Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection leads to various clinical symptoms including anemia. Lipocalin-2 has various biological functions, including defense against bacterial infections through iron sequestration, and it serves as a biomarker for kidney injury. In a human protein array, we observed increased lipocalin-2 expression due to parental SARS-CoV-2 infection in the Calu-3 human lung cancer cell line. The secretion of lipocalin-2 was also elevated in response to parental SARS-CoV-2 infection, and the SARS-CoV-2 Alpha, Beta, and Delta variants similarly induced this phenomenon. In a Calu-3 implanted mouse xenograft model, parental SARS-CoV-2 and Delta variant induced lipocalin-2 expression and secretion. Additionally, the iron concentration increased in the Calu-3 tumor tissues and decreased in the serum due to infection. In conclusion, SARS-CoV-2 infection induces the production and secretion of lipocalin-2, potentially resulting in a decrease in iron concentration in serum. Because the concentration of iron ions in the blood is associated with anemia, this phenomenon could contribute to developing anemia in COVID-19 patients.
Keywords: Anemia, Iron, Lipocalin-2, Lung cancer, SARS-CoV-2
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a pandemic after it was first reported in December 2019 (1). SARS-CoV-2 infects host cells using spike proteins, as previously reported in SARS-CoV. The spike protein of SARS-CoV-2 recognizes angiotensin-converting enzyme 2 (ACE2), a host cell receptor, with a higher affinity compared to the SARS-CoV spike protein, and SARS-CoV-2 invades the host cell (2). After a 5-day incubation period following SARS-CoV-2 infection, nearly all COVID-19 patients exhibit symptoms within approximately 12 days (3). Symptoms of patients include fever, fatigue, dry cough, headache and sputum production (4). Anemia is another common symptom: the prevalence of anemia in COVID-19 patients is higher than control patient group (61% vs 45%) (5).
Lipocalin-2 is one of the lipocalin superfamily members and was originally isolated as a 25 kDa protein associated with human neutrophil gelatinase (6). Lipocalin-2 contributes to the innate immunity against bacterial infection by sequestrating iron. Bacterial infection stimulates the expression and secretion of lipocalin-2 in immune cells through activation of Toll-like receptor (TLR) 4. Lipocalin-2, when secreted, interrupts bacterial growth by capturing the iron-binding siderophore (7). Lipocalin-2 is a potential biomarker for infectious diseases such as bacterial meningitis and chronic liver disease (8, 9). Many studies also suggest lipocalin-2 as a biomarker in various diseases, including arthritic diseases, acute kidney injury, severe acute pancreatitis, obesity-related metabolic diseases and multiple sclerosis (10-14). Lipocalin-2 expression is activated in damaged organs, including the kidneys, heart, brain, pancreas, lungs, and skin (15). Furthermore, the lipocalin-2 expression level is elevated in various cancers and associated with cancer progression; therefore, it was suggested as a therapeutic target or diagnostic marker in several cancers (16, 17). Lipocalin-2 is also associated with virus infection. In human immunodeficiency virus-infected patients, the serum lipocalin-2 level is decreased. When HIV-infected patients take highly active anti-retroviral therapy (HAART), the lipocalin-2 level is significantly increased (18).
Regarding the connection between lipocalin-2 and SARS-CoV-2 infection, an increase in the lipocalin-2 expression has been recently reported in SARS-CoV-2-infected cells and the bronchoalveolar lavage fluid and lungs of COVID-19 patients (19). Investigators also reported that lipocalin-2 can be used as a biomarker to diagnose the occurrence of acute kidney injury and cardiovascular complications in COVID-19 patients (20, 21). However, there has been no report on the functional effect of the increase in lipocalin-2 following infection with SARS-CoV-2. Considering that lipocalin-2 is associated with anemia and reduces red blood cell production by negative regulation (22, 23) and one of the symptoms of COVID-19 patients is anemia (5), it is an interesting issue whether lipocalin-2 is involved in the anemia induced by SARS-CoV-2 infection (24). In this study, we investigated the expression and secretion of lipocalin-2 upon SARS-CoV-2 infection using the ACE2-expressing human lung cancer cell line Calu-3 in vitro and a xenograft mouse model established with Calu-3 cells in vivo. Our results suggest that the lipocalin-2 may contribute to the regulation of iron concentration in vivo.
