Abstract
Colorectal cancer (CRC) is one of the most common tumours worldwide, and 70% of CRC patients are over 65 years of age. However, the scientific evidence available for these patients is poor, as they are underrepresented in clinical trials. Therefore, a group of experts from the Oncogeriatrics Section of the Spanish Society of Medical Oncology (SEOM), the Spanish Cooperative Group for the Treatment of Digestive Tumours, (TTD) and the Multidisciplinary Spanish Group of Digestive Cancer (GEMCAD) have reviewed the scientific evidence available in older patients with CRC. This group of experts recommends a multidisciplinary approach and geriatric assessment (GA) before making a therapeutic decision because GA predicts the risk of toxicity and survival and helps to individualize treatment. In addition, elderly patients with localized CRC should undergo standard cancer resection, preferably laparoscopically. The indication for adjuvant chemotherapy (CT) should be considered based on the potential benefit, the risk of recurrence, the life expectancy and patient comorbidities. When the disease is metastatic, the possibility of radical treatment with surgery, radiofrequency (RF) or stereotactic body radiation therapy (SBRT) should be considered. The efficacy of palliative CT is similar to that seen in younger patients, but elderly patients are at increased risk of toxicity. Clinical trials should be conducted with the elderly population and include GAs and specific treatment plans.
Keywords: Aged, Antineoplastic, Capecitabine, Geriatric oncology, Fluoropyrimidines, Treatment outcome
Introduction
Colorectal cancer (CRC) is one of the most common tumours worldwide [1, 2]. According to GLOBOCAN 2020 data, it is the third most prevalent tumour among both sexes after breast and lung neoplasia and the second leading cause of death from cancer after lung cancer [3]. Incidence and prevalence vary by country depending on lifestyles and screening programmes [2]. The risk of CRC increases with age; population ageing and overcoming other competing causes of death, such as cardiovascular diseases, are important factors [2]. The elderly population, therefore, is at high risk of developing CRC. This poses a challenge for healthcare organizations due to the presence of factors associated with ageing, such as frailty or comorbidities, which increase the risk of toxicity, impacting decision-making, especially as the algorithms for colon cancer treatment increase in complexity with age [2]. The heterogeneity of ageing generates discrepancies between chronological age and physiological age, making it essential to incorporate geriatric tools in decision-making for this population group.
To examine the management of the elderly population with CRC cancer in more detail, a group of experts from the Oncogeriatrics Section of the Spanish Society of Medical Oncology (SEOM), the Spanish Cooperative Group for the Treatment of Digestive Tumours (TTD) and the Multidisciplinary Spanish Group of Digestive Cancer (GEMCAD) have carried out an exhaustive review of the available scientific evidence, from which this publication arises.
Categorization of elderly patients with CRC
Geriatric assessment (GA) is the basic work tool used in geriatrics. It allows a multidimensional evaluation, using different scales and questionnaires, of the health status of elderly patients. As a whole, a GA allows clinicians to identify the functional status of an individual, their state of mind, social and economic situation, and nutritional state, the presence of polypharmacy, and mobility, cognition and geriatric syndromes, among other aspects.
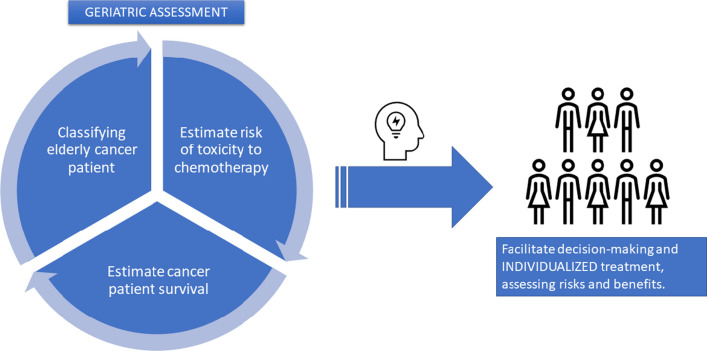
In the field of oncogeriatrics, the International Society of Geriatric Oncology (SIOG) recommends that a GA be performed for cancer patients over 70 years of age [4]. Through this GA, relevant information can be obtained for the development of a treatment plan that allows the most appropriate management for each patient (Fig. 1).
Fig. 1.
Categorization of elderly patients with CRC. CRC colorectal cancer
Since Professor Balducci’s proposal in 2000 to categorize elderly patients with cancer using data from the comprehensive geriatric assessment (CGA) [5], different classifications have been published, all of which have focused on identifying frail patients through the combination of different GA dimensions, such as functional capacity, nutritional status, comorbidities and geriatric syndromes [6]. All these classifications have emerged from consensuses among experts and from clinical experience. However, Ferrat et al. in 2016 used a statistical approach, i.e., latent class analysis (LCA), to identify frail patients from GA components [7].
In theory, frail patients tend to require more attention and care, as they are less able to cope with stressful situations, such as surgery or chemotherapy (CT). In fact, frail patients develop a higher incidence of post-surgical complications and a poorer tolerance to cytostatics [8]. For this reason, adapted treatment should be administered, and close follow-up should be conducted, together with the implementation of other intervention measures [9].
In the specific case of individuals with CRC, on whom this review focuses, the prevalence of frailty in the published series ranges from 20 to 43%; in all series, this population group has poorer survival and a greater number of severe complications after surgery [10, 11]. These results have also been described in a systematic review by Boakye et al. [12] and in a meta-analysis by Chen et al. [13], both in CRC.
However, these classifications would be meaningless if they only served to catalogue patients. In contrast, antitumour treatment can be adapted depending on the presence or absence of frailty in patients with CRC, which favours tolerance to antitumour treatment, increases the probability that these patients will complete the treatment plan initially established, results in less frequent treatment dose reductions and improves patient quality of life [9, 14, 15]. Table 1 summarizes the main treatment options in elderly patients with localized CRC, taking into account their baseline situation.
Table 1.
Therapeutic recommendations for elderly patients with localized CRC depending on their functional status
| Patient | Colon cancer | Rectal cancer |
|---|---|---|
| Fit |
1° Standard surgery 2° Adjuvant CT with fluoropyrimidines Alternative: XELOX for 3 months |
1° 5 × 5 RT or CT with prolonged RT 2° Standard surgery Alternative: total neoadjuvant treatment |
| Pre-fragile |
1° Standard surgery and perioperative rehabilitation 2° Adjuvant CT with fluoropyrimidines at reduced doses |
1° Long course CT-RT and toxicity monitoring 2° Standard surgery and perioperative rehabilitation |
| Fragile |
1° Palliative surgery 2° No adjuvant CT |
1° RT 5 × 5 Alternative: derivative colostomy |
CRC Colorectal cancer, CT chemotherapy, RT radiotherapy, XELOX capecitabine plus oxaliplatin
Treatment of localized disease
Mortality from competing causes is higher among elderly patients than among younger patients with CRC. Therefore, although the time to recurrence is similar in both population groups, other efficacy variables, such as overall survival (OS) or disease-free survival (DFS), are not as favourable. A Swedish population study showed that the risk of recurrence after surgery, adjusted for the administration or not of adjuvant CT, was similar between elderly patients and the global population [16]. Therefore, the benefit of adjuvant CT after CRC surgery to reduce the risk of recurrence in the elderly is the same in elderly patients as in the rest of the population. In the absence of prospective randomized studies conducted specifically in this population, the only available scientific evidence comes from retrospective analyses performed with elderly patients with a good functional status that have been included in larger randomized clinical trials.
Colon cancer
Surgery
Generally, elderly patients with CRC are assigned elective (scheduled) surgery less frequently than are younger patients; palliative intervention is more common. Although standard surgical procedures have proven to be tolerated in the elderly population, postoperative complications are more frequent, especially in frail patients. A comprehensive geriatric assessment (CGA) is therefore essential and should be incorporated into the preoperative assessment of geriatric patients with CRC.
Laparoscopic surgery has gained ground in recent years due to its lower postoperative morbidity, faster recovery of intestinal function and shorter hospital stay. This technique has also demonstrated its efficacy and safety in elderly patients with colon and rectal cancer [17]. Therefore, elderly patients with localized CRC and surgical indications and good functional status should undergo standard cancer resection, preferably laparoscopically. In cases with prefrailty, perioperative multimodal rehabilitation is recommended to reduce morbidity [18].
Adjuvant CT
Elderly patients with CRC are referred to Medical Oncology less frequently than are younger people, receive standard CT scans more rarely and are often given reduced doses or end treatment early.
In two joint analyses of 7 studies that evaluated adjuvant CT with fluoropyrimidines with that of surgery without CT in colon cancer, the benefits were similar between patients older than 70 years and the global population, without a significant increase in toxicity [19, 20]. Subsequently, a significant benefit has been demonstrated by adding oxaliplatin to the adjuvant regimens for CRC in these patients. In a pooled analysis of 4 randomized trials, DFS and OS improved significantly with the administration of oxaliplatin; however, in patients older than 70 years, this benefit was lower, and greater toxicity was observed [21]. One series of noninferiority trials, grouped under the umbrella of the International Duration Evaluation of Adjuvant Chemotherapy (IDEA), compared 3 and 6 months of adjuvant treatment in 12,834 patients with stage III colon cancer [22]. For various subgroups of patients (T3N1), 3 months of CT with capecitabine and oxaliplatin (XELOX) was not less effective than 6 months of the same treatment. The effect of age was not considered in any of these studies.
