Abstract
The accumulation of quaternary ammonium compounds in Lactobacillus plantarum is mediated via a single transport system with a high affinity for glycine betaine (apparent Km of 18 μM) and carnitine and a low affinity for proline (apparent Km of 950 μM) and other analogues. Mutants defective in the uptake of glycine betaine were generated by UV irradiation and selected on the basis of resistance to dehydroproline (DHP), a toxic proline analogue. Three independent DHP-resistant mutants showed reduced glycine betaine uptake rates and accumulation levels but behaved similarly to the wild type in terms of direct activation of uptake by high-osmolality conditions. Kinetic analysis of glycine betaine uptake and efflux in the wild-type and mutant cells is consistent with one uptake system for quaternary ammonium compounds in L. plantarum and a separate system(s) for their excretion. The mechanism of osmotic activation of the quaternary ammonium compound transport system (QacT) was studied. It was observed that the uptake rates were inhibited by the presence of internal substrate. Upon raising of the medium osmolality, the QacT system was rapidly activated (increase in maximal velocity) through a diminished inhibition by trans substrate as well as an effect that is independent of intracellular substrate. We also studied the effects of the cationic amphipath chlorpromazine, which inserts into the cytoplasmic membrane and thereby influences the uptake and efflux of glycine betaine. The results provide further evidence for the notion that the rapid efflux of glycine betaine upon osmotic downshock is mediated by a channel protein that is responding to membrane stretch or tension. The activation of QacT upon osmotic upshock seems to be brought about by a turgor-related parameter other than membrane stretch or tension.
Bacteria protect themselves against high external osmolality by the uptake or synthesis of a limited number of so-called compatible solutes. The predominant compatible solute in many organisms is glycine betaine, which usually is accumulated through an osmoregulated uptake system. Analogues of glycine betaine have been found in several bacteria, and many glycine betaine uptake systems facilitate their uptake as well. The osmotic regulation of the transport systems may occur at the genetic or enzymatic level or both, and these aspects have been studied in most detail with enteric bacteria. In Escherichia coli glycine betaine (and proline) is taken up via a low-affinity secondary transport protein (ProP) and a high-affinity ATP-binding cassette transport system (ProU) (1). The transport activity of both ProP and ProU proteins is stimulated by an increase in external osmolality, although the mechanisms of osmosensing most likely are different (2, 9, 15, 19). Homologues of ProU have been identified in the gram-positive bacterium Bacillus subtilis (6, 7), whereas a homologue of ProP is present in Erwinia chrysanthemi (5). Important structural information regarding the nature of the osmosensing domain has recently been obtained for the BetP protein of Corynebacterium glutamicum, a member of the third family of osmoregulated uptake systems for glycine betaine and proline (18). There is clear evidence that the carboxyl-terminal region (55 amino acids) has a central role in osmosensing.
Glycine betaine is the major compatible solute in the cytoplasm of Lactobacillus plantarum grown in chemically defined high-salt media containing glycine betaine. L. plantarum is unable to synthesize or metabolize glycine betaine, and the final accumulation levels of glycine betaine are thus determined solely by the relative rates of uptake and efflux (3). Previous studies have indicated that osmotic regulation of glycine betaine uptake acted mainly on the transporter activity, whereas changes in protein synthesis were relatively small compared to those for systems such as ProU (13). However, the possibility that more than one system effected the uptake could not be excluded, whereas efflux of glycine betaine upon osmotic downshock seemed to be mediated by more than one efflux system (4). Uptake of glycine betaine in L. plantarum is driven by ATP and is most likely mediated by a binding-protein-dependent system(s) (unpublished results). In this study, mutants defective in glycine betaine uptake were generated and characterized to elucidate the contribution of the transport systems to the overall flux of glycine betaine. We also describe the substrate specificity and the kinetics of the glycine betaine uptake system under high- and low-osmolality conditions, as well as the effect of a cationic amphipath on the uptake and efflux activities in L. plantarum.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and media.
L. plantarum ATCC 14917 was grown at 30°C and pH 6.7 in a chemically defined medium (CDM) or modified CDM (without proline) containing 0.5% (wt/vol) glucose, as described previously (3). High-osmolality media were obtained by adding 0.8 M KCl to the standard CDM.
Isolation of mutants defective in glycine betaine uptake.
A 3-ml aliquot of exponentially growing cells (A660 of 0.2 to 0.6) in low-osmolality CDM was dispersed over a petri dish (diameter, 9 cm) and irradiated for 1 min with UV light (254 nm) at a distance of 24 cm from the petri dishes. The survival rate was around 1% as estimated from the plating of irradiated and nonirradiated samples on MRS agar plates. The irradiated culture was washed and concentrated in CDM without proline and subsequently plated confluently on CDM agar plates without proline. The toxic proline analogue dehydroproline (DHP) (20 μl of a 100 mM solution) was spotted in the middle of the plates (17). After 48 h of incubation, putative DHP-resistant mutants were picked from the colony-free zone around the DHP spots. DHP is a toxic proline analogue that was found to competitively inhibit the uptake of glycine betaine and proline. Transport of leucine and glutamic acid was not affected by DHP (data not shown). Several independently isolated DHP-resistant mutants were able to grow on CDM agar without proline in the presence of 1 mM DHP. The protein patterns of three mutants (DHPR-38.1, -38.2, and -38.3) were analyzed on a Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel and were found to be similar to each other. The patterns of the mutants differed from that of the wild type in four protein bands with apparent molecular masses of 120, 90, 75, and 31 kDa (data not shown). These protein bands are missing, or at least significantly reduced in the three mutants.
