Abstract
The temporomandibular joint is a unique structure composed of the joint capsule, articular disc, mandibular condyles, glenoid fossa of the temporal bone, surrounding ligaments, and associated muscles. The condyle is one of the major components of a functional temporomandibular joint. Reconstruction of large mandibular defects involving the condyle is a surgical challenge for oral and maxillofacial surgeons. To restore large mandibular defects, there are different options for free flap method such as fibula, scapula, and iliac crest. Currently, the vascularized fibula free flap is the gold standard for reconstruction of complex mandibular defects involving the condyle. In the present report, neocondyle regeneration after mandible reconstruction including the condyle head with fibula free flap was evaluated. In this report, two patients were evaluated periodically, and remodeling of the distal end of the free fibula was observed in both cases after condylectomy or mandibulectomy. With preservation of the articular disc, trapezoidal shaping of the neocondyle, and elastic guidance of occlusion, neocondyle bone regeneration occured without ankylosis. Preservation of the articular disc and maintenance of proper occlusion are critical factors in regeneration of the neocondyle after mandible reconstruction.
Keywords: Fibula free flap, Neocondyle regeneration, Mandibular reconstruction, Case reports
I. Introduction
Reconstruction of large mandibular defects involving the condyle is a surgical challenge for oral and maxillofacial surgeons. The reconstruction procedure may present several technical challenges with inherent risk of mandibular deviation, malocclusion, ankylosis, and temporal bone erosion1. Depending on the nature of the mandibular defect, preservation of the native condyle may be considered. However, this option is not recommended due to risk of tumor recurrence and progression of disease2.
Large mandibular resection involving the condyle is indicated for the treatment of squamous cell carcinoma of the oral cavity, osteoradionecrosis, and in cases of congenital or acquired facial dysmorphia2. Various techniques are available to reconstruct the mandibular condyle such as costochondral grafts, distraction osteogenesis, sternoclavicular grafts, and vascularized second metatarsal joint grafts3. Currently, the vascularized fibula free flap (FFF) is the gold standard for reconstruction of complex mandibular defects involving the mandibular condyle2. This flap has many advantages, including a long pedicle length; wide vessel diameter; and the ability to incorporate skin, muscle, and bone components, which are required for mandibular reconstruction4. Moreover, multiple osteotomies of the fibula are feasible to conform to the jaw without devascularization of individual segments5,6. The narrow tubular shape and dense cortical structure of the FFF enable placement into the glenoid fossa without damage to the surrounding hard and soft tissues2. Vascularized bone grafts are more resistant to infection and have a better chance to survive in an irradiated recipient site2. In the present report, reconstruction with the FFF was planned after a pathologic fracture due to multiple myeloma (MM) and after a mandibulectomy due to squamous cell carcinoma. In both cases, remodeling of the distal end of FFF was observed using passive positioning as well as trapezoidal shaping of the neocondyle and elastic guidance of occlusion. The study protocol was reviewed and approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (IRB approval No. S2023-0801-0001). Informed consent was not required for the case report because images do not reveal patient-specific information.
II. Cases Report
1. Case 1
A 60-year-old male patient presented to the Department of Oral and Maxillofacial Surgery at Asan Medical Center with a chief complaint of dull pain in the right lower posterior gingival region and intraoral bleeding. Panoramic radiograph showed large radiolucent lesions on the right ascending ramus of the mandible. The patient underwent cyst enucleation under general anesthesia. During surgery, buccal and lingual cortical perforations were observed around the ascending ramus. Excisional biopsy revealed squamous cell carcinoma. The patient was readmitted to the Department of Oral and Maxillofacial Surgery for a right partial mandibulectomy, supraomohyoid neck dissection, and right FFF.
