Abstract
Neurodegenerative diseases are characterized by distinct protein aggregates, such as those of α-synuclein and tau. Lysosomal defect is a key contributor to the accumulation and propagation of aberrant protein aggregates in these diseases. The discoveries of common proteinopathies in multiple forms of lysosomal storage diseases (LSDs) and the identification of some LSD genes as susceptible genes for those proteinopathies suggest causative links between LSDs and the proteinopathies. The present study hypothesized that defects in lysosomal genes will differentially affect the propagation of α-synuclein and tau proteins, thereby determining the progression of a specific proteinopathy. We established an imaging-based high-contents screening (HCS) system in Caenorhabditis elegans (C. elegans) model, by which the propagation of α-synuclein or tau is measured by fluorescence intensity. Using this system, we performed RNA interference (RNAi) screening to induce a wide range of lysosomal malfunction through knock down of 79 LSD genes, and to obtain the candidate genes with significant change in protein propagation. While some LSD genes commonly affected both α-synuclein and tau propagation, our study identified the distinct sets of LSD genes that differentially regulate the propagation of either α-synuclein or tau. The specificity and efficacy of these LSD genes were retained in the disease-related phenotypes, such as pharyngeal pumping behavior and life span. This study suggests that distinct lysosomal genes differentially regulate the propagation of α-synuclein and tau, and offer a steppingstone to understanding disease specificity.
Keywords: C. elegans, Lysosomal storage disease, Neurodegenerative disease, Protein aggregation
INTRODUCTION
Neurodegenerative diseases (NDs) are a heterogenous group of neurological disorders with the characteristics of progressive neuronal loss and specific types of proteinopathies, which feature accumulation of aberrant protein aggregates. Common proteinopathies that affect a wide range of neurodegenerative diseases include synucleinopathies and tauopathies, which are characterized by the aggregation of α-synuclein and tau, respectively. These pathogenic protein aggregates progressively spread from a few distinct brain regions to broader areas in the brain, and this aggregate spreading is known to drive the progression of disease. Cell-to-cell propagation of protein aggregates is known to be the underlying mechanism of the aggregate spreading in the brain. Therefore, it is important to investigate the mechanism underlying the intercellular propagation of protein aggregates to understand, and thus slow down, the progression of neurodegenerative diseases.
Unwanted proteins are normally degraded by the cell’s protein degrading systems, which involve such machineries as proteasomes and lysosomes. Lysosome is an organelle that has long been regarded as the center of degradation and recycling of biological polymers, and is now also recognized as a regulator of cell homeostasis (1, 2). With mounting evidence for lysosomal dysfunction in human diseases, promising novel opportunities for therapeutic interventions through targeting lysosomes are beginning to emerge. A group of diseases that have the characteristics of excessive substrate accumulation due to lysosomal defects and exhibiting proteinopathy are called lysosomal storage diseases (LSDs) (3). In LSDs, various undigested macromolecules and the secondary products accumulate in multiple organs, often including the brain, explaining prominent neuropathologies and neurological symptoms (4).
Many human genetic studies show the prominent link between LSDs and NDs. GBA1, the gene causing one of the most common LSDs, Gaucher disease, is the prominent genetic risk factor for Parkinson’s disease (PD), which occurs more frequently than other PD-susceptible genes, such as LRRK2, SNCA, and PARK2 (5). It also increases susceptibility for Dementia with Lewy Bodies (DLB), another synucleinopathy (6), while the finding of NPC1 as a genetic risk factor of Alzheimer’s disease (AD) (7) further expands the association between LSDs and NDs.
Lysosomal malfunctions are thought to lead to insufficient degradation of abnormal protein aggregates, and contribute to the cell-to-cell propagation of the aggregates. Considering the discoveries of proteinopathy in LSDs and the findings of some LSD genes as risk factors for those proteinopathies, we hypothesized that specific defects of LSD genes differentially influence the propagation of α-synuclein and tau aggregates. In the present study, to validate the hypothesis, we performed high-contents RNAi screening of LSD genes in C. elegans models that were specifically designed for α-synuclein and tau propagation.