Transport assays.
Transport assays, enzymatic synthesis of [14C]glycine betaine from [N-methyl-14C]choline (40 to 60 mCi/mmol), and assays of uptake of [14C]proline (260 mCi/mmol) were performed as described previously (3, 4). Briefly, the cells were washed and resuspended in 50 mM potassium phosphate (pH 6.5) plus 0.8 M KCl or in 50 mM potassium phosphate (pH 6.5). The latter buffer system was used to release most of the compatible solutes that were accumulated during the growth under high-osmolality conditions. Prior to the transport assays, cells were diluted to a protein concentration of 0.1 to 0.4 mg/ml in 50 mM potassium phosphate (pH 6.5) plus 0.8 M KCl or in 50 mM potassium phosphate (pH 6.5) containing 10 mM glucose, and the mixtures were incubated at 30°C. Following a period of preenergization (see figure legends), radiolabeled substrate was added at time zero, and the reactions were terminated at given time intervals by rapid filtering of 100- to 200-μl samples on 0.45-μm-pore-size cellulose nitrate filters. The filters were washed with 2 ml of LiCl (0.1 to 0.9 M, depending on the osmolality of the assay medium). To monitor the exit of [14C]glycine betaine or [14C]proline upon osmotic downshock, the cells were allowed to accumulate these compounds for a given period of time, after which the media were diluted with buffer (30°C) containing glucose plus [14C]glycine betaine or [14C]proline; care was taken to keep all of the parameters except the osmolality (i.e., KCl concentration) constant. The data from the kinetic experiments were fitted with the Michaelis-Menten equation, from which the apparent affinity constant (Km) and maximal rate of uptake (Vmax) were calculated.
Miscellaneous.
Protein was determined by the method of Lowry et al. (12) with bovine serum albumin as a standard. Total protein extracts of wild-type and DHP-resistant mutants of L. plantarum were subjected to sodium dodecyl sulfate-polyacrylamide (10% wt/vol) gel electrophoresis after lysis of the cells by sonication. The osmolalities of media and buffers were measured by freezing-point depression with an Osmomat 030 apparatus (Gonotec, Berlin, Germany). Growth experiments were performed in sterile low-protein-binding microplates. Plate wells containing 200 μl of culture were sealed by adding 75 μl of sterile silicone oil (1.03 g/ml), and growth rates were determined from A620 increases by using a Multiscan MCC/340 MKII instrument (Flow Laboratories, Lugano, Switzerland). For the calculation of intracellular concentrations, a value of 3 μl/mg of protein for the specific internal volume was used.
RESULTS AND DISCUSSION
Substrate specificity of the glycine betaine uptake system.
In several gram-positive and gram-negative bacteria, proline and glycine betaine are taken up via the same system despite their difference in molecular structure (15, 16, 20). In L. plantarum, a 100-fold excess of unlabeled proline did not affect glycine betaine uptake, whereas proline transport was completely inhibited by a 100-fold excess of unlabeled glycine betaine (Table 1). Experiments with other substrate analogues showed that glycine betaine uptake was strongly inhibited by tetramethylammonium and even by dimethylsulfonium propionic acid. The substrate analogue carnitine abolished the uptake to the same extent as glycine betaine itself. Other substrate analogues, like dimethylglycine, trimethylamine, and 4-aminobutyric acid, had no significant inhibitory effect on the glycine betaine uptake rates. In fact, some of these solutes stimulated the glycine betaine uptake consistently. Since the analogues were added at a concentration of 125 mM, the increased uptake most likely reflects osmotic activation of the glycine betaine uptake system (see below), which may even have masked small inhibitory effects. The osmotic activation of the transport system by 125 nM solute makes the extent of inhibition in all cases an underestimate; e.g., in the case of proline, the osmotic activation and competitive inhibition cancel each other out under these conditions (see below).
TABLE 1.
Substrate specificity of QacT of L. plantarum ATCC 14917a
| Substrate analogue | Rate of glycine betaine uptake (%) | Rate of proline uptake (%) |
|---|---|---|
| 4-Aminobutyric acid | 115 | 55 |
| Dimethylglyine | 90 | <5 |
| Trimethylamine | 105 | <5 |
| Tetramethylammonium | 35 | <5 |
| Proline | 100 | <5 |
| Dimethylsulfonium propionic acid | 30 | <5 |
| Carnitine | <5 | <5 |
| Glycine betaine | <5 | <5 |
The cells were cultured on CDM plus 0.8 M KCl and subsequently washed and resuspended in 50 mM potassium phosphate (pH 6.5) to a final protein concentration of 0.22 mg/ml. Prior to the transport reactions, the cells were incubated for 5 min at 30°C with 10 mM glucose. Uptake was initiated by adding [14C]glycine betaine or [14C]proline (final concentration, 1.25 mM) plus a 100-fold excess of substrate analogue. An uptake activity of 100% corresponds to rates of 33 and 17 nmol/min per mg of protein for glycine betaine and proline, respectively.
