Abstract
Background
To create a combined variable integrating both ventilation and perfusion as measured by preoperative dual‐energy computed tomography (DECT), compare the results with predicted postoperative (PPO) lung function as estimated using conventional methods, and assess agreement with actual postoperative lung function.
Methods
A total of 33 patients with lung cancer who underwent curative surgery after DECT and perfusion scan were selected. Ventilation and perfusion values were generated from DECT data. In the “combined variable method,” these two variables and clinical variables were linearly regressed to estimate PPO lung function. Six PPO lung function parameters (segment counting, perfusion scan, volume analysis, ventilation map, perfusion map, and combined variable) were compared with actual postoperative lung function using an intraclass correlation coefficient (ICC).
Results
The segment counting method produced the highest ICC for forced vital capacity (FVC) at 0.93 (p < 0.05), while the segment counting and perfusion map methods produced the highest ICC for forced expiratory volume in 1 second (FEV1; both 0.89, p < 0.05). The highest ICC value when using the combined variable method was for FEV1/FVC (0.75, p < 0.05) and diffusing capacity of the lung for carbon monoxide (DLco; 0.80, p < 0.05) when using the perfusion map method. Overall, the perfusion map and ventilation map provided the best performance, followed by volume analysis, segment counting, perfusion scan, and the combined variable.
Conclusions
Use of DECT image processing to predict postoperative lung function produced better agreement with actual postoperative lung function than conventional methods. The combined variable method produced ICC values of 0.8 or greater for FVC and FEV1.
Keywords: dual energy computed tomography (DECT), lung cancer, perfusion scintigraphy, predicted postoperative (PPO) lung function, segment counting
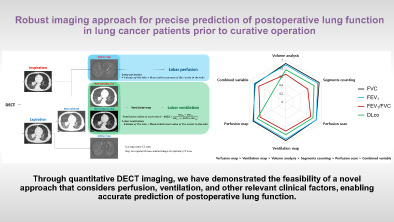
Through quantitative DECT imaging, we have demonstrated the feasibility of a novel approach that considers perfusion, ventilation, and other relevant clinical factors, enabling accurate prediction of postoperative lung function.
INTRODUCTION
Surgery is the primary treatment for operable early‐stage lung cancer. 1 , 2 When determined if a patient can undergo surgery, tumor staging and respiratory function must both be considered. 3 Spirometry and measurement of the diffusing capacity of the lung for carbon monoxide (DLco) are convenient methods to assess lung function: the forced expiratory volume in 1 second (FEV1) or DLco values should exceed 80% of values predicted for patient age, height, gender and body mass. 4 If these values are below the threshold, estimation of postoperative lung function becomes necessary. The risk of postoperative complications increases when either or both of the predicted postoperative (PPO) FEV1 or DLco are less than 40% predicted. 4 , 5 , 6 Accurately predicting postoperative lung function is therefore crucial when managing patients and making surgical decisions, as it helps identify those at a higher risk of postoperative complications and reduces overall morbidity and mortality. 7 With the increasing incidence of lung cancer in the elderly, PPO lung function has become more emphasized over tumor staging in preoperative workup. 8
Postoperative lung function depends mainly on the extent of lung resection and the overall pulmonary function. 9 Conventional methods used to predict postoperative lung function involve perfusion scintigraphy and segment counting. 5 Segment counting, although practical, underestimates actual postoperative lung function by assuming equal contribution from each segment, while disregarding underlying disease factors, heterogeneous distribution, and regional severity. 10 Perfusion scintigraphy is better suited for patients with anatomically diverse lung disease, but accurate estimation is limited by the lack of detailed anatomical differentiation. Furthermore, in the case of many patients in real‐world practice, limited accessibility due to a lack of socioeconomic support hinder its widespread use. In contrast, computed tomography (CT) is almost always carried out for lung cancer work‐up and provides detailed anatomical information, facilitating assessment of underlying lung disease. Recent studies have investigated imaging‐based approaches, such as quantitative CT (QCT), dual‐energy CT (DECT), and four‐dimensional CT (4DCT), as alternative methods to estimate PPO lung function. 11 , 12 , 13 , 14 These methods enable the estimation of regional lung function, including perfusion and ventilation.
There is a still need to develop more accurate and reliable methods to predict postoperative lung function that can reflect the actual postoperative lung function. Accurate prediction of postoperative lung function can aid in proper decision making in terms of selecting and planning optimal treatment for patients with lung cancer. This study aimed to develop a novel method to predict postoperative lung function using DECT combined with additional factors such as ventilation, perfusion, and relevant clinical parameters. Additionally, we aimed to compare the accuracy of these methods with conventional approaches and evaluate the potential utility of preoperative DECT imaging to enhance patient outcomes following lung cancer surgery.
