Abstract
Introduction
Paget's disease of the nipple (PDN) is a rare and often misdiagnosed condition characterized by the infiltration of adenocarcinoma cells into the nipple epidermis. It poses substantial diagnostic and therapeutic challenges due to its similarity to benign dermatological conditions and its association with in situ or invasive carcinoma.
Case presentation
This report details the case of a 47-year-old woman with persistent nipple itching, rash, and occasional bloody discharge. No abnormalities were seen on the mammogram and ultrasound scans; punch biopsy was performed to confirm PDN. A small lesion missed by other imaging methods was detected via breast magnetic resonance imaging (MRI). A second-look ultrasound with needle localization enabled precise surgery. The pathology report after breast-conserving surgery (BCS) revealed invasive ductal carcinoma with no metastasis in the sentinel lymph node biopsy.
Discussion
PDN often mimics benign skin conditions, leading to delayed diagnosis. Furthermore, timely identification is crucial as PDN is frequently associated with underlying breast malignancies. Additional imaging, such as breast MRI, is essential for comprehensive evaluation, as it can reveal hidden lesions previously undetected by conventional mammography and ultrasound. A second-look ultrasound guided needle placement for tumor localization, enhancing surgical precision, aesthetics, and reducing patient harm. Surgical management, including mastectomy, BCS with radiotherapy, and oncoplastic surgery, offers suitable options without affecting recurrence or survival in selected patients.
Conclusion
This case emphasizes the importance of employing additional imaging tools, such as breast MRI and second-look ultrasound for the early detection and surgical management of PDN.
Keywords: Adenocarcinoma, Breast malignancies, Magnetic resonance imaging, Misdiagnosed condition, Paget's disease
Highlights
-
•
Paget's disease of the nipple (PDN) is a rare, often misdiagnosed breast condition characterized by adenocarcinoma cell infiltration into the nipple epidermis.
-
•
PDN poses diagnostic challenges due to its clinical similarity to benign dermatological conditions and frequent association with carcinoma.
-
•
Additional imaging, such as breast MRI and second-look ultrasound, are essential for comprehensive evaluation, revealing previously undetected lesions.
-
•
Surgical management, including mastectomy, breast-conserving surgery (BCS) with radiotherapy, and oncoplastic surgery, offers suitable options without affecting recurrence or survival in selected patients.
1. Introduction
Paget's disease of the nipple (PDN) is a rare and often misdiagnosed breast condition characterized by adenocarcinoma cell infiltration into the nipple epidermis. This condition was first documented by a British surgeon, Sir James Paget, in 1874. PDN typically affects the skin around the nipple in women and is associated with an underlying breast carcinoma, accounting for approximately 1 %–3 % of breast malignancies [[1], [2], [3], [4]]. Despite its relative rarity, PDN poses significant diagnostic and therapeutic challenges due to its clinical similarity to benign dermatological conditions and its frequent association with in situ or invasive carcinoma (observed in 90 %–100 % of cases) [1,2,[5], [6], [7], [8], [9]].
Patients with PDN commonly present with symptoms such as pruritus, erythema, scaling, and, occasionally, sanguineous nipple discharge [1]. However, these nonspecific clinical manifestations often lead to delayed diagnosis and intervention. This report details a PDN case in a female patient with concomitant occult invasive breast cancer. The diagnostic process involved breast magnetic resonance imaging (MRI) and second-look ultrasound to identify suspected lesions, thereby reducing the extent of surgical resection. This case underscores the importance of early diagnosis, additional diagnostic imaging surveys with MRI and second-look ultrasound, and appropriate treatment strategies for managing PDN. This article was written according to the SCARE guidelines [10].
2. Case presentation
A 47-year-old premenopausal woman presented at our outpatient department with a three-month history of persistent pruritus and a rash on her right nipple and areola. She also reported sporadic bloody nipple discharge and mild erythema. The patient had no family history of breast cancer or significant medical history. Upon physical examination, we observed erythema, scaling, and minor excoriation localized to the right nipple and areola. No palpable breast masses, axillary lymphadenopathy, or skin alterations were detected elsewhere on the breast. The contralateral breast appeared unremarkable.
A diagnostic mammogram revealed bilaterally dense breast tissue with multiple benign calcifications. Bilateral breast ultrasound demonstrated fibrocystic changes in both breasts with no concerning findings, effectively ruling out underlying masses or ductal abnormalities.