All of the tested compounds with two or three methyl groups attached to the nitrogen or sulfur atom inhibited the uptake of proline completely. These results are consistent with a single uptake system, with a high affinity for glycine betaine (and carnitine) and a low affinity for proline (and other analogues). To substantiate this conclusion, we determined the kinetic parameters for glycine betaine and proline uptake under various osmotic conditions (see the next section). Of the solutes tested, glycine betaine and carnitine offered the most osmoprotection in growth experiments, proline and dimethylglyine had lesser effects, and 4-aminobutyric acid offered no protection (see also references 3 and 8). The transport system was termed QacT (for quaternary ammonium compound transporter), as the quaternary ammonium compounds are physiologically most relevant for osmoprotection.
Kinetic analysis of glycine betaine and proline uptake.
With cells grown in CDM and uptake assayed at low osmolality, the uptake of glycine betaine was monophasic with an apparent Km of 18 μM and a Vmax of 27 nmol/min per mg of protein (Table 2, line 1). Proline uptake was also monophasic under these conditions, with an apparent Km of 950 μM and a Vmax of 21 nmol/min per mg of protein (Table 2, line 7). The apparent Km for glycine betaine increased to 33 μM, and the Vmax increased to 105 nmol/min per mg of protein, when high-osmolality assay media were used (Table 2, line 1). Similar changes were found for the uptake of proline; the apparent Km and Vmax for proline increased to 1,500 μM and 150 nmol/min per mg of protein, respectively, at high assay osmolality (Table 2, line 7). The uptake of glycine betaine and proline in cells cultured at high osmolality was also studied, but the kinetic parameters were not significantly different from those of low-osmolality-grown cells (Table 2, compare lines 1 and 5). These results indicate that the increased rate of uptake upon an osmotic upshift mainly involves an increased Vmax as a result of activation of QacT. Since the effects of culture and assay conditions on Km and Vmax are similar for glycine betaine and proline uptake and the uptake is monophasic under all conditions tested, the data are best explained by uptake via a single system.
TABLE 2.
Kinetic parameters of glycine betaine and proline uptake in wild-type L. plantarum ATCC 14917 and a DHP-resistant mutanta
| Uptake | Strain | Culture conditions | Low-osmolality assay conditions
|
High-osmolality assay conditions
|
||
|---|---|---|---|---|---|---|
| Km (μM) | Vmax (nmol/min/mg of protein) | Km | Vmax | |||
| Glycine betaine | Wild type | CDM | 18 ± 2 | 27 ± 3 | 33 ± 6 | 105 ± 6 |
| CDM − Pro | 19 ± 4 | 86 ± 5 | 26 ± 6 | 116 ± 6 | ||
| DHPR-38.1 | CDM | 6 ± 2 | 2 ± 0.5 | 12 ± 3 | 9 ± 0.5 | |
| CDM − Pro | 22 ± 6 | 7 ± 0.5 | 22 ± 3 | 9 ± 0.5 | ||
| Wild type | CDM + KCl | 13 ± 3 | 35 ± 2 | 33 ± 6 | 106 ± 6 | |
| CDM − Pro + KCl | 17 ± 4 | 125 ± 9 | 23 ± 7 | 198 ± 20 | ||
| Proline | Wild type | CDM | 950 ± 150 | 21 ± 2 | 1,500 ± 150 | 150 ± 8 |
Following growth in the indicated media (− Pro, proline omitted from CDM; + KCl, 0.8 M KCl added to CDM), the cells were washed and resuspended to a final protein concentration of 0.4 to 0.8 mg/ml in 50 mM potassium phosphate (pH 6.5). The [14C]glycine betaine and [14C]proline concentrations were in the range of 0.9 μM to 10 mM. After 7 min of preenergization with 10 mM glucose, uptake was initiated by the addition of [14C]glycine betaine or [14C]proline with (high-osmolality assay conditions) or without (low-osmolality assay conditions) 0.8 M KCl. After 30 s of uptake, the cells were rapidly filtered and processed further as described in Materials and Methods. The data from two separate experiments were used to calculate the arithmetical average of the uptake rates; standard deviations are also shown.
Effect of internal substrate.