METHODS
Study population
The institutional review board approved this retrospective study (IRB #2021‐08‐137‐003). Between November 2008 and November 2020, patients who underwent both preoperative DECT and lung perfusion scintigraphy followed by curative operation for lung cancer were identified by searching the database of the department of thoracic surgery in Samsung Medical Center (n = 79). Lung perfusion scintigraphy was performed selectively in patients who exhibited severe decline in pulmonary function testing (PFT) results. Patients with the following conditions were excluded; (1) 40 patients who underwent limited resection (segmentectomy or wedge resection) due to the fact that lobe‐based volumetric measurements were not available; and (2) six patients with no postoperative spirometry results. Finally, 33 cases were included for this study (Figure 1). Preoperative and postoperative pulmonary function and smoking history were assessed in all patients.
FIGURE 1.
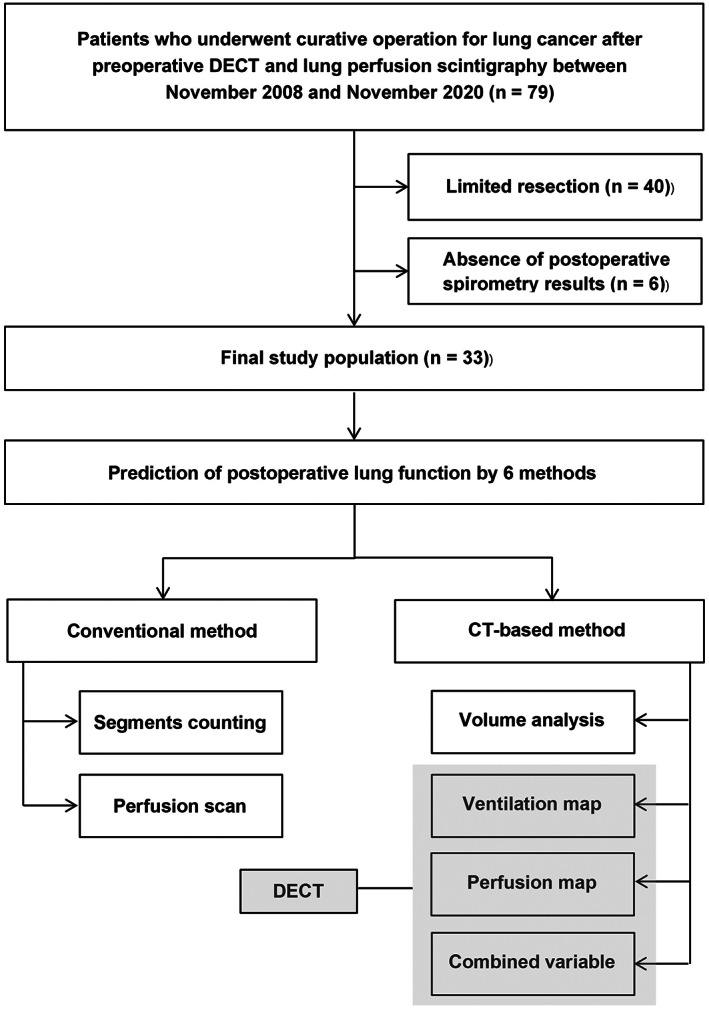
Flow chart of the study population selection process.
Image acquisition
All patients underwent examination using a CT scanner (Somatom Definition Flash, Siemens Healthcare) with a dual‐energy technique before and after contrast medium administration, with images obtained at full inspiration and normal expiration. We used the following parameters for non‐contrast scan: 110 mAs (effective) at 120 kV, 32 × 0.6 mm collimation, 0.7 pitch, 0.5 s rotation time, and 512 × 512 pixel matrix size. All DECT scanning images were obtained 90 s after the administration of iodinated contrast material (100 mL of iopamidol: Iomeron 300; Bracco) via the antecubital vein at a flow rate of 1.5 mL/s by using a power injector, followed by 20 mL saline flushing at a rate of 1.5 mL/s. Scan parameters were as follows: 105 mAs (effective) at 140 kV, 248 mAs (effective) at 80 kV, 32 × 0.6 mm collimation, 0.7 pitch, 0.5 s rotation time, and 512 × 512 pixel matrix (Figure 2).
FIGURE 2.
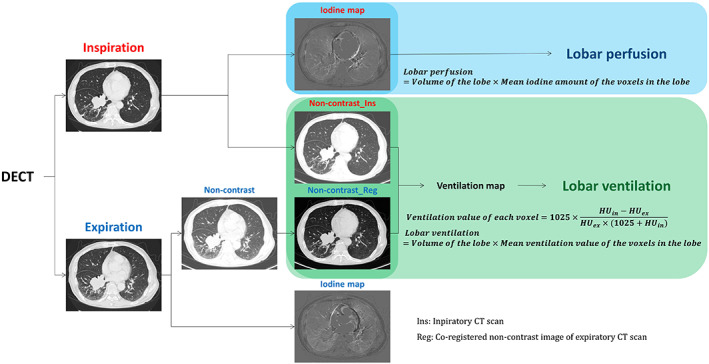
Post processing of preoperative dual‐energy computed tomography (DECT) images.
