Abstract
This case report details the cutaneous findings of a patient with a history of diffuse B-cell lymphoma and SAE-1–positive dermatomyositis who developed an adverse cutaneous reaction after initiation of treatment with hydroxychloroquine. This adds to the sparse literature available detailing the correlation between anti–SAE-1 autoantibodies in dermatomyositis and the unique adverse cutaneous reactions in patients taking hydroxychloroquine. Additionally, our patient developed dermatomyositis years after a diagnosis of lymphoma. This report highlights the utility of the myositis-specific antibody panel to guide diagnosis and management, as well as the potential for developing dermatomyositis years after a lymphoma diagnosis.
Keywords: Adverse cutaneous reactions, B-cell lymphoma, dermatomyositis, hydroxychloroquine
CASE SUMMARY
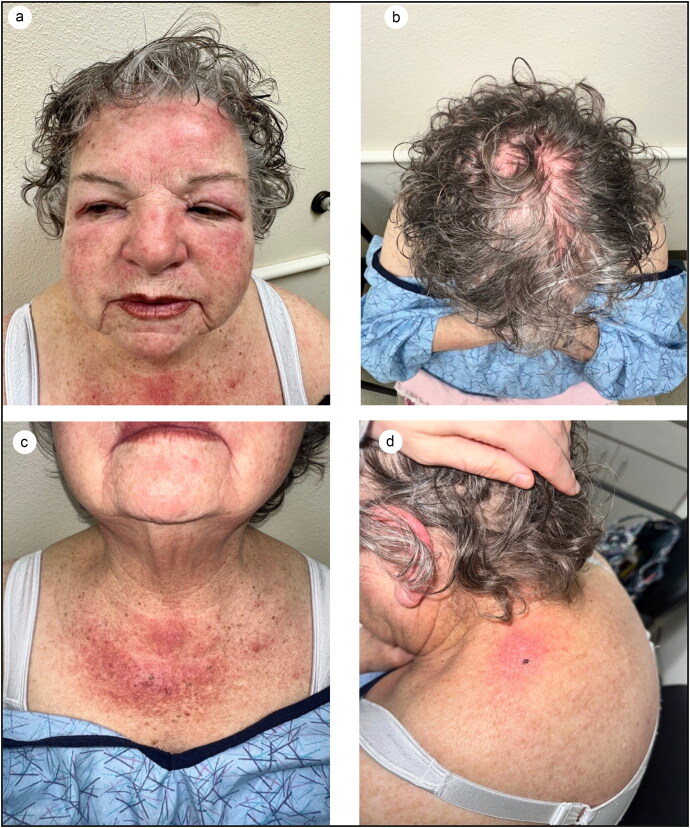
A 75-year-old woman with a history of stage IIA diffuse large B-cell lymphoma (DLBCL) presented to the dermatology clinic with a 5-week history of a rash on her bilateral eyelids with no other associated features. She noted sensitive skin since her lymphoma diagnosis 10 years prior. The patient was initially prescribed tacrolimus 0.1% ointment twice daily for suspected allergic contact dermatitis of the eyelids. Three months later, she returned to the dermatology clinic for the same persistent rash that had spread to the face and chest. She noted associated swelling of her face in the periorbital region and bilateral cheeks, as well as dry mouth and itchy eyes. Thus far, the patient had tried oral antihistamines, a short course of oral prednisone, a 40 mg intramuscular dose of triamcinolone, and a change in medication from lisinopril to losartan to carvedilol. Although the rash improved slightly, the facial swelling persisted. Physical examination revealed mild periorbital edema, impressive bright erythema of the face and scalp, and scaly plaques on the upper chest and shoulders (Figure 1).
Figure 1.
(a) Mild periorbital edema and erythema of the face. (b) Diffuse erythema of the scalp. (c) Scaly erythematous plaques on the chest. (d) Scaly erythematous plaques on the left shoulder (Shawl sign).
Nailfold telangiectasias were present and persistent erythema was noted to the eyelids bilaterally. Two 4-mm punch biopsies were performed on the left upper back and chest. As dermatomyositis was suspected, CK, aldolase, ANA, RF, ESR, CRP, SS-A, SS-B, anti-Jo-1, anti-Smith/RNP, and anti-histone antibodies were ordered, which all returned as negative/normal. Biopsies revealed interface dermatitis, consistent with dermatomyositis. Subsequently, myositis-specific antibody testing was ordered, including anti-SRP, Mi-2, TIF-1-gamma, MDA-5, NXP-2, and SAE-1 antibodies. Anti-SAE-1 autoantibody returned elevated at 98 U. (Anti‐SAE autoantibody status was assigned per the manufacturer’s recommendations, as follows: negative = 0–7 U; borderline = 8–14 U; weak positive = 15–35 U; moderate= 36–70 U; and strong positive = 71–255 U).
