrowing hydrogen or dehydrogenative condensation. ACS Catalysis. 2018;8(9):8525–8530. doi: 10.1021/acscatal.8b02530. [DOI] [Google Scholar]
- 30.Reed-Berendt BG, Polidano K, Morrill LC. Recent advances in homogeneous borrowing hydrogen catalysis using earth-abundant first row transition metals. Organic & Biomolecular Chemistry. 2019;17:1595–1607. doi: 10.1039/C8OB01895B. [DOI] [PubMed] [Google Scholar]
- 31.Balamurugan G, Ramesh R, Malecki JG. Nickel (II)–N˄N˄O pincer type complex-catalyzed N-alkylation of amines with alcohols via the hydrogen autotransfer reaction. The Journal of Organic Chemistry. 2020;85(11):7125–7135. doi: 10.1021/acs.joc.0c00530. [DOI] [PubMed] [Google Scholar]
- 32.Polidano K, Allen BD, Williams JM, Morrill LC. Iron-catalyzed methylation using the borrowing hydrogen approach. ACS Catalysis. 2018;8(7):6440–6445. doi: 10.1021/acscatal.8b02158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llabres-Campaner PJ, Ballesteros-Garrido R, Ballesteros R, Abarca B. β-Amino alcohols from anilines and ethylene glycol through heterogeneous Borrowing Hydrogen reaction. Tetrahedron. 2017;73(37):5552–5561. doi: 10.1016/j.tet.2017.08.006. [DOI] [Google Scholar]
- 34.Montgomery SL, Mangas-Sanchez J, Thompson MP, Aleku GA, Dominguez B, et al. Direct alkylation of amines with primary and secondary alcohols through biocatalytic hydrogen borrowing. Angewandte Chemie International Edition. 2017;129(35):10627–10630. doi: 10.1002/ange.201705848. [DOI] [PubMed] [Google Scholar]
- 35.Thompson MP, Turner NJ. Two-enzyme hydrogen-borrowing amination of alcohols enabled by a cofactor-switched alcohol dehydrogenase. ChemCatChem. 2017;9(20):3833–3836. doi: 10.1002/cctc.201701092. [DOI] [Google Scholar]
- 36.Eka Putra A, Oe Y, Ohta T. Ruthenium-catalyzed enantioselective synthesis of β-amino alcohols from 1, 2-diols by “Borrowing Hydrogen. ” European Journal of Organic Chemistry. 2013;2013; 27:6146–6151. doi: 10.1002/ejoc.201300692. [DOI] [Google Scholar]
- 37.Saidi O, Blacker AJ, Farah MM, Marsden SP, Williams JM. Selective amine cross-coupling using iridium-catalyzed “Borrowing Hydrogen” methodology. Angewandte Chemie International Edition. 2009;121(40):7511–7514. doi: 10.1002/ange.200904028. [DOI] [PubMed] [Google Scholar]
- 38.Kaloğlu M, Gürbüz N, Sémeril D, Özdemir İ. Ruthenium (II)-(p-cymene)-N-heterocyclic carbene complexes for the N-alkylation of amine using the green hydrogen borrowing methodology. European Journal of Organic Chemistry. 2018;10:1236–1243. doi: 10.1002/ejic.201701479. [DOI] [Google Scholar]
- 39.Kaloğlu N, Achard M, Bruneau C, Özdemir İ. Ruthenium (II)-(arene)-N-heterocyclic carbene complexes: efficient and selective catalysts for the N-alkylation of aromatic amines with alcohols. European Journal of Organic Chemistry. 2019;2019(21):2598–2606. doi: 10.1002/ejic.201900191. [DOI] [Google Scholar]
- 40.Şahin N, Özdemir N, Gürbüz N, Özdemir İ. Novel N-alkylbenzimidazole-ruthenium (II) complexes: synthesis and catalytic activity of N-alkylating reaction under solvent-free medium. Applied Organometallic Chemistry. 2019;33(2):e4704. doi: 10.1002/aoc.4704. [DOI] [Google Scholar]
- 41.Çicek M, Gürbüz N, Özdemir N, Özdemir İ, İspir E. Half-sandwich Ru (II) arene complexes bearing benzimidazole ligands for the N-alkylation reaction of aniline with alcohols in a solvent-free medium. New Journal of Chemistry. 2021;45:11075–11085. doi: 10.1039/D1NJ01539G. [DOI] [Google Scholar]
- 42.Podyacheva E, Afanasyev OI, Vasilyev DV, Chusov D. Borrowing hydrogen amination reactions: a complex analysis of trends and correlations of the various reaction parameters. ACS Catalysis. 2022;12(12):7142–7198. doi: 10.1021/acscatal.2c01133. [DOI] [Google Scholar]
- 43.Banerjee D, Kabadwal LM, Bera S. Recent advances in sustainable organic transformations using methanol: expanding the scope of hydrogen borrowing catalysis. Organic Chemistry Frontiers. 2021;8:7077–7096. doi: 10.1039/D1QO01412A. [DOI] [Google Scholar]
- 44.Moutaoukil Z, Serrano-Díez E, Collado IG, Jimenez-Tenorio M, Botubol-Ares JM. N-Alkylation of organonitrogen compounds catalyzed by methylene-linked bis-NHC; half-sandwich ruthenium complexes. Organic & Biomolecular Chemistry. 2022;20:831–839. doi: 10.1039/D1OB02214H. [DOI] [PubMed] [Google Scholar]
- 45.Arduengo AJ, III, Dias HR, Harlow RL, Kline M. Electronic stabilization of nucleophilic carbenes. Journal of the American Chemical Society. 1992;114(14):5530–5534. doi: 10.1021/ja00040a007. [DOI] [Google Scholar]
