Abstract
Prospective evidence regarding the combination of programmed cell death (PD)−1 and angiogenesis inhibitors in treating locally advanced gastric cancer (LAGC) is limited. In this multicenter, randomized, phase 2 trial (NCT04195828), patients with gastric adenocarcinoma (clinical T2-4N + M0) were randomly assigned (1:1) to receive neoadjuvant camrelizumab and apatinib combined with nab-paclitaxel plus S-1 (CA-SAP) or chemotherapy SAP alone (SAP) for 3 cycles. The primary endpoint was the major pathological response (MPR), defined as <10% residual tumor cells in resection specimens. Secondary endpoints included R0 resection rate, radiologic response, safety, overall survival, and progression-free survival. The modified intention-to-treat population was analyzed (CA-SAP [n = 51] versus SAP [n = 53]). The trial has met pre-specified endpoints. CA-SAP was associated with a significantly higher MPR rate (33.3%) than SAP (17.0%, P = 0.044). The CA-SAP group had a significantly higher objective response rate (66.0% versus 43.4%, P = 0.017) and R0 resection rate (94.1% versus 81.1%, P = 0.042) than the SAP group. Nonsurgical grade 3-4 adverse events were observed in 17 patients (33.3%) in the CA-SAP group and 14 (26.4%) in the SAP group. Survival results were not reported due to immature data. Camrelizumab and apatinib combined with chemotherapy as a neoadjuvant regimen was tolerable and associated with favorable responses for LAGC.
Subject terms: Gastric cancer, Cancer immunotherapy, Surgical oncology
Neoadjuvant treatment represents a therapeutic option for locally advanced gastric cancer (LAGC). Here the authors report the results of a randomized phase 2 trial of camrelizumab (anti-PD1) and apatinib (anti-VEGFR2) combined with nab-paclitaxel plus S-1 versus chemotherapy alone as neoadjuvant treatment for LAGC.
Introduction
Gastric cancer (GC) is the fifth most frequently diagnosed malignancy and the fourth leading cause of cancer death worldwide, with the highest incidence and mortality rates reported in Eastern Asia1. Surgical resection is the mainstay of treatment for resectable GC; however, over 30% of patients with locally advanced gastric cancer (LAGC) relapse even after complete resection and adjuvant therapies2,3. Neoadjuvant treatment was introduced and has been widely applied to improve the survival profiles of LAGC patients in the past 20 years4,5. To date, the exploration of the most effective neoadjuvant regimens continues.
A programmed cell death protein 1 (PD-1) inhibitor, which suppresses the interaction between PD-1 and its ligands (programmed cell death protein‒ligand 1 [PD-L1] or PD-L2), has demonstrated encouraging antitumor activity in advanced GC. Based on the results of phase 3 trials, a combination of the PD-1 inhibitor and chemotherapy exhibited extended clinical benefits6,7 in comparison with PD-1 inhibitor monotherapy8,9. Moreover, neoadjuvant administration of PD-1 inhibitors with or without chemotherapy has been explored in two small, nonrandomized trials, with pathological complete response (pCR) rates of 19.4%10 and 3.3%11, respectively. These results suggest that PD-1 inhibitors should be used in combination with other systemic agents to strengthen their effectiveness.
Apatinib, an oral receptor tyrosine kinase inhibitor that selectively targets vascular endothelial growth factor (VEGF) receptor 2, has shown clinically significant efficacy in advanced or metastatic GC12. Our earlier phase 2 study revealed that apatinib combined with chemotherapy was effective and tolerable as a neoadjuvant treatment for LAGC13. Moreover, apatinib plus camrelizumab (a high-affinity humanized IgG4 monoclonal antibody targeting PD-1) has shown promising benefits in various malignancies14,15. We therefore hypothesized that apatinib and camrelizumab combined with chemotherapy might be beneficial in patients with LAGC.
Currently, paclitaxel-based chemotherapy has proven efficacy in LAGC16 and was recommended as the first-line treatment17. Nanoparticle albumin-bound (nab)-paclitaxel, a 130 nm particle formulation consisting of paclitaxel and albumin nanoparticles linked by a noncovalent bond, improves the efficacy and safety of paclitaxel18. In this trial, we prespecified the regimen with nab-paclitaxel plus S-1 (SAP) as a control for two reasons: one was that a higher major pathological regression (MPR) rate and a low incidence of thrombocytopenia with SAP than with oxaliplatin plus S-1 (SOX) were observed in clinical practice19, and the other was that nab-paclitaxel exhibited synergistic effects on both angiogenesis inhibitors and PD-1 inhibitors20,21.
Here we reported the results of Arise-FJ-G005, a phase 2, multicenter, randomized controlled trial, that investigate the efficacy and safety of camrelizumab and apatinib combined with nab-paclitaxel plus S-1 versus nab-paclitaxel plus S-1 alone as neoadjuvant treatment for LAGC.
Results
Patients
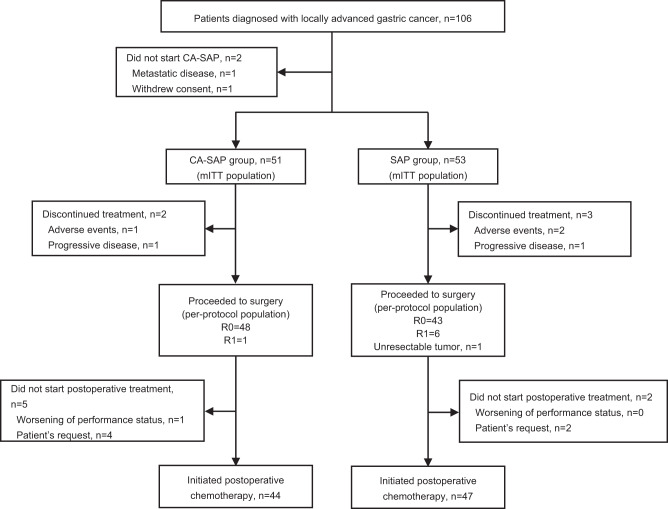
Between June 18, 2020, and March 31, 2022, 106 patients were enrolled and underwent randomization at 5 centers. After excluding 2 patients who withdrew their consent after random assignment, 51 and 53 patients were treated with CA-SAP and SAP, respectively. The modified intention-to-treat (mITT) population consisted of these 104 patients. Two patients in the CA-SAP group and 3 patients in the SAP group did not receive surgery, and the remaining 99 patients comprised the per-protocol population. The flow diagram is provided in Fig. 1.
Fig. 1. Trial profile.
After excluding 2 patients who withdrew their consent after random assignment, 51 and 53 patients were treated with CA-SAP and SAP, respectively, and included in the mITT analysis. CA-SAP camrelizumab, apatinib, nab-paclitaxel, and S-1, SAP nab-paclitaxel and S-1, mITT modified intention-to-treat.
The median age of all patients was 63 years (first quartile-third quartile [Q1–Q3]: 57–68 years); 77 of 104 (74.0%) were men. Most of the patients had diffuse-type tumors (n = 81, 77.9%) and had cT4N+ disease (n = 91, 87.5%). Baseline characteristics are detailed in Table 1.
Table 1.
Baseline characteristics of the modified intention-to-treat population
| Variable | CA-SAP group (n = 51) | SAP group (n = 53) |
|---|---|---|
| Age, years | 63 (57–68) | 63 (56–68) |
| Sex | ||
| Male | 42 (82.4) | 35 (66.0) |
| Female | 9 (17.6) | 18 (34.0) |
| ECOG performance status | ||
| 0 | 33 (64.7) | 36 (67.9) |
| 1 | 18 (35.3) | 17 (32.1) |
| Lauren classification | ||
| Intestinal | 11 (21.6) | 10 (18.9) |
| Diffuse | 39 (76.5) | 42 (79.2) |
| Unknown | 1 (2.0) | 1 (1.9) |
| Tumor location | ||
| Upper 1/3 | 22 (43.1) | 29 (54.7) |
| Middle 1/3 | 10 (19.6) | 6 (11.3) |
| Lower 1/3 | 11 (21.6) | 11 (20.8) |
| Mixed | 8 (15.7) | 7 (13.2) |
| Tumor size, mm | 65 (45–80) | 60 (50–75) |
| Borrmann type | ||
| II-III | 43 (84.3) | 48 (90.6) |
| IV | 8 (15.7) | 5 (9.4) |
| cT stage | ||
| T3 | 5 (9.8) | 8 (15.1) |
| T4 | 46 (90.2) | 45 (84.9) |
| PD-L1 expression (CPS) | ||
| <1 | 23 (45.1) | 23 (43.4) |
| ≥1 | 27 (52.9) | 28 (52.8) |
| Unknown | 1 (2.0) | 2 (3.8) |
| MSI status | ||
| MSS | 47 (92.2) | 48 (90.6) |
| MSI-High | 3 (5.9) | 3 (5.7) |
| Unknown | 1 (2.0) | 2 (3.8) |
Data are No. (%) or median (first quartile-third quartile [Q1–Q3]). Because of rounding, not all percentages add up to 100%.