RESULTS
Establishment of a high-contents screening system for α-synuclein and tau propagation in C. elegans
To investigate candidate genes regulating the propagation of aggregates, we established a high-contents screening (HCS) system in the C. elegans model, which enables monitoring the intercellular propagation of α-synuclein and tau aggregates with fluorescence signals in a highly quantitative manner using bimolecular fluorescence complementation (BiFC) (Fig. 1A) (8). These Venus-BiFC worms showed strong BiFC fluorescence in the pharynx, whereas wild-type N2 worms did not produce BiFC signals on automated screening system (Fig. 1B, C). In BiFC transgenic worms, the intensity of fluorescence was increased with aging, due to the occurrence of continuous transfer of aggregates (Fig. 1D-F).
Fig. 1.
Establishment of the high-contents screening system for protein aggregate propagation. (A) Transgenes used in C. elegans models for synucleinopathy and tauopathy, and generation of BiFC fluorescence via propagation of transgenic proteins between pharyngeal muscles and neurons. (B, C) The Venus-BiFC C. elegans models for α-synuclein (aS) and tau propagation exhibited strong VENUS fluorescence compared to wild-type (WT) N2 worms. (B) Analysis of BiFC signals in WT and transgenic C. elegans models at day 2. All pictures contain DIC and fluorescence images. The red box represents the BiFC signal in the pharynx. (C) Quantification of VENUS fluorescence in (B). More than fifty worms from each line were analyzed. (D) Increase in VENUS fluorescence with aging in transgenic models. (E, F) Quantification of VENUS fluorescence at the different ages in (D). More than fifty worms for each group were analyzed. (G) Alteration of VENUS fluorescence in α-synuclein BiFC models in various mutant backgrounds. (H) Quantification of VENUS fluorescence in (G). More than fifty worms from each line were analyzed. All values are represented as means ± SEM. Statistical significance, ***P < 0.001, was determined by one-way ANOVA with Tukey’s post hoc comparison (E, F) and P values, including *P < 0.05, ***P < 0.001, were calculated by one-way ANOVA with Dunnett’s post hoc test (H).
Based on our previous findings that genetic factors related to aging, lysosomal function, and cell trafficking can regulate the propagation of α-synuclein aggregates, we performed an adaptation process for the established HCS system with the known regulators of α-synuclein propagation. As a result, the BiFC fluorescence intensity was decreased in daf-2 mutant, which has a delay in aging, compared to wild-type worms. Furthermore, the deficiency of lrk-1 gene (human LRRK2 ortholog), a disease-causing factor, and of rab-35 gene, which regulates vesicle trafficking, resulted in downregulation of the fluorescence, whereas lysosomal dysfunction with mutations in asp-1 (human CTSD ortholog), asp-4 (CTSE), and sul-2 (ARSA) genes resulted in a significant increase in fluorescence intensity (Fig. 1G, H). These results suggest that we successfully established a HCS system to identify genetic modifiers that can regulate the propagation of protein aggregates in C. elegans.
Some lysosomal genes specifically regulate the propagation of either α-synuclein or tau, while others regulate both
To investigate the genetic factors that might regulate the propagation of protein aggregates, we performed RNA interference (RNAi) screening in our HCS system. First, we crossed each transgenic model with neuron-sensitized strain, eri-1 mutant worm (9), to create worm models that were suited to enhancing the knock-down effects on neurons and muscle cells in the pharynx. These RNAi-sensitive worms in eri-1 mutant background did not show significant change of fluorescence intensity, compared to the wild-type lines (Supplementary Fig. 1A, B of the Supplementary Information [SI]). Then, we introduced each E. coli bacteria expressing different double-stranded RNAs (dsRNAs) that correspond to genes known to cause LSDs into the C. elegans BiFC models, and screened for the candidates regulating the propagation of α-synuclein or tau protein aggregates.
