Abstract
Following a period of slow progress, the completion of genome sequencing and the paradigm shift relative to the cell of origin for high grade serous ovarian cancer (HGSOC) led to a new perspective on the biology and therapeutic solutions for this deadly cancer. Experimental models were revisited to address old questions, and improved tools were generated. Additional pathways emerging as drivers of ovarian tumorigenesis and key dependencies for therapeutic targeting, in particular, VEGF-driven angiogenesis and homologous recombination deficiency, were discovered. Molecular profiling of histological subtypes of ovarian cancer defined distinct genetic events for each entity, enabling the first attempts toward personalized treatment. Armed with this knowledge, HGSOC treatment was revised to include new agents. Among them, PARP inhibitors (PARPis) were shown to induce unprecedented improvement in clinical benefit for selected subsets of patients. Research on mechanisms of resistance to PARPis is beginning to discover vulnerabilities and point to new treatment possibilities. This Review highlights these advances, the remaining challenges, and unsolved problems in the field.
Epidemiology, risk factors, and histological subtypes
Over the past 10 years, we have made progress in understanding the biology of and improving the therapeutic options for ovarian cancer (OC), a disease that has been described by many as a “silent killer” and recognized as the deadliest of gynecologic cancers (1, 2). It is estimated that more than 150,000 OC-related deaths occur annually worldwide (3), while 19,710 new diagnoses and 13,270 deaths are projected in the United States in 2023. For all stages of OC combined, 5-year survival rates range from 36% in non-Hispanic Black women to 47%–48% in non-Hispanic White, Asian-Pacific Islander, and Hispanic women, highlighting existing disparities in outcomes (4).
Several nongenetic risk factors have been associated with OC, the most prominent being age, with half of women with OC being older than 63 years. Menopause, having a late full-term pregnancy or never being pregnant, and lifetime ovulatory years (5) are additional risk factors, while oral contraceptives, multiple pregnancies, and breast feeding have been linked to lower risk, presumably due inhibition of ovulation (6, 7). Less than 20% of all OCs are due to hereditary cancer syndromes caused by mutations in genes involved in DNA damage response, especially BRCA1, BRCA2, TP53, BRIP1, RAD51C, RAD51D, and others (4); therefore, germline assessment of mutation status for key genes is recommended for all new cases.
Histologically, ovarian tumors are categorized as epithelial, germ cell, and sex-cord stromal. Epithelial tumors are commonly referred to as OC, and the major histological types are low-grade serous, clear cell, endometrioid, mucinous, and transitional cell (type I) or high-grade serous, mixed epithelial/stromal (carcinosarcoma), and undifferentiated (type II). Unique genetic events characterize each subtype (Figure 1) (8–10). High-grade serous ovarian cancer (HGSOC) accounts for 70% of all OC cases and nearly 80% of deaths.
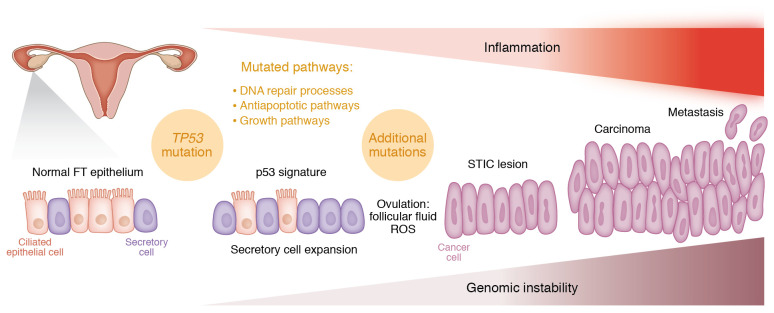
Figure 1. Model of HGSOC initiation from the epithelium of the fallopian tube.
Following initiating TP53 mutation, fallopian tube secretory epithelial cells proliferate and form secretory cell expansion with a TP53 signature. Follicular fluid released during ovulation contains ROS, which induces inflammation and can cause additional mutations and increased genetic instability. Mutated pathways include DNA repair processes, antiapoptotic pathways, and growth pathways. The secretory cells become irregular in size and shape, and the tissue becomes disordered as serous tubal intraepithelial carcinoma lesions develop. Finally, transformed cells begin to dissociate from the precursor lesion leading to metastasis. FT, fallopian tube; STIC, serous tubal intraepithelial carcinoma; SS-DNA, single-stranded DNA; DS-DNA, double-stranded DNA; MAPK, mitogen-activated protein kinase.
Origins of HGSOC
Until the late nineties, OC was thought to originate from the ovarian surface epithelium (OSE), which undergoes repeated cycles of rupture and repair with ovulation. The theory of “incessant ovulation” (11) postulated that OC originates from inclusion cysts, which form as a consequence of repeated rupture of OSE, which allows egg extrusion. Epidemiological studies associating decreased risk of OC with fewer lifetime ovulatory cycles (e.g., due to multiparity, breast feeding, or use of oral contraceptives) (6, 7, 12) supported this concept. The discovery of serous tubal intraepithelial carcinomas (STIC) arising in the distal fimbriae of the fallopian tube and bearing a TP53-mutated signature in women carrying BRCA mutations (13, 14) caused a paradigm shift, supporting the origin of HGSOC from fallopian tube epithelium (FTE), rather than OSE. Since then, STIC lesions have been recognized as potential HGSOC-precursor lesions that share tumor-specific genetic alterations, such as mutations in BRCA1, BRCA2, TP53, and PTEN (15, 16). The concept that HGSOC originates in the secretory cells of the FTE is now at the forefront of the field. Somatic mutation of TP53 is thought to be the first mutagenic event in the fimbria, is identified in more than 95% of cases of HGSOC, and is shared with STIC lesions. Foci of histologically normal epithelial cells bearing TP53 mutations are detected in about one-third of healthy tubes of women with BRCA mutations, and they are associated with γ-H2AX foci (17), indicating DNA breaks and implicating genomic instability in the early stages of tumor initiation. Emerging studies support the role of oxidative stress in causing genomic instability and promoting tumor initiation in FTE (18). Carcinogenic alterations, such as DNA breaks and TP53 accumulation, and inflammatory changes have been detected ex vivo in cultured FTE cells exposed to follicular fluid or to other oxidants (19, 20). Loss of ciliated cells, which is common with increasing age, is also a risk factor for HGSOC (21), supporting a protective role of these cells in the fimbriae (Figure 2). A recent study using single-cell sequencing of epithelial cells dissociated from the fallopian tube fimbriae identified several expression signatures among the secretory FTE cells, which were recapitulated among the subtypes of HGSOC tumors profiled by The Cancer Genome Atlas (TCGA) (22). Other studies using CRISPR genetic engineering of cancer-related genes such as TP53, BRCA1, NF1, and PTEN support the premise that engineered FTE and OSE cells can acquire tumorigenic features, suggesting that HGSOC arises from either the ovary or the fallopian tube (23, 24). However, it should be noted that genetically engineered oviductal cells proliferate and form tumors more aggressively than OSE cells (24). Combined, these data supporting the origin of HGSOC in the fallopian tube fimbriae have led to investigating removal of the tubes (salpingectomy) instead of removal of ovaries (oophorectomy) as preventive surgery for women with high-risk of HGSOC (25). For example, the ongoing SOROCK trial (NCT04251052) compares outcomes of bilateral salpingectomy with delayed oophorectomy to bilateral salpingo-oophorectomy to reduce the risk of OC in women with germline BRCA mutations.
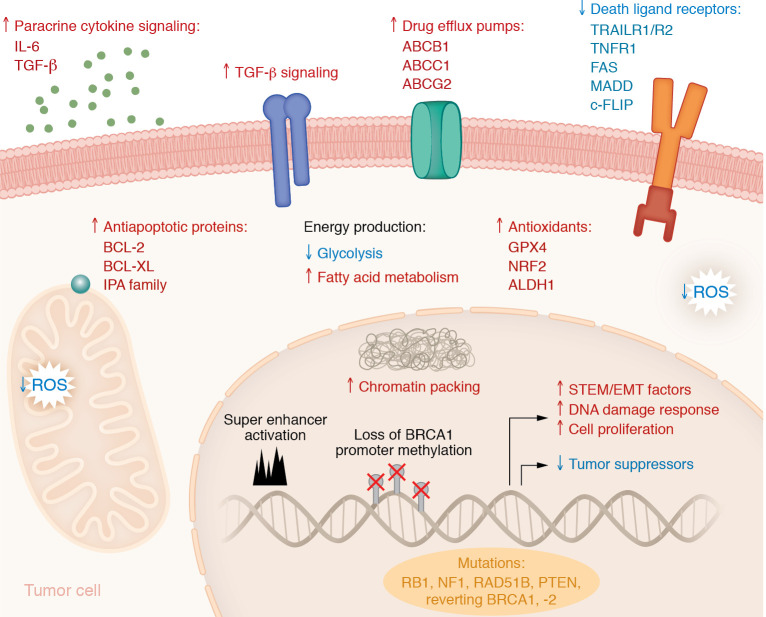
Figure 2. Key mechanisms implicated in emergence of platinum resistance.