Using commercially available dual‐energy postprocessing software (Syngo Dual Energy, Siemens Medical Solutions), weighted‐average images, iodine‐distribution images, and fusion of weighted and iodine images were generated. The weighted‐average images were a combination of 30% of the 80 kV and 70% of the 140 kV image series, which was assumed to be similar to that of the single‐energy 120 kV images. A D30f (medium‐smooth) kernel for the iodine‐enhanced images and a D45f (medium‐sharp) kernel for the noncontrast images were used for image reconstruction with a section thickness of 1 mm. We used reconstructed iodine‐distribution images as the perfusion map (Figure 2).
Five quantitative methods for PPO
Conventional methods for PPO
Perfusion scan
To perform standard lung perfusion scintigraphy, the patients were administered 37 to 370 MBq of radioactive technetium‐99 m‐labeled, macro‐aggregated albumin (99mTc‐MAA) and placed in the supine position. 15 Images were acquired using a dual‐head gamma camera (E‐cam, Siemens Healthcare), with a medium‐energy, general‐purpose collimator. The whole lung was divided into six regions of interest comprising the right upper, right middle, right lower, left upper, left middle, and left lower lung fields, and each of the six regions was considered a lung lobe to obtain the lobar perfusion. In this study, we divided left middle area into two regions (upper and lower) and integrated the left upper and left lower areas.
Segment counting
The segment‐counting method is another conventional method to predict lung function and residual lung volume for patients who may undergo lobectomy, for which we considered a total number of lung segments of 19, including 10 in the right lung (3 in the upper lobe, 2 in the middle lobe, and 5 in the lower lobe) and nine in the left lung (5 in the upper lobe and 4 in the lower lobe). 5
Our proposed methods for PPO and their analytic process
Volumetric analyses
We performed volumetric analysis to segment lung regions and achieve spatial coregistration between expiratory and inspiratory DECT scans. The whole‐lung volume of interest was automatically segmented by excluding pulmonary vessels and airways in order to focus on the lung parenchymal lesions. Additionally, a deep‐learning process was utilized to extract the volume of each lobe based on lung fissure. Paired inspiratory and expiratory CT scans resulted in a pixel‐by‐pixel coregistration of imaging feature that shared the same spatial coordinates. Through this processing, we obtained mask images for five lobar regions, including the right upper, right middle, right lower, left upper, and left lower. All processing was conducted using commercial software (Aview; Coreline Soft, Seoul, South Korea).
Generation of perfusion and ventilation map
To obtain quantitative variables reflecting perfusion and ventilation in parenchymal lung tissue, we established two distinct perfusion and ventilation function maps. To estimate lobar perfusion, the total amount of iodine was calculated by multiplying the volume of the lobe by the mean iodine amount of the total voxels within that lobe.
To generate the ventilation map, we considered the registration outcomes analyzed with the paired inspiratory and expiratory CT images in the previous section, and the ventilation value of each voxel was then calculated by following equation using Hounsfield units from inhalation and exhalation images (). 14
This density‐change based equation corresponds to the change of the air content in each voxel and the lobar ventilation was calculated by multiplying the volume of the lobe by the mean ventilation value of the voxels in each lobe (Figure 2). The areas exceeding a − 250 HU were excluded from the calculations of ventilation value 16 and a few negative ventilation values were also excluded from the final calculation of lobar ventilation.
Creation of combined variables
When calculating only one ventilation or perfusion variable, it is difficult to accurately evaluate the lung field where ventilation/perfusion (V/Q) mismatch is present due to the underlying disease. Therefore, it is necessary to devise a method for calculating lung function by simultaneously considering ventilation and perfusion. A clustering approach was performed by using threshold values in which the input values are composed of two vectors, the ventilation and perfusion values of each voxel. For the lobes remaining after resection, the ventilation and perfusion values of each voxel were classified into one of four quadrants based on the 10th percentile threshold of each value. The first to fourth quadrants (Q1, Q2, Q3, Q4) corresponded to hypoventilation‐hypoperfusion, hypoventilation‐hyperperfusion, hyperventilation‐hypoperfusion and hyperventilation‐hyperperfusion, respectively. An additional Q5 voxel group was created by combining Q1 and Q4, and a Q6 group by combining Q2 and Q3. The volume occupied by each of Q1 to Q6 quadrants or quadrant groupings was considered a variable, and PPO lung function using combined variables was calculated after performing linear regression analyses by adding together four clinical variables—patient's age, gender, smoking history and type of surgery—to these six volumes derived from clustering analysis (Figure 3).
FIGURE 3.