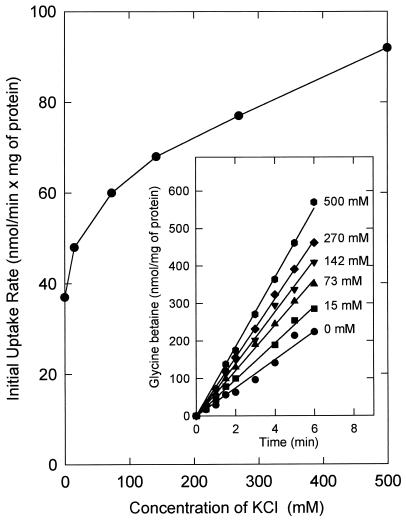
Low- or high-osmolality growth media do not significantly affect the expression of QacT. However, it was observed that the final accumulation levels as well as the rates of glycine betaine uptake were lower when the cells were cultured in the presence of glycine betaine or proline, i.e., under conditions in which the cells contained large amounts of glycine betaine or proline (data not shown). We also observed that the rate of activated uptake above a certain threshold upshock (200 mM KCl) was similar irrespective of the size of the osmolality change but that the extent of uptake (final accumulation level) was in proportion to the shift (3). Thus, the larger the upshock, the longer the system remained in the activated state. These experiments, however, were performed with washed cell suspensions that had already accumulated glycine betaine to about 600 nmol/mg of protein. Consequently, the glycine betaine uptake system might have already been inhibited by internal substrate, and differences in the uptake rates as a function of the size of the osmolality shift may have been overlooked. In a new series of experiments, the initial rate of glycine betaine uptake in cells that did not contain any glycine betaine was measured. Moreover, the washing of these cells in hypotonic medium (50 mM potassium phosphate, pH 6.5) released most compatible solutes, including proline. In these cells the rate of uptake increased with medium osmolality, in particular in the range of 35 to 200 mM (Fig. 1). Thus, although we concluded previously that the activation of the uptake system occurred through an on-off mechanism, this conclusion appears to have been somewhat premature. It was based on the rates of glycine betaine uptake in cells already containing internal glycine betaine and under conditions where the medium osmolality was increased by adding 0.2 to 1.2 M KCl. Since some differences in the uptake rate were also observed in the range above 0.2 M KCl (Fig. 1), which was not seen before, we conclude that the presence or absence of internal glycine betaine might have contributed to these variations.
FIG. 1.
Dependence of the initial rate of glycine betaine uptake on increases in medium osmolality imposed by KCl. Cells of L. plantarum ATCC 14917 were grown on CDM containing 0.8 M KCl, washed, and resuspended in 50 mM potassium phosphate (pH 6.5). The concentrated cell suspensions were diluted in 50 mM potassium phosphate (pH 6.5) to a final protein concentration of 0.13 mg/ml. After 8 min of preenergization with 10 mM glucose, uptake was initiated at time zero by the addition [14C]glycine betaine (final concentration of 1.3 mM) plus KCl as indicated. The initial uptake rates were determined and plotted against the concentration of KCl that was added to the 50 mM potassium phosphate.
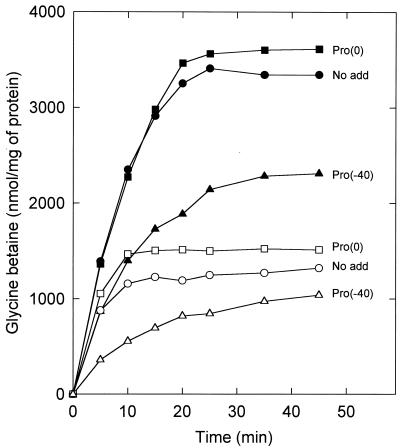
To discriminate between possible effects on the expression of QacT and feedback inhibition by cytoplasmic proline, cells were grown on CDM without proline. Half of the cells were allowed to take up unlabeled proline (final concentration, 1.3 mM) in the presence of 50 μg of chloramphenicol per ml; the other half were treated similarly, but proline was omitted. After 45 min of incubation, [14C]glycine betaine (final concentration, 1.3 mM) was added (time zero in Fig. 2), and the uptake was monitored. It was observed that cells that had accumulated proline in the cytoplasm exhibited a lower rate of uptake of glycine betaine than control cells, under both high- and low osmolality assay conditions (Fig. 2). In other experiments, the cells were energized for 45 min prior to the addition of 1.3 mM unlabeled proline together with [14C]glycine betaine. Under these conditions, the unlabeled proline exerted no significant effect on the uptake of [14C]glycine betaine, as anticipated from the large differences in Km values for these substrates. Thus, the preloading of the cells with proline lowers the uptake rate and final accumulation levels of glycine betaine, which is consistent with a mechanism that involves a specific interaction of internal proline with the QacT system. To test this hypothesis further, the effects of internal proline on the kinetic parameters of glycine betaine uptake under high- and low-osmolality assay conditions in cells that were grown on CDM with or without proline were studied. For cells grown in low-osmolality media, the Vmax of uptake under low-osmolality assay conditions was about fourfold lower when proline was a component of the growth medium, whereas these differences were much smaller under high-osmolality assay conditions, i.e., upon osmotic upshock (Table 2, lines 1 and 2). This indicates that internal proline, and most likely glycine betaine as well, inhibits QacT, an inhibition that is largely relieved upon osmotic upshock. Similar observations were made for cells grown in high-osmolality media, although the increase in Vmax may not entirely be explained by the relief of trans inhibition by internal proline upon osmotic upshock (compare lines 5 and 6 in Table 2). In the mutant DHPR-38.1, exhibiting reduced glycine betaine uptake (see below), the internal proline affected the uptake of glycine betaine in a manner similar to that in the wild type (Table 2, lines 3 and 4).