OC cells develop chemoresistance due to diverse mechanisms, including paracrine release of cytokines from stromal elements in the TME, upregulation of cell membrane ABC transporters to enhance drug efflux, increased cellular antioxidant defense to reduce ROS, promotion of antiapoptotic signaling through increased expression of antiapoptotic proteins and decreased expression of death ligand receptors, metabolic reprogramming, an increase in chromatin packing, genetic and epigenetic inactivation of tumor suppressor and DNA repair genes, modulation of superenhancers that induce transcriptional reprogramming, and acquisition of mutations, including reverting BRCA 1 and 2 mutations. ABCB1, also known as P-glycoprotein (PgP) and multidrug resistance protein 1 (MDR1); ABCC1, multidrug resistance-associated protein 1 (also known as MRP1); ABCG2, breast cancer resistance protein (also known as BCRP); TRAILR1, TNF-related apoptosis-inducing ligand receptor 1; TRAILR2, TNF-related apoptosis-inducing ligand receptor 2; FAS, Fas cell surface death receptor; MADD, MAPK-activating death domain; c-FLIP, cellular FLICE-like inhibitory protein; GPX4, glutathione peroxidase 4; NRF2, nuclear factor erythroid-2 related factor; ALDH1, aldehyde dehydrogenase 1; BRCA1, breast cancer gene 1; EMT, epithelial-mesenchymal transition; RB1, retinoblastoma 1; NF1, neurofibromatosis 1; RAD51B, RAD51 paralog B.
Genetic and nongenetic drivers
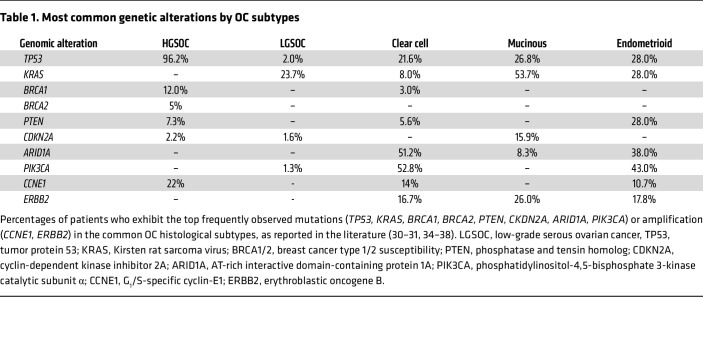
OC is driven by gain of function, copy number changes, and loss-of-function mutations in critical tumor suppressor genes (TSGs). Specifically in HGSOC, the hallmark genetic alterations are mutations in TP53 tumor suppressor and DNA repair genes (26). TP53 is lost in nearly half of human cancers due to loss-of-function mutations. In HGSOC, point mutations are scattered across the gene, indicating mostly loss-of-function alterations. However, hot spot mutations associated with gain of function occur in TP53’s DNA binding domain, such as R175 (~9% of HGSOC), R248 (~6%), and R273 (~6%) (27) (https://www.cbioportal.org/). Interestingly the type of TP53 mutation and TP53 protein levels were shown to have significant effect on drug response in other gynecologic cancers (e.g., endometrial cancer) (28). In addition to TP53 mutations, recurrent loss-of-function mutations are also observed in NF1 (20%), RB1 (17%), and PTEN (29). In addition to loss of TSGs, other hallmark genetic alterations with important therapeutic implications in HGSOC reflect mutations in key DNA repair genes. Among those, germline and somatic BRCA1/2 mutations occur in roughly 17% and 3% of HGSOCs, respectively (30), while several Fanconi anemia genes (FANCA, FANCI, FANCL, and FANCC) and DNA damage response genes involved in homology-directed DNA repair (PALB2, ATM, ATR, CHEK1, and CHEK2) are recurrently mutated in HGSOC (30). Therefore, over 50% of all HGSOC are homologous recombination deficient (HRD), and this biology has critical therapeutic implications, as discussed below. HGSOC is also driven by genomic copy number changes that lead to a gain of function in several critical genes (26, 29). Among these, the most notable are gene level copy number gain in cyclin E1 (CCNE1) (31) and MYC genes (32, 33). Notably, amplification of CCNE1, a critical regulator of the G1/S transition, is directly associated with poor response to chemotherapy (34) and poor overall patient survival (31, 34). Distinct mutations associate with HGSOC (35) and differentiate this subtype from the other OC subtypes (36–38) (Table 1).
Table 1. Most common genetic alterations by OC subtypes.
In addition to genetic alterations, OC pathogenesis is governed by a network of transcription factors (TFs). Growing evidence suggests various TFs as critical regulators of gene expression programs in OC subtypes, overall tumorigenesis, and therapy response (39–41). For example, Li et al. identified that expression levels of 17 TFs associate with overall survival (OS) in HGSOC (41). While some TFs are shared across OC subtypes, a set of TFs are expressed in a subtype-specific manner (40). For example, while several HOX family members, hormonal nuclear receptors, and MYC are commonly expressed in several subtypes of OC, aberrant activity of BRCA1/2, FOXM1, and MECOM is relatively more restricted to HGSOC (40). Through integrative analysis of cancer-type-specific gene expression and chromatin state programs, Reddy et al. nominated several master TFs for various types of cancers (39). The analyses identified known regulators of OC, including SOX17 and PAX8 (39), both of which interact and promote angiogenesis in OC (42). TFs also govern chemoresistance, either directly, as in the case of BRCA1/2, or indirectly by reprogramming the transcriptional state that enables cells to tolerate chemotherapy (43). Because cancer cells tend to become dependent on a specific set of TFs that control the dysregulated transcriptional programs (44), targeting these transcriptional dependencies has critical therapeutic implications. Fortunately, advances in medicinal chemistry and targeted-protein depletion strategies such as proteolysis-targeting chimera (45) tools render TFs potentially targetable.
Experimental models
The completion of the genomic characterization of HGSOC through TCGA and new understanding of the cell of origin have caused a paradigm shift in the use of representative experimental models, with increased focus on platforms that recapitulate the molecular features of HGSOC.
Cell line models
Human cell lines.
Cell lines derived from human OC tumors and ascites are useful models to study the disease. They are easy to culture, maintain, and manipulate, and they have been established from different subtypes of OC (see Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI174013DS1). A study that investigated 47 presumed OC cell lines from the Cancer Cell Line Encyclopedia defined the lines that harbor the closest genetic similarity to HGSOC by comparing copy number changes, mutations, and mRNA expression profiles with tumors profiled in TCGA (46, 47). TP53 was found mutated in 62% of cell lines, while BRCA1 and BRCA2 were mutated in 6% and 9% of cell lines, respectively. Two conventionally used cell lines (SKOV3 and A2780) were molecularly dissimilar from HGSOC (47), limiting their current use. TP53 mutations occur in representative cell lines (KURAMOCHI, OVSAHO, SNU119, COV362, OVCAR4, COV318, JHOS4, TYKNU. OVKATE, CAOV4, OAW28, JHOS2, CAOV3, 59M, ONCODG1, FUOV1, NIH-OVCAR3) (47). BRCA1/2 mutations are detected in KURAMOCHI, COV362, JHOS2, and PEO1 cells lines, while CCNE1 amplification is present in COV318, ONCODG1, FUOV1, and NIH-OVCAR3 cell lines (47).
Murine cell lines.
The ID8 cell line was derived from C57BL/6 mouse ovarian surface epithelial cells (MOSECs) transformed by serial passage in vitro (48). These transformed MOSECs form metastatic tumors in the peritoneal cavity of immunocompetent mice (48) and have been used to study antitumor immunity (48). As the ID8 model does not harbor the common Trp53 mutations, CRISPR/Cas9 gene editing generated cells carrying deletions of Trp53, Brca1, Brca2, and other HGSOC-associated mutations. Various derivative ID8 cells with such alterations were established, including ID8 Trp53−/− (49), ID8 Trp53−/−Brca1−/−, ID8 Trp53–/–BRCA2–/–, ID8 Trp53−/−Pten −/−, and ID8 Trp53−/−Nf1−/− (50, 51). More recently, a panel of murine FTE cells bearing characteristic mutations and able to form tumors with HGSOC histopathology were described previously (52). These cells phenocopy HRD models though combined loss of Trp53, Brca1, Pten, and Nf1 and overexpression of Myc and Trp53R172H and phenocopy homologous recombination proficient (HR-proficient) models through loss of Trp53 and overexpression of Ccne1, Akt2, Trp53 R172H, and Kras G12V or Brd4 or Smarca4 (Supplemental Table 2).
Animal models
Genetically engineered mouse models.
