Abstract
The LEPR (leptin receptor) genotype is associated with obesity. Gut microbiome composition differs between obese and non-obese adults. However, the impact of LEPR genotype on gut microbiome composition in humans has not yet been studied. In this study, the association between LEPR single nucleotide polymorphism (rs1173100, rs1137101, and rs790419) and the gut microbiome composition in 65 non-obese Korean adults was investigated. Leptin, triglyceride, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels were also measured in all participants. Mean ± SD (standard deviation) of age, body mass index, and leptin hormone levels of participants was 35.2 ± 8.1 years, 21.4 ± 1.8 kg/m2, and 7989.1 ± 6687.4 pg/mL, respectively. Gut microbiome analysis was performed at the phylum level by 16S rRNA sequencing. Among the 11 phyla detected, only one showed significantly different relative abundances between LEPR genotypes. The relative abundance of Candidatus Saccharibacteria was higher in the G/A genotype group than in the G/G genotype group for the rs1137101 single nucleotide polymorphism (p=0.0322). Participant characteristics, including body mass index, leptin levels, and other lipid levels, were similar between the rs1137101 G/G and G/A genotypes. In addition, the relative abundances of Fusobacteria and Tenericutes showed significant positive relationship with plasma leptin concentrations (p=0.0036 and p=0.0000, respectively). In conclusion, LEPR genotype and gut microbiome may be associated even in normal-weight Korean adults. However, further studies with a greater number of obese adults are needed to confirm whether LEPR genotype is related to gut microbiome composition.
Keywords: Gastrointestinal microbiome, Leptin, Receptors, leptin, Obesity, Industrial microbiology, Pharmacogenetics
INTRODUCTION
Obesity is an important risk factor of ischemic heart disease, stroke, diabetes, hypertension, dyslipidemia, musculoskeletal disorders, and various cancers. An increase in the prevalence of obesity leads to an increase in various comorbidities and the socioeconomic burden on the country (Tremmel et al., 2017). The obesity rate in the Korean population (the proportion of the population aged 19 years or older with a body mass index (BMI) of 25 kg/m2 or more) has been maintained at approximately 34% since 2015 but has increased by 4.5% from 2019 to 38.3% in 2020 (Korea National Statistical Office, 2023). Globally, a BMI exceeding 30 kg/m2 is classified as obese, according to which the obesity rate in Korean men was 6.2% in 2019, which is very low compared to that in Western developed countries. The obesity rates among men in 2019 were 43.5% in the US, 26.7% in Canada, 31.5% in Australia, 27.0% in the UK, 18.1% in Germany, and 13.5% in France. The obesity rate among Korean women was 5.5% in 2019, which is much lower than that in Western countries (OECD, 2021). Obesity is a structural phenomenon that occurs in tandem with economic development and lifestyle changes. Therefore, its incidence is likely to increase in the future.
LEPR gene encodes leptin receptor protein. It is located on human chromosome 1p31.3 (Vauthier et al., 2012). Leptin receptor plays a crucial role in the binding and signaling of the leptin hormone. LEP gene encodes leptin hormone and is located on human chromosome 7q32.1 (Manju et al., 2022). Leptin acts as a signaling molecule that regulates energy balance, appetite, and metabolism (Zhang and Scarpace, 2006). Genetic variations in these genes can cause disorders such as leptin receptor deficiency or congenital leptin deficiency.
Several previous studies have investigated the correlation between LEPR single nucleotide polymorphisms (SNPs) and obesity. Part et al. reported that K109R among LEPR SNPs was correlated with BMI in 1,463 Koreans (Park et al., 2006). They sequenced the LEPR gene to identify polymorphisms in potential candidate genes associated with obesity and type 2 diabetes mellitus in Koreans. Among the 11 polymorphisms, only K109R in exon 3 showed a marginal association with BMI (p=0.02). S343S in exon 8 needed additional research to confirm association with BMI (p=0.05). In a multiracial Malaysian population, the K109R and Q223R variants were correlated with BMI (Fan and Say, 2014). Fan and Say (2014) investigated the association of LEP SNP (A19G and G2548A) and LEPR SNP (K109R and Q223R) prevalence with fasting plasma leptin hormone concentrations and obesity in the Malaysian suburban population of Kampar. Participants with LEPR K109 and Q223 alleles had significantly high systolic blood pressure and adiposity indices after ethnicity adjustment. That is, when the BMI was higher, the total body fat and subcutaneous fat amounts were higher, and the skeletal muscle ratio was lower. Participants with LEPR 109R allele had lower plasma leptin levels than those with the wild-type allele. Fan and Say (2014) reported that several SNPs of LEP and LEPR contribute to minor but significant changes in obesity-related characteristics. In addition to race, LEPR K109R and Q223R were correlated with birth weight in a twin study (Souren et al., 2008). In addition, the interaction between the two SNPs was statistically significant (p=0.014). Based on the proceeding studies mentioned above, rs1173100 (K109R), rs1137101 (Q223R), and rs790419 (S343S) were selected for exploration in this study.