FIG. 2.
Dependence of glycine betaine uptake on the internal proline concentration. Cells of L. plantarum ATCC 14917 were grown on CDM without proline containing 0.8 M KCl. The cells were washed and resuspended in 50 mM potassium phosphate (pH 6.5) plus 50 μg of chloramphenicol per ml to a final protein concentration of 0.18 mg/ml. After 40 min of preenergization with 10 mM glucose, uptake was initiated at time zero by the addition of 1.3 mM [14C]glycine betaine (no add) or 1.3 mM [14C]glycine betaine plus 1.3 mM unlabeled proline [Pro(0)]. Alternatively, the cells were preenergized for 40 min with 10 mM glucose plus 1.3 mM unlabeled proline, and uptake was initiated at time zero by the addition of 1.3 mM [14C]glycine betaine [Pro(−40)]. The uptake was assayed without (open symbols) or with (closed symbols) 0.8 M KCl (final concentration) added together with the glucose.
From all of these experiments we conclude the following. (i) Glycine betaine and proline are most likely taken up via a single transport system (QacT). (ii) The QacT system is expressed semiconstitutively; neither proline, glycine betaine, nor the osmolality of the medium has a large effect on the expression per se. (iii) Upon osmotic upshift the system is activated through diminished inhibition by trans substrate as well as through a turgor-related increase in the activity. Both effects result in an increase of the Vmax when the medium osmolality is raised. Feedback inhibition of glycine betaine and proline uptake has also been described for Staphylococcus aureus (20, 25). However, from these studies it is not clear whether an osmotic upshift relieves the trans inhibition. Activation of uptake through a relief of trans inhibition has been described for the glycine betaine and carnitine uptake system of Listeria monocytogenes (28).
Isolation and physiological properties of DHP-resistant mutants.
Mutants that are defective in glycine betaine uptake were isolated to test whether we could dissect the glycine betaine uptake activity even more rigorously into one or more systems. In low-osmolality media, the growth of wild-type L. plantarum was severely impaired by the addition of 1 mM DHP to CDM without proline (the specific growth rate [μ] was decreased from 0.17 to 0.04 h−1), whereas the growth of the mutants was not affected (μ of 0.17 h−1) (data not shown). The growth inhibition of wild-type L. plantarum by DHP in low-osmolality media was counteracted by the addition of proline or glycine betaine to the medium, which prevents DHP from entering the cell due to competitive inhibition of DHP uptake. In contrast to the wild-type cells, the mutants did not grow in CDM plus 0.8 M KCl, whereas some growth (μ ∼ 0.02 h−1) was observed when glycine betaine (2.5 mM) was present. In high-osmolality media (CDM plus 0.8 M KCl), wild-type cells grew with a μ of about 0.14 h−1, which increased to 0.17 h−1 when glycine betaine was present. Together, the data clearly show that the DHP-resistant mutants are compromised in their ability to accumulate glycine betaine, which results in an osmosensitive phenotype.
Kinetic characterization of the DHP-resistant mutants.
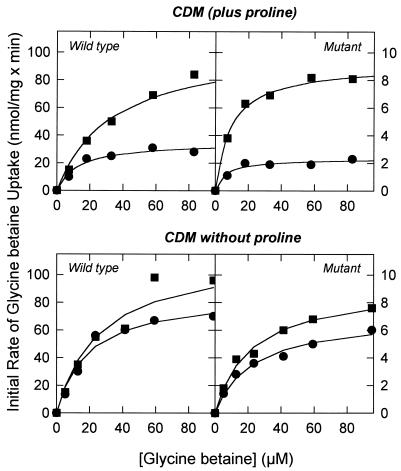
Uptake experiments showed that the mutants were highly defective in their ability to take up glycine betaine under low-osmolality assay conditions, but the system could still be activated upon osmotic upshock (Table 2, lines 3 and 4). In fact, the hyperosmotic activation of glycine betaine uptake by the mutants was comparable to that by the wild type. To establish this point more firmly, we measured the kinetics of uptake in DHPR-38.1 cells grown in CDM with and without proline and under assay conditions of high and low osmolality. As observed for the wild-type cells grown under high- and low-osmolality conditions, an osmotic upshift increased the Vmax three- to fourfold when the cells were grown in the presence of proline, whereas the effect was much smaller for cells lacking proline (Fig. 3; Table 2, lines 3 and 4) (similar results were obtained for the other mutants). Despite the more than 10-fold reduction of Vmax under high-osmolality conditions (Table 2, compare lines 1 and 2 with lines 3 and 4), the mutant cells were still able to accumulate glycine betaine to concentrations of more than 400 nmol/mg of protein, which is only threefold lower than in the wild-type (data not shown). Overall, the results show that glycine betaine and proline uptake is severely impaired in each of the three mutants, most likely as a result of a lowered expression of QacT. Importantly, the residual uptake activity can still be activated upon hyperosmotic shock.