CA-SAP camrelizumab, apatinib, nab-paclitaxel, and S-1, SAP nab-paclitaxel and S-1, ECOG Eastern Cooperative Oncology Group, PD-L1 programmed death-ligand 1, CPS combined positive score, MSI microsatellite instability, MSS microsatellite stable.
Neoadjuvant and adjuvant treatments
Overall, 47 of 51 patients (92.2%) in the CA-SAP group and 48 of 53 patients (90.6%) in the SAP group completed the planned 3 cycles of neoadjuvant treatment; 4 patients in the CA-SAP group and 4 patients in the SAP group completed 2 cycles, and 1 patient in the SAP group completed 1 cycle. Four patients in the CA-SAP group discontinued preoperative treatment, of whom 3 experienced intolerable adverse events (AEs) and 1 had PD; 5 patients in the SAP group discontinued preoperative treatment, of whom 2 experienced intolerable AEs, 2 had PD, and 1 refused to continue the treatment.
Of 98 patients who underwent gastrectomy, 44 of 49 patients (89.8%) in the CA-SAP group and 47 of 49 patients (95.9%) in the SAP group received adjuvant treatment. Reasons for not starting adjuvant treatment in the CA-SAP group were poor performance status (n = 1) and patient request (n = 4). The median time to adjuvant treatment from surgery was 36 days (Q1–Q3: 30–43 days) in the CA-SAP group and 35 days (Q1–Q3: 28–42 days) in the SAP group (P = 0.338). At the last follow-up (August 31, 2022), 22 of 44 patients (50.0%) in the CA-SAP group completed all 5 cycles of adjuvant treatment, 11 (25.0%) were still on treatment, and 11 (25.0%) discontinued the treatment; 22 of 47 patients (46.8%) in the SAP group completed all 5 cycles of adjuvant treatment, 12 (25.5%) were still on treatment, and 13 (27.7%) discontinued the treatment. Reasons for not completing adjuvant treatment in the CA-SAP group were AEs (n = 6), PD (n = 1), and patient request (n = 4). In the SAP group, the reasons were AEs (n = 5), PD (n = 1), and patient request (n = 7).
Surgery
Forty-nine of 51 patients (96.1%) in the CA-SAP group and 50 of 53 patients (94.3%) in the SAP group underwent surgery, including 98 gastrectomies and 1 exploratory laparoscopy (SAP group). The median time between the last cycle of neoadjuvant treatment and surgery was 15 days (Q1–Q3: 14–21 days) in the CA-SAP group and 14 days (Q1–Q3: 14–17 days) in the SAP group (P = 0.100). The surgical characteristics and pathological findings of the patients who underwent gastrectomy are shown in Table 2. Of note, one patient in the CA-SAP group underwent palliative proximal gastrectomy due to acute bleeding.
Table 2.
Surgical and pathology findings
| Variable | CA-SAP group (n = 49) | SAP group (n = 49) |
|---|---|---|
| Surgical technology | ||
| Open | 1 (2.0) | 0 (0.0) |
| Laparoscopic | 45 (91.8) | 48 (98.0) |
| Robotic | 3 (6.1) | 1 (2.0) |
| Type of gastrectomy | ||
| Total | 39 (79.6) | 44 (89.8) |
| Distal | 9 (18.4) | 5 (10.2) |
| Proximal | 1 (2.0) | 0 (0.0) |
| Blood loss, mL | 35 (30–50) | 30 (30–50) |
| No. of lymph node metastasis | 1 (0–7) | 1 (0–7) |
| No. of lymph node harvested | 40 (29–55) | 40 (34–54) |
| Lymphovascular invasion | ||
| No | 25 (51.0) | 28 (57.1) |
| Yes | 24 (49.0) | 21 (42.9) |
| Neural invasion | ||
| No | 21 (42.9) | 19 (38.8) |
| Yes | 28 (57.1) | 30 (61.2) |
| ypT stage | ||
| T0 | 8 (16.3) | 3 (6.1) |
| T1 | 4 (8.2) | 5 (10.2) |
| T2 | 5 (10.2) | 5 (10.2) |
| T3 | 23 (46.9) | 23 (46.9) |
| T4a | 9 (18.4) | 13 (26.5) |
| ypN stage | ||
| N0 | 18 (36.7) | 21 (42.9) |
| N1 | 12 (24.5) | 8 (16.3) |
| N2 | 7 (14.3) | 7 (14.3) |
| N3 | 12 (24.5) | 13 (26.5) |
| ypM stage | ||
| M0 | 49 (100.0) | 47 (95.9) |
| M1 | 0 (0.0) | 2 (4.1) |
| Pathological response | ||
| TRG 1a | 8 (16.3) | 3 (6.1) |
| TRG 1b | 9 (18.4) | 6 (12.2) |
| TRG 2 | 10 (20.4) | 21 (42.9) |
| TRG 3 | 22 (44.9) | 19 (38.8) |
Data are No. (%) or median (first quartile-third quartile [Q1–Q3]). Because of rounding, not all percentages add up to 100%.
CA-SAP camrelizumab, apatinib, nab-paclitaxel, and S-1, SAP nab-paclitaxel and S-1, TRG tumor regression grade.
Efficacy
The results for tumor response are shown in Table 3. In the mITT population, a significantly higher proportion of patients achieved MPR (Tumor regression grade [TRG] 1a/b) in the CA-SAP group (n = 17, 33.3%; 95% CI: 19.9%–46.7%) than in the SAP group (n = 9, 17.0%; 95% CI: 6.5%–27.4%; P = 0.044, FDR-adjusted P = 0.080; Fig. 2a). Eight of 51 patients (15.7%; 95% CI: 5.4%–26.0%) in the CA-SAP group and 3 of 53 patients (5.7%; 95% CI: 0.0%–12.1%) in the SAP group achieved pCR (TRG 1a; P = 0.089, FDR-adjusted P = 0.118). In the per-protocol population, the MPR rate was also higher with CA-SAP (34.7%; 95% CI: 20.9%–48.5%) than with SAP (18.0%; 95% CI: 7.0%–29.0%; P = 0.048, FDR-adjusted P = 0.080). The pCR rates were 16.3% (95% CI: 5.6%–27.1%) and 6.0% (95% CI: 0%–12.8%), respectively, in the CA-SAP and SAP groups (P = 0.094, FDR-adjusted P = 0.118).
Table 3.
