Abstract
Key Clinical Message
Acquired hemophilia A (AHA) can present as life‐threatening bleeding during the postpartum period. Prompt treatment allows patients with AHA to achieve complete remission and have normal subsequent pregnancies.
Abstract
Acquired hemophilia A (AHA) is a rare bleeding disorder caused by the production of autoantibodies against factor VIII (FVIII). AHA can present with severe bleeding, especially in postpartum patient. We report a 38‐year‐old woman who presented in an emergency department with severe postpartum hemorrhage 2 weeks after cesarean section. Her investigation showed an isolated prolongation of partial thromboplastin time (PTT), low factor VIII assay and a factor VIII inhibitor test, resulting in abnormal Bethesda units which consistent with AHA. This case report highlights the importance of early diagnosis and treatment of AHA. With timely and appropriate management, most patients can achieve a good outcome.
Keywords: acquired hemophilia A, bleeding, factor VIII, postpartum hemorrhage

1. INTRODUCTION
Acquired hemophilia A (AHA) is a rare bleeding disorder caused by the production of an autoantibody against factor VIII (FVIII). This autoantibody, also known as an inhibitor, can neutralize FVIII and prevent it from functioning properly, leading to an increased risk of bleeding. 1 The most common manifestation of AHA is subcutaneous bleeding in the skin, soft tissue, or muscles. Other possible bleeding sites include the gastrointestinal tract, genitourinary tract, and retroperitoneum. 2 , 3
The estimated incidence of AHA is 1.34 cases per million people per year. Though rare, prompt management is crucial as AHA can be life‐threatening. While the median age of onset for AHA is 70 years old, it can occur at any age. Around 50% of AHA cases are idiopathic. However, in younger patients, AHA may be associated with an underlying autoimmune disorder or pregnancy. 4
Acquired hemophilia A (PAIHA) is a less common subtype of AHA that primarily affects women after childbirth. PAIHA accounts for 7% of all AHA cases and is often triggered by pregnancy. Most bleeding episodes in PAIHA occur in the peri‐partum and post‐partum periods, with only 2% of cases being diagnosed during pregnancy. 5
In most cases of PAIHA, patients experience a favorable outcome as the autoantibody levels typically decrease spontaneously over time. However, delayed diagnosis and treatment can be dangerous to both mother and child. Patients with PAIHA commonly suffer from postpartum hemorrhage, and the fetus can experience postnatal bleeding due to the transfer of maternal antibodies through the placenta. Studies indicate that the mean duration of bleeding episode is 33 months, with a mean time to achieve remission is 14 months. 5 , 6
We present a case involving a 38‐year‐old woman who presented with delayed postpartum bleeding and subsequently received a diagnosis of AHA. She was effectively treated using activated prothrombin complex concentrate (aPCC) and corticosteroids. Follow‐up over the course of 1 year has shown no recurrence of symptoms.
2. CASE REPORT
A 38‐year‐old female with no significant medical history presented to the emergency department (ED) with vaginal bleeding. The patient had undergone a cesarean section 2 weeks prior to presentation due to her history of cesarean delivery during her first pregnancy. Upon arrival at the ED, she was hypotensive with a blood pressure of 80/50 mmHg and was experiencing active bleeding from the vagina. A complete blood count (CBC) revealed significant anemia, with a hematocrit (Hct) of 22%. The patient received immediate intravenous fluid resuscitation and a blood transfusion.
Evacuation of blood clots and curettage was performed, with an estimated total blood loss of 1200 mL. To address the severity of the vaginal bleeding, a total of 6 units of packed red blood cells and 6 units of fresh frozen plasma were administered. Additionally, the patient was provided with tranexamic acid (1 gram), vitamin K (10 milligrams), intravenous oxytocin (20 units), and intravenous methergine (0.2 mg).