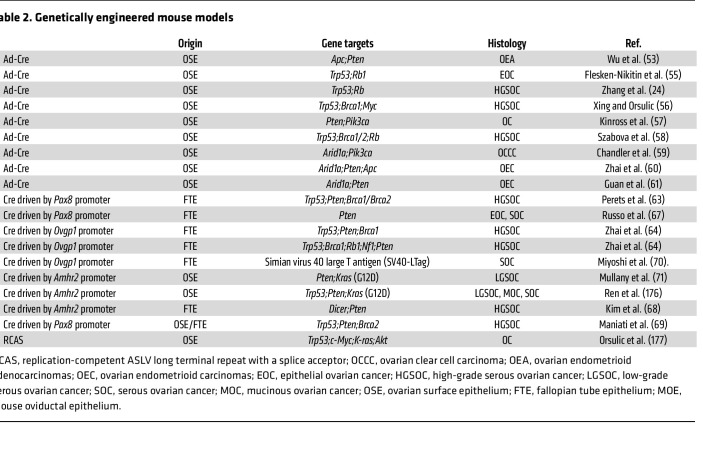
Given the controversies surrounding OC’s cell of origin, genetically engineered mouse models (GEMMs) have been generated by using mutations of driver genes in either FTE or OSE (Table 2). The Cre/loxP system allows tissue-specific gene knockin or knockout either through intrabursal injection of the adenovirus-encoding Cre-recombinase (Ad-Cre), which knocks out LoxP site–flanked alleles in situ (53), or via selective expression of Cre-recombinase using tissue-specific gene promoters (54). Table 2 summarizes key models. Among them, GEMMs resembling ovarian serous carcinomas developed by using recombinant Ad-Cre in MOSECs include Apc–/–Pten–/– (53), Trp53–/–Rb–/– (24, 55), Trp53–/–Brca1–/–Myc–/– (56), Pten–/–Pik3ca(H1047R) (57), and Trp53–/–Brca1–/–Rb–/– (58). GEMMs for other OC subtypes developed using Ad-Cre include ovarian clear cell carcinoma Arid1a–/–Pik3ca(H1047R) (59), ovarian endometrioid cancer Arid1a–/–Pten–/–Apc–/– (60), and Arid1a–/–Pten –/– (61).
Table 2. Genetically engineered mouse models.
Selective expression of Cre-recombinase in the Müllerian duct epithelium can be driven by tissue-specific promoters, such as Amhr2 (62), Pax8 (63), and Ovgp1 (64). Amhr2 is expressed in OSE and the stromal cells of the ovary, oviduct, and other portions of the female genital tract (62); Pax8 is expressed in FTE but also in the endometrial epithelium (65); and Ovgp1 is only expressed in FTE/mouse oviductal epithelium (64). Although Trp53 mutations are a hallmark of HGSOC, Trp53 mutations alone rarely drive OC tumorigenesis (66). However, Pten loss in FTE induces serous tumorigenesis (63), and Pax8-driven Pten loss was sufficient to generate endometrioid and serous borderline tumors (67). Other GEMMs developed using this strategy include Amhr2 promoter–driven, Cre-mediated Dicer1 and Pten double knockout (68); Pax8-driven loss of Brca1, Pten, and Trp53 together or of Brca2, Pten, and Trp53 together (63); and Pax8-Cre–driven Trp53–/–Pten–/–Brca2–/– or Trp53–/–Pten–/–Brca2+/- (69). Oviductal serous tumors develop in mice engineered to express the simian virus 40 large T antigen under the control of Ovgp1 (70). Cho and colleagues developed tamoxifen-regulated Ovgp1-iCreERT2 Trp53–/–, Pten–/–, and Brca1–/– mice, which resulted in tumors resembling HGSOC (64). Amhr2-Cre–driven Pten–/–Kras(G12D) mice expressing the oncogenic mutant form of Kras in OSE cells developed low-grade ovarian serous adenocarcinomas (71). Recently CRISPR/Cas9-mediated gene editing was used to develop a GEMM by intrabursal injection of lentivirus targeting Trp53, Brca1, Pten, Nf1, and overexpression of Myc and the gain-of-function variant Trp53(R172H) (52).
Syngeneic models.
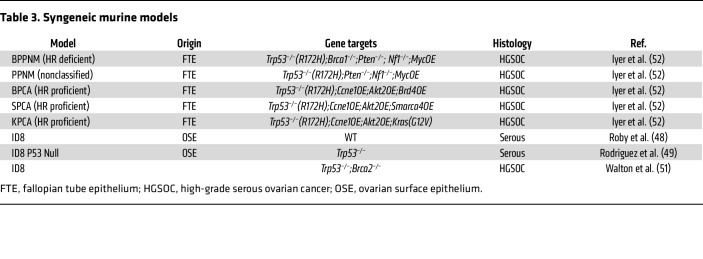
Syngeneic models are useful to study the tumor microenvironment (TME) and antitumor immunity. The common models use murine cell lines (Table 3). Tumors develop i.p. and are associated with ascites, reproducing the clinical features of the disease.
Table 3. Syngeneic murine models.
Xenograft models.
Xenografts employ immunodeficient mice as hosts. Cancer cells implanted subcutaneously, i.p., or orthotopically (intrabursally) yield variable tumorigenicity (72). Intrabursal implantation gives rise to tumors in the ovary and metastatic spread in the peritoneum, reproducing disease development. Because xenograft models grow relatively fast and display little variation, they are commonly used to evaluate effects of gene manipulation or various therapeutic agents. Patient-derived xenografts developed by implanting OC fragments under the renal capsule or i.p. reproduce the original tumor histology and molecular alterations but grow slower, display higher variability, and are subject to drift during serial propagation (73).
Metastasis and TME
HGSOC is characterized by a unique pattern of invasion-metastasis. Dislodged from the primary sites in the fallopian tube or ovary where tumors initiate, OC cells float in the peritoneal fluid, attach to the mesothelial layer, and invade into the submesothelial matrix to establish secondary lesions. The most common sites of metastasis occur along the peritoneal cavity, in the fat-rich omentum, and on the surface of bowel or other abdominal organs. Metastasis to distant sites such as lung, skin, bone, brain, and intraabdominal organs is rare and occurs via hematogenous dissemination (74).
The interactions between cancer cells and other components of the peritoneal environment govern this pattern of metastasis. The preference of OC cells to metastasize to the omentum is attributed to energy requirements, which are facilitated by the symbiotic relationship between cancer cells and adipocytes. This direct interaction allows transfer of fatty acids which are used for β-oxidation in OC cells (75). As peritoneal implants develop, OC cells are in direct contact with mesothelial cells. The OC cell–mesothelial cell interaction activates the anti-Müllerian hormone axis, which activates immunosuppressive signals and enables tumor growth (76). HGSOC cells secrete cytokines, microRNAs, and other growth factors that activate fibroblasts in the peritoneal milieu (77). In turn, cancer-associated fibroblasts secrete matrix proteins that sustain tumor cell proliferation and chemokines (IL-6, CLC5, CXCL14, CXCL12) that promote epithelial-mesenchymal transition and OC cell dissemination.
To reproduce events associated with peritoneal metastasis, 3D organotypic models have been developed by using omentum-derived primary human mesothelial cells, fibroblasts, and patient-derived extracellular matrix (78). This model recapitulates the human peritoneal microenvironment and reproduces the molecular mechanisms of metastasis. The model can be expanded to include other cell types from the TME, such as adipocytes, immune cells, or macrophages (79, 80). A four-cell culture model, including cancer cells, mesothelial cells, omental fibroblasts, and adipocytes was developed to study cell interactions during metastasis (81). The unique pattern of HGSOC dissemination reinforces the rationale for i.p. administration of chemotherapy, which has improved clinical outcomes.
Ascites accumulation in the peritoneal cavity is a common symptom of the disease. This fluid contains nonadherent tumor cells or multicellular aggregates; a wide range of nontumor cells, including fibroblasts, adipocytes, mesothelial, endothelial, and inflammatory cells; and acellular components, such as cell-free DNA, cytokines, and chemokines (82). Single-cell transcriptional profiling shows substantial variability in the composition and functions of ascites cells (83). An important driver of ascites accumulation is VEGF (84, 85), and therapies that target it such as bevacizumab effectively inhibit ascites formation and have advanced to clinical practice.
Immune milieu
HGSOC is one interesting case where most evidence suggests that immune modulators would benefit patients, yet immune-targeting strategies have yielded modest results. Importantly, the majority (~55%) of OC tumors are “immune hot,” meaning that they contain high numbers of tumor-infiltrating T cells (TILs) (86). The 5-year survival rate of patients with TIL-containing tumors is higher compared with those with low number of TILs (74% vs. ~12%) (86). Similarly, a high ratio of cytotoxic CD8+ TILs to immune-suppressive Tregs is a favorable marker for patients with OC (87). Recent reports showed that expression of the programmed death (PD) ligand PD-L1 on immune cells in the tumor milieu is associated with increased total numbers of TILs and better survival in HGSOC (88, 89). Additionally, it was reported that OC immunogenicity is regulated by a small subset of CD8+ TILs that are primed against high-affinity antigens, representing progenitors of tissue-resident memory cells (90). Humoral tumor B cell–produced IgA responses that sensitize tumor cells to killing by T cells were also described previously (91), and tertiary lymphoid structures, detected in HGSOC tumors, predicted response to immune checkpoint inhibitors (ICIs) (92). These clinical findings strongly indicate the value of the antitumor immune response. However, by and large, testing of immune strategies in OC has been disappointing, with modest 5%–15% response rates to single agents (93) and lack of synergy with combination treatments (94).
