Abstract
The present study aimed to investigate the impact of various concentrations of ginger and cinnamon oils as antibiotic substitutes on some blood biochemical parameters, antioxidant capacity, and histopathological profile of the liver and gut of growing Japanese. A total of 900 Japanese quails were randomly allotted into 6 treatment groups. Each group had 5 replicates (30 chicks each). The first group received a basal diet and served as the control, while the second received a basal diet plus 0.5 g of colistin antibiotic/kg diet. The third and fourth groups were supplemented with 0.5 mL and 1.0 mL of ginger oil (GO)/kg diet, respectively. While the fifth and sixth groups received basal diet with 0.5 and 1.0 mL of cinnamon oil (CO)/kg diet, respectively. Results showed that adding herbal oils significantly (P < 0.05) decreased the aspartate aminotransferase (AST) and urea levels compared to control and colistin groups. Various levels of GO and CO significantly (P < 0.05) reduced cholesterol levels compared to control birds. Compared to the control and antibiotic groups, Japanese quails supplemented with various levels of herbal oils (GO and CO) had more extraordinarily significant (P < 0.05) values for total antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GPX), and glutathione reductase (GSR). Regarding histopathologic examination, the jejunum displayed a nearly empty lumen, a few fusions, and mild goblet cell metaplasia. On the other hand, the duodenum looked tall and had a few fusions of villi and remnants of removal in its lumina. It could be concluded that cinnamon and GO improved birds’ blood biochemical parameters, electorate oxidative stress, and enhanced intestinal and hepatic histology of the treated quails. Also, the levels of 0.5 mL CO and 0.5 mL GO may be an acceptable substitute for antibiotics (colistin) in the diets of growing Japanese quail.
Key words: quails, blood chemistry, cinnamon, ginger, histopathology
INTRODUCTION
Poultry production, immunity and health are several factors that challenge the future growth of the poultry industry. Product quality and safety, consumer confidence, types of products, and disease prevalence are considered major challenges to the recent and strategic future of the industry (Abd El-Hack et al., 2022a; Youssef et al., 2023a). According to Salem et al. (2022), there is growing pressure on the poultry business to create superior products in large quantities for consumers. Many years ago, antimicrobial feed additives were utilized as antibiotics to boost the production of meat and eggs by controlling and preventing harmful microbes throughout the digestive system (Abd El-Hack et al., 2022b). Antibiotic used in chicken production has become banned because of residual antibiotics in meat products (Youssef et al., 2018; Jammoul and El Darra, 2019) and the emergence of antibiotic-resistant microbes in humans (Chandrakar et al., 2023). Recently, herbal plant extracts are regarded as substances obtained from herbs that are included in feed for livestock to enhance the health of the animals and product quality (Abd El-Hack et al., 2022c). Extracts, herbs and spices, aromatic oils (lipophilic compounds generated by steam distillation of the oils of grasses), and olives (compounds derived using non-hydrolysis solvents) are the categories used to describe them in terms of their sources and active principles (Windisch et al., 2007).
The gastrointestinal equilibrium of the pre-existing flora is maintained by the use of beneficial plants like ginger (Zingiber officinale) and cinnamon (Cinnamomum verum) in various forms (Ahmed et al., 2019). They also have a functional purpose by promoting the release of particular enzymes (Boyraz and Ozcan, 2006; Londoño et al., 2022). Cold-pressed ginger and cinnamon oils (COs) are excellent sources of dietary components such as organic antioxidants, essential fatty acids, and lipid-soluble bioactive compounds (Wei et al., 2022). They also acted as organic antimicrobials and antioxidants, they may directly bind with free radicals to quench them to avoid lipid peroxidation, enhancing health and avoiding some diseases (Abo El-maati et al., 2016). The bioactive ingredients of cold-pressed ginger and COs positively impact poultry's development, production, immunity, and health (Farag et al., 2014). Medicinal oils from ginger and cinnamon have antibacterial, anti-inflammatory, and antioxidant properties and stimulate the appetite and digestive system (Kamel, 2001; Dragland et al., 2003; Krauze, 2021a).
Exploring the potential effects of ginger and COs on blood biochemical parameters and oxidative status in Japanese quail is essential to understanding energy metabolism and the improvement of the animal's health condition. Therefore, this experiment was performed to study the impact of various concentrations of ginger and COs as antibiotic substitutes on some blood biochemical parameters, antioxidant capacity, and histopathological profile of the liver and gut of growing Japanese quail between the ages of 1 and 5 wk.
