Abstract
Despite the considerable advancements in fabricating polymeric-based scaffolds for tissue engineering, the clinical transformation of these scaffolds remained a big challenge because of the difficulty of simulating native organs/tissues’ microenvironment. As a kind of natural tissue-derived biomaterials, decellularized extracellular matrix (dECM)-based scaffolds have gained attention due to their unique biomimetic properties, providing a specific microenvironment suitable for promoting cell proliferation, migration, attachment and regulating differentiation. The medical applications of dECM-based scaffolds have addressed critical challenges, including poor mechanical strength and insufficient stability. For promoting the reconstruction of damaged tissues or organs, different types of dECM-based composite platforms have been designed to mimic tissue microenvironment, including by integrating with natural polymer or/and syntenic polymer or adding bioactive factors. In this review, we summarized the research progress of dECM-based composite scaffolds in regenerative medicine, highlighting the critical challenges and future perspectives related to the medical application of these composite materials.
Keywords: decellularized extracellular matrix, polymer, bioactive factors, composites, tissue engineering
Graphical Abstract
Introduction
The loss of tissue or organ integrity or function induced by trauma, disease, congenital anomalies and aging are common clinical challenges [1, 2]. Although tissue transplantation is a promising strategy for restoring organ function in patients, severe immune rejection, suitable donor shortages and potential disease transmission remain inevitable challenges, limiting their applicability [3, 4]. To address those challenges, new therapeutical approaches are required to restore damaged tissues or/and organs. In addition, recovering their biofunctions should be addressed to meet the growing demands of patients. Biomaterials have acted as optimal biological substitutes to replace or repair damaged human tissue or organs, providing structural support for cell growth, regulating cellular behavior and mimicking the physical or biochemistry properties of natural tissue [5].
To satisfy clinical requirements, various types of biomaterials, such as organic polymers of synthetic and natural origins, have been widely used as implant scaffolds or wound dressing materials in biomedical fields [6–8]. Using those biomaterials suffers from various limitations, including the potential immunogenic response of natural polymers, the lower biological activity of synthetic polymers and the difficulty in processability of metallic materials [9–11]. With the rapid development in scaffold fabrication processes, composite scaffolds with two or more components have achieved a suitable physical or biological property for tissue engineering based on the synergistic effect [12]. Due to the complex tissue microenvironment, the complex ultrastructure and multiple compositions, and the ongoing reorganization characteristic of natural extracellular matrix (ECM), the design of bioactive scaffold and the fabrication of scaffold with suitable microstructure are still challenging in tissue engineering [13, 14].
Since Poel introduced decellularization in 1948, many studies reported different decellularization technologies to obtain the decellularized ECM (dECM) from cells, tissues or organs [15, 16]. In this context, these decellularization technologies are broadly classified into physical, chemical and enzymatic methods. The physical methods include freeze-thawing, electroporation and mechanical agitation-assisted hydrostatic pressure, among others. To this end, the chemical methods are based on the utilization of detergents, acids/bases, hypotonic/hypertonic solutions, organic solvents and enzymic methods based on DNases and RNases, trypsin to remove cells and maintain the ECM in organs or cells [17, 18]. In addition, different types of dECM have been successfully obtained by supercritical method or the integration of physical, chemical and enzymatic methods [19]. After eliminating the cells and immunogenic factors in decellularization protocols, the native ECM’s original architecture and functional components mainly were preserved [20]. Due to retaining bioactive components and signaling cues, dECM-based biomaterials acted as structures for cell growth and adhesion [21, 22]. They could regulate cell behavior, including cell migration, proliferation and differentiation [23]. More importantly, dECM-based biomaterials have been approved by the Food and Drug Administration (FDA) to fabricate medical products for clinical application, widely investigated as new alternatives for regenerative therapy [24, 25].
Furthermore, the dECM solution could be transformed into hydrogel at an appropriate temperature with three-dimensional (3D) network structures, providing a unique and effective microenvironment for the developing cells and surrounding tissue [26, 27]. However, the unsuitable mechanical rigidity and rapid biodegradability still hinder the application of pure dECM [28, 29]. Bioengineered composite scaffolds composed of dECM with different kinds of biomaterials have provided excellent prospects in regenerative medicine and resulted in hopeful achievements of bioengineered constructions for tissue repair, reconstruction and regeneration. Additionally, considering the requirements of damaged tissue repair, bioactive factors have been utilized effectively as new generations of composite scaffolds to enhance biological activities of dECM.
In this article, we summarized the recent progress on dECM-based composite scaffolds for tissue regeneration (Figure 1). Firstly, we summed up the contemporary designs of dECM-based composite materials containing nature and synthetic polymers. Considering the synergism of effect in tissue regeneration, the composite scaffolds of dECM combined with bioactive factors are introduced and discussed. Finally, we highlighted the prospects and challenges of dECM-based composite scaffolds and presented insights for developing those composite materials in regenerative medicine.
Figure 1.
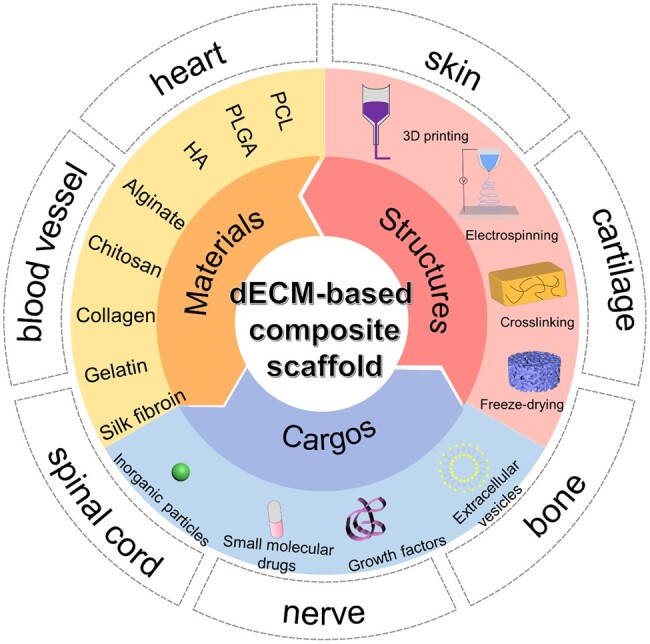
Compositions and applications of dECM-based composite scaffolds for tissue engineering.
dECM–synthetic polymer composite materials
Synthetic polymers with tunable mechanical properties and suitable stability are broadly chosen as fundamental biomaterials for advancing tissue engineering [30]. Moreover, the stable production process, easily reproducibly and ignorable batch difference of those synthetic polymers are sufficient for further clinical and industrial application. Considering the suitable tissue repair requirements, various synthetic polymers have been introduced into prepare dECM-based composite scaffolds with tunable mechanical strength, strong stability in vivo and excellent biomimetic properties for application in tissue engineering. In this section, we reviewed the research efforts on some composite scaffolds composed of dECM and synthetic polymers, such as polylactic acid-co-glycolic acid (PLGA), polycaprolactone (PCL), as well as other polymers in tissue regeneration applications. The fabrication method and the application of dECM–synthetic polymer composite materials are summarized in Table 1.
Table 1.
The fabrication methods and applications of dECM/polymer-based composite scaffolds
| Polymer type | Polymer | dECM type | Fabrication method | Main finding | Reference |
|---|---|---|---|---|---|
| Synthetic polymer | PCL | Rabbit BMSC | Electrospinning | The composite scaffold could stimulate the chondrogenic | [35] |
| PCL | Porcine auricular cartilage | Electrospinning | The composite structure provided a conducive microenvironment for promoting chondrocyte proliferation | [36] | |
| PCL | Human umbilical cords | Electrospinning | The composite nanofibrous membrane achieved tubular tracheal cartilage reconstruction in a rabbit model | [37] | |
| PCL | Porcine femoral | Electrospinning | The composite scaffold could promote bone regeneration by increasing osteogenic-related gene expressions | [38] | |
| PCL | Porcine periosteum | Electrospinning | The core–shell electrospun membrane showed tensile strength and long-term durability with excellent osteogenic properties | [39] | |
| PCL | Porcine knee menisci | 3D printing | The cell-free scaffold displayed favorable biomechanical capacities close to the native meniscus | [43] | |
| PLGA | Porcine skeletal muscle | Electrospinning and 3D printing | 3D-printed scaffold promoted cell orientation and induced myotube formation | [45] | |
| PLGA | Porcine dermis | 3D printing | 3D printing bilayer membranous nanofiber inhibited scar hyperplasia by inhibiting collagen fiber deposition and angiogenesis | [46] | |
| PLGA | Human umbilical cord MSC | 3D printing | The 3D-printed scaffold exhibited remarkable bone regeneration capabilities by enhancing the accumulation of M2 macrophages in vivo | [47] | |
| PLGA, PCL | Porcine cartilage | Double emulsion method | The microsphere significantly accelerated chondrogenesis and maintained osteochondral regeneration activity after being incorporated into the filament | [49] | |
| PLGA | Rat thoracic spinal cord | Electrospinning | The implanted scaffold increased the deposition of collagen and promoted neuronal differentiation | [52] | |
| PLLA | Porcine sciatic nerves | Electrospinning | The coating of dECM hydrogel accelerated the nerve tissue recovery | [59] | |
| PLCL | Human adipose tissue | 3D printing | This 3D scaffold provided proper mechanical properties and a suitable microenvironment for adipose tissue formation | [61] | |
| Thiol-Ene PEG | Human placenta | Crosslinking | The composite hydrogel showed tunable storage moduli from 1080 ± 290 to 51 400 ± 200 Pa | [62] | |
| 4-arm-PEG-NHS | Bovine pericardia | Modification | The integrated scaffold showed an anti-adhesion effect after cardiac surgery | [63] | |
| Genipin-terminated 4-arm-PEG | Porcine urinary bladder, heart, liver, pancreas and small intestine | Crosslinking | The urinary bladder-derived dECM showed the lowest gelation time and the highest shear modulus, exhibiting the most elevated burst pressure | [64] | |
| Polyurethane | Porcine spinal cord | Freeze-drying | The scaffold showed peripheral nerve regeneration potential in the nerve-transected injury model | [66] | |
| Polyurethane | Porcine femoral condyles | 3D printing | The hierarchical macro–microporous structure almost filled the defect site in vivo and exhibited excellent integration to cartilage tissue | [67] | |
| Polyurethane, PCL | Porcine lateral and medial menisci | 3D printing | The controllability bioink with durable architectural integrity provided 3D cell-printed meniscus construction to facilitate tissue regeneration | [68] | |
| MAP-PNIPAM | Human adipose | Crosslinking | The adipose-derived stem cells-laden hydrogel showed better therapeutic effects in soft tissue regeneration | [70] | |
| Natural polysaccharides | HA-NB | Porcine skin | Crosslinking | The composite hydrogels exhibited strong adhesion to the skin and accelerated irregular wound healing | [75] |
| HA functionalized with thiol groups | Porcine joint | 3D printing | The layered cartilage scaffold showed bulk compressive and surface mechanics that were similar to the native cartilage | [76] | |
| HA-MA | Porcine corneal stroma | Crosslinking | The hydrogel showed cornea-matching transparency, slow degradation and good mechanical properties that were suitable for cornea regeneration | [77] | |
| HA-ADH, HA-CHO | Porcine fat | Crosslinking | The injectable hydrogel provided a tissue-specific microenvironment for resident adipose stromal cells and adipogenic differentiation | [78] | |
| Sodium alginate, HAT | Bovine aorta | 3D printing | The 3D printed construct presented a significant angiogenesis promotion activity and anti-inflammatory responses | [79] | |
| Alginate | Porcine kidney | Crosslinking | The cell-laden hydrogel showed a potential role in kidney regeneration | [82] | |
| Alginate | Porcine lung | Crosslinking | 3D hybrid scaffold provided a stable environment for achieving the synergistic effect to support neovascularization in airway tissue engineering | [87] | |
| Alginate | Porcine bone | 3D printing | The cell-laden composite microstructure exhibited outstanding osteogenic differentiation activity | [88] | |
| Alginate | Porcine sciatic nerves | Microfluidics | The gradient microtube based on dECM and alginate guided cell migration and axonal outgrowth for sciatic nerve regeneration | [89] | |
| Sodium alginate, gelatin | Porcine coronary artery | Crosslinking | The bilayer hybrid scaffold exhibited pore structure and mechanical properties similar to porcine coronary arteries | [90] | |
| Chitosan | Porcine decellularized nerve tissue | Freeze-drying | The antibacterial scaffold simulated the neural microenvironment for Schwann cells | [95] | |
| Chitosan | Porcine cardiac extracellular | Crosslinking | The injectable scaffold improved the cardiac function of acute and long-term chronic myocardial infarction in vivo | [97] | |
| Chitosan, PCL | Bovine pericardium | Electrospinning | The composite material showed strong mechanical strength and created a suitable microenvironment for cardiovascular tissue regeneration | [99] | |
| Oleoyl chitosan | Porcine cancellous bone segments | Crosslinking | The hybrid hydrogel exhibited excellent antimicrobial potential, stronger mechanical strength and showed superior proliferation/differentiation ability | [100] | |
| Natural proteins | Collagen | Bovine eyeballs | Crosslinking | The composite scaffold exhibited a uniform structure with excellent elastic modulus and tensile strength | [104] |
| Collagen-MA | Porcine ear, joint, meniscus cartilage and bone | Crosslinking | The composite scaffold reconstructed biphasic cartilage–bone biomimetic microenvironment for osteochondral regeneration | [105] | |
| Collagen | Porcine brain | Crosslinking | The hydrogel promoted functional recovery in the spinal cord injury model | [106] | |
| Collagen | Porcine cadaveric join | Crosslinking | dECM solution functionalized scaffold achieved better subchondral bone repair than dECM powder functionalized scaffold | [107] | |
| Collagen | Rabbit’s joint cartilage | Temperature-induced phase separation | The addition of cartilage dECM with different developmental stages in scaffolds resulted in the different chondrogenic inducibilities with BMSC | [108] | |
| Gelatin | Rabbit’s femur | 3D printing | The 3D scaffold enhanced the mechanical strength, degradation rate and was suitable for bone tissue engineering | [117] | |
| Gelatin | Human amniotic membrane | Electrospinning | The nanofiber membrane enhanced mechanical properties, and reduced degradation rate, and showed better functional recovery in peripheral nerve injury repair | [118] | |
| Gelatin, PCL | Porcine knee joint | Electrospinning and freeze-drying | The composite 3D cartilage scaffold with biomimetic microstructure promoted chondrocyte proliferation | [119] | |
| Gelatin, PLGA | Cow scapular cartilage | Electrospinning and 3D printing | 3D-printed fiber-reinforced scaffold enhanced repair articular cartilage effect in rabbits defect model | [120] | |
| Gelatin, HA-NB | Rabbit auricular cartilage | Crosslinking | The photo-crosslinked hydrogel provided a tissue-specific microenvironment for promoting cartilage repair | [121] | |
| Gelatin, chitosan | Porcine skin | Freeze-drying method | The biological functional hybrid scaffold showed excellent antibacterial activity for wound healing | [122] | |
| GelMA | Porcine trabecular | Crosslinking | The scaffold induced human stem cells’ osteogenic differentiation efficiently | [129] | |
| GelMA | Dental pulp | Crosslinking | Human dental pulp stem cells loaded microcarrier exhibited favorable plasticity and biological performances for endodontic regeneration | [131] | |
| GelMA | Human placenta | Crosslinking | The photo-crosslinked hydrogel with outstanding mechanical properties could act as a promising material for skin defect healing | [132] | |
| GelMA | Porcine dental follicles | 3D printing | The fabricated scaffold could carry DFUs to produce periodontal modules for subsequent orthotopic transplantation | [134] | |
| TGUPy | Porcine urinary bladder | Crosslinking | The scaffold showed excellent tissue-adhesive properties, which could be used for abdominal wall defect repair | [135] | |
| o-Nitrobenzene-modified gelatin | Small intestine submucosa | Crosslinking | The composite hydrogel effectively promoted angiogenesis and collagen deposition for wound healing | [136] | |
| Silk fibroin | Rat adipose | Crosslinking | The composite hydrogel showed a similar mechanical property to natural adipose tissue | [146] | |
| Silk fibroin | Porcine knee joint | Customized mold and freeze-drying | The ring-shaped porous scaffold displayed suitable pore size and porosity for cell colonization | [147] | |
| Silk fibroin, PCL | Human umbilical cord MSC | Electrospinning | The extracellular matrix-modified electrospun fibers could promote axon regeneration of Schwann cells | [148] | |
| Silk fibroin, PCL | Human amniotic membrane | Electrospinning | The graft provided a native-like mechanical structure and biocompatible microenvironment for cellular infiltration | [149] |
BMSC, bone mesenchymal stem cells; Collagen-MA, collagen methacryloyl; DFUs, human dental follicle cells; GelMA, gelatin methacryloyl; HA, hyaluronic acid; HA-ADH, hyaluronic acid grafted adipic acid dihydrazide; HA-CHO, hyaluronic acid grafted aldehyde; HA-MA, hyaluronic acid methacryloyl; HA-NB, nitrobenzene-modified hyaluronic acid; HAT, tyramine-modified hyaluronic acid; MAP-PNIPAM, poly(N-isopropyl acrylamide) grafted mussel adhesive protein; MSC, mesenchymal stem cells; PCL, polycaprolactone; PEG, polyethylene glycol; PLCL, poly(l-lactide-co-caprolactone); PLGA, polylactic acid-co-glycolic acid; PLLA, poly-l-lactic acid; TGUPy, 2-ureido-4[1H]-pyrimidinone unit modified with gelatin; Thiol-Ene PEG, thiol terminated 3-mercaptopropionic acid functioned poly(ethylene glycol); 4-arm-PEG-NHS, 4-arm poly(ethylene glycol) succinimidyl glutarate.
Polycaprolactone
PCL is a hydrophobic FDA-approved synthetic polymer widely used in biomedical applications [31]. Considering dECM-based scaffolds possess unsatisfactory mechanical properties and rapid degradation, PCL can overcome those problems and combine fascinating characteristics, including prolonged degradation kinetic, and excellent rheological, and viscoelastic properties [32].