RESULTS
Protein expression patterns in Calu-3 cells induced by SARS-CoV-2 infection
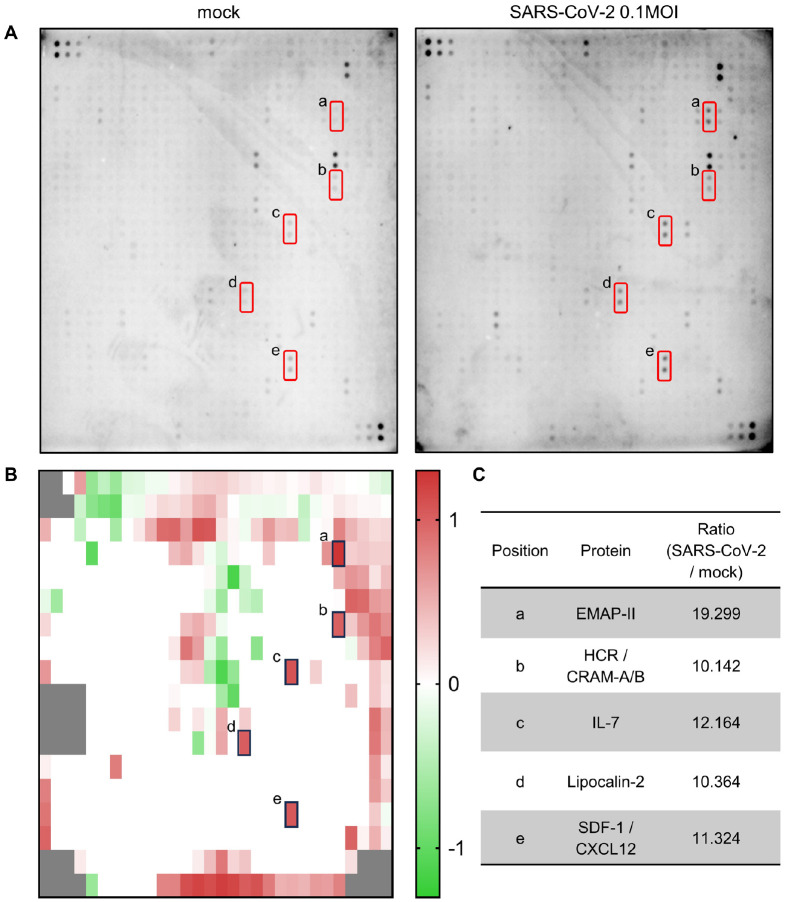
SARS-CoV-2 infection initiates many changes in the gene expression of host cells and patients, including interferons and cytokines (19, 25, 26). To investigate SARS-CoV-2 infection-induced change of expression pattern at a protein level, we performed a dot blot assay capable of identifying 507 proteins simultaneously with an antibody array. When Calu-3 cells were infected with 0.1 MOI of parental SARS-CoV-2, the expression levels of several proteins changed at 48 h after infection (Fig. 1A, B). The dot density of the membrane blots incubated with the lysates of mock-infected and SARS-CoV-2-infected cells, respectively, was measured, and the ratio of SARS-CoV-2 infection to mock control was calculated for each dot using an analysis tool. Candidate proteins were sorted when the ratio was higher than 10. The sorted proteins were Cerberus 1, Chem R23, CXCR3, EMAP-II, FGF-BP, HCR/CRAM-A/B, IL-4, IL-7, lipocalin-2, SDF-1/CXCL12, uPAR, Vasorin, VCAM-1 (CD106), VE-Cadherin, VEGF, VEGF R2 (KDR) and VEGF R3. Among them, EMAP-II, HCR/CRAM-A/B, IL-7, lipocalin-2, and SDF-1/CXCL12 (Fig. 1A, red square) were identified as proteins with significant differences. As shown in Fig. 1C and Supplementary Table 1, most of the proteins are cytokine and chemokine-related proteins except for lipocalin-2. Especially, EMAP-II was most highly induced (Fig. 1C). EMAP-II is a proinflammatory cytokine whose expression increases under stresses including virus infection and can be used as a marker for severity and mortality in COVID-19 patients (27, 28). Here, we focused on the regulation of lipocalin-2 expression in response to SARS-CoV-2 infection in cells and a xenograft mouse model.