MATERIALS AND METHODS
The Poultry Research Farm was the site of the current investigation. All experimental procedures, bird rearing, and management were conducted upon approval from animal resources at the Faculty of Agriculture, Zagazig University, Egypt, according to the rules of the Institutional Animal Care and Use Committee.
Experimental Birds, Diets, and Management
Nine hundred 1-wk-old Japanese quail chicks were randomly assigned into 6 groups, each group consisting of 150 chicks and divided into five replicates (30 chicks/replicate). The first group received only the basic diet (as a control), while the second group received the basal diet plus 0.50 g of colistin as an antibiotic/kg of diet. The third group received the basal diet and was supplemented with 0.5 mL of ginger oil (GO)/kg diet. The fourth group received the basal diet and was supplemented with 1.0 mL of GO/kg diet. The fifth group received the basal diet and was supplemented with 0.5 mL of CO/kg diet. The sixth group received the basal diet and was supplemented with 1.0 mL of CO/kg diet. The basal diet was designed following NRC (1994). Table 1 displays the chemical composition and the structure of the basal experimental diet.
Table 1.
Chemical and compositional evaluation of the experimental diets.
| Ingredients | (%) |
|---|---|
| Yellow corn (8.5%) | 52.84 |
| Soybean meal (44%) | 38.69 |
| Gluten meal (62%) | 3.20 |
| Soybean oil | 1.67 |
| Di calcium phosphate | 0.81 |
| Limestone | 0.30 |
| Vit-min premix* | 0.30 |
| NaCl | 0.3 |
| DL-methionine (58%) | 0.39 |
| L-lysine HCl (119%) | 1.50 |
| Total | 100 |
| Calculated analysis⁎⁎ | |
| CP % | 23.51 |
| ME Kcal/kg | 2,881 |
| Ca % | 0.85 |
| P (available) % | 0.45 |
| Lysine % | 1.60 |
| Meth. + Cys. % | 0.88 |
| CF % | 3.92 |
Growth Vitamin and Mineral premix Each 2 kg consists of: Vit A 12000, 000 IU; Vit D3, 2000, 000 IU; Vit. E. 10g; Vit k3 2 g; Vit B1, 1,000 mg; Vit B2, 49g; Vit B6, 105 g; Vit B12, 10 mg; Pantothenic acid, 10 g; Niacin, 20 g, Folic acid, 1,000 mg; Biotin, 50 g; Choline Chloride, 500 mg, Fe, 30 g; Mn, 40 g; Cu, 3 g; Co, 200 mg; Si, 100 mg and Zn, 45 g.
Calculated according to NRC (1994).
Quail chicks were grown in a battery brooder cage. Using gas heaters, the house temperature was kept at 35°C for the first 3 d, then decreased to 32°C on the fourth day and then decreased by 2°C every week until it was kept at 24°C during the rest of the experimental period. Chicks received continuous light during the first 24 h, followed by 23 h per day throughout the 1 to 5 wk of age trial. Throughout the trial, every cage received unlimited access to fresh water and mash-based feed.
The Investigated Measurement
Blood Biochemical Parameters
At the end of the experimental period, 10 blood samples were collected from 10 birds randomly selected from each group. Samples were collected in sterilized tubes with rubber stoppers, which were utilized for the various analyses. Samples were centrifuged at 4,000 rpm for 10 min to collect serum. The collected serum was used to estimate aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, urea, total cholesterol, triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), total antioxidant capacity (TAC), superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GPX), and glutathione reductase (GSR) using available commercial kits as described by the manufacture companies (Spectrum, Diagnostics, Egypt, Co. for Biotechnology, S. A. E.).
Histopathological Study
Samples from quail liver and intestine were taken, fixed in a 10% neutral buffed formalin solution for 48 h, dehydrated in ascending concentrations of ethanol (70, 80, 95, and 100%), and embedded in paraffin after being washed with several changes of xylene. Five-micron thick paraffin sections were made by microtome (Leica RM 2155, London, England). Following, hematoxylin and eosin stains were routinely used to stain sections, where the prepared slides were examined under a light microscope (BX51, Olym-pus, Tokyo, Japan) (Suvarna et al., 2013). Morphometric analyses were performed on several representative photomicrographs to estimate the thickness of the submucosal layer, area % of goblet cells and intraepithelial leukocytic infiltration, and villus height from the villus’ tip to its base (mm).
Statistical Analysis
Utilized SAS software's general linear model (GLM) techniques, the data was subjected to the one-way analysis of variance (ANOVA) process for a completely randomized design (SAS, 2004). Statistical analysis was:
Where Yij = the value of an observation, μ = the overall mean, Ti = effect of treatment and eijk= random error. The post hoc Newman–Keuls test was applied to identify the variations in means. Statistics are deemed significant when P < 0.05 is met.
