Abstract
The RecBCD enzyme has a powerful duplex DNA exonuclease activity in vivo. We found that this activity decreased strongly when cells were irradiated with UV light (135 J/m2). The activity decrease was seen by an increase in survival of phage T4 2− of about 200-fold (phage T4 2− has defective duplex DNA end-protecting gene 2 protein). The activity decrease depended on excision repair proficiency of the cells and a postirradiation incubation. During this time, chromosome fragmentation occurred as demonstrated by pulsed-field gel electrophoresis. In accord with previous observations, it was concluded that the RecBCD enzyme is silenced during interaction with duplex DNA fragments containing Chi nucleotide sequences. The silencing was suppressed by induction or permanent derepression of the SOS system or by the overproduction of single-strand DNA binding protein (from a plasmid with ssb+) which is known to inhibit degradation of chromosomal DNA by cellular DNases. Further, mutations in xonA, recJ, and sbcCD, particularly in the recJ sbcCD and xonA recJ sbcCD combinations, impeded RecBCD silencing. The findings suggest that the DNA fragments had single-stranded tails of a length which prevents loading of RecBCD. It is concluded that in wild-type cells the tails are effectively removed by single-strand-specific DNases including exonuclease I, RecJ DNase, and SbcCD DNase. By this, tailed DNA ends are processed to entry sites for RecBCD. It is proposed that end blunting functions to direct DNA ends into the RecABCD pathway. This pathway specifically activates Chi-containing regions for recombination and recombinational repair.
Mutations in the recB and recC genes greatly reduce chromosomal recombination in Escherichia coli and render cells sensitive to UV irradiation, gamma rays, and other DNA-damaging agents, which suggests that these genes function in recombinational repair of DNA damage, particularly of DNA double-strand breaks (8, 44). Together with recD, these genes encode the three protein subunits of the RecBCD enzyme. In vitro, the enzyme has exonuclease activity for duplex DNA (therefore also termed exonuclease V), exo- and endonucleolytic activities for single-stranded DNA, and a DNA helicase activity, all of which require ATP (for references, see reference 18). From early studies, it is known that the rapid degradation of linear duplex DNA in E. coli cells is mainly caused by the RecBCD enzyme, irrespective of whether the DNA has entered the cell during phage infection (43), transfection (51), or transformation (33, 52). Accordingly, the gene 2 mutant of phage T4 which lacks the protective protein attached to the ends of the T4 genome has a very low chance of surviving in wild-type cells as a result of genome degradation (34) but multiplies unimpaired in recB or recC mutants. Therefore, the efficiency of plating (EOP) of T4 2− has been used to monitor the level of RecBCD enzyme in a variety of experiments (1, 7, 11, 17, 40).
Although in vivo the RecBCD enzyme effectively degrades linear double-stranded DNA, it is nevertheless essential for the recombinational repair of DNA double-strand breaks (41). These apparently conflicting observations have been explained by the findings that the octanucleotide sequence 5′-GCTGGTGG-3′ termed Chi which is present in the E. coli genome about once per 5 kb can block the nucleolytic activity of the RecBCD enzyme and stimulate recombination in their vicinity (11, 14, 32, 44). How the switch at Chi from the DNA-degrading activity of RecBCD to a recombination-initiating function is achieved in vivo is not yet known in detail. It has been proposed (32, 46) and some evidence provided by in vitro and in vivo experiments (13, 17, 31) that the RecD subunit may play a key role in the switch process. Before RecBCD can encounter a properly oriented Chi sequence in a duplex DNA molecule, the enzyme must bind to the end of that DNA. In vitro, effective binding requires that the DNA is blunt ended or has single-stranded tails of only a few nucleotides (37, 45).
We have previously observed that the EOP of the T4 2− mutant strongly increased when E. coli wild-type cells were irradiated with UV prior to phage infection (48). This indicated a decrease of RecBCD enzyme activity. Here we report experiments which led us to conclude that the activity decrease occurs in the UV-irradiated E. coli cells as a result of the interaction of RecBCD with duplex DNA fragments. These fragments are produced in an excision repair-dependent process at high UV doses. Results of further experiments suggest that the ends of the DNA fragments require processing by other DNases to make them a substrate for RecBCD enzyme. The possibility that processing functions direct tailed linear DNA into the RecA- and RecBCD-dependent recombination repair process is discussed.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli strains used are listed in Table 1. Except for WA485, all strains are AB1157 and its derivatives obtained by P1 transduction or plasmid transformation using standard procedures. Strain WA235 was used for propagation of T4 2−. Table 1 lists the EOP of T4 2− on all strains employed. Details of strain constructions can be provided upon request.
TABLE 1.