Efficacy analysis in the modified intention-to-treat population
| Variable | CA-SAP group (n = 51) | SAP group (n = 53) | P value | FDR-adjusted P value |
|---|---|---|---|---|
| Pathological response | ||||
| TRG 0 (Complete) | 8 (15.7) | 3 (5.7) | ||
| TRG 1 (Subtotal) | 9 (17.6) | 6 (11.3) | ||
| TRG 2 (Partial) | 10 (19.6) | 21 (39.6) | ||
| TRG 3 (Minimal or none) | 22 (43.1) | 19 (35.8) | ||
| No gastrectomy | 2 (3.9) | 4 (7.5) | ||
| Major pathological response rate (%, 95% CI) | 33.3 (19.9–46.7) | 17.0 (6.5–27.4) | 0.044 | 0.080 |
| Complete response rate (%, 95% CI) | 15.7 (5.4–26.0) | 5.7 (0–12.1) | 0.089 | 0.118 |
| Radiologic response | ||||
| CR | 3 (5.9) | 0 (0.0) | ||
| PR | 30 (58.8) | 23 (43.4) | ||
| SD | 16 (31.4) | 28 (52.8) | ||
| PD | 1 (2.0) | 2 (3.7) | ||
| Unidentified | 1 (2.0) | 0 (0.0) | ||
| Objective response rate (%, 95% CI) | 66.0 (52.4–79.6) | 43.4 (29.2–57.6) | 0.017 | 0.080 |
| Disease control rate (%, 95% CI) | 96.1 (90.6–100) | 96.2 (90.0–100) | 0.677 | 0.677 |
| Tumor downstaging | ||||
| cT stage | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
| T1 | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| T2 | 0 (0.0) | 10 (19.6) | 0 (0.0) | 5 (9.4) |
| T3 | 5 (9.8) | 17 (33.3) | 8 (15.1) | 19 (35.8) |
| T4 | 46 (90.2) | 22 (43.1) | 45 (84.9) | 29 (54.7) |
| Unidentified | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| T downstaging (%) | 27 (52.9) | 17 (32.1) | 0.025 | 0.080 |
| cN stage | Pre-treatment | Post-treatment | Pre-treatment | Post-treatment |
| N0 | 0 (0.0) | 13 (25.5) | 0 (0.0) | 9 (17.0) |
| N+ | 51 (100.0) | 36 (70.6) | 53 (100.0) | 44 (83.0) |
| Unidentified | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| N downstaging (%) | 13 (25.5) | 9 (17.0) | 0.206 | 0.229 |
| Surgical Fingdings | ||||
| R0 resection rate (%, 95% CI) | 94.1 (87.4–100) | 81.1 (70.2–92.0) | 0.042 | 0.080 |
Data are No. (%). Because of rounding, not all percentages add up to 100%. P values were one-sided for efficacy analyses in Fisher’s exact test and adjusted by controlling for the false discovery rate (FDR) using the Benjamini–Hochberg procedure.
CA-SAP camrelizumab, apatinib, nab-paclitaxel, and S-1, SAP nab-paclitaxel and S-1, TRG tumor regression grade, CI confidence interval, CR complete response, PR partial response, SD stable disease, PD progressive disease.
Fig. 2. Treatment response.
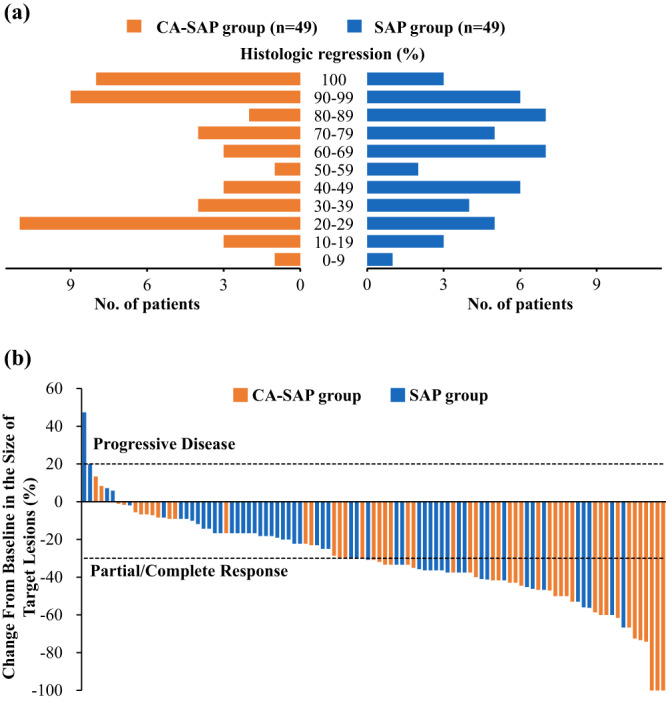
Results of pathological regression (a) and radiologic response assessed using the Response Evaluation Criteria in Solid Tumours (version 1.1) (b) in patients eligible for assessments (n = 98 and 103, respectively). CA-SAP camrelizumab, apatinib, nab-paclitaxel, and S-1, SAP nab-paclitaxel and S-1. Source data are provided as a Source data file.
One hundred and three patients had evaluable radiologic results (Table 3), and one patient treated with CA-SAP did not receive radiologic assessment after neoadjuvant treatment. In the mITT population, an objective response was achieved in 33 of 51 patients (66.0%; 95% CI: 52.4%–79.6%) in the CA-SAP group and 23 of 53 patients (43.4%; 95% CI: 29.6%–57.2%) in the SAP group (P = 0.017, FDR-adjusted P = 0.080; Fig. 2b). The disease control rate (DCR) rate was 96.1% in the CA-SAP group and 96.2% in the SAP group (P = 0.677). In a comparison between the pretreatment and posttreatment clinical staging, T downstaging occurred in 52.9% of patients (n = 27) with CA-SAP and 32.1% of patients (n = 17) with SAP (P = 0.025, FDR-adjusted P = 0.080). N downstaging occurred in similar proportions of patients in both groups (25.5% versus 17.0%; P = 0.206, FDR-adjusted P = 0.229).
R0 resection was achieved in 48 of 51 patients (94.1%; 95% CI: 87.4%–100%) in the CA-SAP group and 43 of 53 patients (81.1%; 95% CI: 70.2%–92.0%) in the SAP group (P = 0.042, FDR-adjusted P = 0.080; Table 3). In the per-protocol population, the R0 resection rate was also significantly higher in the CA-SAP group (98.0%; 95% CI: 93.9%–100%) than in the SAP group (86.0%; 95% CI: 76.0%–96.0%; P = 0.032, FDR-adjusted P = 0.080).
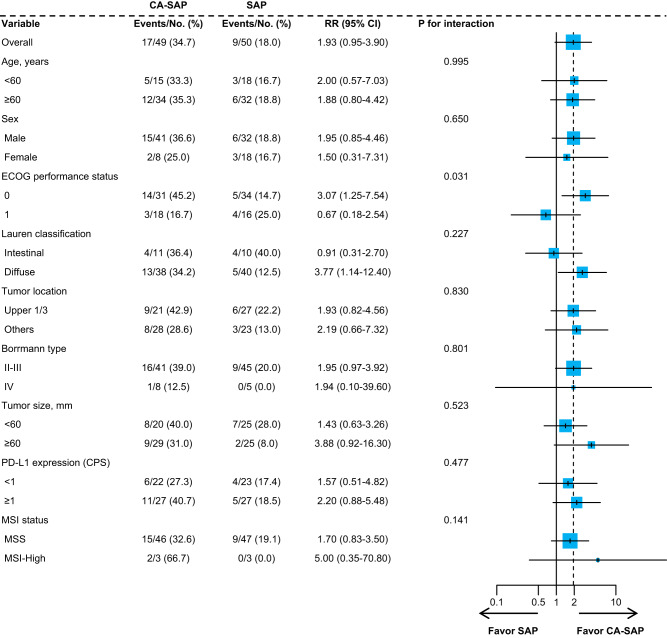
Subgroup analysis
We prespecified a set of subgroup analyses for the primary endpoint according to baseline characteristics in the per-protocol population (Fig. 3). Patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 had significantly higher MPR rates with CA-SAP than with SAP (45.2% versus 14.7%; P = 0.007; P for interaction = 0.031). For intestinal-type tumors, the MPR rates were 36.4% and 40.0% in the CA-SAP and SAP groups, respectively (P = 0.608). For diffuse-type tumors, the CA-SAP group showed a significantly higher MPR rate than the SAP group (34.2% vs. 12.5%, P = 0.022). However, this interaction did not reach statistical significance (P for interaction = 0.227).
Fig. 3. Subgroup analysis of major pathological response in per-protocol population (CA-SAP [n = 49] vs. SAP [n = 50]).
Forest plots show the risk ratios (RRs) as centers, the upper and lower hinges represent the corresponding 95% confidence intervals (CIs). Interaction between agents was evaluated by likelihood ratio test, and P values were two sided at the 5% significance level. No adjustments were made for multiple comparisons. CA-SAP camrelizumab, apatinib, nab-paclitaxel, and S-1, SAP nab-paclitaxel and S-1, ECOG Eastern Cooperative Oncology Group, PD-L1 programmed death-ligand 1, CPS combined positive score, MSI microsatellite instability, MSS microsatellite stable. Source data are provided as a Source Data file.
