Abstract
Background:
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) blood pressure (BP) guideline lowered the BP thresholds for defining hypertension compared with the Seventh Report of the Joint National Committee (JNC7).
Methods:
We analyzed clinic and ambulatory BP monitoring data from 717 Coronary Artery Risk Development in Young Adults study participants and compared the prevalence of clinic and out-of-clinic BP phenotypes using thresholds from the 2017 ACC/AHA and JNC7 guidelines.
Results:
Among participants not taking antihypertensive medication and according to the JNC7 and 2017 ACC/AHA guidelines, 11.1% and 30.1% of participants had clinic hypertension, 37.5% and 57.9% had awake hypertension, 35.7% and 58.1% had asleep hypertension, and 35.7% and 58.6% had 24-hour hypertension, respectively. According to the JNC7 and 2017 ACC/AHA guideline definitions, 1.9% and 3.2% had white coat hypertension, 28.2% and 31.0% had masked hypertension and 9.3% and 26.9% had sustained hypertension, respectively. Among participants taking antihypertensive medication and when defined using the JNC7 and 2017 ACC/AHA guideline BP thresholds, 18.6% and 45.6% had uncontrolled clinic BP, 48.1% and 62.5% had uncontrolled awake BP, 48.1% and 70.2% had uncontrolled asleep BP and, 47.7% and 65.3% had uncontrolled 24-hour BP, respectively. Using JNC7 and 2017 ACC/AHA guideline BP thresholds, the prevalence was 1.4% and 5.2% for white coat effect, 30.9% and 22.5% for masked uncontrolled hypertension, and 17.2% and 40.0% for sustained uncontrolled BP, respectively.
Conclusion:
The 2017 ACC/AHA guideline results in a substantially higher prevalence of awake, asleep, 24-hour, and sustained hypertension.
Keywords: ambulatory blood pressure, prevalence, blood pressure thresholds, guideline
Introduction
Based on blood pressure (BP) measured in the clinic setting, hypertension in the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline for the Prevention, Detection, Evaluation and Management of High BP is defined by systolic BP (SBP) ≥130 mm Hg or diastolic BP (DBP) ≥80 mm Hg [1]. These BP levels are lower than those used to define hypertension in the Seventh Report of the Joint National Committee (JNC7) [SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg [1, 2]. The lower BP thresholds in the 2017 ACC/AHA guideline has been estimated to increase the prevalence of hypertension from 31.9% to 45.6% among US adults [3].
Prior studies have demonstrated that BP measured outside of the clinic setting has a stronger association with target organ damage compared with BP measured in the clinic [4–7]. The lower clinic BP thresholds used to define hypertension in the 2017 ACC/AHA guideline correspond with lower out-of-clinic BP thresholds (Table 1). As with hypertension defined solely by clinic BP, the prevalence of hypertension phenotypes derived using BP measurements obtained outside of the clinic with ambulatory BP monitoring (ABPM), alone or in combination with clinic measurements, may be substantially different when using clinic and ABPM thresholds from the 2017 ACC/AHA guideline versus the JNC7 guideline. Therefore, we compared the prevalence of hypertension and uncontrolled BP based on clinic and out-of-clinic BP measurements defined using thresholds corresponding to clinic SBP/DBP thresholds of 130/80 mm Hg (2017 ACC/AHA guideline) and 140/90 mm Hg (JNC7 guideline) in the population-based Coronary Artery Risk Development in Young Adults (CARDIA) study. In addition, we compared the association between each of these hypertension phenotypes, defined using the 2017 ACC/AHA versus the JNC7 guideline, with left ventricular mass index (LVMI).
Table 1.
Systolic and diastolic blood pressure thresholds on ambulatory blood pressure monitoring corresponding with clinic blood pressure thresholds for defining phenotypes in the 2017 ACC/AHA and JNC7 blood pressure guidelines.
| Type of blood pressure phenotypes | 2017 ACC/AHA guideline | JNC7 guideline | |
|---|---|---|---|
| Not taking antihypertensive medication | Taking antihypertensive medication | Systolic/Diastolic blood pressure, mm Hg | Systolic/Diastolic blood pressure, mm Hg |
| Clinic hypertension | Uncontrolled clinic blood pressure | ≥130/80 | ≥140/90 |
| Awake hypertension | Uncontrolled awake blood pressure | ≥130/80 | ≥135/85 |
| Asleep hypertension | Uncontrolled asleep blood pressure | ≥110/65 | ≥120/70# |
| 24-hour hypertension | Uncontrolled 24-hour blood pressure | ≥125/75 | ≥130/80 |
| Sustained normotension‡‡ | Sustained controlled BP‡‡ | Clinic <130/80 and awake <130/80 | Clinic <140/90 and awake <135/85 |
| White coat hypertension* | White coat effect* | Clinic ≥130/80 and awake <130/80 | Clinic ≥140/90 and awake <135/85 |
| Masked hypertension† | Masked uncontrolled hypertension† | Clinic <130/80 and awake ≥130/80 | Clinic <140/90 and awake ≥135/85 |
| Sustained hypertension‡ | Sustained uncontrolled BP‡ | Clinic ≥130/80 and awake ≥130/80 | Clinic ≥140/90 and awake ≥135/85 |
ACC/AHA: American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults [1].
JNC7: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [2].
The systolic and diastolic blood pressure thresholds for asleep hypertension and uncontrolled asleep blood pressure according to the Seventh Report of the Joint National Committee guideline are 120/75 mm Hg. However, guidelines and scientific statements have since adopted asleep systolic/diastolic blood pressure thresholds of 120/70 mm Hg to correspond with clinic-measured systolic/diastolic blood pressure of 140/90 mm Hg. The systolic and diastolic blood pressure thresholds for awake, asleep, and 24-hour hypertension, and uncontrolled awake, asleep, and 24-hour blood pressure corresponding to clinic systolic and diastolic blood pressure levels of 140 mm Hg and 90 mm Hg and 130 mm Hg and 80 mm Hg were published in the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults guideline.
Sustained normotension and sustained controlled BP were evaluated among all participants.