RESULTS
Liver and Kidney Functions
The impacts of antibiotic and herbal oils supplementation on liver and kidney functions in Japanese quails are displayed in Table 2. Results revealed that adding herbal oils (GO or CO) significantly (P < 0.05) decreased AST and urea levels compared to control and colistin supplementing quails. The lowest AST value (P < 0.05) was recorded in the third group that received a 0.5 mL GO/kg diet compared to other treated and control groups. In addition, the lowest values for urea level (P < 0.05) were recorded in the sixth group that was supplemented with a 1.0 mL CO/kg diet compared to other experimental groups.
Table 2.
Liver and kidney functions ( ± SE) for growing Japanese quails as affected by dietary supplementation.
| Liver function |
Kidney function |
|||
|---|---|---|---|---|
| Items | AST (U/L) | ALT (U/L) | Creatinin (mg/dL) | Urea (mg/dL) |
| Control | 27.67 ± 0.88a | 26.00 ± 2.61 | 0.577 ± 0.03 | 24.00 ± 1.15a |
| Colistin (0.5 g/kg diet) | 25.67 ± 2.98a | 21.33 ± 2.01 | 0.467 ± 0.04 | 20.67 ± 0.88a |
| 0.5 mL GO/kg diet | 10.67 ± 1.76d | 24.67 ± 1.18 | 0.397 ± 0.04 | 16.67 ± 1.53b |
| 1.0 mL GO/kg diet | 13.00 ± 1.00c | 23.67 ± 1.76 | 0.437 ± 0.07 | 14.00 ± 1.15b |
| 0.5 mL CO/kg diet | 11.67 ± 1.86c | 21.00 ± 2.23 | 0.383 ± 0.02 | 15.33 ± 1.76b |
| 1.0 mL CO/kg diet | 21.00 ± 2.69b | 22.67 ± 1.67 | 0.403 ± 0.06 | 10.67 ± 0.33c |
This means in the same column bearing different letters are significantly different at (P < 0.05).
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; CO: cinnamon oil; GO: ginger oil.
Regarding to ALT and creatinine levels, there were no significant differences (P > 0.05) by supplementation of colistin or various levels of GO and CO to Japanese Quail compared to the control group.
Lipid Profile
Results of supplementation of colistin and various levels of ginger and COs to Japanese quail on serum lipid profile are listed in Table 3. The cholesterol levels were significantly (P < 0.05) decreased when colistin and different levels of GO and CO were supplemented to Japanese quail than in control birds. Triglyceride, HDL, and LDL levels were not affected (P > 0.05) by supplementation of colistin and various levels of ginger and COs to Japanese quail compared to the control group.
Table 3.
Serum lipid profile for growing Japanese quails as affected by dietary supplementation.
| Items | Total cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL (mg/dL) | LDL (mg/dL) |
|---|---|---|---|---|
| Control | 249.33 ± 4.48a | 774.00 ± 15.14 | 60.67 ± 9.94 | 188.67 ± 6.36 |
| Colistin (0.5 g/kg diet) | 206.33 ± 2.19b | 440.00 ± 16.77 | 62.33 ± 7.88 | 179.67 ± 5.06 |
| 0.5 mL GO/kg diet | 214.00 ± 8.33b | 331.67 ± 19.42 | 53.67 ± 9.39 | 163.00 ± 8.14 |
| 1.0 mL GO/kg diet | 221.00 ± 2.08b | 339.33 ± 16.39 | 62.00 ± 5.11 | 166.33 ± 6.84 |
| 0.5 mL CO/kg diet | 205.00 ± 4.51b | 326.67 ± 17.75 | 55.20 ± 4.33 | 161.00 ± 6.66 |
| 1.0 mL CO/kg diet | 206.67 ± 12.02b | 366.67 ± 15.19 | 60.40 ± 5.77 | 160.67 ± 6.94 |
This means in the same column bearing different letters are significantly different at (P < 0.05).
Abbreviations: CO: cinnamon oil; GO: ginger oil; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Antioxidative Status
Table 4 illustrates the effects of dietary supplementation of colistin and different levels of GO and CO on the antioxidant parameters of Japanese quails. In comparison to the control and antibiotic groups, Japanese quails supplemented with various levels of herbal oils (GO and CO) had greater significant (P < 0.05) values for TAC, SOD, GPX, and GSR. In contrast, the herbal oil groups recorded significantly lower (P < 0.05) levels of MDA compared to the control and antibiotic-supplemented groups.