Several studies have reported that LEP and LEPR have specific effects on the composition of gut microbiota (Duranti et al., 2017). According to Ranji et al. (2019), the oral intake of Lactobacillus acidophilus and Bifidobacterium bifidum reduces leptin receptor gene expression in mouse rectal cancer cells (Ranji et al., 2019). In another study, LEPR loss in obese rats increased the abundance of Halomonas and Sphingomonas species and decreased the abundance of Bifidobacterium species in the fecal microbiome (Waldram et al., 2009).
However, no studies have been conducted to examine the correlation between LEPR genotypes and the gut microbiome composition in humans. Therefore, the objective of this study was to investigate whether there are differences in the composition of the gut microbiota based on the LEPR genotype among normal-weight healthy Koreans.
MATERIALS AND METHODS
The study protocol and informed consent forms were approved by the Institutional Review Board of Chungnam National University Hospital (IRB number: CNUH 2022-07-043). All procedures were performed in compliance with the Korean Good Clinical Practice Guidelines and the Declaration of Helsinki. Written informed consent was obtained from all participants before participating in the study. This study was conducted at the Clinical Trials Center of the Chungnam National University Hospital (Daejeon, Korea).
Study design
This study was a prospective, descriptive, single-center, observational study. Each participant was provided with a stool collection kit during the screening visit. Participants who successfully passed the screening visited the study site once to submit their stool and blood samples to the investigators. Blood samples were collected to analyze the genotype of the leptin receptor, measure leptin hormone concentration, and assess the lipid panel, which included triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels. Throughout the study period, all participants were allowed to restrict their alcohol consumption.
Study population
The study included only eligible volunteers who were healthy, normal-weight Korean adults. The inclusion criteria were as follows: between 19 and 50 years of age and a BMI ranging from 18.0 to 25.0 kg/m2. Male participants had to weigh at least 55.0 kg, while female participants had to weigh at least 45.0 kg. Participants who had been administered antibiotics within one month prior to the screening date were excluded. The health status of each participant was assessed through a physical examination, measurements of vital signs, and clinical laboratory tests.
Gut microbiome analysis with stool samples
Stool samples were collected using a stool collection kit (NBgene-GUT kit; Noble Biosciences, Seoul, Korea) containing preservatives. They were kept frozen below –70°C until analysis proceeded.
Gut microbiome analysis was conducted using 16S ribosomal RNA (rRNA) sequencing performed by GC Genome. Upon the arrival of the samples, DNA was immediately extracted using a Chemagic DNA Stool Kit (PerkinElmer, MA, USA), which included a modified bead-beating pretreatment step. Each sample was then aliquoted into a bead tube (Lysing Matrix E; MP Biomedical, CA, USA) and homogenized for 1 min using a Fastprep-24 homogenizer. The V4 hypervariable region of the 16S rRNA gene was amplified using the NEXTflex 16S V4 Amplicon-Seq Kit (BioO Scientific, Austin, TX, USA). The amplified DNA was subsequently sequenced using the Illumina MiSeq Reagent Kit v2 (500 cycles) (Illumina, CA, USA) following the manufacturer’s protocol.
At least 20,000 reads were obtained for each sample. Sequence reads were analyzed using the QIIME 2 framework (Bolyen et al., 2019). The demultiplexed and primer-trimmed data were quality-filtered and denoised using the DADA2 plugin (Callahan et al., 2016). To ensure robust data analysis, amplicon sequence variants (ASVs) that had fewer than 10 reads or were found in only one sample were excluded from the analysis. Each remaining ASV was assigned a taxonomic classification using naive Bayes machine-learning taxonomy classifiers available in the q2-feature-classifier software (Bokulich et al., 2018). The classifiers were trained using the NCBI refseq database, enabling accurate taxonomic assignment of the ASVs.
Leptin receptor genotyping
Genomic DNA was isolated using a QIAamp DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. LEPR rs790419 SNP in 65 participants was genotyped via polymerase chain reaction (PCR) amplification and direct sequencing. The forward primer was 5′-TCCAAACAAGACAATAGCGGC-3′, and the reverse primer was 5′-CCTCGAGGTTTGGTTTCATTCA-3′. The PCR products (674 bp) were sequenced using the same PCR primers. LEPR rs1173100 and rs1137101 SNPs in 65 participants were genotyped using the Applied Biosystems (MA, USA) TaqMan SNP Genotyping Assay with predesigned primer/probe sets (C_7586955_10 and C_8722581_10, respectively). PCR was performed using the StepOnePlus Real-time PCR System (Applied Biosystems) according to the following conditions: one cycle at 95°C for 10 min and 45 cycles at 92°C for 15 s and 60°C for 90 s.
Leptin hormone measurement
To measure the leptin hormone levels, the collected blood samples were centrifuged at 3,000 rpm for 10 min at 4°C. Following centrifugation, 1 mL of the resulting plasma was immediately transferred into Eppendorf tubes, which were then stored at –70°C until further analysis. The measurement of leptin hormone levels was performed using an enzyme-linked immunosorbent assay (ELISA) using Human Leptin Immunoassay Kit from R&D Systems® (MN, USA). An absorbance microplate reader (VersaMax, Molecular Devices, LLC., CA, USA) was used to read and quantify the absorbance values. The analysis was carried out by GCCL Co., Ltd (Yongin, Korea).