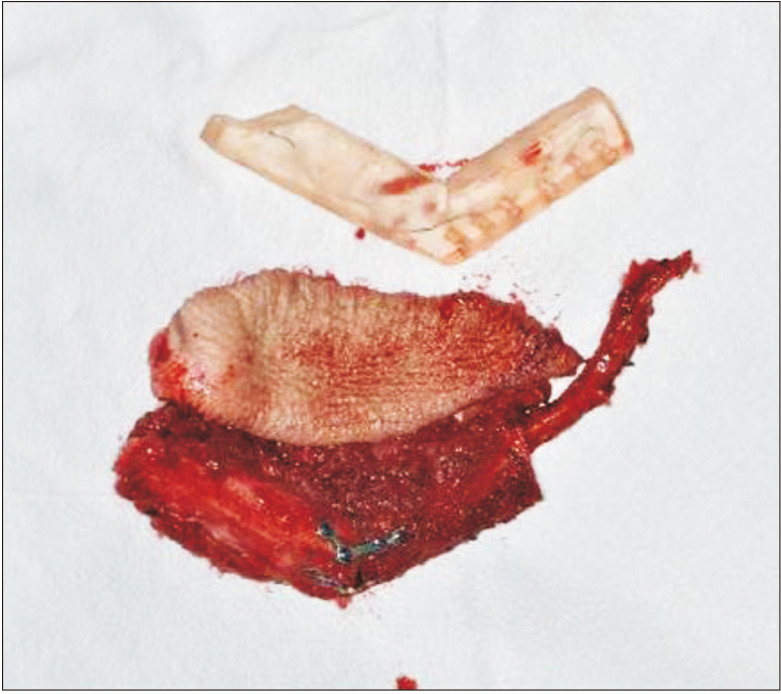
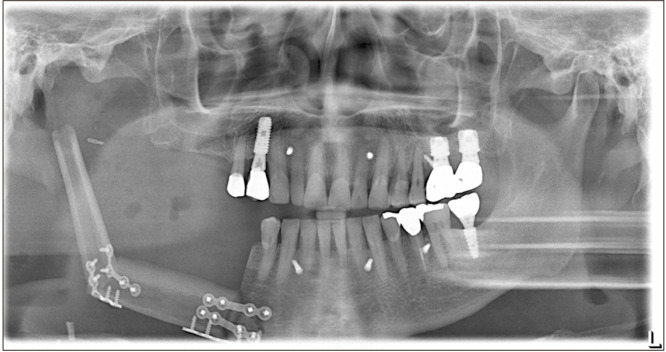
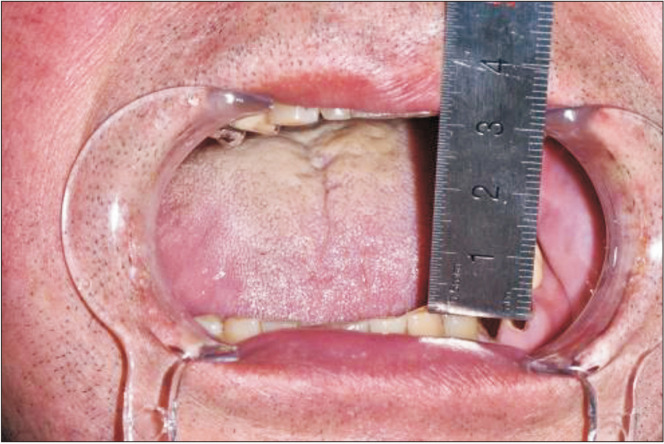
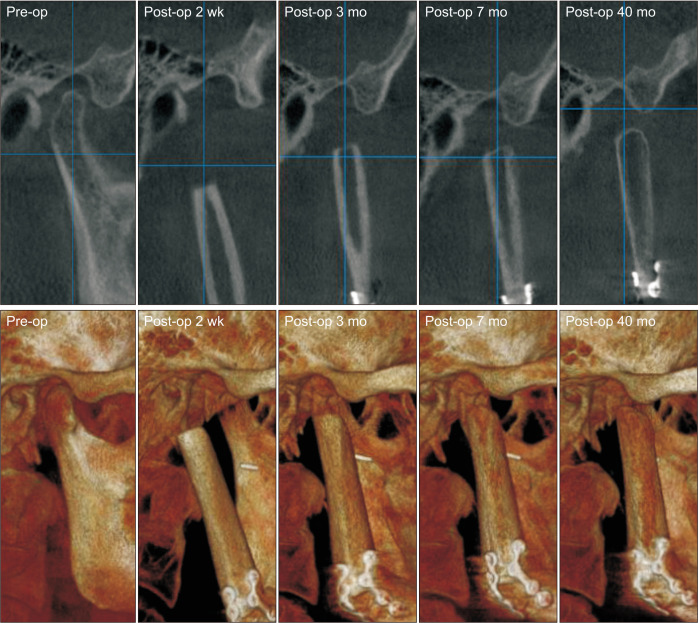
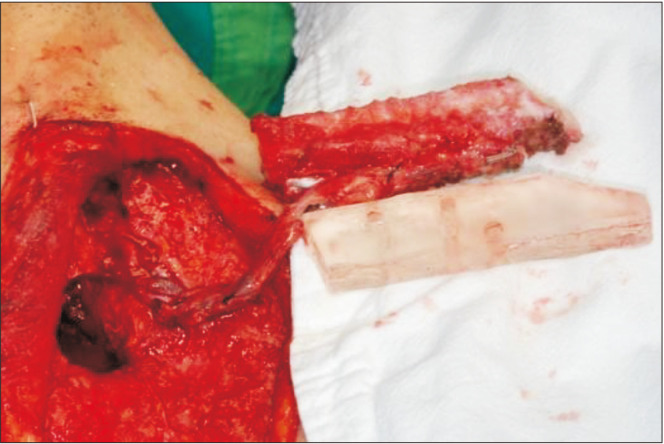
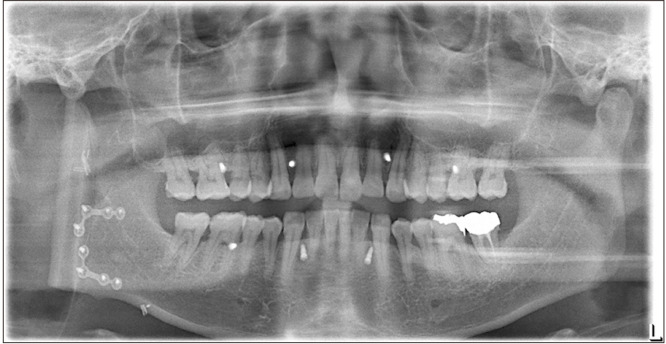
Preoperatively, facial and neck computed tomography (CT) images were obtained, and lower leg angiography confirmed the patency of the peroneal circulation. The mandibular body and angle including the condyle were resected based on preoperative planning. The temporomandibular joint disc was preserved during the surgery. The right fibula bone was removed except for 7 cm proximally and distally to stabilize the ankle and protect the peroneal nerve7. The fibula bone was prepared in an L-shape with a trapezoidal distal end using a prefabricated surgical template.(Fig. 1) A microvascular anastomosis was completed with end-to-end anastomosis of the peroneal artery to the superior thyroid artery and the vena comitans to the superior thyroid vein and external jugular vein. After microvascular anastomosis, the vascularized FFF was fixed with two L-shaped semi-rigid mini plates and three four-hole mini plates.(Fig. 2) The distal end of the neocondyle was passively seated into the glenoid fossa. In the early recovery period, a wide joint space was observed due to postoperative edema and oozing. To guide proper occlusion, guided elastics were applied for six weeks. At the 40-month follow-up, the patient showed a 35-mm maximum mouth opening (MMO).(Fig. 3) Also, remodeling of the distal end of the FFF was observed without any recurrence.(Fig. 4) Upon mouth opening, mandibular deviation toward the affected side was observed.