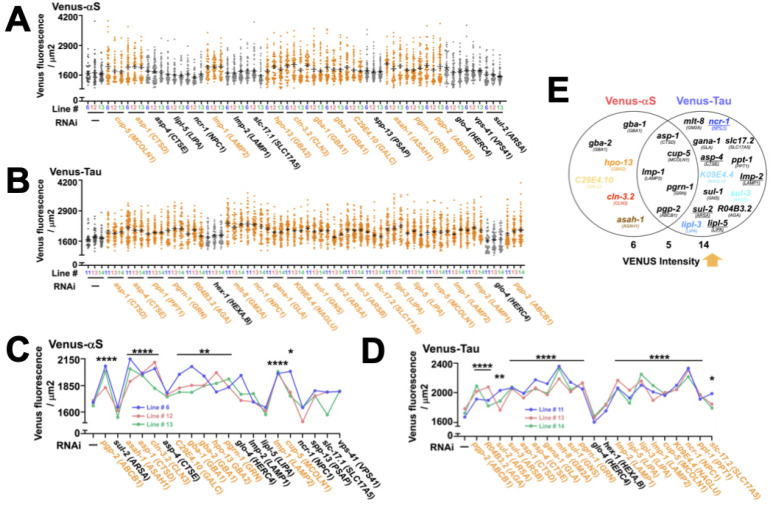
We carried out RNAi screening in the first and second stages, and in the primary screening, examined 79 LSD genes in a single line of each proteinopathy model. From the initial screening, we obtained 30 genes with significant increase of BiFC fluorescence (Fig. 2A, B). Among them, 9 and 11 LSD genes that distinctively regulate only the transfer of α-synuclein or tau protein aggregates, respectively, were detected, while 10 genes could upregulate the propagation of both protein aggregates (Fig. 2).
Fig. 2.
Primary RNAi screening to identify candidate genes that regulate the propagation of pathological proteins. (A, B) RNAi screening with dsRNA containing each gene associated with LSDs in the α-synuclein (A) and Tau (B) BiFC models. More than fifty worms form each group were analyzed. Orange letters indicate genes with significantly increased fluorescence intensity compared to control transgenic worms, and green letters designate genes with no change in the level of BiFC signals. (C) Classification of genes showing changes in fluorescence intensity identified in initial screening. All values shown in the figures are represented as mean ± SEM. Statistical significance, including *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, was determined by one-way ANOVA with Dunnett’s post hoc test (A, B).
To assess the reproducibility of the results obtained from primary screening, the candidate genes upregulating either α-synuclein and/or tau protein propagation with statistical significance underwent secondary screening (Fig. 3A-D). This was performed in triplicate, as three independent lines in each proteinopathy model were used. The secondary screening results showed that 9 of the total 20 genes, which upregulated α-synuclein propagation in the primary screening, were reproducible, while 16 out of 21 genes were reproduced to increase the propagation of tau protein (Fig. 3E). Among the above genes with significance in secondary screening, asp-1, cup-5 (MCOLN1), lmp-1 (LAMP2), pgrn-1 (GRN), and pgp-2 (ABCB1) regulated both α-synuclein and tau propagation, while gba-1 (GBA1), gba-2 (GBA1), hpo-13 (GBA2), C29E4.10 (GALC), cln-3.2 (CLN3), and asah-1 (ASAH1) genes, and mlt-8 (GM2A), ncr-1 (NPC1), gana-1 (GLA), slc17.2 (SLC17A5), asp-4, ppt-1 (PPT1), K09E4.4 (NAGLU), lmp-2 (LAMP1), sul-1 (GNS), sul-2, sul-3 (ARSB), R04B3.2 (AGA), lipl-3 (LIPA), and lipl-5 (LIPA) genes, exclusively regulated either α-synuclein and tau propagation, respectively (Fig. 3E).
Fig. 3.
Secondary RNAi-based screening to verify the primary candidate genes. (A, B) Validation of primary candidates in three independent lines of each BiFC proteinopathy model. More than fifty worms for each line were examined. Orange letters indicate the genes that consistently increase the propagation of the disease proteins. Black letters indicate the genes that fail to reproduce the results of the primary screening. (C, D) Comparison of the mean VENUS fluorescence of three lines between WT and proteinopathy models. Orange: genes whose primary test results are confirmed to be reproduced. (E) Venn-diagram representing the validated genes by secondary screening. Black or colored: genes reproduced in the secondary screening. Underlined: genes enhanced both tau and α-synuclein propagation in the primary screening but enhanced only tau propagation in the secondary screening. Colored: genes selected for the further validation studies. All data are presented in the figures are represented as mean ± SEM. Statistical significance, including *P < 0.05, **P < 0.01, ****P < 0.0001, was determined by one-way ANOVA with Dunnett’s post hoc test (C, D).