E. coli strains used in this studya
| Strain | Relevant genotype | Relative EOP of T4 2− | Source or reference |
|---|---|---|---|
| AB1157 | F−argE3 hisG4 leuB6 proA2 Δthr-1 thi-1 ara-14 galK2 lacY1 mtl-1 xyl-5 tsx-33 rpsL31 supE44 | 5.4 × 10−4 | 16 |
| WA632 | As AB1157, but recB21 arg+ | 1b | 39 |
| BT136 | As AB1157, but lexA3 malE::Tn5 | 5.7 × 10−4 | This work |
| WA618 | As AB1157, but uvrA277::Tn10 | 5.1 × 10−4 | R. Lloyd |
| WA778 | As AB1157, but uvrC279::Tn10 | 5.5 × 10−4 | R. Lloyd |
| WA818 | As AB1157, but xonA2 his+ arg+ | 5.5 × 10−4 | 39 |
| BT122 | As AB1157, but recJ284::Tn10 | 6.7 × 10−4 | 39 |
| BT384 | As AB1157, but ΔsbcDC::Kmr | 8.8 × 10−4 | This work |
| BT421 | As AB1157, but ΔsbcDC::KmrxonA2 his+ arg+ | 8.6 × 10−4 | This work |
| BT386 | As AB1157, but ΔsbcDC::KmrrecJ284::Tn10 | 1.1 × 10−3 | This work |
| BT422 | As AB1157, but ΔsbcDC::KmrrecJ284::Tn10 xonA2 his+ arg+ | 1.0 × 10−3 | This work |
| BT244 | As AB1157, but lexA71::Tn5 sulA::MudBx::Tn9 | 4.7 × 10−4 | This work |
| BT346 | As AB1157, but lexA3 recAo281 srl-350::Tn10 | 8.3 × 10−4 | This work |
| BT389 | As AB1157, but lexA71::Tn5 Δ(recA-srl)::Tn10 | 3.6 × 10−3 | This work |
| BT330 | As AB1157, but lexA71::Tn5 ssb-1 | 4.7 × 10−4 | This work |
| BT335 | As AB1157, but lexA71::Tn5 ssb-113 | 1.5 × 10−4 | This work |
| WA235 | recB21 recA56 strA sup+ | 38 | |
| WA485 | polA1 endA1 thyA | R. Eichenlaub | |
| WA691 | AB1157 with pBR322 | 7.1 × 10−4 | This work |
| WA692 | AB1157 with pDW1 (recB+C+D+) | 3.0 × 10−4 | This work |
| BT305 | AB1157 with pJF118EH | 5.3 × 10−4 | This work |
| BT306 | AB1157 with pSK1 (recD+) | 5.5 × 10−4 | This work |
| BT161 | AB1157 with pUC19 | 9.0 × 10−4 | This work |
| BT168 | AB1157 with pJA40 (ssb+) | 5.7 × 10−4 | This work |
| BT230 | BT136 with pUC19 | 1.2 × 10−3 | This work |
| BT231 | BT136 with pJA40 (ssb+) | 2.4 × 10−3 | This work |
| BT241 | WA618 with pUV1 (uvrA+) | 5.9 × 10−4 | This work |
Plasmids employed were the cloning vectors pBR322 (3), pUC19 (57), and pJF118EH (15). Plasmid pDW1 is pBR322 with a 17-kbp fragment (obtained after partial Sau3AI digestion of E. coli DNA) cloned into the BamHI site spanning the recC+, recB+, and recD+ gene region of E. coli (40). Plasmid pSK1 is pJF118EH with a 3.8-kbp PstI fragment covering the recD+ gene of E. coli (17). Plasmid pJA40 is pUC9 with a 664-bp HaeIII fragment covering ssb+ of E. coli (obtained by J. Brandsma, Leiden, The Netherlands). Plasmid pUV1 is the circularized large HindIII fragment of pJA501 (6) containing the vector pBR322 and the uvrA+ gene with its promoter. Plasmids and their relevant genotypes are included in the genotypes of strains in Table 1.
Media and growth conditions.
Bacterial cultures were grown in TBY (10 g of Bacto Tryptone, 5 g of Bacto yeast extract, 5 g of NaCl per 1,000 ml) at 30°C. Plates contained TBY agar (TBY solidified by 1.5% Bacto Agar). The soft agar consisted of TBY with 0.5% Bacto Agar. Phosphate buffer consisted of 0.04 M Na2HPO4, 0.02 M KH2PO4, 0.07 M NaCl, and 0.002 M MgSO4.
Determination of the EOP of T4 2−.
Phages (in 0.1 ml of phosphate buffer) were added to 0.2 ml of log-phase culture (2 × 108 cells/ml). After 15 min at 30°C for phage adsorption, the suspension was poured together with 3 ml of soft agar on a plate. Plaques were counted after 24 h at 30°C. The titer of the T4 2− lysate was determined with the recB mutant strain WA632. The EOP is the plaque count determined on a given strain divided by the plaque count on WA632. The relative EOP is the EOP on irradiated cells divided by the EOP on unirradiated cells of the same strain determined in the same experiment. As a control of the cellular capacity for phage propagation, the EOP of T4+ was determined in parallel. When the EOPs of T4 2− and T4+ were determined with irradiated cells, 0.01 ml of a culture grown overnight (AB1157) was added after phage adsorption and before plating. With some strains, a decline of the T4+ plating upon irradiation with the highest doses was observed (up to 0.3 compared to the unirradiated cells). The T4 2− EOP was corrected accordingly. Phage adsorption was always more than 95%.
UV irradiation.
The cells of 5 ml of a log-phase culture were sedimented and resuspended in 4.5 ml of phosphate buffer. Cells were irradiated in a glass petri dish at room temperature with stirring as described previously (47). After irradiation, 0.5 ml of 10-fold concentrated TBY was added and the suspension was incubated at 30°C in a 100-ml Erlenmeyer flask on a shaker for a time period given for each experiment.
Measurement of T4 2− DNA degradation.
Phage T4 2− labelled with [3H]thymidine was prepared by standard procedures. The phages had a specific radioactivity of 2 × 10−5 cpm per infective center. The quantification of intracellular DNA degradation was performed as described previously (47) and corrected for the efficiency of phage adsorption (39).
Pulsed-field gel electrophoresis.
Cells were washed in 10 mM Tris-HCl (pH 7.5)–1 mM EDTA and resuspended in 100 mM NaCl–10 mM Tris-HCl (pH 7.5)–25 mM EDTA. The cells were concentrated, embedded in 1% agarose (type III; Biometra, Göttingen, Germany) lysed, and treated with proteinase K essentially as described previously (24). Electrophoresis was performed in 1% agarose at 13°C for 16 h at 180 V with a Rotaphor R22 (Biometra) and using a pulse time of 3 to 30 s (linear) and an angle of 120 to 95° (log). The electrophoresis markers were λ DNA concatemers and HindIII-digested λ DNA. The gel was stained with ethidium bromide and photographed.
RESULTS
Increased EOP of phage T4 2− on UV-irradiated cells.
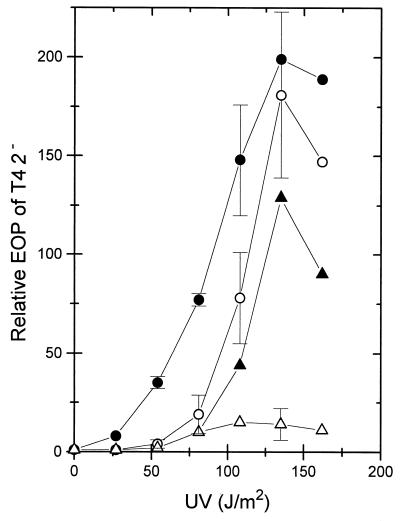
Compared to recB mutant cells (WA632), the EOP of T4 2− on wild-type cells (AB1157) is only about 5 × 10−4 (Table 1) due to RecBCD-dependent exonucleolytic phage genome destruction (34, 39). When wild-type cells were irradiated with UV and aerated in broth at 30°C for various time periods before infection with T4 2−, a time-dependent increase of the EOP of T4 2− was observed, reaching a maximum after about 90 to 120 min. This phenomenon was termed increase of T4 2− EOP (ITE). Its dependence on the UV dose showed a typically concave-shaped curve (Fig. 1), with an increase in the EOP of about 200-fold at a UV dose of 135 J/m2. The level of cell survival at the various UV doses of the wild-type cells (and of other strains in later experiments) is not relevant since the capacity to propagate T4 was largely maintained even at the highest doses (see Materials and Methods).