White coat hypertension and white coat effect were evaluated among all participants and among participants with clinic systolic/diastolic blood pressure in the hypertension range.
Masked hypertension and masked uncontrolled hypertension were evaluated among all participants and among participants with clinic systolic/diastolic blood pressure not in the hypertension range.
Sustained hypertension and sustained uncontrolled BP were evaluated among all participants.
Methods
Study population
The CARDIA study was designed to examine the development and determinants of cardiovascular disease (CVD) and its risk factors [8]. In 1985–86, 5115 black and white adults aged 18 to 30 years old were recruited and enrolled into the CARDIA study at four field centers (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). The current analysis was restricted to the 825 CARDIA study participants who underwent ABPM in an ancillary study following the Year 30 exam at the Birmingham and Chicago field centers. We excluded 44 participants without a complete ABPM recording (defined below), one participant missing data on antihypertensive medication use and 63 participants without echocardiography data. After these exclusions, data for 717 participants were analyzed. The CARDIA study protocol was approved by the Institutional Review Boards at the participating centers. Written informed consent was provided by all participants.
Data collection
Participants’ date of birth collected at baseline was used to calculate age at exam Year 30. Information on sex and race was collected at baseline by questionnaires, with the remaining variables used in the current analysis collected as part of the Year 30 exam. Highest level of education attained, current smoking status, and antihypertensive and antidiabetes medication use were ascertained by self-administered questionnaires. Using height and weight measured during the exam, body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Diabetes was defined as a fasting (≥8 hours) serum glucose ≥126 mg/dL or current use of antidiabetes medication. Total and high-density lipoprotein (HDL) cholesterol were quantified by precipitation with dextran sulfate-magnesium chloride and triglycerides were quantified enzymatically. Low-density lipoprotein (LDL) cholesterol among participants with triglycerides ≤400 mg/dL was calculated using the Friedewald equation [9]. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [10]. Reduced eGFR was defined by levels <60 mL/min/1.73m2. Urinary albumin and creatinine were quantified using the nephelometric immunoassay and enzymatic methods, respectively [11]. Albuminuria was defined as a urine albumin to urine creatinine ratio ≥30 mg/g.
BP measurement
Clinic SBP and DBP were measured on each participant’s right arm by trained and certified staff following standardized protocols using an automated oscillometric device (Omron model® HEM907XL). An appropriate cuff size was selected after measuring participants’ arm circumference at the midpoint between the acromion and olecranon. Participants rested for five minutes in a seated position with their feet flat on the floor, back supported and right arm positioned at heart level before having their clinic BP measured. Three BP measurements, each separated by at least 30 seconds, were recorded. The second and third BP measurements were averaged for analysis.
Following the Year 30 study visit, participants were fitted with an ABPM device (Spacelabs Model 90227; Spacelabs Healthcare, Snoqualmie, WA) on their non-dominant arm. SBP and DBP were measured every 30 minutes for 24 hours. If the device failed to obtain a valid reading, it tried once more 3 minutes later. To be considered to have a complete ABPM recording, participants were required to have at least ten valid awake and five valid asleep SBP and DBP readings [12]. Mean SBP and DBP were each calculated using readings while participants were awake and asleep, separately. Periods when participants were awake and asleep were determined using actigraphy supplemented by sleep diaries. Mean 24-hour SBP and DBP were calculated using all of the readings from the ABPM recording period weighted to the amount of time each participant was awake and asleep [13]. For participants not taking antihypertensive medication, clinic, awake, asleep and 24-hour hypertension, sustained normotension and white-coat, masked and sustained hypertension were defined using clinic SBP and DBP thresholds corresponding with the 2017 ACC/AHA BP guideline and the JNC7 guideline (Table 1) [1, 2]. For participants taking antihypertensive medication, uncontrolled clinic, awake, asleep and 24-hour BP and sustained controlled BP, white coat effect, masked uncontrolled hypertension and sustained uncontrolled BP were calculated according to thresholds from each guideline.
Echocardiography
Sonographers were trained and certified to conduct 2D-guided M-mode echocardiography using an Artida cardiac ultrasound scanner (Toshiba Medical Systems, Otawara, Japan) following standardized protocols [14]. The quality of sonographers’ echocardiograms was monitored, and retraining was provided as needed. Interventricular septal thickness at end-diastole (IVSD), left ventricular end-diastolic diameter (LVEDD), and posterior wall thickness at end-diastole (PWTD) were measured in accordance with the 2015 American Society of Echocardiography (ASE) and European Society of Cardiovascular Imaging recommendations [15]. LVM was quantified as 0.8 × (1.04 × ((IVSD + LVEDD + PWTD)3 – (LVEDD)3)) + 0.6 and indexed to height2.7 [15].
Statistical analysis
Characteristics were calculated as mean ± standard deviation or percentage for participants who attended the Year 30 CARDIA study visit at the Birmingham and Chicago field centers. We compared the characteristics of participants who were and were not included in the current analysis (i.e., those who did and did not have complete ABPM recordings and valid echocardiography data). Among participants who had complete ABPM and echocardiography data and were included in this analysis, characteristics were calculated overall and among participants taking and not taking antihypertensive medication, separately.
The analyses described below for awake hypertension were repeated for clinic, asleep and 24-hour hypertension, and sustained normotension and white-coat, masked, sustained hypertension, uncontrolled awake, asleep and 24-hour BP and sustained controlled BP, white coat effect, masked uncontrolled hypertension and sustained uncontrolled BP defined using the JNC7 and 2017 ACC/AHA guidelines, separately. The prevalence of awake hypertension, defined using BP thresholds from the JNC7 and 2017 ACC/AHA guidelines, was calculated. Additionally, the prevalence of awake hypertension was calculated in subgroups further stratified by sex and by race. The difference in the prevalence of awake hypertension defined using BP thresholds from the JNC7 and 2017 ACC/AHA guidelines was calculated with the 95% confidence interval (CI) estimated using a 1,000 iteration bootstrap with bias correction [16].