The efficacy and specificity of candidate genes are validated with disease-related phenotype analyses
Eight genes were selected among the LSD genes that passed the reproducibility assessment and ensured responsibility for the specific protein’s propagation. The choices were made based on the presence of the previous studies supporting the specific links between LSD genes and either α-synuclein or tau protein. The candidate genes selected for further study with α-synuclein specificity were CLN3, GBA2, GALC and ASAH1, while those chosen for tau-specific candidate genes were NPC1, LIPA, NAGLU, and ARSB (Fig. 3E).
These candidate genes were then validated for their specificity through the efficacy assessment of disease-related phenotypes, such as pharyngeal pumping behavior and life span. The results showed that CLN3, GBA2, GALC and ASAH1 significantly decreased the pumping behavior and mean life span only in synucleinopathy transgenic worms, but not in tauopathy worms. On the other hand, tauopathy worms showed significant downregulation of the pumping behavior and life span only upon the knock-down of the following genes: NPC1, LIPA, NAGLU, and ARSB, while in synucleinopathy worms, their effects were not observed (Fig. 4, see also Supplementary Fig. 2 of the SI). In accordance with the screening results, the LSD genes that specifically upregulated either α-synuclein or tau propagation led to the specific disease-related phenotypes in either the synucleinopathy or tauopathy model, supporting the specificity of the knock-down effect of the LSD genes.
Fig. 4.
Validation of efficacy of the candidate genes on disease-related phenotypes. (A, B) Mean pharyngeal pumping rates at day 2 after RNAi induction. Twenty worms for each line were analyzed (N = 3). (C, D) Mean life span. One hundred worms for three independent lines were analyzed (N = 3). All values shown in the figures are represented as mean ± SEM. All statistical significance, including **P < 0.01, ***P < 0.001, ****P < 0.0001, was determined by one-way ANOVA with Tukey’s post hoc comparison (A-D).
DISCUSSION
Here, we discovered that distinct sets of LSD genes, one set of CLN3, GBA2, GALC, and ASAH-1, and the other set of NPC1, LIPA, NAGLU, and ARSB, specifically upregulate the propagation of α-synuclein, and tau protein, respectively. Furthermore, the validation assay of disease-related phenotypes showed the specificity and efficacy of distinct LSD gene sets were retained in the pharyngeal pumping behavior and life span assessments. These findings collectively led to the idea that differential defects of lysosomal function may act as a determinant of a specific proteinopathy to develop, providing disease specificity.
Among the set of genes for synucleinopathy, CLN3 is a gene in which its mutation causes the most common form of recessively inherited neurodegenerative disorder, juvenile Neuronal ceroid lipofuscinosis (JNCL) (10). JNCL patients are reported to have parkinsonian features, such as synucleinopathy (11), and extrapyramidal signs (12). Also, the finding of enhanced α-synuclein oligomerization in JNCL patients supports the connection between CLN3 and α-synuclein (13). GBA2 is an isotype of the well-known PD susceptible gene, GBA1. Its link with α-synuclein is evidenced by the studies presenting the accumulation of α-synuclein in GBA2 knock-out medaka brain (14), and the downregulation of GBA2 activity in PD patients (15).
GALC is another LSD gene to have a link with α-synuclein protein, and its mutations cause Krabbe disease, which results in the accumulation of psychosine, a lipid raft-associated neurotoxin (16). The GWAS finding represents GALC as a risk locus for PD (17), and an in vitro study discovered the direct interaction between α-synuclein and psychosine, which leads to the conformational change into an aggregation-prone form (18). Also, the recent finding of α-synuclein pathology in Krabbe infants supports the potential primary importance of GALC in synucleinopathy (19). ASAH1 is another candidate gene, evidenced with the whole exome sequencing analysis, revealing to be a PD-susceptible gene (20).