Therefore, the indication for adjuvant CT should be considered in the context of potential benefits, risk of recurrence, and life expectancy in relation to age and comorbidities. Elderly patients with resected stage III CRC (or stage II with poor prognosis) and good functional status should receive adjuvant CT with fluoropyrimidines for 6 months (or XELOX for 3 months). In prefrail patients, although there are no specific studies to support this approach, the administration of capecitabine at reduced doses with close monitoring for toxicity might be an option to improve tolerance. Frail patients are candidates for follow-up without complementary therapy. There are no published studies of adjuvant treatment in elderly patients; however, the French PRODIGE 34 ADAGE trial is under way.
Rectal cancer
The management of localized rectal cancer is complex and requires a specialized multidisciplinary team. Standard treatment consists of long-term radiotherapy (RT) concomitant with fluoropyrimidines, followed by surgical resection (total excision of the mesorectum). This neoadjuvant treatment is associated with better local control and less toxicity [23]. The value of adjuvant CT after neoadjuvant CT and surgery is more questionable. Short-course RT (5 × 5) is a simpler alternative and does not require concomitant CT. However, it is not the best option in elderly patients who are candidates for surgery because it is associated with a higher rate of complications [24]. Recently, the value of total neoadjuvant treatment in conjunction with induction or preferably consolidation CT has been demonstrated, with the option of preserving the rectum provided there is close monitoring [25]. However, data for the elderly population are very limited.
In a review of the Swedish Rectal Cancer Registry, patients older than 75 years received preoperative RT and resection surgery less frequently. In addition, when this was carried out, the Hartmann technique (with definitive colostomy) was the approach that was used most frequently [26]. In general, after a preoperative assessment of faecal continence and a discussion of the potential risks of defecation urgency and faecal losses, the option of sphincter-conserving surgery should be offered to elderly patients with lower-third rectal cancer [27]. As the function of the anal sphincter may be lost with aging, sphincter-preserving surgery may not be considered in some cases. Importantly, all cases must be evaluated within a multidisciplinary team.
For nonfrail elderly patients, standard neoadjuvant treatment (long-course CT or short-course RT) is recommended, followed by conventional surgery (sphincter-sparing or abdominoperineal amputation, depending on baseline sphincter tone). Furthermore, in prefrailty situations, care should be taken to identify early signs of toxicity and adjust the treatment dose in a timely manner. In frail patients, the possible options are (i) discharge colostomy; (ii) palliative RT (5 × 5 technique); and (iii) symptomatic treatment.
Treatment of advanced disease
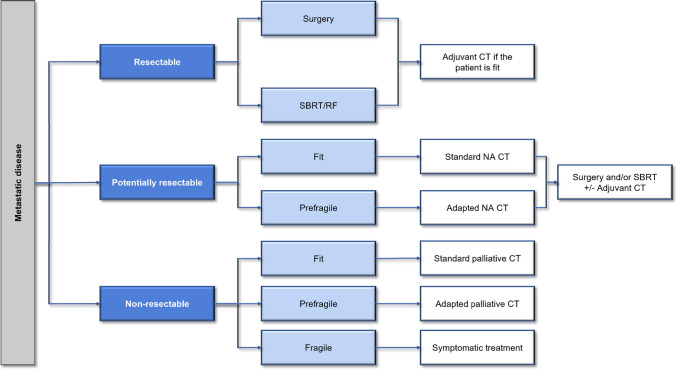
Approximately 15–30% of CRC patients present with metastatic disease, and 20–40% of patients will metachronously develop metastases. The most frequent metastatic sites are the liver, lung, peritoneum, and distant lymphadenopathy. It is vitally important to assess and discuss the situation of each patient within a multidisciplinary committee of experts to establish the best individualized therapeutic option [28]. In addition, it is important to define whether the metastatic disease is resectable, potentially resectable after induction treatment, or unresectable; if unresectable, only palliative CT should be considered [29] (Fig. 2).
Fig. 2.
Decision algorithm for the treatment of elderly patients with metastatic CRC based on resectability. CRC colorectal cancer, CT chemotherapy, NA neoadjuvant, RF radiofrequency, SBRT stereotactic body radiation therapy
First-line treatment with CT
Elderly patients with metastatic CRC have a lower OS than do younger patients because of multiple factors, including more advanced stage at diagnosis, more comorbidities and a greater frequency of suboptimal treatments [30, 31]. International guidelines, such as those released by the European Society for Medical Oncology (ESMO), prioritize establishing patient status during decision-making; in elderly patients, status is closely linked, although not exclusively, with comorbidities and functional reserves. In this sense, 3 subgroups of patients can be defined: fit, vulnerable or prefrail, and fragile [32].
Fit elderly patients
The ESMO guidelines recommend that chemotherapy treatment for fit elderly patients should be conducted in a similar way as that for younger patients, on the basis of results obtained in phase II studies and in age subgroup analyses in phase III trials, most of which include a fit elderly patient subgroup.
Some retrospective studies have evaluated 5-fluorouracil (5FU) and leucovorin (LV) with or without irinotecan (IRI) in elderly patients. Notably, a meta-analysis was conducted of 4 first-line treatment studies comparing 5FU/LV/IRI versus 5FU/LV in 2691 patients (599 ≥ 70 years). The same benefits in response rate and progression-free survival (PFS) were observed with the addition of IRI as in the control group, with a nonsignificant tendency towards higher OS in the elderly group. Toxicity was similar, except in patients older than 75 years, who had a higher incidence of severe neutropenia with monotherapy and a higher incidence of nausea, vomiting and diarrhoea in the IRI arm [33].
A subanalysis of the BICC-C study, which included 1,430 patients randomized to receive irinotecan with 5FU and folinic acid in continuous infusion (FOLFIRI), irinotecan with 5FU-bolus and folinic acid (mIFL) or irinotecan and capecitabine (CapeIRI), showed that the response and PFS for patients older than 70 years were similar to those for younger patients, although with a higher incidence of asthenia and dehydration related mainly to capecitabine [34]. Other prospective studies in which irinotecan regimens have been evaluated have confirmed their feasibility and efficacy [35].
In analyses by population subgroup, the efficacy/safety relationship has been shown to remain positive in fit elderly patients treated with oxaliplatin-based regimens, as demonstrated by a meta-analysis of 3742 patients (614 ≥ 70 years) in 4 phase III studies that evaluated the combination of folinic acid, 5FU and oxaliplatin (FOLFOX) as first- and second-line adjuvant treatment for advanced disease, detecting no differences in terms of efficacy or toxicity (except in some haematological parameters) with respect to the global population of the study [36]. A subanalysis of the phase III study 03/TTD/01, which compared 5FU and oxaliplatin (FUOX) with XELOX in 348 patients (109 ≥ 70 years), also did not show differences between the elderly population and the younger population in terms of efficacy or toxicity [37]. Prospective studies carried out in the elderly population confirm that the activities of the FOLFOX or XELOX combinations in elderly patients are similar to those in the younger population [38–40].
Two randomized phase III studies of elderly populations evaluated whether combined treatment provides any benefit compared with the administration of fluoropyrimidines as monotherapy. The FOCUS2 study recruited 459 patients not eligible for full-dose CT [41]. With a 2 × 2 factorial design, it included two arms with fluoropyrimidines in monotherapy and two arms with fluoropyrimidines in combination with oxaliplatin at reduced initial doses. The addition of oxaliplatin increased the response rate without an increase in PFS (5.8 vs. 4.5 months; p = 0.07) or OS. Substitution of 5FU for capecitabine did not improve patient quality of life. The addition of oxaliplatin did not significantly increase the incidence of grade 3/4 toxicity (38% vs. 32%; p = 0.17), which was observed when capecitabine was administered instead of 5FU (40% vs. 30%; p = 0.03). The FFCD 2001-02 trial compared FOLFIRI versus 5FU/LV administered in a classic or simplified way, without observing a benefit in terms of PFS or OS; however, greater toxicity was observed in the arms with irinotecan [42].
Therefore, clinicians need to carefully assess the results of GA in patients with metastatic CRC. Although the guidelines recommend administering similar treatments for nonelderly patients and fit elderly patients, in the presence of toxicity or a negative impact on quality of life, there is no clear evidence that polychemotherapy provides a clear survival rate benefit when compared to monotherapy.
Prefrail or vulnerable elderly patients
The benefit of administering fluoropyrimidines rather than supportive treatment has been clearly demonstrated in past decades [43]. Capecitabine offers an activity greater than 5FU-bolus and has a similar toxicity profile to 5FU administered as a continuous infusion, although with a better quality of life. A phase II study of patients ≥ 70 years of age treated with capecitabine as monotherapy reported a response rate of 24%, PFS of 7 months and OS of 11 months. There were no significant differences in the incidence of grade 3–4 toxicities between the general study population and patients older than 80 years [44].