Mean LVMI was calculated among participants with and without awake hypertension according to the JNC7 and 2017 ACC/AHA guideline definitions, separately. The mean difference in LVMI associated with having versus not having awake hypertension comparing the JNC7 and 2017 ACC/AHA guideline definitions was calculated using linear regression. After an unadjusted model, we conducted a model adjusted for the field center, age, sex, race, education, current smoking, BMI, HDL cholesterol, LDL cholesterol, diabetes, eGFR and albuminuria. The statistical significance of differences in the association of awake hypertension with LVMI, comparing the JNC7 with 2017 ACC/AHA definitions for awake hypertension was determined by 1,000 iteration bias-corrected bootstraps [16]. Also, the mean LVMI was calculated for three mutually exclusive groups defined by (1) not having awake hypertension according to the 2017 ACC/AHA or the JNC7 guideline, (2) having awake hypertension according to the 2017 ACC/AHA guideline but not the JNC7 guideline and (3) having awake hypertension according to both guidelines.
The main analyses for the prevalence of white coat hypertension and masked hypertension were conducted among all participants not taking antihypertensive medication with those for white coat effect and masked uncontrolled hypertension were conducted among all participants taking antihypertensive medication. We also calculated the prevalence of these phenotypes conditioned on clinic BP levels (e.g., the prevalence of white coat hypertension among participants with clinic-measured BP in the hypertension range and the prevalence of masked hypertension among participants with clinic-measured BP not in the hypertension range). P-values <0.05 were considered statistically significant. All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of participants
Among participants from the Birmingham and Chicago CARDIA field centers who attended the Year 30 study visit, those included in this analysis were less likely to be men and more likely to be black and taking antihypertensive medication than their counterparts who were not included in this analysis (Supplemental Table 1). The mean age of study participants meeting the inclusion criteria for the current analysis was 54.7 years, 41.0% were men, 62.8% were black, and 39.8% were taking antihypertensive medication (Table 2). Participants taking antihypertensive medication were less likely to be men, more likely to be black and to have diabetes, reduced eGFR, and albuminuria than their counterparts not taking antihypertensive medication. Additionally, mean LDL and HDL cholesterol were lower and mean BMI and clinic, asleep, and 24-hour SBP and DBP were higher among participants taking versus not taking antihypertensive medication. Mean awake SBP was higher and mean awake DBP was the same among participants taking versus not taking antihypertensive medication.
Table 2.
Characteristics of Coronary Artery Risk Development in Young Adults participants included in the current analysis, overall and, by antihypertensive medication use.
| Characteristic | Overall | Antihypertensive medication use | |
|---|---|---|---|
| (N= 717) | No | Yes | |
| (n=432) | (n=285) | ||
| Birmingham field center, n (%) | 420 (58.6) | 236 (54.6) | 184 (64.6) |
| Age, years | 54.7 ± 3.7 | 54.5 ± 3.8 | 55.0 ± 3.7 |
| Men, n (%) | 294 (41.0) | 190 (44.0) | 104 (36.5) |
| Black, n (%) | 450 (62.8) | 223 (51.6) | 227 (79.7) |
| Education less than high school, n (%) | 27 (3.8) | 15 (3.5) | 12 (4.2) |
| Current smoking, n (%) | 113 (15.8) | 62 (14.4) | 51 (17.9) |
| Body mass index, kg/m2 | 31.3 ± 7.0 | 29.3 ± 6.1 | 34.3 ± 7.1 |
| LDL cholesterol, mg/dL | 111.7 ± 33.4 | 115.0 ± 32.4 | 106.6 ± 34.3 |
| HDL cholesterol, mg/dL | 59.5 ± 19.6 | 61.1 ± 19.5 | 57.2 ± 19.6 |
| Diabetes, n (%) | 121 (16.9) | 32 (7.4) | 89 (31.2) |
| Reduced eGFR, n (%) | 27 (3.8) | 6 (1.4) | 21 (7.4) |
| Albuminuria, n (%) | 61 (8.6) | 20 (4.7) | 41 (14.5) |
| Systolic blood pressure, mm Hg | |||
| Clinic | 122 ± 17 | 120 ± 16 | 124 ± 18 |
| Awake | 130 ± 15 | 128 ± 15 | 132 ± 15 |
| Asleep | 112 ± 16 | 110 ± 15 | 116 ± 16 |
| 24-hour | 124 ± 15 | 123 ± 14 | 127 ± 15 |
| Diastolic blood pressure, mm Hg | |||
| Clinic | 74 ± 11 | 73 ± 11 | 76 ± 11 |
| Awake | 81 ± 9 | 81 ± 9 | 81 ± 10 |
| Asleep | 67 ± 9 | 66 ± 9 | 68 ± 10 |
| 24-hour | 77 ± 9 | 76 ± 8 | 77 ± 9 |
The numbers in the table are number (percentage) or mean ± standard deviation.
LDL: low-density lipoprotein.
HDL: high-density lipoprotein.
eGFR: estimated glomerular filtration rate, reduced eGFR was defined as < 60 mL/min/1.73m2.
Albuminuria: urine albumin-to-creatinine ratio ≥ 30 mg/g.
Awake, asleep and 24-hour BP
Among participants not taking antihypertensive medication, the prevalence of clinic, awake, asleep, and 24-hour hypertension were between 19.0 and 22.9 percentage points higher when defined using the BP thresholds in the 2017 ACC/AHA versus JNC7 guideline (Figure 1 and Supplemental Table 2). Among participants taking antihypertensive medication, the prevalence of uncontrolled clinic, awake, asleep, and 24-hour BP were between 14.4 and 26.7 percentage points higher when defined using the BP thresholds in the 2017 ACC/AHA versus JNC7 guideline (Figure 1 and Supplemental Table 2). Results were similar for men and women (Supplemental Table 3), and in whites and blacks (Supplemental Table 4).
Figure 1.