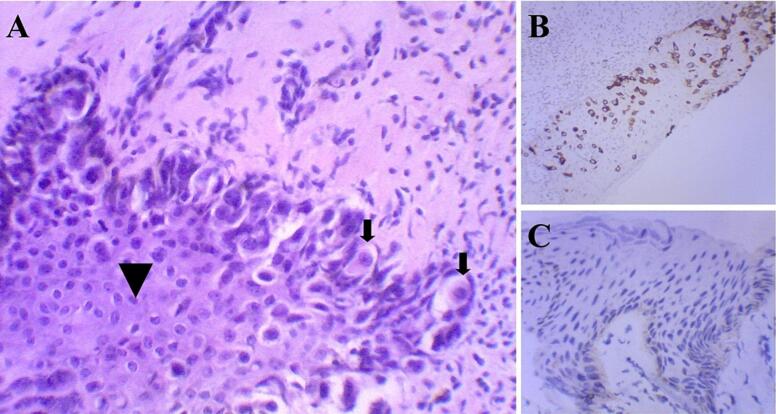
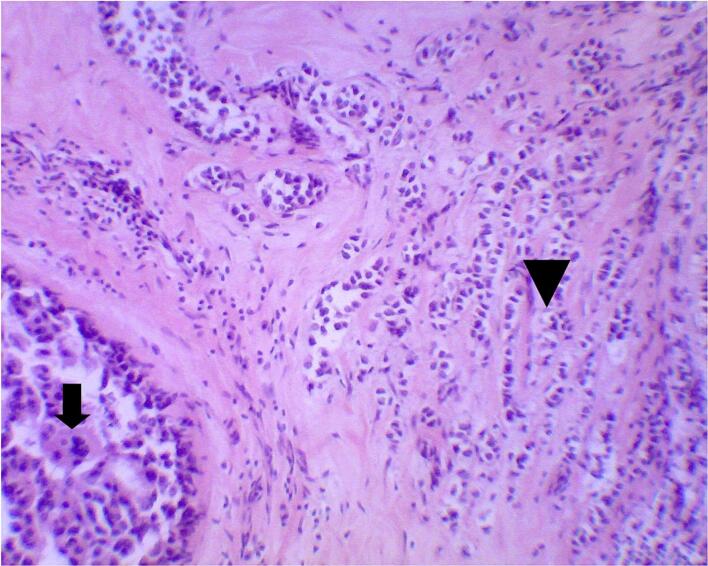
Given the persistence of the symptoms and the clinical suspicion of PDN, a punch biopsy of the right nipple and areola was performed. Histopathological examination identified a population of atypical large clear cells within the squamous epithelium, raising the suspicion of Paget's disease (Fig. 1A). Immunohistochemical staining revealed tumor cells positive for cytokeratin-7, a marker commonly associated with mammary glandular cells, and negative for S-100 protein, different from melanoma, thus confirming the diagnosis of PDN (Fig. 1B and C). In addition, intraductal proliferation testing revealed positivity for estrogen receptors (ER). P63 staining positive demonstrates preserved myoepithelial cells, confirming the diagnosis of ductal carcinoma in situ (DCIS).
Fig. 1.
Micrographs showing the section histopathology from the nipple and areola.
(A) Microscopic appearance of Paget's disease. The large clear tumor cells (arrows) are distinct from the malpighian layer (arrowhead) (H&E ×200). (B) Strong CK7 immunoreactivity in the malignant intraepithelial cells in Paget's disease (CK7, ×100). (C) The malignant intraepithelial cells in Paget's disease are negative for S-100 protein (S-100, ×200).
H&E = hematoxylin and eosin.