Lipid panel analysis
The lipid panels included TG, LDL-C, and HDL-C. Serum samples were collected at the clinical laboratory of the Chungnam National University Hospital according to the institution’s standard operating procedure.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism software version 9.5.1 for Windows 64-bit (GraphPad Software, La Jolla, CA, USA). Significance was set at p<0.05.
For the comparison of three groups, one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was utilized. When there were five or fewer non-zero values and only two comparison groups, the Mann–Whitney test was employed. Otherwise, unpaired t-tests with Welch’s correction were conducted. Correlation analysis was performed using Pearson’s correlation coefficient (r) to assess the strength and direction of correlations between variables.
RESULTS
Participants’ characteristics and LEPR genotypes
A total of 65 participants were enrolled in this clinical study (Table 1), including 7 male and 58 female participants. Mean ± SD (standard deviation) of age, BMI, leptin hormone levels, TG levels, HDL-C levels, and LDL-C levels of all participants was 35.2 ± 8.1 years, 21.4 ± 1.8 kg/m2, 7989.1 ± 6687.4 pg/mL, 101.4 ± 75.7 mg/dL, 72.9 ± 18.1 mg/dL, and 102.9 ± 27.8 mg/dL, respectively. The normal ranges of TG, HDL-C, and LDL-C levels are 45-150 mg/dL, <40 mg/dL, and 0-140 mg/dL, respectively.
Table 1.
Characteristics of the participants according to their LEPR genotypes
| Parameter | Total | rs1173100 | rs1137101 | rs790419 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G/G | G/A | A/A | G/G | G/A | T/T | T/C | ||||
| n | 65 | 17 | 37 | 11 | 52 | 13 | 58 | 7 | ||
| Age (yr) | 35.2 ± 8.1 | 36.6 ± 8.6 | 34.0 ± 7.5 | 36.7 ± 9.3 | 35.6 ± 8.2 | 33.3 ± 7.8 | 35.0 ± 8.2 | 36.7 ± 7.8 | ||
| Sex [male:female(n)] | 7:58 | 1:16 | 6:31 | 0:11 | 6:46 | 1:12 | 6:52 | 1:06 | ||
| Body Mass Index (kg/m2) | 21.4 ± 1.8 | 21.4 ± 2.0 | 21.6 ± 1.7 | 20.7 ± 1.7 | 21.4 ± 1.8 | 21.0 ± 1.7 | 21.2 ± 1.7 | 22.6 ± 2.2 | ||
| Leptin (pg/mL) | 7989.1 ± 6687.4 | 7074.3 ± 2697.8 | 8959.5 ± 8211.5 | 6227.1 ± 5086.0 | 8415.0 ± 7332.5 | 6318.3 ± 2639.8 | 7804.1 ± 6727.5 | 9495.2 ± 6647.1 | ||
| Triglyceride (mg/dL) | 101.4 ± 75.7 | 92.8 ± 46.2 | 109.8 ± 89.1 | 86.6 ± 63.1 | 102.7 ± 82.6 | 96.2 ± 38.6 | 98.3 ± 72.4 | 127.4 ± 102.0 | ||
| HDL-C (mg/dL) | 72.9 ± 18.1 | 74.8 ± 16.1 | 76.6 ± 18.8 | 67.8 ± 19.5 | 73.5 ± 19.0 | 70.6 ± 14.0 | 74.2 ± 17.7 | 62.9 ± 19.6 | ||
| LDL-C (mg/dL) | 102.9 ± 27.8 | 93.1 ± 21.8 | 105.5 ± 30.3 | 109.5 ± 25.5 | 104.4 ± 28.8 | 96.9 ± 23.6 | 102.3 ± 26.6 | 108.3 ± 38.6 | ||
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Data presented as mean ± standard deviation.
There were 17, 37, and 11 participants in the rs1173100 G/G, G/A, and A/A genotype groups, respectively. For rs1137101, 52 participants had the G/G genotype, and 13 participants had the G/A genotype. None of the participants had the A/A genotype. For rs790419, 58 participants had the T/T genotype, and 7 participants had the T/C genotype. None of the participants had the C/C genotype. When the participants’ characteristics were classified by LEPR genotype, there were no significant differences.