A wide range of PCL and dECM-based scaffolds in different forms have been developed using electrospinning techniques, possessing great potential in tissue engineering [33, 34]. For instance, Xu et al. [35] cultured chondrocytes on the PCL electrospinning fibrous scaffold, and then obtained the dECM–PCL composite scaffold by decellularization treatment. This composite scaffold was a promising candidate for cartilage regeneration by stimulating the chondrogenic. Feng et al. [36] constructed a nanofibrous electrospinning membrane composed of cartilage dECM particles and PCL. Compared with PCL membranes, these hybrid nanofibers with uniform and smooth structures showed excellent mechanical properties and better wettability. The results indicated that the composite structures provided a conducive microenvironment for promoting chondrocyte proliferation. By electrospinning, Xu et al. [37] fabricated three membranes with different dECM and PCL ratios and investigated the physicochemical properties. After optimizing the composition in vivo, the author constructed a 3D trachea-shaped scaffold based on the composite nanofibrous membrane, which achieved tubular tracheal cartilage reconstruction in vitro.
In addition, Dong et al. [38] mixed bone dECM and PCL in hexafluoroisopropanol to get a homogenous solution and then obtained nanofibrous scaffolds by electrospinning. The bone dECM and PCL scaffolds showed the enhancement in regulation the osteogenic differentiation of mesenchymal stem cells (MSCs) over the PCL scaffold alone. Overall, the composite scaffold showed the smallest existing defect area in the rat defect model by increasing osteogenic-related gene expressions. Li et al. [39] synthesized core–shell composites with periosteal dECM–PCL using co-axial electrospinning. Similarly, Deng et al. [40] also designed core–shell structured nanofibers using peripheral nerve dECM modified on the surface of PCL, which could be rolled up into a cylinder-like tube with highly elevated toughness. Compared with PCL-based nanofibers, dECM–PCL-based nanofibers promoted neurite outgrowth, and regulated Schwann cell behaviors. To improve the structural flexibility of PCL scaffolds, Liu et al. [41] added silk fibroin directly with PCL and human amniotic dECM to produce bioactive scaffolds. The composite mesh fabricated by electrospinning showed similar mechanical elongation and physiological stiffness compared with the native abdominal wall. Moreover, the hybrid platform inhibited transforming growth factor-β1 (TGF-β1) and collagen expressions in the abdominal wall defects model.
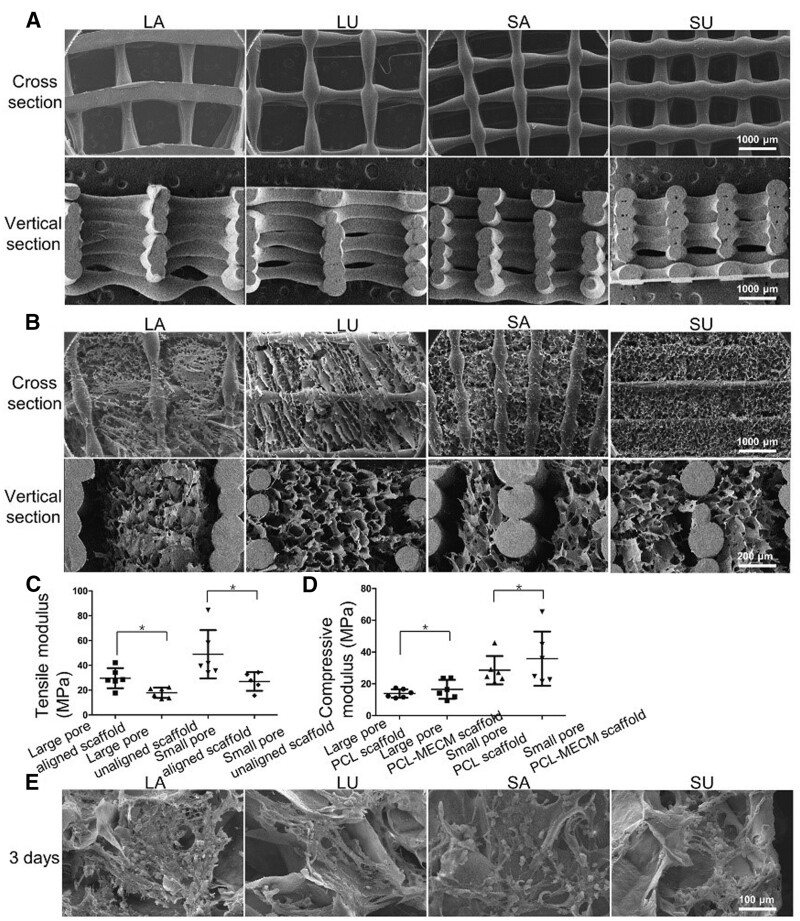
3D printing produced lots of composite scaffolds with customized shapes and structures based on dECM and PCL. Wiggenhauser et al. [42] generated a 3D printable scaffold containing dECM and PCL to enhance the cartilage regenerative capacity. Guo et al. [43] combined PCL scaffolds with meniscal dECM (PCL-MECM) to produce a composite 3D printing scaffold for simulating the tissue microstructure and environment. Moreover, scaffolds with different pores and structures were constructed to assess the chondrogenic differentiation (Figure 2). Compared to small-pore scaffolds, the collagen type II (COL-II) and SOX 9 expression in large-pore scaffolds were higher after cultured with meniscus fibrochondrocytes. In the rabbit and sheep models, PCL-MECM composite scaffolds protected the tibial plateau from osteoarthritis development and promoted functional neo-meniscus regeneration.
Figure 2.
(A, B) SEM images of PCL and PCL-MECM scaffolds. (C, D) Biomechanical assessment. (E) SEM images of meniscal fibrochondrocytes after 3 days on various scaffolds. Adapted with permission from Ref. [43].
Polylactic acid-co-glycolic acid
PLGA is a biodegradable polymer approved by the FDA, which was widely applied to regenerate damaged tissues [44]. Lee et al. [45] utilized an electrospinning technique to obtain dECM nanofibrous and combined it with a microscale 3D-printed PLGA construct to promote cell orientation and induce myotube formation. Fang et al. [46] built a bilayer membranous nanofiber scaffold containing PLGA electrospinning nano nanofiber as the bottom layer and dECM hydrogel as the top layer. This dual-layered scaffold showed a high tensile strength and degradation rate, significantly enhancing the matrix metalloproteinases 1 (MMP-1) and collagen type I (COL-I) expression to inhibit hypertrophic scars formation. Deng et al. [47] fabricated PLGA scaffolds and cultured them with human umbilical cord MSC by 3D printing, and then decellularized by physical methods to achieve the sustainable release of proteins in dECM. The 3D-printed scaffolds exhibited remarkable bone regeneration capabilities in vivo by enhancing the accumulation of M2 macrophages.
Due to the lower degradation rate, dECM-incorporated PLGA-based scaffolds were used to maintain the stability of dECM and achieve sustained release of bioactive compounds [48]. Ghosh et al. [49] loaded cartilage dECM microspheres via double emulsion and then incorporated them in a PCL filament to form 3D scaffolds. The microspheres significantly accelerated chondrogenesis by increasing the COL-II, aggrecan, and SOX 9 gene expressions and maintained osteochondral regeneration activity after being incorporated into the filament. Similarly, Gupta et al. [50] prepared novel microspheres based on PLGA for carrying cartilage dECM and bone dECM, which possessed repair tissue effects in the rabbit osteochondral defects model.
The combination of PLGA and dECM could also improve the mechanical strength of dECM for cell growth and adhesion support. Lih et al. [51] used an ice particle leaching method to prepare a biomimetic scaffold based on kidney dECM incorporated PLGA at different ratios. Enhancing the dECM contents in PLGA scaffolds decreased the compressive moduli and compressive stress, and the composite scaffold with 10% dECM showed a soft-mechanical property for kidney tissue regeneration. In addition, Ma et al. [52] obtained spinal cord dECM and coated it by electrospinning to fabricate a mechanically matched scaffold for neural repair. Compared with the compressive modulus of the spinal cord dECM scaffold of 3.2 ± 0.768 kPa, PLGA coated dECM (PLGA-DSC) scaffold was successfully reinforced 10 times with a compressive modulus of 29.8 ± 5.805 kPa. The PLGA-DSC scaffold possessed brilliant mechanical strength and good cytocompatibility, creating a homeostatic microenvironment for neural stem cell migration, residence and differentiation (Figure 3). Moreover, the scaffold showed translational potential to repair function after spinal cord injury with immunoregulation activity.
Figure 3.
(A, B) Stress–strain curve and compressive modulus values of PLGA-DSC and DSC scaffolds. (C, D) H&E staining images of the SCI area at 7 days after implantation. (E) High-magnification H&E staining images of PLGA-DSC and (F) DSC scaffolds. (G) Immunostaining images of the PLGA-DSC and (H) DSC scaffolds. (I) The quantification of α-SMA staining density. Adapted with permission from Ref. [52].
Other kinds of polymers
Besides the aforementioned synthetic biomaterials, some synthetic biomaterials with superior biocompatibility and excellent mechanical strength (such as poly-l-lactic acid (PLLA), polyethylene glycol (PEG), poly(l-lactide-co-caprolactone) (PLCL), polyurethane) have also been studied for enhancing the dECM biofunction in tissues engineering [53–58].
For instance, Zheng et al. [59] designed PLLA electrospun nanofiber scaffold encapsulated with dECM hydrogel, they analyzed the tissue regeneration effect in rat sciatic nerve defect model, and the post-transplantation results showed that the coating of dECM hydrogel could accelerate nerve tissue recovery than the raw PLLA electrospun scaffold, showing more neurites, and thicker axons thicker myelin. Kim et al. [60] reported PLCL and heart dECM-based electrospun nanofibrous for providing a favorable microenvironment conducive to angiogenesis for wound healing. In addition, Lee et al. [61] designed a 3D scaffold based on PLCL and human adipose dECM for large-volume fat tissue regeneration application via dual-nozzle 3D printing technique. This 3D scaffold provided proper mechanical properties and a suitable microenvironment for enhancing angiogenesis and adipose tissue formation.
Owing to the facile modifiability and high stability, PEG-based hydrogels for loading dECM or PEG functional dECM hydrogels have shown great promise in medical applications. Fan et al. [62] developed a human placenta dECM powder-loaded thiolene PEG-based hydrogel based on UV-induced thiolene click cross-linking reactions. By incorporating dECM at 8 wt%, this composite hydrogel could match the soft tissue moduli, which showed the highest storage moduli at 51 411 ± 199 Pa and exhibited great potential in tissue regeneration. Hashimoto et al. [63] functionalized bovine pericardia dECM with four-arm PEG-NHS due to the high excluded interactions with protein. This integrated dECM had flexibility properties and sufficient suturable strength and showed an anti-adhesion effect after cardiac surgery. In another work, Nishiguchi et al. [64] synthesized genipin modified with four-arm PEG as a crosslinker to design a dECM-based tissue adhesive. This novel crosslinker was used to combinate with different kinds of dECM hydrogel. The urinary bladder-derived dECM showed the lowest gelation time and the highest shear modulus, exhibiting the most elevated burst pressure.
Polyurethanes is a promising biomaterial for medical applications such as medical device and implantation because of their high mechanical strength, elasticity, fatigue resistance and durability [65]. Wang et al. [66] employed polyurethane and spinal cord dECM to build 3D scaffolds, which showed peripheral nerve regeneration potential in the nerve-transected injury model. Chen et al. [67] constructed a waterborne polyurethane and cartilage dECM 3D hybrid scaffold with a hierarchical macro–microporous structure. The addition of dECM not only significantly enhanced the porosity of this scaffold but also improved the expression of COL 2A1, SOX 9 and ACAN was significantly increased after being cultured with adipose-derived stem cells at 14 days than polyurethane scaffold. After subcutaneous implantation with cartilage defect in a rabbit model for 3 months, the polyurethane–dECM scaffold almost filled the defect site in vivo, it exhibited excellent integration with surrounding cartilage tissue. In addition, Chae et al. obtained different kinds of dECM bioink for cell-loading and printed on the polyurethane and PCL frame. This composite scaffold with excellent mechanical properties provided an appropriate biochemical microenvironment for regulating cell behavior [68, 69]. Jeon et al. [70] designed poly(N-isopropyl acrylamide) grafted mussel adhesive protein (MAP-PNIPAM) and incorporated it with adipose dECM powder to form hydrogel network. Moreover, the hydrogel with injectable and tissue adhesive activity could promote the integration of adipose-derived stem cells in soft tissue defect sites for long term.
dECM–natural polymer composite materials
In contrast to synthetic polymers, natural polymers are regarded as effective biomaterials to promote tissue repair with higher biocompatibility, sufficient biodegradability and lower toxicity. Considering the exciting results from dECM-based scaffolds, natural polymers were used to obtain new-generation functional composite scaffolds by combining with dECM for tissue regeneration. Therefore, the recent developments of composite scaffolds formed by the combination of natural polysaccharides (alginate, hyaluronic acid (HA) and chitosan) and natural proteins (collagen, gelatin and silk fibroin) with dECM will be discussed. The applications of dECM–natural polymer composite materials are also summarized in Table 1.
Natural polysaccharides
HA derivatives
HA is a natural hydrophilic polysaccharide, an important ECM component around cells and tissue [71]. Due to the essential biofunction in the tissue regeneration process, HA has been widely studied as a component of scaffold, such as hydrogels, sponges and films. Although HA could not form hydrogels alone, HA derivatives based on chemical reactions have been introduced in HA-based hydrogel to achieve a synergistic effect with dECM [72–74].
Among the crosslinking methods, photo-crosslinking is a highly versatile method that could produce hydrogel by controlling crosslinking time and strength using light. Bo et al. [75] reported a photo-crosslinking hydrogel composed of nitrobenzene-modified HA (HA-NB) and dECM. Human adipose-derived MSCs were loaded into the composite hydrogel system, exhibiting more satisfactory therapeutic effects than other groups in irregular wounds. Barthold et al. [76] functionalized HA with thiol groups to form a disulfide bond-related hydrogel network with dECM particles and used it as a bioink to construct a 3D hydrogel with high biocompatibility. In another study, Shen et al. [77] reported a dual-crosslinked hybrid hydrogel by using methacrylated HA (HA-MA) and corneal stroma dECM (Figure 4). The dual-crosslinked composite hydrogel exhibited not only high transparency with a stable structure but also showed excellent force-resistance and slow degradation under enzyme environment. In vitro results showed that dual-crosslinked hydrogel significantly enhanced keratocan and lumican expressions and decreased the expression of myofibroblast-related genes compared to tissue culture plates. Moreover, the implantation of HA-MA/dECM scaffolds in corneal defect model greatly reduced corneal scarring and promoted the stromal wound healing process, providing great potential for long-term corneal tissue regeneration.
Figure 4.
Schematic illustrations and results of the hybrid hydrogel applied onto corneal defect in situ for long-term regenerative repair. Adapted with permission from Ref. [77].
Lin et al. [78] used HA-aldehyde (HA-CHO) and adipic acid dihydrazide grafted HA (HA-ADH) as raw materials to incorporate fat dECM. This injectable composite hydrogel provided a better microenvironment for loading adipose stromal cells and including adipogenic differentiation. Isik et al. [79] constructed perfusable dECM-loaded hydrogel with high adhesion, self-healing properties and enhanced bioactivity, which were formed by the fast gelation of sodium alginate with tyramine-modified HA. By controlling the composition ratios of hydrogel and gelation induce factors, a 3D scaffold with impressive mechanical robustness and elasticity for cell growth. Furthermore, the printed construct presented a significant angiogenesis promotion activity and anti-inflammatory responses, evidencing the potential in cardiovascular surgery application.
Alginate
Alginate, a naturally available polysaccharide obtained from seaweeds, provides remarkable biocompatible and biodegradable characteristics, particularly interesting for biomedical applications [80, 81]. Alginate-based materials with different forms have been utilized with dECM to mimic the tissue microenvironment [82–84]. Curley et al. [85] improved the mechanical properties of dECM hydrogel by mixing alginate and heart dECM, which showed storage modulus of 1.6 kPa and compressive modulus of 29 kPa. Park et al. [86] optimized the bioink composition based on alginate and heart dECM for cell bioprinting, which provided a cell-friendly microenvironment for cell growth and maturation promotion.
Due to alginate properties, De Santis et al. [87] designed alginate and dECM-based bioink to build hybrid human airways tissue engineering scaffolds by 3D printing. In vivo results demonstrated that this hybrid scaffold provided a stable environment to achieve the synergistic effect for supporting neovascularization with a lower inflammatory response. In another work, Lee et al. [88] used methacrylate-modified dECM (dECM-MA) and mixed it with sodium alginate as bioink to 3D scaffold fabrication, which induced excellent osteogenic activities in vivo. Additionally, Jin et al. [89] generated a microtube containing sciatic nerve dECM and alginate using microfluidic technology via polyvinyl alcohol solution as sacrificing core. The microtube with a homogeneous core–shell structure and controllable gradient encapsulation was successfully obtained by controlling the component and flow ratio of the fluid. Moreover, this composite microtube enhanced the Schwann cell proliferation and migration for nerve repair and also inhibited muscular atrophy in vitro. Similarly, Du et al. [90] reported a bilayer hybrid scaffold that made of alginate and gelatin mixed hydrogel coated on the surface of coronary artery dECM. The core–shell scaffold possessed a pore structure and showed similar mechanical properties to native porcine coronary arteries, which provided a suitable environment for cell proliferation.
Chitosan
Chitosan is an abundant natural polysaccharide obtained from chitin, widely exists in crustacean shells [91]. Owing to the advantages, such as antibacterial, biodegradable, anti-inflammatory and biocompatible, the chitosan-based scaffolds have broad medical applications in tissue regeneration and repair [92–94]. Kong et al. [95] constructed porous nerve dECM–chitosan scaffolds by freeze-drying technique, exhibiting excellent antibacterial activity, and simulated the neural microenvironment for Schwann cells. Similarly, Ye et al. [96] designed a dECM-covered chitosan globule for bone regeneration. In another study, Efraim et al. [97] prepared a dECM injectable scaffold with different amounts of chitosan for acute myocardial infarction treatment, which provided mechanical support during cardiac tissue repair. The hemodynamics results showed that this composite scaffold could improve cardiac function and alleviate damage and myocardial infarction after treatment.
Combining chitosan with other polymer or chitosan derivative-based scaffolds, such as hydrogels, membranes and sponges, has attracted significant attention for loading dECM [98, 99]. For instance, Datta et al. [100] prepared a novel biohybrid hydrogel using fatty acid-modified chitosan and bone dECM to deliver human amnion-derived MSCs. The composite hydrogel demonstrated high mechanical strength, excellent antimicrobial potential and superior cell proliferation activity. Furthermore, the hydrogels significantly improved the osteogenic-related gene expressions, including COL-I, osteocalcin (OCN) and osteopontin (OPN). The cell-laden hydrogel positively impacted newly formed bone tissues with surrounding tissues at the defect site. Chu et al. [101] prepared chitosan and gelatin-based composites spongy scaffold with various compositions and then modified with liver dECM. The optimized spongy scaffold demonstrated a great blood absorption rate and fast blood clotting, demonstrating good application potential as potential hemostatic material.