Among the gene set promoting tau propagation, NPC1 is a cholesterol transporter-encoding gene, and its mutation causes autosomal recessive lysosomal lipidosis, Niemann-Pick type C (NPC) disease (21). It is noteworthy that NPC brains have a constant feature of neurofibrillary tangles (NFTs) with the immunological and ultrastructural similarity of that found in AD (22). Also, constant findings of the increase of phosphorylated tau in murine NPC models (23, 24) and human patients (25, 26) remark the association between NPC1 and tauopathy. LIPA is a lysosomal acid lipase-encoding gene that locates between regions with increased LOD scores for AD on chromosome 10 (27). Although the biological function and gene location of LIPA propose its possibility as a candidate for AD susceptibility gene, controversy exists. While some studies suggested polymorphisms in LIPA gene do not influence AD risk (28, 29), there is also evidence conferring significant susceptibility for AD (30).
Another LSD gene regulating the propagation of tauopathy is NAGLU, mutations of which cause lysosomal accumulation of heparan sulfate. The link between heparan sulfate and tau has arisen as these molecules coexist in the AD brain (31). Under physiological conditions in vitro, incubation with heparan sulfate resulted in even non-phosphorylated recombinant tau isoforms with three microtubule-binding repeats to form paired helical filament (PHF) (31). Also, the intracerebroventricular administration of the NAGLU enzyme to Mucopolysaccharidosis IIIB mice reduced the levels of heparan sulfate and phosphorylated tau to control levels (32), indicating the link between NAGLU and tau protein. ARSB is a gene causing an autosomal recessive LSD, Mucopolysaccharidosis type VI (MPS VI) (33). Although in this study, ARSB is suggested to be responsible for the enhancement of tauopathy, MPS VI patients generally lack neurodegeneration and the associated cognitive impairment, unlike most other MPS disorders. The CNS findings of MPS VI are mental retardation (34), increased cranial pressure and hydrocephalus (35), and high cerebral volume (36).
There were common LSD genes, which upregulated both α-synuclein and tau protein propagation. They were CTSD (Cathepsin D), MCOLN1 (Mucolipin 1), LAMP2 (lysosomal associated membrane protein 2), GRN (progranulin), and ABCB1 (ATP binding cassette subfamily B member 1). Although MCOLN1 and ABCB1 were not found to be involved in either synucleinopathy or tauopathy, GRN has well-established evidence showing the link with α-synuclein and tau proteins. Patients with GRN mutations showed both α-synuclein and tau pathology (37), while progranulin reduction in tau transgenic mice accelerated tau phosphorylation (38). Also, the downregulation of both mRNA and protein levels of LAMP2A in association with the increase in α-synuclein level in PD patients indicates the link between LAMP2 and α-synuclein (39). CTSD is also linked to α-synuclein, evidenced by study showing the increase of α-synuclein aggregation upon CTSD deficiency (10).
In conclusion, we found the distinct sets of lysosomal genes that define synucleinopathy and tauopathy in terms of regulating protein propagation, and this remarkable specificity of gene sets was preserved through to their efficacy on disease-related phenotypes. The specificity of the genes to either α-synuclein and tau is consistent with the previously identified pathophysiological functions of these genes, and needs further validation in different experimental models. Furthermore, future research on the genes identified in the current study, determining the mechanism by which the specific lysosomal genes regulate the propagation of specific pathological aggregates, would pave the way to the understanding of disease specificity. We anticipate that expansion of our study to the understanding of the mechanism by which each lysosomal gene delivers specificity to either synucleinopathy or tauopathy would contribute to the discovery of novel diagnostic and therapeutic strategies.
MATERIALS AND METHODS
Further detailed information is provided in the Supplementary Information.