Gut microbiome composition
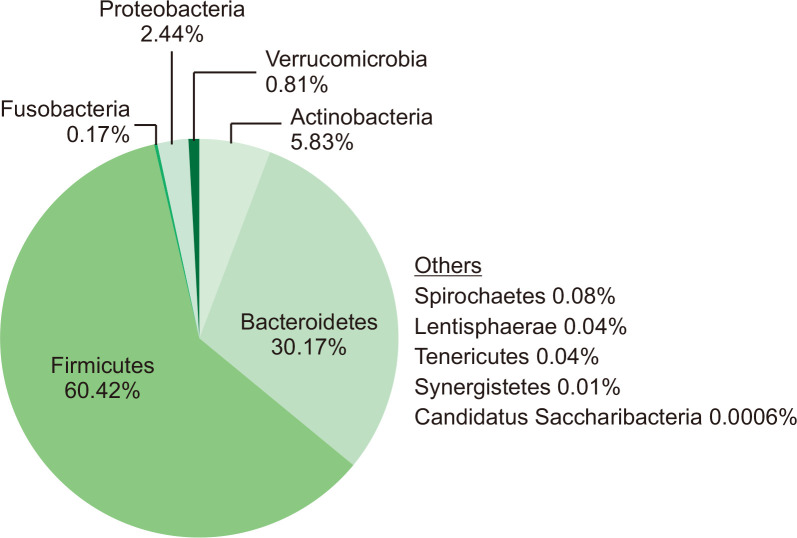
The mean ± SD Shannon diversity (alpha diversity) of the gut microbiome in all participants was 5.38 ± 0.59, ranging from 4.19 to 6.13. The relative abundance (RA) of the gut microbiota at the phylum level was calculated and averaged for all participants. The results are presented in Fig. 1. A total of eleven phyla were detected in the gut microbiome: Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia, Fusobacteria, Spirochaetes, Lentisphaerae, Tenericutes, Synergistetes, and Candidatus Saccharibacteria. The mean RA of these phyla was as follows: Firmicutes, 60.42%; Bacteroidetes, 30.17%; Actinobacteria, 5.83%; and Proteobacteria, 2.44%. The remaining phyla had a mean RA of less than 1%.
Fig. 1.
The average phylum distribution of the gut microbiome in non-obese Korean adults (N=65).
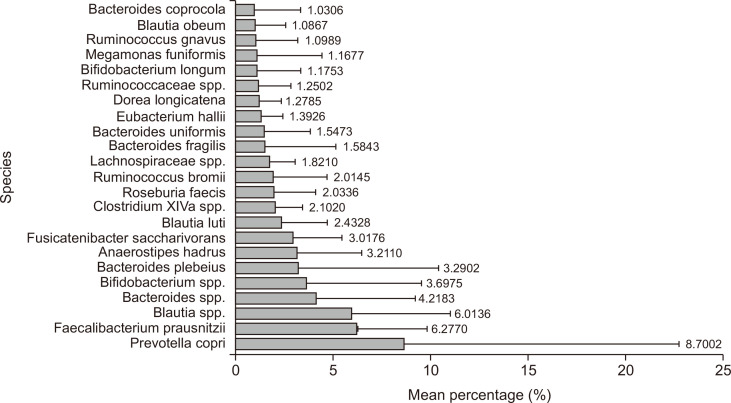
The RAs at the species level were averaged and plotted (Fig. 2). Data are shown only when the mean percentage is greater than 1%. A total of 23 species were identified. The three species with the highest RA were Prevotella copri, Faecalibacterium prausnitzii, and Blautia spp. It should be noted that “spp.” refers to multiple species within the genus Blautia. Among these species, Bacteroides plebeius had the third-highest RA. However, the exact species represented by “spp.” could not be determined due to technical limitations.
Fig. 2.
The average species distribution of the gut microbiome in non-obese Korean adults, based on species with a mean percentage above 1% (N=65).
Association between LEPR SNP and gut microbiome
ANOVA or an unpaired t-test was performed for each LEPR SNP (rs1173100, rs1137101, and rs790419) and the RA of 11 phyla. Only Candidatus Saccharibacteria showed statistically significant differences between the rs1137101 G/G and G/A genotypes in healthy non-obese Korean adults (Fig. 3).
Fig. 3.
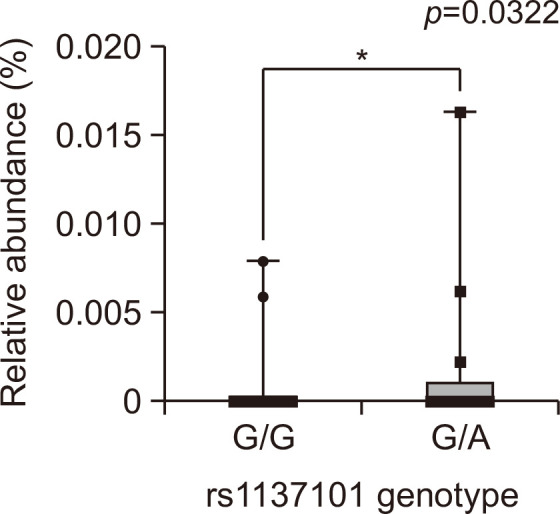
Comparison of relative abundance of Candidatus Saccharibacteria according to LEPR rs1137101 genotype (Mann–Whitney test).
Association between leptin hormone concentration and gut microbiome
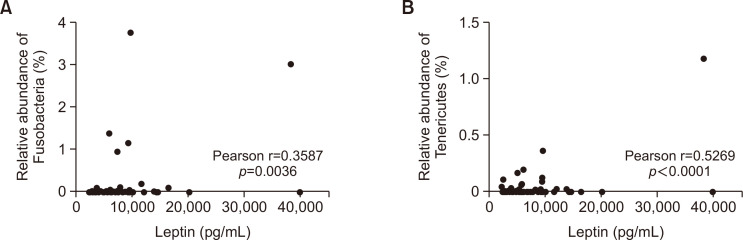
Pearson’s r was calculated for the RA of the 11 phyla and leptin concentrations. The RA of Fusobacteria and Tenericutes showed a statistically significant positive correlation with leptin concentration (Fig. 4). Other phyla did not show significant correlations.