Natural proteins
Collagen
Collagen is the most abundant protein in vertebrate animals and a prevalent component found in ECM [102]. Moreover, collagen could promote cell adhesion and migration, as well as regulate cellular growth, which plays a dominant role in maintaining ECM homeostasis [103]. Due to its abundance source and facility of collagen-based scaffold, the combination with dECM and collagen composite scaffold also attracted lots of attention from researchers. For instance, Hong et al. [104] mixed compressed collagen with cornea dECM to construct a dense collagenous scaffold for mimicking biomimetic corneal stroma analog. Due to the reduction of fibril diameter, the addition of cornea dECM significantly improved the optical transparency of the collagen scaffold and maintained the native keratocyte morphology and functions. Hua et al. prepared a photo-cross-linkable cartilage–bone integrated hydrogel using methacryloyl derivative (collagen-MA) and cartilage dECM-MA hydrogel. Then, they incorporated bone dECM particles to effectively promote osteochondral regeneration [105]. The bone MSC (BMSC) laden integrated scaffolds that contain bone and cartilage dECM induced chondrogenesis in the early stage and initiated bone regeneration. In vivo studies on rabbits with osteochondral defects demonstrated that this composite scaffold could achieve satisfactory cartilage–bone interface integration.
Researchers also discussed the tissue regeneration effect of collagen-based scaffold combined with dECM at different ratios, tissue types or forms. Hong et al. produced collagen-based hydrogels that contained various concentrations of dECM (1, 5 and 8 mg/ml) [106]. Adding dECM could enhance the cortical neuron's length, whereas the length of hippocampal neurons was improved in dECM concentration at 5 and 8 mg/ml. Lu et al. [107] fabricated collagen scaffolds that functionalized cartilage dECM in solution and particle forms. Compared with dECM particle-functionalized scaffolds, the growth factors were more dispersed in dECM-functionalized scaffolds, thus facilitating the BMSC’ proliferation and chondrogenic differentiation. More importantly, the dECM composite scaffold induced the neocartilage tissue formation on the surface of articular, and the dECM solution functionalized scaffolds achieved neocartilage formation with better structure and smoother surface after implantation. Cao et al. [108] obtained rabbit articular dECM cartilage at 3, 100 and 200 days (Col 3d-S, Col 100d-S and Col 200d-S) and crosslinked with collagen to investigate the chondrogenic inducibilities. The degradation rate result of collagen–cartilage dECM scaffolds indicated the cartilage dECM with higher matured could prolong the degradation time of collagen–cartilage composite scaffold. Moreover, In vivo and vitro results demonstrated that Col 3d-S efficiently accelerated subchondral bone regeneration, while Col 100d-S exhibited exceptional chondrogenic performance (Figure 5).
Figure 5.
(A) The immunohistochemistry staining, (B) the semiquantitative analysis of COL-II after culturing BMSC on various scaffolds. Quantitative determination of (C) GAG and (D) DNA. The chondrogenic genes expression of (E) COL-II, (F) SOX 9, (G) AGG and (H) COL X. Adapted with permission from Ref. [108].
Gelatin
Gelatin is an essential protein obtained from native collagen by hydrolysis, which can be obtained by disrupting the intermolecular bonds and breaking the α-helix conformation of collagen [109, 110]. Compared with collagen, gelatin maintains the RGD (Arg–Gly–Asp) sequence to promote cell adhesion and also shows lower immunogenicity [111]. Due to their biocompatibility and acceptable toxicity, gelatin-based medical products (such as Cutanplast, Surgiflo and Floseal) have been used for hemostasis in surgeries [112]. Moreover, gelatin can act as an excellent candidate to produce biomedical materials, such as hydrogels, scaffolds or films for promoting tissue repair [113, 114]. Thus, gelatin is also commonly used to fabricate hybrid scaffolds with dECM for tissue generation [115, 116]. For instance, Kara et al. [117] fabricated bone dECM particle-reinforced gelatin as bioink to build scaffold for mimicking the bone tissue. Compared with pure gelatin 3D scaffold, this composite scaffold increased the roughness of the scaffold surface and improved the elastic modulus. Chen et al. [118] integrated the gelatin nanofiber membrane into the dECM membrane based on the interfacial covalent bonding to maintain the biological activity of dECM. In vitro results showed that this hybrid membrane enhanced the adhesion and proliferation of Schwann cells and macrophage polarization. Moreover, implanting gelatin/dECM membrane greatly promoted axon regeneration and myelination and improved muscle function in a rat model.
Moreover, some researchers have paid attention to the hybridization of gelatin with other biomaterials to achieve improved mechanical properties or other performances. For example, Li et al. [119] indicated that the gelatin/PCL nanofiber in the dECM scaffold could increase its mechanical properties and structural stability. The results proved that combining electrospun nanofibers and a dECM-based scaffold could promote chondrocyte proliferation and facilitate early maturation of the cartilage. Similarly, Chen et al. [120] prepared electrospinning fiber based on gelatin and PLGA and then mixed it with dECM to form a 3D scaffold. The histological results after implantation for 12 weeks demonstrated that the electrospinning fiber-assisted 3D scaffolds improved the outcomes of cartilage repair in vivo. Xu et al. [121] designed a photo-crosslinked hydrogel system consisting of HA-NB/gelatin/dECM for loading chondrocytes. The composite system showed satisfactory mechanical properties, and the addition of dECM demonstrated enhanced cartilage regeneration activity. Xu et al. [122] added chitosan into dECM/gelatin scaffolds, which exhibited high porosity, appropriate elastic modulus, and degradability for wound healing. Moreover, the chitosan imparted hybrid scaffolds with high antibacterial properties.
Due to the poor mechanical strength and mismatched degradation of the gelatin-based scaffold, the chemical modification of gelatin with methacryloyl (GelMA) has been increasingly developed [123, 124]. GelMA maintained functional sequences of gelatin (Arg–Gly–Asp and the matrix metalloproteinase) and could form hydrogel with tunable physicochemical properties by photo-crosslinking [125]. The GelMA-combined dECM scaffold with different forms has shown extraordinary behaviors in regenerating various tissues [126–128]. For instance, Gao et al. [129] incorporated bone dECM particles into GelMA hydrogel for bone defect reconstruction. With increasing the dECM content, the compressive strength and stiffness of the hydrogel were also improved. He et al. [130] prepared spinal cord dECM and GelMA hydrogel and achieved a synergistic effect with human menstrual blood-derived stem cells for spinal cord injury treatment. Zheng et al. [131] developed GelMA microspheres and conjugated dental pulp dECM to mimic pulp-specific microenvironment (Figure 6). The multifunctional hydrogel microspheres could maintain the activity and ability proliferation of human dental pulp stem cells (hDPSCs). Notably, after cultured the cells in scaffold for 14 days, these composite microspheres significantly enhanced the expression of dentin phosphophorym and dentin matrix protein1 and reduced the runt-related transcription factor 2 in hDPSCs compared with other groups. Moreover, the addition of dECM in this multifunctional hydrogel microspheres also resulted in the odontogenic, angiogenic and neurogenic differentiation of cells. In addition, Zhang et al. [132] designed a visible light cross-linkable hydrogel based on MA-modified human placenta dECM and GelMA for wound healing. Besides enhancing the tensile strength, the cross-linkable hydrogel also possessed water-absorption capacity. Moreover, the hybrid hydrogel could support the proliferation of fibroblasts by simulating the extracellular microenvironment.
Figure 6.
(A) Schematic diagram showing the fabrication of hydrogel microspheres functionalized with dental pulp dECM and their application in regenerative endodontic treatment. (B, C) Dentin phosphophorym immunofluorescence analysis of hDPSCs. (D, E) Odontogenic-related gene mRNA expression levels. Adapted with permission from Ref. [131].
Recently, 3D printing techniques have been introduced to fabricate dECM and GelMA composite hydrogel for mimicking the 3D microstructure in tissue engineering. Chen et al. [133] fabricated GelMA and dECM-based scaffolds to understand the influence of tensile stimulation on chondrocytes. Yang et al. [134] prepared a 3D biomimetic periodontal module that containing GelMA, dECM and human dental follicle cells (DFUs). In vivo results demonstrated that the fabricated 3D biomimetic periodontal module could promote fibrogenesis and osteogenic of DFUs, and could carry DFUs to produce periodontal modules for subsequent orthotopic transplantation. Moreover, the study in periodontal defect of a beagle model confirmed their effectiveness in alveolar bone recovery, which was highly desirable for periodontal regeneration.
To further improve the tissue-adhesive activity, Nishiguchi et al. [135] functionalized gelatin with the 2-ureido-4[1H]-pyrimidinone unit (TGUPy) and mixed it with urinary bladder dECM to obtain a composite patch. Compared to the dECM patches, TGUPy–dECM composite patch enhanced the strain (8-fold) and stress (10-fold) that did not influence the porous structures and swelling behavior, showing an outstanding tissue-adhesive property, which could be used for abdominal wall defect repair. In another work, Wang et al. [136] designed o-nitrobenzene-modified gelatin coated with dECM hydrogel as a multifunctional wound dressing. In vivo experiments of the rat model demonstrated that the composite hydrogel effectively promoted angiogenesis and collagen deposition, indicating its good effect for accelerating epidermal regeneration in the wound healing process.
Silk fibroin
Compared to collagen and gelation-derived biomaterials, silk fibroin purified from silkworm cocoons has achieved superior mechanical performance [137]. In recent years, owing to the excellent biocompatibility, tunable degradability and abundant sources of silk fibroin, silk fibroin-based bio-scaffolds have received considerable attention for tissue regeneration [138, 139].
Based on the unique propriety, the silk fibroin–dECM composite scaffold with different shapes was constructed by previous studies, which provided a new approach to tissue regeneration [140, 141]. Liu et al. [142] cultured cardiac fibroblasts in silk fibroin scaffold and decellularized to obtain the cell dECM-coated scaffold to mimic the myocardial microenvironment. Gholipourmalekabadi et al. [143] designed electrospun nanofibrous silk fibroin that coated with human amniotic dECM membrane to seeded adipose-tissue-derived MSC, which could accelerate wound healing process regeneration and significantly reduces scars formation in burn wound. Dhasmana et al. [144] modified silk fibroin on the acellular dermal matrix scaffold to prolong the degradation time and lower immune response in wound healing.
Zhu et al. [145] fabricated a hybrid nanofiber scaffold silk fibroin and dECM using the electrospinning method for islet transplantation. Kayabolen et al. [146] prepared silk fibroin/dECM matrix hydrogel with different ratios via crosslinking to mimic the native tissue microenvironment. The composite hydrogel with similar mechanical properties to natural adipose tissue promoted the vascularization process for adipose tissue engineering. Gao et al. [147] fabricated silk fibroin and dECM scaffold with a ring-shaped porous structure via freeze-drying method. The hybrid scaffold displayed suitable water absorption, degradation rate and proper mechanical strength, which accelerated the chondrogenesis by enhancing the cartilage-related expression of SOX9, ACAN and COL-II. Moreover, the BMSC-embedded silk fibroin–dECM scaffold demonstrated excellent cartilaginous ring regeneration activity after subcutaneous implantation for 4 weeks, along with comparable mechanical strength and components to the normal trachea in a rabbit model.
In addition, other polymers have been combined with silk fibroin and dECM-based scaffolds for tissue engineering [148]. Typically, Liu et al. [149] constructed human amniotic membrane dECM-based grafts with tubular structures and rolled with PCL/silk fibroin nanofiber to form the luminal surface. The core–shell graft integrating dECM with PCL/silk fibroin exhibited better endothelial cell proliferation, inhibition of collagen secretion and suitable mechanical strength, remodeling the similar native structure over 24 weeks of implantation (Figure 7). In another work, Lee et al. [150] developed a 3D porous scaffold that consisted of collagen, dECM and silk fibroin using low-temperature printing technology. Moreover, the porous composite scaffold showed highly improved compressive modulus for hard tissue regeneration.
Figure 7.
(A) Schematic illustration of a vascular graft implanted into the descending abdominal aortas of Sprague–Dawley rats. (B) Color doppler ultrasound images after implantation. (C) The patency analysis. (D) SEM and CD31 immunofluorescent staining images of the luminal surface of the vascular grafts. Adapted with permission from Ref. [149].
dECM–bioactive factor composite materials
Combining scaffold fabrication technologies, the bioactive factors are incorporated into biomaterials to create a multifunctional cell-supporting structure to show a better tissue repair effect than a single scaffold due to the synergistic effect. A wide range of bioactive factors, such as growth factors, small molecular drugs, inorganic particles and bioactive factors are obtained for cells, have been combined with the dECM scaffold to promote damaged tissue or organ healing. The application in tissue engineering of bioactive factors loaded dECM-based scaffolds are summarized in Table 2.
Table 2.
Bioactive factor loaded dECM-based composite scaffolds for tissue application
| Scaffold | dECM type | Bioactive factor | Applications | Reference |
|---|---|---|---|---|
| dECM | Calf nose | IGF-1 | Cartilage regeneration | [152] |
| dECM | Rabbit skeletal muscle | IGF-1 | Muscle regeneration | [153] |
| dECM | Porcine heart | Stromal cell-derived factor 1 | Myocardial infarction treatment | [154] |
| dECM | Porcine spinal cord | bFGF | Spinal cord injury treatment | [155] |
| dECM | Rat brain | bFGF | Parkinson’s disease treatment | [156] |
| Chitosan, dECM | Rabbit annulus fibrosus | bFGF | Annulus fibrosus tissue engineering | [157] |
| PCL | Rabbit adipose | bFGF | Breast tissue engineering | [158] |
| Aptamer-functionalized GelMA, dECM, dECM | Porcine articular cartilage | TGF-β3 | Cartilage regeneration | [160] |
| dECM | Osteogenic-differentiated human MSC | BMP-2 | Bone regeneration | [161] |
| dECM | Porcine small intestine | BMP-2 | Bone defect regeneration | [162] |
| PEGDA, dECM | Porcine spine | TGF-β1 | Promote annulus fibrosus repair | [163] |
| Silk fibroin, dECM | Goat articular cartilage | TGF-β3 | Cartilage tissue engineering | [164] |
| PCL, GelMA | Sheep knee | TGF-β3 | Cartilage regeneration | [165] |
| Silk fibroin, dECM | Goat cartilage and bone | TGF-β, BMP-2 | Osteochondral repair | [166] |
| PEGDA, dECM | Porcine meniscus | TGF-β1, BMP-7 and IGF-1 | Cartilage regeneration | [167] |
| PCL | Rat abdominal artery | Heparin, VEGF | Vascular grafts fabrication | [168] |
| dECM | Porcine adipose | VEGF | Enhancing angiogenesis | [169] |
| Silk fibroin, dECM | Porcine small intestinal submucosa | VEGF and TGF-β1 | Vascular tissue engineering | [170] |
| HA-MA, dECM | Porcine aortas | VEGF and HGF | Cerebral angiogenesis promotion | [171] |
| dECM | Goat small intestine | Curcumin | Wound healing | [173] |
| dECM, PCL | Porcine skin | Usnic acid | Wound healing | [174] |
| dECM, decellularized kelp | Porcine liver | Coumaric acid | Wound healing | [175] |
| GelMA, dECM | Porcine tendon tissues | Asiaticoside | Wound healing | [177] |
| dECM | Bovine pericardia | Copper@tea polyphenol nanoparticles | Wound healing | [178] |
| dECM | Human adipose | Simvastatin, hydroxyapatite | Bone regeneration | [179] |
| Silk fibroin methacryloyl, dECM | Porcine skeletal muscle | Quercetin | Muscle regeneration | [180] |
| HA, dECM | Porcine stomach | Omeprazole | Repairing gastric ulcer | [181] |
| PLGA, dECM | Porcine small intestinal submucosa | Pifithrin-alpha | Bone regeneration | [182] |
| PGCL | BMSC | Icariin | Bone regeneration | [183] |
| dECM/oleoyl chitosan | Porcine bone | Alendronate and BMP-2 | Bone regeneration | [184] |
| HA-heparin | Human great saphenous vein | Heparin | Vascular grafts | [185] |
| Oxidized chondroitin sulfate | Porcine aortas | Adenine | Vascular regeneration | [186] |
| Alginate, dECM | Porcine aortic tissue | Atorvastatin | Ischemic diseases treatment | [187] |
| PLCL | Porcine thoracic aorta | Salidroside | Vascular regeneration | [189] |
| PCL | Rat aorta | Rapamycin | Vascular regeneration | [188] |
| dECM | Bovine achilles and neck tendons | Hydroxyapatite | Bone regeneration | [193] |
| PCL, dECM | Bovine small intestinal submucosa | Hydroxyapatite | Bone regeneration | [194] |
| PLLA, dECM | Porcine thigh bone | Hydroxyapatite | Bone regeneration | [195] |
| dECM | Porcine small intestine | Sr2+/Fe3+ co-doped hydroxyapatite | Bone regeneration | [196] |
| dECM | Porcine femoral condyle | Graphene oxide | Cartilage regeneration | [198] |
| dECM | Human placental and umbilical cord | Graphene oxide | Vascular graft | [199] |
| dECM | Rat liver | Nano-graphene oxide | Liver regeneration | [200] |
| dECM | Porcine adipose | Reduced graphene oxide | Neural tissue engineering | [201] |
| dECM | Porcine myocardial | Reduced graphene oxide | Heart tissue engineering | [202] |
| PLGA, dECM | Porcine liver | Magnesium-enriched graphene oxide nanoscrolls | Bone regeneration | [203] |
| Chitosan, dECM | Rat skin | Carbon nanodot | Wound healing | [204] |
| dECM | Goat small intestine submucosa | Nanoceria | Skin tissue engineering | [205] |
| dECM | Goat small intestine submucosa | Nanoceria, curcumin | Wound healing | [206] |
| GelMA, dECM | Fish scale | Black phosphorus | Bone defect | [209] |
| PLGA, dECM | Porcine liver | Copper oxide nanozymes | Acute liver failure treatment | [210] |
| dECM | Fish scale | Osteogenic BMSC exosomes | Bone regeneration | [214] |
| dECM | Porcine nucleus pulposus | Adipose-derived MSC exosomes | Intervertebral disc degeneration treatment | [215] |
| Pluronic F127, dECM | Human nucleus pulposus | Human MSC-derived small extracellular vesicles | Intervertebral disc regeneration treatment | [216] |
| GelMA, HA-DA, OHA, dECM | Porcine knee cartilage and cancellous bone | Rat BMSC exosomes | Cartilage and subchondral bone regeneration | [217] |
| Gelatin, quaterinized chitosan, dECM | Porcine skin | Human adipose MSC exosomes, nano-hydroxyapatite | Osteogenesis and vascularity regeneration | [218] |
| dECM | Small intestinal submucosa | Rat BMSC exosomes, mesoporous bioactive glass | Wound healing | [219] |
| HA, dECM | Rat brain tissue | TNF-α/IFN-γ- primed human umbilical cord MSC-derived extracellular vesicles, polydeoxyribonucleotide | Repair of spinal cord injury | [220] |
| PLGA, dECM | Porcine kidney | TNF-α/IFN-γ-primed umbilical cord MSC-derived extracellular vesicles, polydeoxyribonucleotide, magnesium hydroxide | Kidney regeneration | [221] |
bFGF, basic fibroblast growth factor; BMP-2, bone morphogenetic protein-2; BMP-7, bone morphogenetic protein-7; BMSC, bone mesenchymal stem cells; GelMA, gelatin methacryloyl; HA, hyaluronic acid; HA-DA, dopamine-conjugated hyaluronic acid; HA-MA, hyaluronic acid methacryloyl; HGF, hepatocyte growth factor; IFN-γ, interferon-γ; MSC, mesenchymal stem cells; IGF-1, insulin growth factor-1; OHA, oxidative hyaluronic acid; PCL, polycaprolactone; PEGDA, polyethylene glycol diacrylate; PGCL, poly(glycolide-co-caprolactone); PLCL, poly(l-lactide-co-caprolactone); PLGA, polylactic acid-co-glycolic acid; PLLA, poly-l-lactic acid; TGF-β1, transforming growth factor-β1; TGF-β3, transforming growth factor-β3; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factors.