Fig. 1.
Analysis of protein expression pattern after parental SARS-CoV-2 infection in Calu-3 cells. (A) Calu-3 cells were infected with mock (A, left) or 0.1 MOI of parental SARS-CoV-2 (A, right) for 48 h. Cell lysates were collected, biotinylated and then incubated with the Human Antibody Array 507 Membrane. The protein-antibody interactions were detected with HRP-conjugated streptavidin and developed using a chemiluminescence imaging system. Red squares indicate that the protein expression ratio changed more than 10 times. (B) The protein expression ratio upon SARS-CoV-2 infection compared to the mock control was calculated and then visualized as a heatmap after converting the values in a logarithmic scale. Gray boxes are blanks or controls. (C) Selected proteins based on the ratio of change. The lowercase letters represent the corresponding dots on the Human Antibody Array 507 Membrane.
Analysis of lipocalin-2 expression by SARS-CoV-2 infection in Calu-3 cells
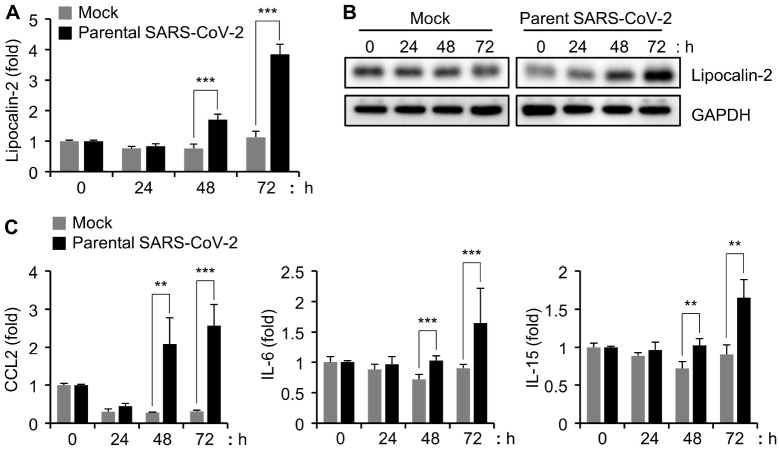
To investigate the pattern of lipocalin-2 expression caused by SARS-CoV-2 infection, we infected Calu-3 cells with 0.1 MOI of parental SARS-CoV-2 as previously described (29) and analyzed the expression of lipocalin-2 in a time-dependent manner. The quantitative real-time PCR (qRT-PCR) analysis revealed that the mRNA level of lipocalin-2 was increased at 48 h and further increased at 72 h after infection (Fig. 2A). According to the western blot analysis, protein levels of lipocalin-2 were also increased from 48 h after parental SARS-CoV-2 infection in Calu-3 cells (Fig. 2B). In addition to lipocalin-2, CCL2/MCP1, IL-15, and IL-16 have been also reported as soluble biomarkers associated with mortality of COVID19 patients (19). Therefore, we additionally investigated their mRNA levels in Calu-3 cells after SARS-CoV-2 infection and found increased expression of those genes in a time-dependent manner (Fig. 2C). Although the dot blot we used included CCL2/MCP1, IL-15, and IL-16, there was no significant change in the expression levels of these proteins in the parental SARS-CoV-2-infected Calu-3 cell lysates (Fig. 1). This result could be associated with the differential kinetics of protein production and secretion: we monitored the protein expression pattern of the host cell while a previous study investigated the sera of SARS-CoV-2 infected-patients (19). Importantly, we confirmed that the analysis of SARS-CoV-2-infected Calu-3 cells can partially explain the biomarker expression profiles observed in the blood of COVID-19 patients. Moreover, the expression of lipocalin-2 was increased in Calu-3 cells after SARS-CoV-2 infection.
Fig. 2.
Time-dependent increase of lipocalin-2 protein and mRNA expression by SARS-CoV-2 infection in Calu-3 cells. (A, C) Calu-3 cells were infected with 0.1 MOI of parental SARS-CoV-2, and the cells were collected at the indicated time points. Lipocalin-2 (A) and CCL2, IL-5, and IL-6 (C) mRNA levels were quantified by qRT-PCR analysis. The GAPDH mRNA level was used for normalization of the gene expression. **P < 0.01, ***P < 0.001 vs. mock control. (B) Cell lysates were prepared at the indicated time points after parental SARS-CoV-2 infection in Calu-3 cells. The cell lysates were analyzed by western blotting with the indicated antibodies.