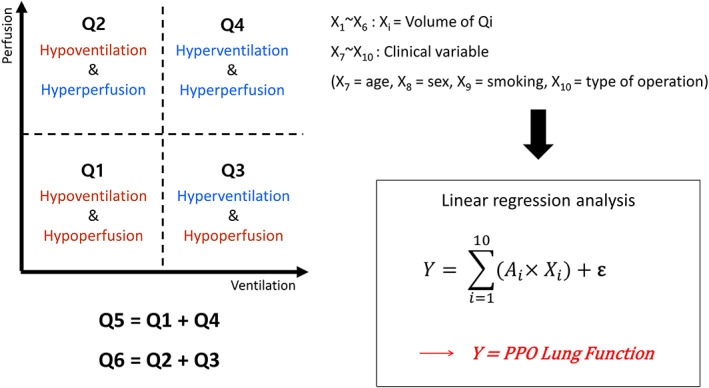
Analysis of postoperative lung function as predicted when using a combined variable comprising perfusion and ventilation mapping results obtained by dual‐energy computed tomography (DECT).
Prediction of postoperative lung function
Postoperative lung function was predicted using five different methods: ventilation and perfusion mapping, volume analysis, perfusion scintigraphy, and segment counting. For each patient, we predicted postoperative lung function on the basis of four types of preoperative PFT results: forced vital capacity (FVC), FEV1, FEV1/FVC ratio, and DLco. The PPO lung function was generated by multiplying the ratio of the lung region remaining postoperatively to the whole lung area using the variables derived from the five different methods by preoperative PFT results. The formulas for calculating PPO lung function are presented in Table 1.
TABLE 1.
Formulas used to predict postoperative lung function in the study patient population.
| Method | Lung function variable | Formula used to predict postoperative lung function | |
|---|---|---|---|
| Ventilation map | Lobar ventilation |
|
|
| Perfusion map | Lobar perfusion |
|
|
| Volume analysis | Functional volume |
|
|
| Perfusion scan | Radioactivity |
|
|
| Segment counting | The number of segments |
|
Note: *PFT results (FVC, FEV1, FEV1/FVC, DLco).
Note: **The sum of lobar ventilation values for each individual lobe.
Note: ***The sum of lobar perfusion values for each individual lobe.
Abbreviations: DLco, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PFT, pulmonary function test.
Statistical analysis
The agreement between the PPO lung function results obtained using the aforementioned six methods (volume analysis, segment counting, perfusion scintigraphy, ventilation map, perfusion map, and combined variable) and the actual postoperative lung function measured 6 months after surgery were evaluated using intraclass correlation coefficient (ICC) analyses. All statistical analyses were performed with MedCalc version 20.218 (MedCalc Software). A p‐value <0.05 was considered statistically significant.
RESULTS
Clinical characteristics of patients
Of 33 patients, analyses of diffusing‐capacity‐result‐based PPO lung function was conducted on a subset of 30 patients. The mean age of the population was 68.03 years with a standard deviation (SD) of 6.62 years. Of these, 11 patients were over the age of 70 (33.3%). Preoperative DECT and lung perfusion scintigraphy were performed at mean intervals of 8.8 days (range, 1–94 days) and 29.3 days (range, 1–141 days) prior to surgery, respectively. Each patient underwent PFT after surgery (mean interval to testing 147 ± 51.5 days). The demographics and clinical characteristics of the study population are summarized in Table 2.
TABLE 2.
Demographics and clinical characteristics of 33 lung cancer patients who underwent lobectomy or pneumonectomy.
| Parameter | Mean (SD) or number (%) |
|---|---|
| Age, years | 68.03 ± 6.62 |
| Sex | |
| Male | 26 (78.8) |
| Female | 7 (21.2) |
| Smoking history | |
| Ever | 28 (84.8) |
| Never | 5 (15.2) |
| Operation type | |
| Lobectomy | 28 (84.8) |
| LLL | 9 (32.1) |
| LUL | 6 (21.4) |
| RLL | 6 (21.4) |
| RML | 1 (3.6) |
| RUL | 5 (17.8) |
| Bilobectomy | 1 (3.6) |
| Pneumonectomy | 5 (15.2) |
| Left | 5 (100) |
| Lung volume (L) | 4.29 ± 1.27 |
| Preoperative PFT | |
| FVC (L) | 3.41 ± 0.92 |
| FEV1 (L) | 2.16 ± 0.52 |
| FEV1/FVC (%) | 66.02 ± 10.57 |
| DLco (mL/min/mmHg) | 13.24 ± 3.14 |
| Postoperative PFT | |
| FVC (L) | 2.74 ± 0.79 |
| FEV1 (L) | 1.90 ± 0.42 |
| FEV1/FVC (%) | 68.47 ± 15.07 |
| DLco (mL/min/mmHg) | 10.70 ± 2.71 |
Abbreviations: DLco, diffusing capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LLL, left lower lobe; LUL, left upper lobe; PFT, pulmonary function test; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SD, standard deviation.