FIG. 1.
Increase in the EOP of T4 2− with different UV doses applied to various E. coli strains. Postirradiation aeration in broth was for 120 min at 30°C. The relative EOPs were determined as described in Materials and Methods. The data are means from two to three independent experiments. For clarity, only some points are given with standard deviations or with the range (strain WA692). The strains were wild type (AB1157; ○), lexA3 (BT136; •), wild type with pDW1 carrying the recB+C+D+ genes (WA692; ▵), and wild type with vector pBR322 (WA691; ▴).
The degradation of [3H]thymidine-labelled T4 2− DNA to acid-soluble material decreased from about 50% acid-soluble material in nonirradiated cells to 33% in irradiated cells (135 J/m2) (Table 2), suggesting that the EOP increase was related to lower duplex DNA-degrading activity in irradiated cells. This was supported by the finding that in cells with an about 15-fold-higher RecBCD enzyme activity due to the multicopy plasmid pDW1 (with recB+ recC+ recD+ [40]), induction of ITE was weak (Fig. 1). Apparently, in strongly UV-irradiated cells, less RecBCD enzyme is available for attacking T4 2− genomes. A possible reason could be (i) that the enzyme is inhibited by an UV-induced protein (ExoV inhibitor [56]) or (ii) that the enzyme interacts with UV-damaged DNA or repair intermediates which sequester the enzyme. The facts that in a lexA3 mutant (in which induction of SOS proteins is blocked) [53]), full induction of ITE was observed at high doses and hyperinduction was observed at lower doses (Fig. 1) and the data in the next section are all in favor of the second possibility.
TABLE 2.
Degradation of [3H]thymidine-labelled T4 2− DNA in UV-irradiated cells
| Strain | Relevant genotype | % Acid-soluble material from cells irradiated with the following UV dose (J/m2)a:
|
|||
|---|---|---|---|---|---|
| 0 | 27 | 54 | 135 | ||
| AB1157 | Wild-type | 48.7 (±1.2) | ND | 41.5 (±1.0) | 34.3 (±0.5) |
| WA618 | uvrA277::Tn10 | 46.8 (±1.6) | 44.3 (±4.4) | ND | 45.7 (±1.3) |
| WA485 | polA1 | 52.6 (±2.0) | 17.7 (±5.8) | ND | 9.7 (±0.2) |
a Log-phase cells were irradiated with the UV doses indicated and aerated at 30°C for 90 min before infection with labelled T4 2− phage at 30°C. The amount of cold acid-soluble products was determined 60 min after infection. The data are means from two independent experiments given with the range. ND, not determined.
The increase of T4 2− EOP depends on excision repair.
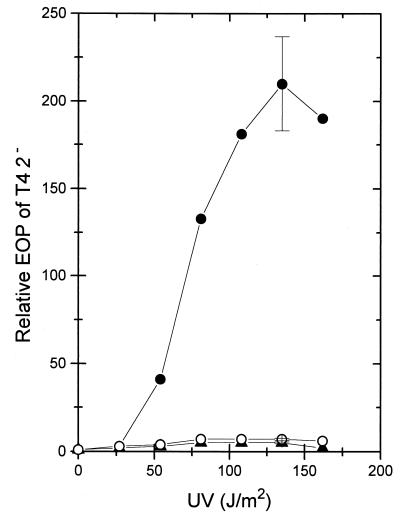
Mutations in uvrA block the incision step of the excision repair pathway of UV photoproducts and other DNA damages (50). Compared to wild type, in a uvrA mutant induction of ITE was strongly reduced at low and high UV doses (Fig. 2) and after postirradiation incubation periods between 0.5 and 4 h (data not shown). Results similar to those with the uvrA mutant were also obtained with uvrC (Fig. 2) and uvrB (not shown) mutants. A plasmid expressing uvrA+ restored inducible ITE in the uvrA mutant (Fig. 2). In contrast to UV-irradiated wild-type cells, a decrease of T4 2− DNA degradation was not observed in UV-irradiated uvrA cells (Table 2). In polA1 cells, in which incision at photoproducts is normal but closing of excision repair gaps is defective (50), UV-induced ITE was seen (data not shown) and the degradation of T4 2− DNA was reduced (Table 2). Crossing of a uvrC279::Tn10 mutation into the polA1 strain abolished UV-induced ITE (data not shown). From these observations, it was concluded that incision of DNA at photoproducts is required for ITE and that a higher survival of T4 2− phages is correlated with less degradation of phage DNA. It should be pointed out that the increase in T4 2− survival of about 200-fold at 135 J/m2 (Fig. 1) represents the rescue of only about 10% of the infecting phage. Correspondingly, the production of acid-soluble material from phage DNA was only partially lowered upon UV irradiation (Table 2).
FIG. 2.
Increase in the EOP of T4 2− with UV doses applied to a uvrA strain (WA618; ○), a uvrC strain (WA778; ▴), and a uvrA strain with plasmid pUV1 carrying the uvrA+ gene (BT241; •). For details, see the legend to Fig. 1. The data are means from two independent experiments, the range is shown only for the points after the UV dose of 135 J/m2.
The notion that ITE became effective only in uvr+ cells and particularly following high UV doses (Fig. 1) suggested that excision repair of closely spaced photoproducts on opposite strands leads to the formation of DNA double-strand breaks (4, 42). In excision-proficient E. coli, Bonura and Smith (4) observed 20 to 30 double-strand breaks per genome after 100 J/m2 and postirradiation incubation of 80 min. Using pulsed-field gel electrophoresis, the UV dose-dependent fragmentation of chromosomal DNA was detected in wild-type cells, which became very apparent after 135 J/m2 and 90 min of postirradiation incubation (Fig. 3). The time dependence of DNA fragmentation could indicate that breakage is also a result of incision plus replication. Chromosome fragmentation was not found in the uvrA mutant (Fig. 3). It was, however, seen in the polA strain (not shown), showing that ITE depends on incising but not on gap closure.