Fig. 4.
Association between leptin concentration and relative abundance of Fusobacteria (A) and Ternericutes (B) in non-obese Korean adults. Each black circle in the graph represents an individual participant (N=65).
Relative abundance association between each phylum
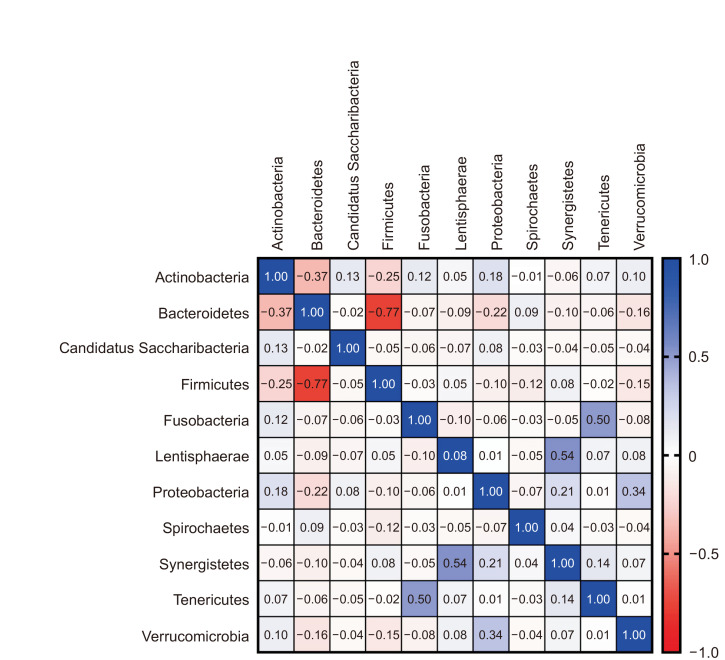
Pearson’s r was calculated to determine whether there was an association between the RA of each phylum in non-obese Korean adults (Fig. 5). The RAs of Firmicutes and Bacteroidetes were negatively correlated with each other (r=–0.77). The RAs of Synergistetes positively correlated with that of Lentisphaerae (r=0.54), and the RA of Fusobacteria positively correlated with that of Tenericutes (r=0.50).
Fig. 5.
Relative abundance association between each phylum in non-obese Korean adults (N=65).
Association between lipid panels
Pearson’s r was calculated to explore the association among LDL-C, HDL-C, and TG levels in non-obese Korean adults (Fig. 6). HDL-C levels negatively correlated with both LDL-C and TG levels (r=–0.20 and –0.46, respectively). LDL-C and TG levels were positively correlated with HDL-C levels (r=0.42).
Fig. 6.
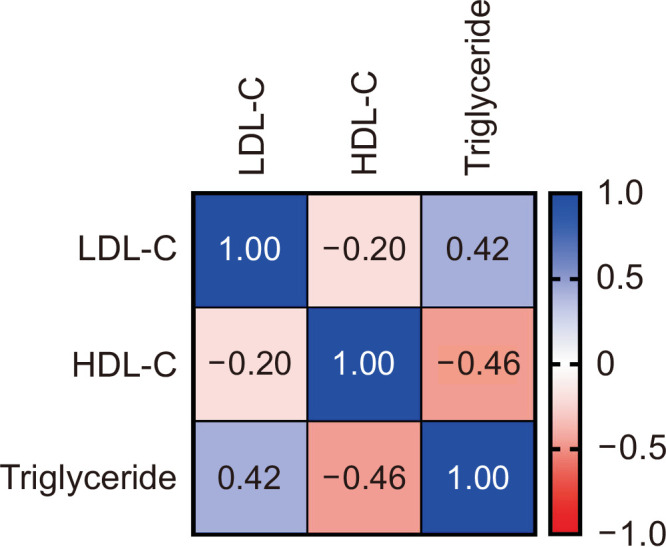
Association between low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride levels in non-obese Korean adults (N=65).
DISCUSSION
This study is the first to explore the correlations between LEPR SNPs, leptin concentration, and gut microbiome composition in non-obese Korean adults. This study is an intersection of genomics and microbiology. A healthy, non-obese Korean adult cohort was recruited. This cohort information can be used in future studies.
Candidatus Saccharibacteria is a gram-positive, uncultivated bacterium. It is mainly associated with mild-to-severe periodontitis (McLean et al., 2020). The RA of Candidatus Saccharibacteria was higher in LEPR rs1137101 heterozygous (G/A) individuals than in those with other genotypes. Some studies have reported that rs1137101 A allele carriers have a higher BMI than do G allele-only carriers (Kang et al., 2014; El Fessikh et al., 2022). However, no previous studies have investigated whether rs1137101 A allele carriers are more likely to harbor Candidatus Saccharibacteria than are carriers of other genotypes. Candidatus Saccharibacteria has been reported to be strongly associated with all obesity markers, including BMI, weight, fat mass, lean mass, waist circumference, and lipid accumulation (Gomes et al., 2020). Based on these facts, the RA of Candidatus Saccharibacteria may be higher in the gut microbiome of obese Korean adults with the rs1137101 A allele than in that of those with other genotypes.