Despite these aggressive resuscitative measures, her hematocrit continued to trend downward, reaching 17%. Consultation with a gynecologist was sought. Bedside ultrasound was recommended, which detected free fluid in the hepatorenal and splenorenal spaces. A computed tomography angiography (CTA) showed a retroperitoneal mass compressing the right kidney, with consequent mild right hydronephrosis and hydroureter (Figures 1A,B and 2). This mass was also associated with a significant amount of ascites, connecting with an intermuscular fluid collection located near the anterior abdominal wall and the cesarean surgical site.
FIGURE 1.
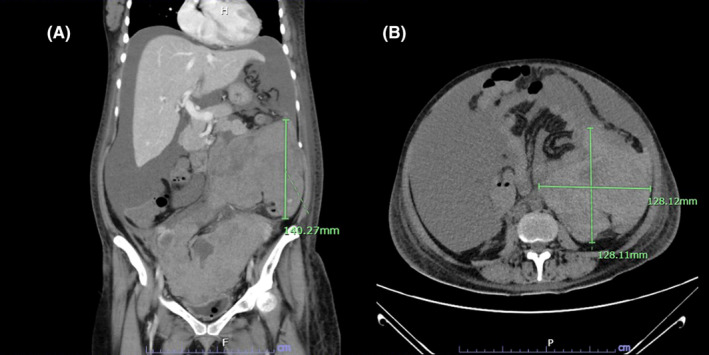
(A, B) Coronal view and axial view of retroperitoneal mass. The presence of this lesion resulted in the displacement of the small bowel in an anteromedial direction and the kidney in a posterior direction due to its mass effect. The present lesion exhibited an extension that resulted in the compression of the right ureter, with consequent mild right hydronephrosis.
FIGURE 2.
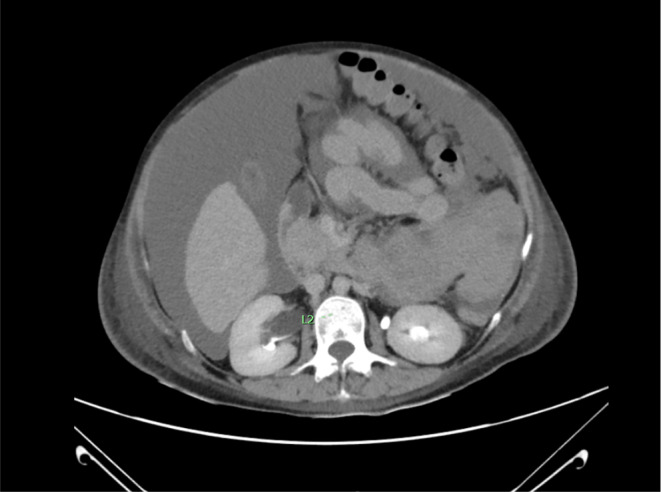
Axial view showed right hydronephrosis due to mass effect of large hematoma.
Based on these findings, an emergency total abdominal hysterectomy was expeditiously performed. During intra‐operative assessment, a uterine rupture measuring 2 × 5 × 8 cm was identified, with the tear extending deep into the lower segment of the uterus and involving the right broad ligament and anterior portion of the uterus. An estimated 3500 mL of blood was lost during the procedure.
The patient's medical history contained no underlying diseases or previous bleeding disorders. The initial cesarean section during her first pregnancy had taken place 3 years prior, and at that time, no incidents of post‐partum hemorrhage had been recorded. Furthermore, she denied any family history of bleeding disorders and denied usage of anti‐coagulants or anti‐platelet medications.
Further investigation revealed normal kidney, liver, and thyroid functions. However, her CBC indicated a hemoglobin level of 4.4 g/dL, a white blood cell count of 20,050 cells/cu.mm, and a platelet count of 321,000 cells/cu.mm. The coagulation profile showed an isolated prolongation of partial thromboplastin time (PTT) at 69.6 s (reference range: 28.8–36.4 s), alongside a normal prothrombin time (PT) of 10.7 s (reference range: 9.9–12.3 s).