Growth factors
The supplementation of growth factors in the scaffold could promote tissue regeneration by promoting cell proliferation and differentiation [151]. In recent research, different kinds of growth factors, including basic fibroblast growth factor (bFGF), TGF-β superfamily, vascular endothelial growth factors (VEGF) and other types of growth factors were incorporated into dECM-based materials [152–154].
Basic fibroblast growth factor
As the physiological activities in organogenesis and tissue homeostasis, bFGF has been used to combine with dECM to build the composite scaffold [155]. For instance, Lin et al. [156] dispensed bFGF in the dECM hydrogel for central nervous system disease treatment, showing sustained bFGF release behavior in 3 days to enhance the neuroprotective effect. Liu et al. [157] fabricated a dECM and chitosan-based hydrogel with bFGF for supporting annulus fibrosus-derived stem cell growth. Compared with the dECM/chitosan hydrogels, after culture with the hybrid hydrogels that contained bFGF, the expressions of COL-I, COL-II and aggrecan in stem cells were significantly enhanced. In particular, Zhang et al. [158] designed a miniaturized porous PCL chamber by 3D printing and filled it with bFGF-loaded adipose tissue dECM hydrogel. This 3D-printed composite scaffold showed a highly cumulative bFGF release rate in 14 days, supporting the generation of new adipose tissue in vivo. Magnetic resonance imaging and histological results of the chamber revealed that the bFGF-loaded 3D scaffold significantly enhanced the large volume of adipose tissue regeneration and showed adipose regenerative properties for 6 mouths.
TGF-β superfamily
TGF-β superfamily has been widely used in tissue engineering because they could stimulate multiple cell functions, including TGF-β1/2/3 and bone morphogenetic proteins (BMPs) [159]. To this end, several studies applied the TGF-β superfamily to design multifunctional systems combined with dECM [160]. For example, Larochette et al. [161] developed bone morphogenetic protein-2 (BMP-2)-incorporated osteogenic-differentiated MSC-derived dECM scaffolds for ectopic bone formation. Tan et al. [162] impregnated BMP-2 into dECM hydrogel, aiming to achieve bone defects repair in rat model. In vivo results demonstrated that the BMP-2-loaded dECM hydrogel could modulate macrophage polarization and accelerate bone regeneration of promoting angiogenesis and osteogenesis.
In another study, Wei et al. [163] fabricated a multifunctional system made by polyethylene glycol diacrylate (PEGDA) and annulus fibrosus dECM capable of continuously releasing TGF-β1 in 7 days. Compared with other groups, the TGF-β1-loaded injectable hydrogel promoted the migration of annulus fibrosus cells, enhancing ECM deposition to alleviate disc degeneration. Zhang et al. [164] prepared silk fibroin–dECM bioink encapsulated with TGF-β3 by 3D printing for cartilage tissue engineering. The hybrid bioink with suitable mechanical strength for cell growth could control the release of TGF-β3 for chondrogenic differentiation promotion. Similarly, Yang et al. [165] developed PLGA microspheres to control the TGF-β3 releasement and then loaded them with a functional cartilage scaffold based on PCL, dECM and GelMA. In vitro analysis indicated that TGF-β3-loaded microspheres containing bioink improved the MSC migration and chondrogenic differentiation. Furthermore, in a full-thickness articular cartilage defects sheep model, this composite hydrogel showed a well-organized collagen orientation, indicating the cartilage regeneration properties (Figure 8).
Figure 8.
(A) Representative macroscopic images of the repaired tissues. (B) Macroscopic total scores, (C) macroscopic color, (D) defect filling (E) and macroscopic surface at 6 months. (F) Macroscopic total scores, (G) macroscopic color, (H) defect filling and (I) macroscopic surface at 12 months. Adapted with permission from Ref. [165].
In addition, Zhang et al. [166] designed a silk fibroin and dECM composite scaffold as an integrated 3D platform to deliver TGF-β and BMP-2 to achieve osteochondral regeneration. Han et al. [167] incorporated TGF-β1, BMP-2 and insulin growth factor-1 (IGF-1) into PEGDA–dECM composite scaffold using high-precision 3D printing technology. In this platform, the TGF-β1 and BMP-7 loaded hydrogel was acted as the upper layer to reduce cartilage surface friction. In contrast, the downer layer contained TGF-β1 and IGF-1, promoting the production of collagen and proteoglycans. The multiple growth factors loaded hybrid scaffold could successfully guide cartilage regeneration by promoting chondrogenic differentiation and alleviating the inflammatory response.
Vascular endothelial growth factor
VEGF, an essential growth factor regulating neovascularization and angiogenesis, has also been processed into a dECM-based scaffold for tissue regeneration [168]. Liu et al. [169] found that the combination of dECM and VEGF hydrogel could enhance tube formation and accelerate wound healing process. In another case, Liu et al. [170] combined SF membrane encapsulating different growth factors (TGF-β1 and VEGF) with dECM membrane as a composite vascular graft by coaxial aqueous electrospinning. The combination of dual growth factors in one membrane was used for promoting endothelialization and preventing collagen over-deposition, indicating the potential for vascular tissue engineering applications. Hwang et al. [171] designed a new generation of 3D dECM-based scaffolds to support tissue regeneration that explores the incorporation of VEGF and FGF. In detail, the outer layer of the 3D patch was made by VEGF-laden HA-MA and dECM ink, in which the layer contained hepatocyte growth factor (HGF)-laden HA-MA and dECM inks. Due to the sequential and sustained release of VEGF and HGF, this patch significantly promoted angiogenesis and induced neovascularization in the brain after implantation.
Small molecular drugs
By adding small molecular drugs with special functions into scaffolds, the hybrid scaffold can be used to achieve drug release in demand to improve the activities of drugs [172]. Several types of scaffolds composed of dECM and small molecular drugs were recently designed to accelerate tissue repair and regeneration.
Antibacterial drugs
Although dECM-based materials are widely studied as wound dressings, eradicating bacterial infections is crucial in wound healing. Following the previous research, antibacterial agents were loaded onto dECM to fabricate new-generation dressings [173]. For instance, Chandika et al. [174] mixed usnic acid with PCL and dECM to develop novel composite nanofibrous using electrospinning technology. Arin et al. [175] used decellularized kelp as crosslinking material to strengthen the physical structure of liver dECM and then loaded it with p-coumaric acid (EK-20). Compared with the control group, the composite wound dressing achieved superior wound healing in vivo. On the 14th day, the wounds of the EK-20 group with 98.75% of wound closure percentage, while the wounds of the control group showed 26.82% wound closure rate (Figure 9). Additionally, the immuno-histological and biochemical phenotyping of macrophages results concluded that the released p-coumaric acid could regulate the immune response and switch the macrophages from M1 to M2 phenotype.
Figure 9.
(A) Representative optical images of the zone of inhibition against bacteria. (B) Quantitative analysis of zone of inhibition. (C) Confocal images of the rBMSC cells. (D) The rBMSCs viability in different scaffolds. Adapted with permission from Ref. [175].
To achieve sustained drug release, voriconazole-loaded PLGA microspheres were produced by using emulsion evaporation technique before being mixed with dECM and gelatin composite hydrogel [176]. The drug-loaded hybrid hydrogel showed excellent antifungal properties in a rabbit corneal defect model, which could prevent fungal infection and promote corneal regeneration. Similarly, Liu et al. [177] fabricated polydopamine nanoparticles loaded with asiaticoside and mixed with dECM and GelMA composite hydrogel, which has the potential for scarless wound healing application. Li et al. [178] functionalized bovine pericardia dECM scaffold with copper@tea polyphenol self-assembly nanoparticles based on the coordination of tea polyphenol with copper ions. As expected, the scaffold maintained the antimicrobial activity of nanoparticles, which also exhibited anti-calcification, remodeling and integration properties for cardiovascular applications.
Other drugs
Apart from the antibacterial drugs, other drugs, such as anti-resorptive agents and antioxidants, among others, were also combinated with dECM-based scaffolds in biomedical applications, including bone and vascular tissue engineering, among others [179–181]. For example, Xie et al. [182] fabricated the nanofibers composed of dECM and PLGA-loaded pifithrin-α acting as bilayer bone scaffold for enhancing osteoinductivity. Zhou et al. [183] fabricated icariin-loaded poly(glycolide-co-caprolactone) microparticles with suitable porous structures and coated with BMSC-derived dECM. Subsequently, the sustained release of the icariin from the microcarriers showed excellent synergistic effects with dECM in the rat models. In another study, Datta et al. [184] designed alendronate-loaded gelatin microspheres embedded in bone dECM/oleoyl chitosan hydrogel for loading BMP-2. In the rabbit tibial bone defects model, the dual drug-loaded composite hydrogel showed 76% defect closure, whereas the bone dECM/oleoyl chitosan hydrogel had a 56% defect closure rate after 4 weeks of implantation.
Inhibition of thrombus formation and intimal hyperplasia, proangiogenic, have positive impacts on enhancing the performance of vascular grafts in vivo. Thus, different drug agent was incorporated in dECM-based vascular grafts to achieve better performance in tissue regeneration [185, 186]. Gao et al. [187] produced atorvastatin-loaded PLGA microspheres for delivering endothelial progenitor cells and mixed with dECM and alginate-based bioink to construct bio-blood-vessel. The cumulative release of atorvastatin constantly improved the neovascularization of endothelial progenitor cells and avoided the risk of overdose, exhibiting synergistic therapeutic effects on mice’s hind limb ischemia model. Yang et al. [188] fabricated vascular grafts with small diameter that consisted of rapamycin-loaded PCL nanofibrous as an outer layer to coat on the rat aorta dECM layer. The novel hybrid vascular grafts significantly reduced neo-intimal hyperplasia after abdominal aorta transplantation at 12 weeks. Shi et al. [189] developed a tri-layered vascular graft via electrospun by sandwiching media dECM powders loaded in PLCL between outer layers that loaded intima powders and inner layers that salidroside. In this platform, the salidroside of the inner layer inhibited thrombus formation and reduced the adhesion effect of platelets, whereas the dECM powder significantly promoted smooth muscle regeneration and endothelialization.
Inorganic particles
Inorganic particles have shown unique properties in medical applications, which have emerged as one of the probable agents in tissue regeneration [190]. Adding the inorganic particles into biomaterials could remedy the mechanical shortcomings and increase their bioactivity for tissue regeneration [191]. Thus, to integrate both advantages of inorganic particles and dECM, the dECM–inorganic particles composite scaffold with outstanding mechanical properties and multi-functionalities have been developed.
Hydroxyapatite
Hydroxyapatite is widely used as an inorganic material for bone regeneration due to its similar mineral composition to bone. Researchers developed lots of dECM/hydroxyapatite composite scaffolds by different manufacturing processes, achieving a better bone repair effect than using those materials alone [192, 193]. Parmaksiz et al. [194] prepared a multilayer composite scaffold using small intestinal submucosa dECM as layer and PCL-hydroxyapatite microparticles as a binder. By co-culturing with rat bone marrow MSCs, this composite scaffold showed higher osteocalcin and alkaline phosphatase expression, proving the potential for new bone formation. Hwangbo et al. [195] reported a hydroxyapatite-loaded PLLA–dECM bone tissue scaffold by adopting 3D printing technology. By adjusting the weight fraction of hydroxyapatite in scaffold and the particle size of hydroxyapatite, the composite scaffold obtained 96.3–136.8 MPa flexural strengths and flexural moduli around 1.4 GPa. After culturing with human adipose stem cells, Runt-related transcription factor 2 (Runx2) and alkaline phosphatase expressions in the hydroxyapatite loaded PLLA–dECM group were significantly improved than the hydroxyapatite/PLLA scaffold. In addition, Yang et al. [196] fabricated Sr2+/Fe3+ doped hydroxyapatite modified dECM scaffold with a rough surface and suitable mechanical strength using extrusion cryogenic 3D printing technology. The hydroxyapatite modified dECM scaffold provided a desirable microenvironment for bone regeneration, promoting the angiogenesis and osteogenesis properties of cells, enhancing biomineralization ability and manipulating the immunoregulation process.
Graphene-based particles
Based on the unique mechanical and electronic properties, graphene oxide functioned scaffold has attracted increased attention [197, 198]. For example, Pereira et al. [199] coated the graphene oxide on decellularized umbilical cord arteries to enhance mechanical performance. Compared to the dECM scaffold, the maximum force, burst pressure, strain and compliance of graphene oxide modified dECM scaffold significantly enhanced. Likely, Kim et al. [200] crosslinked the dECM scaffold with nano-graphene oxide to alleviate the enzymatic degradation activity and enhance the mechanical rigidity. Integrating nano-graphene oxide within dECM scaffolds has shown high stability after implantation for 60 days, alleviating the graft-elicited inflammation responses by regulating macrophage polarity. More importantly, the bioengineered scaffold showed superior hepatic regeneration activity after implantation in rat acute or chronic liver failure models.
Barroca et al. [201] found that incorporating reduced graphene oxide into adipose dECM scaffolds could induce neuron differentiation. In another work, Tsui et al. [202] developed a reduced graphene oxide-contained dECM scaffold to mimic the cardiac microenvironment. By regulating the reduction degree of reduced graphene oxide and the concentration in the hydrogel, the mechanical properties and conductivity of scaffolds could be tuned. After cultured the cardiomyocytes in this composite hydrogel, the genes associated with regulating contractile function were significantly enhanced, indicating the synergistic effect of dECM and graphene oxide reduced in tissue engineering. Zheng et al. [203] synthesized magnesium-enriched graphene oxide nanoparticles for modulating the inflammatory responses and then combined them with bone dECM scaffold for bone regeneration. Compared to the bone dECM scaffold, the 3 months post-implantation results showed that the magnesium-enriched graphene oxide nanoparticles-based dECM hydrogel showed significantly higher neovascularization lever.
Other inorganic nanoparticles
Combining the outstanding merits of other inorganic nanoparticles (nanoceria, laponite, metal or non-metal particles) with dECM, the hybrid scaffold exhibited excellent performance in tissue regeneration [204]. For example, nanoceria can be used as an antibacterial/antioxidant agent in hydrogel to offer multifunctional properties. Singh et al. [205] found that the activity accelerates tissue regeneration by combining nanoceria and dECM. Furthermore, they functionalized goat small intestine dECM composite scaffolds with nanoceria and nanoemulsion-loaded curcumin [206]. Additionally, the composite scaffold prolonged curcumin release with antibacterial properties and showed excellent free radicals scavenging activity, which significantly enhanced the wound healing rate.
Based on the 2D structure and special composition, Laponite has been used to enhance the mechanical properties and degradation time of dECM in tissue engineering [207]. Shin et al. [208] bioprinted a PEGDA and cardiac dECM hydrogel reinforced with Laponite to model cardiac tissue. In addition, some novel inorganic nanoparticles with extraordinary characteristics were used to enhance the bioactivity of dECM. In recent, Shen et al. [209] designed a black phosphorus nanosheets degradation loaded photothermal scaffold based on fish scale dECM and GelMA to accelerate bone regeneration. Combined with the photothermal therapy, the photothermal scaffold promoted osteocalcin accumulation and facilitated angiogenesis. Jin et al. [210] developed a synergistic platform based on copper oxide nanoparticles-loaded PLGA nanofibers and dECM hydrogel with human adipose-derived MSC. In this system, copper oxide nanoparticles with reactive oxygen species elimination ability significantly promoted cell migration and proliferation. Moreover, the copper oxide nanoparticles loaded in nanofiber-reinforced hydrogel could promote liver regeneration and benefit liver function recovery, which showed outstanding synergistic effects in acute liver failure treatment.
Extracellular vesicles
Extracellular vesicles, a kind of paracrine factor obtained for cells, contain proteins, nucleic acids and growth factors. Due to the biological activities, the bioactive properties of extracellular vesicles have been used to cooperate with scaffolds in tissue regeneration [211–213]. To combine the exosomes and dECM for achieving tissue repair and regeneration, Wang et al. [214] designed fish skin dECM hydrogel to bind to BMSCs, leading to a stronger synergistic effect on promoting bone regeneration. Xing et al. [215] innovatively incorporated adipose-derived MSC-exosomes into dECM hydrogels to modulate the microenvironment of intervertebral disc degeneration. In this system, exosomes could accumulate the ECM in the microenvironment by regulating matrix metalloproteinases and inhibit pyroptosis by decreasing the expression of inflammatory factor. Meanwhile, the dECM-based injectable hydrogel provided a tissue-specific environment for nucleus pulposus cell growth. According to the result of the tail vertebral disc degeneration model, this composite hydrogel exhibited higher levers of aggrecan and collagen than other groups, which provided a promising strategy for intervertebral disc degeneration treatment.