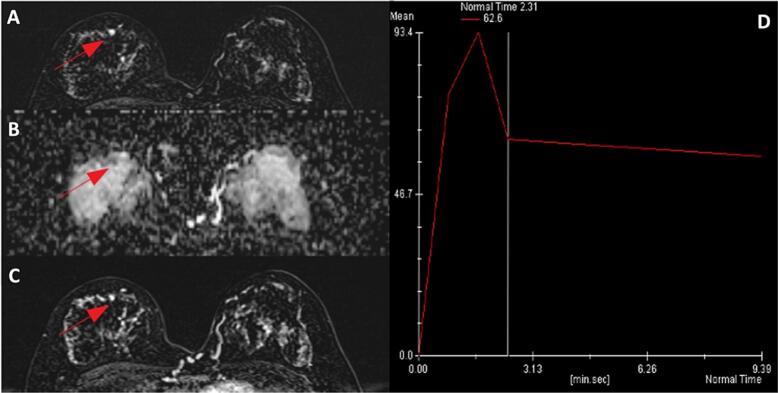
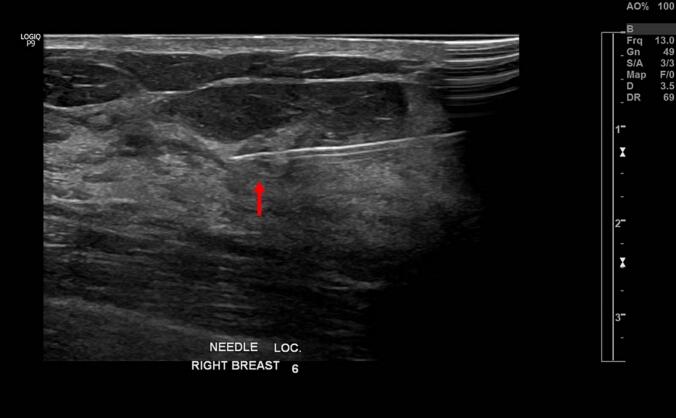
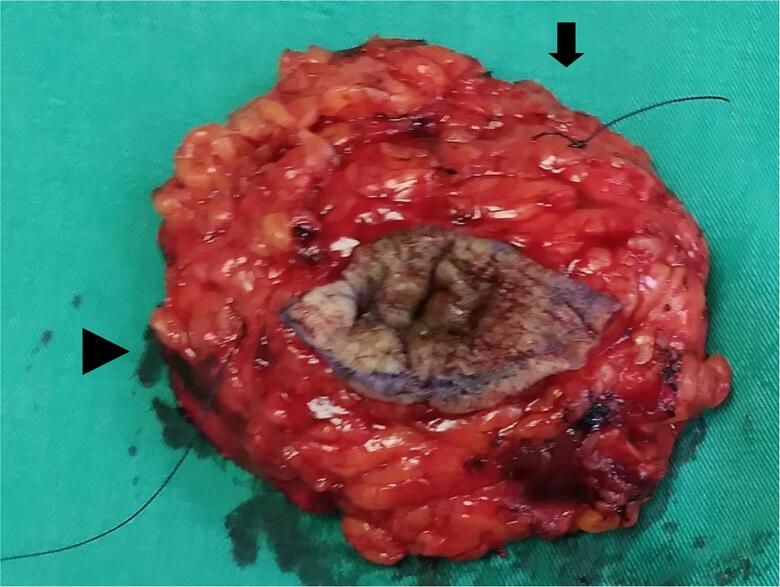
Breast MRI, which was performed to rule out any underlying breast carcinoma, demonstrated a small enhancing focus in the 12 o'clock position in the right breast (Fig. 2). Based on the clinical presentation and pathological findings, a diagnosis of both PDN and DCIS was established. Further surgical intervention was recommended; however, a second-look ultrasound with focused needle localization was performed preoperatively due to challenges in precisely identifying the suspicious lesion noted on the breast MRI scan (Fig. 3). Breast-conserving surgery (BCS) involving the removal of the nipple-areola complex (NAC) was performed (Fig. 4). The postoperative pathological report indicated DCIS and invasive ductal carcinoma, measuring approximately 0.4 cm in size (Fig. 5). The deep fascia, skin, and soft tissue margins were tumor-free. Immunohistochemical staining revealed ER positivity in 10 % of the cells; however, the cells were negative for progesterone receptor and Her-2/neu.
Fig. 2.
Breast contrast-enhanced MRI showing a small enhancing nodule in the right subareolar region (arrow); (A) Lesion DWI reveals high-signal intensity, (B) whereas ADC shows signal drop. (C) The subareolar nodule demonstrates early-phase enhancement, (D) and is followed by contrast washout in the later phase.
DWI = diffusion-weighted imaging; ADC = apparent diffusion coefficient.
Fig. 3.
Image taken after a second-look ultrasound with focused needle localization to precisely identify the suspicious lesion seen on the breast MRI scan (arrow).
Fig. 4.
Gross photograph of the specimen post breast-conserving surgery, including the excision of the nipple-areola complex. Short thread (arrow) indicates the superior margin, and long thread (arrowhead) denotes the lateral margin.
Fig. 5.
Micrographs showing the histopathology of sections from the breast tissue following breast-conserving surgery, which included the removal of the nipple-areola complex. The diagnosis revealed in situ (arrow) and invasive components of ductal carcinoma (arrowhead) (H&E ×100).