Given the abnormal coagulation test results, a mixing test for PTT was conducted. The APTT mixing study revealed values of 33 s and 44.6 s at 0 and 2 h, respectively, indicating an inability to correct after the 2‐h mark.
Considering the information above, the patient's potential diagnosis pointed towards AHA. This was confirmed through a factor 8 assay, yielding a result of 5.4% (normal range 70–150), and a factor 8 inhibitor test, resulting in 12 Bethesda units. Factor 9 and factor 11 assays were conducted, both yielding normal results.
Following the hysterectomy, the patient experienced a persistent decline in hematocrit. She was treated with the first dose of FEIBA (activated prothrombin complex concentrate) at 2500 units (50 units/kg) and Dexamethasone at 5 mg IV every 6 h, along with transfusion support. Sheehan's syndrome was also diagnosed by an endocrinologist due to evidence of severe postpartum hemorrhage and a decrease of all axes of pituitary gland hormones.
Over the next 2 days, a CT angiogram was performed due to progressive abdominal distention, while the patient's vital signs remained stable. It showed a large retroperitoneal hematoma in the lower left abdomen and active extravasation from the left ovarian artery. The patient did not display any signs of gross hematuria. The patient underwent trans‐arterial embolization of the left ovarian artery. A second dose of FEIBA 2500 units was administered before the procedure to stop the bleeding.
After the trans‐arterial embolization procedure, the patient's clinical condition improved, with no active bleeding observed in the drain. Consequently, the drain was removed, and all transfusions were discontinued. The patient was discharged 7 days after surgery while receiving prednisolone at a dosage of 50 mg/day (1 mg/kg/d).
During the 2‐week follow‐up, the patient's factor 8 assay had increased to 19%, and her factor 8 inhibitor level had decreased to 0.7 Bethesda units. The patient was given Rituximab at a dose of 600 mg (375 mg/m2) weekly for four doses. After the fourth dose of Rituximab, the patient's factor 8 assay measured 53.4% and her factor 8 inhibitor level was negative. Her bleeding issues resolved, and she continued to follow‐up with the hematology clinic.
3. DISCUSSION
This case report addressed AHA within the context of a postpartum patient. The patient presented with postpartum hemorrhage and an unexpected retroperitoneal hematoma, distinctive complications stemming from cesarean surgery. These findings should prompt consideration that the patient might have an acquired coagulation disorder.
The severity of the patient's bleeding was pronounced and life‐threatening, as she experienced hypovolemic shock due to active intraperitoneal bleeding. Immediate administration of a bypassing agent was therefore deemed essential. The repertoire of bypassing agents for AHA encompasses aPCC or recombinant factor VIIa, both of which expedite thrombin formation. 7
Simultaneously, corticosteroids are part of the primary treatment approach for AHA and were thus administered to the patient. Moreover, she underwent treatment with Rituximab, a second‐line intervention known to be efficacious in managing AHA. The patient tolerated these interventions well, and ultimately she achieved complete remission.
A look at EACH2 registry data reveals that women with AHA who underwent steroid‐based first‐line treatment invariably achieved complete remission. However, a notable enhancement in the rate of complete remission (100%) and a corresponding diminution in the relapse rate (0%) were observed among patients who received combination therapy involving steroids and Rituximab. 5 These data imply that a combined therapeutic stratagem could present a promising option for patients with AHA. Importantly, rituximab's minimal excretion into breast milk substantiates its appropriateness for administration to breastfeeding women. 8 , 9
Temporal nuances play a pivotal role in prognosticating the outcome for patients with acquired hemophilia. In the present case, the patient underwent total hysterectomy prior to the diagnostic determination, a sequence that might have exacerbated bleeding complications during the procedure, culminating in profound shock. Nevertheless, prompt resuscitation coupled with the administration of bypassing agents ensued due to suspicions of a bleeding disorder. Prior investigations indicate that the median interval between the onset of bleeding and formal diagnosis of AHA is approximately 6 days. 5 For this patient, a similar amount of time passed before a diagnosis was made. Had the diagnosis been determined sooner, timely administration of bypassing agents might have arrested the postpartum hemorrhage, averting progression to severe bleeding and shock.