FIG. 3.
Kinetics of glycine betaine uptake in wild-type and DHPR-38.1 L. plantarum ATCC 14917. The cells were grown on CDM or CDM without proline, washed, and resuspended in 50 mM potassium phosphate (pH 6.5). After 7 min of preenergization with 10 mM glucose, uptake was initiated by the addition of [14C]glycine betaine with (squares) or without (circles) 0.8 M KCl. Further details are described in the footnote to Table 2.
The DHPR mutants behaved similarly to the wild type with regard to the rapid and slow components of efflux when an osmotic downshock was applied (data not shown; see also reference 4 and the next section). The uptake system for glycine betaine (QacT), which is significantly hampered in the mutants (lower Vmax and unaltered Km), is thus not involved in the efflux of glycine betaine upon osmotic downshock. This clearly establishes the independence of QacT and the efflux systems.
Effect of membrane strain on the uptake and efflux of glycine betaine.
Previously, we have shown that L. plantarum releases glycine betaine and other compatible solutes in response to an osmotic downshock (3, 4). Under these conditions, the uptake of glycine betaine is virtually completely inhibited. The observed rates of efflux upon osmotic downshock are far higher than the highest rates of uptake, implying that the net efflux upon osmotic downshock cannot be explained by the diminished uptake. The net efflux is largely due to an increased exit of glycine betaine via a system with properties resembling those of mechanosensitive ion channels (19, 27). Efflux of glycine betaine and other compounds in response to a hypo-osmotic shock has also been observed in E. coli, S. aureus, C. glutamicum, and several other bacteria (9, 10, 21, 22).
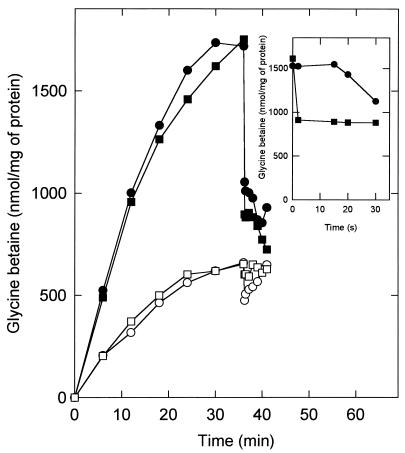
For many of these systems, membrane strain is thought to trigger their activation or to increase the open probability or the duration of the open state in the case of channel proteins. The partitioning of amphipaths into the cytoplasmic membrane also affects the membrane strain. Cationic amphipaths insert into the more negatively charged inner leaflet of the membrane, causing the cells to form cups (convex shapes), whereas anionic amphipaths insert into the less negatively charged outer leaflet, forming concave shapes (23, 24). Chlorpromazine is an cationic amphipath that increases the open probability of mechanosensitive ion channels in E. coli (14), triggers glutamate excretion in C. glutamicum (11), and stimulates the activity of the KdpD protein (26). The addition of 0.1 mM chlorpromazine to cells of L. plantarum which had accumulated glycine betaine under high-osmolality conditions resulted in a rapid efflux of glycine betaine that mimics the efflux elicited by an osmotic downshock (Fig. 4). An important difference between the effluxes triggered by an osmotic downshock and by chlorpromazine is that the former is instantaneous (within 1 s), whereas a lag time of about 15 s was observed for the efflux triggered by chlorpromazine (Fig. 4, inset). This lag time may reflect the time that chlorpromazine needs to partition into the lipid bilayer. Nevertheless, the observation that rapid glycine betaine exit can be elicited by osmotic downshock as well as by chlorpromazine (an amphipatic compound) is consistent with the idea that the system is directly regulated via membrane stretch or tension. In contrast, under low-osmolality conditions, chlorpromazine elicited only a small, transient exit of glycine betaine (Fig. 4). Thus, membrane strain evoked by chlorpromazine alone is not sufficient to activate the efflux system(s) for long periods of time; rather, a high intracellular osmolality is required in order to observe the effect of chlorpromazine.
FIG. 4.
Hypo-osmotic shock and chlorpromazine trigger efflux of [14C]glycine betaine. Cells of L. plantarum ATCC 14917 were grown on CDM containing 0.8 M KCl, washed, and resuspended in 50 mM potassium phosphate (pH 6.5). The concentrated cell suspensions were diluted in 50 mM potassium phosphate (pH 6.5) to a final protein concentration of 0.39 mg/ml. After 6 min of preenergization with 10 mM glucose, uptake was initiated at time zero by the addition of [14C]glycine betaine (final concentration of 1.3 mM) without (open symbols) or with (closed symbols) 0.8 M KCl (final concentration). After 36.5 min, 0.1 mM chlorpromazine (final concentration) was added (circles), or the samples were diluted fivefold with 50 mM potassium phosphate (pH 6.5) containing 10 mM glucose plus 1.3 mM [14C]glycine betaine (squares). The inset shows the lag time of the efflux triggered by the addition of 0.1 mM chlorpromazine (circles); the efflux after hypo-osmotic shock (squares) is instantaneous, since 2 s is the time resolution of the experiment.