Fig. 1.
On the day 6 postoperative panoramic radiograph, the vascularized fibula free flap was fixated with two L-shaped semi-rigid mini plates and three four-hole mini plates.
Fig. 2.
The fibula bone was prepared in an L-shape according to the three-dimensional simulation surgical template.
Fig. 3.
At the 40-month follow-up, the patient showed 35-mm maximum mouth opening.
Fig. 4.
Rounding off at the distal end of the fibula was observed at the three-month postoperative follow-up.
2. Case 2
A 49-year-old male patient was referred by his oncologist to the Department of Oral and Maxillofacial Surgery at Asan Medical Center. The patient presented to the office with a chief complaint of persistent pain upon biting and right posterior premature contact. The patient had facial swelling on the right side that subsided after antibiotic treatment for one week. The patient had been diagnosed with MM about three years prior and had been undergoing treatment. Traditional treatment for MM includes autologous stem cell transplant and chemotherapy8. In addition, radiation therapy can be used as palliative treatment for painful bone lesions9. Patients may experience pathologic fractures caused by destruction of bone due to tumor9. The treatment of a mandibular pathologic fracture associated with a solitary MM lesion usually includes a segmental resection of the mandibular bone10. Panoramic radiograph and CT scan showed pathologic fracture of the right mandibular condyle with severe bony resorption. After a thorough evaluation, right condylectomy and reconstruction of the mandibular condyle with left FFF were planned.
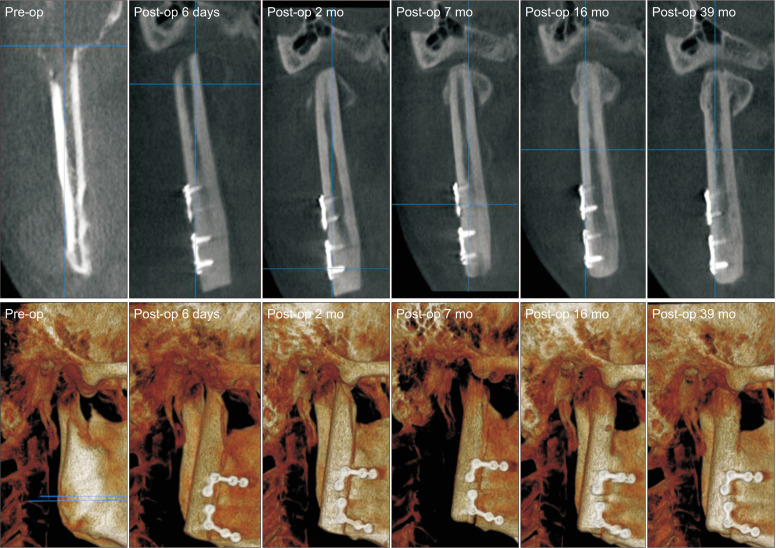
During the surgery, tumor lesion was exposed via a retromandibular incision. The mandible was resected according to the preoperative planning. A single osteotomy from the right mandibular angle to the middle of the sigmoid notch was performed, and the condyle was removed with caution to preserve the temporomandibular joint disc. The distal end of the fibula bone was prepared in the same manner as in Case 1.(Fig. 5) Microvascular anastomosis was completed with end-to-end anastomosis of the peroneal artery to the facial artery and the vena comitans to the facial vein. After microvascular anastomosis, the vascularized FFF was fixated with two L-shaped semi-rigid mini plates on the native mandible.(Fig. 6) To guide proper occlusion, intermaxillary fixation (IMF) was applied with skeletal anchorage system and rubber rings for six weeks. At the 39-month follow-up, 45 mm of MMO with slight right deviation upon opening was observed. Radiographic exam showed neocondyle bone growth in the direction of the lateral pterygoid traction (DLPT).(Fig. 7) Upon mouth opening, mandibular deviation toward the affected side was observed.