We explored the associations of MPR with PD-L1 expression and microsatellite instability (MSI) status. In the combined positive score (CPS) < 1% subgroup (n = 45), the MPR rates were 27.3% and 17.4% in the CA-SAP and SAP groups, respectively (P = 0.331). Among the 54 patients with a CPS ≥ 1%, the MPR rates were 40.7% and 18.5%, respectively, in the CA-SAP and SAP groups (P = 0.068). Among the 27 patients with a CPS ≥ 5%, the MPR rates were 50.0% and 27.3%, respectively, in the CA-SAP and SAP groups (P = 0.107; Supplementary Table 1). Compared with the SAP group, the CA-SAP group showed a trend toward a higher MPR rate in patients with MSI-H (66.7% [2 of 3 patients with CA-SAP] versus 0.0% [0 of 3 patients with SAP]) than in those with microsatellite stability (MSS; 32.6% [15 of 46 patients with CA-SAP] versus 19.1% [9 of 47 patients with SAP]).
Safety
During the neoadjuvant treatment periods, the most common nonsurgical AEs was leukopenia (CA-SAP: 72.5%; SAP: 71.7%) in both groups (Table 4). Seventeen of 51 patients (33.3%) in the CA-SAP group and 14 of 53 patients (26.4%) in the SAP group experienced at least one grade 3-4 AE (P = 0.441). The most common grade 3-4 AEs were leukopenia (n = 7, 13.7%), neutropenia (n = 5, 9.8%), and alanine transaminase (ALT) elevation (n = 5, 9.8%) in the CA-SAP group and leukopenia (n = 4, 7.5%), neutropenia (n = 3, 5.7%), ALT elevation (n = 3, 5.7%), and aspartate aminotransferase (AST) elevation (n = 3, 5.7%) in the SAP group. Immune-related adverse events occurred in 10 patients (19.6%) in the CA-SAP group and in 1 patient (1.9%) in the SAP group, of which the most common event was hypothyroidism (Supplementary Table 2). All immune-related adverse events were grade 1 or 2.
Table 4.
Summary of non-surgical adverse events
| Adverse events | CA-SAP group (n = 51) | SAP group (n = 53) | ||
|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Hematologic | ||||
| Neutropenia | 21 (41.2) | 5 (9.8) | 27 (50.9) | 3 (5.7) |
| Leukopenia | 30 (58.9) | 7 (13.7) | 35 (66.0) | 4 (7.5) |
| Thrombocytopenia | 7 (13.7) | 1 (2.0) | 0 (0.0) | 2 (3.8) |
| Anemia | 15 (29.4) | 1 (2.0) | 21 (39.6) | 0 (0.0) |
| Gastrointestinal | ||||
| Nausea | 19 (37.3) | 0 (0.0) | 23 (43.4) | 0 (0.0) |
| Vomiting | 11 (21.6) | 0 (0.0) | 13 (24.5) | 0 (0.0) |
| Anorexia | 10 (19.6) | 0 (0.0) | 11 (20.8) | 1 (1.9) |
| Diarrhea | 14 (27.5) | 2 (3.9) | 8 (15.1) | 2 (3.8) |
| Bleeding | 8 (15.7) | 1 (2.0) | 8 (15.1) | 1 (1.9) |
| Liver | ||||
| AST elevation | 11 (21.6) | 3 (5.9) | 8 (15.1) | 3 (5.7) |
| ALT elevation | 9 (17.6) | 5 (9.8) | 10 (18.9) | 3 (5.7) |
| Bilirubin increased | 8 (15.7) | 0 (0.0) | 8 (15.1) | 0 (0.0) |
| Cardio-renal | ||||
| Hypertension | 10 (19.6) | 0 (0.0) | 2 (3.8) | 0 (0.0) |
| Proteinuria | 8 (15.7) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Respiratory | ||||
| Immune pneumonitis | 2 (3.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dermatologic | ||||
| Rash | 9 (17.6) | 0 (0.0) | 5 (9.4) | 0 (0.0) |
| Hand–foot syndrome | 8 (15.7) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Systemic | ||||
| Fatigue | 15 (29.4) | 1 (2.0) | 19 (35.8) | 1 (1.9) |
| Fever | 10 (19.6) | 0 (0.0) | 5 (9.4) | 0 (0.0) |
| Others | ||||
| Peripheral sensory neuropathy | 6 (11.8) | 0 (0.0) | 7 (13.2) | 1 (1.9) |
| Hypothyroidism | 7 (13.7) | 0 (0.0) | 1 (1.9) | 0 (0.0) |
| Stomatitis | 9 (17.6) | 1 (2.0) | 4 (7.5) | 0 (0.0) |
Data are No. (%).
CA-SAP camrelizumab, apatinib, nab-paclitaxel, and S-1, SAP nab-paclitaxel and S-1.
Of 98 patients who underwent gastrectomy, postoperative recovery (all P > 0.05) and morbidity (20.4% [10 of 49 patients with CA-SAP] versus 12.2% [6 of 49 patients with SAP]; P = 0.295) were similar between the two groups (Supplementary Table 3). Most of the complications were categorized as Clavien‒Dindo grade II. No reoperation or mortality within 30 days was observed.
Discussion
The Arise-FJ-G005 study is a multicenter, randomized controlled trial evaluating the efficacy and safety of neoadjuvant anti–PD-1 immunotherapy and antiangiogenic therapy combined with chemotherapy versus chemotherapy alone in patients with LAGC. The study achieved the prespecified primary endpoint with a significantly higher MPR rate in the CA-SAP group (33.3%; 95% CI: 19.9%–46.7%) than in the SAP group (17.0%; 95% CI: 6.5%–27.4%). Analysis of secondary endpoints also revealed a significantly higher objective response rate (ORR) (66.0%) and R0 resection rate (94.1%) with an acceptable safety profile in patients with CA-SAP.
A neoadjuvant approach can downstage the tumor, improve the resectability, provide survival benefits22, and has been widely used for the treatment of LAGC in Eastern and Western countries23–25. Pathological response is commonly used to evaluate the short-term effectiveness of neoadjuvant treatment26. Neoadjuvant FLOT has become a standard regimen in Europe due to the high pCR (16%) and MPR (37%) rates based on results from FLOT4-AIO16. However, differences in pharmacokinetics and tumor biology exist between Western and Asian populations27, which may limit the application of FLOT in Asian populations. Although perioperative chemotherapy with SOX (RESOLVE trial) and DOS (PRODIGY trial) both improved progression-free survival (PFS) compared with adjuvant chemotherapy, the pCR rates of these two regimens (5.6% and 10.4%, respectively) were unsatisfactory28,29. Thus, there is an urgent need for a tolerable and more effective combination therapeutic regimen. Our results demonstrated that the CA-SAP group had higher MPR and pCR rates (33.3% and 17.0%, respectively) than the SAP group (17.0% and 5.7%, respectively). However, several nonrandomized trials of neoadjuvant immunochemotherapy have reported higher pCR and MPR rates than those in the CA-SAP group10,30,31, while others have reported lower rates32,33. To explore the additional effect of anti-angiogenesis therapy on neoadjuvant immunochemotherapy, we reviewed historical control patients receiving neoadjuvant camrelizumab plus SAP (C-SAP) during the same period (from 2020 to 2022) and met the eligibility criteria of this trial (Supplementary Table 4). The MPR (24.4%) and pCR rates (6.7%) of the C-SAP cohort was both lower than the CA-SAP group but higher than the SAP group (Supplementary Table 5). This finding suggested that the addition of apatinib to neoadjuvant immunochemotherapy might further improve the antitumor activity. CA-SAP also exhibited a higher pCR rate than apatinib plus SOX (6.3%) in our earlier study13, indicating the synergistic antitumor activity of camrelizumab and apatinib. The immune suppressive nature of the tumor microenvironment is one of the most important reasons for primary resistance to immune checkpoint inhibitors and can be explained in part by the effects of neoangiogenesis34,35. Anti-angiogenesis therapy can reverse this immune suppressive nature and has the potential to improve the therapeutic response to immunotherapy36–38. A two-by-two factorial randomized controlled trial should be conducted to further confirm this synergistic effect.
Paclitaxel-based chemotherapy has shown satisfactory efficacy and safety in the treatment of advanced gastric cancer39–55 and shown non-inferior efficacy as compared with platinum-based chemotherapy in several randomized controlled trials56–58. A meta-analysis involving 1407 patients also supported the clinical efficacy of paclitaxel combined with S-159. According to the Japanese gastric cancer treatment guidelines 2018 (5th edition), paclitaxel combined with S-1 or 5-FU, as well as platinum-based chemotherapy, were all considered as “Recommended regimens17”. Our preliminary study also demonstrated a higher MPR rate with SAP than with SOX in clinical practice19. In addition, this trial aimed to explore the feasibility of immune checkpoint inhibitors (camrelizumab) and angiogenesis inhibitors (apatinib) in combination with chemotherapy as a neoadjuvant treatment for LAGC. Although neoadjuvant apatinib plus SOX has shown favorable efficacy in previous prospective studies, this regimen was associated with a high risk of thrombocytopenia13,60,61. This increased risk can be partly attributed to the use of oxaliplatin62 and may lead to frequent treatment discontinuation63. Thus, this trial prespecified SAP as the chemotherapy regimen due to its low incidence of thrombocytopenia and high MPR rate.