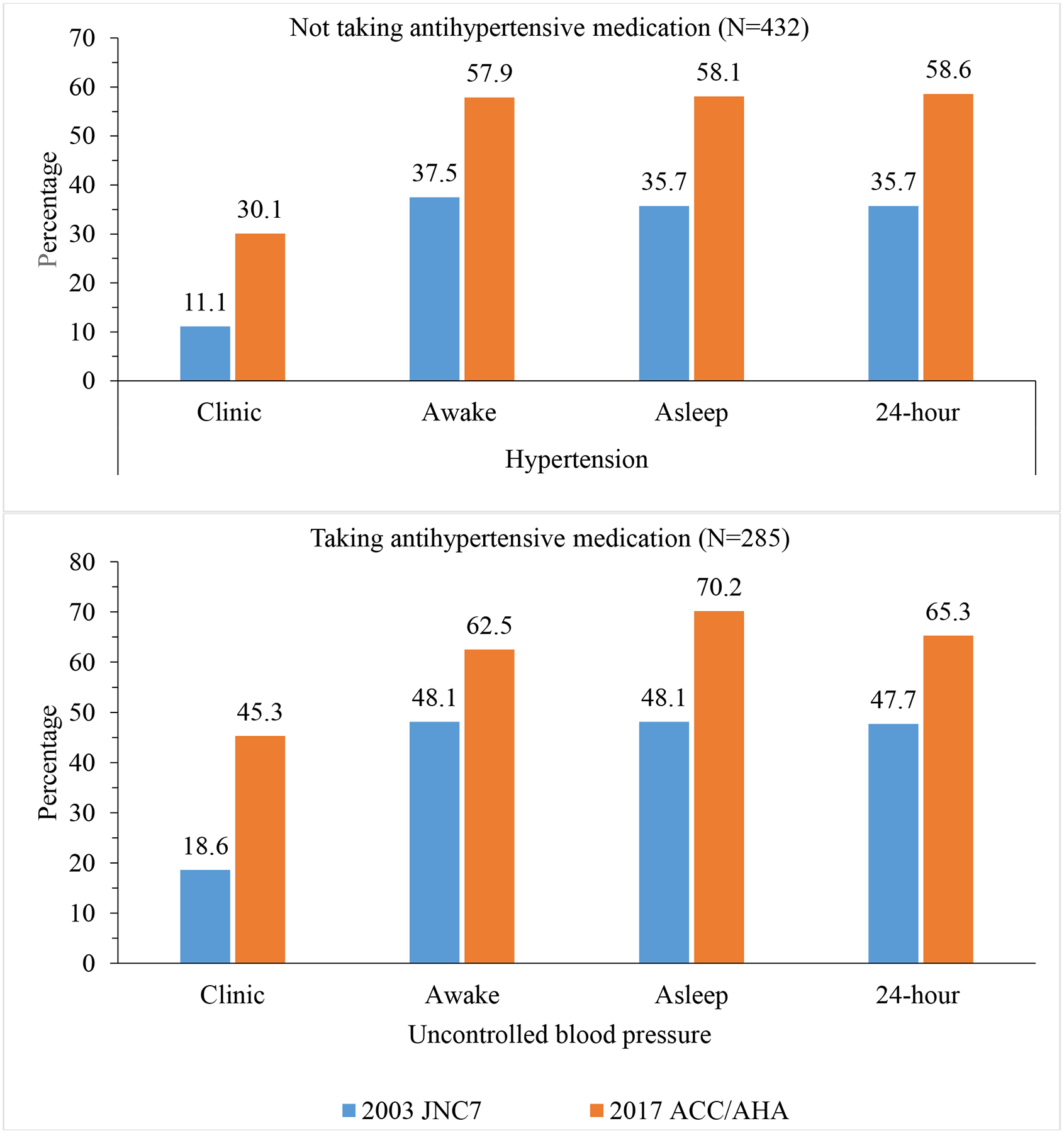
Prevalence of clinic, awake, asleep and 24-hour hypertension and uncontrolled clinic, awake, asleep, and 24-hour blood pressure according to blood pressure threshold in the 2017 ACC/AHA and JNC7 guidelines.
ACC/AHA: American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults [1].
JNC7: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [2].
Association of awake, asleep and 24-hour BP with LVMI
For participants not taking antihypertensive medication, mean LVMI was higher among participants with versus without clinic, awake, asleep, and 24-hour hypertension defined using the 2017 ACC/AHA guideline or the JNC7 guideline. Similarly for those taking antihypertensive medication, mean LVMI was higher among those with versus without uncontrolled clinic, awake and 24-hour BP defined using either guideline (Table 3). These associations were present before and after multivariable adjustment and were not statistically significantly different when the phenotypes were defined using the 2017 ACC/AHA guideline versus the JNC7 guideline. There was no evidence that mean LVMI differed for participants with awake, asleep, and 24-hour hypertension and uncontrolled awake, asleep, and 24-hour BP, according to the 2017 ACC/AHA guideline but not the JNC7 guideline compared with participants without hypertension by both the 2017 ACC/AHA and JNC7 (Supplemental Table 5).
Table 3.
Mean and mean difference in left ventricular mass index among participants with and without clinic, awake, asleep and 24-hour hypertension, and with and without uncontrolled clinic, awake, asleep and 24-hour blood pressure using blood pressure thresholds in the 2017 ACC/AHA and JNC7 guidelines.