FIG. 3.
Pulsed-field gel electrophoresis of chromosomal DNA from UV-irradiated cells of the wild type (AB1157) and a uvrA mutant (WA618). The two leftmost lanes contain a λ DNA multimer ladder and HindIII-digested λ DNA fragments. The sizes of the fragments are indicated as kilobase pairs (kb) to the left of the gel. The other lanes were loaded with agar blocks, with each containing about 3 × 108 cells. The UV doses applied to cells and the time spans of postirradiation incubation at 30°C (min) are indicated at the top.
Influence of SOS derepression on UV-induced ITE.
Since several components of the excision repair system are coded by genes under SOS regulation (see reference 50), we asked whether derepression of the SOS regulon would stimulate the subsequent UV induction of ITE. This was not the case. Rather, induction of ITE was suppressed when wild-type cells had received an SOS-inducing UV dose (54 J/m2) 90 min before the ITE-inducing irradiation (135 J/m2) and further incubation (Table 3). In a lexA71::Tn5 mutant in which the SOS system is permanently and fully derepressed, ITE was hardly inducible with UV (without or with prior SOS-inducing treatment [Table 3]), although chromosomal DNA fragmentation occurred, as in UV-irradiated wild-type cells (Fig. 3 and data not shown). In contrast, pretreatment of a lexA3 mutant with UV (54 J/m2) did not prevent subsequent UV induction of ITE (Table 3). These findings show that (i) a gene under the control of the lexA repressor can counteract UV induction of ITE and (ii) that the chromosome fragmentation following high UV doses is necessary but not sufficient to trigger ITE.
TABLE 3.
Inhibition of the UV-induced increase of T4 2− EOP by derepression of the SOS system
| Strain | Relevant genotype | SOS-inducing pre-treatment of cells (54 J/m2)a | Relative EOP of T4 2−
|
|
|---|---|---|---|---|
| After pretreatment | After induction (135 J/m2)b | |||
| AB1157 | Wild-type | − | 1 | 220 |
| + | 2.1 | 19 | ||
| BT244 | lexA71::Tn5 | − | 1 | 2.5 |
| + | 4 | 12 | ||
| BT136 | lexA3 | − | 1 | 183 |
| + | 40 | 390 | ||
| BT346 | lexA3 recAo281 | − | 194 | |
| BT389 | lexA71::Tn5 ΔrecA | − | 7 | |
a The cells were irradiated (+) or not irradiated (−) with the indicated UV dose followed by 90 min of aeration at 30°C. About 10% of wild-type and lexA71::Tn5 cells survived the dose of 54 J/m2, while only about 0.0001% of the lexA3 cells survived.
b Cells with or without SOS-inducing pretreatment were irradiated with the indicated UV dose and aerated for 120 min at 30°C before determination of the EOP. The data are means from two or three independent experiments.
Overexpression of recA in a lexA3 mutant caused by the recAo298 operator mutation (9) did not prevent induction of ITE (Table 3), suggesting that recA is not the SOS gene counteracting induction of ITE. In an attempt to find the counteracting SOS gene, defective alleles of SOS genes were crossed into the lexA71::Tn5 (plus sulA) genetic background including Δ(recA-srl)::Tn10, dinA::Mud, dinB::Mud, dinD::Mud, umuC::Mud, recN::Tn5, ruvA::Tn10, and recQ::Tn3. None of these crosses restored the inducibility of ITE (data not shown). However, when an ssb-1 allele was crossed into the lexA71::Tn5 (plus sulA) background (giving strain BT330), induction of ITE by UV was partially restored (the EOP of T4 2− increased after 135 J/m2 and 90 min at 30°C by a factor of 25.3 ± 5.5; n = 3). This was not observed with the ssb-113 allele (strain BT335; 2.9 ± 0.8; n = 4).
The single-strand binding protein (SSB) coded by ssb-1 has a 1,000-fold-lower single-stranded DNA affinity than the wild-type protein, whereas the ssb-113 protein is not impaired in DNA binding (28, 54, 55). The ssb gene has a lexA-regulated promoter (5), and an increased rate of SSB synthesis was observed in cells treated with nalidixic acid (35). Meyer and Laine (28) concluded that the ssb gene of E. coli is inducible by conditions derepressing the SOS operon. Since in the lexA71::Tn5 background, only the weakly DNA-binding SSB-1 protein allowed induction of ITE whereas the wild-type SSB and the SSB-113 did not, it was hypothesized that increased amounts of SSB with normal DNA-binding activity would prevent induction of ITE.
Effect of ssb+ overexpression.
In cells with the multicopy plasmid pJA40 overproducing SSB (6), induction of ITE was absent (Table 4). A similar result was obtained using the ssb+ gene cloned in pSY343 (58), a runaway replicon (data not shown) and also with pJA40 in lexA3 cells (Table 4). These observations suggest that overexpression of ssb+ suppresses the induction of ITE by UV. The results also imply that single-stranded DNA influences the induction of ITE. Since chromosomal DNA fragmentation in the SSB-overproducing cells was as strong as in wild-type cells (Fig. 3 and data not shown), excess SSB probably prevents induction of ITE by interfering with a step occurring subsequent to chromosome breakage.
TABLE 4.
Influence of SSB overproduction from the multicopy plasmid pJA40 (with ssb+) on the UV-induced increase of T4 2− EOP
| Strain | Relevant genotype | Relative EOPa of T4 2− |
|---|---|---|
| BT161 | Wild-type pUC19 | 212 ± 9.3 |
| BT168 | Wild-type pJA40 | 5.1 ± 0.8 |
| BT230 | lexA3 pUC19 | 184 ± 16 |
| BT231 | lexA3 pJA40 | 15.7 ± 8.9 |
a The EOP on irradiated cells (135 J/m2) followed by aeration at 30°C for 120 min is given relative to the EOP on unirradiated cells. The data are means ± standard deviations (n = 3).
Gene functions required for full induction of ITE.