After discussion with her oncology team, the patient was started on hydroxychloroquine 200 mg twice a day. Two weeks later, she developed a new rash from head to toe with severe itching, noting that her skin felt “on fire” (Figure 2). Topical corticosteroids and calcineurin inhibitors provided no relief. She denied systemic symptoms including muscle weakness or shortness of breath. Exam revealed diffuse, ill-defined erythematous papules on the scalp, face, trunk, and extremities with a total body surface area of approximately 70%. The rash was attributed to hydroxychloroquine, so the patient was instructed to stop the medication and begin a 1-month taper of oral prednisone and topical corticosteroids. The rate of true adverse drug reactions to hydroxychloroquine is 31% in patients with dermatomyositis.1
Figure 2.
Erythematous eruptions after hydroxychloroquine initiation: (a) papular eruption on the left buttock, (b) papular eruption on the back, and (c) eruption on the face.
Approximately 6 weeks after diagnosis, the patient reported difficulty swallowing that had been ongoing for the past month. Due to her dysphagia, the patient underwent esophagogastroduodenoscopy, which revealed erosive gastritis unrelated to her dermatomyositis diagnosis, as well as a colonoscopy that was unremarkable. A barium swallow was also performed, which showed no aspiration. Additionally, given the association of SAE-1–positive dermatomyositis with interstitial lung disease and dysphagia,2 pulmonary function tests were ordered, which revealed mild ventilatory impairment that did not meet criteria for restriction.
After coordination with her primary care provider, the patient was started on two doses of 1 g of rituximab 2 weeks apart as well as mycophenolate mofetil at 1500 mg orally twice a day per recent dermatomyositis management guidelines3 and a prednisone taper starting at 40 mg, which led to resolution of her rash and dysphagia over a few months.
Clinical questions
-
What is the myositis-specific antibody that may predispose patients to have a greater risk of an adverse cutaneous reaction to hydroxychloroquine?
Anti-MDA
Anti-Tif-1-gamma
Anti-NXP-2
Anti-SAE
-
Which of the following has not been associated with anti-SAE–positive dermatomyositis?
Lung involvement/interstitial lung disease
Heart failure
Dysphagia
Cancer
Joint involvement
Correct answers are given at the end of the article.
Discussion
Dermatomyositis is a multisystem, autoimmune inflammatory disorder with a variable clinical picture. Patients with dermatomyositis commonly present with proximal muscle weakness and pathognomonic cutaneous findings such as the heliotrope sign, Gottron’s papules, shawl sign, and dilated capillary loops of the proximal nail fold. Less commonly, patients may present with a rash without muscle involvement, known as amyopathic dermatomyositis, as seen in our patient. An amyopathic clinical picture can be seen in patients with both anti-SAE and MDA-5 antibodies.4 However, anti-SAE positivity is rare and represents 1.5% to 8% of patients with dermatomyositis.5 Patients with this antibody may also present with lung disease, dysphagia, varying degrees of muscle and joint involvement and, less commonly, cancer.6 In addition to classic skin findings, SAE-antibody–positive patients can also present with a unique dark red rash, as seen in our patient.2 Further complicating the picture is the fact that SAE-1 antibody–positive patients are three times more likely to develop skin eruptions compared to those without the antibody.7 Many studies to date have noted that patients with dermatomyositis experience adverse cutaneous reactions after initiating hydroxychloroquine (an antimalarial agent commonly used in dermatomyositis), including morbilliform drug eruptions, similar to that seen in our patient, as well as hyperpigmentation, pruritus, Steven Johnson syndrome, toxic epidermal necrolysis, and acute generalized erosive pustulosis.8 Certain dermatomyositis-specific autoantibodies like SAE-1 antibody can play a role in the development of these adverse reactions.7 Thus, hydroxychloroquine will be avoided in the future given that mycophenolate mofetil or methotrexate are the preferred therapeutic options in this cohort of patients.3
Another important association with dermatomyositis is malignancy; particularly, SAE-1 antibodies have been shown to be associated with malignancies of the gastrointestinal tract.5,9 Our patient had a history of DLBCL diagnosed 12 years prior and treated with R-CHOP chemotherapy and radiation therapy. She followed up with oncology regularly and had had no recurrence of her disease to date. She recently underwent an esophagogastroduodenoscopy, colonoscopy, and a mammogram, none of which showed any evidence of malignancy. While the association between dermatomyositis and malignancy has been well established, few reports to date have discussed dermatomyositis associated with B-cell lymphoma. A retrospective review of 32 patients found that most patients with polymyositis and dermatomyositis with hematologic malignancy had B-cell lymphoma, with many of these malignancies paralleling their disease course or presenting soon after diagnosis.10 This is in contrast to our patient whose diagnosis of DLBCL preceded the onset of dermatomyositis by over a decade.