Funding Statement
ACKNOWLEDGEMENTS This work was supported by National Research Foundation (NRF) grants funded by the Korean Government (MSIT) (NRF-2018R1A5A2025964 to S.-J.L. and 2022R1I1A1A01070958 to D.-K.K.). K.W.O. received a scholarship from the BK21-FOUR education program.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim CY, Zoncu R. The lysosome as a command-and-control center for cellular metabolism. J Cell Biol. 2016;214:653–664. doi: 10.1083/jcb.201607005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroemer G, Jäättelä M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 4.Ballabio A, Gieselmann V. Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta - Mol Cell Res. 2009;1793:684–696. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira C, Ottman R, Orr-Urtreger A. Multi-center analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mata IF, Samii A, Schneer SH, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol. 2008;65:379–382. doi: 10.1001/archneurol.2007.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kresojević N, Dobričić V, Svetel M, Kostić V. Mutations in Niemann Pick type C gene are risk factor for Alzheimer's disease. Med Hypotheses. 2014;83:559–562. doi: 10.1016/j.mehy.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Kim DK, Lim HS, Kawasaki I, et al. Anti-aging treatments slow propagation of synucleinopathy by restoring lysosomal function. Autophagy. 2016;12:1849–1863. doi: 10.1080/15548627.2016.1207014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadiya P, Nazir A. A pre-and co-knockdown of RNAseT enzyme, Eri-1, enhances the efficiency of RNAi induced gene silencing in Caenorhabditis elegans. PLoS One. 2014;9:e87635. doi: 10.1371/journal.pone.0087635.3b4d598b18514f8ab87c64cb90ab940d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IBD Consortium, author. Isolation of a novel gene underlying Batten disease. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 11.Järvelä I, Sainio M, Rantamäki T, et al. Biosynthesis and intracellular targeting of the CLN3 protein defective in Batten disease. Hum Mol Genet. 1998;7:85–90. doi: 10.1093/hmg/7.1.85. [DOI] [PubMed] [Google Scholar]
- 12.Järvelä I, Autti T, Lamminranta S, Åberg L, Raininko R, Santavuori P. Clinical and magnetic resonance imaging findings in batten disease: analysis of the major mutation (1.02-Kb Deletion) Ann Neurol. 1997;42:799–802. doi: 10.1002/ana.410420517. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Heo TH, Kim SJ. Altered levels of α-synuclein and sphingolipids in Batten disease lymphoblast cells. Gene. 2014;539:181–185. doi: 10.1016/j.gene.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi E, Uemura N, Akiyama H, et al. Impact of Gba2 on neuronopathic Gaucher's disease and α-synuclein accumulation in medaka (Oryzias latipes) Mol Brain. 2021;14:1–15. doi: 10.1186/s13041-021-00790-x.4dcda80e219b4d179a2bdf0bf1201e92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huebecker M, Moloney EB, van der Spoel AC, et al. Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson's disease. Mol Neurodegener. 2019;14:1–21. doi: 10.1186/s13024-019-0339-z.8a3bdc7450e34016b365323d7a34ab17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- 17.Chang D, Nalls MA, Hallgrímsdóttir IB, et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet. 2017;49:1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelkarim H, Marshall MS, Scesa G, et al. α-Synuclein interacts directly but reversibly with psychosine: implications for α-synucleinopathies. Sci Rep. 2018;8:1–19. doi: 10.1038/s41598-018-30808-9.4adc82051b0144b0a3f11de190f2a080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatton C, Ghanem SS, Koss DJ, et al. Prion-like α-synuclein pathology in the brain of infants with Krabbe disease. Brain. 2022;145:1257–1263. doi: 10.1093/brain/awac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robak LA, Jansen IE, Van Rooij J, et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson's disease. Brain. 2017;140:3191–3203. doi: 10.1093/brain/awx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanier MT, Rodriguez-Lafrasse C, Rousson R, et al. Type C Niemann-Pick disease: spectrum of phenotypic variation in disruption of intracellular LDL-derived cholesterol processing. Biochim Biophys Acta Mol Basis Dis. 1991;1096:328–337. doi: 10.1016/0925-4439(91)90069-L. [DOI] [PubMed] [Google Scholar]