H&E = hematoxylin and eosin.
Further staging was achieved through sentinel lymph node biopsy (SLNB), which demonstrated negative results for lymph node metastasis. Subsequently, the patient received postoperative radiotherapy and hormonal therapy to minimize the risk of disease recurrence. No adverse events were reported during the 18-month follow-up period.
3. Discussion
PDN typically presents with clinical signs and symptoms that mimic benign dermatological conditions, such as eczema or contact dermatitis. Common clinical features include pruritus, erythema, scaling, and, occasionally, nipple discharge [1]. Consequently, a delayed diagnosis is common, with patients often seeking treatment from dermatologists before a correct diagnosis is made. The differential diagnosis for PDN includes various dermatological conditions affecting the nipple and areola, such as contact dermatitis, eczema, psoriasis, and Pagetoid melanoma. A timely diagnosis can significantly impact the treatment and prognosis of the disease; therefore, distinguishing PDN from these benign conditions is crucial [11].
Patients with Paget's disease exhibit distinctive characteristics that should heighten the suspicion for diagnosis. More than 90 % of Paget's disease cases are associated with an underlying breast malignancy. Therefore, even when traditional mammography and ultrasound do not reveal abnormalities, additional imaging studies such as breast MRI should be considered for further evaluation, especially in patients with PDN [3]. Lim et al. [8] underscored the significance of MRI in Paget's disease and reported its importance in assessing the nipple-areolar complex and detecting potential underlying breast malignancies. In the current case study, a small suspicious lesion that had gone unnoticed on mammography and ultrasound was detected in the breast MRI scan. Subsequently, a second-look ultrasound was conducted to identify the relative position of the potential lesion and guide the placement of a localization needle, thereby ensuring the precise localization of the tumor during surgery and minimizing the extent of the surgical procedure. This approach not only improved the aesthetic outcome but also reduced the potential harm to the patient.
Surgical management remains the primary approach for PDN. Typically, total or skin-sparing mastectomy, with or without breast reconstruction, is performed for PDN associated with multifocal disease. However, advances in imaging and patient selection have led to increased use of breast-conserving therapy for unifocal PDN limited to the nipple-areolar region. The surgical procedures include central lumpectomy for nipple-areolar complex removal and oncoplastic techniques like Grisotti mastopexy [1,12] and Wise-Pattern mammaplasty [1,13,14] for aesthetic outcomes. Nipple-areolar reconstruction and medical tattooing may enhance the post-surgery appearance and psychological well-being [1].
The utility of SLNB for PDN currently remains inconclusive. Given that PDN pathology typically remains localized to the NAC, the propensity for metastatic involvement of the axillary lymph nodes is comparatively low [7]. In this case, the patient initially underwent BCS with the removal of the NAC and a small lesion via needle localization. The decision for this surgical intervention was prompted by the suspicion of a PDN in conjunction with DCIS without clinical evidence of nodal involvement. Postoperative pathology confirmed a diagnosis of invasive cancer, staged as T1a according to the American Joint Committee on Cancer (AJCC) 8th edition staging system. Subsequent SLNB revealed no nodal metastasis.
Considering oncological outcome, Lin et al. [7] reported that Mastectomy and BCS with radiotherapy have significantly lower local recurrence rates than BCS alone in patients with PDN with underlying invasive carcinoma and DCIS. Additionally, a palpable mass, underlying invasive carcinoma, and a positive lymph node status may lead to a poorer prognosis. Helme et al. [15] reached a similar conclusion, emphasizing that the outcomes obtained after achieving the surgical margins and administering adjuvant radiotherapy were equivalent to those obtained after mastectomy. Moreover, a Brazilian study [16] indicated that oncoplastic breast surgery for Paget's disease expands treatment options without adversely affecting recurrence or survival rates.
4. Conclusion
PDN is a rare and often misdiagnosed breast condition requiring a high suspicion index for prompt recognition. Enhancing awareness among the general population and healthcare professionals is vital for early disease detection and intervention. Given the frequent association with underlying malignancies, additional diagnostic imaging examinations are imperative. MRI and second-look ultrasound play pivotal roles in uncovering potential lesions, enabling a more precise surgical approach with minimal surgical extension.
Consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Registration of research studies
Not applicable.
Ethical approval
Ethical approval was deemed unnecessary by our institutional ethical committee, as the paper is reporting a single case that emerged during normal practice.