Cancer cells develop complex mechanisms to evade immune surveillance. To this end, induction of PD-L1 on cancer cells in response to proinflammatory cytokines such as IFN-γ and TNF-α released from T and NK cells is a crucial immune escape mechanism (95–97). The engagement of PD-L1 on antigen-presenting cells and cancer cells with PD-1 receptor on T cells dampen T cell cytotoxicity, leading to exhaustion (98, 99). Importantly, in addition to its immune inhibitory functions, PD-L1 may also contribute to radio- and chemoresistance (95, 100). Although the mechanism of PD-L1–mediated chemoresistance is poorly understood, recent studies highlight a previously unappreciated role of PD-L1 in DNA repair. Recent findings implicate intracellular PD-L1 as a stabilizer of mRNAs from DNA damage–related genes (101), indicating that it could aid the repair of damaged DNA and, hence, contribute to chemoresistance. Biologics that target PD-L1 and PD-1 interactions have elicited impressive antitumor responses and clinical benefits in many cancer types (100) but not in OC.
It has been postulated that potent immunosuppressive signals dominate the HGSOC TME. Key inducers of the “cold” milieu remain controversial. It was reported that T cell function and IFN-γ secretion are blunted in the OC peritoneal milieu; one of the driving mechanisms involved exposure to malignant ascites, which activates the transcription factor XBP1 and induces the ER-stress response (102). Additionally, XBP1 is activated in dendritic cells, causing functional inhibition (103). Lysophosphatidic acid secreted in OC-associated ascites directly affects dendritic cell function (104), contributing to impaired immune responses. Other T cell inhibitory signals come from myeloid cells, including myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages, which are detected in tumor tissue, ascites, and peripheral blood of women with OC (105). Ascites fluid stimulates expansion of monocytic-MDSCs through a mechanism dependent on IL-6, IL-10, and STAT3 (106), contributing to immunosuppression. In preclinical models, epigenetic modulators, such as combination of histone deacetylase and DNA methyltransferase inhibitors, enhanced response to anti-PD1 antibodies by depleting MDSCs (107). However, epigenetic priming did not induce robust clinical responses to ICIs in early clinical trials (92). Other strategies targeting MDSCs and tumor-associated macrophages are under clinical investigation.
Advances in therapy
Standard treatment.
Women with OC are typically approached with tumor cytoreductive surgery, involving removal of gynecologic organs (total abdominal hysterectomy, bilateral salpingo-oophorectomy), lymph nodes, and omentum followed by platinum (Pt)/taxane-based chemotherapy. Cytoreductive surgery that removes most of the tumor mass, leaving behind microscopic or less than 1 mm tumor implants, is known as “optimal debulking” and affects survival (108, 109). Following surgery, the standard regimen of carboplatin and paclitaxel (CP) has withstood the passage of time (110) with minimal modifications. The clinical effect of Pt on disease control is explained by the DNA repair defects and HRD features of HGSOC (46). Substitution of cisplatin with less toxic carboplatin (111) and other modifications of the regimen have been tested. Dose-dense administration of paclitaxel (weekly vs. every three weeks) demonstrated benefit in some but not all populations and was associated with greater hematological toxicity (112, 113). Rooted in the biology of the disease and taking advantage of its abdominal distribution, i.p. administration of chemotherapy (114) and heated i.p. chemotherapy (115) deliver higher doses of chemotherapy in the peritoneal space, where tumors reside. The i.p. treatment induces improved responses and increased survival compared with intravenous drug delivery. Several ongoing trials are evaluating the impact of heated i.p. chemotherapy after cytoreductive surgery. However, difficulty administering i.p. chemotherapy and controversies surrounding optimal patient selection have remained topics of debate, with current clinical practice trends continuing to favor the standard intravenous administration of the CP regimen every three weeks (116).
Biology-driven advances in treatment.
Treatment advances over the past decade have stemmed from the successful targeting of two key biological drivers of OC: VEGF-driven tumor angiogenesis and HRD. This success followed decade-long attempts to target other signaling pathways ultimately deemed irrelevant, such as the EGFR, HER-2 neu, HER-3, PDGFR, mTOR, HDAC, and RAF pathways, and others (117).
Development of effective VEGF/VEGFR blockade inhibitors led to the initial testing of the humanized neutralizing antibody bevacizumab in recurrent OC. After noting that bevacizumab had remarkable single-agent activity in recurrent OC (118), particularly in patients with ascites (119), several randomized studies tested its effects in combination with chemotherapy in the upfront and recurrent setting. Bevacizumab plus CP induced improvement in progression-free survival (PFS) in women with newly diagnosed OC (120, 121) and prolonged OS in the high-risk groups, e.g., women who underwent suboptimal surgery and those with stage IV disease (121). Bevacizumab also improved the response rate to chemotherapy and the PFS in women with recurrent Pt-sensitive (122) or Pt-resistant HGSOC (123), leading to its FDA approval and widespread use for both upfront and recurrent OC.
On the other hand, the discovery of synthetic lethality induced by PARP inhibitors (PARPi) in BRCA-mutated cancers (124, 125) and the recognition that approximately half of HGSOC tumors harbor genomic features of HRD (46) led to the fervent investigation of PARPi (126–128), resulting in the approval of three agents (olaparib, rucaparib, and niraparib). Although it has been suggested that all patients with HGSOC benefit from PARPi after response to Pt (126), the highest gain was observed in women with BRCA-mutated or HRD tumors (129, 130). The completion of the SOLO-1 trial, which randomized women with BRCA1/2-mutated HGSOC to olaparib versus placebo for 2 years after completion of standard treatment (131, 132), represents a major step toward cure. Maintenance olaparib reduced the risk of progression by 70% (131), and close to half the women treated on this trial were alive and free of disease recurrence at 7 years (132). The magnitude and the duration of benefit from PARPi in this subset of patients remains unprecedented.
Building upon this success, design of effective combination treatments and exploration of mechanisms of resistance advanced to the forefront. The combination of PARPi and antiangiogenic agents was active in preclinical models and in clinical trials (130). Olaparib and bevacizumab combination was approved as a 2-year maintenance strategy after standard treatment in women with newly diagnosed HRD HGSOC. The additive therapeutic effects of the two drugs were attributed to enhanced HRD induced by bevacizumab, leading to improved responses to PARPi. The observation that PARPi elicits release of cytosolic double-stranded DNA in BRCA-mutated OC cells to induce STING activation and enhance antitumor immunity (133) led to speculation that PARPi could enhance the activity of ICIs. The combination of niraparib and pembrolizumab was found to be moderately active in the nonrandomized phase I/II Topacio trial (134), and results of randomized studies (ATHENA, NCT03522246; DUO-O, NCT03737643; and FIRST, NCT03602859) testing combination PARPi and ICIs in the upfront treatment setting for HGSOC are pending. Other combinations of PARPi with ATR kinase, PIK3/AKT kinase, or RAS/RAF/MEK inhibitors are in early phases of development. These agents in combination may circumvent mechanisms of resistance or accentuate HRD (135).
PARPi resistance.
Much interest has been devoted to understanding mechanisms of resistance to PARPi. Multiple pathways to resistance exist, including restoration of HR capacity through genetic or epigenetic mechanisms or HR-independent mechanisms affecting efflux mechanisms, PARP trapping, and replication fork stabilization. Initial studies identified reverting somatic BRCA1 and BRCA2 mutations as a modality of restoring HR function and a mechanism of resistance to both Pt and PARPi (136, 137). Secondary mutations that restore RAD51C and RAD51D function were also detected in tumors progressing during PARPi and linked to resistance (138). Epigenetic restoration of HR function also occurs through loss of BRCA1 promoter methylation and associates with Pt and PARPi resistance (139, 140). Another mechanism by which BRCA1-mutated cancer cells regain HR function involves the loss of the protein 53BP1, which mediates the switch between repair of double-stranded DNA breaks from HR to nonhomologous end joining. In BRCA-deficient cells also lacking 53BP, ATM-dependent repair of DNA is activated, HR is restored, and cells become resistant to PARPi (141). CRISPR/Cas9 synthetic lethality screens in BRCA1-mutated cells treated with PARPi identified loss of elements of the Shieldin complex as mediators of PARPi resistance (142). The Shieldin complex, acting downstream of 53BP to promote nonhomologous end joining–dependent double-stranded DNA break repair, sensitizes BRCA-deficient cells to Pt and PARPi (143).
Other mechanisms of resistance that do not depend on restoration of HR function include depletion of the E3 ligase TRIP12, which was shown to limit PARP-1 availability (144), or mutations of the enzyme PARP-1 (145). Both situations limit cancer cell killing by restricting PARPi from trapping PARP-1 on damaged DNA. The efforts to understand mechanisms of resistance are critical to finding new ways to target tumors with innate or acquired PARPi resistance. Based on these findings, ongoing studies are testing emerging drugs or PARPi combinations for tumors predicted to be less responsive to PARP inhibition. For example, HR-deficient OC cells, including cells resistant to PARPi, are highly dependent on polymerase θ. Inhibitors of this enzyme, such as novobiocin, an antibiotic developed in the 1950s, induce synthetic lethality either alone or in combination with PARPi (146).