- 46.Sanford MS, Love JA, Grubbs RH. A versatile precursor for the synthesis of new ruthenium olefin metathesis catalysts. Organometallics. 2021;20(25):5314–5318. doi: 10.1021/om010599r. [DOI] [Google Scholar]
- 47.Herrmann WA, Köcher C. N-Heterocyclic carbenes. Angewandte Chemie International Edition. 1997;36(20):2162–2187. doi: 10.1002/anie.199721621. [DOI] [Google Scholar]
- 48.Meeniga I, Gokanapalli A, Peddiahgari VGR. Synthesis of environmentally benign new ionic liquids for the preparation of 2-aryl/heteroaryl benzimidazoles/benzoxazoles under ultrasonication. Sustainable Chemistry and Pharmacy. 2022;30:100874–100879. doi: 10.1016/j.scp.2022.100874. [DOI] [Google Scholar]
- 49.Wang WQ, Wang ZQ, Sang W, Zhang R, Cheng H, et al. Dehydrogenative amide synthesis from alcohols and amines utilizing N-heterocyclic carbene-based ruthenium complexes as efficient catalysts: the influence of catalyst loadings, ancillary and added ligands. Polyhedron. 2021;195:114979–114981. doi: 10.1016/j.poly.2020.114979. [DOI] [Google Scholar]
- 50.Huang M, Li Y, Lan XB, Liu J, Zhao C, et al. Ruthenium (II) complexes with N-heterocyclic carbene–phosphine ligands for the N-alkylation of amines with alcohols. Organic & Biomolecular Chemistry. 2021;19:3451–3461. doi: 10.1039/D1OB00362C. [DOI] [PubMed] [Google Scholar]
- 51.Shan SP, Xiaoke X, Gnanaprakasam B, Dang TT, Ramalingam B, et al. Benzimidazolin-2-ylidene N-heterocyclic carbene complexes of ruthenium as a simple catalyst for the N-alkylation of amines using alcohols and diols. RSC Advances. 2015;5:4434–4442. doi: 10.1039/C4RA15398G. [DOI] [Google Scholar]
- 52.Şahin Z, Gürbüz N, Özdemir İ, Şahin O, Büyükgüngör O, et al. N-Alkylation and N, C-dialkylation of amines with alcohols in the presence of ruthenium catalysts with chelating N-heterocyclic carbene ligands. Organometallics. 2015;34(11):2296–2304. doi: 10.1021/om501066n. [DOI] [Google Scholar]
- 53.Nawaz Z, Ullah H, Gürbüz N, Zafar MN, Verpoort F, et al. Benzimidazole-based N-heterocyclic carbene silver complexes as catalysts for the formation of carbonates from carbon dioxide and epoxides. Molecular Catalysis. 2022;526(60):112369–112380. doi: 10.1016/j.mcat.2022.112369. [DOI] [Google Scholar]
- 54.Burla MC, Caliandro R, Carrozzini B, Cascarano GL, Cuocci C, et al. Crystal structure determination and refinement via SIR2014. Journal of Applied Crystallography. 2015;48:306–309. doi: 10.1107/S1600576715001132. [DOI] [Google Scholar]
- 55.Sheldrick GM. A short history of SHELX. Acta Crystallographica Section A. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 56.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JA, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. Journal of Applied Crystallography. 2009;42:339–341. doi: 10.1107/S0021889808042726. [DOI] [Google Scholar]
- 57.Nawaz Z, Gürbüz N, Zafar MN, Tahir MN, Ashfaq M, et al. Direct arylation (hetero-coupling) of heteroarenes via unsymmetrical palladium-PEPPSI-NHC type complexes. Polyhedron. 2021;208:115412–115410. doi: 10.1016/j.poly.2021.115412. [DOI] [Google Scholar]
- 58.Hackenberg F, Müller-Bunz H, Smith R, Streciwilk W, Zhu X, et al. Novel ruthenium (II) and gold (I) NHC complexes: synthesis, characterization, and evaluation of their anticancer properties. Organometallics. 2013;32(19):5551–5560. doi: 10.1021/om400819p. [DOI] [Google Scholar]
- 59.Tay BY, Wang C, Phua PH, Stubbs LP, Huynh HV. Selective hydrogenation of levulinic acid to γ-valerolactone using in situ generated ruthenium nanoparticles derived from Ru–NHC complexes. Dalton Transactions. 2016;45:3558–3563. doi: 10.1039/C5DT03366G. [DOI] [PubMed] [Google Scholar]
- 60.Lam NY, Truong D, Burmeister H, Babak MV, Holtkamp HU, et al. From catalysis to cancer: toward structure–activity relationships for benzimidazol-2-ylidene-derived N-heterocyclic-carbene complexes as anticancer agents. Inorganic Chemistry. 2018;57(22):14427–14434. doi: 10.1021/acs.inorgchem.8b02634. [DOI] [PubMed] [Google Scholar]
- 61.Wang WQ, Yuan Y, Miao Y, Yu BY, Wang HJ, et al. Well-defined N-heterocyclic carbene/ruthenium complexes for the alcohol amidation with amines: the dual role of cesium carbonate and improved activities applying an added ligand. Applied Organometallic Chemistry. 2020;34(2):e5323–5333. doi: 10.1002/aoc.5323. [DOI] [Google Scholar]