Fusobacteria abundance is well known to be increased in the gut microbiome of patients with colon cancer (King et al., 2020), with Fusobacterium nucleatum being the most important species (Abed et al., 2020). Furthermore, the RA of Fusobacteria was found to be significantly higher in obese Japanese individuals than in lean Japanese individuals (Andoh et al., 2016). Leptin level is well-known to be elevated in the obese population (Obradovic et al., 2021). Leptin is secreted by fat cells, and many obese individuals exhibit reduced leptin sensitivity. The Fusobacterial virulence gene, Fusobacterium adhesin A (FadA), promotes the gastrointestinal translocation of Fusobacterium (Wang and Fang, 2022). If FadA-induced changes in gut permeability occur, there is a possibility that the hypothalamus detects these changes and influences leptin receptor and leptin levels. Leptin receptors are expressed in various tissues’ cell membranes, but they are most abundantly expressed in hypothalamic neurons. The hypothalamus is responsible for regulating bodily homeostasis, including hunger, body temperature, sleep, and more (Hajdarovic et al., 2022). Our study showed that higher leptin levels were correlated with a higher RA of Fusobacteria, even when the BMI was within the normal range. Whether the cause of obesity is high leptin levels or high RA of Fusobacteria is currently unknown. However, simultaneously targeting both leptin and the gut microbiome for obesity treatment may be effective for weight loss.
Tenericutes have yet to be reported for their association with human obesity. However, several studies have reported that the RA Tenericutes in the animal gut microbiome is correlated with obesity or leptin concentration (Pedersen et al., 2013; Huang et al., 2020). The RA of Tenericutes is found to be significantly decreased in metabolically unhealthy obese Chinese children compared to that in healthy normal-weight Chinese children (Yuan et al., 2021). We found no reports demonstrating a direct relationship between RA of Tenericutes and obesity in adults. However, the RA of Tenericutes tends to be low in the gut microbiome of healthy adults (Panta and Dasb, 2022).
In this study, we explored the correlation among the RA of gut microbes to infer the type of interaction among gut microbes inside the human gut. Little is known about how the gut microbiome interacts with or affects the human host or other microbes (D’Argenio and Salvatore, 2015; Gao et al., 2017).
It has been reported that the LEPR rs1137101 variant is associated with several disease. The G/G genotype of rs1137101 is known to have a higher risk of obesity compared to the A/G or A/A genotypes (Mahmoudi et al., 2016). Male multiple sclerosis patients with the G allele had higher multiple sclerosis severity scores compared to female patients (Kolić et al., 2021). In addition, it has been suggested that the genetic variation of rs1137101 is associated with the pathophysiology of both obesity and knee osteoarthritis in Egyptian female patients (Abdel-Rahman et al., 2020). While there have been reports linking rs1137101 to various conditions, no definitive causal relationship with any specific disease has been established yet. However, there is a substantial amount of research suggesting its association with obesity. In this study, demonstrating the association between rs1137101 genotype and Candidatus Saccharibacteria may contribute to mechanistically explaining the effects of LEPR SNPs on the human body.
HDL-C levels negatively correlated with both LDL-C and TG levels. This is consistent with the results of previous studies (Assmann and Schulte, 1992; Jungner et al., 1992; Choi et al., 2003). The primary focus of our study is that it specifically targeted normal-weight healthy Korean adults, as opposed to individuals who are obese. Hence, our findings suggest that even non-obese individuals need to manage their blood lipid levels steadily.
This study has several limitations. First, the number of participants was insufficient to detect the recessive genotype of LEPR. None of the participants harbored the A/A genotype of rs1137101 or the C/C genotype of rs790419. Second, almost all the participants were female, and the male-to-female ratio was not representative of the general population. Third, the participants were healthy, non-obese adults. As LEPR is closely related to obesity, further studies should be conducted on obese Korean adults. Fourth, as this study was essentially a cohort study, only cross-sectional correlations between genotypes, leptin level and the RA of gut microbiota could be observed. Additionally, due to large variations in the RA of gut microbiota among individuals, it was challenging to provide integrated interpretation.
In conclusion, associations between LEPR SNP and the gut microbiome composition and between leptin concentration and gut microbiome composition were found in non-obese Korean adults. If consistent results are obtained in follow-up studies targeting obese Korean adults, this concept could be applied to the treatment of obesity.