| Mean and mean difference by blood pressure guideline | Antihypertensive medication use | |||
|---|---|---|---|---|
| No (n=432) | Yes (n=285) | |||
| Clinic hypertension | Uncontrolled clinic blood pressure | |||
| Mean (standard deviation) | No | Yes | No | Yes |
| 2017 ACC/AHA | 34.8 (8.7) | 39.7 (9.5) | 41.6 (12.8) | 47.4 (14.3) |
| JNC7 | 35.8 (8.8) | 40.2 (11.7) | 42.9 (13.2) | 50.0 (15.0) |
| Adjusted mean differencea (95% confidence interval) | ||||
| 2017 ACC/AHA | 0 (ref) | 3.00 (1.04, 4.94) | 0 (ref) | 5.10 (2.00, 8.20) |
| JNC7 | 0 (ref) | 3.24 (0.48, 6.00) | 0 (ref) | 6.27 (2.41, 10.13) |
| p-valueb | 0.887 | 0.567 | ||
| Awake hypertension | Uncontrolled awake blood pressure | |||
| Mean (standard deviation) | No | Yes | No | Yes |
| 2017 ACC/AHA | 34.0 (8.0) | 38.0 (9.7) | 42.0 (12.7) | 45.5 (14.3) |
| JNC7 | 34.5 (8.6) | 39.3 (9.5) | 42.1 (11.9) | 46.5 (15.3) |
| Adjusted mean differencea (95% confidence interval) | ||||
| 2017 ACC/AHA | 0 (ref) | 3.20 (1.40, 5.00) | 0 (ref) | 2.47 (−0.88, 5.82) |
| JNC7 | 0 (ref) | 3.75 (1.93, 5.57) | 0 (ref) | 3.00 (−0.18, 6.18) |
| p-valueb | 0.502 | 0.650 | ||
| Asleep hypertension | Uncontrolled asleep blood pressure | |||
| Mean (standard deviation) | No | Yes | No | Yes |
| 2017 ACC/AHA | 34.5 (8.0) | 37.6 (9.8) | 40.5 (10.0) | 45.8 (14.9) |
| JNC7 | 34.8 (8.0) | 39.1 (10.5) | 41.5 (10.8) | 47.1 (15.9) |
| Adjusted mean differencea (95% confidence interval) | ||||
| 2017 ACC/AHA | 0 (ref) | 1.09 (−0.79, 2.97) | 0 (ref) | 2.66 (−0.93, 6.25) |
| JNC7 | 0 (ref) | 2.69 (0.72, 4.66) | 0 (ref) | 2.51 (−0.68, 5.70) |
| p-valueb | 0.642 | 0.966 | ||
| 24-hour hypertension | Uncontrolled 24-hour blood pressure | |||
| Mean (standard deviation) | No | Yes | No | Yes |
| 2017 ACC/AHA | 34.2 (8.0) | 37.8 (9.7) | 40.8 (10.0) | 46.0 (15.1) |
| JNC7 | 34.5 (8.0) | 39.6 (10.3) | 41.7 (12.0) | 46.9 (15.1) |
| Adjusted mean differencea (95% confidence interval) | ||||
| 2017 ACC/AHA | 0 (ref) | 2.60 (0.83, 4.45) | 0 (ref) | 2.83 (−0.54, 6.20) |
| JNC7 | 0 (ref) | 3.97 (2.09, 5.85) | 0 (ref) | 2.98 (−0.25, 6.21) |
| p-valueb | 0.096 | 0.838 | ||
ACC/AHA: American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.
JNC7: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Adjusted for field center, age, sex, race, education less than high school, current smoking, body mass index, low and high-density lipoprotein cholesterol, diabetes, reduced estimated glomerular filtration rate, and albuminuria.
P-value comparing the difference in left ventricular mass index among participants with versus without hypertension and with versus without uncontrolled blood pressure according to the 2017 ACC/AHA guideline versus the JNC7 guideline.
Phenotypes defined by clinic and out-of-clinic BP
The prevalence of sustained normotension and sustained controlled BP were lower and the prevalence of sustained hypertension and sustained uncontrolled BP were higher when defined using thresholds from the 2017 ACC/AHA guideline versus the JNC7 guideline (Figure 2 and Supplemental Table 6). The prevalence of white coat hypertension and white coat effect among all participants (i.e., not conditioned on clinic BP) were both higher when defined using BP thresholds from the 2017 ACC/AHA guideline versus the JNC7 guideline. Overall population (i.e., not conditioned on clinic BP), the prevalence of masked hypertension was not statistically significantly different but the prevalence of masked uncontrolled hypertension was lower when defined using the 2017 ACC/AHA versus JNC7 guideline. Results are presented by sex in Supplemental Table 7 and by race in Supplemental Table 8. The prevalence of white coat hypertension, masked hypertension, white coat effect and masked uncontrolled hypertension, conditioned on clinic BP-based hypertension status are presented in Supplemental Table 9 and Supplemental Figure 1 and by sex and race in Supplemental Tables 10 and 11, respectively.
Figure 2.
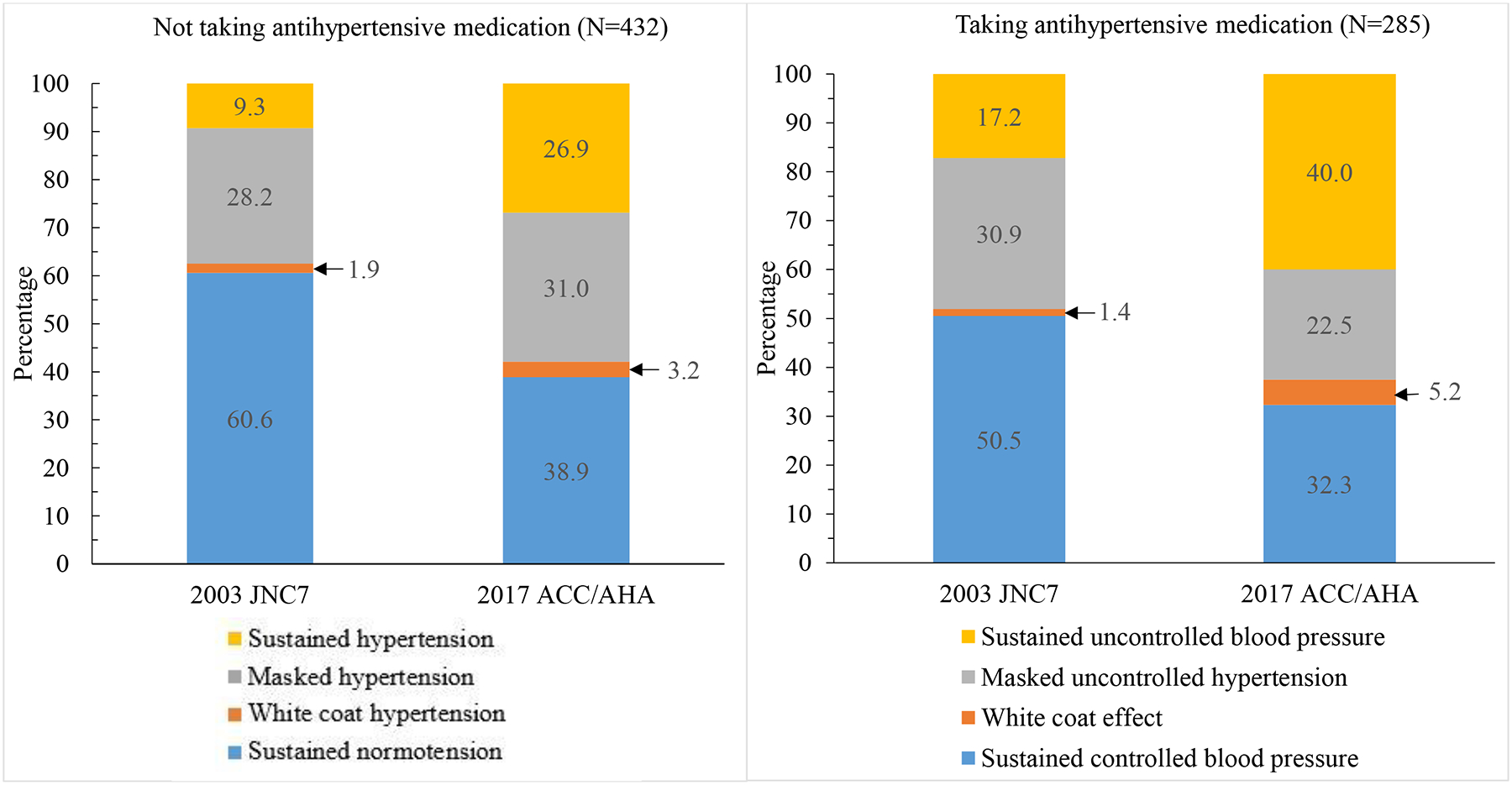