For achieving sustained release of extracellular vesicles, Liao et al. [216] fabricated a thermo-responsive hydrogel that composed of Pluronic F127 and nucleus pulposus dECM for human MSC-derived extracellular vesicle delivery. Additionally, Li et al. [217] designed a 3D double-network hydrogel by using GelMA, oxidative HA, dopamine-conjugated HA, cartilage dECM and bone dECM as raw materials, encapsulating with human adipose MSC exosomes. This composite system showed 80% exosome release efficiency in 24 days. After being implanted in a rat model for 12 weeks, the spatial microenvironment-biomimetic 3D printed scaffolds filled with cartilage-defected tissue and displayed higher trabecular thickness (Figure 10). In addition, in vivo results have shown that his combined 3D hydrogel-exosomes significantly reduced inflammatory reaction by inflammatory factor interleukin-1β expression.
Figure 10.
(A) An overview illustrating 3D-printed different scaffolds implanted in rat knee osteochondral defects. (B) Representative MRI images. (C) Representative 3D reconstructed micro-CT images at defect sites. Quantitative analysis of (D) bone volume/total volume, (E) trabecular number and (F) trabecular thickness for regenerated bone tissues in the defects. Adapted with permission from Ref. [217].
In addition, extracellular vesicles were combined with other bioactive factors in dECM-based scaffold forms, which exerted synergistic effects in tissue engineering [218]. Hu et al. [219] designed a 3D scaffold based on dECM, mesoporous bioactive glass and exosomes by cryogenic printing technology. The exosomes-loaded composite scaffold exhibited hemostasis ability in the liver hemorrhaging model and enabled skin regeneration in 14 days in the diabetic wound healing model. Roh et al. [220] incorporated MSC-derived extracellular vesicles and polydeoxyribonucleotide into HA and dECM-based scaffold, studying its spinal cord regeneration activity. In this system, combining extracellular vesicles and polydeoxyribonucleotide reduced inflammation in the tissue microenvironment during repair and promoted angiogenesis, axonal regrowth and anti-apoptosis of neurons. In vivo study demonstrated that integrating exosomes and polydeoxyribonucleotide conduits was a useful therapeutic intervention in treating acute or chronic spinal cord injury. Likely, Ko et al. [221] integrated the biochemical cues of exosomes and polydeoxyribonucleotide into PLGA-based scaffolds containing magnesium hydroxide and kidney dECM. Moreover, the multifunctional scaffold system provided an optimal microenvironment for kidney regeneration promotion.
Conclusion and outlook
dECM has gained popularity in tissue regeneration applications due to its biochemical and biophysical components from native tissues, which can mimic the complexity of organs and excellent biocompatibility. Moreover, the dECM solution tended to form 3D hydrogel at 37°C based on the thermal crosslinking mechanism, which provided a tissue-specific structure to support cell growth and remodel the tissue microenvironment. Nonetheless, the poor mechanical properties and rapid degradation of pure dECM hydrogel remain an inherent challenge for further application. Fortunately, the multiphasic designs of dECM-based biomaterials, including additive bioactive factors and integration of other biomaterials in a multifunctional system, have enhanced the preclinical tissue regeneration outcomes of dECM. In this article, we summarized the recent researches in designing and fabricating dECM-based composite scaffolds (nature polymers, synthetic polymers, bioactive factors) and their applications in regenerative medicines. Although designing multifunctional dECM-based composite materials is an essential research area in tissue engineering, several challenges remain in building integrated dECM-based composite scaffolds for successful clinical translation.
Along with the charming development of decellularized methods recently, dECM from different organs was obtained by physical, chemical, biological and a combination of these techniques. The agents used in traditional decellularized processes would induce potential toxicity, which may be an essential challenge in the future. Moreover, the unstable dECM yield, unavoidable bioactive factor loss and heterogeneity between different batches still need to be addressed. Due to the lack of standard protocol and infrastructure, the high production cost and the time-consuming dECM production are unavoidable problems faced in the industrial scale-up. Therefore, the researchers should focus on developing novel decellularized methods to rapidly obtain dECM that maintain a high degree of bioactive factors in different tissue/organs.
Despite the outstanding functional reconstruction potential of dECM-based scaffolds, choosing suitable sterilization methods after decellularization is still an existent challenge that hindered the clinical applications of dECM. Recently, researchers demonstrated several sterilization and disinfection methods, such as ultraviolet irradiation, ethylene oxide and supercritical carbon dioxide. However, due to the different compositions and 3D structures of native tissue or organs, those sterilization methods may not be suitable for obtaining different kinds of dECM. Therefore, it is undoubtedly a great research future direction to develop appropriate sterilization and disinfection methods thought over synthetically the properties and structure of various dECMs.
Clinical results on dECM-based commercial products suggested its great potential in tissue regeneration. Thus, the clinic application of dECM-based composite scaffolds is meaningful but challenging. Although emerging research focused on the tissue generation activity of dECM-based composite scaffolds, the long-time biosafety and potential toxicity of these composite scaffolds rarely receive attention. As the degradation products of adding polymers or bioactive factors might influence the tissue regeneration microenvironment, the current preclinical studies are insufficient for supporting the successful clinical application of dECM-based composite scaffolds. As a result, researchers should consider the clinical use and potential toxicity to humans while designing dECM-based composite systems for tissue regeneration.
Despite the significant development, there exist several scientific challenges hindering the clinical application of dECM-based composite scaffolds. The animal trials of dECM-based composite scaffolds have been widely investigated on small animal models, such as mouse or rat models, lacking comprehensive investigations of their therapeutic effects on large animal models. More importantly, even beagle models and goat models showed similar implant sizes to humans, and those models were not suitable for replicating the physiological environment and tissue forces of humans. In addition, the scaling up production of scaffolds remains a challenge for the clinical application of dECM-based composite scaffolds.
An engineered composite scaffold made of dECM or combined with other polymers can be useful for delivering various therapeutic agents to achieve tissue regeneration. However, tissue regeneration is a dynamic and complex process that involves cell recruitment, adhesion, proliferation, differentiation, ECM formation and remodeling. Although single growth factors and multiple growth factors enhanced the biological functions of dECM, the unstable bioactivity and burst release of growth factors still hinder the medicine application. Therefore, the addition of exosome and platelet-rich plasma obtained from the body in a dECM-based scaffold should be considered. Moreover, combining different agents has shown synergistic effects in tissue regeneration promotion. Thus, the fabrication of dECM composite scaffolds with on-demand drug delivery and multifunctional and controllable shapes will become a research hot point in the future.
In conclusion, despite the remaining problems, tissue engineering using dECM-based multiphasic scaffolds is an ideal and effective strategy for tissue defect repair in clinical applications. With the cross-disciplinary development in innovative biofabrication technology, novel materials designing and cell engineering, we believe that dECM-based composite materials would be promising alternatives for achieving defective tissue reconstruction and regeneration.
Contributor Information
Peiyao Xu, Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen, Fujian 361021, PR China; Fujian Provincial Key Laboratory of Biochemical Technology (Huaqiao University), Xiamen, Fujian 361021, PR China.
Ranjith Kumar Kankala, Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen, Fujian 361021, PR China; Fujian Provincial Key Laboratory of Biochemical Technology (Huaqiao University), Xiamen, Fujian 361021, PR China.
Shibin Wang, Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen, Fujian 361021, PR China; Fujian Provincial Key Laboratory of Biochemical Technology (Huaqiao University), Xiamen, Fujian 361021, PR China.
Aizheng Chen, Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen, Fujian 361021, PR China; Fujian Provincial Key Laboratory of Biochemical Technology (Huaqiao University), Xiamen, Fujian 361021, PR China.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC 32271410, 32071323 and 81971734), the Science and Technology Projects in Fujian Province (2022FX1, 2023Y4008), Scientific Research Funds of Huaqiao University (21BS113) and the Open Research Fund of Academy of Advanced Carbon Conversion Technology, Huaqiao University (AACCT0004).
Conflicts of interest statement. The authors declare no competing financial interest.
References
- 1. Fu Q, Xia B, Huang X, Wang FP, Chen ZM, Chen GB.. Pro-angiogenic decellularized vessel matrix gel modified by silk fibroin for rapid vascularization of tissue engineering scaffold. J Biomed Mater Res A 2021;109:1701–13. [DOI] [PubMed] [Google Scholar]
- 2. Peirsman A, Nguyen HT, Van Waeyenberge M, Ceballos C, Bolivar J, Kawakita S, Vanlauwe F, Tirpakova Z, Van Dorpe S, Van Damme L, Mecwan M, Ermis M, Maity S, Mandal K, Herculano R, Depypere B, Budiharto L, Van Vlierberghe S, De Wever O, Blondeel P, Jucaud V, Dokmeci MR, Khademhosseini A.. Vascularized adipose tissue engineering: moving towards soft tissue reconstruction. Biofabrication 2023;15:032003. [DOI] [PubMed] [Google Scholar]
- 3. Nikolopoulos VK, Augustine R, Camci-Unal G.. Harnessing the potential of oxygen-generating materials and their utilization in organ-specific delivery of oxygen. Biomater Sci 2023;11:1567–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu XM, Wu K, Gao L, Wang LP, Shi XT.. Biomaterial strategies for the application of reproductive tissue engineering. Bioact Mater 2022;14:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rai V, Dilisio MF, Dietz NE, Agrawal DK.. Recent strategies in cartilage repair: a systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A 2017;105:2343–54. [DOI] [PubMed] [Google Scholar]
- 6. Guo BL, Ma PX.. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Sci China Chem 2014;57:490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koons GL, Diba M, Mikos AG.. Materials design for bone-tissue engineering. Nat Rev Mater 2020;5:584–603. [Google Scholar]
- 8. Guo LQ, Liang ZH, Yang L, Du WY, Yu T, Tang HY, Li CD, Qiu HB.. The role of natural polymers in bone tissue engineering. J Control Release 2021;338:571–82. [DOI] [PubMed] [Google Scholar]
- 9. Li YL, Rodrigues J, Tomas H.. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev 2012;41:2193–221. [DOI] [PubMed] [Google Scholar]
- 10. Alven S, Peter S, Mbese Z, Aderibigbe BA.. Polymer-based wound dressing materials loaded with bioactive agents: potential materials for the treatment of diabetic wounds. Polymers (Basel) 2022;14:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janani G, Kumar M, Chouhan D, Moses JC, Gangrade A, Bhattacharjee S, Mandal BB.. Insight into silk-based biomaterials: from physicochemical attributes to recent biomedical applications. ACS Appl Bio Mater 2019;2:5460–91. [DOI] [PubMed] [Google Scholar]
- 12. Chen R, Pye JS, Li J, Little CB, Li JJ.. Multiphasic scaffolds for the repair of osteochondral defects: outcomes of preclinical studies. Bioact Mater 2023;27:505–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev 2008;60:184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jana S, Levengood SKL, Zhang MQ.. Anisotropic materials for skeletal-muscle-tissue engineering. Adv Mater 2016;28:10588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poel WE. Preparation of acellular homogenates from muscle samples. Science 1948;108:390–1. [DOI] [PubMed] [Google Scholar]
- 16. Xiao H, Chen X, Liu X, Wen G, Yu Y.. Recent advances in decellularized biomaterials for wound healing. Mater Today Bio 2023;19:100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choudhury D, Yee M, Sheng ZLJ, Amirul A, Naing MW.. Decellularization systems and devices: state-of-the-art. Acta Biomater 2020;115:51–9. [DOI] [PubMed] [Google Scholar]
- 18. Mokhtarinia K, Masaeli E.. Post-decellularized printing of cartilage extracellular matrix: distinction between biomaterial ink and bioink. Biomater Sci 2023;11:2317–29. [DOI] [PubMed] [Google Scholar]
- 19. Casali DM, Handleton RM, Shazly T, Matthews MA.. A novel supercritical CO2-based decellularization method for maintaining scaffold hydration and mechanical properties. J Supercrit Fluids 2018;131:72–81. [Google Scholar]
- 20. Nie XL, Wang DA.. Decellularized orthopaedic tissue-engineered grafts: biomaterial scaffolds synthesised by therapeutic cells. Biomater Sci 2018;6:2798–811. [DOI] [PubMed] [Google Scholar]
- 21. KC P, Hong Y, Zhang G.. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: advantages and challenges. Regen Biomater 2019;6:185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu LY, Yuhan J, Yu H, Zhang BY, Huang KL, Zhu LJ.. Decellularized extracellular matrix for remodeling bioengineering organoid's microenvironment. Small 2023;19:e2207752. [DOI] [PubMed] [Google Scholar]
- 23. Brown M, Li JY, Moraes C, Tabrizian M, Li-Jessen NYK.. Decellularized extracellular matrix: new promising and challenging biomaterials for regenerative medicine. Biomaterials 2022;289:121786. [DOI] [PubMed] [Google Scholar]
- 24. Brown-Etris M, Milne CT, Hodde JP.. An extracellular matrix graft (oasis (R) wound matrix) for treating full-thickness pressure ulcers: a randomized clinical trial. J Tissue Viability 2019;28:21–6. [DOI] [PubMed] [Google Scholar]
- 25. Tan YH, Helms HR, Nakayama KH.. Decellularization strategies for regenerating cardiac and skeletal muscle tissues. Front Bioeng Biotechnol 2022;10:831300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bing P, Du LL, Zhang TX, Chen JP, Xu BS.. Research progress in decellularized extracellular matrix hydrogels for intervertebral disc degeneration. Biomater Sci 2023;11:1981–93. [DOI] [PubMed] [Google Scholar]
- 27. Kang B, Park Y, Hwang DG, Kim D, Yong U, Lim KS, Jang J.. Facile bioprinting process for fabricating size-controllable functional microtissues using light-activated decellularized extracellular matrix-based bioinks. Adv Mater Technol 2022;7:2270001. [Google Scholar]
- 28. Yeleswarapu S, Dash A, Chameettachal S, Pati F.. 3D bioprinting of tissue constructs employing dual crosslinking of decellularized extracellular matrix hydrogel. Biomater Adv 2023;152:213494. [DOI] [PubMed] [Google Scholar]
- 29. Sun WY, Yang YY, Wang L, Tang H, Zhang L, She YL, Xiao XF, Hu XF, Feng Q, Chen C.. Utilization of an acellular cartilage matrix-based photocrosslinking hydrogel for tracheal cartilage regeneration and circumferential tracheal repair. Adv Funct Mater 2022;32:2201257. [Google Scholar]
- 30. De Witte T-M, Fratila-Apachitei LE, Zadpoor AA, Peppas NA.. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater 2018;5:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li ZB, Tan BH.. Towards the development of polycaprolactone based amphiphilic block copolymers: molecular design, self-assembly and biomedical applications. Mater Sci Eng C Mater Biol Appl 2014;45:620–34. [DOI] [PubMed] [Google Scholar]
- 32. Yadav A, Ghosh S, Samanta A, Pal J, Srivastava RK.. Emulsion templated scaffolds of poly(epsilon-caprolactone) - a review. Chem Commun (Camb) 2022;58:1468–80. [DOI] [PubMed] [Google Scholar]
- 33. Patel KH, Dunn AJ, Talovic M, Haas GJ, Marcinczyk M, Elmashhady H, Kalaf EG, Sell SA, Garg K.. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed Mater 2019;14:035010. [DOI] [PubMed] [Google Scholar]