Lipocalin-2 is secreted by SARS-CoV-2 infection in Calu-3 cells
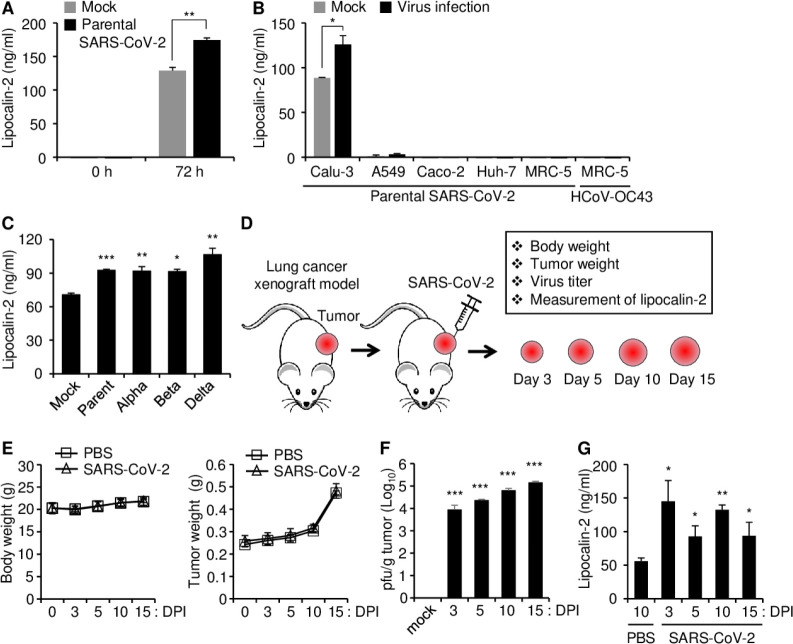
Calu-3 cells were infected with SARS-CoV-2 (0.1 MOI) to investigate the effect of SARS-CoV-2 infection on the expression of lipocalin-2 and its secretion into the culture medium, and the levels of secreted lipocalin-2 in the cell culture supernatant at 72 h after infection were measured by ELISA using the human Lipocalin-2/NGAL immunoassay kit. Lipocalin-2 secretion was increased by parental SARS-CoV-2 infection compared to the mock control in Calu-3 cells (Fig. 3A). To determine whether this phenomenon also occurs in other cell lines, we tested three SARS-CoV-2 susceptible cell lines: human lung epithelial carcinoma cell line A549, human colorectal epithelial adenocarcinoma cell line Caco-2, and human hepatocellular carcinoma cell line Huh-7 and an HCoV OC43-permissive control cell line MRC-5 (human lung fibroblast cell) (30). Lipocalin-2 secretion was absent or very weak independent of SARS-CoV-2 infection in these cells (Fig. 3B). SARS-CoV-2, as an RNA virus, has high mutation rates, and there are many SARS-CoV-2 variants (31). Therefore, we analyzed whether lipocalin-2 expression is commonly induced by infection of SARS-CoV-2 variants, including Alpha (B.1.1.7), Beta (B1.351) and Delta (B1.617.2) in Calu-3 cells. Lipocalin-2 secretion was increased at 72 h in Calu-3 cells after infection of all the tested variants of SARS-CoV-2, and the levels of secreted lipocalin-2 did not significantly differ among the viruses (Fig. 3C).
Fig. 3.