Table 4.
Serum antioxidative status for growing Japanese quails as affected by dietary supplementation.
| Items | TAC (ng/mL) | SOD (U/L) | MDA (nmol/mL) | GPX (ng/mL) | GSR (ng/mL) |
|---|---|---|---|---|---|
| Control | 0.090 ± 0.01c | 0.077 ± 0.00d | 0.443 ± 0.01a | 0.097 ± 0.02d | 0.103 ± 0.01c |
| Colistin (0.5 g/kg diet) | 0.101 ± 0.01c | 0.147 ± 0.00c | 0.300 ± 0.01b | 0.170 ± 0.01a | 0.090 ± 0.01c |
| GO (0.5 mL/kg diet) | 0.130 ± 0.01b | 0.160 ± 0.01b | 0.267 ± 0.04bc | 0.157 ± 0.01b | 0.140 ± 0.00a |
| GO (1.0 mL/kg diet) | 0.160 ± 0.02a | 0.163 ± 0.03b | 0.230 ± 0.00c | 0.167 ± 0.00a | 0.153 ± 0.02a |
| CO (0.5 mL/kg diet) | 0.133 ± 0.00b | 0.157 ± 0.00b | 0.250 ± 0.01bc | 0.113 ± 0.02c | 0.140 ± 0.00a |
| CO (1.0 mL/kg diet) | 0.170 ± 0.01a | 0.190 ± 0.01a | 0.220 ± 0.01c | 0.150 ± 0.02b | 0.137 ± 0.01b |
This means in the same column bearing different letters are significantly different at (P < 0.05).
Abbreviations: CO: cinnamon oil; GO: ginger oil; GPX: glutathione peroxidase; GSR: glutathione reductase; MDA: malondialdehyde; TAC: total antioxidant capacity; SOD: superoxide dismutase.
Histopathological Findings
Histological evolutions of liver tissues as affected by treatments are demonstrated in Figure 1. Histopathological examination of liver tissues from the control group (a) revealed normal histological structures of the hepatic parenchyma, hepatic cords, portal triad, central vein, and sinusoids. However, the liver section from quail in group 2 (b) revealed vascular congestion, focal interstitial lymphocytic aggregation, and mild to moderate degenerated hepatocytes. Moreover, the histologic examination of liver tissues in group 3 (c) revealed fatty infiltration within the centrilobular hepatocytes associated with mild focal interstitial lymphocytic aggregations. On the other hand, birds from group 4 (d) showed multifocal interstitial lymphocytic aggregation surrounded by mild to moderate degenerated hepatocytes. In addition, sinusoidal spaces were absent due to acute cellular swelling in most liver-examined sections. The examined liver sections from quails in group 5 (e) showed diffuse fatty infiltration without inflammatory cell aggregations. Also, the histologic examination for the liver section collected from quail in group 6 (f) revealed micro and macro fat vacuoles within the centrilobular hepatocytes, besides minute focal inflammatory cell aggregations.
Figure 1.
Representative photomicrograph of the quail liver of (A) negative control, (B) 0.5 g colistin antibiotic, (C) 0.5 mL ginger oil/kg diet, (D) 1 mL ginger oil/kg diet, (E) 0.5 mL cinnamon oil/kg diet, and (F) 1 mL cinnamon oil/kg diet (H&E stain; X100 except for B) showing: (A) normal vasculature, hepatic parenchyma and hepatic cords (star). (B) Focal interstitial infiltration of mononuclear inflammatory cells (arrow, X400). (C) Diffuse fatty infiltration (star) and focal interstitial lymphocytic aggregation (arrow). (D) Nearly normal hepatocytes with multifocal lymphocytic aggregation (arrow). (E) Diffuse centrilobular fatty infiltration (star). (F) Centro-lobular fatty changes (stars) beside minute inflammatory cell infiltration (arrow).