Correlation between predicted and measured postoperative lung function
With respect to agreement between predicted and measured postoperative lung function for FVC, the ICC values ranged from 0.847 to 0.93 across all methods, with the segment‐counting method demonstrating the best performance. Regarding the FEV1, the segment‐counting and perfusion‐map methods produced the highest ICC values. The FEV1/FVC ratio yielded moderate to high agreement. Despite the relatively low ICC values obtained for all six methods, the combined‐variable method resulted in the highest ICC value overall. For DLco, all methods except for the combined‐variable method produced high agreement, with ICC values ranging from 0.76 to 0.796; however, the ICC values for DLco were lower than those for FVC or FEV1 (Figure 4). All ICC values were statistically significant (p < 0.001) except for the combined‐variable analysis of the DLco. Consequently, the methods ranked in order of high concordance were perfusion map, ventilation map, volume analysis, segment counting, perfusion scan, and combined variable (Figure 5).
FIGURE 4.
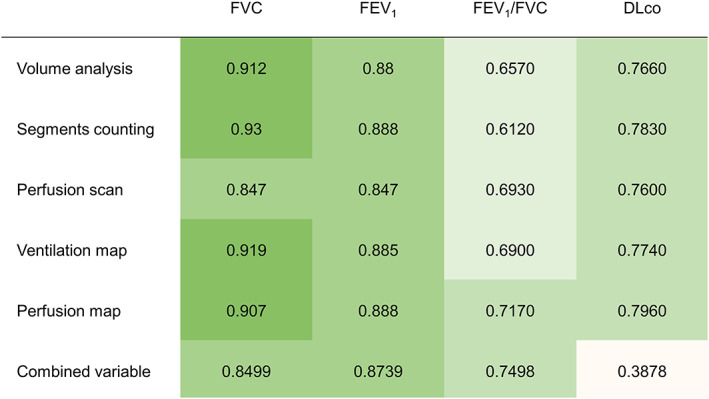
Agreement between predicted and measured postoperative lung function.
FIGURE 5.
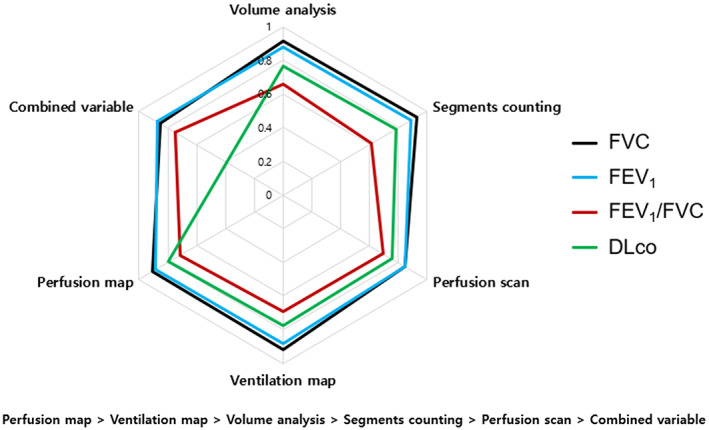
The level of agreement between predicted and measured postoperative lung function for each parameter obtained by the six methods of lung function assessment.
DISCUSSION
In recent years, there has been a growing emergence of 3D anatomical approaches that utilize CT images. Such innovative approaches provide a more comprehensive and accurate prediction of postoperative lung function by incorporating detailed anatomical information obtained from CT scans. The QCT method is fast, technically simple and available in all cases as preoperative CT is routinely performed on lung cancer patients: QCT assesses radiologically defined functional lung volume by excluding nonfunctioning spaces, such as emphysematous, fibrotic or atelectatic tissue, and tumor‐related air space loss. 17 The PPO lung function values derived from QCT correlate well with actual lung function measured after surgery. 12 Generally, QCT has demonstrated good agreement and comparable or improved accuracy compared with conventional segment counting or perfusion scintigraphy when predicting postoperative lung function in lung cancer patients. 11 , 17 , 18 , 19 However, there may be discrepancies between QCT and perfusion scintigraphy results, which may be attributed to regional V/Q mismatch, particularly in cases with hilar tumors. It is important to note that QCT may underestimate postoperative lung function in regions with air‐trapping, such as obstructive bronchitis or airway involvement of tumor, if lung attenuation is not as low as in emphysema despite functional impairment. 11 , 17 Therefore, it is necessary to evaluate both inspiratory and expiratory CT scans to obtain a comprehensive assessment of lung function.