Several phase II trials with modified treatment or dosage regimens have evaluated the administration of fluoropyrimidine-based polychemotherapy in this subgroup of patients. Low-dose FOLFOX regimens resulted in response rates of 43–51% and OS of 13–20 months [45, 46]. The administration of oxaliplatin at a reduced dose in the first cycle (85 mg/m2) and higher doses in the second cycle (100 mg/m2) together with capecitabine at standard doses resulted in toxicity and escalation in 51% of elderly patients with metastatic CRC at the initial assessment, with a response rate of 40% and an OS of 14 months [47].
The results from the NORDIC-9 randomized phase II trial suggest that, compared with a sequence of monotherapy with full doses, the sequence of first and second lines of polychemotherapy at a reduced dose leads to an increase in PFS, less toxicity and improved quality of life in the vulnerable elderly [48, 49].
Frail elderly patients
Current treatment guidelines advise against the use of CT in frail elderly patients with metastatic CRC [33].
First-line treatment with CT and targeted therapy
The most relevant studies for this topic are shown in Table 2.
Table 2.
Evaluation of first-line treatments in the elderly patient with metastatic CRC
| Study | N Age | Treatment scheme | GA | Efficacy |
|---|---|---|---|---|
|
Phase III AVEX [50] |
N = 280 ≥ 70 |
A: Capecitabine B: Capecitabine + bevacizumab |
No |
PFS: 5.2 vs. 9.1 m; p < 0.0001 OS: 20.7 vs. 16.8 m; p = 0.18 RR: 19% vs. 10%; p = 0.04 |
|
Phase III |
N = 856 435 ≥ 75 (Fragile) |
A: TAS-102 + bevacizumab B: Capecitabine + bevacizumab |
Yes |
PFS: 9.4 vs. 9.3 m; p = 0.0464 (superiority p < 0.021) OS: Pending RR: 36% vs. 42%; p = 0.0942 |
|
Phase III [53] JCOG1018 |
N = 251 ≥ 70 |
A: FP + bevacizumab B: FP + oxaliplatin + bevacizumab |
Yes |
PFS: 9.4 vs. 10 m; p = 0.086 OS: 21.3 vs. 19.7 m RR: 30% vs. 48% |
|
Phase II-III AGITG MAX [54] |
N = 99 Subgroup ≥ 75 |
C: Capecitabine CB: Capecitabine + bevacizumab CBM: Capecitabine + bevacizumab + Mitomycin C |
No |
PFS CB vs. C: 0.52 (0.32–0.86); p = 0.01 PFS CBM vs. C: 0.38 (0.23–0.64); p = 0.001 OS CB vs. C: 0.80 (0.47–1.36); p = 0.41 OS CBM vs. C: 0.78 (0.46–1.34); p = 0.36 RR C vs. CB vs. CBM: 28% vs. 23% vs. 57%; p = 0.08 |
|
Phase II [55] PRODIGE 20 |
N = 102 ≥ 75 |
A: FOLFOX/FOLFIRI/ 5FU-LV B: FOLFOX/FOLFIRI/5FU-LV + bevacizumab |
No |
PFS: 7.8 vs. 9.7 m; HR 0.79 [95% Cl 0.53–1.17] OS: 19.8 vs. 21.7 m; HR = 0.73 [95% Cl 0.48–1.11] RR: 33% vs. 37% |
|
Phase III-II AVF2107-AVF2192 pooled [56] |
N = 439 ≥ 65 |
A: 5FU-LV bolus B: 5FU-LV bolus + bevacizumab |
No |
PFS: 6.2 vs. 9.2 m; p < 0.001 OS: 14.3 vs. 19.3 m; p = 0.006 RR: 29% vs. 34%; p = ns |
|
Phase II Sastre et al. [57] |
N = 66 ≥ 70 |
Capecitabine + cetuximab | No |
PFS: 8.4 RAS WT vs. 6 RAS mut; p = 0.024 OS: 8.4 RAS WT vs. 6 RAS mut; p = 0.024 RR: 48% RAS WT vs. 21% RAS mut; p = 0.027 |
|
Phase II PANDA [58] |
N = 185 ≥ 70 |
A: FOLFOX + panitumumab B: 5FU/LV + panitumumab |
Yes |
PFS: 9.6 vs. 9.1 m OS: Pending RR: 65% vs. 57%; p = ns |
5FU 5-fluorouracil, CRC colorectal cancer, FOLFIRI 5FU and folinic acid with irinotecan, FOLFOX 5FU and folinic acid with oxaliplatin, FP fluoropirimidina (5FU/capecitabina, GA geriatric assessment, LV leucovorin, m month, mut mutant, OS overall survival, PFS progression-free survival, RAS rat sarcoma virus, TAS-102 trifluridine/tipiracil, WT wild-type, RR response rate
CT plus antiangiogenic treatment
The phase III AVEX study evaluated the administration of capecitabine with or without bevacizumab in 280 patients with metastatic CRC ≥ 70 years of age who were not candidates to receive oxaliplatin- or irinotecan-based regimens. The average age of the patients was 76 years. The median PFS was higher in the arm treated with capecitabine and bevacizumab than in the arm treated with capecitabine alone (9.1 vs. 5.2 months; p < 0.0001), with a trend towards higher OS (20.7 vs. 16.8 months; p = 0.18) and a higher response rate (19% vs. 10%; p = 0.04). The benefit was seen in both older and younger patients. The combination also caused higher grade 3–4 toxicity (40% vs. 22%) [50].
In vulnerable patients, the phase III SOLSTICE study compared the administration of bevacizumab and capecitabine with bevacizumab and trifluridine/tipiracil (TAS-102). However, no benefit was observed in the experimental arm; the PFS was 9.4 months versus 9.3 months, with a different toxicity profile (more neutropenia with TAS-102 and more hand-foot syndrome with capecitabine). The G8 scale was included for patients ≥ 70 years of age, and a trend towards greater toxicity was observed in patients with a score < 14 in the TAS-102 arm [51, 52].
The benefit of adding oxaliplatin to fluoropyrimidine and bevacizumab treatment for patients ≥ 70 years of age with metastatic CRC was evaluated in the phase III JCOG1018 (RESPECT) study, which included 251 patients. In the oxaliplatin arm, the PFS was 9.4 (10.0 months in the other arm; p = 0.086), the OS was 21.3 (19.7 months in the other arm), and the response rate was 29.5% (47.7% in the other arm), without differences in the quality of life of patients in the two arms. The authors did not recommend the addition of oxaliplatin to the combination of fluoropyrimidines and bevacizumab as a first-line treatment [53].
CT plus anti-EGFR treatment
Combinations of CT with epidermal growth factor receptor inhibitors (anti-EGFR), such as cetuximab and panitumumab, are less studied than are combinations with bevacizumab in elderly patients.
Papamichael et al. evaluated, from 7 clinical trials, the safety and efficacy of adding anti-EGFR treatment to first-line CT in 1920 patients (≥ 70 and < 70 years) with metastatic CRC without a RAS gene mutation. Older patients who received CT plus anti-EGFR had a PFS similar to younger patients (8.7 vs. 10.3 months; p = 0.107) with a lower OS (21.3 vs. 26.3 months; p = 0.011) and without significant differences regarding the incidence of toxicity. In the group of patients ≥ 70 years of age, when comparing CT plus anti-EGFR versus CT alone, no significant differences were observed in terms of PFS and OS. This may be due to the poorer prognosis of patients ≥ 70 years of age, with a higher percentage of patients with an Eastern Cooperative Oncology Group (ECOG) score > 1, a higher incidence of primary tumours located in the right colon, and a higher presence of pulmonary metastases [59].
A phase II study with 66 patients ≥ 70 years of age with metastatic CRC evaluated the combination of cetuximab and capecitabine. The response rate was 48.3% in patients without a KRAS mutation. The PFS was 8.4 months and the OS was 18.8 months. After the first 27 patients were included, a dose reduction on capecitabine was implemented due to toxicity [57].
In the retrospective REVOLT study, 118 vulnerable patients ≥ 70 years of age with metastatic CRC without RAS or BRAF mutations (B-Raf proto-oncogene, BRAF) who received FOLFOX or FOLFIRI at a reduced dose plus an anti-EGFR (cetuximab or panitumumab) were evaluated. The G8 score was ≤ 14 for all patients, the response rate was 57.3%, the PFS was 10.0 months, and the OS was 18.0 months. The most frequent grade 3–4 toxicities were neutropenia (11.8%) and skin toxicity (11%). These results support the administration of reduced-dose treatments to vulnerable populations [60].