Previous studies have demonstrated the predictive value of PD-L1 expression in response to anti–PD-1 immunotherapy in advanced GC. In the KEYNOTE-062 and CheckMate 649 trials, survival benefits in the addition of PD-1 inhibitors to chemotherapy were only demonstrated in patients with a higher CPS6,9. Our results also showed a trend toward a higher MPR rate in patients with a higher CPS (≥1% or ≥5%) than in those with a lower CPS in the CA-SAP group. Patients with CA-SAP who had a CPS of <1% showed an MPR rate (27.3%) similar to that reported with apatinib plus SOX (25.0%) in our earlier study13. These results suggest that adding PD-1 inhibitors to other antitumor agents might provide no benefit in patients with a lower CPS. Moreover, there is still no consensus regarding the association between PD-L1 expression and response to neoadjuvant chemotherapy64–66. Although the MPR rate was higher in the CPS ≥ 5% subgroup than in the CPS ≥ 1% and <1% subgroups in the SAP group, these differences did not reach statistical significance. Future studies are needed to confirm this relation. In addition, MSI status is a potential biomarker for GC treatment. In a retrospective study of 535 patients with LAGC, the MPR rate was significantly lower in patients with MSI-H (0%) than in those with MSS (16%)67. In the NEONIPIGA trial evaluating neoadjuvant immunotherapy in patients with MSI-H LAGC, 72.4% and 58.6% of patients, respectively, achieved MPR and pCR68. In our trial, only 6 patients had MSI-H tumors, with MPR rates of 66.7% and 0.0% in the CA-SAP and SAP groups, respectively. These results suggest the potential value of MSI status for selecting patients who may benefit more from anti–PD-1 immunotherapy; however, this prediction warrants further investigation due to the limited sample size.
The unique biological characteristics and tumor microenvironment of diffuse-type gastric cancer make it less sensitive to chemotherapy and immunotherapy69,70. The FLOT-4 trial demonstrated that patients with diffuse-type tumors exhibited lower pCR rates (both <3%) than those with intestinal-type tumors in both arms16. Likewise, among patients who were treated with neoadjuvant SAP, the MPR rate was significantly lower in diffuse-type tumors (12.5%) than in intestinal-type tumors (40.0%). In comparison, patients with diffuse-type tumors derived the highest benefit from neoadjuvant CA-SAP, with an MPR rate of 34.2%. A feasible explanation was that the introduction of apatinib altered the resistance profile of diffuse-type tumors. On one hand, anti-angiogenic therapy can improve the local hypoxia of diffuse-type tumors, thereby increasing sensitivity to chemotherapy and immunotherapy71. On the other hand, the immune-modulating properties of angiogenesis inhibitors may induce an immune-activated tumor microenvironment and enhance the efficacy of immunotherapy72. This finding might facilitate more individualized decision-making based on Lauren type. In addition, prespecified subgroup analysis showed a significant interaction between ECOG performance status and treatment regimen; patients with an ECOG performance status of 0 had a significantly higher MPR rate with CA-SAP than those who had a performance status of 1 (45.2% versus 14.7%). In the KEYNOTE-059 and KEYNOTE-061 trials, better ECOG performance status was also associated with better overall survival with pembrolizumab9,73. Further investigation is needed to determine the potential predictive value of performance status on response to anti–PD-1 immunotherapy.
Although pathological response was the primary endpoint of this trial, the surrogacy of this pathological endpoint remains hotly debated74,75. In the FLOT4 trial, the superiority of FLOT in terms of pCR rates eventually translated into survival benefits16. The KEYNOTE 585 trial also demonstrated a statistically significant improvement in pCR rates in the chemotherapy plus pembrolizumab group; however, results did not meet statistical significance for event-free survival. Thus, active follow-up is needed to provide further insight into our findings. Nevertheless, pathological response could help to accelerate the process of testing new therapies as an early endpoint for predicting efficacy. Additionally, pathological response could be less susceptible to selection bias and less dependent on the quality of surgical resection compared with other endpoints. We therefore believe that pathological response could serve as an appropriate endpoint for neoadjuvant phase 2 trials.
Secondary efficacy endpoints included radiologic response and R0 resection rate. Because of the poor prognostic value of Response Evaluation Criteria in Solid Tumors (RECIST) response in patients with LAGC76, both ORR and clinical downstaging were evaluated in this trial. We observed a higher ORR and a higher proportion of patients achieving T downstaging (66.0% and 52.9%, respectively) in the CA-SAP group than in the SAP group (43.4% and 32.1%, respectively). As previously reported, significant downstaging could provide favorable conditions for curative surgery77. In addition to the promising tumor response results, a remarkable improvement in the R0 resection rate was observed with CA-SAP. These results further support the favorable tumor response of neoadjuvant CA-SAP. Given the prognostic value of R0 resection and tumor downstaging77–79, the advantages of CA-SAP in these efficacy endpoints were expected to translate into improved survival outcomes.
Our results demonstrated a favorable safety profile of CA-SAP. The most common overall AE and grade 3-4 AE were both hematologic in patients with CA-SAP, which is in line with results reported with sintilimab plus CapeOx10 and with apatinib plus SOX13. All AEs with potential immune etiology were categorized as grade 1–2 and were manageable according to the known safety management algorithm. No thromboembolism events were observed in the CA-SAP group, which was consistent with previous studies12,80. This finding showed a relatively low toxicity profile for apatinib, particularly in vascular toxicity. Chemotherapy may have direct or indirect effects on immune cells, leading to immune-related adverse reactions81,82. Similar to the KEYNOTE-061, KEYNOTE-062, and ATTRACTION-4 trials7–9, one immune-related adverse reaction was also observed in the SAP group, but its incidence was obviously lower than the CA-SAP group.
Surgical outcomes were also manageable in both groups and comparable between them. Although an increased incidence of anastomotic leakage was observed in the CA-SAP group (8.2% versus 2.0%), this difference did not reach statistical significance (P = 0.201; Supplementary Table 3). In several prospective studies, apatinib plus neoadjuvant chemotherapy did not show a significant increase in the risk of anastomotic leakage13,60,61. Given the negative impact of VEGF inhibitors on anastomotic healing83, we recommended stopping apatinib treatment at least 14 days before surgery and correcting hypoalbuminemia/anemia during the perioperative course.
Some limitations should be considered. First, our study was performed in an Asian population, and therefore, the effectiveness of CA-SAP should be validated in other populations. Second, although CA-SAP was demonstrated to be more effective than SAP, it remains unclear whether this superiority can translate into survival benefits. Active follow-up is needed to provide further insight into our findings. Third, the SAP chemotherapy regimen is not widely accepted as it was validated only in the Asian population, but we thought this regimen could be non-inferior to the standard regimens (e.g., oxaliplatin plus S-1). For example, the MPR and pCR rates (33.3% and 15.7%, respectively) in the CA-SAP group were similar to those reported by a recent nonrandomized trial investigating the neoadjuvant combination of camrelizumab, apatinib, and S-1 with or without oxaliplatin (26.3% and 15.8%, respectively)84. Nevertheless, a randomized controlled trial is needed to confirm the feasibility of camrelizumab and apatinib combined with platinum-based chemotherapy. Fourth, due to the two-arm design, it was unconvincing to demonstrate benefit of adding apatinib in the neoadjuvant treatment even with a historical control. Finally, the clinical response observed in this study should be further accompanied and explained with biomarkers and translational studies. These analyses are still ongoing in a post-hoc analysis. Nevertheless, we believe that our results can provide important information for further research and serve as preliminary data for a larger phase 3 trial.
In conclusion, camrelizumab and apatinib combined with nab-paclitaxel plus S-1 significantly increased the proportions of patients achieving pathological response, radiologic response, and R0 resection with acceptable safety compared with nab-paclitaxel plus S-1. This regimen might be a promising neoadjuvant treatment for patients with LAGC in the future, particularly in subpopulations with good performance status or diffuse-type tumors. An international, randomized phase 3 trial is needed to confirm our conclusions.