Fig. 5.
The distal end of the fibula was prepared in a right trapezoidal shape using a surgical template.
Fig. 6.
On the day 4 postoperative panoramic radiograph, the vascularized fibula free flap was fixed with two L-shaped semi-rigid mini plates on the native mandible.
Fig. 7.
Neocondyle bone growth in the direction of the lateral pterygoid traction started two months after surgery.
III. Discussion
Patients with osteomyelitis, trauma, or tumor may require a mandibulectomy involving the condyle4. Depending on the nature of pathologies such as temporomandibular joint (TMJ) ankylosis, TMJ prosthetic devices can be an effective treatment option11,12. In our cases, vascularized FFF was recommended to reconstruct the mandible involving the condyle. To connect the resected bone segments of the FFF, the use of metal plates longer than 2.3 mm is recommended13. However, the use of reconstruction plates has the disadvantages of causing small injuries on the fibula segment and may lead to metal plate exposure13. In the present report, the flap was fixed to the mandible with miniplates to decrease the risk of vascular impairment, stress shielding, osteoporosis, and osteoradionecrosis6. Reconstruction with FFF can generate devastating complications, such as ankylosis and malposition of the neocondyle2. Some studies have found that disc damage and traumatic temporomandibular (TMJ) bony ankylosis are closely related14,15. For this reason, the TMJ disc was preserved in both cases. Additionally, positional and angular changes in FFF can occur with healing between the bone segments13. Muscle atrophy around the condyle can also cause neocondylar movement due to an imbalance between inner and outer neocondyle pressures3. Moreover, neocondyle malposition can result from long-term postoperative tissue resorption3. In order to minimize the risk of ankylosis, positioning of the fibula in the joint space without direct contact and early postoperative mobilization guiding elastics are recommended2. According to Callahan et al.16, IMF with wires for a minimum of two weeks could achieve stable occlusion and oral function, while avoiding significant temporomandibular pain. Additionally, IMF can have a positive effect on the soft tissue envelope of the vascularized FFF. In the present report, the trapezoidal shaping of the distal end of the fibula created space for the articular disc, and passive seating of the neocondyle helped in avoiding ankylosis without damaging surrounding tissues. If the disc is removed, precise fibula positioning with a planned 1-cm gap and postoperative range of motion exercise with guided elastics are required to achieve an appropriately positioned and functional condylar reconstruction2. Engroff17 proposed direct suturing of the masseter muscle to the angle of the reconstruction plate for proper seating and positioning of the fibula into the glenoid fossa. In the present case, stable posterior occlusion helped to position the neocondyle into the fossa without suturing of the masseter muscle.
In Case 1, rounding off at the distal end of fibula was observed at the three-month postoperative follow-up. Also the neocondyle was gradually repositioned in the fossa. This finding was consistent with Guyot et al.’s report18 on long-term radiologic findings following reconstruction of the condyle with FFF17. Remodeling of the neocodyle was observed in 10 of 11 previous patients. Patients younger than 45 years of age showed more considerable remodeling, and radiation therapy may impair remodeling and temporomandibular function18. Compared to Case 2, minimal bone remodeling was observed in Case 1 due to postoperative radiation therapy and more invasive surgery.
In Case 2, neocondyle bone growth in the DLPT was observed two months after surgery. According to three-dimensional morphological analysis of neocondyle bone growth after FFF reconstruction, neocondyle bone growth occurred in two directions, the DLPT and toward the glenoid fossa4. Neocondyle bone growth in the direction of the DLPT is closely connected with distraction osteogenesis of the lateral pterygoid muscle4,19,20. Postoperatively, early mobilization of the joint was encouraged to maintain function and to facilitate patient rehabilitation17. In the present case, patients were advised to begin mouth opening exercise two weeks after surgery.