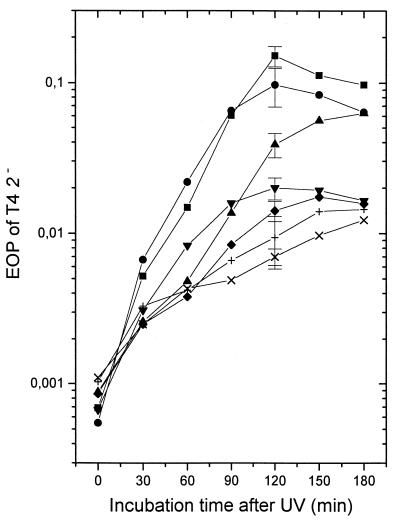
We assumed that the chromosomal fragments produced after high UV doses contain single-stranded tails of various length and of 5′ and 3′ polarity. Tails of both polarities longer than about 25 nucleotides are known to prevent loading of RecBCD enzyme on the DNA fragment (37, 45). Thus, long tails would have to be removed or shortened by single-strand-specific exo- and endonucleases to make the duplex DNA ends available to RecBCD. To address this point, UV-induced ITE was determined at different times after irradiation of isogenic strains defective for various DNases (Fig. 4). A xonA2 mutation abolishing exonuclease I (36) had only a minor, if any, effect. A stronger reduction was observed in a recJ::Tn10 mutation eliminating RecJ DNase. A xonA recJ double mutant and the recJ single mutant gave similar results (not shown). We also examined an sbcCD deletion mutant which lacks the two subunit genes of an enzyme that has besides duplex DNA exonuclease activity a single-strand endonuclease activity (10). As shown in Fig. 4, lack of the SbcCD endonuclease strongly decreased induction of ITE up to 60 min after irradiation, but the effect was almost lost 120 to 180 min after irradiation. An sbcCD xonA double mutant showed a further decrease of ITE, particularly 120 to 180 min after irradiation. The lowest UV-induced ITE values were obtained in the sbcCD recJ double mutant and the sbcCD recJ xonA triple mutant. In these strains, induced ITE after 2 h of incubation was about 5% of that observed in wild-type cells.
FIG. 4.
Effect of postincubation time after UV irradiation (135 J/m2) in broth at 30°C on the EOP of T4 2− on strains deficient for various DNases. The strains were wild type (AB1157; ■), xonA (WA818; •), ΔsbcCD (BT384; ▴), recJ (BT122; ▾), ΔsbcCD xonA (BT421; ⧫), ΔsbcCD recJ (BT386; ×), and ΔsbcCD xonA recJ (BT422; +). The data are means from three to four independent experiments. For clarity, standard deviations are given only for the 120-min incubation values.
Influence of recD+ overexpression.
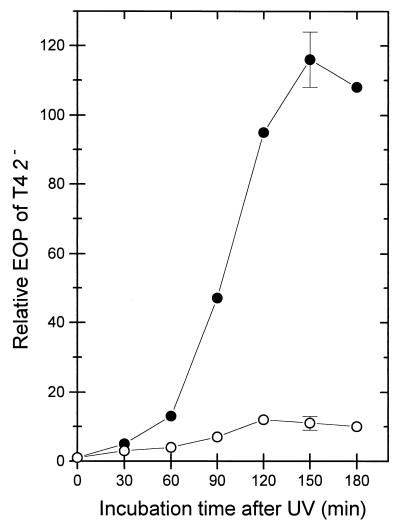
Two mechanisms have been proposed to explain how chromosomal DNA fragments could lower the RecBCD enzyme activity in cells: (i) the interaction of RecBCD with the DNA (i.e., binding followed by DNA degradation) may temporarily sequester the enzyme on the substrate DNA (7) or (ii) the duplex DNA-degrading activity is silenced irreversibly during DNA degradation at properly oriented Chi sites (17). Previously it was demonstrated that overexpression of recD+ partially alleviated the silencing (17). As shown in Fig. 5, derepression of recD+ located on an expression plasmid also measurably alleviated induction of ITE by UV.
FIG. 5.
Influence of recD+ overexpression from pSK1 (recD+) on the increase of the EOP of T4 2− in UV-irradiated (135 J/m2) cells. Strains were wild type with vector plasmid pJF118EH (BT305; •) and wild type with pSK1 (BT306; ○). The cells were grown with IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM). The data are means from three independent experiments. The standard deviations are given only for the 150-min point.
DISCUSSION
Irradiation with relatively high UV doses (producing thousands of pyrimidine dimers per chromosome) leads to the fragmentation of the E. coli chromosomal DNA in an excision repair-dependent process (4) (Fig. 3). Here we have shown that with this DNA fragmentation the EOP of T4 2− increased about 200-fold. This finding is reminiscent of the observation that the EOP of T4 2− increased strongly on E. coli cells treated with agents producing DNA double-strand breaks, such as gamma ray (7) or bleomycin (17). Brcic-Kostic et al. (7) concluded that the concentration of free RecBCD enzyme in the cytoplasm was depleted because the enzyme was loaded on the duplex DNA ends and that this helped infecting T4 2− genomes to survive. In the studies with gamma ray or bleomycin, ITE was seen immediately after treatment of cells. In contrast, in the UV-experiments reported here, ITE required a postirradiation incubation of about 1 to 2 h at 30°C. The time span appears to be required for the excision repair-dependent and possibly replication-dependent chromosome fragmentation following UV irradiation. The necessity of relatively high UV doses and the use of stationary-phase cells in earlier experiments may be the reasons why an effect of UV on the T4 2− survival was not detected in a previous study (7).
Several conditions besides overexpression of recB+ recC+ recD+ were identified which strongly suppressed induction of ITE. Two were related to the SOS system. These conditions were the prior induction of the SOS response by a small UV dose or the permanent SOS derepression by a lexA71::Tn5 mutation. Among several defective SOS genes screened for no longer causing suppression of ITE in a lexA71 background, only the ssb-1 allele coding for an SSB protein with a low DNA single-strand affinity turned out to partially alleviate the suppression. The ssb-113 allele coding for an SSB with normal DNA binding had no such effect. The third condition preventing ITE, in accord with the previous finding, was the overexpression of ssb+ from a plasmid. These observations pointed to a role of single-stranded DNA in the response of the RecBCD enzyme level to high UV doses. Since excess SSB did not interfere with the chromosome fragmentation following UV irradiation, we assumed that SSB associated with single-stranded tails present on the DNA fragments. In vitro covering of single strands with SSB makes the DNA refractory to the single-strand-specific endo- and exonucleolytic activities of the RecBCD enzyme (27). The lengths of the tails on the fragments in the UV-irradiated cells are not known. It is conceivable that excision repair gaps (12.4 nucleotides [50]) in close proximity on opposite strands could result in chromosome breakage leaving tails of well over 25 nucleotides. Tails of a few nucleotides covered with SSB and tails longer than 25 nucleotides with or without SSB all prevent loading of RecBCD on the DNA in vitro (27, 37, 45). Thus, if the DNA fragments in UV-irradiated cells have tails and SSB is overproduced, then the RecBCD enzyme could not bind to DNA and the level of free enzyme would remain high. This was observed. In accord with these considerations is the notion that at low UV doses in lexA3 cells, in which the SOS system is not inducible and therefore the level of SSB cannot increase, higher levels of induced ITE were observed than in the wild type (Fig. 1 and Table 3). If induction of the SOS response occurred in the wild type after application of ITE-inducing UV doses, it occurred apparently too late or was too weak (as a consequence of the high UV doses) to suppress ITE.