Methods
Trial design
We conducted a multicenter, randomized, open-label, phase 2 trial (Arise-FJ-G005) at 5 centers in China (Supplementary Fig. 1). The study protocol and all amendments were approved by the institutional review boards of the Fujian Medical University Union Hospital, Second Affiliated Hospital of Fujian Medical University, Zhongshan Hospital of Xiamen University, Zhangzhou Municipal Hospital of Fujian Province, and The Affiliated Hospital of Putian University. All patients provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. This study is registered with ClinicalTrials.gov, number NCT04195828. This study was reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Guidelines. The original study protocol is available in the Supplementary Information as Supplementary Note 2.
Participants
Patients were eligible for enrollment if they were aged 18–75 years with at least one measurable lymph node with a short axis of ≥15 mm according to the RECIST (version 1.1)85, histologically confirmed locally advanced gastric adenocarcinoma that was clinically T2 to T4 and M0 according to the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging Manual, an ECOG performance status of 0 or 1, and adequate organ function. The main exclusion criteria were previous cancer therapy, history of malignancy within the past 5 years, or history of concurrent autoimmune disease. Complete inclusion and exclusion criteria are listed in Supplementary Table 6. The first patient was enrolled on June 18, 2020, and the last was recruited on March 31, 2022.
Randomization and blinding
A blinded statistician performed randomization with a list of randomly ordered treatment identifiers generated by SAS software, version 9.2 (SAS Institute). The randomized sequence was created for 1:1 allocation of 106 cases, 53 cases in each group, and was concealed from the investigators who screened and enrolled participants. The assignment was made by telephone contact or text messages after the patient met the eligibility criteria and signed the informed consent form. The study was open-label and no blinding was required. For randomization to be successfully implemented, the randomization sequence was concealed so that the investigators who screened and enrolled participants were not aware of the upcoming assignment. Patients and caregivers were not blinded to the treatment received. Outcome assessment for the primary endpoint was performed by two blinded pathologists. All statistical analyses were also performed by a blinded investigator.
Treatments
Eligible patients were randomly assigned to receive camrelizumab (200 mg intravenously on day 1) and apatinib (250 mg orally once daily on days 1–21) combined with chemotherapy (nab-paclitaxel 125 mg/m2 intravenously on days 2 and 9, S-1 40 to 60 mg orally twice daily depending on body surface area on days 1–14) or chemotherapy alone every 3 weeks for 3 preoperative cycles followed by 5 postoperative cycles. Dose modifications (e.g., dose interruption, delay, or reduction) were permitted in the presence of grade ≥3 hematologic or grade ≥2 nonhematologic AEs. The criteria for stopping treatment were patient refusal, tumor progression, intolerable toxicity, or investigator’s decision. An Independent Data Monitoring Committee (IDMC) monitored patient safety and study conduct.
After enrollment, tumor tissue samples were evaluated for PD-L1 expression and MSI status by a central laboratory in a blinded manner. PD-L1 expression was measured using the CPS, defined as the number of PD-L1–positive cells (tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells multiplied by 100, with the Ventana PD-L1 (SP263) immunohistochemistry assay. The MSI-high (MSI-H) status was defined as the loss of expression of at least one mismatch repair protein (MLH1, MSH1, MSH6, and PMS2). We performed MLH1 (ab92312, Abcam, 1: 250), MSH2 (ab52266, Abcam, 1: 250), MSH6 (ab92471, Abcam, 1: 250), PMS2 (ab110638, Abcam, 1: 250) immunohistochemical staining on the tissue.
Tumor assessments by means of contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) were performed after completion of the second cycle and before surgery. If tumor progression was demonstrated, surgery or other antitumor treatment could be administered at the investigator’s discretion. Total or distal gastrectomy was scheduled 2 to 4 weeks after completion of the last cycle of neoadjuvant treatment. All surgical procedures, including the extent of gastric resection and D2 lymph node dissection, were performed according to the guidelines of the Japanese Research Society for the Study of Gastric Cancer17. All surgeons performed at least 200 gastrectomies for GC annually. Adjuvant treatment started 3 to 8 weeks after operation.
Endpoints and assessments
The primary endpoint was the MPR rate, defined as the proportion of patients with <10% residual tumor cells in resection specimens86. Secondary endpoints included the pCR rate, R0 resection rate, radiologic response, safety, and survival.
Tumor regression grade was evaluated centrally using the Becker regression criteria, which are based on the percentage of vital tumor cells in the tumorous area and include the following categories: TRG 1a (no residual tumor cells), TRG 1b (<10% residual tumor cells), TRG 2 (10–50% residual tumor cells) and TRG 3 (>50% residual tumor cells)86. Radiologic response was evaluated using RECIST (version 1.1) by local radiologists, which is based on the short axis of the target lymph node(s) measured by CT or MRI findings and includes complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD)85. The ORR was defined as the proportion of patients with CR and PR, and the DCR was defined as the proportion of patients with CR, PR, and SD. R0 resection was defined as complete resection without macroscopic or microscopic residual disease, whereas R1 resection was defined as gross removal of tumors with microscopic resection margin involvement. Nonsurgical AEs were evaluated according to the Common Terminology Criteria for Adverse Events, version 5.0. Postoperative morbidity was evaluated according to the Clavien‒Dindo classification87. Other secondary endpoints including overall survival and progression-free survival were not analyzed because the follow-up time was insufficient.
Sample size and statistical analysis
Based on the assumption of MPR rates of 15% in the SAP group and 35% in the CA-SAP group, a sample size of 53 patients per group was required to detect improvement with 80% power and a type I error rate of 0.1 (Fisher’s exact test), including a 5% dropout rate. The mITT population included all patients who were randomly assigned and received at least one dose of allocated treatment. The per-protocol population included patients in the mITT population who did not present major deviations from protocol. Efficacy analyses were performed in the mITT population and per-protocol population. Safety analyses were performed in all patients who received at least one dose of allocated treatment.
Continuous variables are presented as medians and interquartile ranges (Q1–Q3) and were compared using the Wilcoxon rank sum test. Categorical variables are presented as frequencies and percentages and were compared using the χ2 test or Fisher’s exact test. Notably, the significance level was set to be 10% for efficacy analyses and 5% for other analyses. P values were one-sided for efficacy analyses in Fisher’s exact test and were two-sided for other analyses. To address the issue of multiplicity, P values were adjusted by controlling for the false discovery rate (FDR) using the Benjamini–Hochberg procedure88. This post hoc adjustment was made for efficacy analyses, and no adjustment was made for other analyses which should be considered as explorative or descriptive. The study protocol prespecified a set of subgroup analysis according to baseline characteristics in the per-protocol population (Supplementary Note 2). Interaction between agents was evaluated by likelihood ratio test. Statistical significance of the interaction between baseline characteristics and treatment effect was assessed by comparing the logistic regression models with and without the interaction term. All statistical analyses were conducted with SPSS statistical software (version 21.0; SPSS Inc.) and R software (version 4.1.2; R Foundation for Statistical Computing).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We thank who have devoted a lot to this study, including nurses, pathologists, further-study doctors, and statisticians. This study was supported by the Construction Funds for “High-level Hospitals and Clinical Specialties” of Fujian Province (No. [2021]76, H.C.M.). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
C.M.H. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C.M.H. and J.X.L. obtained funding, conceived of and designed the study, and supervised the whole study. H.L.Z., K.Y., J.C.C., L.S.C., W.L., J.W.X., J.B.W., J.L., Q.Y.C., L.L.C., C.H.Z., and P.L. acquired the data, were responsible for study administration, and provided technical and material support. Y.H.T. interpreted and analyzed the data. Y.H.T. drafted the manuscript. C.M.H., J.X.L., C.H.Z., and P.L. critically revised the manuscript. All authors reviewed the manuscript and agreed to submit it for publication.
Peer review
Peer review information
Nature Communications thanks Lin Shen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings in this study are available under controlled access due to data privacy laws related to patient consent for data sharing and the data should be used for research purposes only. All the original clinical data will be made available on request from the corresponding author (Huang CM) at any time in a de-identified manner. Request for data sharing will be handled in line with the data access and sharing policy of Fujian Medical University Union Hospital, which can be found in Supplementary Note 1. The original study protocol is available as Supplementary Note 2 in the Supplementary information file. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jian-Xian Lin, Yi-Hui Tang, Hua-Long Zheng.