Pt resistance.
After initial response to Pt-based therapy (147), most women experience relapse, and tumors become Pt resistant and ultimately fatal (147). In recent years, Pt resistance was recognized as the best predictor of resistance to PARPi, further underscoring the associated clinical adverse outcomes (148). Pt causes intrastrand and interstrand DNA cross-links, which trigger cell death if left unrepaired. Mechanisms of resistance have been studied for decades and include altered membrane transport (149), drug-metabolizing enzymes (149), upregulation of antiapoptotic mechanisms, mechanisms of DNA repair or trans-lesion synthesis, activation of epithelial-mesenchymal transition programs (150), enhanced oxidative defense (151), enrichment in cancer stem cell population (151, 152), or induction of metabolic reprogramming (e.g., a shift from glycolysis to increased fatty acids uptake and oxidation; refs. 153, 154) (Figure 2).
One of the major mechanisms contributing to chemoresistance is upregulation of membrane transporter proteins, such as the adenosine triphosphate-binding cassette (ABC) superfamily transporters (155), which enhance drug efflux (149). Within this family, ABCB1 (also known as P-glycoprotein [PgP] and multidrug resistance protein 1 [MDR1]), ABCC1 (also known as multidrug resistance-associated protein 1 [MRP1]), and ABCG2 (also known as breast cancer resistance protein [BCRP]) are three major isoforms associated with chemoresistance (155). Enhancement of antiapoptotic mechanisms related to either the intrinsic or the extrinsic pathways also contribute to chemoresistance (149). For example, activation of the antiapoptotic proteins BCL-2 and BCL-XL, or of inhibitors of apoptosis proteins, such as the IAP family members (XIAP, survivin), prevent activation of the caspase cascade, promoting cell survival and chemoresistance (149).
Accumulated genomic and epigenomic alterations have been described as key contributors to resistance (156, 157). Whole-genome sequencing of tumor and germline DNA samples from 92 patients with primary refractory and paired sensitive and resistant tumors reported inactivating mutations of TSGs, including RB1, NF1, RAD51B, and PTEN in resistant tumors (158). Other genomic changes included reversion mutations of germline BRCA1 or BRCA2 mutations, loss of BRCA1 promoter methylation, and promoter fusion induced overexpression of the drug efflux pump MDR1 (158). CCNE1 amplification, observed in about 19% HGSOC tumors (158), is mutually exclusive with BRCA1/2 mutations and common in primary resistant or refractory tumors. A recent proteogenomic analysis of Pt-sensitive and Pt-refractory HGSOC tumors identified chromosome 17 (Chr17) loss of heterozygosity (LOH) as the most robust marker of sensitivity to Pt (159). Chr17 LOH was associated with mutant TP53 transcriptional signature and responsiveness to Pt, while WT TP53 activity correlated with Pt refractoriness (159). The study proposed a 64-protein panel as a predictive model of Pt resistance (159).
Epigenome alterations can cause transcriptional silencing of TSGs and of genes associated with apoptotic responses to chemotherapy, leading to resistance. Mapping of H3K4me3 (active) and H3K27me3 (repressive) histone marks in primary and recurrent HGSOC identified genes marked by bivalent histone marks in primary tumors (160). This set of genes was enriched in known Polycomb complex target genes from embryonic stem cells and prone to acquiring CpG island methylation in recurrent tumors. It was proposed that acquisition of bivalent chromatin marks contributes to a stem cell–like phenotype that provides tumors with a mechanism for rapid adaptation to Pt (160). Increased CpG island methylation in tumor cells was shown to occur via direct response to hits inflicted by Pt (161) or through signals conveyed from the TME. In response to Pt, fibroblasts secrete cytokines (IL-6, TGF-β) that promote epigenetically mediated cancer cell plasticity and transition to a resistant state (152). In other cancer models, multiscale models combining molecular mapping (Hi-C, scRNA-Seq) with live-cell partial wave spectroscopy showed that cancer cells with high chromatin packing scaling were resistant to Pt (162), supporting the role of the state of chromatin in determining responsiveness to chemotherapy.
From a cell population standpoint, two models have been proposed. One model speculates that Pt eliminates sensitive cells, leaving behind cells tolerant to oxidative stress. Such cells may be cancer stem cells or stem-like cells, both of which typically upregulate aldehyde dehydrogenase (ALDH) and are capable of removing ROS, allowing them to survive chemotherapy (163, 164). This population possesses high ALDH expression, the ability to form spheres, increased expression of stemness-associated TFs, and antioxidant capacity (152, 165). Key antioxidant molecules, including glutathione peroxidase 4 (GPX4), nuclear factor erythroid 2-related factor 2 (NRF2) (166), and ALDH1 (167), are upregulated in cancer stem cells and in resistant cells and tumors (151). Notably, small molecules that block ALDH activity (168, 169) resensitize OC cells to chemotherapy by reducing the antioxidant defense and suppressing Pt-induced senescence and stemness features. Likewise, small-molecule inhibitors targeting GPX4 eliminated Pt-resistant cells via ferroptosis, an iron and lipid peroxidation-dependent form of cell death (151).
The second model assumes that any cell within a tumor can undergo reprogramming to become Pt resistant. This assumption is based on recent genome mapping of H3K27ac, which marks enhancer regions in Pt-sensitive and Pt-resistant cell lines (43). Integrated analysis revealed that distal enhancers, superenhancers, and their gene targets govern transcriptional programs in resistant HGSOC, resulting in the upregulation of key cell signaling pathways (e.g., NF-κB, IL-2/STAT5, TGF-β, and WNT) and downregulation of major metabolic pathways (e.g., oxidative phosphorylation, fatty acid metabolism, TCA cycle). The analysis identified known (e.g., ZEB2, E2F7, MYC, KLF6, ELK3) and novel (SOX9, HLX, MYBL1, ZNF430, ZNF502) superenhancer-regulated master TFs as drivers of Pt resistance. Small-molecule epigenetic inhibitors (e.g., bromodomain inhibitor JQ1) targeted these TFs, reversing the resistant phenotype and supporting the reprogramming concept. Epigenetic interventions using hypomethylating agents to reverse Pt resistance have had moderate success in clinical trials for women with recurrent HGSOC (170, 171).
Exceptional survivors.
On the flip side, there are rare patients with HGSOC who are not cured but who respond repeatedly to Pt and other lines of chemotherapy and survive longer than 10 years (172). The biological determinants of these exceptional survivors could provide clues to improve outcomes for the reminder of the patients. In a recent study, three key factors associated with survival greater than 10 years were the germline genome, presence of tumor somatic mutations, and antitumor immune response (172). Patients whose tumors exhibited co-occurring alterations in DNA repair pathway genes, such as co-occurrence of BRCA1 and BRCA2 mutations, or RB1 and BRCA1 or BRCA2 loss-of-function mutations, lived longer (172). Surprisingly, enhanced proliferation marked by overexpression of the cell proliferation–related genes PCNA and Ki67 was observed in tumors from some long-term survivors, probably because increased proliferation rendered tumor cells more susceptible to chemotherapy and reduced their ability to become quiescent (172). Long-term survivors also harbored a high tumor-mutation burden and a higher quantity of predicted neoantigens compared with short and medium-term survivors (172). An active immune TME was noted in some exceptional survivors, including in rare examples in which patients possessed CCNE1-amplified and HR-proficient tumors, demonstrating the power of the immune system in harnessing the progression of potentially resistant tumors (172).
Future directions
Important advances during the past decade have honed on defining the cell of origin of HGSOC, identifying genomic vulnerabilities to therapies (PARPi), classifying HGSOC tumors as HR deficient or HR proficient for treatment selection, and identifying subsets of ovarian tumors with unique genomic features for which targeted treatment is still evolving, such as with cyclin E–amplified HGSOC and Arid1A-mutated clear cell ovarian carcinoma. Given the accelerated pace of discovery in the field, we anticipate progress in addressing the remaining unmet needs of women with advanced stage OC. There are several areas of interest that remain unsolved and require solutions to diagnostic and treatment dilemmas in OC. First, early diagnosis and prevention of OC continues to be an unresolved issue that has a profound effect on the deadly course of the disease. The development of highly sensitive methods for detecting cell-free DNA and cancer-specific mutations or patterns of tagmentation in systemic circulation (173) open possibilities for detecting cancer with higher accuracy at an early stage, but testing in prospective studies is lagging. Second, while PARPi have affected the outcomes of women with BRCA-mutated or HRD tumors, there remain limited therapeutic options for women with HR-proficient cancers. This subgroup of patients should be subtyped and approached differently, by identifying and blocking other targets, such as cyclin E or c-Myc. For example, clinical testing of CDK2 inhibitors is underway in patients with cyclin E–amplified tumors (174). Third, despite recent advances in therapy, most women with advanced disease relapse and acquire Pt resistance. Development of strategies for this population is critically needed. Recent advances include antibody drug conjugates (ADCs), such as mirvetuximab soravtansine that targets the folate receptor α to deliver an antitubulin toxin. Mirvetuximab soravtansine induced potent antitumor effects and improved PFS and OS compared with standard chemotherapy in women with Pt-resistant OC (175). Other specific surface proteins in OC considered for development of ADCs include mesothelin (MSLN), tumor-associated calcium signal transducer 2 (TROP-2), sodium-dependent phosphate transport protein 2B (NaPi2b), tissue factor (TF), MUC-16 (CA125), activated leukocyte cell adhesion molecule (CD166), Her-2 neu, and others. Observations that the Her-2 neu–targeting ADC trastuzumab deruxtecan is clinically active not only in high Her-2 neu–expressing breast cancer, but also in patients with breast cancer expressing low levels of the receptor (160), have led to interest in testing it for HGSOC. Finally, because strategies targeting immune checkpoints have failed to make an impact in OC, efforts are underway to identify key inducers of the cold milieu and to design combinatorial therapeutic interventions. With biological discoveries driving therapies, the needle is finally moving for deadly HGSOC.