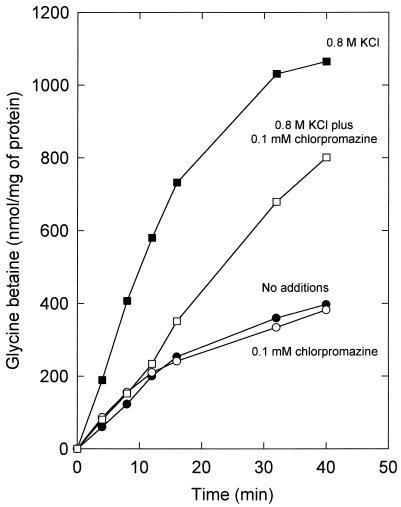
As reasoned for the efflux upon osmotic downshock (see the beginning of this section), the very rapid net efflux of glycine betaine that is triggered by chlorpromazine cannot be due solely to an inhibition of uptake. Nevertheless, it was important to establish whether chlorpromazine affected the uptake under osmostatic and hyperosmotic conditions. Chlorpromazine had no effect on the uptake under osmostatic conditions, but in the presence of the amphipath the rates of uptake no longer increased upon osmotic upshock (Fig. 5). The final level of accumulation of glycine betaine under hyperosmotic conditions was not significantly affected by chlorpromazine, suggesting that the system is still responding to the lowered turgor pressure. Thus, hyperosmotic conditions are sensed in the presence of chlorpromazine, and as a result, the lowered rate of uptake does not level off as quickly as it does under osmostatic conditions (Fig. 5).
FIG. 5.
Effect of chlorpromazine on the uptake of [14C]glycine betaine. Cells of L. plantarum ATCC 14917 were grown on CDM containing 0.8 M KCl, washed, and resuspended in 50 mM potassium phosphate (pH 6.5). The concentrated cell suspensions were diluted in 50 mM potassium phosphate (pH 6.5) to a final protein concentration of 0.17 mg/ml. After 6 min of preenergization with 10 mM glucose, uptake was initiated at time zero by the addition of [14C]glycine betaine (final concentration of 1.3 mM) without (closed symbols) or with (open symbols) 0.1 mM chlorpromazine (final concentration). The uptake was assayed without (circles) or with (squares) 0.8 M KCl (final concentration) added together with the [14C]glycine betaine.
Concluding remarks.
L. plantarum possesses a transport system (QacT) that is regulated by turgor and has a broad specificity for a wide range of compatible solutes. The phenotypes of three DHP-resistant mutants were identical in terms of kinetics of uptake of glycine betaine and proline under osmostatic and hyperosmotic conditions. It is concluded that the expression of the QacT system is decreased in the mutants, thereby preventing the cells from accumulating DHP to toxic levels. Neither the kinetic analysis of uptake in the wild type nor the properties of the mutants provide any indication for more than one major uptake system for quaternary ammonium compounds and proline. If more than one transporter is operative, then all of the systems should have very similar kinetic and regulatory properties. For instance, if the basal uptake (osmostatic conditions) and the activated uptake (after osmotic upshift) were mediated by separate systems, the relative contributions of these activities to the overall flow would be different in the wild type and DHPR mutants.
The mutant analysis clearly shows that the QacT system is also distinct from the osmoregulated systems that mediate the efflux of compatible solutes. The QacT system and the putative channel protein not only are affected in opposing manners by osmolality changes in the medium but also are affected differently by the cationic amphipath chlorpromazine. Chlorpromazine mimics an osmotic downshock in terms of evoking glycine betaine efflux, which is most likely due to an increase in the open probability or the duration of the open state of the putative channel protein in response to membrane strain. The data for the regulation of QacT are more ambiguous but indicate that membrane strain is not directly involved in the regulation of QacT. Finally, kinetic analysis of QacT activity reveals a dual mode of regulation, since a hyperosmotic shock seems to affect the Vmax through a diminishing of the trans inhibition as well as through an effect that is independent of internal substrate.
ACKNOWLEDGMENTS
This research was funded by Unilever Research Laboratories, Vlaardingen, The Netherlands.
We thank P. F. ter Steeg and J. P. P. M. Smelt for stimulating discussions.