The conclusion that single-stranded tails are initially present on the DNA fragments produced in UV-irradiated cells and prevent direct loading of RecBCD is also supported by the finding that mutational elimination of single-strand-specific DNases is a further condition which counteracted induction of ITE (Fig. 4). Apparently, the activities of exonuclease I (specific for 3′ single strands [22]), RecJ DNase (specific for 5′ single strands [26]) and SbcCD DNase (single-stranded DNA endonuclease [10]) all contribute to high ITE by acting in the rapid removal of tails from duplex DNA fragments. Perhaps the long tails are particularly sensitive to shortening by endonucleolytic cutting, whereas the exonucleases act in the further trimming. Apparently, when sufficient time is provided, exonucleases can achieve extensive blunting of DNA ends in the ΔsbcCD mutant, leading to full induction of ITE (Fig. 4). Since overproduction of SSB apparently blocks tail removal, the responsible nucleases must be inhibited by SSB. It is not known whether SSB affects SbcCD and RecJ. In vitro exonuclease I is not inhibited, whereas protection of DNA by SSB against various other DNases has been described (29). In vivo, the ssb-1 mutation was shown to eliminate the protection of DNA against RecBCD and one or more other DNases that is conferred by wild-type SSB (23). The function of single-strand-specific DNases such as RecJ in recombination is probably not limited to the early step of producing entry sites for RecBCD but is also necessary for later steps and for RecBCD-independent recombination (18, 26).
What happens to RecBCD when it has gained access to a chromosomal duplex DNA fragment after removal of tails? We believe that the short time sequestration of RecBCD as a result of interacting with DNA is not the main cause of ITE. Rather the exposure of RecBCD during its tracking along DNA to properly oriented Chi sites will silence the exonucleolytic activity for duplex DNA as observed in vitro (14) and in vivo (11, 17, 21, 31). In vivo, the silencing appears to be irreversible since RecBCD activity (determined as ATP-dependent duplex DNA exonuclease) was no longer detectable in extracts made from bleomycin-treated cells (17). The silencing of RecBCD is partially alleviated by overexpression of recD+ (17, 31). Overexpression of recD+ also partially alleviated UV-induced ITE (Fig. 5), suggesting that the mechanism of RecBCD silencing in heavily UV-irradiated cells is the same as that in cells in which the chromosome is fragmented by gamma rays (7) or bleomycin (17) or in cells in which linear duplex plasmid DNA with Chi sites is presented (31). The detailed mechanism of RecBCD silencing is not yet known. The heavy UV irradiation in our experiments was necessary to obtain an excess of presumably tailed DNA ends and an almost synchronous silencing of a large part of RecBCD in the cell population in order to study factors that lead to silencing or prevent it. We believe that our conclusions drawn for the bulk of RecBCD in UV-irradiated cells are also true for single enzyme molecules interacting with DNA broken as a result of other impacts.
Recent in vitro experiments show that the 3′ single strand that is exposed at Chi by RecBCD is simultaneously recognized by RecA with high efficiency in an apparently coordinated reaction and transferred to a homologous duplex (2). This suggested a physical cooperation of RecA with RecBCD in Chi-specific recombination (2). It fits with the observation that in vivo the highest recombination frequencies and the most effective DNA repair were achieved by RecA and RecBCD components from the same species instead of interspecific combinations (12). The necessity of blunt ends for the loading of RecBCD on DNA would make single-strand-specific DNases important enzymes because their activity would direct tailed DNA ends at a double-strand break into the RecABCD recombination repair process. In this, RecA protein is specifically targeted to the “recombination island” sequences surrounding the Chi sites which strongly stimulate strand transfer (2, 49). Random single-stranded tails at DNA ends would not specifically expose such sequences. The overlapping activities of the various single-strand-specific DNases in processing tailed DNA into blunt ends would give mutants defective in one or two of the DNases, probably not a recombination- or repair-deficient phenotype. In contrast, absence of the single-strand-degrading activities exonuclease I and SbcCD DNase restores recombination and repair to recB or recC mutants (20, 25). In these mutants, stabilization, not removal, of single-stranded tails by the sbcB and sbcCD mutations, perhaps supported by increased production of SSB, appears to make recombination possible which is no longer focused to Chi sites.
ACKNOWLEDGMENTS
We thank Jourica Brandsma, Rudi Eichenlaub, David Leach, Bob Lloyd, and Ralph Meyer for providing bacterial strains and plasmids and Regina Rinken and Johann de Vries for help with some of the experiments.
This work was supported by the Fonds der Chemischen Industrie and the Volkswagen-Stiftung (Lower Saxony-Israel joint project).