Prevalence of phenotypes based on clinic and out-of-clinic blood pressure levels according to the blood pressure thresholds in the 2017 ACC/AHA and JNC7 guidelines.
ACC/AHA: American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults [1].
JNC7: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [2].
Association of phenotypes defined by clinic and out-of-clinic BP with LVMI
Using the BP thresholds in the 2017 ACC/AHA or JNC7 guidelines, participants with sustained hypertension versus sustained normotension and sustained uncontrolled BP versus sustained controlled BP had higher LVMI (Table 4). White coat hypertension and white coat effect were not associated with LVMI. Participants with masked hypertension had higher LVMI when compared to their counterparts with sustained normotension. Masked uncontrolled hypertension was not associated with higher LVMI when compared to sustained controlled BP. These associations did not differ when each phenotype was defined using BP thresholds from the 2017 ACC/AHA versus JNC7 guidelines (all p-values >0.50). Mean LVMI was not statistically significantly different between participants without white coat hypertension, with white coat hypertension by only the 2017 ACC/AHA guideline and with white coat hypertension by both the guidelines versus sustained normotension or with white coat effect by only the 2017 ACC/AHA or by both the guidelines versus sustained controlled BP. (Supplemental Table 12). Among participants not taking antihypertensive medication, mean LVMI was higher for participants with masked hypertension according to the 2017 ACC/AHA guideline only and both guidelines versus those with sustained normotension by both guidelines (Supplemental Table 13). There was no evidence of a difference in LVMI between participants without masked hypertension, with masked hypertension by only the 2017 ACC/AHA guideline versus sustained normotension or without masked uncontrolled hypertension by both guidelines, with masked uncontrolled hypertension by only the 2017 ACC/AHA guideline, and with masked uncontrolled hypertension by both guidelines versus sustained controlled BP.
Table 4.
Mean and mean difference in left ventricular mass index among all participants with sustained normotension, white coat, masked, or sustained hypertension and among all participants with sustained controlled blood pressure, white coat effect, masked uncontrolled hypertension, and sustained uncontrolled blood pressure according to blood pressure thresholds in the 2017 ACC/AHA and JNC7 guidelines.
| Not taking antihypertensive medication (n=432) | Taking antihypertensive medication (n=285) | ||||
|---|---|---|---|---|---|
| Phenotype by guideline | Mean (SD) | Adjusted mean differencea (95% confidence interval) |
Phenotype by guideline | Mean (SD) | Adjusted mean differencea (95% confidence interval) |
| 2017 ACC/AHA | 2017 ACC/AHA | ||||
| Sustained normotension | 33.9 (8.1) | 0 (ref) | Sustained controlled BP | 41.3 (12.6) | 0 (ref) |
| White coat hypertension | 35.5 (7.1) | −0.90 (−5.68, 3.88) | White coat effect | 46.6 (12.6) | 3.17 (−3.84, 10.18) |
| Masked hypertension | 36.0 (9.4) | 1.96 (−0.16, 4.07) | Masked uncontrolled hypertension | 42.0 (13.1) | −0.43 (−4.66, 3.81) |
| Sustained hypertension | 40.2 (9.6) | 4.51 (2.27, 6.74) | Sustained uncontrolled BP | 47.5 (14.6) | 5.18 (1.37, 8.99) |
| JNC7 | JNC7 | ||||
| Sustained normotension | 34.5 (8.7) | 0 (ref) | Sustained controlled BP | 42.1 (11.9) | 0 (ref) |
| White coat hypertension | 33.1 (6.3) | −2.06 (−7.96, 3.84) | White coat effect | 41.1 (12.2) | 2.78 (−9.61, 15.17) |
| Masked hypertension | 38.6 (8.4) | 3.00 (0.97, 5.02) | Masked uncontrolled hypertension | 44.2 (15.0) | 1.01 (−2.56, 4.57) |
| Sustained hypertension | 41.6 (12.1) | 5.57 (2.53, 8.61) | Sustained uncontrolled BP | 50.7 (15.1) | 7.08 (2.76, 11.40) |
| p-valuesb | |||||
| White coat hypertension | 0.469 | White coat effect | 0.937 | ||
| Masked hypertension | 0.398 | Masked uncontrolled hypertension | 0.389 | ||
| Sustained hypertension | 0.500 | Sustained uncontrolled BP | 0.338 | ||
BP: blood pressure.