- 34. Kim HS, Mandakhbayar N, Kim H-W, Leong KW, Yoo HS.. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials 2021;269:120214. [DOI] [PubMed] [Google Scholar]
- 35. Xu JZ, Fang Q, Liu YL, Zhou Y, Ye ZY, Tan WS.. In situ ornamenting poly(epsilon-caprolactone) electrospun fibers with different fiber diameters using chondrocyte-derived extracellular matrix for chondrogenesis of mesenchymal stem cells. Colloids Surf B Biointerfaces 2021;197:111374. [DOI] [PubMed] [Google Scholar]
- 36. Feng B, Ji TJ, Wang XG, Fu W, Ye LC, Zhang H, Li F.. Engineering cartilage tissue based on cartilage-derived extracellular matrix cECM/PCL hybrid nanofibrous scaffold. Mater Des 2020;193:108773. [Google Scholar]
- 37. Xu Y, Duan H, Li YQ, She YL, Zhu JJ, Zhou GD, Jiang GN, Yang Y.. Nanofibrillar decellularized Wharton's jelly matrix for segmental tracheal repair. Adv Funct Mater 2020;30:1910067. [Google Scholar]
- 38. Dong CJ, Qiao FY, Chen GB, Lv YG.. Demineralized and decellularized bone extracellular matrix-incorporated electrospun nanofibrous scaffold for bone regeneration. J Mater Chem B 2021;9:6881–94. [DOI] [PubMed] [Google Scholar]
- 39. Li SY, Deng RL, Zou XN, Rong Q, Shou JL, Rao ZL, Wu WQ, Wu G, Quan DP, Zhou M, Forouzanfar T.. Development and fabrication of co-axially electrospun biomimetic periosteum with a decellularized periosteal ECM shell/PCL core structure to promote the repair of critical-sized bone defects. Compos Pt B Eng 2022;234:109620. [Google Scholar]
- 40. Deng RL, Luo ZL, Rao ZL, Lin ZD, Chen SH, Zhou J, Zhu QT, Liu XL, Bai Y, Quan DP.. Decellularized extracellular matrix containing electrospun fibers for nerve regeneration: a comparison between core-shell structured and preblended composites. Adv Fiber Mater 2022;4:503–19. [Google Scholar]
- 41. Liu ZN, Liu JJ, Liu N, Zhu XQ, Tang R.. Tailoring electrospun mesh for a compliant remodeling in the repair of full-thickness abdominal wall defect-The role of decellularized human amniotic membrane and silk fibroin. Mater Sci Eng C Mater Biol Appl 2021;127:112235. [DOI] [PubMed] [Google Scholar]
- 42. Wiggenhauser PS, Schwarz S, Koerber L, Hoffmann TK, Rotter N.. Addition of decellularized extracellular matrix of porcine nasal cartilage improves cartilage regenerative capacities of PCL-based scaffolds in vitro. J Mater Sci Mater Med 2019;30:121. [DOI] [PubMed] [Google Scholar]
- 43. Guo WM, Chen MX, Wang ZY, Tian Y, Zheng JX, Gao S, Li YY, Zheng YF, Li X, Huang JX, Niu W, Jiang SP, Hao CX, Yuan ZG, Zhang Y, Wang MJ, Wang ZH, Peng J, Wang AY, Wang Y, Sui X, Xu WJ, Hao LB, Zheng XF, Liu SY, Guo QY.. 3D-printed cell-free PCL-MECM scaffold with biomimetic micro-structure and micro-environment to enhance in situ meniscus regeneration. Bioact. Mater 2021;6:3620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang X, Li Y, Liu X, Huang Q, Zhang R, Feng Q.. Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells. Regen Biomater 2018;5:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee H, Kim W, Lee J, Yoo JJ, Kim GH, Lee SJ.. Effect of hierarchical scaffold consisting of aligned dECM nanofibers and poly(lactide-co-glycolide) struts on the orientation and maturation of human muscle progenitor cells. ACS Appl Mater Interfaces 2019;11:39449–58. [DOI] [PubMed] [Google Scholar]
- 46. Fang Y, Han Y, Wang S, Chen J, Dai K, Xiong Y, Sun B.. Three-dimensional printing bilayer membranous nanofiber scaffold for inhibiting scar hyperplasia of skin. Biomater Adv 2022;138:212951. [DOI] [PubMed] [Google Scholar]
- 47. Deng MY, Tan JL, Hu CS, Hou TY, Peng W, Liu J, Yu B, Dai QJ, Zhou JL, Yang YS, Dong R, Ruan CS, Dong SW, Xu JZ.. Modification of PLGA scaffold by MSC-derived extracellular matrix combats macrophage inflammation to initiate bone regeneration via TGF-beta-induced protein. Adv Healthc Mater 2020;9:2100872. [DOI] [PubMed] [Google Scholar]
- 48. Chen Y, Chen L-F, Wang Y, Duan Y-Y, Luo S-C, Zhang J-T, Kankala RK, Wang S-B, Chen A-Z.. Modeling dECM-based inflammatory cartilage microtissues in vitro for drug screening. Compos Pt B Eng 2023;250:110437. [Google Scholar]
- 49. Ghosh P, Gruber SMS, Lin CY, Whitlock PW.. Microspheres containing decellularized cartilage induce chondrogenesis in vitro and remain functional after incorporation within a poly(caprolactone) filament useful for fabricating a 3D scaffold. Biofabrication 2018;10:025007. [DOI] [PubMed] [Google Scholar]
- 50. Gupta V, Lyne DV, Laflin AD, Zabel TA, Barragan M, Bunch JT, Pacicca DM, Detamore MS.. Microsphere-based osteochondral scaffolds carrying opposing gradients of decellularized cartilage and demineralized bone matrix. ACS Biomater Sci Eng 2017;3:1955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lih E, Park KW, Chun SY, Kim H, Kwon TG, Joung YK, Han DK.. Biomimetic porous PLGA scaffolds incorporating decellularized extracellular matrix for kidney tissue regeneration. ACS Appl Mater Interfaces 2016;8:21145–54. [DOI] [PubMed] [Google Scholar]
- 52. Ma YH, Shi HJ, Wei QS, Deng QW, Sun JH, Liu Z, Lai BQ, Li G, Ding Y, Niu WT, Zeng YS, Zeng X.. Developing a mechanically matched decellularized spinal cord scaffold for the in situ matrix-based neural repair of spinal cord injury. Biomaterials 2021;279:121192. [DOI] [PubMed] [Google Scholar]
- 53. Li C, Zhou Y, Liu SJ, Guo RQ, Lu CF, Yin D, Zhang YH, Xu X, Dong NG, Shi JW.. Surface modification of decellularized heart valve by the POSS-PEG hybrid hydrogel to prepare a composite scaffold material with anticalcification potential. ACS Appl Bio Mater 2022;5:3923–35. [DOI] [PubMed] [Google Scholar]
- 54. Hewawasam RS, Blomberg R, Serbedzija P, Magin CM.. Chemical modification of human decellularized extracellular matrix for incorporation into phototunable hybrid-hydrogel models of tissue fibrosis. ACS Appl Mater Interfaces 2023;15:15071–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrou CL, D'Ovidio TJ, Bolukbas DA, Tas S, Brown RD, Allawzi A, Lindstedt S, Nozik-Grayck E, Stenmark KR, Wagner DE, Magin CM.. Clickable decellularized extracellular matrix as a new tool for building hybrid-hydrogels to model chronic fibrotic diseases in vitro. J Mater Chem B 2020;8:6814–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Song ES, Park JH, Ha SS, Cha PH, Kang JT, Park CY, Park K.. Novel corneal endothelial cell carrier couples a biodegradable polymer and a mesenchymal stem cell-derived extracellular matrix. ACS Appl Mater Interfaces 2022;14:12116–29. [DOI] [PubMed] [Google Scholar]
- 57. Suhaeri M, Subbiah R, Kim SH, Kim CH, Oh SJ, Kim SH, Park K.. Novel platform of cardiomyocyte culture and coculture via fibroblast-derived matrix-coupled aligned electrospun nanofiber. ACS Appl Mater Interfaces 2017;9:224–35. [DOI] [PubMed] [Google Scholar]
- 58. Young BM, Shankar K, Allen BP, Pouliot RA, Schneck MB, Mikhaiel NS, Heise RL.. Electrospun decellularized lung matrix scaffold for airway smooth muscle culture. ACS Biomater Sci Eng 2017;3:3480–92. [DOI] [PubMed] [Google Scholar]
- 59. Zheng CS, Yang ZH, Chen SH, Zhang F, Rao ZL, Zhao CL, Quan DP, Bai Y, Shen J.. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics 2021;11:2917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim TH, Jung Y, Kim SH.. Nanofibrous electrospun heart decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng Part A 2018;24:830–48. [DOI] [PubMed] [Google Scholar]
- 61. Lee S, Lee HS, Chung JJ, Kim SH, Park JW, Lee K, Jung Y.. Enhanced regeneration of vascularized adipose tissue with dual 3D-printed elastic polymer/dECM hydrogel complex. Int J Mol Sci 2021;22:2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fan YM, Luchow M, Badria A, Hutchinson DJ, Malkoch M.. Placenta powder-infused thiol-Ene PEG hydrogels as potential tissue engineering scaffolds. Biomacromolecules 2023;24:1617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hashimoto Y, Yamashita A, Negishi J, Kimura T, Funamoto S, Kishida A.. 4-Arm PEG-functionalized decellularized pericardium for effective prevention of postoperative adhesion in cardiac surgery. ACS Biomater Sci Eng 2022;8:261–72. [DOI] [PubMed] [Google Scholar]
- 64. Nishiguchi A, Taguchi T.. A pH-driven genipin gelator to engineer decellularized extracellular matrix-based tissue adhesives. Acta Biomater 2021;131:211–21. [DOI] [PubMed] [Google Scholar]
- 65. Singh S, Paswan KK, Kumar A, Gupta V, Sonker M, Khan MA, Kumar A, Shreyash N.. Recent advancements in polyurethane-based tissue engineering. ACS Appl Bio Mater 2023;6:327–48. [DOI] [PubMed] [Google Scholar]
- 66. Wang Y, Lin J, Chen J, Liang R, Zhang Q, Li J, Shi M, Li L, He X, Lan T, Hui X, Tan H.. Biodegradable polyurethane-incorporating decellularized spinal cord matrix scaffolds enhance Schwann cell reprogramming to promote peripheral nerve repair. J Mater Chem B 2023;11:2115–28. [DOI] [PubMed] [Google Scholar]
- 67. Chen MX, Li YY, Liu SY, Feng ZX, Wang H, Yang DJ, Guo WM, Yuan ZG, Gao S, Zhang Y, Zha KK, Huang B, Wei F, Sang XY, Tian QY, Yang X, Sui X, Zhou YX, Zheng YF, Guo QY.. Hierarchical macro-microporous WPU-ECM scaffolds combined with microfracture promote in situ articular cartilage regeneration in rabbits. Bioact Mater 2021;6:1932–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chae S, Lee SS, Choi YJ, Hong D, Gao G, Wang JH, Cho DW.. 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 2021;267:120466. [DOI] [PubMed] [Google Scholar]
- 69. Chae S, Sun Y, Choi YJ, Ha DH, Jeon IH, Cho DW.. 3D cell-printing of tendon-bone interface using tissue-derived extracellular matrix bioinks for chronic rotator cuff repair. Biofabrication 2021;13:035005. [DOI] [PubMed] [Google Scholar]
- 70. Jeon EY, Joo KI, Cha HJ.. Body temperature-activated protein-based injectable adhesive hydrogel incorporated with decellularized adipose extracellular matrix for tissue-specific regenerative stem cell therapy. Acta Biomater 2020;114:244–55. [DOI] [PubMed] [Google Scholar]
- 71. Zamboni F, Wong CK, Collins MN.. Hyaluronic acid association with bacterial, fungal and viral infections: can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioact Mater 2023;19:458–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dimitrievska S, Wang J, Lin T, Weyers A, Bai HL, Qin LF, Li GX, Cai C, Kypson A, Kristofik N, Gard A, Sundaram S, Yamamoto K, Wu W, Zhao LP, Kural MH, Yuan YF, Madri J, Kyriakides TR, Linhardt RJ, Niklason LE.. Glycocalyx-like hydrogel coatings for small diameter vascular grafts. Adv Funct Mater 2020;30:1908963. [Google Scholar]
- 73. Zhang D, Fu Q, Fu H, Zeng J, Jia L, Chen M.. 3D-bioprinted human lipoaspirate-derived cell-laden skin constructs for healing of full-thickness skin defects. Int J Bioprint 2023;9:718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guo P, Jiang N, Mini C, Miklosic G, Zhu SS, Vernengo AJ, D'Este M, Grad S, Alini M, Li Z.. Decellularized extracellular matrix particle-based biomaterials for cartilage repair applications. J Mater Sci Technol 2023;160:194–203. [Google Scholar]
- 75. Bo QT, Yan L, Li H, Jia ZH, Zhan AQ, Chen J, Yuan ZQ, Zhang W, Gao BW, Chen R.. Decellularized dermal matrix-based photo-crosslinking hydrogels as a platform for delivery of adipose derived stem cells to accelerate cutaneous wound healing. Mater Des 2020;196:110966. [Google Scholar]
- 76. Barthold JE, McCreery KP, Martinez J, Bellerjeau C, Ding YF, Bryant SJ, Whiting GL, Neu CP.. Particulate ECM biomaterial ink is 3D printed and naturally crosslinked to form structurally-layered and lubricated cartilage tissue mimics. Biofabrication 2022;14:025021. [DOI] [PubMed] [Google Scholar]
- 77. Shen XR, Li SQ, Zhao X, Han JD, Chen JX, Rao ZL, Zhang KX, Quan DP, Yuan J, Bai Y.. Dual-crosslinked regenerative hydrogel for sutureless long-term repair of corneal defect. Bioact Mater 2023;20:434–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin CY, Liu TY, Chen MH, Sun JS, Chen MH.. An injectable extracellular matrix for the reconstruction of epidural fat and the prevention of epidural fibrosis. Biomed Mater 2016;11:035010. [DOI] [PubMed] [Google Scholar]
- 79. Isik M, Karakaya E, Arslan TS, Atila D, Erdogan YK, Arslan YE, Eskizengin H, Eylem CC, Nemutlu E, Ercan B, D'Este M, Okesola BO, Derkus B.. 3D printing of extracellular matrix-based multicomponent, all-natural, highly elastic, and functional materials toward vascular tissue engineering. Adv Healthc Mater 2023;12:e2203044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER.. Alginate-based composite materials for wound dressing application: a mini review. Carbohydr Polym 2020;236:116025. [DOI] [PubMed] [Google Scholar]
- 81. Zhou J, Wu Y, Tang Z, Zou K, Chen J, Lei Z, Wan X, Liu Y, Zhang H, Wang Y, Blesch A, Lei T, Liu S.. Alginate hydrogel cross-linked by Ca2+ to promote spinal cord neural stem/progenitor cell differentiation and functional recovery after a spinal cord injuryhh. Regen Biomater 2022;9:rbac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chu TL, Tripathi G, Park M, Bae SH, Lee BT.. In-vitro and in-vivo biocompatibility of dECM-alginate as a promising candidate in cell delivery for kidney regeneration. Int J Biol Macromol 2022;211:616–25. [DOI] [PubMed] [Google Scholar]
- 83. Ahn M, Cho WW, Kim BS, Cho DW.. Engineering densely packed adipose tissue via environmentally controlled in-bath 3D bioprinting. Adv Funct Mater 2022;32:2200203. [Google Scholar]
- 84. Guagliano G, Volpini C, Sardelli L, Bloise N, Briatico-Vangosa F, Cornaglia AI, Dotti S, Villa R, Visai L, Petrini P.. Hep3Gel: a shape-shifting extracellular matrix-based, three- dimensional liver model adaptable to different culture systems. ACS Biomater Sci Eng 2022;9:211–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Curley CJ, Dolan EB, Otten M, Hinderer S, Duffy GP, Murphy BP.. An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug Deliv Transl Res 2019;9:1–13. [DOI] [PubMed] [Google Scholar]
- 86. Park Y, Ji ST, Yong U, Das S, Jang WB, Ahn G, Kwon SM, Jang J.. 3D bioprinted tissue-specific spheroidal multicellular microarchitectures for advanced cell therapy. Biofabrication 2021;13:045017. [DOI] [PubMed] [Google Scholar]
- 87. De Santis MM, Alsafadi HN, Tas S, Bolukbas DA, Prithiviraj S, Da Silva IAN, Mittendorfer M, Ota C, Stegmayr J, Daoud F, Konigshoff M, Sward K, Wood JA, Tassieri M, Bourgine PE, Lindstedt S, Mohlin S, Wagner DE.. Extracellular-matrix-reinforced bioinks for 3D bioprinting human tissue. Adv Mater 2021;33:e2005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee J, Hong J, Kim W, Kim GH.. Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering. Carbohydr Polym 2020;250:116914. [DOI] [PubMed] [Google Scholar]
- 89. Jin B, Yu Y, Chen X, Yang Y, Xiong Y, Im YJ, Zhao Y, Xiao J.. Microtubes with gradient decellularized porcine sciatic nerve matrix from microfluidics for sciatic nerve regeneration. Bioact Mater 2023;21:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Du J, Hu X, Su Y, Wei T, Jiao Z, Liu T, Wang H, Nie Y, Li X, Song K.. Gelatin/sodium alginate hydrogel-coated decellularized porcine coronary artery to construct bilayer tissue engineered blood vessels. Int J Biol Macromol 2022;209:2070–83. [DOI] [PubMed] [Google Scholar]
- 91. Fh T, Yx C, Xw S, Hf Z, Ym D, Xiang W, Hb D.. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr Polym 2020;230:115658. [DOI] [PubMed] [Google Scholar]
- 92. Zhang R, Chang SJ, Jing Y, Wang L, Chen C-J, Liu J-T.. Application of chitosan with different molecular weights in cartilage tissue engineering. Carbohydr Polym 2023;314:120890. [DOI] [PubMed] [Google Scholar]
- 93. Yang Y, Zhang YY, Yan YS, Ji Q, Dai YT, Jin SY, Liu YX, Chen JH, Teng LP.. A sponge-like double-layer wound dressing with chitosan and decellularized bovine amniotic membrane for promoting diabetic wound healing. Polymers (Basel) 2020;12:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu PY, Kankala RK, Li YW, Wang SB, Chen AZ.. Synergistic chemo-/photothermal therapy based on supercritical technology-assisted chitosan-indocyanine green/luteolin nanocomposites for wound healing. Regen Biomater 2022;9:rbac072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kong Y, Wang D, Wei QF, Yang YM.. Nerve decellularized matrix composite scaffold with high antibacterial activity for nerve regeneration. Front Bioeng Biotechnol 2021;9:840421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ye YH, Pang YC, Zhang Z, Wu CC, Jin JF, Su MZ, Pan JL, Liu YB, Chen L, Jin KK.. Decellularized periosteum-covered chitosan globule composite for bone regeneration in rabbit femur condyle bone defects. Macromol. Biosci 2018;18:1700424. [DOI] [PubMed] [Google Scholar]