In conclusion, with preservation of the articular disc and trapezoidal shaping, neocondyle bone remodeling after FFF reconstruction did not develop into temporofibular ankylosis4. Additionally, passive seating of the neocondyle and elastic guidance of occlusion improved the surgical outcome. Distraction osteogenesis of the lateral pterygoid muscle seems to be a key factor for neocondyle bone growth in the DLPT direction4,19,20. FFF is a reliable option in reconstruction of mandibular defects involving the condyle as it provides a good long-term functional outcome.
Funding Statement
Funding No funding to declare.
Footnotes
Authors’ Contributions
All authors have read and approved the manuscript. H.I.P. reviewed articles on neocondyle regeneration after mandible reconstruction and was a major contributor in writing manuscript. H.J.C. reviewed articles on functional reconstruction of the mandibular condyle defect. J.H.L. shared clinical and surgical data.
Ethics Approval and Consent to Participate
The study protocol was reviewed and approved by the Institutional Review Board of Asan Medical Center, Seoul, Korea (IRB approval No. S2023-0801-0001). Informed consent was not required for the case report because images do not reveal patient-specific information.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Schmelzeisen R, Voss PJ, Neukam FW. Microvascular reconstruction of the condyle and the ascending ramus. In: Greenberg AM, Schmelzeisen R, editors. Craniomaxillofacial reconstructive and corrective bone surgery. 2nd ed. Springer; 2019. pp. 427–47. [DOI] [Google Scholar]
- 2.Lee ZH, Avraham T, Monaco C, Patel AA, Hirsch DL, Levine JP. Optimizing functional outcomes in mandibular condyle reconstruction with the free fibula flap using computer-aided design and manufacturing technology. J Oral Maxillofac Surg. 2018;76:1098–106. doi: 10.1016/j.joms.2017.11.008. https://doi.org/10.1016/j.joms.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Yu Y, Zhang WB, Liu XJ, Guo CB, Yu GY, Peng X. Regeneration of the neocondyle after free fibular flap reconstruction of the mandibular condyle. J Oral Maxillofac Surg. 2020;78:479–87. doi: 10.1016/j.joms.2019.11.009. https://doi.org/10.1016/j.joms.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Soh HY, Bai S, Zhang WB, Wang Y, Peng X. Three-dimensional morphological analysis of neocondyle bone growth after fibula free flap reconstruction. Int J Oral Maxillofac Surg. 2021;50:1429–34. doi: 10.1016/j.ijom.2021.03.005. https://doi.org/10.1016/j.ijom.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Peled M, El-Naaj IA, Lipin Y, Ardekian L. The use of free fibular flap for functional mandibular reconstruction. J Oral Maxillofac Surg. 2005;63:220–4. doi: 10.1016/j.joms.2004.06.052. https://doi.org/10.1016/j.joms.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Chaine A, Pitak-Arnnop P, Hivelin M, Dhanuthai K, Bertrand JC, Bertolus C. Postoperative complications of fibular free flaps in mandibular reconstruction: an analysis of 25 consecutive cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:488–95. doi: 10.1016/j.tripleo.2009.05.043. https://doi.org/10.1016/j.tripleo.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Wax MK, Winslow CP, Hansen J, MacKenzie D, Cohen J, Andersen P, et al. A retrospective analysis of temporomandibular joint reconstruction with free fibula microvascular flap. Laryngoscope. 2000;110:977–81. doi: 10.1097/00005537-200006000-00018. https://doi.org/10.1097/00005537-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Wei H, Pinting L, Enyi T, Zhiyong W. Initial finding of mandible mass in multiple myeloma. J Craniofac Surg. 2012;23:e599–600. doi: 10.1097/SCS.0b013e31826bf44c. https://doi.org/10.1097/scs.0b013e31826bf44c. [DOI] [PubMed] [Google Scholar]