Supplementary Material
Acknowledgments
Funding for this study was contributed by the National Cancer Institute (1R01CA224275 and U54 CA268084 to DM and 1R01CA267544-01 to MA) and the US Department of Veterans Affairs (IO1BX000792 to DM).
Version 1. 01/02/2024
Electronic publication
Footnotes
Conflict of interest: DM declares research funding from PinotBio and consultant fees from GlaxoSmithKline, CVS Health, Elsevier, and Gynecologic Oncology Group Partners Foundation.
Copyright: © 2024, Wang et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2024;134(1):e174013. https://doi.org/10.1172/JCI174013.
Contributor Information
Yinu Wang, Email: yinu.wang@northwestern.edu.
Mazhar Adli, Email: adli@northwestern.edu.
Daniela Matei, Email: daniela.matei@northwestern.edu.
References
- 1.Spriggs DR, Zivanovic O. Ovarian cancer treatment - are we getting warmer? N Engl J Med. 2018;378(3):293–294. doi: 10.1056/NEJMe1714556. [DOI] [PubMed] [Google Scholar]
- 2.Bowtell DD, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15(11):668–679. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu Z, et al. Lifetime ovulations and epithelial ovarian cancer risk and survival: A systematic review and meta-analysis. Gynecol Oncol. 2022;165(3):650–663. doi: 10.1016/j.ygyno.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Lurie G, et al. Combined oral contraceptive use and epithelial ovarian cancer risk: time-related effects. Epidemiology. 2008;19(2):237–243. doi: 10.1097/EDE.0b013e31816334c5. [DOI] [PubMed] [Google Scholar]
- 7.Adami HO, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344(8932):1250–1254. doi: 10.1016/S0140-6736(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 8.Noske A, et al. Characterization of the 19q12 amplification including CCNE1 and URI in different epithelial ovarian cancer subtypes. Exp Mol Pathol. 2015;98(1):47–54. doi: 10.1016/j.yexmp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Cheasley D, et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun. 2019;10(1):3935. doi: 10.1038/s41467-019-11862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koopman T, et al. HER2 immunohistochemistry in endometrial and ovarian clear cell carcinoma: discordance between antibodies and with in-situ hybridisation. Histopathology. 2018;73(5):852–863. doi: 10.1111/his.13704. [DOI] [PubMed] [Google Scholar]
- 11.Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971;2(7716):163. doi: 10.1016/S0140-6736(71)92335-X. [DOI] [PubMed] [Google Scholar]
- 12.Fu Z, et al. Lifetime ovulatory years and risk of epithelial ovarian cancer: a multinational pooled analysis. J Natl Cancer Inst. 2023;115(5):539–551. doi: 10.1093/jnci/djad011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson JW, et al. Serous tubal intraepithelial carcinoma: diagnostic reproducibility and its implications. Int J Gynecol Pathol. 2010;29(4):310–314. doi: 10.1097/PGP.0b013e3181c713a8. [DOI] [PubMed] [Google Scholar]
- 14.Jarboe E, et al. Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol. 2008;27(1):1–9. doi: 10.1097/pgp.0b013e31814b191f. [DOI] [PubMed] [Google Scholar]
- 15.Labidi-Galy SI, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8(1):1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xian W, et al. The Li-Fraumeni syndrome (LFS): a model for the initiation of p53 signatures in the distal Fallopian tube. J Pathol. 2010;220(1):17–23. doi: 10.1002/path.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 18.King SM, et al. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18(5):627–642. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahar-Shany K, et al. Exposure of fallopian tube epithelium to follicular fluid mimics carcinogenic changes in precursor lesions of serous papillary carcinoma. Gynecol Oncol. 2014;132(2):322–327. doi: 10.1016/j.ygyno.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Huang HS, et al. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: initiation of fimbria carcinogenesis. Carcinogenesis. 2015;36(11):1419–1428. doi: 10.1093/carcin/bgv132. [DOI] [PubMed] [Google Scholar]
- 21.Tao T, et al. Loss of tubal ciliated cells as a risk for “ovarian” or pelvic serous carcinoma. Am J Cancer Res. 2020;10(11):3815–3827. [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, et al. The Repertoire of serous ovarian cancer non-genetic heterogeneity revealed by single-cell sequencing of normal fallopian tube epithelial cells. Cancer Cell. 2020;37(2):226–242. doi: 10.1016/j.ccell.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Lohmussaar K, et al. Assessing the origin of high-grade serous ovarian cancer using CRISPR-modification of mouse organoids. Nat Commun. 2020;11(1):2660. doi: 10.1038/s41467-020-16432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, et al. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat Commun. 2019;10(1):5367. doi: 10.1038/s41467-019-13116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nebgen DR, et al. Bilateral salpingectomy with delayed oophorectomy for ovarian cancer risk reduction: A pilot study in women with BRCA1/2 mutations. Gynecol Oncol. 2018;150(1):79–84. doi: 10.1016/j.ygyno.2018.04.564. [DOI] [PubMed] [Google Scholar]
- 26.Ciriello G, et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–1133. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song H, et al. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol. 2007;9(5):573–580. doi: 10.1038/ncb1571. [DOI] [PubMed] [Google Scholar]
- 28.Thiel KW, et al. TP53 sequencing and p53 immunohistochemistry predict outcomes when bevacizumab is added to frontline chemotherapy in endometrial cancer: an NRG oncology/gynecologic oncology group study. J Clin Oncol. 2022;40(28):3289–3300. doi: 10.1200/JCO.21.02506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patch AM, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research N, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farley J, et al. Cyclin E expression is a significant predictor of survival in advanced, suboptimally debulked ovarian epithelial cancers: a Gynecologic Oncology Group study. Cancer Res. 2003;63(6):1235–1241. [PubMed] [Google Scholar]
- 32.Baker VV, et al. c-myc amplification in ovarian cancer. Gynecol Oncol. 1990;38(3):340–342. doi: 10.1016/0090-8258(90)90069-W. [DOI] [PubMed] [Google Scholar]
- 33.Zeng M, et al. Targeting MYC dependency in ovarian cancer through inhibition of CDK7 and CDK12/13. Elife. 2018;7:e39030. doi: 10.7554/eLife.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etemadmoghadam D, et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009;15(4):1417–1427. doi: 10.1158/1078-0432.CCR-08-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamulla RJ, et al. Most commonly mutated genes in high-grade serous ovarian carcinoma are nonessential for ovarian surface epithelial stem cell transformation. Cell Rep. 2020;32(9):108086. doi: 10.1016/j.celrep.2020.108086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ElNaggar A, et al. Genomic profiling in low grade serous ovarian cancer: Identification of novel markers for disease diagnosis and therapy. Gynecol Oncol. 2022;167(2):306–313. doi: 10.1016/j.ygyno.2022.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Ryland GL, et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015;7(1):87. doi: 10.1186/s13073-015-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollis RL, et al. Molecular stratification of endometrioid ovarian carcinoma predicts clinical outcome. Nat Commun. 2020;11(1):4995. doi: 10.1038/s41467-020-18819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy J, et al. Predicting master transcription factors from pan-cancer expression data. Sci Adv. 2021;7(48):eabf6123. doi: 10.1126/sciadv.abf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nameki R, et al. Transcription factors in epithelial ovarian cancer: histotype-specific drivers and novel therapeutic targets. Pharmacol Ther. 2021;220:107722. doi: 10.1016/j.pharmthera.2020.107722. [DOI] [PubMed] [Google Scholar]
- 41.Li H, et al. Development of a novel transcription factors-related prognostic signature for serous ovarian cancer. Sci Rep. 2021;11(1):7207. doi: 10.1038/s41598-021-86294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaves-Moreira D, et al. The transcription factor PAX8 promotes angiogenesis in ovarian cancer through interaction with SOX17. Sci Signal. 2022;15(728):eabm2496. doi: 10.1126/scisignal.abm2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang S, et al. Chemotherapy-induced distal enhancers drive transcriptional programs to maintain the chemoresistant state in ovarian cancer. Cancer Res. 2019;79(18):4599–4611. doi: 10.1158/0008-5472.CAN-19-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradner JE, et al. Transcriptional addiction in cancer. Cell. 2017;168(4):629–643. doi: 10.1016/j.cell.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bekes M, et al. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domcke S, et al. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roby KF, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21(4):585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez GM, et al. The tumor immune profile of murine ovarian cancer models: an essential tool for ovarian cancer immunotherapy research. Cancer Res Commun. 2022;2(6):417–433. doi: 10.1158/2767-9764.CRC-22-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walton JB, et al. CRISPR/Cas9-derived models of ovarian high grade serous carcinoma targeting Brca1, Pten and Nf1, and correlation with platinum sensitivity. Sci Rep. 2017;7(1):16827. doi: 10.1038/s41598-017-17119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walton J, et al. CRISPR/Cas9-Mediated Trp53 and Brca2 knockout to generate improved murine models of ovarian high-grade serous carcinoma. Cancer Res. 2016;76(20):6118–6129. doi: 10.1158/0008-5472.CAN-16-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iyer S, et al. Genetically defined syngeneic mouse models of ovarian cancer as tools for the discovery of combination immunotherapy. Cancer Discov. 2020;11(2):384–407. doi: 10.1158/2159-8290.CD-20-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11(4):321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24(1):71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- 55.Flesken-Nikitin A, et al. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63(13):3459–3463. [PubMed] [Google Scholar]