Lipocalin-2 secretion in Calu-3 cells and lipocalin-2 production in a lung tumor xenograft mouse model after SARS-CoV-2 infection. (A) Calu-3 cells were infected with 0.1 MOI of parental SARS-CoV-2 for 72 h, and then, cell culture supernatants were collected. (B) Parental SARS-CoV-2 or HCoV-OC43 were infected into the indicated cells at 0.1 MOI for 72 h, and then, cell culture supernatants were collected. (C) Calu-3 cells were infected with 0.1 MOI of parental SARS-CoV-2, Alpha variant, Beta variant, or Delta variant for 72 h, and then, cell culture supernatants were collected. Lipocalin-2 concentration in the supernatants was determined by ELISA using the Human Lipocalin-2/NGAL immunoassay kit (A-C). (D-G) Lipocalin-2 production after parental SARS-CoV-2 infection in a lung tumor xenograft mouse model. (D) Schematic diagram of parental SARS-CoV-2 infection and the experimental contents. Calu-3 cells were subcutaneously injected into the right flank of NRGA mice (n = 3/group). Parental SARS-CoV-2 was intratumorally infected after the tumor volume reached approximately 100 mm3. (E) Body weight (left panel) and tumor weight (right panel) were measured at 3, 5, 10, and 15 DPI. (F) Replication of SARS-CoV-2 in lung tumors. Supernatants of tumor homogenates were collected at 3, 5, 10, and 15 DPI, and then, viral titers were determined by plaque formation assay. (G) Measurement of lipocalin-2 in lung tumors. Cell lysates of the tumor homogenates were collected at 3, 5, 10, and 15 DPI, and then, the lipocalin-2 concentration was determined by ELISA. DPI, days post-infection. pfu, plaque forming unit. Values are the mean ± SEM. *P < 0.05, **P < 0.01, ***P <0.001 vs. each mock or PBS control.
Lipocalin-2 expression induced by long-term infection of SARS-CoV-2 in a lung cancer xenograft mouse model
Previously, we established a xenograft mouse model by transplanting Calu-3 cells into the flanks of immunodeficient NOD/ShiLtJ-Rag2em1AMCIl2rgem1AMC (NRGA) mice to induce long-term infection by SARS-CoV-2 (32). To investigate whether lipocalin-2 expression is increased by SARS-CoV-2 infection in vivo, the mice with Calu-3 xenograft tumors were infected with parental SARS-CoV-2 after the tumor developed a diameter of approximately 100 mm3 (Fig. 3D). The mice were monitored 3, 5, 10, and 15 days after infection. The tumor volume and body weight were not influenced by parental SARS-CoV-2 infection (Fig. 3E). Long-term replication of SARS-CoV-2 in the tumors was confirmed by the plaque formation assay (Fig. 3F), which is consistent with previously reported results (32). Lipocalin-2 protein levels were measured by ELISA, and the results showed that lipocalin-2 levels were increased compared to mock infection at all the tested time points after infection (Fig. 3G). These results suggest that SARS-CoV-2 infection induces lipocalin-2 expression in vivo and in vitro.
Inverse relationship between the lipocalin-2 level and iron content in serum
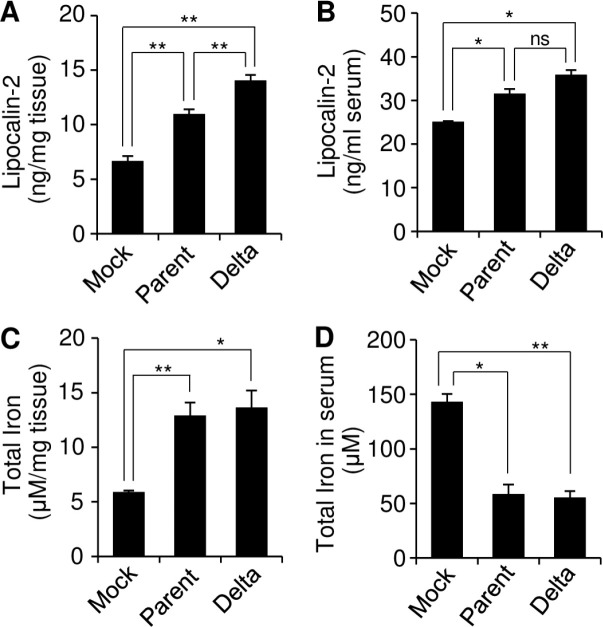
To investigate a potential effect of lipocalin-2 expression on the iron content in vivo, we measured the total amount of iron in the tumor tissues and sera at 15 days after infection with parental SARS-CoV-2 or SARS-CoV-2 Delta in a lung cancer xenograft mice model. The tumor tissues were homogenized, and the supernatants were collected to analyze the tumor tissues. While the lipocalin-2 expression in the tumor tissues was increased by both virus infection with significantly higher extent by the Delta variant (Fig. 4A), the lipocalin-2 levels in the sera were similarly increased by the two viruses (Fig. 4B) suggesting that the level of lipocalin-2 secretion was not significantly affected by the type of virus. On the other hand, the iron contents in the tumor tissues and the sera were similarly increased and decreased, respectively, by parental SARS-CoV-2 and SARS-CoV-2 Delta infection (Fig. 4C, D). These data suggest that SARS-CoV-2 infection induces iron accumulation in the tissues and reduces iron content in the sera by modulating lipocalin-2 secretion.