REFERENCES
- 1.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faatz E, Middendorf A, Bremer E. Cloned structural genes for the osmotically regulated binding-protein-dependent glycine betaine transport system (ProU) of Escherichia coli K-12. Mol Microbiol. 1988;2:265–279. doi: 10.1111/j.1365-2958.1988.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 3.Glaasker E, Konings W N, Poolman B. Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J Bacteriol. 1996;178:575–582. doi: 10.1128/jb.178.3.575-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glaasker E, Konings W N, Poolman B. Glycine-betaine fluxes in Lactobacillus plantarum during osmostasis and hyper- and hypoosmotic shock. J Biol Chem. 1996;271:10060–10065. doi: 10.1074/jbc.271.17.10060. [DOI] [PubMed] [Google Scholar]
- 5.Gouesbet G, Trautwetter A, Bonassie S, Wu L F, Blanco C. Characterization of the Erwinia chrysanthemi osmoprotectant transporter gene ousA. J Bacteriol. 1996;178:447–455. doi: 10.1128/jb.178.2.447-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappes R M, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempf B, Bremer E. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J Biol Chem. 1995;270:16701–16713. doi: 10.1074/jbc.270.28.16701. [DOI] [PubMed] [Google Scholar]
- 8.Kets E P W, Galinski E A, de Bont J A M. Carnitine: a novel compatible in Lactobacillus plantarum. Arch Microbiol. 1994;162:243–248. [Google Scholar]
- 9.Koo S-P, Higgins C F, Booth I R. Regulation of compatible solute accumulation in Salmonella typhimurium: evidence for a glycine betaine efflux system. J Gen Microbiol. 1991;137:2617–2625. doi: 10.1099/00221287-137-11-2617. [DOI] [PubMed] [Google Scholar]
- 10.Lamark T, Styrvold O B, Strôm A R. Efflux of choline and glycine betaine from osmoregulating cells of Escherichia coli. FEMS Microbiol Lett. 1992;96:149–154. doi: 10.1016/0378-1097(92)90395-5. [DOI] [PubMed] [Google Scholar]
- 11.Lambert C, Erdmann A, Eikmanns M, Krämer R. Triggering glutamate excretion in Corynebacterium glutamicum by modulating the membrane state with local anesthetics and osmotic gradients. Appl Environ Microbiol. 1995;61:4334–4342. doi: 10.1128/aem.61.12.4334-4342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Lucht J M, Bremer E. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Rev. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinac B, Adler J, Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 15.Milner J L, McClellan D J, Wood J M. Factors reducing and promoting the effectiveness of proline as an osmoprotectant in Escherichia coli K12. J Gen Microbiol. 1987;133:1851–1860. doi: 10.1099/00221287-133-7-1851. [DOI] [PubMed] [Google Scholar]
- 16.Molenaar D, Hagting A, Alkema H, Driessen A J M, Konings W N. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patchett R A, Kelly A F, Kroll R G. Effect of sodium chloride on the intracellular solute pools of Listeria monocytogenes. Appl Environ Microbiol. 1992;58:3959–3963. doi: 10.1128/aem.58.12.3959-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peter H, Burkovski A, Krämer R. Osmo-sensing by N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum. J Biol Chem. 1998;273:2567–2574. doi: 10.1074/jbc.273.5.2567. [DOI] [PubMed] [Google Scholar]
- 19.Poolman B, Glaasker E. Regulation of compatible solute accumulation in bacteria. Mol Microbiol. 1998;29:397–407. doi: 10.1046/j.1365-2958.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 20.Pourkomalian B, Booth I R. Glycine betaine transport by Staphylococcus aureus: evidence for feed back regulation of the activity of two transport systems. Microbiology. 1994;140:3131–3138. doi: 10.1099/13500872-140-11-3131. [DOI] [PubMed] [Google Scholar]
- 21.Ruffert S, Lambert C, Peter H, Wendisch V F, Kramer R. Efflux of compatible solutes in Corynebacterium glutamicum mediated by osmoregulated channel activity. Eur J Biochem. 1997;247:572–580. doi: 10.1111/j.1432-1033.1997.00572.x. [DOI] [PubMed] [Google Scholar]
- 22.Schleyer M, Schmid R, Bakker E P. Transient, specific and extremely rapid release of osmolytes from growing cells of Escherichia coli K-12 exposed to hypoosmotic shock. Arch Microbiol. 1993;160:424–431. doi: 10.1007/BF00245302. [DOI] [PubMed] [Google Scholar]
- 23.Sheetz M P, Painter R G, Singer S J. Biological membranes as bilayer couples. III. Compensatory shape changes induced in membranes. J Cell Biol. 1976;70:193–203. doi: 10.1083/jcb.70.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheetz M P, Singer S J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stimeling K W, Graham J E, Kaenjak A, Wilkinson B J. Evidence for feed back (trans) regulation of, and two systems for, glycine betaine transport by Staphylococcus aureus. Microbiology. 1994;140:3139–4144. doi: 10.1099/13500872-140-11-3139. [DOI] [PubMed] [Google Scholar]
- 26.Sugiura A, Hirokawa K, Nakashima K, Mizuno T. Signal-sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Mol Microbiol. 1994;14:929–938. doi: 10.1111/j.1365-2958.1994.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 27.Sukharev S I, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 28.Verheul A, Glaasker E, Poolman B, Abee T. Betaine and l-carnitine transport in response to osmotic signals in Listeria monocytogenes Scott A. J Bacteriol. 1997;179:6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]