REFERENCES
- 1.Amundsen S K, Neiman A M, Thibodeaux S M, Smith G R. Genetic dissection of the biochemical activities of RecBCD enzyme. Genetics. 1990;126:25–40. doi: 10.1093/genetics/126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D G, Kowalczykowski S C. The translocating RecBCD enzyme stimulates recombination by directing RecA protein onto ssDNA in a χ-regulated manner. Cell. 1997;90:77–86. doi: 10.1016/s0092-8674(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 3.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heynecker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 4.Bonura T, Smith K C. Enzymatic production of deoxyribonucleic acid double-strand breaks after ultraviolet irradiation of Escherichia coli K-12. J Bacteriol. 1975;121:511–517. doi: 10.1128/jb.121.2.511-517.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandsma J A, Bosch D, de Ruyter M, van de Putte P. Analysis of the regulatory region of the ssb gene of Escherichia coli. Nucleic Acids Res. 1985;13:5095–5109. doi: 10.1093/nar/13.14.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandsma J A, Stoorvogel J, van Sluis C A, van de Putte P. Effect of lexA and ssb genes, present on a uvrA recombinant plasmid, on the UV survival of Escherichia coli K-12. Gene. 1982;18:77–85. doi: 10.1016/0378-1119(82)90058-0. [DOI] [PubMed] [Google Scholar]
- 7.Brcic-Kostic K, Salaj-Smic E, Maršic N, Kajic S, Stojiljkovic I, Trgovcevic Z. Interaction of RecBCD enzyme with DNA damaged by gamma radiation. Mol Gen Genet. 1991;228:136–142. doi: 10.1007/BF00282458. [DOI] [PubMed] [Google Scholar]
- 8.Clark A J. Recombination-deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- 9.Clark A J. recA operator mutations and their usefulness. Biochimie. 1982;64:669–675. doi: 10.1016/s0300-9084(82)80108-9. [DOI] [PubMed] [Google Scholar]
- 10.Connelly J C, Leach D R F. The sbcC and sbcD genes of Escherichia coli encode a nuclease involved in palindrome inviability and genetic recombination. Genes Cells. 1996;1:285–291. doi: 10.1046/j.1365-2443.1996.23024.x. [DOI] [PubMed] [Google Scholar]
- 11.Dabert P, Ehrlich S D, Gruss A. χ sequence protects against RecBCD degradation of DNA in vivo. Proc Natl Acad Sci USA. 1992;89:12073–12077. doi: 10.1073/pnas.89.24.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries J, Wackernagel W. Recombination and UV resistance of Escherichia coli with the cloned recA and recBCD genes of Serratia marcescens and Proteus mirabilis: evidence for an advantage of intraspecies combination of P. mirabilis RecA and RecBCD enzyme. J Gen Microbiol. 1992;138:31–38. doi: 10.1099/00221287-138-1-31. [DOI] [PubMed] [Google Scholar]
- 13.Dixon D A, Churchill J J, Kowalczykowski S C. Reversible inactivation of the Escherichia coli RecBCD enzyme by the recombination hotspot χ in vitro: evidence for functional inactivation or loss of the RecD subunit. Proc Natl Acad Sci USA. 1994;91:2980–2984. doi: 10.1073/pnas.91.8.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon D A, Kowalczykowski S C. The recombination hotspot χ is a regulatory sequence that acts by attenuating the nuclease activity of the E. coli RecBCD enzyme. Cell. 1993;73:87–96. doi: 10.1016/0092-8674(93)90162-j. [DOI] [PubMed] [Google Scholar]
- 15.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 16.Howard-Flanders P, Theriot L. Mutants of E. coli defective in DNA repair and genetic recombination. Genetics. 1966;53:1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Köppen A, Krobitsch S, Thoms B, Wackernagel W. Interaction with the recombination hot spot χ in vivo converts the RecBCD enzyme of Escherichia coli into a χ-independent recombinase by inactivation of the RecD subunit. Proc Natl Acad Sci USA. 1995;92:6249–6253. doi: 10.1073/pnas.92.14.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Biochemistry of homologous recombination in Escherichia coli. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger J H, Elledge S J, Walker G C. Isolation and characterization of Tn5 insertion mutations in the lexA gene of Escherichia coli. J Bacteriol. 1983;153:1368–1378. doi: 10.1128/jb.153.3.1368-1378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushner S R, Nagaishi H, Clark A J. Indirect suppression of recB and recC mutations by exonuclease I deficiency. Proc Natl Acad Sci USA. 1972;69:1366–1370. doi: 10.1073/pnas.69.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuzminov A, Schabtach E, Stahl F W. χ sites in combination with RecA protein increase the survival of linear DNA in Escherichia coli by inactivating exoV activity of RecBCD nuclease. EMBO J. 1994;13:2764–2776. doi: 10.1002/j.1460-2075.1994.tb06570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehman I R, Nussbaum R. The deoxyribonuclease of Escherichia coli. V. On the specificity of exonuclease I (phosphodiesterase) J Biol Chem. 1964;239:2628–2636. [PubMed] [Google Scholar]
- 23.Lieberman H B, Witkin E M. DNA degradation, UV sensitivity and SOS-mediated mutagenesis in strains of Escherichia coli deficient in single-strand DNA binding protein: effects of mutations and treatments that alter levels of exonuclease V or RecA protein. Mol Gen Genet. 1983;190:92–100. doi: 10.1007/BF00330329. [DOI] [PubMed] [Google Scholar]
- 24.Linhardt F, Ziebuhr W, Meyer P, Witte W, Hacker J. Pulsed-field gel electrophoresis of genomic restriction fragments as a tool for the epidemiological analysis of Staphylococcus aureus and coagulase-negative staphylococci. FEMS Microbiol Lett. 1992;95:181–186. doi: 10.1016/0378-1097(92)90426-o. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd R G, Buckman C. Identification and genetic analysis of sbcC mutations in commonly used recBC sbcB strains of Escherichia coli K-12. J Bacteriol. 1985;164:836–844. doi: 10.1128/jb.164.2.836-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovett S T, Kolodner R D. Identification and purification of a single-stranded DNA-specific exonuclease encoded by the recJ gene of Escherichia coli. Proc Natl Acad Sci USA. 1989;86:2627–2631. doi: 10.1073/pnas.86.8.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKay V, Linn S. Selective inhibition of the DNase activity of the recBC enzyme by the DNA binding protein from Escherichia coli. J Biol Chem. 1976;251:3716–3719. [PubMed] [Google Scholar]
- 28.Meyer R R, Laine P S. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990;54:342–380. doi: 10.1128/mr.54.4.342-380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molineux I J, Gefter M L. Properties of the Escherichia coli DNA-binding (unwinding) protein interaction with nucleolytic enzymes and DNA. J Mol Biol. 1975;98:811–825. doi: 10.1016/s0022-2836(75)80012-x. [DOI] [PubMed] [Google Scholar]