The randomized phase II PANDA study compared the administration of panitumumab and FOLFOX with panitumumab and 5FU/LV (eliminating the 5FU bolus in both arms), followed by maintenance treatment with panitumumab in patients ≥ 70 years without RAS or BRAF mutations. G8 and CRASH were applied. The PFS was 9.6 versus 9.1 months in the FOLFOX and 5FU/LV arms, respectively, and the response rate was 65% versus 57% in the FOLFOX and 5FU/LV arms, respectively. A higher incidence of grade 3–4 toxicity was observed in the oxaliplatin arm (neutropenia, diarrhoea, and neurotoxicity). The OS results have not yet been communicated. The authors recommended administering the panitumumab with 5FU/LV regimen followed by a maintenance treatment with panitumumab as the best option for these patients until the results of the phase III studies are reported [58].
Second-line or successive treatments with CT
There are no second-line or successive studies aimed at the elderly population; therefore, data are taken from analyses of subgroups by age, from which it is extracted that fit patients can receive the same CT regimen as younger patients (Table 3). The regimen should be chosen based on the first-line treatment administered. However, in prefrail and frail patients, monotherapy should be administered to reduce toxicity [61]. Second-line irinotecan in patients ≥ 70 years has shown efficacy and toxicity similar to that in younger patients [62].
Table 3.
Evaluation of second-line and successive treatments for elderly patients with metastatic CRC
| Study N | Treatment | Age, median (range) | Efficacy | Toxicity |
|---|---|---|---|---|
| Second-line treatment | ||||
|
Phase III [62] Chau I et al. N = 339 |
Irinotecan monotherapy | 62 (29–80) |
All: OS: 9.1 m; < 70 vs. ≥ 70: no differences; p = 0.74 < 70: RR: 9.0%; 95% CI 5.6–12.4 ≥ 70: RR: 11.1%: 95% CI 4.9–20.7; p = 0.585 |
No differences < 70 vs. ≥ 70: 38% vs. 46%; p = 0.218 |
| < 70 yr: 79% | ||||
| ≥ 70 yr: 21% | ||||
|
VELOUR N = 1226 |
Ratio 1:1 FOLFIRI + A or B A: Aflibercept B: Placebo |
61 (19–86) |
A vs. B OS: 13.50 vs. 12.06 m; HR: 0.82; p = 0.0032 OS < 65: HR: 0.80; 95% CI 0.67–0.95 OS ≥ 65: HR: 0.85; 95% CI 0.68–1.07 PFS: 6.90 vs. 4.67 m; p < 0.0001 PFS < 65: HR: 0.77; 95% CI 0.55–1.08 PFS ≥ 65: HR: 0.75; 95% CI 0.48–1.17 RR: 19.8% vs. 11.1%; p = 0.29 |
A vs. B ≥ G3: 84% vs. 63% |
| < 65 yr: 783 (64%) | ||||
| ≥ 65 yr: 443 (36%) | ||||
|
Phase III [72] RAISE N = 1072 |
Ratio 1:1 FOLFIRI + A or B A: Ramucirumab B: Placebo |
62 (21–87) |
A vs. B OS: 13.3 vs. 11.7 m; HR: 0.84; p = 0.0219 OS < 65: HR: 0.86; 95% CI 0.72–1.03 OS ≥ 65: HR: 0.85; 95% CI 0.68–1.07 PFS: 5.7 vs. 4.5 m; p < 0.0005 PFS < 65: HR: 0.82 95% CI 0.67–1.00 PFS ≥ 65: HR: 0.78 95% CI 0.67–0.92 RR: 13.4% vs. 12.5%; p = 0.63 |
A vs. B G3: 79% vs. 62% |
| < 65 yr: 645 (60%) | ||||
| ≥ 65 yr: 427 (40%) | ||||
| Second-line and third-line treatment | ||||
|
Phase III [73] RECOURSE N = 800 |
Ratio 2:1 A: TAS-102 B: Placebo |
63 (27–82) |
A vs. B OS: 7.1 vs. 5.3 m; HR: 0.68; p < 0.001 OS < 65: HR: 0.74; 95% CI 0.59–0.94 OS ≥ 65: HR: 0.62; 95% CI 0.48–0.80 PFS: 2.0 vs. 1.7 m; HR: 0.48; p < 0.001 PFS < 65: HR: 0.52; 95% CI 0.42–0.65 PFS ≥ 65: HR: 0.41; 95% CI 0.32–0.52 RR: 1.6% vs. 0.4%; p = 0.29 |
A vs. B ≥ G3: 69% vs. 52% |
| < 65 yr: 448 (56%) | ||||
| ≥ 65 yr: 352 (44%) | ||||
|
Phase III [74] CORRECT N = 760 |
Ratio 2:1 A: Regorafenib B: Placebo |
61 (54–68) |
A vs. B OS: 6.4 vs. 5.0 m; HR: 0,77; p = 0.0052 OS < 65: HR: 0.72; 95% CI 0.56–0.91 OS ≥ 65: HR: 0.86; 95% CI 0.61–1.19 PFS: 1.9 vs. 1.7 m; p < 0.0001 PFS < 65: HR: 0.42; 95% CI 0.34–0.51 PFS ≥ 65: HR: 0.65; 95% CI 0.50–0.86 RR: 1.0% vs. 0.4%; p = 0.19 |
A vs. B ≥ G3: 54% vs. 14% |
| < 65 yr: 475 (63%) | ||||
| ≥ 65 yr: 285 (37%) | ||||
|
Phase III [75] BEACON N = 665 |
Ratio 1:1:1 Cetuximab + A/B/C A: Encorafenib + Binimetinib B: Encorafenib C: Irinotecan or FOLFIRI |
61 (26–91) |
A vs. C OS: 9.3 vs. 5.9 m; HR: 0.60 OS < 65: HR: 0.61; 95% CI 0.45–0.81 OS ≥ 65: HR: 0.56; 95% CI 0.38–0.82 PFS: 4.5 vs. 1.5 m; HR: 0.42; 95% CI 0.33–0.53 RR: 26.8% vs. 1.8%; p < 0.0001 B vs. C OS: 9.3 vs. 5.9 m; HR: 0.61 OS < 65: HR: 0.56; 95% CI 0.42–0.76 OS ≥ 65: HR: 0.65; 95% CI 0.44–0.95 PFS: 4.3 vs. 1.5 m; HR: 0.44; 95% CI 0.35–0.55 RR: 19.5% vs. 1.8%; p < 0.0001 |
G ≥ 3 A vs. B vs. C: 66% vs. 57% vs. 64% |
| < 65 yr: 290 (65%) | ||||
| ≥ 65 yr: 155 (35%) | ||||
CI Confidence interval, CRC colorectal cancer, FOLFIRI irinotecan with 5-fluorouracil and folinic acid, G grade, HR hazard ratio, m month, OS overall survival, PFS progression free survival, RR response rate. TAS-102 trifluridine/tipiracil, yr year
The retrospective STREAM study included 873 patients with metastatic CRC between November 2016 and April 2017 in 33 Spanish hospitals. The treatment regimens most frequently received by patients > 75 years (15.2% of the total) were second line: (i) irinotecan (13%); (ii) capecitabine (10%); and (iii) FOLFIRI (10%). As third-line treatments, they received (i) FOLFOX (18%); (ii) FOLFIRI (8%); (iii) irinotecan plus cetuximab (8%); (iv) capecitabine (8%); and (v) panitumumab (8%). Fourth-line treatments included (i) capecitabine (32%); (ii) FOLFOX (16%); and (iii) regorafenib (16%) [63].
TAS-102 is an oral fluoropyrimidine indicated in patients previously treated with CT based on fluoropyrimidines, oxaliplatin and irinotecan, vascular endothelial growth factor inhibitors (anti-VEGF) and, in patients without KRAS mutations, with anti-EGFR treatments. In a subgroup analysis of a post hoc phase III study, the benefits of this therapy in elderly patients were confirmed. In addition, in other analyses carried out in real clinical practice, there were no differences either in efficacy or in the safety profile as a function of patient age [64, 65].
Second-line or successive treatments with CT and targeted therapy
In a review reported by Altshuler E et al. it was observed that tumours of younger patients were less likely to have a microsatellite instability-high/deficient mismatch repair (MSI-H/dMMR) than tumours of older patients (12.5% vs. 21.4%, p = 0.013), similarly to BRAF mutation (1.5% vs. 16%, p = 0.002). BRAF mutation status was highly associated with MMR status. Thus, BRAF mutated tumours were 29.7 times more likely than B-RAF-WT tumours to be MSI-H/dMMR (p < 0.001) [66].
Patients with native RAS and BRAF CRC who have not received anti-EGFR therapy (cetuximab or panitumumab) as a first-line treatment can receive it alone or in combination with CT in successive lines, with acceptable tolerance in the elderly [67]. Evaluation of the RAS mutation status by analysing circulating tumour DNA (ctDNA) may be useful in identifying patients who are candidates for retreatment with anti-EGFR drugs in successive lines [68, 69] (Table 3).