DECT is a technique that allows for the extraction of the iodine‐contrast component from enhanced lung parenchyma using material decomposition theory 20 and allows detailed assessment of lung perfusion through the acquisition of an iodine map, making it a promising tool for predicting postoperative lung function. Iodine distribution images reflect the blood volume at a specific timepoint and can be used to calculate the lobar perfusion ratio. This imaging technique may also provide information regarding perfusion alterations that are associated with parenchymal abnormalities, such as emphysema or air‐trapping. 21 , 22 A previous study reported that dual‐energy perfusion CT is a more accurate method for prediction of postoperative lung function than perfusion scintigraphy, likely attributable to the precise lobar segmentation. 13 The PPO lung function values obtained from dual‐energy perfusion CT tended to be lower than the actual postoperative lung function, possibly due to variations in spirometry results upon repeated examination. Other factors such as chest wall mechanics or respiratory rehabilitation after surgery are also thought to influence postoperative lung function, leading to discrepancies between predicted and measured lung function. However, this study did not consider additional compounding factors beyond lobe resection that might affect postoperative lung function. 13
More recently, a novel imaging modality has been developed in the field of radiation oncology to assess lung function, which utilizes 4DCT to generate ventilation maps. This method uses an equation based on the change in Hounsfield units, as described in previous studies, to calculate ventilation values. 16 , 23 The PPO lung function using these ventilation values demonstrated good agreement when compared with nuclear medicine ventilation‐perfusion imaging. Overall, 4DCT offers several advantages over nuclear medicine, including reduced cost and imaging time, improved spatial resolution, and elimination of the need for a radioactive contrast agent. 14 However, it should be noted that while 4DCT is commonly used in thoracic radiation oncology, it is not a widely adopted modality in general radiology practice. Alternatively, breath‐hold inspiratory and expiratory CT scans can also be utilized to calculate CT‐based ventilation values. To evaluate both inspiratory and expiratory CT scans, we applied image registration technique to generate ventilation map to both scans. The technique involves registering the CT images acquired at different lung volumes using a deformable image registration algorithm to accurately align corresponding lung structures. The registration technique enables assessment of functional lung ventilation through mapping the ratio of intensity change between inspiratory and expiratory lung images. 24 , 25 Furthermore, breath‐hold CT scans are generally less susceptible to motion artifact compared with those obtained using 4DCT. However, ventilation‐based methods may not accurately reflect postoperative lung function in patients with V/Q mismatch caused by invasion of pulmonary vasculature by central lung cancer and/or hilar lymph node metastases.
Dynamic‐perfusion magnetic resonance imaging (MRI) has been found to be useful to predict postoperative lung function. Several studies have indicated that dynamic‐perfusion MRI can serve as an alternative to perfusion scintigraphy and provide comparable accuracy with QCT. 18 , 26 , 27 Despite being a safe diagnostic modality that does not employ ionizing radiation, it is not commonly used as a routine diagnostic tool for patients with lung cancer or other conditions necessitating lung resection.
There are several differences between our study and previous studies in this field. We recognized some limitations of segment counting, perfusion scintigraphy, QCT, dual‐energy perfusion CT, and CT‐based ventilation imaging when calculating PPO lung function. Therefore, we developed a novel method that considers perfusion, ventilation, and other relevant clinical factors in a very simple and practical way. We directly compared the PPO lung function values obtained by all six methods with the actual lung function measured after surgery. In addition, both spirometry parameters and DLco were considered in the calculation of the lung function. For FVC and FEV1, all six methods yielded similar values to the actual postoperative lung function. The combined variable also demonstrated accurate prediction compared to other methods, and FEV1/FVC ratio exhibited the highest correlation with postoperative lung function. Current methods for measuring PPO lung function tend to underestimate actual pulmonary function, which improves during a stabilization period between 6 and 12 months after surgery. 28 Therefore, accurately predicting lung function during this stabilization period is essential. In our study, the majority of postoperative physiological indices were assessed 6 months after surgery, while some cases were assessed earlier. Moreover, it is important to consider that postoperative lung function varies depending on the specific lobectomy area, highlighting the importance of analyzing lung function separately for each lobe based on the tumor location. 29 , 30
Our study had several limitations. First, the sample size may not have been large enough to conduct a robust analysis. However, this limitation was unavoidable due to the limited use of perfusion scintigraphy in patients with poor preoperative pulmonary function. Hence, it is necessary to validate our findings in a large‐scale study. Second, the validation of our novel approach, which incorporates a combined variable comprising multiple factors, may be limited due to the absence of prior studies utilizing such a variable. Nevertheless, our objective is to introduce this new method for precise prediction of postoperative lung function, and further improvements can be made by refining the implementation of the combined variable in future research. Additionally, it is important to note that lung resection itself can enhance overall pulmonary function in patients with severe emphysema, similar to the effects observed with lung‐volume‐reduction procedures. 31 , 32 Elimination of nonperfused dead space within the ventilated lung leads to compensatory expansion of residual viable lung tissue. 33 , 34 , 35 Such postoperative improvements in lung elastic recoil and chest wall mechanics may pose challenges in accurately predicting postoperative lung function.