- 97. Efraim Y, Sarig H, Cohen Anavy N, Sarig U, de Berardinis E, Chaw S-Y, Krishnamoorthi M, Kalifa J, Bogireddi H, Duc TV, Kofidis T, Baruch L, Boey FYC, Venkatraman SS, Machluf M.. Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater 2017;50:220–33. [DOI] [PubMed] [Google Scholar]
- 98. Zhan A, Chen L, Sun W, Tang Y, Chen J, Yu D, Zhang W.. Enhancement of diabetic wound healing using a core-shell nanofiber platform with sequential antibacterial, angiogenic, and collagen deposition activities. Mater Des 2022;218:110660. [Google Scholar]
- 99. Mudigonda J, Onohara D, Amedi A, Suresh KS, Kono T, Corporan D, Padala M.. In vivo efficacy of a polymer layered decellularized matrix composite as a cell honing cardiovascular tissue substitute. Mater Today Bio 2022;17:100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Datta S, Rameshbabu AP, Bankoti K, Roy M, Gupta C, Jana S, Das AK, Sen R, Dhara S.. Decellularized bone matrix/oleoyl chitosan derived supramolecular injectable hydrogel promotes efficient bone integration. Mater Sci Eng C Mater Biol Appl 2021;119:111604. [DOI] [PubMed] [Google Scholar]
- 101. Chu TL, Tripathi G, Bae SH, Lee BT.. In-vitro and in-vivo hemostat evaluation of decellularized liver extra cellular matrix loaded chitosan/gelatin spongy scaffolds for liver injury. Int J Biol Macromol 2021;193:638–46. [DOI] [PubMed] [Google Scholar]
- 102. Sarrigiannidis SO, Rey JM, Dobre O, González-García C, Dalby MJ, Salmeron-Sanchez M.. A tough act to follow: collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater Today Bio 2021;10:100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Altinova H, Hammes S, Palm M, Gerardo-Nava J, Achenbach P, Deumens R, Hermans E, Führmann T, Boecker A, van Neerven SGA, Bozkurt A, Weis J, Brook GA.. Fibroadhesive scarring of grafted collagen scaffolds interferes with implant–host neural tissue integration and bridging in experimental spinal cord injury. Regen Biomater 2019;6:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hong H, Kim H, Han SJ, Jang J, Kim HK, Cho DW, Kim DS.. Compressed collagen intermixed with cornea-derived decellularized extracellular matrix providing mechanical and biochemical niches for corneal stroma analogue. Mater Sci Eng C Mater Biol Appl 2019;103:109837. [DOI] [PubMed] [Google Scholar]
- 105. Hua YJ, Huo YY, Bai BS, Hao JX, Hu GH, Ci Z, Wu XD, Yu MY, Wang X, Chen H, Ren WJ, Zhang YX, Wang X, Zhou GD.. Fabrication of biphasic cartilage-bone integrated scaffolds based on tissue-specific photo-crosslinkable acellular matrix hydrogels. Mater Today Bio 2022;17:100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hong JY, Seo Y, Davaa G, Kim HW, Kim SH, Hyun JK.. Decellularized brain matrix enhances macrophage polarization and functional improvements in rat spinal cord injury. Acta Biomater 2020;101:357–71. [DOI] [PubMed] [Google Scholar]
- 107. Lu Y, Wang YX, Zhang HJ, Tang ZZ, Cui XL, Li X, Liang J, Wang QG, Fan YJ, Zhang XD.. Solubilized cartilage ECM facilitates the recruitment and chondrogenesis of endogenous BMSCs in collagen scaffolds for enhancing microfracture treatment. ACS Appl Mater Interfaces 2021;13:24553–64. [DOI] [PubMed] [Google Scholar]
- 108. Cao HF, Wang XY, Chen MY, Liu YH, Cui XL, Liang J, Wang QG, Fan YJ, Zhang XD.. Childhood cartilage ECM enhances the chondrogenesis of endogenous cells and subchondral bone repair of the unidirectional collagen–dECM scaffolds in combination with microfracture. ACS Appl Mater Interfaces 2021;13:57043–57. [DOI] [PubMed] [Google Scholar]
- 109. Santoro M, Tatara AM, Mikos AG.. Gelatin carriers for drug and cell delivery in tissue engineering. J Control Release 2014;190:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Aldana AA, Abraham GA.. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int J Pharm 2017;523:441–53. [DOI] [PubMed] [Google Scholar]
- 111. Rose JB, Pacelli S, El Haj AJ, Dua HS, Hopkinson A, White LJ, Rose F.. Gelatin-based materials in ocular tissue engineering. Materials (Basel) 2014;7:3106–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lukin I, Erezuma I, Maeso L, Zarate J, Desimone MF, Al-Tel TH, Dolatshahi-Pirouz A, Orive G.. Progress in gelatin as biomaterial for tissue engineering. Pharmaceutics 2022;14:1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Andreazza R, Morales A, Pieniz S, Labidi J.. Gelatin-based hydrogels: potential biomaterials for remediation. Polymers (Basel) 2023;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen SW, Zhang Q, Nakamoto T, Kawazoe N, Chen GP.. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue engineering. Tissue Eng Part C Methods 2016;22:189–98. [DOI] [PubMed] [Google Scholar]
- 115. Kim MK, Jeong W, Lee SM, Kim JB, Jin S, Kang HW.. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2020;12:025003. [DOI] [PubMed] [Google Scholar]
- 116. Baiguera S, Del Gaudio C, Lucatelli E, Kuevda E, Boieri M, Mazzanti B, Bianco A, Macchiarini P.. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014;35:1205–14. [DOI] [PubMed] [Google Scholar]
- 117. Kara A, Distler T, Polley C, Schneidereit D, Seitz H, Friedrich O, Tihminlioglu F, Boccaccini AR.. 3D printed gelatin/decellularized bone composite scaffolds for bone tissue engineering: fabrication, characterization and cytocompatibility study. Mater Today Bio 2022;15:100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chen L, Song XB, Yao ZC, Zhou CL, Yang JJ, Yang QM, Chen JR, Wu JR, Sun ZY, Gu LL, Ma Y, Lee SJ, Zhang C, Mao HQ, Sun L.. Gelatin nanofiber-reinforced decellularized amniotic membrane promotes axon regeneration and functional recovery in the surgical treatment of peripheral nerve injury. Biomaterials 2023;300:122207. [DOI] [PubMed] [Google Scholar]
- 119. Li YQ, Liu YQ, Xun XW, Zhang W, Xu Y, Gu DY.. Three-dimensional porous scaffolds with biomimetic microarchitecture and bioactivity for cartilage tissue engineering. ACS Appl Mater Interfaces 2019;11:36359–70. [DOI] [PubMed] [Google Scholar]
- 120. Chen WM, Xu Y, Li YQ, Jia LT, Mo XM, Jiang GN, Zhou GD.. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem Eng J 2020;382:122986. [Google Scholar]
- 121. Xu Y, Wang Z, Hua Y, Zhu X, Wang Y, Duan L, Zhu L, Jiang G, Xia H, She Y, Zhou G.. Photocrosslinked natural hydrogel composed of hyaluronic acid and gelatin enhances cartilage regeneration of decellularized trachea matrix. Mater Sci Eng C Mater Biol Appl 2021;120:111628. [DOI] [PubMed] [Google Scholar]
- 122. Xu J, Fang H, Zheng SS, Li LY, Jiao ZR, Wang H, Nie Y, Liu TQ, Song KD.. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int J Biol Macromol 2021;187:840–9. [DOI] [PubMed] [Google Scholar]
- 123. Wang Y, Wang Q, Luo S, Chen Z, Zheng X, Kankala RK, Chen A, Wang S.. 3D bioprinting of conductive hydrogel for enhanced myogenic differentiation. Regen Biomater 2021;8:rbab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Choi E, Kim D, Kang D, Yang GH, Jung B, Yeo M, Park M-J, Lee AS, Kim K, Kim JS, Jeong JC, Yoo W, Jeon HH.. H. 3D-printed gelatin methacrylate (GelMA)/silanated silica scaffold assisted by two-stage cooling system for hard tissue regeneration. Regen Biomater 2021;8:rbab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kurian AG, Singh RK, Patel KD, Lee JH, Kim HW.. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact Mater 2022;8:267–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tavares-Negrete JA, Pedroza-González SC, Frías-Sánchez AI, Salas-Ramírez ML, de Santiago-Miramontes M, Luna-Aguirre CM, Alvarez MM, Trujillo-de Santiago G.. Supplementation of GelMA with minimally processed tissue promotes the formation of densely packed skeletal-muscle-like tissues. ACS Biomater Sci Eng 2023;9:3462–75. [DOI] [PubMed] [Google Scholar]
- 127. Mao QJ, Wang YF, Li Y, Juengpanich S, Li WH, Chen MY, Yin J, Fu JZ, Cai XJ.. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C Mater Biol Appl 2020;109:110625. [DOI] [PubMed] [Google Scholar]
- 128. Yu YK, Wang Y, Zhang WD, Wang H, Li JY, Pan LB, Han FX, Li B.. Biomimetic periosteum-bone substitute composed of preosteoblast-derived matrix and hydrogel for large segmental bone defect repair. Acta Biomater 2020;113:317–27. [DOI] [PubMed] [Google Scholar]
- 129. Gao CY, Sow WT, Wang YY, Wang YL, Yang DJ, Lee BH, Maticic D, Fang L, Li HQ, Zhang CW.. Hydrogel composite scaffolds with an attenuated immunogenicity component for bone tissue engineering applications. J Mater Chem B 2021;9:2033–41. [DOI] [PubMed] [Google Scholar]
- 130. He WH, Zhang XX, Li XZ, Ju DY, Mao TT, Lu Y, Gu Y, Qi LJ, Wang QH, Wu QF, Dong CM.. A decellularized spinal cord extracellular matrix-gel/GelMA hydrogel three-dimensional composite scaffold promotes recovery from spinal cord injury via synergism with human menstrual blood-derived stem cells. J Mater Chem B 2022;10:5753–64. [DOI] [PubMed] [Google Scholar]
- 131. Zheng LW, Liu YX, Jiang L, Wang XP, Chen YQ, Li L, Song MY, Zhang HM, Zhang YS, Zhang XM.. Injectable decellularized dental pulp matrix-functionalized hydrogel microspheres for endodontic regeneration. Acta Biomater 2023;156:37–48. [DOI] [PubMed] [Google Scholar]
- 132. Zhang Q, Chang CW, Qian CY, Xiao WS, Zhu HJ, Guo J, Meng ZB, Cui WG, Ge ZL.. Photo-crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater 2021;125:197–207. [DOI] [PubMed] [Google Scholar]
- 133. Chen YW, Lin YH, Lin TL, Lee KXA, Yu MH, Shie MY.. 3D-biofabricated chondrocyte-laden decellularized extracellular matrix-contained gelatin methacrylate auxetic scaffolds under cyclic tensile stimulation for cartilage regeneration. Biofabrication 2023;15:045007. [DOI] [PubMed] [Google Scholar]
- 134. Yang XT, Ma Y, Wang XT, Yuan SM, Huo FJ, Yi GZ, Zhang JY, Yang B, Tian WD.. A 3D-bioprinted functional module based on decellularized extracellular matrix bioink for periodontal regeneration. Adv Sci 2023;10:2205041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nishiguchi A, Ito S, Nagasaka K, Taguchi T.. Tissue-adhesive decellularized extracellular matrix patches reinforced by a supramolecular gelator to repair abdominal wall defects. Biomacromolecules 2023;24:1545–54. [DOI] [PubMed] [Google Scholar]
- 136. Wang L, Liu FL, Zhai XR, Dong W, Wei W, Hu Z.. An adhesive gelatin-coated small intestinal submucosa composite hydrogel dressing aids wound healing. Int J Biol Macromol 2023;241:124622. [DOI] [PubMed] [Google Scholar]
- 137. Wu RJ, Li HT, Yang YL, Zheng QJ, Li SL, Chen YF.. Bioactive silk fibroin-based hybrid biomaterials for musculoskeletal engineering: recent progress and perspectives. ACS Appl Bio Mater 2021;4:6630–46. [DOI] [PubMed] [Google Scholar]
- 138. Gholipourmalekabadi M, Sapru S, Samadikuchaksaraei A, Reis RL, Kaplan DL, Kundu SC.. Silk fibroin for skin injury repair: where do things stand? Adv Drug Deliv Rev 2020;153:28–53. [DOI] [PubMed] [Google Scholar]
- 139. Liu Y, Fan J, Lv M, She K, Sun J, Lu Q, Han C, Ding S, Zhao S, Wang G, Zhang Y, Zang G.. Photocrosslinking silver nanoparticles–aloe vera–silk fibroin composite hydrogel for treatment of full-thickness cutaneous wounds. Regen Biomater 2021;8:rbab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jeyakumar V, Amraish N, Niculescu-Morsza E, Bauer C, Pahr D, Nehrer S.. Decellularized cartilage extracellular matrix incorporated silk fibroin hybrid scaffolds for endochondral ossification mediated bone regeneration. Int J Mol Sci 2021;22:4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gholipourmalekabadi M, Samadikuchaksaraei A, Seifalian AM, Urbanska AM, Ghanbarian H, Hardy JG, Omrani MD, Mozafari M, Reis RL, Kundu SC.. Silk fibroin/amniotic membrane 3D bi-layered artificial skin. Biomed Mater 2018;13:035003. [DOI] [PubMed] [Google Scholar]
- 142. Liu W, Sun YF, Dong XH, Yin Q, Zhu HM, Li SW, Zhou J, Wang CY.. Cell-derived extracellular matrix-coated silk fibroin scaffold for cardiogenesis of brown adipose stem cells through modulation of TGF-beta pathway. Regen Biomater 2020;7:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gholipourmalekabadi M, Seifalian AM, Urbanska AM, Omrani MD, Hardy JG, Madjd Z, Hashemi SM, Ghanbarian H, Milan PB, Mozafari M, Reis RL, Kundu SC, Samadikuchaksaraei A.. 3D protein-based bilayer artificial skin for the guided scarless healing of third-degree burn wounds in vivo. Biomacromolecules 2018;19:2409–22. [DOI] [PubMed] [Google Scholar]
- 144. Dhasmana A, Singh L, Roy P, Mishra NC.. Silk fibroin protein modified acellular dermal matrix for tissue repairing and regeneration. Mater Sci Eng C Mater Biol Appl 2019;97:313–24. [DOI] [PubMed] [Google Scholar]
- 145. Zhu Y, Wang DZ, Yao XH, Wang MM, Zhao YH, Lu YH, Wang ZW, Guo YB.. Biomimetic hybrid scaffold of electrospun silk fibroin and pancreatic decellularized extracellular matrix for islet survival. J Biomater Sci Polym Ed 2021;32:151–65. [DOI] [PubMed] [Google Scholar]
- 146. Kayabolen A, Keskin D, Aykan A, Karslioglu Y, Zor F, Tezcaner A.. Native extracellular matrix/fibroin hydrogels for adipose tissue engineering with enhanced vascularization. Biomed Mater 2017;12:035007. [DOI] [PubMed] [Google Scholar]
- 147. Gao E, Li G, Cao RF, Xia HT, Xu Y, Jiang GN, Xiao KY, Chen J, Chen R, Duan L.. Bionic tracheal tissue regeneration using a ring-shaped scaffold comprised of decellularized cartilaginous matrix and silk fibroin. Compos Pt B Eng 2022;229:109470. [Google Scholar]
- 148. Guan YJ, Ren ZQ, Yang BY, Xu WJ, Wu WJ, Li XL, Zhang TY, Li DD, Chen SF, Bai J, Xy S, Jia ZB, Xiong X, He SL, Li CC, Meng FQ, Wu T, Zhang J, Liu XZ, Meng HY, Peng J, Wang Y.. Dual-bionic regenerative microenvironment for peripheral nerve repair. Bioact Mater 2023;26:370–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Liu JJ, Chen D, Zhu XQ, Liu N, Zhang HB, Tang R, Liu ZN.. Development of a decellularized human amniotic membrane-based electrospun vascular graft capable of rapid remodeling for small-diameter vascular applications. Acta Biomater 2022;152:144–56. [DOI] [PubMed] [Google Scholar]
- 150. Lee H, Yang GH, Kim M, Lee J, Huh J, Kim G.. Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl 2018;84:140–7. [DOI] [PubMed] [Google Scholar]
- 151. Dai Y, Han XH, Hu LH, Wu HW, Huang SY, Lü YP.. Efficacy of concentrated growth factors combined with mineralized collagen on quality of life and bone reconstruction of guided bone regeneration. Regen Biomater 2020;7:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sani M, Hosseinie R, Latifi M, Shadi M, Razmkhah M, Salmannejad M, Parsaei H, Talaei-Khozani T.. Engineered artificial articular cartilage made of decellularized extracellular matrix by mechanical and IGF-1 stimulation. Biomater Adv 2022;139:213019. [DOI] [PubMed] [Google Scholar]
- 153. Lee H, Ju YM, Kim I, Elsangeedy E, Lee JH, Yoo JJ, Atala A, Lee SJ.. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods 2020;171:77–85. [DOI] [PubMed] [Google Scholar]
- 154. Wu K, Wang YY, Yang H, Chen YH, Lu KY, Wu Y, Liu CX, Zhang HX, Meng HY, Yu Q, Zhang YX, Shen ZY.. Injectable decellularized extracellular matrix hydrogel containing stromal cell-derived factor 1 promotes transplanted cardiomyocyte engraftment and functional regeneration after myocardial infarction. ACS Appl Mater Interfaces 2023;15:2578–89. [DOI] [PubMed] [Google Scholar]
- 155. He WL, Shi CY, Yin J, Huang FF, Yan WJ, Deng J, Zhang B, Wang B, Wang HP.. Spinal cord decellularized matrix scaffold loaded with engineered basic fibroblast growth factor-overexpressed human umbilical cord mesenchymal stromal cells promoted the recovery of spinal cord injury. J Biomed Mater Res B Appl Biomater 2023;111:51–61. [DOI] [PubMed] [Google Scholar]
- 156. Lin Q, Wong HL, Tian FR, Huang YD, Xu J, Yang JJ, Chen PP, Fan ZL, Lu CT, Zhao YZ.. Enhanced neuroprotection with decellularized brain extracellular matrix containing bFGF after intracerebral transplantation in Parkinson’s disease rat model. Int J Pharm 2017;517:383–94. [DOI] [PubMed] [Google Scholar]
- 157. Liu C, Jin ZX, Ge X, Zhang Y, Xu HG.. Decellularized annulus fibrosus matrix/chitosan hybrid hydrogels with basic fibroblast growth factor for annulus fibrosus tissue engineering. Tissue Eng Part A 2019;25:1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang G, Ci H, Ma CG, Li ZP, Jiang WB, Chen LF, Wang ZX, Zhou MR, Sun JM.. Additively manufactured macroporous chambers facilitate large volume soft tissue regeneration from adipose-derived extracellular matrix. Acta Biomater 2022;148:90–105. [DOI] [PubMed] [Google Scholar]
- 159. Chen L, Liu JX, Guan M, Zhou TQ, Duan X, Xiang Z.. Growth factor and its polymer scaffold-based delivery system for cartilage tissue engineering. Int J Nanomedicine 2020;15:6097–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yang Z, Zhao TY, Gao CJ, Cao FY, Li H, Liao ZY, Fu LW, Li PX, Chen W, Sun ZQ, Jiang SP, Tian Z, Tian GZ, Zha KK, Pan TT, Li X, Sui X, Yuan ZG, Liu SY, Guo QY.. 3D-bioprinted difunctional scaffold for in situ cartilage regeneration based on aptamer-directed cell recruitment and growth factor-enhanced cell chondrogenesis. ACS Appl Mater Interfaces 2021;13:23369–83. [DOI] [PubMed] [Google Scholar]