As a second-line antiangiogenic treatment, CT plus bevacizumab is feasible, even in patients who have previously progressed on bevacizumab and with native RAS tumours [76]. In the VELOR trial, which randomized patients to receive FOLFIRI with or without aflibercept as a second-line treatment after progression on an oxaliplatin regimen, 34% of patients ≥ 65 years were included in the combination arm with aflibercept, among whom 5% were ≥ 75 years. A survival benefit was observed with the combination of FOLFIRI plus aflibercept in patients ≥ 65 years of age, although with a higher incidence of diarrhoea, dehydration, asthenia and weight loss and permanent discontinued treatment due to adverse events [70]. However, studies carried out in real clinical practice showed contradictory data regarding the safety profile in elderly patients [77, 78].
The phase III RAISE trial, which administered FOLFIRI with or without ramucirumab after progression on first-line CT with bevacizumab, included 427 patients (40%) ≥ 65 years of age. In the subgroup analysis, the survival benefit was similar regardless of age [72]. In the phase III CORRECT trial, compared to placebo, the administration of the multikinase inhibitor regorafenib led to an increase in survival in patients previously treated with CT based on fluoropyrimidines, oxaliplatin, irinotecan, anti-VEGF and, in native KRAS patients, with anti-EGFR treatment. Subgroup analysis suggested a lower benefit of regorafenib in patients > 65 years of age [74]. In the single-arm phase IIIb CONSIGN trial, no differences in efficacy and toxicity were observed as a function of age. However, other studies have suggested less benefit in patients > 65 years of age with a worse safety profile [79, 80]. The results of the phase III SUNLIGHT study have recently been published [81]. In 492 patients with metastatic CRC treated with 1–2 lines of previous CT, a survival benefit was demonstrated for patients treated with TAS-102 plus bevacizumab versus TAS-102 alone. A total of 44.1% of the patients included in the study were ≥ 65 years of age, and the subgroup analysis favoured the combination in this subgroup of patients.
Regarding treatment in patients with BRAF V600E-mutated tumours, the phase III BEACON trial with patients with BRAF-mutated CRC randomized patients who had progressed on first- and second-line treatments to receive encorafenib, an oral inhibitor of BRAF V600E, with cetuximab with or without binimetinib versus CT (FOLFIRI or irinotecan) plus cetuximab. The experimental arms exhibited benefits in OS compared to the control arm, establishing the encorafenib-cetuximab combination as the preferred regimen for these patients. In this study, 34.8% of the patients were ≥ 65 years of age (age range: 30–91 years in the encorafenib-cetuximab arm), and the subgroup analysis indicated a benefit in OS for patients ≥ 65 years of age in the experimental branch [75].
In patients with human epidermal growth factor receptor 2 (HER2) overexpression, i.e., between 3 and 5% of CRC patients, anti-HER2 treatments (trastuzumab plus lapatinib and trastuzumab plus pertuzumab) can be used, such as tucatinib or trastuzumab-deruxtecan.
Immunotherapy has shown greater efficacy than CT in patients with metastatic CRC with microsatellite instability or DNA repair deficiencies (dMMR). The phase II KEYNOTE 164 study evaluated efficacy and safety in two cohorts (cohort A with two previous lines and cohort B with one previous line). Twenty-eight percent of the patients were over 65 years of age in cohort A and 38% in cohort B; the response rate, which was the main outcome of the study, was 33% in both cohorts; and there were 13% and 16% grade 3 adverse events in each cohort, respectively [82]. Nivolumab, an anti-programmed cell death protein 1 (anti-PD-1) antibody, both in monotherapy and in combination with ipilimumab (cytotoxic T-lymphocyte antigen 4, anti-CTLA-4), demonstrated efficacy in pretreated patients. In both studies, more than 30% of the patients were older than 65 years of age. The percentage of treatment-related grade 3 adverse events was higher with the combination treatment [83, 84].
Radical treatment of oligometastatic disease
Local ablative treatments (LAT) as well as locoregional therapies offer local tumour control and extensive cytoreduction with low morbidity and mortality. In oligometastatic disease, LAT can achieve long-term disease control by complete tumour ablation in patients not eligible for surgery [85]. Locoregional therapies such as Y90 radioembolization (RE) may contribute to the OS of selected patients by improving the local response in liver-dominant disease or by providing a salvage treatment in chemo-refractory disease [86]. These aspects have been included in the main international clinical guidelines for CRC with oligometastatic disease or liver dominant chemo-refractory metastases [87, 88], but in the context of elderly patients, efficacy data are still rare.
Yang S. et al. built a Markiv-decision model to examine the effect on life expectancy and quality concluding that, in older patients with comorbidities, RT may provide better results [89]. Another study reported by Tanis E. et al. in the phase II Clocc trial [90] included 119 patients with non-resectable colorectal liver metastases randomized to systemic treatment or systemic treatment plus radiofrequency, which met the primary end point of 30 months OS > 38%, with the absence of a subanalysis in older patients.
Chemoembolization (CE) is another LAT, including hepatic transarterial CE (TACE) therapy with drug-eluting beads loaded with irinotecan (DEBIRI), that has been used in several prospective studies that demonstrated improved OS (22 vs. 15 months; p = 0.031) with an acceptable toxicity profile [91]. Other studies have shown benefits with the simultaneous administration of CT and DEBIRI in response rate (78% vs. 54% at 2 months; p = 0.02) [92].
Considering locoregional therapies such as radioembolization, Seidenstickler et al. reported the results of a cohort study including 266 patients who received radiofrequency, high-dose rate brachytherapy (HDR-BT) or Y90-RE. Median OS was 14 months and age > 70 years did not influence survival after local therapies [93].
Finally, SBRT offers promising results. Recently, the SABR-COMET study showed a median OS of 3.2 months compared with 1.6 months in the control group of elderly patients treated with SBRT with a response rate per adverse events grade ≥ 2 of 22.2% compared with 21.4% in the control group [94]. There are no results of phase III trials at this point, but several phase II studies are ongoing, and results are expected in the near future.
In conclusion, LAT can be selected for elderly CRC patients with liver and lung metastases who are not suitable for surgery. CE and RE are reasonable options in selected elderly patients with CRC and predominantly hepatic metastasis.
Geriatric interventions
After performing a CGA, an intervention plan must be developed considering compromised or vulnerable domains that have been detected. Several domains are considered very important from the oncological point of view (Fig. 3). One domain is nutrition, and the objective of nutrition is to avoid malnutrition syndrome, sarcopenia and cachexia. For this, there are 3 possible interventions, which are not mutually exclusive: (i) individualized nutritional advice; (ii) nutritional support with oral supplements; and (iii) pharmaconutrients. To establish the composition of the supplements and their content in pharmaconutrients, the catabolic-inflammatory situation of patients should be taken into account [95, 96].
Fig. 3.
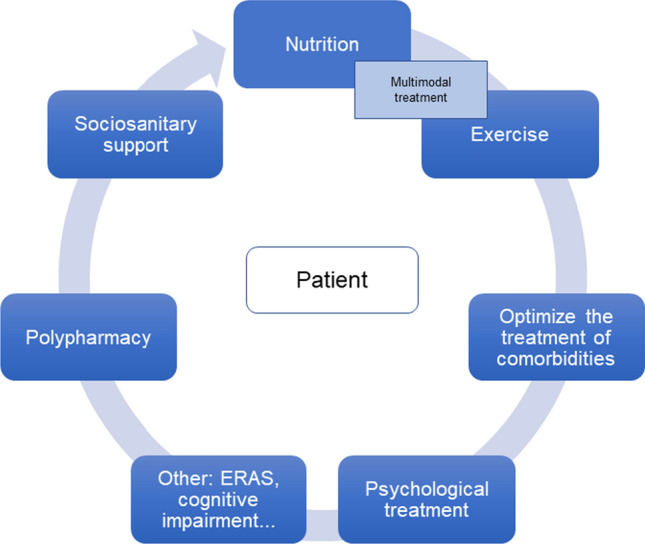
GA in elderly patients with CRC. CRC colorectal cancer, ERAS enhanced recovery after surgery, GA geriatric assessment
The functionality and independence of a patient improves with physical exercise [97]. The Vivifrail team provides a list of exercises to be performed based on the functional situation of each patient [98]. Different studies have shown that the combination of multimodal exercises with nutritional support leads to better results in the recovery of lean mass and in functionality; therefore, both are recommended to be provided together.
Prehabilitation is another domain that involves a multimodal process and that allows functional recovery in stressful situations. Patients should not be excluded from subsequent rehabilitation [99]. In most centres, in digestive pathology, and therefore in colon pathology, enhanced recovery after surgery (ERAS) care protocols are followed as presurgical prehabilitation programmes [100]. In addition, the treatment of chronic diseases before starting cancer treatment should be optimized [14].
Patients usually have complex therapeutic requirements, with visits to multiple specialists and for tests, for which they need optimal social and family support. It must be taken into account that in many cases, patients may have only one main caregiver who may also be elderly. Other important domains in which it is necessary to intervene are polypharmacy, adapting to treatment, the management of depression-anxiety, and reducing the risk of cognitive impairment or delirium, which also play important roles from an oncological point of view.