- 62.Sarı Y, Gürses C, Celepci DB, Keleştemur Ü, Aktaş A, et al. 4-Vinylbenzyl and 2-morpholinoethyl substituted ruthenium (II) complexes: design, synthesis, and biological evaluation. Journal of Molecular Structure. 2020;1202:127355–127363. doi: 10.1016/j.molstruc.2019.127355. [DOI] [Google Scholar]
- 63.Kathuria L, Din Reshi NU, Samuelson AG. N-Heterocyclic carbene (NHC)-stabilized Ru0 nanoparticles: in situ generation of an efficient transfer hydrogenation catalyst. Chemistry A European Journal. 2020;26(34):7622–7630. doi: 10.1002/chem.202000142. [DOI] [PubMed] [Google Scholar]
- 64.Dang TT, Ramalingam B, Seayad AM. Efficient ruthenium-catalyzed N-methylation of amines using methanol. ACS Catalysis. 2015;5(7):4082–4088. doi: 10.1021/acscatal.5b00606. [DOI] [Google Scholar]
- 65.Liu Z, Yang Z, Yu X, Zhang H, Yu B, et al. Efficient cobalt-catalyzed methylation of amines using methanol. Advanced Synthesis & Catalysis. 2017;359(24):4278–4283. doi: 10.1002/adsc.201701044. [DOI] [Google Scholar]
- 66.Lin WH, Chang HF. A study of ethanol dehydrogenation reaction in a palladium membrane reactor. Catalysis Today. 2004;97(2–3):181–188. doi: 10.1016/j.cattod.2004.03.068. [DOI] [Google Scholar]
- 67.Illam PM, Rit A. Electronically tuneable orthometalated RuII–NHC complexes as efficient catalysts for C–C and C–N bond formations via borrowing hydrogen strategy. Catalysis Science & Technology. 2022;12:67–74. doi: 10.1039/D1CY01767E. [DOI] [Google Scholar]
- 68.Donthireddy S, Mathoor Illam P, Rit A. Ruthenium (II) complexes of heteroditopic N-heterocyclic carbene ligands: efficient catalysts for C–N bond formation via a hydrogen-borrowing strategy under solvent-free conditions. Inorganic Chemistry. 2020;59(3):1835–1847. doi: 10.1021/acs.inorgchem.9b03049. [DOI] [PubMed] [Google Scholar]
- 69.Biswas N, Srimani D. Ru-catalyzed selective catalytic methylation and methylenation reaction employing methanol as the C1 source. The Journal of Organic Chemistry. 2021;86(15):10544–10554. doi: 10.1021/acs.joc.1c01185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H NMR spectrum of ruthenium–BNHC complex 1a (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1a (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1a.
1H NMR spectrum of ruthenium–BNHC complex 1b (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1b (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1b.
1H NMR spectrum of ruthenium–BNHC complex 1c (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1c (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1c.
1H NMR spectrum of ruthenium–BNHC complex 1d (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1d (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1d.
1H NMR spectrum of ruthenium–BNHC complex 1e (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1e (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1e.
1H NMR spectrum of ruthenium–BNHC complex 1f (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1f (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1f.
1H NMR spectrum of ruthenium–BNHC complex 1g (in CDCl3, 25 °C, TMS, 400 MHz).
13C NMR spectrum of ruthenium–BNHC complex 1g (in CDCl3, 25 °C, TMS, 101 MHz).
FT-IR spectrum of ruthenium–BNHC complex 1g.
1H NMR and 13C NMR spectrum of 2a (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 2b (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 2c (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 2d (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 2e (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 2f (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 2g (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 2h (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3a (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3b (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3c (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3d (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3e (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3f (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3g (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 3h (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR and 13C NMR spectrum of 4a (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR spectrum of 4b (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR spectrum of 4c (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR spectrum of 4d (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR spectrum of 4e (in CDCl3, 25 °C, TMS, 400 MHz).
1H NMR spectrum of 4f (in CDCl3, 25 °C, TMS, 400 MHz).