Contributor Information
Ping Li, Email: pingli811002@163.com.
Chang-Ming Huang, Email: hcmlr2002@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-44309-5.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Noh S, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389–1396. doi: 10.1016/S1470-2045(14)70473-5. [DOI] [PubMed] [Google Scholar]
- 3.Sasako M, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011;29:4387–4393. doi: 10.1200/JCO.2011.36.5908. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 5.Ychou M, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 6.Janjigian Y, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang Y, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–247. doi: 10.1016/S1470-2045(21)00692-6. [DOI] [PubMed] [Google Scholar]
- 8.Shitara K, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 9.Shitara K, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J. Immunother. Cancer. 2022;10:e003635. doi: 10.1136/jitc-2021-003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa H, et al. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric Cancer. 2022;25:619–628. doi: 10.1007/s10120-022-01286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 13.Lin JX, et al. Effectiveness and safety of apatinib plus chemotherapy as neoadjuvant treatment for locally advanced gastric cancer: a nonrandomized controlled trial. JAMA Netw. Open. 2021;4:e2116240. doi: 10.1001/jamanetworkopen.2021.16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin. Cancer Res. 2019;25:515–523. doi: 10.1158/1078-0432.CCR-18-2484. [DOI] [PubMed] [Google Scholar]
- 15.Liang L, et al. Safety and efficacy of PD-1 blockade-activated multiple antigen-specific cellular therapy alone or in combination with apatinib in patients with advanced solid tumors: a pooled analysis of two prospective trials. Cancer Immunol. Immunotherapy. 2019;68:1467–1477. doi: 10.1007/s00262-019-02375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Batran SE, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 17.Japanese gastric cancer treatment guidelines 2018. 5th ed. Gastric Cancer24, 1–21 (2021). [DOI] [PMC free article] [PubMed]
- 18.Shitara K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017;2:277–287. doi: 10.1016/S2468-1253(16)30219-9. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, et al. Safety and efficacy of camrelizumab in combination with nab-paclitaxel plus S-1 for the treatment of gastric cancer with serosal invasion. Front. Immunol. 2021;12:783243. doi: 10.3389/fimmu.2021.783243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steins A, et al. Systemic effects of angiogenesis inhibition alter pharmacokinetics and intratumoral delivery of nab-paclitaxel. Drug Deliv. 2017;24:1801–1810. doi: 10.1080/10717544.2017.1406559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, et al. Nab-paclitaxel promotes the cancer-immunity cycle as a potential immunomodulator. Am. J. Cancer Res. 2021;11:3445–3460. [PMC free article] [PubMed] [Google Scholar]
- 22.Miao Z, et al. Effect of neoadjuvant chemotherapy in patients with gastric cancer: a PRISMA-compliant systematic review and meta-analysis. BMC Cancer. 2018;18:118. doi: 10.1186/s12885-018-4027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang FH, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021;41:747–795. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajani J, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 2022;20:167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 25.Lordick F, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33:1005–1020. doi: 10.1016/j.annonc.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi M, Fujita T, Matsushita H. Prognostic value of tumor regression grade following the administration of neoadjuvant chemotherapy as treatment for gastric/gastroesophageal adenocarcinoma: a meta-analysis of 14 published studies. Eur. J. Surg. 2021;47:1996–2003. doi: 10.1016/j.ejso.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Huang R, et al. One size does not fit all: marked heterogeneity in incidence of and survival from gastric cancer among Asian American Subgroups. Cancer Epidemiol. Biomarkers Prev. 2020;29:903–909. doi: 10.1158/1055-9965.EPI-19-1482. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081–1092. doi: 10.1016/S1470-2045(21)00297-7. [DOI] [PubMed] [Google Scholar]
- 29.Kang Y, et al. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J. Clin. Oncol. 2021;39:2903–2913. doi: 10.1200/JCO.20.02914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo H, et al. Efficacy and safety of sintilimab plus XELOX as a neoadjuvant regimen in patients with locally advanced gastric cancer: a single-arm, open-label, phase II trial. Front. Oncol. 2022;12:927781. doi: 10.3389/fonc.2022.927781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin Y, et al. Neoadjuvant tislelizumab and tegafur/gimeracil/octeracil (S-1) plus oxaliplatin in patients with locally advanced gastric or gastroesophageal junction cancer: early results of a phase 2, single-arm trial. Front. Oncol. 2022;12:959295. doi: 10.3389/fonc.2022.959295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, et al. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: updated results of efficacy and safety. J. Clin. Oncol. 2021;39:4036–4036. doi: 10.1200/JCO.2021.39.15_suppl.4036. [DOI] [Google Scholar]
- 33.Liu Z, et al. Efficacy and safety of camrelizumab combined with FLOT versus FLOT alone as neoadjuvant therapy in patients with resectable locally advanced gastric and gastroesophageal junction adenocarcinoma who received D2 radical gastrectomy: data update. J. Clin. Oncol. 2022;40:e16044–e16044. doi: 10.1200/JCO.2022.40.16_suppl.e16044. [DOI] [Google Scholar]
- 34.Binnewies M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giraldo N, et al. The clinical role of the TME in solid cancer. Br. J. Cancer. 2019;120:45–53. doi: 10.1038/s41416-018-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabrilovich D, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 38.Maenhout S, Thielemans K, Aerts J. Location, location, location: functional and phenotypic heterogeneity between tumor-infiltrating and non-infiltrating myeloid-derived suppressor cells. Oncoimmunology. 2014;3:e956579. doi: 10.4161/21624011.2014.956579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, et al. Phase II study of docetaxel and capecitabine in patients with metastatic or recurrent gastric cancer. Oncology. 2005;68:190–195. doi: 10.1159/000086773. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida K, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin. Cancer Res. 2006;12:3402–3407. doi: 10.1158/1078-0432.CCR-05-2425. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi K, et al. Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br. J. Cancer. 2006;94:1803–1808. doi: 10.1038/sj.bjc.6603196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Im C, et al. A phase II study of paclitaxel combined with infusional 5-fluorouracil and low-dose leucovorin for advanced gastric cancer. Cancer Chemother. Pharmacol. 2008;61:315–321. doi: 10.1007/s00280-007-0508-6. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, Lee H, Park E, Jang J, Lee S. A phase II study of leucovorin, 5-FU and docetaxel combination chemotherapy in patients with inoperable or postoperative relapsed gastric cancer. Cancer Res. Treat. 2008;40:11–15. doi: 10.4143/crt.2008.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S, et al. Phase I/II study of S-1 combined with weekly docetaxel in patients with metastatic gastric carcinoma. Br. J. cancer. 2008;98:1305–1311. doi: 10.1038/sj.bjc.6604312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J, et al. A multi-center phase II study of S-1 plus paclitaxel as first-line therapy for patients with advanced or recurrent unresectable gastric cancer. Cancer Chemother. Pharmacol. 2009;63:1083–1090. doi: 10.1007/s00280-008-0818-3. [DOI] [PubMed] [Google Scholar]
- 46.Zang D, et al. Phase I/II trial with docetaxel and S-1 for patients with advanced or recurrent gastric cancer with consideration to age. Cancer Chemother. Pharmacol. 2009;63:509–516. doi: 10.1007/s00280-008-0768-9. [DOI] [PubMed] [Google Scholar]
- 47.Kakeji Y, et al. Phase II study of biweekly docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Oncology. 2009;77:49–52. doi: 10.1159/000226111. [DOI] [PubMed] [Google Scholar]
- 48.Kunisaki C, et al. Phase II study of biweekly docetaxel and S-1 combination chemotherapy as first-line treatment for advanced gastric cancer. Cancer Chemother. Pharmacol. 2011;67:1363–1368. doi: 10.1007/s00280-010-1433-7. [DOI] [PubMed] [Google Scholar]
- 49.Shigeyasu K, et al. Multicenter phase II study of S-1 and docetaxel combination chemotherapy for advanced or recurrent gastric cancer patients with peritoneal dissemination. Cancer Chemother. Pharmacol. 2013;71:937–943. doi: 10.1007/s00280-013-2086-0. [DOI] [PubMed] [Google Scholar]