Future research
There are various ongoing clinical trials and projects, either active or recently completed, related to the systemic treatment of older patients with CRC.
In the adjuvant setting, the ADAGE-PRODIGE randomized phase III trial is comparing, for patients ≥ 70 years of age and in terms of PFS at 3 years, treatment with fluoropyrimidines and oxaliplatin versus only fluoropyrimidines in physically fit patients (group 1) and administration of fluoropyrimidines versus only observation in frail patients (group 2). Thus far, only preliminary tolerance data have been published for 50% of the patients included, showing that grade 3–5 toxicities were more common in fit individuals treated with oxaliplatin or in frail patients treated with fluoropyrimidines than in fit patients treated with fluoropyrimidines, and that early discontinuations were more frequent among frail patients [101].
For advanced disease, a multicentre controlled phase II clinical trial (NCT03530267), in which 196 elderly or frail patients with metastatic CRC have been included, is evaluating the administration of aflibercept with 5FU or FOLFOX (1:1) as the first line of treatment with an initial dose reduced to 80%. The main outcome is the rate of progression-free survival at 6 months.
In the phase II clinical trial CAIRO7 (NCT05092880), expected completion date October 2028, elderly or frail patients with CRC and unresectable liver metastases are randomized to receive a liver ER with holmium-166 microspheres as a first-line treatment or combined capecitabine and anti-VEGF treatment. The main outcome is PFS.
The SOLSTICE clinical trial (NCT03869892), already mentioned, includes patients with metastatic CRC who are not candidates for or who do not require intensive treatment.
All these investigations will help to improve the therapeutic options available for elderly or frail patients with CRC.
Conclusions
CRC is the most common cancer in Europe, with 70% of patients older than 65 years of age. A multidisciplinary approach and performing a GA before each therapeutic decision is essential. GA predicts the risk of toxicity and survival and is of great help to tailor individualized treatment. In addition, it allows detection of patient vulnerabilities, allowing interventions aimed at achieving less morbidity and toxicity of the treatments administered. Elderly patients with localized CRC should undergo standard cancer resection, preferably laparoscopically.
There are few studies aimed at this population; therefore, the therapeutic recommendations are based on the extrapolation of the results obtained for the general population. The indication for adjuvant CT should be considered in the context of potential benefits, risk of recurrence and life expectancy in relation to age and comorbidities, as well as patient preferences. In fit elderly patients with stage III CRC, adjuvant CT with fluoropyrimidines can be administered for 6 months. In prefrail patients, capecitabine can be administered at reduced doses, and the patient should be closely monitored for toxicity.
When disease is metastatic, radical treatment with surgery or radiofrequency (RF) or SBRT should be considered. Regarding palliative CT, if a patient is fit, it is recommended that the same treatment as that for younger patients be used, and dose reductions assessed to minimize the incidence of toxicity while maintaining similar efficacy. In prefrail patients, if they do not have RAS and BRAF gene mutations, the combination of fluoropyrimidines with bevacizumab or anti-EGFR is a good option. In contrast, frail patients are candidates for symptomatic palliative treatment without CT.
In the future, specific clinical trials in the elderly population that include a GA and specific objectives for this population should be conducted. The Oncogeriatrics Section of the SEOM as well as other scientific associations are working on the dissemination and implementation of projects to strengthen the evidence and improve the assessment of elderly patients with cancer.
Acknowledgements
The authors are grateful for the editorial assistance of Pablo Tous of HealthCo Trials (Madrid, Spain) in the drafting of this manuscript.
Author contributions
All authors contributed to the study concept and design. All authors participating in the first draft of the manuscript commented on subsequent draft versions and approved the final version of the manuscript.
Funding
SEOM, TTD and GEMCAD acknowledge the financial support for this project in the form of unrestricted collaboration in the logistics from Merck, S.L.U., Spain, an affiliate of Merck KGaA, Darmstadt, Germany and Servier.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that, when writing and revising the text, they did not know the names of the pharmaceutical companies that provided financial support for this project, so this support has not influenced the content of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Informed consent
Not applicable.
Footnotes
The original online version of this article was revised to correct the funder name to Merck, S.L.U., Spain, an affiliate of Merck KGaA, Darmstadt, Germany.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/29/2023
A Correction to this paper has been published: 10.1007/s12094-023-03351-x
References
- 1.Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–1305. doi: 10.1016/j.annonc.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, et al. Treatment of colorectal cancer in older patients: international society of geriatric oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26(3):463–476. doi: 10.1093/annonc/mdu253. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol. 2000;35(3):147–154. doi: 10.1016/s1040-8428(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 6.Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5(3):224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 7.Ferrat E, Audureau E, Paillaud E, Liuu E, Tournigand C, Lagrange JL, et al. Four distinct health profiles in older patients with cancer: latent class analysis of the prospective ELCAPA cohort. J Gerontol A Biol Sci Med Sci. 2016;71(12):1653–1660. doi: 10.1093/gerona/glw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, Selby PJ, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 9.Retornaz F, Guillem O, Rousseau F, Morvan F, Rinaldi Y, Nahon S, et al. Predicting chemotherapy toxicity and death in older adults with colon cancer: results of MOST study. Oncologist. 2020;25(1):e85–e93. doi: 10.1634/theoncologist.2019-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessems SAM, Konsten JLM, Vogelaar JFJ, Csepán-Magyar R, Maas H, van de Wouw YAJ, et al. Frailty screening by Geriatric-8 and 4-meter gait speed test is feasible and predicts postoperative complications in elderly colorectal cancer patients. J Geriatr Oncol. 2021;12(4):592–598. doi: 10.1016/j.jgo.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Procaccio L, Bergamo F, Gatti M, Chiusole B, Tierno G, Bergo E, et al. The oncological multidimensional prognostic index is a promising decision-making tool: a real-world analysis in older patients with metastatic colorectal cancer. Eur J Cancer. 2022;177:112–119. doi: 10.1016/j.ejca.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Boakye D, Rillmann B, Walter V, Jansen L, Hoffmeister M, Brenner H. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: a systematic review and meta-analysis. Cancer Treat Rev. 2018;64:30–39. doi: 10.1016/j.ctrv.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Ma T, Cui W, Li T, Liu D, Chen L, et al. Frailty and long-term survival of patients with colorectal cancer: a meta-analysis. Aging Clin Exp Res. 2022;34(7):1485–1494. doi: 10.1007/s40520-021-02072-x. [DOI] [PubMed] [Google Scholar]
- 14.Lund CM, Vistisen KK, Olsen AP, Bardal P, Schultz M, Dolin TG, et al. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: a randomised trial (GERICO) Br J Cancer. 2021;124(12):1949–1958. doi: 10.1038/s41416-021-01367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feliu J, Espinosa E, Basterretxea L, Paredero I, Llabrés E, Jiménez-Munárriz B, et al. Prediction of chemotoxicity, unplanned hospitalizations and early death in older patients with colorectal cancer treated with chemotherapy. Cancers. 2022;14(1):127. doi: 10.3390/cancers14010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire swedish population. Dis Colon Rectum. 2018;61(9):1016–1025. doi: 10.1097/DCR.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 17.Fujii S, Tsukamoto M, Fukushima Y, Shimada R, Okamoto K, Tsuchiya T, et al. Systematic review of laparoscopic vs open surgery for colorectal cancer in elderly patients. World J Gastrointest Oncol. 2016;8(7):573–582. doi: 10.4251/wjgo.v8.i7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Senac NM, Mayordomo-Cava J, Macías-Valle A, Aldama-Marín P, Majuelos González S, Cruz Arnés ML, et al. Colorectal cancer in elderly patients with surgical indication: state of the art, current management, role of frailty and benefits of a geriatric liaison. Int J Environ Res Public Health. 2021;18(11):6072. doi: 10.3390/ijerph18116072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 20.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 21.Haller DG, O'Connell MJ, Cartwright TH, Twelves CJ, McKenna EF, Sun W, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol. 2015;26(4):715–724. doi: 10.1093/annonc/mdv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177–1188. doi: 10.1056/NEJMoa1713709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 24.Maas HA, Lemmens VE, Nijhuis PH, de Hingh IH, Koning CC, Janssen-Heijnen ML. Benefits and drawbacks of short-course preoperative radiotherapy in rectal cancer patients aged 75 years and older. Eur J Surg Oncol. 2013;39(10):1087–1093. doi: 10.1016/j.ejso.2013.07.094. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;40(23):2546–2556. doi: 10.1200/JCO.22.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung B, Påhlman L, Johansson R, Nilsson E. Rectal cancer treatment and outcome in the elderly: an audit based on the Swedish rectal cancer registry 1995–2004. BMC Cancer. 2009;9:68. doi: 10.1186/1471-2407-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hathout L, Maloney-Patel N, Malhotra U, Wang SJ, Chokhavatia S, Dalal I, et al. Management of locally advanced rectal cancer in the elderly: a critical review and algorithm. J Gastrointest Oncol. 2018;9(2):363–376. doi: 10.21037/jgo.2017.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prades J, Borras JM. Shifting sands: adapting the multidisciplinary team model to technological and organizational innovations in cancer care. Future oncol (London, England) 2014;10(13):1995–1998. doi: 10.2217/fon.14.125. [DOI] [PubMed] [Google Scholar]
- 29.Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41(9):729–741. doi: 10.1016/j.ctrv.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Berrino F, Capocaccia R, Estève J, Gatta G, Hakulinen T, Micheli A, et al. Survival of cancer patients in Europe: The EUROCARE-2 study: International agency for research on cancer; 1999.