In summary, our study is the first to directly compare the PPO lung function values calculated using conventional methods, radiologically‐based methods and a novel method with combined variable. We also implemented perfusion and ventilation maps using the isotropic volumetric CT data obtained through the DECT and image registration technique. Our methods have the practical advantage of utilizing only the imaging data obtained from CT scans routinely performed before surgery, without the need for additional examinations. This study confirmed that prediction of postoperative lung function using DECT image processing (ventilation mapping, perfusion mapping) showed comparable or better agreement with actual postoperative lung function compared with conventional methods, suggesting the possibility of improving the accuracy of PPO lung function. In addition, the combined‐variable method produced ICC values of 0.8 or greater in FVC and FEV1, and these parameters may also prove useful when predicting postoperative lung function in clinical practice.
AUTHOR CONTRIBUTIONS
SK, UJ, SG‐P and HY‐L contributed to conceptualization, methodology, and funding acquisition. UJ, JK, and YJ‐O were involved in statistical analyses and data collection. SK, JK, and UJ contributed to literature searching and writing. HY‐L and JK contributed to manuscript review and editing. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT; NRF‐2021R1A4A5032806) and partly supported by a grant from the Institute of Information & Communications Technology Planning & Evaluation (IITP) funded by the MSIT (2021‐0‐02068, Artificial Intelligence Innovation Hub).
Kim S, Kim J, Jeong U, Oh YJ, Park SG, Lee HY. Robust imaging approach for precise prediction of postoperative lung function in lung cancer patients prior to curative operation. Thorac Cancer. 2024;15(1):35–43. 10.1111/1759-7714.15153
Suho Kim and Jonghoon Kim contributed equally to this work.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- 1. Greenlee RT, Hill‐Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51(1):15–36. [DOI] [PubMed] [Google Scholar]
- 2. Shields TW. Surgical therapy for carcinoma of the lung. Clin Chest Med. 1993;14(1):121–147. [PubMed] [Google Scholar]
- 3. Beckles MA, Spiro SG, Colice GL, Rudd RM. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2003;123(1 Suppl):105s–114s. [DOI] [PubMed] [Google Scholar]
- 4. Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced‐based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):161s–177s. [DOI] [PubMed] [Google Scholar]
- 5. Brunelli A, Kim AW, Berger KI, Addrizzo‐Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5 Suppl):e166S–e190S. [DOI] [PubMed] [Google Scholar]
- 6. Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123(6):2096–2103. [DOI] [PubMed] [Google Scholar]
- 7. Ferguson MK, Watson S, Johnson E, Vigneswaran WT. Predicted postoperative lung function is associated with all‐cause long‐term mortality after major lung resection for cancer. Eur J Cardiothorac Surg. 2014;45(4):660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venuta F, Diso D, Onorati I, Anile M, Mantovani S, Rendina EA. Lung cancer in elderly patients. J Thorac Dis. 2016;8(Suppl 11):S908–S914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pierce RJ, Copland JM, Sharpe K, Barter CE. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med. 1994;150(4):947–955. [DOI] [PubMed] [Google Scholar]
- 10. Smulders SA, Smeenk FW, Janssen‐Heijnen ML, Postmus PE. Actual and predicted postoperative changes in lung function after pneumonectomy: a retrospective analysis. Chest. 2004;125(5):1735–1741. [DOI] [PubMed] [Google Scholar]
- 11. Ueda K, Tanaka T, Li TS, Tanaka N, Hamano K. Quantitative computed tomography for the prediction of pulmonary function after lung cancer surgery: a simple method using simulation software. Eur J Cardiothorac Surg. 2009;35(3):414–418. [DOI] [PubMed] [Google Scholar]
- 12. Wu MT, Chang JM, Chiang AA, Lu JY, Hsu HK, Hsu WH, et al. Use of quantitative CT to predict postoperative lung function in patients with lung cancer. Radiology. 1994;191(1):257–262. [DOI] [PubMed] [Google Scholar]
- 13. Chae EJ, Kim N, Seo JB, Park JY, Song JW, Lee HJ, et al. Prediction of postoperative lung function in patients undergoing lung resection: dual‐energy perfusion computed tomography versus perfusion scintigraphy. Invest Radiol. 2013;48(8):622–627. [DOI] [PubMed] [Google Scholar]
- 14. Vinogradskiy Y, Jackson M, Schubert L, Jones B, Castillo R, Castillo E, et al. Assessing the use of 4DCT‐ventilation in pre‐operative surgical lung cancer evaluation. Med Phys. 2017;44(1):200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Markos J, Mullan BP, Hillman DR, Musk AW, Antico VF, Lovegrove FT, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139(4):902–910. [DOI] [PubMed] [Google Scholar]