SD: standard deviation.
ACC/AHA: American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.
JNC7: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Adjusted for field center, age, sex, race, education less than high school, current smoking, body mass index, low and high-density lipoprotein cholesterol, diabetes, reduced estimated glomerular filtration rate, and albuminuria.
P-value comparing the difference in left ventricular mass index among participants with white coat, masked, and sustained hypertension, and white coat effect, masked uncontrolled hypertension, and sustained uncontrolled BP defined using the 2017 ACC/AHA guideline versus the JNC7 guideline.
Discussion
In the current population-based study, the prevalence of clinic, awake, asleep, 24-hour hypertension, and uncontrolled clinic, awake, asleep, 24-hour BP, and sustained hypertension and sustained uncontrolled BP were substantially higher and the prevalence of sustained normotension and sustained controlled BP was lower when defined according to the lower BP thresholds in the 2017 ACC/AHA guideline compared with the JNC7 guideline. Among all participants, the prevalence of white coat hypertension was higher when defined using BP thresholds in the 2017 ACC/AHA versus JNC7 guideline, but the difference was not statistically significant for those not taking antihypertensive medication. The prevalence of masked hypertension was higher while the prevalence of masked uncontrolled hypertension was lower when defined using the 2017 ACC/AHA versus JNC7 BP thresholds. The associations of clinic, awake, asleep, 24-hour, white coat, masked, and sustained hypertension, and uncontrolled clinic, awake, asleep, and 24-hour BP, white coat effect, masked uncontrolled hypertension, and sustained uncontrolled BP with LVMI were not statistically significantly different when defined using BP thresholds in the 2017 ACC/AHA versus JNC7 guidelines.
The United States Preventive Services Task Force (USPSTF) and 2017 ACC/AHA BP guideline recommend performing ABPM in adults with high clinic BP to confirm the diagnosis of hypertension [1, 17]. A USPSTF review of 27 studies found the prevalence of white coat hypertension, among adults with clinic BP in the hypertensive range, to be between 15% and 30% when using the JNC7 BP thresholds [17, 18]. In many studies, white coat hypertension has been associated with at most only a modestly increased risk for CVD [18, 19] and people with white coat hypertension may receive little cardiovascular risk reduction benefit from initiating antihypertensive medication [20]. In the current study, the prevalence of white coat hypertension and white coat effect in the overall population were relatively low when defined using BP thresholds in the 2017 ACC/AHA guideline. The prevalence of these phenotypes among adults with clinic BP in the hypertensive range were not statistically significantly different between guidelines. The low prevalence was partially due to relatively few participants with clinic BP in the hypertension range. However, when conditioned on having BP in hypertension range, the prevalence of white coat hypertension was consistent with prior studies [18]. A prior study estimated that 13.7% of US adults (~31.1 million) have clinic-measured SBP between 130 and 139 mm Hg or clinic-measured DBP between 80 and 89 mm Hg, and therefore have hypertension according to the 2017 ACC/AHA guideline but not JNC7 guideline [3]. The lower BP thresholds used to define hypertension in the 2017 ACC/AHA versus JNC7 guideline will result in more adults with BP in the hypertension range (i.e., more adults have SBP/DBP ≥ 130/80 mm Hg than SBP/DBP ≥ 140/90 mm Hg). For this reason, a larger number of US adults may have white coat hypertension according to the 2017 ACC/AHA guideline compared with the JNC7 guideline. This finding supports the use of ABPM for confirming the diagnosis of hypertension and excluding white coat hypertension as recommended by the USPSTF.
The 2017 ACC/AHA guideline recommends screening for masked hypertension [1]. Masked hypertension is associated with a two times higher risk for CVD compared with sustained normotension [21]. Prior studies have reported the prevalence of masked hypertension among adults with clinic-measured BP not in the hypertensive range between 15% and 30% when using the JNC7 BP thresholds [22, 23]. Also, it has been estimated that 16 million US adults have masked hypertension [24]. Although the prevalence of masked hypertension was not higher when defined using the 2017 ACC/AHA guideline versus the JNC7 guideline, over 40% of participants without clinic-measured BP in the hypertension range had masked and masked uncontrolled hypertension according to the 2017 ACC/AHA guideline. Currently, there are no large-scale outcome trials testing whether adults with masked hypertension receive CVD risk reduction benefits from initiating antihypertensive medication. Randomized trials are needed to test the feasibility, sustainability, benefits and harms of non-pharmacological interventions and initiation of antihypertensive medication for adults with masked hypertension.
The associations of higher awake, asleep, and 24-hour BP with higher LVM and increased risk for CVD, independent of clinic-measured BP, has been repeatedly reported [17, 25–27]. In the current study, these associations were not statistically significantly different when the hypertension phenotypes were defined using BP thresholds from the 2017 ACC/AHA and JNC7 guidelines. These data demonstrating associations of hypertension phenotypes, when defined according to the 2017 ACC/AHA guideline, with end-organ damage confirm the lower BP thresholds are appropriate for identifying adults with a higher prevalence of subclinical CVD. Future studies are needed to determine the association of hypertension phenotypes defined using out-of-clinic BP thresholds from the 2017 ACC/AHA guideline with CVD events. Given the strong association between awake, asleep, 24-hour hypertension, and uncontrolled awake, asleep, and 24-hour BP with CVD, and the high prevalence of these phenotypes, the population attributable risk associated with awake, asleep, and 24-hour hypertension may be substantial.