Funding Statement
ACKNOWLEDGMENTS This research was supported by the Basic Science Research Program through the National Research Foundation of Korea and was funded by the Ministry of Education (grant number: NRF-2022R1I1A1A01053088). We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- Abdel-Rahman A. A., Hamouda H. E., Zeid I. F., Amal M., Al-Ashwah A. A. Impact of obesity on leptin, leptin receptor gene polymorphism, and some adipokines in egyptian patients with knee osteoarthritis. Indian J. Rheumatol. 2020;15:84–91. doi: 10.4103/injr.injr_71_19.4f0f1a80334b4a3f9b2d95b8241dcd1c [DOI] [Google Scholar]
- Abed J., Maalouf N., Manson A. L., Earl A. M., Parhi L., Emgård J. E., Klutstein M., Tayeb S., Almogy G., Atlan K. A., Chaushu S. Colon cancer-associated Fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front. Cell Infect. Microbiol. 2020;10:400. doi: 10.3389/fcimb.2020.00400.dae2d4d255ff4eeabc3b471e35de357d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A., Nishida A., Takahashi K., Inatomi O., Imaeda H., Bamba S., Kito K., Sugimoto M., Kobayashi T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016;59:65–70. doi: 10.3164/jcbn.15-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G., Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience) Am. J. Cardiol. 1992;70:733–737. doi: 10.1016/0002-9149(92)90550-I. [DOI] [PubMed] [Google Scholar]
- Bokulich N. A., Kaehler B. D., Rideout J. R., Dillon M., Bolyen E., Knight R., Huttley G. A., Gregory Caporaso J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2's q2-feature-classifier plugin. Microbiome. 2018;6:90. doi: 10.1186/s40168-018-0470-z.551b94192d324bc7b48c97a0fc6d7a3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E., Rideout J. R., Dillon M. R., Bokulich N. A., Abnet C. C., Al-Ghalith G. A., Alexander H., Alm E. J., Arumugam M., Asnicar F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., Holmes S. P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. W., Choe H.-W., Pai S. H. Serum lipid concentrations correlate more strongly with total body fat than with body mass index in obese humans. Clin. Chim. Acta. 2003;329:83–87. doi: 10.1016/S0009-8981(03)00018-4. [DOI] [PubMed] [Google Scholar]
- D'Argenio V., Salvatore F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta. 2015;451:97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Duranti S., Ferrario C., Van Sinderen D., Ventura M., Turroni F. Obesity and microbiota: an example of an intricate relationship. Genes Nutr. 2017;12:18. doi: 10.1186/s12263-017-0566-2.76a8e94dc84a418b9fb5561713bba462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fessikh M., Elkarhat Z., Flatters D., Camproux A.-C., Belghiti H., Guerinech H., Bakri Y., Dakka N., El Baghdadi J. Association study of leptin receptor polymorphisms in women with obesity and their impact on protein domains: a case-control study and in silico analyses. J. Biomol. Struct. Dyn. 2022 doi: 10.1080/07391102.2022.2109755. doi: 10.1080/07391102.2022.2109755 [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Fan S.-H., Say Y.-H. Leptin and leptin receptor gene polymorphisms and their association with plasma leptin levels and obesity in a multi-ethnic Malaysian suburban population. J. Physiol. Anthropol. 2014;33:15. doi: 10.1186/1880-6805-33-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Chi L., Mahbub R., Bian X., Tu P., Ru H., Lu K. Multi-omics reveals that lead exposure disturbs gut microbiome development, key metabolites, and metabolic pathways. Chem. Res. Toxicol. 2017;30:996–1005. doi: 10.1021/acs.chemrestox.6b00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A. C., Hoffmann C., Mota J. F. Gut microbiota is associated with adiposity markers and probiotics may impact specific genera. Eur. J. Nutr. 2020;59:1751–1762. doi: 10.1007/s00394-019-02034-0. [DOI] [PubMed] [Google Scholar]
- Hajdarovic K. H., Yu D., Webb A. E. Understanding the aging hypothalamus, one cell at a time. Trends Neurosci. 2022;45:942–954. doi: 10.1016/j.tins.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-C., Huang L.-T., Sheen J.-M., Hou C.-Y., Yeh Y.-T., Chiang C.-P., Lin I.-C., Tiao M.-M., Tsai C.-C., Lin Y.-J. Resveratrol treatment improves the altered metabolism and related dysbiosis of gut programed by prenatal high-fat diet and postnatal high-fat diet exposure. J. Nutr. Biochem. 2020;75:108260. doi: 10.1016/j.jnutbio.2019.108260. [DOI] [PubMed] [Google Scholar]
- Jungner I., Walldius G., Holme I., Kolar W., Steiner E. Apolipoprotein B and A-I in relation to serum cholesterol and triglycerides in 43 000 Swedish males and females. Int. J. Clin. Lab. Res. 1992;21:247–255. doi: 10.1007/BF02591655. [DOI] [PubMed] [Google Scholar]
- Kang S. H., Lee J., Han H. R., Soh M., Hong J. P. Polymorphisms of the leptin and HTR2C genes and clozapine-induced weight change and baseline BMI in patients with chronic schizophrenia. Psychiatr. Genet. 2014;24:249–256. doi: 10.1097/YPG.0000000000000053. [DOI] [PubMed] [Google Scholar]