Funding
This study received no funding.
CRediT authorship contribution statement
H.M. Li: Study conception, production of initial manuscript, collection of data.
T.N. Wen: Production of initial manuscript, revision of the manuscript, proofreading.
T.Y Huang: Pathology imaging, revision of the manuscript, proofreading.
T.H. Chang: Radiology imaging, revision of the manuscript, proofreading.
Guarantor
Tzu-Ning Wen.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT to improve readability and language. After using this service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of competing interest
There is no conflict of interest.
Acknowledgments
Not applicable.
References
- 1.Markarian S., Holmes D.R. Mammary Paget’s disease: an update. Cancers (Basel). 2022:14. doi: 10.3390/cancers14102422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piras A., Boldrini L., Venuti V., Sanfratello A., La Vecchia M., Gennari R., et al. Mammary Paget’s disease and radiotherapy: a systematic literature review. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1821–1827. doi: 10.26355/eurrev_202102_25076. [DOI] [PubMed] [Google Scholar]
- 3.Samreen N., Madsen L.B., Chacko C., Heller S.L. Magnetic resonance imaging in the evaluation of pathologic nipple discharge: indications and imaging findings. Br. J. Radiol. 2021;94:20201013. doi: 10.1259/bjr.20201013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollmorgen D.R., Varanasi J.S., Edge S.B., Carson W.E., 3rd. Paget’s disease of the breast: a 33-year experience. J. Am. Coll. Surg. 1998;187:171–177. doi: 10.1016/s1072-7515(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 5.Dubar S., Boukrid M., Bouquet de Joliniere J., Guillou L., Vo Q.D., Major A., et al. Paget’s breast disease: a case report and review of the literature. Front Surg. 2017;4:51. doi: 10.3389/fsurg.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakorafas G.H., Blanchard K., Sarr M.G., Farley D.R. Paget’s disease of the breast. Cancer Treat. Rev. 2001;27:9–18. doi: 10.1053/ctrv.2000.0203. [DOI] [PubMed] [Google Scholar]
- 7.Lin C.W., Chiang M.H., Tam K.W. Treatment of mammary Paget disease: a systematic review and meta-analysis of real-world data. Int. J. Surg. 2022;107 doi: 10.1016/j.ijsu.2022.106964. [DOI] [PubMed] [Google Scholar]
- 8.Lim H.S., Jeong S.J., Lee J.S., Park M.H., Kim J.W., Shin S.S., et al. Paget disease of the breast: mammographic, US, and MR imaging findings with pathologic correlation. RadioGraphics. 2011;31:1973–1987. doi: 10.1148/rg.317115070. [DOI] [PubMed] [Google Scholar]
- 9.Ashikari R., Park K., Huvos A.G., Urban J.A. Paget’s disease of the breast. Cancer. 1970;26:680–685. doi: 10.1002/1097-0142(197009)26:3<680::aid-cncr2820260329>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Group S. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Lopes Filho L.L., Lopes I.M., Lopes L.R., Enokihara M.M., Michalany A.O., Matsunaga N. Mammary and extramammary Paget’s disease. An. Bras. Dermatol. 2015;90:225–231. doi: 10.1590/abd1806-4841.20153189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantürk N.Z., Şimşek T., Özkan Gürdal S. Oncoplastic breast-conserving surgery according to tumor location. Eur J Breast Health. 2021;17:220–233. doi: 10.4274/ejbh.galenos.2021.2021-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J., Wechter D., Grumley J. Neoareolar wise pattern reduction in patients requiring central partial mastectomy. Ann. Surg. Oncol. 2013;20:3351. doi: 10.1245/s10434-013-3149-5. [DOI] [PubMed] [Google Scholar]
- 14.Chopra K., Tadisina K.K., Singh D.P. Breast reduction mammaplasty. Eplasty. 2013;13:ic59. [PMC free article] [PubMed] [Google Scholar]
- 15.Helme S., Harvey K., Agrawal A. Breast-conserving surgery in patients with Paget’s disease. Br. J. Surg. 2015;102:1167–1174. doi: 10.1002/bjs.9863. [DOI] [PubMed] [Google Scholar]
- 16.Pelorca R.J.F., de Oliveira-Junior I., da Costa Vieira R.A. Oncoplastic surgery for Paget’s disease of the breast. Front. Oncol. 2023;13:1151932. doi: 10.3389/fonc.2023.1151932. [DOI] [PMC free article] [PubMed] [Google Scholar]