- 56.Xing D, Orsulic S. A mouse model for the molecular characterization of brca1-associated ovarian carcinoma. Cancer Res. 2006;66(18):8949–8953. doi: 10.1158/0008-5472.CAN-06-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinross KM, et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J Clin Invest. 2012;122(2):553–557. doi: 10.1172/JCI59309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szabova L, et al. Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 2012;72(16):4141–4153. doi: 10.1158/0008-5472.CAN-11-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandler RL, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat Commun. 2015;6:6118. doi: 10.1038/ncomms7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhai Y, et al. Arid1a inactivation in an Apc- and Pten-defective mouse ovarian cancer model enhances epithelial differentiation and prolongs survival. J Pathol. 2016;238(1):21–30. doi: 10.1002/path.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guan B, et al. Roles of deletion of Arid1a, a tumor suppressor, in mouse ovarian tumorigenesis. J Natl Cancer Inst. 2014;106(7):dju146. doi: 10.1093/jnci/dju146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arango NA, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75(7):1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 63.Perets R, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24(6):751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhai Y, et al. High-grade serous carcinomas arise in the mouse oviduct via defects linked to the human disease. J Pathol. 2017;243(1):16–25. doi: 10.1002/path.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ordóñez NG. Value of PAX 8 immunostaining in tumor diagnosis: a review and update. Adv Anat Pathol. 2012;19(3):140–151. doi: 10.1097/PAP.0b013e318253465d. [DOI] [PubMed] [Google Scholar]
- 66.Perets R, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24(6):751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russo A, et al. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene. 2018;37(15):1976–1990. doi: 10.1038/s41388-017-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J, et al. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A. 2012;109(10):3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maniati E, et al. Mouse ovarian cancer models recapitulate the human tumor microenvironment and patient response to treatment. Cell Rep. 2020;30(2):525–540. doi: 10.1016/j.celrep.2019.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyoshi I, et al. Mouse transgenic for murine oviduct-specific glycoprotein promoter-driven simian virus 40 large T-antigen: tumor formation and its hormonal regulation. Mol Reprod Dev. 2002;63(2):168–176. doi: 10.1002/mrd.10175. [DOI] [PubMed] [Google Scholar]
- 71.Mullany LK, et al. Molecular and functional characteristics of ovarian surface epithelial cells transformed by KrasG12D and loss of Pten in a mouse model in vivo. Oncogene. 2011;30(32):3522–3536. doi: 10.1038/onc.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw TJ, et al. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol Ther. 2004;10(6):1032–1042. doi: 10.1016/j.ymthe.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 73.Dong R, et al. Histologic and molecular analysis of patient derived xenografts of high-grade serous ovarian carcinoma. J Hematol Oncol. 2016;9(1):92. doi: 10.1186/s13045-016-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomakos N, et al. Rare distant metastatic disease of ovarian and peritoneal carcinomatosis: a review of the literature. Cancers (Basel) 2019;11(8):1044. doi: 10.3390/cancers11081044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chauvin M, et al. Cancer-associated mesothelial cells are regulated by the anti-Müllerian hormone axis. Cell Rep. 2023;42(7):112730. doi: 10.1016/j.celrep.2023.112730. [DOI] [PubMed] [Google Scholar]
- 77.Mitra AK, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2(12):1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kenny HA, et al. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer. 2007;121(7):1463–1472. doi: 10.1002/ijc.22874. [DOI] [PubMed] [Google Scholar]
- 79.Raghavan S, et al. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J Immunother Cancer. 2019;7(1):190. doi: 10.1186/s40425-019-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delaine-Smith RM, et al. Modelling TGFβR and Hh pathway regulation of prognostic matrisome molecules in ovarian cancer. iScience. 2021;24(6):102674. doi: 10.1016/j.isci.2021.102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malacrida B, et al. A human multi-cellular model shows how platelets drive production of diseased extracellular matrix and tissue invasion. iScience. 2021;24(6):102676. doi: 10.1016/j.isci.2021.102676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ford CE, et al. The untapped potential of ascites in ovarian cancer research and treatment. Br J Cancer. 2020;123(1):9–16. doi: 10.1038/s41416-020-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Izar B, et al. A single-cell landscape of high-grade serous ovarian cancer. Nat Med. 2020;26(8):1271–1279. doi: 10.1038/s41591-020-0926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghosh S, Maity P. Isolation and purification of vascular endothelial growth factor (VEGF) from ascitic fluid of ovarian cancer patients. Pathol Oncol Res. 2004;10(2):104–108. doi: 10.1007/BF02893464. [DOI] [PubMed] [Google Scholar]
- 85.Hu L, et al. Paracrine VEGF/VE-cadherin action on ovarian cancer permeability. Exp Biol Med (Maywood) 2006;231(10):1646–1652. doi: 10.1177/153537020623101010. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 87.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 88.Darb-Esfahani S, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7(2):1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Webb JR, et al. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141(2):293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Anadon CM, et al. Ovarian cancer immunogenicity is governed by a narrow subset of progenitor tissue-resident memory T cells. Cancer Cell. 2022;40(5):545–557. doi: 10.1016/j.ccell.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biswas S, et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature. 2021;591(7850):464–470. doi: 10.1038/s41586-020-03144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S, et al. Epigenetic priming enhances antitumor immunity in platinum-resistant ovarian cancer. J Clin Invest. 2022;132(14):e158800. doi: 10.1172/JCI158800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamanishi J, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 94.Pujade-Lauraine E, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22(7):1034–1046. doi: 10.1016/S1470-2045(21)00216-3. [DOI] [PubMed] [Google Scholar]
- 95.Cha JH, et al. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76(3):359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan LC, et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest. 2019;129(8):3324–3338. doi: 10.1172/JCI126022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim SO, et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30(6):925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Callahan MK, Wolchok JD. Recruit or reboot? How does anti-PD-1 therapy change tumor-infiltrating lymphocytes? Cancer Cell. 2019;36(3):215–217. doi: 10.1016/j.ccell.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 99.Waldman AD, et al. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zou W, et al. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tu X, et al. PD-L1 (B7-H1) Competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Mol Cell. 2019;74(6):1215–1226. doi: 10.1016/j.molcel.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song M, et al. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562(7727):423–428. doi: 10.1038/s41586-018-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cubillos-Ruiz JR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chae CS, et al. Tumor-derived lysophosphatidic acid blunts protective type I interferon responses in ovarian cancer. Cancer Discov. 2022;12(8):1904–1921. doi: 10.1158/2159-8290.CD-21-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okla K, et al. Clinical relevance and immunosuppressive pattern of circulating and infiltrating subsets of myeloid-derived suppressor cells (MDSCs) in epithelial ovarian cancer. Front Immunol. 2019;10:691. doi: 10.3389/fimmu.2019.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu L, et al. Ascites-derived IL-6 and IL-10 synergistically expand CD14+HLA-DR-/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget. 2017;8(44):76843–76856. doi: 10.18632/oncotarget.20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stone ML, et al. Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden. Proc Natl Acad Sci U S A. 2017;114(51):E10981–E10990. doi: 10.1073/pnas.1712514114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hacker NF, van der Burg ME. Advanced ovarian cancer. Debulking and intervention surgery. Ann Oncol. 1993;4 Suppl 4:17–22. [PubMed] [Google Scholar]
- 109.du Bois A, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 110.McGuire WP, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 111.Alberts DS, et al. Improved therapeutic index of carboplatin plus cyclophosphamide versus cisplatin plus cyclophosphamide: final report by the Southwest Oncology Group of a phase III randomized trial in stages III and IV ovarian cancer. J Clin Oncol. 1992;10(5):706–717. doi: 10.1200/JCO.1992.10.5.706. [DOI] [PubMed] [Google Scholar]
- 112.Chan JK, et al. Weekly vs. Every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016;374(8):738–748. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Katsumata N, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 114.Armstrong DK, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 115.van Driel WJ, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 116.Armstrong DK, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17(8):896–909. doi: 10.6004/jnccn.2019.0039. [DOI] [PubMed] [Google Scholar]