- 9.Neville BW, Damm DD, Allen CM, Chi AC. Hematologic disorders. In: Neville BW, Damm DD, Allen CM, Chi AC, editors. Oral and maxillofacial pathology. 4th ed. Elsevier; 2016. pp. 563–5. [Google Scholar]
- 10.Boffano P, Viterbo S, Barreca A, Berrone S. Pathologic mandibular fracture as the presenting manifestation of multiple myeloma. J Craniofac Surg. 2011;22:1312–5. doi: 10.1097/SCS.0b013e31821c6cbe. https://doi.org/10.1097/scs.0b013e31821c6cbe. [DOI] [PubMed] [Google Scholar]
- 11.Gerbino G, Zavattero E, Bosco G, Berrone S, Ramieri G. Temporomandibular joint reconstruction with stock and custom-made devices: indications and results of a 14-year experience. J Craniomaxillofac Surg. 2017;45:1710–5. doi: 10.1016/j.jcms.2017.07.011. https://doi.org/10.1016/j.jcms.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Amarista FJ, Jones JP, Brown Z, Rushing DC, Jeske NA, Perez DE. Outcomes of total joint alloplastic reconstruction in TMJ ankylosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134:135–42. doi: 10.1016/j.oooo.2021.12.121. https://doi.org/10.1016/j.oooo.2021.12.121. [DOI] [PubMed] [Google Scholar]
- 13.Kang SH, Lee S, Nam W. Condyle dislocation following mandibular reconstruction using a fibula free flap: complication cases. Maxillofac Plast Reconstr Surg. 2019;41:14. doi: 10.1186/s40902-019-0197-1. https://doi.org/10.1186/s40902-019-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng FW, Zhao JL, Hu KJ, Liu YP. A new hypothesis of mechanisms of traumatic ankylosis of temporomandibular joint. Med Hypotheses. 2009;73:92–3. doi: 10.1016/j.mehy.2009.01.024. https://doi.org/10.1016/j.mehy.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Liu CK, Liu P, Meng FW, Deng BL, Xue Y, Mao TQ, et al. The role of the lateral pterygoid muscle in the sagittal fracture of mandibular condyle (SFMC) healing process. Br J Oral Maxillofac Surg. 2012;50:356–60. doi: 10.1016/j.bjoms.2011.05.015. https://doi.org/10.1016/j.bjoms.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Callahan N, Patel M, Dyalram D, Lubek JE. Is the prevention of condylar sag with maxillomandibular fixation the key to functional reconstruction of a mandibular disarticulation resection? Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134:317–22. doi: 10.1016/j.oooo.2022.02.012. https://doi.org/10.1016/j.oooo.2022.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Engroff SL. Fibula flap reconstruction of the condyle in disarticulation resections of the mandible: a case report and review of the technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:661–5. doi: 10.1016/j.tripleo.2005.03.016. https://doi.org/10.1016/j.tripleo.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Guyot L, Richard O, Layoun W, Cheynet F, Bellot-Samson V, Chossegros C, et al. Long-term radiological findings following reconstruction of the condyle with fibular free flaps. J Craniomaxillofac Surg. 2004;32:98–102. doi: 10.1016/j.jcms.2003.11.003. https://doi.org/10.1016/j.jcms.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Yang XJ, Cheng P, Deng TG, Jiang X, Liu P, et al. The lateral pterygoid muscle affects reconstruction of the condyle in the sagittal fracture healing process: a histological study. Int J Oral Maxillofac Surg. 2015;44:1010–5. doi: 10.1016/j.ijom.2015.02.004. https://doi.org/10.1016/j.ijom.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Deng TG, Liu CK, Liu P, Zhang LL, Wu LG, Zhou HZ, et al. Influence of the lateral pterygoid muscle on traumatic temporomandibular joint bony ankylosis. BMC Oral Health. 2016;16:62. doi: 10.1186/s12903-016-0220-1. https://doi.org/10.1186/s12903-016-0220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]