Fig. 4.
Effect of SARS-CoV-2 infection on the iron concentration in a lung tumor xenograft mouse model. Calu-3 cells were subcutaneously injected into the right flank of NRGA mice (n = 3/group). Parental SARS-CoV-2 or SARS-CoV-2 Delta was intratumorally infected. (A, C) Supernatants of tumor homogenates were collected at 15 DPI, and then, the Lpocalin-2 concentration was determined by ELISA (A). The total iron concentration in the tissues was determined using the supernatants of the tumor homogenates by the Iron Colorimetric assay kit (C). (B, D) Sera were collected at 15 DPI in a lung tumor xenograft mouse model, and then, the lipocalin-2 concentration was determined by ELISA (B). The total iron concentration in sera was determined by the Iron Colorimetric assay kit (D). Values are the mean ± SEM. *P < 0.05, **P < 0.01 vs. mock control.
DISCUSSION
Lipocalin-2 contributes to innate immunity against bacterial infection, and its expression is increased upon organ damage, enabling it to be used as a biomarker (7-9, 15). It was reported that lipocalin-2 gene expression is increased by SARS-CoV-2 infection (19), and lipocalin-2 protein level is decreased by HIV infection (18). In this study, we found an increase in lipocalin-2 protein expression and secretion by SARS-CoV-2 infection in cellular and long-term SARS-CoV-2 replication models in a lung cancer xenograft mouse. Importantly, we suggest a functional role of lipocalin-2 expression related to iron regulation.
Regulation of iron balance through lipocalin-2 secretion is one of the important functions of lipocalin-2 (33). Lipocalin-2 can bind with iron. When lipocalin-2 is loaded with iron, it can be taken up by cells after interacting with a 24p3 cell-surface receptor (24p3R). After the iron-loaded lipocalin-2 is internalized into cells, the iron concentration is increased in the cell. Conversely, when lipocalin-2 lacks iron, it can result in a decrease in intracellular iron (34). Furthermore, in patients with systemic inflammation and anemia, the levels of serum lipocalin-2 were elevated compared to patients without anemia. Interestingly, in those anemic patients with higher levels of serum lipocalin-2, the concentration of iron in their blood was lower compared to anemic patients with lower levels of serum lipocalin-2 (35). Therefore, lipocalin-2 regulates iron homeostasis, and the lipocalin-2-mediated iron deprivation in plasma may be related to anemia development. In this regard, our results may explain the functional role of lipocalin-2 related with anemia of COVID-19 patients (5). We found that SARS-CoV-2 infection increased the expression and secretion of lipocalin-2 in the infected cells and tissues. In addition, the iron concentration was increased in the tissue samples but decreased in the serum by SARS-CoV-2 infection. It suggests a possibility that the secreted lipocalin-2 captures iron in the serum and reenters the cells to release iron in the cytoplasm. This phenomenon follows previous reports related to lipocalin-2-mediated anemia. Iron deprivation in the serum is a factor contributing to sideropenic anemia (36). In a specific animal model involving anemia induced by chronic infection of Mycobacterium avium, it was suggested that lipocalin-2 may be involved in the development of anemia (37). Therefore, our results suggest that SARS-CoV-2-mediated anemia in COVID-19 patients may be caused by increased expression of lipocalin-2 in the infected tissues and its secretion to the serum.
Since the emergence of SARS-CoV-2, numerous SARS-CoV-2 variants have been continuously reported. SARS-CoV-2 variants are grouped into three categories: those of concern (VOC), those of interest (VOI), and those being observed or monitored (VUM). This categorization relies on multiple considerations, encompassing factors such as contagiousness, transmissibility, severity, alterations in clinical manifestation, impact on the efficacy of public health measures, and the availability of diagnostics, vaccines, and treatments (38-40). Therefore, it is necessary to investigate whether several variants of SARS-CoV-2 commonly induce lipocalin-2. Lipocalin-2 secretion was induced by all the tested SARS-CoV-2 VOCs, including Alpha, Beta, and Delta variants. Therefore, it is presumed that the mutations of SARS-CoV-2 do not affect the secretion of lipocalin-2. Recently, Omicrons have been the most prevailing variant. Because changes in the expression and secretion of lipocalin-2 caused by other mutations cannot be excluded, additional studies may provide more information.