In conclusion, our patient presented with some classic cutaneous findings of dermatomyositis along with unique characteristics that are in line with her antibody profile. Myositis-specific antibody testing should be considered in newly diagnosed dermatomyositis patients, as the results can provide prognostic value and dictate management.
Answers to Clinical Questions
Question 1, d. Many studies have noted that patients with dermatomyositis experience adverse cutaneous reactions after initiating hydroxychloroquine. These reactions include morbilliform drug eruptions, similar to that seen in our patient, as well as hyperpigmentation, pruritus, Steven Johnson syndrome, toxic epidermal necrolysis, and acute generalized erosive pustulosis. The presence of the dermatomyositis-specific autoantibody SAE-1 antibody can play a role in the development of an adverse cutaneous reaction to hydroxychloroquine in these patients.
Question 2, b. The presence of anti-SAE antibodies in patients with dermatomyositis may help clinicians better predict the clinical course and manage patients in a timely manner. Studies to date have shown that anti-SAE positivity correlates with both cutaneous and extracutaneous manifestations, including rash, lung involvement (commonly interstitial lung disease), dysphagia, cancer, and joint involvement. Heart failure is not a clinical feature associated with the presence of the anti-SAE antibody in patients with dermatomyositis.
Conflict of Interest
The authors report no funding or conflicts of interest.
Disclosure statement
Permission was obtained from the patient for publication of this case and use of clinical photos, including those of her face.
References
- 1.Pelle MT, Callen JP.. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol. 2002;138(9):1231–1233; discussion 1233. doi: 10.1001/archderm.138.9.1231. [DOI] [PubMed] [Google Scholar]
- 2.Ge Y, Lu X, Shu X, Peng Q, Wang G.. Clinical characteristics of anti-SAE antibodies in Chinese patients with dermatomyositis in comparison with different patient cohorts. Sci Rep. 2017;7(1):188. doi: 10.1038/s41598-017-00240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldman R, DeWane ME, Lu J.. Dermatomyositis: diagnosis and treatment. J Am Acad Dermatol. 2020;82(2):283–296. doi: 10.1016/j.jaad.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 4.Okiyama N, Fujimoto M.. Cutaneous manifestations of dermatomyositis characterized by myositis-specific autoantibodies. F1000Res. 2019;8:1951. doi: 10.12688/f1000research.20646.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia E, Wei J, Geng H, et al. Diffuse pruritic erythema as a clinical manifestation in anti-SAE antibody-associated dermatomyositis: a case report and literature review. Clin Rheumatol. 2019;38(8):2189–2193. doi: 10.1007/s10067-019-04562-w. [DOI] [PubMed] [Google Scholar]
- 6.Albayda J, Mecoli C, Casciola-Rosen L, et al. A North American cohort of anti-SAE dermatomyositis: clinical phenotype, testing, and review of cases. ACR Open Rheumatol. 2021;3(5):287–294. doi: 10.1002/acr2.11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolstencroft PW, Casciola-Rosen L, Fiorentino DF.. Association between autoantibody phenotype and cutaneous adverse reactions to hydroxychloroquine in dermatomyositis. JAMA Dermatol. 2018;154(10):1199–1203. doi: 10.1001/jamadermatol.2018.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma AN, Mesinkovska NA, Paravar T.. Characterizing the adverse dermatologic effects of hydroxychloroquine: a systematic review. J Am Acad Dermatol. 2020;83(2):563–578. doi: 10.1016/j.jaad.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Muro Y, Sugiura K, Nara M, Sakamoto I, Suzuki N, Akiyama M.. High incidence of cancer in anti-small ubiquitin-like modifier activating enzyme antibody-positive dermatomyositis. Rheumatology (Oxford). 2015;54(9):1745–1747. doi: 10.1093/rheumatology/kev247. [DOI] [PubMed] [Google Scholar]
- 10.Marie I, Guillevin L, Menard JF, et al. Hematological malignancy associated with polymyositis and dermatomyositis. Autoimmun Rev. 2012;11(9):615–620. doi: 10.1016/j.autrev.2011.10.024. [DOI] [PubMed] [Google Scholar]