The current study has several strengths. These analyses were conducted in a large US population-based cohort of whites and blacks. Clinic and ambulatory BP were measured following a standardized protocol. The conduct of high-quality echocardiograms permitted the assessment of the impact of BP thresholds from the 2017 ACC/AHA guideline on target organ damage. The results should also be interpreted in the context of limitations of the study. ABPM data were collected at only two of the four CARDIA study field centers. The CARDIA study did not enroll Hispanic and Asian participants, limiting the generalizability of the present results. The prevalence estimates from the current study may not be generalizable to the US population. In addition, clinic and ambulatory BP were measured at only one visit for each participant. Finally, some characteristics differed between participants who did and did not complete the CARDIA ABPM ancillary study.
In conclusion, among participants not taking and taking antihypertensive medication, the prevalence of clinic, awake, asleep, 24-hour, and sustained hypertension, and uncontrolled clinic, awake, asleep, 24-hour blood pressure, and sustained uncontrolled BP were each substantially higher when defined using BP thresholds from the 2017 ACC/AHA BP guideline versus the JNC7 guideline. Among all participants, the prevalence of white coat hypertension was higher when defined using the 2017 ACC/AHA guideline versus JNC7 guideline whereas the prevalence of masked uncontrolled hypertension was lower for those taking antihypertensive medication. The high prevalence of white coat and masked hypertension using BP thresholds in the 2017 ACC/AHA guideline supports the recommendation for out-of-clinic BP monitoring.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the CARDIA study for their valuable contributions.
Disclosure of funding:
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). The funding to conduct ambulatory blood pressure monitoring in the CARDIA study was provided by grant 15SFRN2390002 from the American Heart Association. This manuscript has been reviewed by CARDIA for scientific content. Drs. Booth, Moran, Schwartz, Shimbo, Shikany and Muntner receive support through 15SFRN2390002 from the American Heart Association. Dr. Shimbo received support through K24-HL125704 from NIH/NHLBI.
Footnotes
Conflict of interest: None.
References:
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al. : 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex : 1979) 2018, 71(6):1269–1324. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr. et al. : The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama 2003, 289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr., Whelton PK: Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation 2018, 137(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K: Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertension 2008, 21(4):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G: Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005, 111(14):1777–1783. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, Zanchetti A, Agabiti-Rosei E, Benemio G, De Cesaris R, Fogari R, Pessina A, Porcellati C, Rappelli A, Salvetti A et al. : Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation 1997, 95(6):1464–1470. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Pickering TG, Harshfield GA, Kleinert HD, Denby L, Clark L, Pregibon D, Jason M, Kleiner B, Borer JS et al. : Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation 1983, 68(3):470–476. [DOI] [PubMed] [Google Scholar]
- 8.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR, Liu K, Orden S, Pirie P et al. : Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) study. Controlled Clinical Trials 1987, 8(4, Supplement 1):68–73. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry 1972, 18(6):499–502. [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T et al. : A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009, 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JDR, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC: Gender- and Race-specific Determination of Albumin Excretion Rate using Albumin-to-Creatinine Ratio in Single, Untimed Urine Specimens:The Coronary Artery Risk Development in Young Adults Study. American Journal of Epidemiology 2002, 155(12):1114–1119. [DOI] [PubMed] [Google Scholar]
- 12.Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Li Y, Dolan E, Tikhonoff V, Seidlerova J, Kuznetsova T, Stolarz K et al. : The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood pressure monitoring 2007, 12(4):255–262. [DOI] [PubMed] [Google Scholar]
- 13.Octavio JA, Contreras J, Amair P, Octavio B, Fabiano D, Moleiro F, Omboni S, Groppelli A, Bilo G, Mancia G et al. : Time-weighted vs. conventional quantification of 24-h average systolic and diastolic ambulatory blood pressures. J Hypertens 2010, 28(3):459–464. [DOI] [PubMed] [Google Scholar]
- 14.Echocardiography Manual of Procedures. Coronary Artery Risk Development in Young Adults (CARDIA) Study; Accessed from [https://www.cardia.dopm.uab.edu/images/more/pdf/MooY30/chapter10.pdf]; Accessed on September 4, 2018.
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS et al. : Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2005, 18(12):1440–1463. [DOI] [PubMed] [Google Scholar]
- 16.DiCiccio TJ, Efron B: Bootstrap confidence intervals. Statist Sci 1996, 11(3):189–228. [Google Scholar]
- 17.Siu AL: Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Annals of internal medicine 2015, 163(10):778–786. [DOI] [PubMed] [Google Scholar]
- 18.Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP: Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine 2015, 162(3):192–204. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Huang W, Mai W, Cai X, An D, Liu Z, Huang H, Zeng J, Hu Y, Xu D: White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. Journal of hypertension 2017, 35(4):677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagard RH, Staessen JA, Thijs L, Gasowski J, Bulpitt CJ, Clement D, de Leeuw PW, Dobovisek J, Jaaskivi M, Leonetti G et al. : Response to antihypertensive therapy in older patients with sustained and nonsustained systolic hypertension. Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Circulation 2000, 102(10):1139–1144. [DOI] [PubMed] [Google Scholar]
- 21.Fagard RH, Cornelissen VA: Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. Journal of hypertension 2007, 25(11):2193–2198. [DOI] [PubMed] [Google Scholar]
- 22.Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D: Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens 2014, 28(9):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz KM, Veerabhadrappa P, Brown MD, Whited MC, Dubbert PM, Hickson DA: Prevalence, Determinants, and Clinical Significance of Masked Hypertension in a Population-Based Sample of African Americans: The Jackson Heart Study. American journal of hypertension 2015, 28(7):900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YC, Shimbo D, Muntner P, Moran AE, Krakoff LR, Schwartz JE: Prevalence of Masked Hypertension Among US Adults With Nonelevated Clinic Blood Pressure. Am J Epidemiol 2017, 185(3):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K: Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. American journal of hypertension 2008, 21(4):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen TW, Kikuya M, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Jeppesen J et al. : Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. Journal of hypertension 2007, 25(8):1554–1564. [DOI] [PubMed] [Google Scholar]
- 27.Drawz PE, Abdalla M, Rahman M: Blood Pressure Measurement: Clinic, Home, Ambulatory, and Beyond. American journal of kidney diseases : the official journal of the National Kidney Foundation 2012, 60(3):449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