- 31.Surbone A, Kagawa-Singer M, Terret C, Baider L. The illness trajectory of elderly cancer patients across cultures: SIOG position paper. Ann Oncol. 2007;18(4):633–638. doi: 10.1093/annonc/mdl178. [DOI] [PubMed] [Google Scholar]
- 32.Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 33.Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, Stephens RJ, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26(9):1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 34.Jackson NA, Barrueco J, Soufi-Mahjoubi R, Marshall J, Mitchell E, Zhang X, et al. Comparing safety and efficacy of first-line irinotecan/fluoropyrimidine combinations in elderly versus nonelderly patients with metastatic colorectal cancer: findings from the bolus, infusional, or capecitabine with camptostar-celecoxib study. Cancer. 2009;115(12):2617–2629. doi: 10.1002/cncr.24305. [DOI] [PubMed] [Google Scholar]
- 35.Sastre J, Marcuello E, Masutti B, Navarro M, Gil S, Antón A, et al. Irinotecan in combination with fluorouracil in a 48-hour continuous infusion as first-line chemotherapy for elderly patients with metastatic colorectal cancer: a Spanish cooperative group for the treatment of digestive tumors study. J clin Oncol. 2005;23(15):3545–3551. doi: 10.1200/JCO.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J clin Oncol. 2006;24(25):4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 37.Sastre J, Aranda E, Massutí B, Tabernero J, Chaves M, Abad A, et al. Elderly patients with advanced colorectal cancer derive similar benefit without excessive toxicity after first-line chemotherapy with oxaliplatin-based combinations: comparative outcomes from the 03-TTD-01 phase III study. Crit Rev Oncol Hematol. 2009;70(2):134–144. doi: 10.1016/j.critrevonc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Benavides M, Pericay C, Valladares-Ayerbes M, Gil-Calle S, Massutí B, Aparicio J, et al. Oxaliplatin in combination with infusional 5-fluorouracil as first-line chemotherapy for elderly patients with metastatic colorectal cancer: a phase II study of the Spanish cooperative group for the treatment of digestive tumors. Clin Colorectal Cancer. 2012;11(3):200–206. doi: 10.1016/j.clcc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Comella P, Natale D, Farris A, Gambardella A, Maiorino L, Massidda B, et al. Capecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal carcinoma: final results of the Southern Italy Cooperative Oncology Group Trial 0108. Cancer. 2005;104(2):282–289. doi: 10.1002/cncr.21167. [DOI] [PubMed] [Google Scholar]
- 40.Feliu J, Salud A, Escudero P, Lopez-Gómez L, Bolaños M, Galán A, et al. XELOX (capecitabine plus oxaliplatin) as first-line treatment for elderly patients over 70 years of age with advanced colorectal cancer. Br J Cancer. 2006;94(7):969–975. doi: 10.1038/sj.bjc.6603047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet (London, England) 2011;377(9779):1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aparicio T, Lavau-Denes S, Phelip JM, Maillard E, Jouve JL, Gargot D, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001–02) Ann Oncol. 2016;27(1):121–127. doi: 10.1093/annonc/mdv491. [DOI] [PubMed] [Google Scholar]
- 43.Folprecht G, Cunningham D, Ross P, Glimelius B, Di Costanzo F, Wils J, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol. 2004;15(9):1330–1338. doi: 10.1093/annonc/mdh344. [DOI] [PubMed] [Google Scholar]
- 44.Feliu J, Escudero P, Llosa F, Bolaños M, Vicent JM, Yubero A, et al. Capecitabine as first-line treatment for patients older than 70 years with metastatic colorectal cancer: an oncopaz cooperative group study. J Clin Oncol. 2005;23(13):3104–3111. doi: 10.1200/JCO.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Oh DY, Kim YJ, Han SW, Choi IS, Kim DW, et al. Reduced dose intensity FOLFOX-4 as first line palliative chemotherapy in elderly patients with advanced colorectal cancer. J Korean Med Sci. 2005;20(5):806–810. doi: 10.3346/jkms.2005.20.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattioli R, Massacesi C, Recchia F, Marcucci F, Cappelletti C, Imperatori L, et al. High activity and reduced neurotoxicity of bi-fractionated oxaliplatin plus 5-fluorouracil/leucovorin for elderly patients with advanced colorectal cancer. Ann Oncol. 2005;16(7):1147–1151. doi: 10.1093/annonc/mdi222. [DOI] [PubMed] [Google Scholar]
- 47.Comella P, Gambardella A, Farris A, Maiorino L, Natale D, Massidda B, et al. A tailored regimen including capecitabine and oxaliplatin for treating elderly patients with metastatic colorectal carcinoma Southern Italy cooperative oncology group trial 0108. Crit Rev Oncol Hematol. 2005;53(2):133–139. doi: 10.1016/j.critrevonc.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Liposits G, Eshøj HR, Möller S, Winther SB, Skuladottir H, Ryg J, et al. Quality of life in vulnerable older patients with metastatic colorectal cancer receiving palliative chemotherapy-the randomized NORDIC9-study. Cancers. 2021;13(11):2604. doi: 10.3390/cancers13112604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winther SB, Liposits G, Skuladottir H, Hofsli E, Shah CH, Poulsen L, et al. Reduced-dose combination chemotherapy (S-1 plus oxaliplatin) versus full-dose monotherapy (S-1) in older vulnerable patients with metastatic colorectal cancer (NORDIC9): a randomised, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2019;4(5):376–388. doi: 10.1016/S2468-1253(19)30041-X. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(11):1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 51.André T, Falcone A, Shparyk Y, Moiseenko F, Polo-Marques E, Csöszi T, et al. Trifluridine-tipiracil plus bevacizumab versus capecitabine plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer ineligible for intensive therapy (SOLSTICE): a randomised, open-label phase 3 study. Lancet Gastroenterol Hepatol. 2022 doi: 10.1016/S2468-1253(22)00334-X. [DOI] [PubMed] [Google Scholar]
- 52.Andre T, Falcone A, Shparyk Y, Moiseenko F, Polo-Marques E, Csoszi T, et al. Trifluridine-tipiracil plus bevacizumab versus capecitabine plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer ineligible for intensive therapy (SOLSTICE): a randomised, open-label phase 3 study. Lancet Gastroenterol Hepatol. 2023;8(2):133–144. doi: 10.1016/S2468-1253(22)00334-X. [DOI] [PubMed] [Google Scholar]
- 53.Hamaguchi T, Takashima A, Mizusawa J, Shimada Y, Nagashima F, Ando M, et al. A randomized phase III trial of mFOLFOX7 or CapeOX plus bevacizumab versus 5-FU/l-LV or capecitabine plus bevacizumab as initial therapy in elderly patients with metastatic colorectal cancer: JCOG1018 study (RESPECT) J Clin Oncol. 2020;40(4_suppl):10. [Google Scholar]
- 54.Price TJ, Zannino D, Wilson K, Simes RJ, Cassidy J, Van Hazel GA, et al. Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: a subgroup analysis from the AGITG MAX trial: an international randomised controlled trial of Capecitabine, Bevacizumab and Mitomycin C. Ann Oncol. 2012;23(6):1531–1536. doi: 10.1093/annonc/mdr488. [DOI] [PubMed] [Google Scholar]
- 55.Aparicio T, Bouché O, Taieb J, Maillard E, Kirscher S, Etienne PL, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol. 2018;29(1):133–138. doi: 10.1093/annonc/mdx529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S, Rosen O. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol. 2009;27(2):199–205. doi: 10.1200/JCO.2008.17.7931. [DOI] [PubMed] [Google Scholar]
- 57.Sastre J, Grávalos C, Rivera F, Massuti B, Valladares-Ayerbes M, Marcuello E, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD group study. Oncologist. 2012;17(3):339–345. doi: 10.1634/theoncologist.2011-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lonardi S, Schirripa M, Buggin F, Antonuzzo L, Merelli B, Boscolo G, et al. First-line FOLFOX plus panitumumab versus 5FU plus panitumumab in RAS-BRAF wild-type metastatic colorectal cancer elderly patients: the PANDA study. J Clin Oncol. 2020;38(15_suppl):4002. [Google Scholar]