- 22.Auer IA, Schmidt ML, Lee VY, et al. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer's disease. Acta Neuropathol. 1995;90:547–551. doi: 10.1007/BF00318566. [DOI] [PubMed] [Google Scholar]
- 23.Ohm TG, Treiber-Held S, Distl R, et al. Cholesterol and tau protein-findings in Alzheimer's and Niemann Pick C's disease. Pharmacopsychiatry. 2003;36:120–126. doi: 10.1055/s-2003-43060. [DOI] [PubMed] [Google Scholar]
- 24.Sawamura N, Gong JS, Garver WS, et al. Site-specific phosphorylation of tau accompanied by activation of mitogen-activated protein kinase (MAPK) in brains of Niemann-Pick type C mice. J Cell Biol. 2001;276:10314–10319. doi: 10.1074/jbc.M009733200. [DOI] [PubMed] [Google Scholar]
- 25.Bu B, Klunemann H, Suzuki K, et al. Niemann-Pick disease type C yields possible clue for why cerebellar neurons do not form neurofibrillary tangles. Neurobiol Dis. 2002;11:285–297. doi: 10.1006/nbdi.2002.0551. [DOI] [PubMed] [Google Scholar]
- 26.Saito Y, Suzuki K, Hulette CM, Murayama S. Aberrant phosphorylation of α-synuclein in human Niemann-Pick type C1 disease. J Neuropathol. 2004;63:323–328. doi: 10.1093/jnen/63.4.323. [DOI] [PubMed] [Google Scholar]
- 27.Myers A, Holmans P, Marshall H, et al. Susceptibility locus for Alzheimer's disease on chromosome 10. Science. 2000;290:2304–2305. doi: 10.1126/science.290.5500.2304. [DOI] [PubMed] [Google Scholar]
- 28.Von Trotha KT, Heun R, Schmitz S, Lütjohann D, Maier W, Kölsch H. Influence of lysosomal acid lipase polymorphisms on chromosome 10 on the risk of Alzheimer's disease and cholesterol metabolism. Neurosci Lett. 2006;402:262–266. doi: 10.1016/j.neulet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Riemenschneider M, Mahmoodzadeh S, Eisele T, et al. Association analysis of genes involved in cholesterol metabolism located within the linkage region on chromosome 10 and Alzheimer's disease. Neurobiol Aging. 2004;25:1305–1308. doi: 10.1016/j.neurobiolaging.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Papassotiropoulos A, Wollmer MA, Tsolaki M, et al. A cluster of cholesterol-related genes confers susceptibility for Alzheimer's disease. J Clin Psychiatry. 2005;66:940. doi: 10.4088/JCP.v66n0720. [DOI] [PubMed] [Google Scholar]
- 31.Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 32.Kan SH, Aoyagi-Scharber M, Le SQ, et al. Delivery of an enzyme-IGFII fusion protein to the mouse brain is therapeutic for mucopolysaccharidosis type IIIB. Proc Natl Acad Sci U S A. 2014;111:14870–14875. doi: 10.1073/pnas.1416660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litjens T, Baker EG, Beckmann KR, Morris CP, Hopwood JJ, Callen DF. Chromosomal localization of ARSB, the gene for human N-acetylgalactosamine-4-sulphatase. Hum Genet. 1989;82:67–68. doi: 10.1007/BF00288275. [DOI] [PubMed] [Google Scholar]
- 34.Vestermark S, Tønnesen T, Andersen MS, Güttler F. Mental retardation in a patient with Maroteaux-Lamy. Clin Genet. 1987;31:114–117. doi: 10.1111/j.1399-0004.1987.tb02779.x. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GP, Cohen EJ. Hydrocephalus in Maroteaux-Lamy syndrome. Arch Ophthalmol. 1998;116:400. doi: 10.32388/gc13xd. [DOI] [PubMed] [Google Scholar]
- 36.Vedolin L, Schwartz IVD, Komlos M, et al. Brain MRI in mucopolysaccharidosis: effect of aging and correlation with biochemical findings. Neurology. 2007;69:917–924. doi: 10.1212/01.wnl.0000269782.80107.fe. [DOI] [PubMed] [Google Scholar]
- 37.Leverenz JB, Yu CE, Montine TJ, et al. A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain. 2007;130:1360–1374. doi: 10.1093/brain/awm069. [DOI] [PubMed] [Google Scholar]
- 38.Hosokawa M, Arai T, Masuda-Suzukake M, et al. Progranulin reduction is associated with increased tau phosphorylation in P301L tau transgenic mice. J Neuropathol. 2015;74:158–165. doi: 10.1097/NEN.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 39.Murphy KE, Gysbers AM, Abbott SK, et al. Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson's disease. Mov Disord. 2015;30:1639–1647. doi: 10.1002/mds.26141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.