To overcome the COVID-19 pandemic, many researchers have investigated SARS-CoV-2, and many things have been revealed, but there are still many unknowns about the virus. In this study, we investigated the regulation of lipocalin-2 upon SRS-CoV-2 infection and its possible role in iron regulation and anemia in patients. These results provide additional information on the host response to SARS-CoV-2 infection. We believe that our approach may contribute to a better understanding and efficacious treatment of SARS-CoV-2 infection by suggesting a possible mechanism that can cause symptoms during SARS-CoV-2 infection.
MATERIALS AND METHODS
Materials and methods are available in supplementary material.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by grants from the National Research Foundation (NRF-2022M3A9I2082292, NRF-2021R1A2C1006767) funded by the Ministry of Science and ICT in the Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergamaschi G, Borrelli de Andreis F, Aronico N, et al. Anemia in patients with COVID-19: pathogenesis and clinical significance. Clin Exp Med. 2021;21:239–246. doi: 10.1007/s10238-020-00679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triebel S, Blaser J, Reinke H, Tschesche H. A 25 Kda alpha-2-microglobulin-related protein is a component of the 125-Kda form of human gelatinase. FEBS Lett. 1992;314:386–388. doi: 10.1016/0014-5793(92)81511-J. [DOI] [PubMed] [Google Scholar]
- 7.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 8.Behairy Bel S, Salama EI, Allam AA, Ali MA, Elaziz AM. Lipocalin-2 as a marker of bacterial infections in chronic liver disease: a study in Egyptian children. Egypt J Immunol. 2011;18:31–36. [PubMed] [Google Scholar]
- 9.Thanh TT, Casals-Pascual C, Ny NTH, et al. Value of lipocalin 2 as a potential biomarker for bacterial meningitis. Clin Microbiol Infect. 2021;27:724–730. doi: 10.1016/j.cmi.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson R, Belluoccio D, Little CB, Fosang AJ, Bateman JF. Proteomic characterization of mouse cartilage degradation in vitro. Arthritis Rheum. 2008;58:3120–3131. doi: 10.1002/art.23789. [DOI] [PubMed] [Google Scholar]
- 11.Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang Y, Lee JH, Wang Y, Sweeney G. Emerging clinical and experimental evidence for the role of lipocalin-2 in metabolic syndrome. Clin Exp Pharmacol Physiol. 2012;39:194–199. doi: 10.1111/j.1440-1681.2011.05557.x. [DOI] [PubMed] [Google Scholar]
- 14.Berard JL, Zarruk JG, Arbour N, et al. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60:1145–1159. doi: 10.1002/glia.22342. [DOI] [PubMed] [Google Scholar]
- 15.Asimakopoulou A, Weiskirchen S, Weiskirchen R. Lipocalin 2 (LCN2) Expression in hepatic malfunction and therapy. Front Physiol. 2016;7:430. doi: 10.3389/fphys.2016.00430.ec8aa28d4ad648c59b72af9734d2a874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. Onco Targets Ther. 2018;11:8099–8106. doi: 10.2147/OTT.S181223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary N, Choudhary BS, Shah SG, et al. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int J Cancer. 2021;149:1495–1511. doi: 10.1002/ijc.33711. [DOI] [PubMed] [Google Scholar]
- 18.Landro L, Damas JK, Flo TH, et al. Decreased serum lipocalin-2 levels in human immunodeficiency virus-infected patients: increase during highly active anti-retroviral therapy. Clin Exp Immunol. 2008;152:57–63. doi: 10.1111/j.1365-2249.2008.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abers MS, Delmonte OM, Ricotta EE, et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. Jci Insight. 2021;6:e144455. doi: 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L, Zhang QZ, Li Z, et al. Incorporation of urinary neutrophil gelatinase-associated lipocalin and computed tomography quantification to predict acute kidney injury and in-hospital death in COVID-19 patients. Kidney Dis 7. 2021;(Basel):120–130. doi: 10.1159/000511403.6aead572e82145098453a84edd627a09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Al-Tamimi AO, Halwani R, Alsaidi H, Kannan M, Ahmad F. Lipocalin-2, S100A8/A9, and cystatin C: potential predictive biomarkers of cardiovascular complications in COVID-19. Exp Biol Med 247. 