The patient's case serves as a didactic illustration that both corticosteroids and rituximab wield efficacy as treatment modalities for acquired hemophilia in breastfeeding patients. These interventions elicited a gradual elevation in the patient's factor VIII levels, concomitant with the obliteration of the inhibitor within a month. In comparison with instances of AHA stemming from alternative etiologies, the trajectory toward attaining negative levels of factor VIII inhibitors in this patient were attained more quickly. Such a trajectory augments the probability of a favorable outcome within this patient subset.
4. CONCLUSION
This report documents the rare case of acquired hemophilia A (AHA) characterized by profound bleeding during the postpartum phase. This case underscores the importance of timely diagnosis and intervention. If caught quickly and treated appropriately, individuals with AHA can achieve full remission, paving the way for uneventful subsequent pregnancies and deliveries.
AUTHOR CONTRIBUTIONS
Chanakarn Kanitthamniyom: Formal analysis; investigation; project administration; resources; writing – original draft. Pharit Siladech: Data curation; funding acquisition; resources. Natchaya Polpichai: Conceptualization; validation; visualization; writing – review and editing. Maireigh McCullough: Data curation; validation; writing – review and editing. Sakditad Saowapa: Data curation; formal analysis; investigation; methodology; project administration; writing – review and editing.
FUNDING INFORMATION
Authors state no funding involved.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Kanitthamniyom C, Siladech P, Polpichai N, McCullough M, Saowapa S. Combined life‐threatening internal organ bleeding and postpartum hemorrhage associated with acquired hemophilia A. Clin Case Rep. 2024;12:e8399. doi: 10.1002/ccr3.8399
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Hay CR. Acquired haemophilia. Baillieres Clin Haematol. 1998;11(2):287‐303. [DOI] [PubMed] [Google Scholar]
- 2. Collins P, Macartney N, Davies R, Lees S, Giddings J, Majer R. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Br J Haematol. 2004;124(1):86‐90. [DOI] [PubMed] [Google Scholar]
- 3. Acquired hemophilia – symptoms, causes, treatment: Nord. 2023. https://rarediseases.org/rare‐diseases/acquired‐hemophilia/. Accessed September, 30.
- 4. Borg JY, Guillet B, Le Cam‐Duchez V, Goudemand J, Lévesque H, SACHA Study Group . Outcome of acquired haemophilia in France: the prospective SACHA (surveillance des auto antiCorps au cours de l'Hémophilie Acquise) registry. Haemophilia. 2013;19(4):564‐570. [DOI] [PubMed] [Google Scholar]
- 5. Tengborn L, Baudo F, Huth‐Kühne A, et al. Pregnancy‐associated acquired haemophilia A: results from the European acquired haemophilia (EACH2) registry. BJOG. 2012;119(12):1529‐1537. [DOI] [PubMed] [Google Scholar]
- 6. Michiels JJ. Acquired hemophilia a in women postpartum: clinical manifestations, diagnosis, and treatment. Clin Appl Thromb Hemost. 2000;6(2):82‐86. [DOI] [PubMed] [Google Scholar]
- 7. Franchini M, Vaglio S, Marano G, et al. Acquired hemophilia A: a review of recent data and new therapeutic options. Hematology. 2017;22(9):514‐520. [DOI] [PubMed] [Google Scholar]
- 8. Bragnes Y, Boshuizen R, de Vries A, Lexberg Å, Østensen M. Low level of rituximab in human breast milk in a patient treated during lactation. Rheumatology (Oxford). 2017;56(6):1047‐1048. [DOI] [PubMed] [Google Scholar]
- 9. Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117(5):1499‐1506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