- 161. Larochette N, El-Hafci H, Potier E, Setterblad N, Bensidhoum M, Petite H, Logeart-Avramoglou D.. Osteogenic-differentiated mesenchymal stem cell-secreted extracellular matrix as a bone morphogenetic protein-2 delivery system for ectopic bone formation. Acta Biomater 2020;116:186–200. [DOI] [PubMed] [Google Scholar]
- 162. Tan J, Zhang QY, Song YT, Huang K, Jiang YL, Chen J, Wang R, Zou CY, Li QJ, Qin BQ, Sheng N, Nie R, Feng ZY, Yang DZ, Yi WH, Xie HQ.. Accelerated bone defect regeneration through sequential activation of the M1 and M2 phenotypes of macrophages by a composite BMP-2@SIS hydrogel: an immunomodulatory perspective. Compos Pt B Eng 2022;243:110149. [Google Scholar]
- 163. Wei Q, Liu DC, Chu GL, Yu QF, Liu Z, Li JY, Meng QC, Wang WS, Han FX, Li B.. TGF-β1-supplemented decellularized annulus fibrosus matrix hydrogels promote annulus fibrosus repair. Bioact Mater 2023;19:581–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhang X, Liu Y, Luo CY, Zhai CJ, Li ZX, Zhang Y, Yuan T, Dong SL, Zhang JY, Fan WM.. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl 2021;118:111388. [DOI] [PubMed] [Google Scholar]
- 165. Yang Z, Cao FY, Li H, He SL, Zhao TY, Deng HY, Li JW, Sun ZQ, Hao CX, Xu JZ, Guo QY, Liu SY, Guo WM.. Microenvironmentally optimized 3D-printed TGFβ-functionalized scaffolds facilitate endogenous cartilage regeneration in sheep. Acta Biomater 2022;150:181–98. [DOI] [PubMed] [Google Scholar]
- 166. Zhang X, Liu Y, Zuo Q, Wang QY, Li ZX, Yan K, Yuan T, Zhang Y, Shen K, Xie R, Fan WM.. 3D bioprinting of biomimetic bilayered scaffold consisting of decellularized extracellular matrix and silk fibroin for osteochondral repair. Int J Bioprint 2021;7:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Han Y, Lian M, Zhang C, Jia B, Wu Q, Sun B, Qiao Z, Sun B, Dai K.. Study on bioactive PEGDA/ECM hybrid bi-layered hydrogel scaffolds fabricated by electro-writing for cartilage regeneration. Appl Mater Today 2022;28:101547. [Google Scholar]
- 168. Cuenca JP, Kang HJ, Al Fahad MA, Park M, Choi M, Lee HY, Lee BT.. Physico-mechanical and biological evaluation of heparin/VEGF-loaded electrospun polycaprolactone/decellularized rat aorta extracellular matrix for small-diameter vascular grafts. J Biomater Sci Polym Ed 2022;33:1664–84. [DOI] [PubMed] [Google Scholar]
- 169. Liu KT, Zhao M, Li Y, Luo L, Hu DH.. VEGF loaded porcine decellularized adipose tissue derived hydrogel could enhance angiogenesis in vitro and in vivo. J Biomater Sci Polym Ed 2022;33:569–89. [DOI] [PubMed] [Google Scholar]
- 170. Liu Z, Rütten S, Buhl EM, Zhang M, Liu J, Rojas-González DM, Mela P.. Development of a silk fibroin-small intestinal submucosa small-diameter vascular graft with sequential VEGF and TGF-beta 1 inhibitor delivery for in situ tissue engineering. Macromol Biosci 2023;23:e2300184. [DOI] [PubMed] [Google Scholar]
- 171. Hwang SH, Kim J, Heo C, Yoon J, Kim H, Lee SH, Park HW, Heo MS, Moon HE, Kim C, Paek SH, Jang J.. 3D printed multi-growth factor delivery patches fabricated using dual-crosslinked decellularized extracellular matrix-based hybrid inks to promote cerebral angiogenesis. Acta Biomater 2023;157:137–48. [DOI] [PubMed] [Google Scholar]
- 172. Bharathi R, Ganesh SS, Harini G, Vatsala K, Anushikaa R, Aravind S, Abinaya S, Selvamurugan N.. Chitosan-based scaffolds as drug delivery systems in bone tissue engineering. Int J Biol Macromol 2022;222:132–53. [DOI] [PubMed] [Google Scholar]
- 173. Singh H, Purohit SD, Bhaskar R, Yadav I, Bhushan S, Gupta MK, Mishra NC.. Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J Biomed Mater Res B Appl Biomater 2022;110:210–9. [DOI] [PubMed] [Google Scholar]
- 174. Chandika P, Khan F, Heo S-Y, Kim Y-M, Yi M, Jung W-K.. Enhanced wound-healing capability with inherent antimicrobial activities of usnic acid incorporated poly(ε-caprolactone)/decellularized extracellular matrix nanofibrous scaffold. Biomater Adv 2022;140:213046. [DOI] [PubMed] [Google Scholar]
- 175. Arin A, Rahaman MS, Farwa U, Lee BT.. Faster and protective wound healing mechanistic of para-coumaric acid loaded liver ECM scaffold cross-linked with acellular marine kelp. Adv Funct. Mater 2023;33:2212325. [Google Scholar]
- 176. Wang FY, Zhao L, Song FY, Wu JY, Zhou QJ, Xie LX.. Hybrid natural hydrogels integrated with voriconazole-loaded microspheres for ocular antifungal applications. J Mater Chem B 2021;9:3377–88. [DOI] [PubMed] [Google Scholar]
- 177. Liu S, Zhao YS, Li M, Nie L, Wei QQ, Okoro OV, Jafari H, Wang S, Deng J, Chen JH, Shavandi A, Fan LH.. Bioactive wound dressing based on decellularized tendon and GelMA with incorporation of PDA-loaded asiaticoside nanoparticles for scarless wound healing. Chem Eng J 2023;466:143016. [Google Scholar]
- 178. Li Q, Gao Y, Zhang JJ, Tang YF, Sun YY, Wu LJ, Wu H, Shen MF, Liu XH, Han L, Xu ZY.. Crosslinking and functionalization of acellular patches via the self-assembly of copper@tea polyphenol nanoparticles. Regen Biomater 2022;9:rbac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kesim MG, Durucan C, Atila D, Keskin D, Tezcaner A.. Decellularized adipose tissue matrix-coated and simvastatin-loaded hydroxyapatite microspheres for bone regeneration. Biotechnol Bioeng 2022;119:2574–89. [DOI] [PubMed] [Google Scholar]
- 180. Wang Y, Wei X, Wang L, Qian Z, Liu H, Fan Y.. Quercetin-based composite hydrogel promotes muscle tissue regeneration through macrophage polarization and oxidative stress attenuation. Compos Pt B Eng 2022;247:110311. [Google Scholar]
- 181. Ni R, Luo Y, Jiang L, Mao X, Feng Y, Tuersun S, Hu Z, Zhu Y.. Repairing gastric ulcer with hyaluronic acid/extracellular matrix composite through promoting M2-type polarization of macrophages. Int J Biol Macromol 2023;245:125556. [DOI] [PubMed] [Google Scholar]
- 182. Xie XB, Wang WS, Cheng J, Liang HF, Lin ZF, Zhang T, Lu Y, Li Q.. Bilayer pifithrin-alpha loaded extracellular matrix/PLGA scaffolds for enhanced vascularized bone formation. Colloids Surf B Biointerfaces 2020;190:110903. [DOI] [PubMed] [Google Scholar]
- 183. Zhou MY, Guo M, Shi XC, Ma J, Wang ST, Wu S, Yan WQ, Wu F, Zhang PB.. Synergistically promoting bone regeneration by icariin-incorporated porous microcarriers and decellularized extracellular matrix derived from bone marrow mesenchymal stem cells. Front Bioeng Biotechnol 2022;10:824025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Datta S, Rameshbabu AP, Bankoti K, Jana S, Roy S, Sen R, Dhara S.. Microsphere embedded hydrogel construct—binary delivery of alendronate and BMP-2 for superior bone regeneration. J Mater Chem B 2021;9:6856–69. [DOI] [PubMed] [Google Scholar]
- 185. Bai HL, Wang ZW, Li MX, Liu YF, Wang W, Sun P, Wei SB, Wang ZJ, Li JA, Dardik A.. Hyaluronic acid-heparin conjugated decellularized human great saphenous vein patches decrease neointimal thickness. J Biomed Mater Res B Appl Biomater 2020;108:2417–25. [DOI] [PubMed] [Google Scholar]
- 186. Wang X, Gu ZP, Wan JY, Zhou X, Zhu K, Wang X, Cao X, Yu XX, Peng X, Tang Y.. dECM based dusal-responsive vascular graft with enzyme-controlled adenine release for long-term patency. Int J Biol Macromol 2023;242:124618. [DOI] [PubMed] [Google Scholar]
- 187. Gao G, Lee JH, Jang J, Lee DH, Kong JS, Kim BS, Choi YJ, Jang WB, Hong YJ, Kwon SM, Cho DW.. Tissue engineered bio-blood-vessels vonstructed using a tissue-specific bioink and 3D coaxial cell printing technique: a novel therapy for ischemic disease. Adv Funct Mater 2017;27:1700798. [Google Scholar]
- 188. Yang Y, Lei D, Zou HX, Huang SX, Yang Q, Li S, Qing FL, Ye XF, You ZW, Zhao Q.. Hybrid electrospun rapamycin-loaded small-diameter decellularized vascular grafts effectively inhibit intimal hyperplasia. Acta Biomater 2019;97:321–32. [DOI] [PubMed] [Google Scholar]
- 189. Shi J, Teng YJ, Li D, He J, Midgley AC, Guo XQ, Wang XD, Yang XR, Wang SF, Feng YK, Lv Q, Hou S.. Biomimetic tri-layered small-diameter vascular grafts with decellularized extracellular matrix promoting vascular regeneration and inhibiting thrombosis with the salidroside. Mater Today Bio 2023;21:100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang YC, Tang Y, Wang Y, Zhang LY.. Nanomaterials for cardiac tissue engineering application. Nano-Micro Lett 2011;3:270–7. [Google Scholar]
- 191. Mehrali M, Thakur A, Pennisi CP, Talebian S, Arpanaei A, Nikkhah M, Dolatshahi-Pirouz A.. Nanoreinforced hydrogels for tissue engineering: biomaterials that are compatible with load-bearing and electroactive tissues. Adv Mater 2017;29:1603612. [DOI] [PubMed] [Google Scholar]
- 192. Kumar A, Nune KC, Misra RDK.. Biological functionality of extracellular matrix-ornamented three-dimensional printed hydroxyapatite scaffolds. J Biomed Mater Res A 2016;104:1343–51. [DOI] [PubMed] [Google Scholar]
- 193. Ko E, Alberti K, Lee JS, Yang K, Jin Y, Shin J, Yang HS, Xu Q, Cho SW.. Nanostructured tendon-derived scaffolds for enhanced bone regeneration by human adipose-derived stem cells. ACS Appl Mater Interfaces 2016;8:22819–29. [DOI] [PubMed] [Google Scholar]
- 194. Parmaksiz M, Elçin AE, Elçin YM.. Decellularized bovine small intestinal submucosa-PCL/hydroxyapatite-based multilayer composite scaffold for hard tissue repair. Mater Sci Eng C Mater Biol Appl 2019;94:788–97. [DOI] [PubMed] [Google Scholar]
- 195. Hwangbo H, Lee J, Kim G.. Mechanically and biologically enhanced 3D-printed HA/PLLA/dECM biocomposites for bone tissue engineering. Int J Biol Macromol 2022;218:9–21. [DOI] [PubMed] [Google Scholar]
- 196. Yang L, Jin S, Shi L, Ullah I, Yu K, Zhang W, Bo L, Zhang X, Guo X.. Cryogenically 3D printed biomimetic scaffolds containing decellularized small intestinal submucosa and Sr2+/Fe3+ co-substituted hydroxyapatite for bone tissue engineering. Chem Eng J 2022;431:133459. [Google Scholar]
- 197. Kuang ZH, Dai M.. Application of graphene oxide-based hydrogels in bone tissue engineering. ACS Biomater Sci Eng 2022;7:2849–57. [DOI] [PubMed] [Google Scholar]
- 198. Gong M, Sun JC, Liu GM, Li L, Wu S, Xiang Z.. Graphene oxide-modified 3D acellular cartilage extracellular matrix scaffold for cartilage regeneration. Mater Sci Eng C Mater Biol Appl 2021;119:111603. [DOI] [PubMed] [Google Scholar]
- 199. Pereira AT, Schneider KH, Henriques PC, Grasl C, Melo SF, Fernandes IP, Kiss H, Martins MCL, Bergmeister H, Goncalves IC.. Graphene oxide coating improves the mechanical and biological properties of decellularized umbilical cord arteries. ACS Appl Mater Interfaces 2021;13:32662–72. [DOI] [PubMed] [Google Scholar]
- 200. Kim D-H, Kim M-J, Kwak S-Y, Jeong J, Choi D, Choi SW, Ryu J, Kang K-S.. Bioengineered liver crosslinked with nano-graphene oxide enables efficient liver regeneration via MMP suppression and immunomodulation. Nat Commun 2023;14:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Barroca N, da Silva DM, Pinto SC, Sousa JPM, Verstappen K, Klymov A, Fernández-San-Argimiro F-J, Madarieta I, Murua O, Olalde B, Papadimitriou L, Karali K, Mylonaki K, Stratakis E, Ranella A, Marques PAAP.. Interfacing reduced graphene oxide with an adipose-derived extracellular matrix as a regulating milieu for neural tissue engineering. Biomater Adv 2023;148:213351. [DOI] [PubMed] [Google Scholar]
- 202. Tsui JH, Leonard A, Camp ND, Long JT, Nawas ZY, Chavanachat R, Smith AST, Choi JS, Dong Z, Ahn EH, Wolf-Yadlin A, Murry CE, Sniadecki NJ, Kim D-H.. Tunable electroconductive decellularized extracellular matrix hydrogels for engineering human cardiac microphysiological systems. Biomaterials 2021;272:120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Zheng ZW, Chen YH, Hong H, Shen Y, Wang Y, Sun J, Wang XS.. The "yin and yang" of immunomodulatory magnesium-enriched graphene oxide nanoscrolls decorated biomimetic scaffolds in promoting bone regeneration. Adv Healthc Mater 2021;10:2000631. [DOI] [PubMed] [Google Scholar]
- 204. Bankoti K, Rameshbabu AP, Datta S, Roy M, Goswami P, Roy S, Das AK, Ghosh SK, Dhara S.. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J Mater Chem B 2020;8:9277–94. [DOI] [PubMed] [Google Scholar]
- 205. Singh H, Purohit SD, Bhaskar R, Yadav I, Bhushan S, Gupta MK, Gautam S, Showkeen M, Mishra NC.. Biomatrix from goat-waste in sponge/gel/powder form for tissue engineering and synergistic effect of nanoceria. Biomed Mater 2021;16:025008. [DOI] [PubMed] [Google Scholar]
- 206. Singh H, Bashir SM, Purohit SD, Bhaskar R, Rather MA, Ali SI, Yadav I, Makhdoomi DM, Din Dar MU, Gani MA, Gupta MK, Mishra NC.. Nanoceria laden decellularized extracellular matrix-based curcumin releasing nanoemulgel system for full-thickness wound healing. Biomater Adv 2022;137:212806. [DOI] [PubMed] [Google Scholar]
- 207. Kafili G, Tamjid E, Niknejad H, Simchi A.. Development of injectable hydrogels based on human amniotic membrane and polyethyleneglycol-modified nanosilicates for tissue engineering applications. Eur Polym J 2022;179:111566. [Google Scholar]
- 208. Shin YJ, Shafranek RT, Tsui JH, Walcott J, Nelson A, Kim DH.. 3D bioprinting of mechanically tuned bioinks derived from cardiac decellularized extracellular matrix. Acta Biomater 2021;119:75–88. [DOI] [PubMed] [Google Scholar]
- 209. Shen S, Liu R, Song C, Shen T, Zhou Y, Guo J, Kong B, Jiang Q.. Fish scale-derived scaffolds with MSCs loading for photothermal therapy of bone defect. Nano Res 2023;16:7383–92. [Google Scholar]
- 210. Jin YY, Zhang JB, Xu YT, Yi K, Li FF, Zhou HC, Wang HX, Chan HF, Lao YH, Lv SX, Tao Y, Li MQ.. Stem cell-derived hepatocyte therapy using versatile biomimetic nanozyme incorporated nanofiber-reinforced decellularized extracellular matrix hydrogels for the treatment of acute liver failure. Bioact Mater 2023;28:112–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Nagelkerke A, Ojansivu M, van der Koog L, Whittaker TE, Cunnane EM, Silva AM, Dekker N, Stevens MM.. Extracellular vesicles for tissue repair and regeneration: evidence, challenges and opportunities. Adv Drug Deliv Rev 2021;175:113775. [DOI] [PubMed] [Google Scholar]
- 212. Ju YK, Hu Y, Yang P, Xie XY, Fang BR.. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio 2023;18:100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Liu X, Zhang J, Cheng X, Liu P, Feng Q, Wang S, Li Y, Gu H, Zhong L, Chen M, Zhou L.. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regen Biomater 2022;10:rbac085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Wang YYF, Kong B, Chen X, Liu R, Zhao YJ, Gu ZX, Jiang Q.. BMSC exosome-enriched acellular fish scale scaffolds promote bone regeneration. J Nanobiotechnology 2022;20:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Xing HY, Zhang ZJ, Mao QJ, Wang CG, Zhou YL, Zhou XP, Ying LW, Xu HB, Hu SJ, Zhang N.. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnology 2021;19:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Liao ZW, Ke WC, Liu H, Tong BD, Wang K, Feng XB, Hua WB, Wang BJ, Song Y, Luo RJ, Liang HZ, Zhang WF, Zhao KC, Li S, Yang C.. Vasorin-containing small extracellular vesicles retard intervertebral disc degeneration utilizing an injectable thermoresponsive delivery system. J Nanobiotechnology 2022;20:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Li Q, Yu HL, Zhao FY, Cao CX, Wu T, Fan YF, Ao YF, Hu XQ.. 3D printing of microenvironment-specific bioinspired and exosome-reinforced hydrogel scaffolds for efficient cartilage and subchondral bone regeneration. Adv Sci 2023;10:2303650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Kang Y, Xu J, Meng LA, Su Y, Fang H, Liu JQ, Cheng YY, Jiang DQ, Nie Y, Song KD.. 3D bioprinting of dECM/gel/QCS/nHAp hybrid scaffolds laden with mesenchymal stem cell-derived exosomes to improve angiogenesis and osteogenesis. Biofabrication 2023;15:024103. [DOI] [PubMed] [Google Scholar]
- 219. Hu YQ, Wu B, Xiong Y, Tao RY, Panayi AC, Chen L, Tian WQ, Xue H, Shi L, Zhang XL, Xiong LM, Mi BB, Liu GH.. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem Eng J 2021;426:130634. [Google Scholar]
- 220. Roh EJ, Kim DS, Kim JH, Lim CS, Choi H, Kwon SY, Park SY, Kim JY, Kim HM, Hwang DY, Han DK, Han I.. Multimodal therapy strategy based on a bioactive hydrogel for repair of spinal cord injury. Biomaterials 2023;299:122160. [DOI] [PubMed] [Google Scholar]
- 221. Ko KW, Park SY, Lee EH, Yoo YI, Kim DS, Kim JY, Kwon TG, Han DK.. Integrated bioactive scaffold with polydeoxyribonucleotide and stem-cell-derived extracellular vesicles for kidney regeneration. ACS Nano 2021;15:7575–85. [DOI] [PubMed] [Google Scholar]