2022;(Maywood):1205–1213. doi: 10.1177/15353702221091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005;19:1881–1883. doi: 10.1096/fj.05-3809fje. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Constante M, Santos MM. Anemia upregulates lipocalin 2 in the liver and serum. Blood Cells Mol Dis. 2008;41:169–174. doi: 10.1016/j.bcmd.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suriawinata E, Mehta KJ. Iron and iron-related proteins in COVID-19. Clin Exp Med. 2022;23:969–991. doi: 10.1007/s10238-022-00851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neufeldt CJ, Cerikan B, Cortese M, et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-kappaB. Commun Biol. 2022;5:45. doi: 10.1038/s42003-021-02983-5.e74d2975b29b4848bc2c7e84d04fd670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saber MM, Nomair AM, Osman AM, Nomeir HM, Farag NM. Endothelial monocyte-activating polypeptide-ii is an indicator of severity and mortality in COVID-19 patients. Vaccines 10. 2022;(Basel):2177. doi: 10.3390/vaccines10122177.269a314878c94aeab64a1df02bf62bb1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller CA, Richt JA, Meyermann R, Deininger M, Schluesener H. Accumulation of the proinflammatory cytokine endothelial-monocyte-activating polypeptide II in ramified microglial cells in brains of Borna virus infected Lewis rats. Neurosci Lett. 2003;339:215–218. doi: 10.1016/S0304-3940(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Maharjan S, Kim J, et al. MUC1-C influences cell survival in lung adenocarcinoma Calu-3 cells after SARS-CoV-2 infection. BMB Rep. 2021;54:425–430. doi: 10.5483/BMBRep.2021.54.8.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Kim M, Kim D, et al. Targeting the interaction between spike protein and nucleocapsid protein for suppression and detection of human coronavirus OC43. Front Immunol. 2022;13:835333. doi: 10.3389/fimmu.2022.835333.cced431965734a6abe6cf909d417cc20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanjuan R, Domingo-Calap P. Mechanisms of viral mutation. Cell Mol Life Sci. 2016;73:4433–4448. doi: 10.1007/s00018-016-2299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D, Kim J, Kim M, et al. Analysis of spike protein variants evolved in a novel in vivo long-term replication model for SARS-CoV-2. Front Cell Infect Microbiol. 2023;13:1280686. doi: 10.3389/fcimb.2023.1280686.26dba6440dc54c4ba66d83a7f0d09017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 34.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 35.Choi JW, Fujii T, Fujii N. Elevated plasma neutrophil gelatinase-associated lipocalin level as a risk factor for anemia in patients with systemic inflammation. Biomed Res Int. 2016;2016:9195219. doi: 10.1155/2016/9195219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolignano D, Coppolino G, Donato V, Lacquaniti A, Bono C, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL): a new piece of the anemia puzzle? Med Sci Monit. 2010;16:RA131–135. [PubMed] [Google Scholar]
- 37.Rodrigues PN, Gomes SS, Neves JV, et al. Mycobacteria-induced anaemia revisited: a molecular approach reveals the involvement of NRAMP1 and lipocalin-2, but not of hepcidin. Immunobiology. 2011;216:1127–1134. doi: 10.1016/j.imbio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Chavda VP, Patel AB, Vaghasiya DD. SARS-CoV-2 variants and vulnerability at the global level. J Med Virol. 2022;94:2986–3005. doi: 10.1002/jmv.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, Zhang S, Tang YP, et al. Clinical characteristics, transmissibility, pathogenicity, susceptible populations, and re-infectivity of prominent COVID-19 variants. Aging Dis. 2022;13:402–422. doi: 10.14336/AD.2021.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO, author. Historical working definitions and primary actions for SARS-CoV-2 variants. 2023. [2023, Oct 10]. https://www.who.int/publications/m/item/historical-working-definitions-and-primary-actions-for-sars-cov-2-variants.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.