- 30.Mount D W, Low K B, Edmiston S J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet light-induced mutations. J Bacteriol. 1972;112:886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers R S, Kuzminov A, Stahl F W. The recombination hot spot χ activates RecBCD recombination by converting Escherichia coli to a recD mutant phenocopy. Proc Natl Acad Sci USA. 1995;92:6244–6248. doi: 10.1073/pnas.92.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers R S, Stahl F W. χ and the RecBCD enzyme of Escherichia coli. Annu Rev Genet. 1994;28:49–70. doi: 10.1146/annurev.ge.28.120194.000405. [DOI] [PubMed] [Google Scholar]
- 33.Oishi M, Cosloy S D. The genetic and biochemical basis of the transformability of Escherichia coli K-12. Biochem Biophys Res Commun. 1972;49:1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- 34.Oliver B D, Goldberg E B. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J Mol Biol. 1977;116:877–881. doi: 10.1016/0022-2836(77)90276-5. [DOI] [PubMed] [Google Scholar]
- 35.Perrino F W, Rein D C, Bobst A M, Meyer R R. The relative rate of synthesis and levels of single-stranded DNA binding protein during induction of SOS repair in Escherichia coli. Mol Gen Genet. 1987;209:612–614. doi: 10.1007/BF00331171. [DOI] [PubMed] [Google Scholar]
- 36.Phillips G J, Prasher D C, Kushner S R. Physical and biochemical characterization of cloned sbcB and xonA mutations from Escherichia coli K-12. J Bacteriol. 1988;170:2089–2094. doi: 10.1128/jb.170.5.2089-2094.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prell A, Wackernagel W. Degradation of linear and circular DNA with gaps by the recBC enzyme of Escherichia coli. Eur J Biochem. 1980;105:109–116. doi: 10.1111/j.1432-1033.1980.tb04480.x. [DOI] [PubMed] [Google Scholar]
- 38.Rinken R, de Vries J, Weichenhan D, Wackernagel W. The recA-recBCD dependent recombination pathways of Serratia marcescens and Proteus mirabilis in Escherichia coli: functions of hybrid enzymes and hybrid pathways. Biochimie. 1991;73:375–384. doi: 10.1016/0300-9084(91)90104-9. [DOI] [PubMed] [Google Scholar]
- 39.Rinken R, Thoms B, Wackernagel W. Evidence that recBC-dependent degradation of duplex DNA in Escherichia coli recD mutants involves DNA unwinding. J Bacteriol. 1992;174:5424–5429. doi: 10.1128/jb.174.16.5424-5429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romanowski G, Weichenhan D, Gram M, Wackernagel W. Effect of recBCD enzyme overproduction in Escherichia coli on recombination, repair of UV-damage and propagation of phages λ, T7 and T4. Mol Gen (Life Sci Adv) 1987;6:71–74. [Google Scholar]
- 41.Sargentini N J, Smith K C. Quantitation of the involvement of the recA, recB, recC, recF, recJ, recN, lexA, radA, radB, uvrD, and umuC genes in the repair of X-ray-induced DNA double-strand breaks in Escherichia coli. Radiat Res. 1986;107:58–72. [PubMed] [Google Scholar]
- 42.Sedgwick S G. Genetic and kinetic evidence for different types of postreplication repair in Escherichia coli B. J Bacteriol. 1975;123:154–161. doi: 10.1128/jb.123.1.154-161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simmon V F, Lederberg S. Degradation of bacteriophage lambda deoxyribonucleic acid after restriction by Escherichia coli K-12. J Bacteriol. 1972;112:161–169. doi: 10.1128/jb.112.1.161-169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith G R. Homologous recombination in procaryotes. Microbiol Rev. 1988;52:1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor A F, Smith G R. Substrate specificity of the DNA unwinding activity of the RecBC enzyme of Escherichia coli. J Mol Biol. 1985;185:431–443. doi: 10.1016/0022-2836(85)90414-0. [DOI] [PubMed] [Google Scholar]
- 46.Thaler D S, Sampson E, Siddiqi I, Rosenberg S M, Stahl F W, Stahl M. A hypothesis: Chi-activation of RecBCD enzyme involves removal of the RecD subunit. In: Friedberg E, Hanawalt P, editors. Mechanisms and consequences of DNA damage processing. New York, N.Y: Liss; 1988. pp. 413–422. [Google Scholar]
- 47.Thoms B, Wackernagel W. UV-induced alleviation of λ restriction in Escherichia coli K-12: kinetics of induction and specificity of this SOS function. Mol Gen Genet. 1982;186:111–117. doi: 10.1007/BF00422921. [DOI] [PubMed] [Google Scholar]
- 48.Thoms B, Wackernagel W. Interaction of the RecBCD enzyme with DNA excision repair intermediates (double strand breaks) in UV-irradiated Escherichia coli. BioEngineering. 1993;9:100. doi: 10.1128/jb.180.21.5639-5645.1998. . (Abstract P4179.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tracy R B, Chédin F, Kowalczykowski S C. The recombination hot spot Chi is embedded within islands of preferred DNA pairing sequences in the E. coli genome. Cell. 1997;90:205–206. doi: 10.1016/s0092-8674(00)80328-1. [DOI] [PubMed] [Google Scholar]
- 50.van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990;54:18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wackernagel W. An improved spheroplast assay for λ-DNA and the influence of the bacterial genotype on the transfection rate. Virology. 1972;48:94–103. doi: 10.1016/0042-6822(72)90117-1. [DOI] [PubMed] [Google Scholar]
- 52.Wackernagel W. Genetic transformation in E. coli: the inhibitory role of the recBC DNase. Biochem Biophys Res Commun. 1973;51:306–311. doi: 10.1016/0006-291x(73)91257-6. [DOI] [PubMed] [Google Scholar]
- 53.Walker G C. Mutagenesis and inducible response to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984;48:60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whittier R F, Chase J W. DNA repair properties of Escherichia coli tif-1, recAo281 and lexA1 strains deficient in single-strand DNA binding protein. Mol Gen Genet. 1983;190:101–111. doi: 10.1007/BF00330330. [DOI] [PubMed] [Google Scholar]
- 55.Williams K R, Murphy J B, Chase J W. Characterization of the structural and functional defect in the Escherichia coli single-stranded DNA binding protein encoded by the ssb-1 mutant gene. Expression of the ssb-1 gene under λPL regulation. J Biol Chem. 1984;259:11804–11811. [PubMed] [Google Scholar]
- 56.Witkin E M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976;40:869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda S, Takagi T. Overproduction of Escherichia coli replication proteins by the use of runaway-replication plasmids. J Bacteriol. 1983;154:1153–1161. doi: 10.1128/jb.154.3.1153-1161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]