- 16. Guerrero T, Sanders K, Castillo E, Zhang Y, Bidaut L, Pan T, et al. Dynamic ventilation imaging from four‐dimensional computed tomography. Phys Med Biol. 2006;51(4):777–791. [DOI] [PubMed] [Google Scholar]
- 17. Wu MT, Pan HB, Chiang AA, Hsu HK, Chang HC, Peng NJ, et al. Prediction of postoperative lung function in patients with lung cancer: comparison of quantitative CT with perfusion scintigraphy. AJR Am J Roentgenol. 2002;178(3):667–672. [DOI] [PubMed] [Google Scholar]
- 18. Ohno Y, Koyama H, Nogami M, Takenaka D, Matsumoto S, Yoshimura M, et al. Postoperative lung function in lung cancer patients: comparative analysis of predictive capability of MRI, CT, and SPECT. AJR Am J Roentgenol. 2007;189(2):400–408. [DOI] [PubMed] [Google Scholar]
- 19. Fernández‐Rodríguez L, Torres I, Romera D, Galera R, Casitas R, Martínez‐Cerón E, et al. Prediction of postoperative lung function after major lung resection for lung cancer using volumetric computed tomography. J Thorac Cardiovasc Surg. 2018;156(6):2297–2308.e5. [DOI] [PubMed] [Google Scholar]
- 20. Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, et al. Material differentiation by dual energy CT: initial experience. Eur Radiol. 2007;17(6):1510–1517. [DOI] [PubMed] [Google Scholar]
- 21. Kim BH, Seo JB, Chae EJ, Lee HJ, Hwang HJ, Lim C. Analysis of perfusion defects by causes other than acute pulmonary thromboembolism on contrast‐enhanced dual‐energy CT in consecutive 537 patients. Eur J Radiol. 2012;81(4):e647–e652. [DOI] [PubMed] [Google Scholar]
- 22. Lee CW, Seo JB, Lee Y, Chae EJ, Kim N, Lee HJ, et al. A pilot trial on pulmonary emphysema quantification and perfusion mapping in a single‐step using contrast‐enhanced dual‐energy computed tomography. Invest Radiol. 2012;47(1):92–97. [DOI] [PubMed] [Google Scholar]
- 23. Castillo R, Castillo E, Martinez J, Guerrero T. Ventilation from four‐dimensional computed tomography: density versus Jacobian methods. Phys Med Biol. 2010;55(16):4661–4685. [DOI] [PubMed] [Google Scholar]
- 24. Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography‐based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18(11):1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pompe E, Galbán CJ, Ross BD, Koenderman L, Ten Hacken NH, Postma DS, et al. Parametric response mapping on chest computed tomography associates with clinical and functional parameters in chronic obstructive pulmonary disease. Respir Med. 2017;123:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohno Y, Hatabu H, Higashino T, Takenaka D, Watanabe H, Nishimura Y, et al. Dynamic perfusion MRI versus perfusion scintigraphy: prediction of postoperative lung function in patients with lung cancer. AJR Am J Roentgenol. 2004;182(1):73–78. [DOI] [PubMed] [Google Scholar]
- 27. Ohno Y, Koyama H, Nogami M, Takenaka D, Onishi Y, Matsumoto K, et al. State‐of‐the‐art radiological techniques improve the assessment of postoperative lung function in patients with non‐small cell lung cancer. Eur J Radiol. 2011;77(1):97–104. [DOI] [PubMed] [Google Scholar]
- 28. Kim HK, Lee YJ, Han KN, Choi YH. Pulmonary function changes over 1 year after lobectomy in lung cancer. Respir Care. 2016;61(3):376–382. [DOI] [PubMed] [Google Scholar]
- 29. Yokoba M, Ichikawa T, Harada S, Naito M, Sato Y, Katagiri M. Postoperative pulmonary function changes according to the resected lobe: a 1‐year follow‐up study of lobectomized patients. J Thorac Dis. 2018;10(12):6891–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ueda K, Tanaka T, Hayashi M, Li TS, Kaneoka T, Tanaka N, et al. Compensation of pulmonary function after upper lobectomy versus lower lobectomy. J Thorac Cardiovasc Surg. 2011;142(4):762–767. [DOI] [PubMed] [Google Scholar]
- 31. Laghi F, Jubran A, Topeli A, Fahey PJ, Garrity ER Jr, de Pinto DJ, et al. Effect of lung volume reduction surgery on diaphragmatic neuromechanical coupling at 2 years. Chest. 2004;125(6):2188–2195. [DOI] [PubMed] [Google Scholar]
- 32. Ueda K, Murakami J, Sano F, Hayashi M, Kobayashi T, Kunihiro Y, et al. Assessment of volume reduction effect after lung lobectomy for cancer. J Surg Res. 2015;197(1):176–182. [DOI] [PubMed] [Google Scholar]
- 33. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung‐volume‐reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. [DOI] [PubMed] [Google Scholar]
- 34. Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363(13):1233–1244. [DOI] [PubMed] [Google Scholar]
- 35. Hunsaker AR, Ingenito EP, Reilly JJ, Costello P. Lung volume reduction surgery for emphysema: correlation of CT and V/Q imaging with physiologic mechanisms of improvement in lung function. Radiology. 2002;222(2):491–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy and ethical restrictions.