- 59.Papamichael D, Lopes GS, Olswold CL, Douillard JY, Adams RA, Maughan TS, et al. Efficacy of anti-epidermal growth factor receptor agents in patients with RAS wild-type metastatic colorectal cancer ≥ 70 years. Eur J Cancer. 2022;163:1–15. doi: 10.1016/j.ejca.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Rosati G, Corsi D, Avallone A, Brugnatelli S, Dell'Aquila E, Cinausero M, et al. Reduced-dose of doublet chemotherapy combined with anti-EGFR antibodies in vulnerable older patients with metastatic colorectal cancer: data from the REVOLT study. J Geriatr Oncol. 2022;13(3):302–307. doi: 10.1016/j.jgo.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Chau I, Norman AR, Cunningham D, Waters JS, Topham C, Middleton G, et al. Elderly patients with fluoropyrimidine and thymidylate synthase inhibitor-resistant advanced colorectal cancer derive similar benefit without excessive toxicity when treated with irinotecan monotherapy. Br J Cancer. 2004;91(8):1453–1458. doi: 10.1038/sj.bjc.6602169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aranda E, Polo E, Camps C, Carrato A, Díaz-Rubio E, Guillem V, et al. Treatment patterns for metastatic colorectal cancer in Spain. Clin Transl Oncol. 2020;22(9):1455–1462. doi: 10.1007/s12094-019-02279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayer RJ, Hochster HS, Cohen SJ, Winkler R, Makris L, Grothey A. Safety of trifluridine/tipiracil in an open-label expanded-access program in elderly and younger patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2018;82(6):961–969. doi: 10.1007/s00280-018-3686-5. [DOI] [PubMed] [Google Scholar]
- 65.Stavraka C, Pouptsis A, Synowiec A, Angelis V, Satterthwaite L, Khan S, et al. Trifluridine/tipiracil in metastatic colorectal cancer: a UK multicenter real-world analysis on efficacy, safety, predictive and prognostic factors. Clin Colorectal Cancer. 2021;20(4):342–349. doi: 10.1016/j.clcc.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Altshuler E, Franke AJ, Skelton WPT, Feely M, Wang Y, Lee JH, et al. Impact of institutional universal microsatellite-instability (MSI) reflex testing on molecular profiling differences between younger and older patients with colorectal cancer. Clin Colorectal Cancer. 2023;22(1):153–159. doi: 10.1016/j.clcc.2022.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Una Cidon E, Tamas H, Pilar A. Cetuximab as monotherapy in elderly patients with metastatic colorectal cancer. Ann Oncol. 2014;25:ii91. [Google Scholar]
- 68.Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5(3):343–350. doi: 10.1001/jamaoncol.2018.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuji A, Nakamura M, Watanabe T, Manaka D, Matsuoka H, Kataoka M, et al. Phase II study of third-line panitumumab rechallenge in patients with metastatic wild-type KRAS colorectal cancer who obtained clinical benefit from First-line panitumumab-based chemotherapy: JACCRO CC-09. Target Oncol. 2021;16(6):753–760. doi: 10.1007/s11523-021-00845-y. [DOI] [PubMed] [Google Scholar]
- 70.Ruff P, Van Cutsem E, Lakomy R, Prausova J, van Hazel GA, Moiseyenko VM, et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: an age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol. 2018;9(1):32–39. doi: 10.1016/j.jgo.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 72.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 73.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 74.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet (London, England) 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 75.Tabernero J, Grothey A, Van Cutsem E, Yaeger R, Wasan H, Yoshino T, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39(4):273–284. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bennouna J, Hiret S, Bertaut A, Bouché O, Deplanque G, Borel C, et al. Continuation of bevacizumab vs cetuximab plus chemotherapy after first progression in KRAS wild-type metastatic colorectal cancer: the UNICANCER PRODIGE18 randomized clinical trial. JAMA Oncol. 2019;5(1):83–90. doi: 10.1001/jamaoncol.2018.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chau I, Fakih M, García-Alfonso P, Linke Z, Ruiz Casado A, Marques EP, et al. Safety and effectiveness of aflibercept + fluorouracil, leucovorin, and irinotecan (FOLFIRI) for the treatment of patients with metastatic colorectal cancer (mCRC) in current clinical practice OZONE study. Cancers. 2020;12(3):657. doi: 10.3390/cancers12030657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fernández Montes A, López López C, Argilés Martínez G, Páez López D, López Muñoz AM, García Paredes B, et al. Prognostic nomogram and patterns of use of FOLFIRI-aflibercept in advanced colorectal cancer: a real-world data analysis. Oncologist. 2019;24(8):e687–e695. doi: 10.1634/theoncologist.2018-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casadei-Gardini A, Vagheggini A, Gelsomino F, Spallanzani A, Ulivi P, Orsi G, et al. Is there an optimal choice in refractory colorectal cancer? A network meta-analysis. Clin Colorectal Cancer. 2020;19(2):82–90.e9. doi: 10.1016/j.clcc.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Van Cutsem E, Ciardiello F, Seitz JF, Hofheinz R, Verma U, Garcia-Carbonero R, et al. LBA-05 results from the large, open-label phase 3b CONSIGN study of regorafenib in patients with previously treated metastatic colorectal cancer. Ann Oncol. 2015;26:iv118. [Google Scholar]
- 81.Tabernero J, Prager GW, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine/tipiracil plus bevacizumab for third-line treatment of refractory metastatic colorectal cancer: the phase 3 randomized SUNLIGHT study. J Clin Oncol. 2023;41(4_suppl):4. [Google Scholar]
- 82.Diaz L, Marabelle A, Kim TW, Geva R, Van Cutsem E, André T, et al. Efficacy of pembrolizumab in phase 2 KEYNOTE-164 and KEYNOTE-158 studies of microsatellite instability high cancers. Ann Oncol. 2017;28:v128–v129. [Google Scholar]
- 83.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 84.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Baere T, Tselikas L, Yevich S, Boige V, Deschamps F, Ducreux M, et al. The role of image-guided therapy in the management of colorectal cancer metastatic disease. Eur J Cancer. 2017;75:231–242. doi: 10.1016/j.ejca.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy A, Cohn M, Coldwell DM, Drooz A, Ehrenwald E, Kaiser A, et al. Updated survival outcomes and analysis of long-term survivors from the MORE study on safety and efficacy of radioembolization in patients with unresectable colorectal cancer liver metastases. J Gastrointest Oncol. 2017;8(4):614–624. doi: 10.21037/jgo.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. 10.1016/j.annonc.2022.10.003. PMID: 36307056. [DOI] [PubMed]
- 88.Network NCC. National comprehensive cancer network [Available from: https://www.nccn.org]. Accessed 16 Dec 2022.
- 89.Yang S, Alibhai SM, Kennedy ED, El-Sedfy A, Dixon M, Coburn N, et al. Optimal management of colorectal liver metastases in older patients: a decision analysis. HPB (Oxford) 2014;16(11):1031–1042. doi: 10.1111/hpb.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruers T, Punt C, Van Coevorden F, Pierie J, Borel-Rinkes I, Ledermann JA, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23(10):2619–2626. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32(4):1387–1395. [PubMed] [Google Scholar]
- 92.Martin RC, 2nd, Scoggins CR, Schreeder M, Rilling WS, Laing CJ, Tatum CM, et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121(20):3649–3658. doi: 10.1002/cncr.29534. [DOI] [PubMed] [Google Scholar]
- 93.Seidensticker R, Damm R, Enge J, Seidensticker M, Mohnike K, Pech M, et al. Local ablation or radioembolization of colorectal cancer metastases: comorbidities or older age do not affect overall survival. BMC Cancer. 2018;18(1):882. doi: 10.1186/s12885-018-4784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Las PR, Majem M, Perez-Altozano J, Virizuela JA, Cancer E, Diz P, et al. SEOM clinical guidelines on nutrition in cancer patients (2018) Clin Transl Oncol. 2019;21(1):87–93. doi: 10.1007/s12094-018-02009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Hooper L, Kiesswetter E, et al. ESPEN practical guideline: clinical nutrition and hydration in geriatrics. Clin Nutr (Edinburgh, Scotland) 2022;41(4):958–989. doi: 10.1016/j.clnu.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 97.Idorn M, Thor SP. Exercise and cancer: from "healthy" to "therapeutic"? Cancer Immunol Immunother. 2017;66(5):667–671. doi: 10.1007/s00262-017-1985-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vivifrail. Vivifrail – Exercise for elderly adults [Available from: https://vivifrail.com. Accessed 16 Dec 2022.
- 99.Santa Mina D, van Rooijen SJ, Minnella EM, Alibhai SMH, Brahmbhatt P, Dalton SO, et al. Multiphasic prehabilitation across the cancer continuum: a narrative review and conceptual framework. Front Oncol. 2020;10:598425. doi: 10.3389/fonc.2020.598425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Society E. enhanced recovery after surgery [Available from: https://erassociety.org/. Accessed 16 Dec 2022.
- 101.Aparicio T, Bouché O, Etienne PL, Barbier E, Mineur L, Desgrippes R, et al. Preliminary tolerance analysis of adjuvant chemotherapy in older patients after resection of stage III colon cancer from the PRODIGE 34-FFCD randomized trial. Dig Liver Dis. 2022 doi: 10.1016/j.dld.2022.08.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.