- 117.Bast RC., Jr Molecular approaches to personalizing management of ovarian cancer. Ann Oncol. 2011;22 Suppl 8(suppl 8):viii5–viii15. doi: 10.1093/annonc/mdr516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burger RA, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(33):5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 119.Ferriss JS, et al. Ascites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube and peritoneal cancers: an NRG Oncology/GOG study. Gynecol Oncol. 2015;139(1):17–22. doi: 10.1016/j.ygyno.2015.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 121.Perren TJ, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 122.Coleman RL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):779–791. doi: 10.1016/S1470-2045(17)30279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pujade-Lauraine E, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 124.Fong PC, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 125.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 126.Gonzalez-Martin A, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 127.Ledermann J, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 128.Ray-Coquard I, et al. PARP inhibitors in ovarian cancer. Reply. N Engl J Med. 2020;382(16):1574–1575. doi: 10.1056/NEJMc2000644. [DOI] [PubMed] [Google Scholar]
- 129.Mirza MR, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 130.Ray-Coquard I, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–2428. doi: 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 131.Moore K, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 132.DiSilvestro P, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial. J Clin Oncol. 2023;41(3):609–617. doi: 10.1200/JCO.22.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ding L, et al. PARP inhibition elicits STING-dependent antitumor immunity in brca1-deficient ovarian cancer. Cell Rep. 2018;25(11):2972–2980. doi: 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Konstantinopoulos PA, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5(8):1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim H, et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat Commun. 2020;11(1):3726. doi: 10.1038/s41467-020-17127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sakai W, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451(7182):1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin KK, et al. BRCA reversion mutations in circulating tumor DNA Predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019;9(2):210–219. doi: 10.1158/2159-8290.CD-18-0715. [DOI] [PubMed] [Google Scholar]
- 138.Kondrashova O, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7(9):984–998. doi: 10.1158/2159-8290.CD-17-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kondrashova O, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018;9(1):3970. doi: 10.1038/s41467-018-05564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Swisher EM, et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2) Nat Commun. 2021;12(1):2487. doi: 10.1038/s41467-021-22582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bunting SF, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141(2):243–254. doi: 10.1016/j.cell.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dev H, et al. Shieldin complex promotes DNA end-joining and counters homologous recombination in BRCA1-null cells. Nat Cell Biol. 2018;20(8):954–965. doi: 10.1038/s41556-018-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gupta R, et al. DNA repair network analysis reveals shieldin as a key regulator of NHEJ and PARP inhibitor sensitivity. Cell. 2018;173(4):972–988. doi: 10.1016/j.cell.2018.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gatti M, et al. The ubiquitin ligase TRIP12 limits PARP1 trapping and constrains PARP inhibitor efficiency. Cell Rep. 2020;32(5):107985. doi: 10.1016/j.celrep.2020.107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pettitt SJ, et al. Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat Commun. 2018;9(1):1849. doi: 10.1038/s41467-018-03917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou J, et al. A first-in-class polymerase theta inhibitor selectively targets homologous-recombination-deficient tumors. Nat Cancer. 2021;2(6):598–610. doi: 10.1038/s43018-021-00203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vaughan S, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choi YE, et al. Platinum and PARP inhibitor resistance due to overexpression of microRNA-622 in BRCA1-mutant ovarian cancer. Cell Rep. 2016;14(3):429–439. doi: 10.1016/j.celrep.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alatise KL, et al. Mechanisms of drug resistance in ovarian cancer and associated gene targets. Cancers (Basel) 2022;14(24):6246. doi: 10.3390/cancers14246246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marchini S, et al. Resistance to platinum-based chemotherapy is associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Eur J Cancer. 2013;49(2):520–530. doi: 10.1016/j.ejca.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 151.Wang Y, et al. Frizzled-7 identifies platinum-tolerant ovarian cancer cells susceptible to ferroptosis. Cancer Res. 2021;81(2):384–399. doi: 10.1158/0008-5472.CAN-20-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, et al. IL-6 mediates platinum-induced enrichment of ovarian cancer stem cells. JCI Insight. 2018;3(23):e122360. doi: 10.1172/jci.insight.122360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang Y, et al. Metabolic dependencies and targets in ovarian cancer. Pharmacol Ther. 2023;245:108413. doi: 10.1016/j.pharmthera.2023.108413. [DOI] [PubMed] [Google Scholar]
- 154.Tan Y, et al. Metabolic reprogramming from glycolysis to fatty acid uptake and beta-oxidation in platinum-resistant cancer cells. Nat Commun. 2022;13(1):4554. doi: 10.1038/s41467-022-32101-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Huang Y, Sadée W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006;239(2):168–182. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 156.Watts GS, et al. DNA methylation changes in ovarian cancer are cumulative with disease progression and identify tumor stage. BMC Med Genomics. 2008;1:47. doi: 10.1186/1755-8794-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Barton CA, et al. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecol Oncol. 2008;109(1):129–139. doi: 10.1016/j.ygyno.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 158.Patch AM, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 159.Chowdhury S, et al. Proteogenomic analysis of chemo-refractory high-grade serous ovarian cancer. Cell. 2023;186(16):3476–3498. doi: 10.1016/j.cell.2023.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Curry E, et al. Genes predisposed to DNA hypermethylation during acquired resistance to chemotherapy are identified in ovarian tumors by bivalent chromatin domains at initial diagnosis. Cancer Res. 2018;78(6):1383–1391. doi: 10.1158/0008-5472.CAN-17-1650. [DOI] [PubMed] [Google Scholar]
- 161.Flanagan JM, et al. Platinum-based chemotherapy induces methylation changes in blood DNA associated with overall survival in patients with ovarian cancer. Clin Cancer Res. 2017;23(9):2213–2222. doi: 10.1158/1078-0432.CCR-16-1754. [DOI] [PubMed] [Google Scholar]
- 162.Virk RKA, et al. Disordered chromatin packing regulates phenotypic plasticity. Sci Adv. 2020;6(2):eaax6232. doi: 10.1126/sciadv.aax6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Silva IA, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71(11):3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang S, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang Y, et al. Epigenetic targeting of ovarian cancer stem cells. Cancer Res. 2014;74(17):4922–4936. doi: 10.1158/0008-5472.CAN-14-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kahroba H, et al. The Role of Nrf2 signaling in cancer stem cells: From stemness and self-renewal to tumorigenesis and chemoresistance. Life Sci. 2019;239:116986. doi: 10.1016/j.lfs.2019.116986. [DOI] [PubMed] [Google Scholar]
- 167.Kim D, et al. High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis. 2018;9(9):896. doi: 10.1038/s41419-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Muralikrishnan V, et al. A Novel ALDH1A1 inhibitor blocks platinum-induced senescence and stemness in ovarian cancer. Cancers (Basel) 2022;14(14):3437. doi: 10.3390/cancers14143437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nwani NG, et al. A novel ALDH1A1 inhibitor targets cells with stem cell characteristics in ovarian cancer. Cancers (Basel) 2019;11(4):502. doi: 10.3390/cancers11040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Matei D, et al. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res. 2012;72(9):2197–2205. doi: 10.1158/0008-5472.CAN-11-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oza AM, et al. A randomized phase II trial of epigenetic priming with guadecitabine and carboplatin in platinum-resistant, recurrent ovarian cancer. Clin Cancer Res. 2020;26(5):1009–1016. doi: 10.1158/1078-0432.CCR-19-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Garsed DW, et al. The genomic and immune landscape of long-term survivors of high-grade serous ovarian cancer. Nat Genet. 2022;54(12):1853–1864. doi: 10.1038/s41588-022-01230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cristiano S, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570(7761):385–389. doi: 10.1038/s41586-019-1272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Au-Yeung G, et al. Selective targeting of cyclin E1-amplified high-grade serous ovarian cancer by cyclin-dependent kinase 2 and AKT inhibition. Clin Cancer Res. 2017;23(7):1862–1874. doi: 10.1158/1078-0432.CCR-16-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Matulonis UA, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41(13):2436–2445. doi: 10.1200/JCO.22.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ren YA, et al. Mutant p53 promotes epithelial ovarian cancer by regulating tumor differentiation, metastasis, and responsiveness to steroid hormones. Cancer Res. 2016;76(8):2206–2218. doi: 10.1158/0008-5472.CAN-15-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Orsulic S, et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell. 2002;1(1):53–62. doi: 10.1016/S1535-6108(01)00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.