- King M., Hurley K. R., Davidson E. C., Dempsey M. A., Barron E. D., Chan A., Frey The link between Fusobacteria and colon cancer: a fulminant example and review of the evidence. Immune Netw. 2020;20:e30. doi: 10.4110/in.2020.20.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolić I., Stojković L., Stankovic A., Stefanović M., Dinčić E., Zivkovic M. Association study of rs7799039, rs1137101 and rs8192678 gene variants with disease susceptibility/severity and corresponding LEP, LEPR and PGC1A gene expression in multiple sclerosis. Gene. 2021;774:145422. doi: 10.1016/j.gene.2021.145422. [DOI] [PubMed] [Google Scholar]
- Mahmoudi T., Farahani H., Nobakht H., Dabiri R., Zali M. R. Genetic variations in leptin and leptin receptor and susceptibility to colorectal cancer and obesity. Iran. J. Cancer. Prev. 2016;9:e7013. doi: 10.17795/ijcp-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manju S. K., Anilkumar T. R., Vysakh G., Leena B. K., Lekshminarayan V., Kumar P. G., Shenoy T. K. A case-control study of the association of leptin gene polymorphisms with plasma leptin levels and obesity in the kerala population. J. Obes. 2022;2022:1040650. doi: 10.1155/2022/1040650.c710d1cfdebc44dc86a51a2ca7697d3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J. S., Bor B., Kerns K. A., Liu Q., To T. T., Solden L., Hendrickson E. L., Wrighton K., Shi W., He X. Acquisition and adaptation of ultra-small parasitic reduced genome bacteria to mammalian hosts. Cell Rep. 2020;32:107939. doi: 10.1016/j.celrep.2020.107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic M., Sudar-Milovanovic E., Soskic S., Essack M., Arya S., Stewart A. J., Gojobori T., Isenovic E. R. Leptin and obesity: role and clinical implication. Front. Endocrinol. 2021;12:585887. doi: 10.3389/fendo.2021.585887.077a6caec21d40eabbee5c932289bd2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, author. OECD Health Statistics 2021. 2021. [Google Scholar]
- Korea National Statistical Office, author. Obesity Rate. 2023. [retrieved 2023 Jun 12]. Available from: https://www.index.go.kr/unify/idx-info.do?idxCd=8021/
- Panta A., Dasb B. Microbiome-based therapeutics: opportunity and challenges. Prog. Mol. Biol. Transl. Sci. 2022;191:229–262. doi: 10.1016/bs.pmbts.2022.07.006. [DOI] [PubMed] [Google Scholar]
- Park K. S., Shin H. D., Park B. L., Cheong H. S., Cho Y. M., Lee H. K., Lee J.-Y., Lee J.-K., Oh B., Kimm K. Polymorphisms in the leptin receptor (LEPR)-putative association with obesity and T2DM. J. Hum. Genet. 2006;51:85–91. doi: 10.1007/s10038-005-0327-8. [DOI] [PubMed] [Google Scholar]
- Pedersen R., Ingerslev H.-C., Sturek M., Alloosh M., Cirera S., Christoffersen B. Ø., Moesgaard S. G., Larsen N., Boye M. Characterisation of gut microbiota in Ossabaw and Göttingen minipigs as models of obesity and metabolic syndrome. PLoS One. 2013;8:e56612. doi: 10.1371/journal.pone.0056612.89d2d1e7ccc3497e9ef32b7e2e6081a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranji P., Agah S., Heydari Z., Rahmati-Yamchi M., Alizadeh A. M. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the serum biochemical parameters, and the vitamin D and leptin receptor genes on mice colon cancer. Iran. J. Basic Med. Sci. 2019;22:631–636. doi: 10.22038/ijbms.2019.32624.7806.1fde790d89684cbaa2af0996d510aa2d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souren N., Paulussen A., Steyls A., Loos R., Stassen A., Gielen M., Smeets H., Beunen G., Fagard R., Derom C. Common SNPs in LEP and LEPR associated with birth weight and type 2 diabetes-related metabolic risk factors in twins. Int. J. Obes. (Lond.) 2008;32:1233–1239. doi: 10.1038/ijo.2008.68. [DOI] [PubMed] [Google Scholar]
- Tremmel M., Gerdtham U.-G., Nilsson P. M., Saha S. Economic burden of obesity: a systematic literature review. Int. J. Environ. Res. Public Health. 2017;14:435. doi: 10.3390/ijerph14040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauthier V., Jaillard S., Journel H., Dubourg C., Jockers R., Dam J. Homozygous deletion of an 80 kb region comprising part of DNAJC6 and LEPR genes on chromosome 1P31.3 is associated with early onset obesity, mental retardation and epilepsy. Mol. Genet. Metab. 2012;106:345–350. doi: 10.1016/j.ymgme.2012.04.026. [DOI] [PubMed] [Google Scholar]
- Waldram A., Holmes E., Wang Y., Rantalainen M., Wilson I. D., Tuohy K. M., McCartney A. L., Gibson G. R., Nicholson J. K. Top-down systems biology modeling of host metabotype− microbiome associations in obese rodents. J. Proteome Res. 2009;8:2361–2375. doi: 10.1021/pr8009885. [DOI] [PubMed] [Google Scholar]
- Wang N., Fang J. Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2022;31:159–172. doi: 10.1016/j.tim.2022.08.010. [DOI] [PubMed] [Google Scholar]
- Yuan X., Chen R., McCormick K. L., Zhang Y., Lin X., Yang X. The role of the gut microbiota on the metabolic status of obese children. Microb. Cell Fact. 2021;20:53. doi: 10.1186/s12934-021-01548-9.a74de7623a14479691ce0994c3a32949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Scarpace P. J. The role of leptin in leptin resistance and obesity. Physiol. Behav. 2006;88:249–256. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]