- 50.Kosaka T, et al. Preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer. Cancer Chemother. Pharmacol. 2014;73:281–285. doi: 10.1007/s00280-013-2350-3. [DOI] [PubMed] [Google Scholar]
- 51.Koizumi W, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START) J. Cancer Res. Clin. Oncol. 2014;140:319–328. doi: 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oki E, et al. Phase II study of docetaxel and S-1 (DS) as neoadjuvant chemotherapy for clinical stage III resectable gastric cancer. Ann. Surg. Oncol. 2014;21:2340–2346. doi: 10.1245/s10434-014-3594-9. [DOI] [PubMed] [Google Scholar]
- 53.Jiang H, et al. A phase II study of biweekly S-1 and paclitaxel (SPA) as first-line chemotherapy in patients with metastatic or advanced gastric cancer. Cancer Chemother. Pharmacol. 2015;76:197–203. doi: 10.1007/s00280-015-2782-z. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y, et al. A phase II study of perioperative S-1 combined with weekly docetaxel in patients with locally advanced gastric carcinoma: clinical outcomes and clinicopathological and pharmacogenetic predictors for survival. Gastric Cancer. 2016;19:586–596. doi: 10.1007/s10120-015-0490-3. [DOI] [PubMed] [Google Scholar]
- 55.Kosaka T, et al. Outcomes of preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer. Cancer Chemother. Pharmacol. 2019;83:1047–1055. doi: 10.1007/s00280-019-03813-6. [DOI] [PubMed] [Google Scholar]
- 56.Thuss-Patience P, et al. Docetaxel and continuous-infusion fluorouracil versus epirubicin, cisplatin, and fluorouracil for advanced gastric adenocarcinoma: a randomized phase II study. J. Clin. Oncol. 2005;23:494–501. doi: 10.1200/JCO.2005.02.163. [DOI] [PubMed] [Google Scholar]
- 57.Mochiki E, et al. Phase II multi-institutional prospective randomised trial comparing S-1+paclitaxel with S-1+cisplatin in patients with unresectable and/or recurrent advanced gastric cancer. Br. J. Cancer. 2012;107:31–36. doi: 10.1038/bjc.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai Y, et al. versusNab-paclitaxel plus S-1 oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study) Therapeutic Adv. Med. Oncol. 2022;14:17588359221118020. doi: 10.1177/17588359221118020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bian N, Wang Y, Min G. S-1 combined with paclitaxel may benefit advanced gastric cancer: Evidence from a systematic review and meta-analysis. Int. J. Surg. 2019;62:34–43. doi: 10.1016/j.ijsu.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Zheng Y, et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: a single-arm, open-label, phase II trial. Eur. J. Cancer. 2020;130:12–19. doi: 10.1016/j.ejca.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Tang Z, et al. Neoadjuvant apatinib combined with oxaliplatin and capecitabine in patients with locally advanced adenocarcinoma of stomach or gastroesophageal junction: a single-arm, open-label, phase 2 trial. BMC Med. 2022;20:107. doi: 10.1186/s12916-022-02309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jardim D, Rodrigues C, Novis Y, Rocha V, Hoff P. Oxaliplatin-related thrombocytopenia. Annals of oncology: official journal of the European Society for. Med. Oncol. 2012;23:1937–1942. doi: 10.1093/annonc/mds074. [DOI] [PubMed] [Google Scholar]
- 63.Al-Samkari H, Soff G. Clinical challenges and promising therapies for chemotherapy-induced thrombocytopenia. Expert Rev. Hematol. 2021;14:437–448. doi: 10.1080/17474086.2021.1924053. [DOI] [PubMed] [Google Scholar]
- 64.Hashimoto T, et al. Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer. 2019;22:785–792. doi: 10.1007/s10120-018-00918-4. [DOI] [PubMed] [Google Scholar]
- 65.Ribeiro H, et al. PD-L1 expression in gastric and gastroesophageal junction cancer patients treated with perioperative chemotherapy. J. Surg. Oncol. 2022;126:150–160. doi: 10.1002/jso.26929. [DOI] [PubMed] [Google Scholar]
- 66.Zurlo I, et al. Predictive value of NLR, TILs (CD4+/CD8+) and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Cancer Immunol. Immunotherapy. 2022;71:45–55. doi: 10.1007/s00262-021-02960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vos, E. et al. Survival of locally advanced msi-high gastric cancer patients treated with perioperative chemotherapy: a retrospective cohort study. Ann. Surg.277, 798–805 (2022). [DOI] [PMC free article] [PubMed]
- 68.André, T., et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA Phase II study. J. Clin. Oncol.41, 255–265 (2023). [DOI] [PMC free article] [PubMed]
- 69.Jinawath N, et al. Comparison of gene-expression profiles between diffuse- and intestinal-type gastric cancers using a genome-wide cDNA microarray. Oncogene. 2004;23:6830–6844. doi: 10.1038/sj.onc.1207886. [DOI] [PubMed] [Google Scholar]
- 70.Kim T, da Silva E, Coit D, Tang L. Intratumoral immune response to gastric cancer varies by molecular and histologic subtype. Am. J. Surg. Pathol. 2019;43:851–860. doi: 10.1097/PAS.0000000000001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mander K, Finnie J. Tumour angiogenesis, anti-angiogenic therapy and chemotherapeutic resistance. Aust. Vet. J. 2018;96:371–378. doi: 10.1111/avj.12747. [DOI] [PubMed] [Google Scholar]
- 72.Shigeta K, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71:1247–1261. doi: 10.1002/hep.30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuchs C, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conforti F, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375:e066381. doi: 10.1136/bmj-2021-066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huynh C, et al. Pathological complete response as a surrogate endpoint after neoadjuvant therapy for lung cancer. Lancet Oncol. 2021;22:1056–1058. doi: 10.1016/S1470-2045(21)00405-8. [DOI] [PubMed] [Google Scholar]
- 76.Kurokawa Y, et al. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A) Gastric Cancer. 2014;17:514–521. doi: 10.1007/s10120-013-0294-2. [DOI] [PubMed] [Google Scholar]
- 77.Prasad P, et al. Significance of neoadjuvant downstaging in gastric adenocarcinoma. Surgery. 2022;172:593–601. doi: 10.1016/j.surg.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Tu R, et al. Pathological features and survival analysis of gastric cancer patients with positive surgical margins: a large multicenter cohort study. Eur. J. Surg. 2019;45:2457–2464. doi: 10.1016/j.ejso.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 79.Levenson G, et al. Tumor downstaging after neoadjuvant chemotherapy determines survival after surgery for gastric adenocarcinoma. Surgery. 2021;170:1711–1717. doi: 10.1016/j.surg.2021.08.021. [DOI] [PubMed] [Google Scholar]
- 80.Li J, et al. Safety and efficacy of apatinib in patients with advanced gastric or gastroesophageal junction adenocarcinoma after the failure of two or more lines of chemotherapy (AHEAD): a prospective, single-arm, multicenter, phase IV study. BMC Med. 2023;21:173. doi: 10.1186/s12916-023-02841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen G, Emens L. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol. Immunotherapy. 2013;62:203–216. doi: 10.1007/s00262-012-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dias Costa A, et al. Neoadjuvant chemotherapy is associated with altered immune cell infiltration and an anti-tumorigenic microenvironment in resected pancreatic cancer. Clin. Cancer Res. 2022;28:5167–5179. doi: 10.1158/1078-0432.CCR-22-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakamura H, et al. The effects of bevacizumab on intestinal anastomotic healing in rabbits. Surg. Today. 2016;46:1456–1463. doi: 10.1007/s00595-016-1342-4. [DOI] [PubMed] [Google Scholar]
- 84.Li S, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat. Commun. 2023;14:8. doi: 10.1038/s41467-022-35431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eisenhauer E, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 86.Becker K, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann. Surg. 2011;253:934–939. doi: 10.1097/SLA.0b013e318216f449. [DOI] [PubMed] [Google Scholar]
- 87.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laffel L, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323:2388–2396. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings in this study are available under controlled access due to data privacy laws related to patient consent for data sharing and the data should be used for research purposes only. All the original clinical data will be made available on request from the corresponding author (Huang CM) at any time in a de-identified manner. Request for data sharing will be handled in line with the data access and sharing policy of Fujian Medical University Union Hospital, which can be found in Supplementary Note 1. The original study protocol is available as Supplementary Note 2 in the Supplementary information file. The remaining data are available within the Article, Supplementary Information, or Source Data file. Source data are provided with this paper.