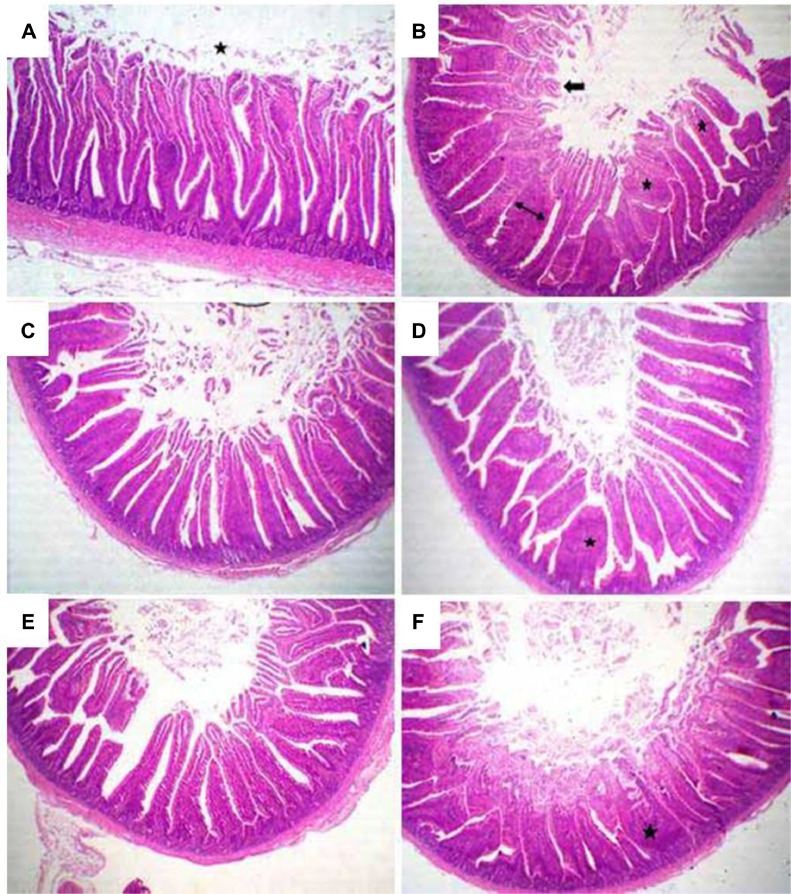
On the other side, histological evolutions of duodenum as affected by treatments are demonstrated in Figure 2. Examination of duodenum sections from the control group (a) showed normal leaf-like intestinal villi and free lumen. However, examination of duodenum tissues from quail in group 2 (b) showed shortening and fusion of the intestinal villi with most examined tissues. In addition, there was moderate necrosis in villus tips and desquamation in their lumina. The duodenum tissues from quails in the third and fourth groups (c and d) showed nearly normal apparent length of villi, with mild fusions and desquamation of villus epithelium in the lumen. Meanwhile, all examined sections from quail's duodenum in group 5 (e) revealed normal villus lengths, free lumen, and metaplasia without denudations. Duodenum sections from quails in group 6 (f) showed marked fusion of villi and mild necrosis of their tips.
Figure 2.
Representative photomicrograph of the quail duodenum of (A) negative control, (B) 0.5 g colistin antibiotic, (C) 0.5 mL ginger oil/kg diet, (D) 1 mL ginger oil/kg diet, (E) 0.5 mL cinnamon oil/kg diet, and (F) 1 mL cinnamon oil/kg diet (H&E stain; X100) showing: (A) normal leaf-like intestinal villi with free lumen (star). (B) Short fused intestinal villi (2-sided arrow), with necrotic epithelium (stars) and necrotic tips (arrow). (C) Apparently long villi with few fusions and desquamated epithelium in the lumen. (D) Apparently long and a slight fusion (star). (E) Apparently long villi with free lumen. (F) Marked villi fusion (stars) and necrosis of its tips.
Regarding the examination of jejunum segments (Figure 3), tissues from the control group (a) showed normal finger-like intestinal villi with a limited number of goblet cells. While second group (b) tissues showed denuded villus tips with desquamated parts in their lumina. Moreover, jejunum from the third group (c) showed nearly free lumen and a few fusions with slightly goblet cell metaplasia. While the jejunum tissues from the fourth group (d) examination showed long and thin villi with mild denuded villus tips. Additionally, all examined sections from quail jejunum in the fifth group (e) revealed healthy long villi, free lumen, limited goblet cells, and metaplasia without denudations. Also, jejunum sections from the sixth group (f) showed long villi without fusion or denudation.
Figure 3.
Representative photomicrograph of the quail jejunum of (A) negative control, (B) 0.5 g colistin antibiotic, (C) 0.5 mL ginger oil/kg diet, (D) 1 mL ginger oil/kg diet, (E) 0.5 mL cinnamon oil/kg diet, and (F) 1 mL cinnamon oil/kg diet (H&E stain; X100) showing: (A) normal finger like intestinal villi (arrow) with numerous goblet cells (arrowheads). (B) Denuded villus tips (arrow) with desquamated parts in the lumen (star). (C) Slightly goblet cell metaplasia (arrow), a few fusions (star) with nearly free lumen. (D) Apparently long and thin villi with mild denuded villus tips (arrow). (E) Apparently long and free lumen. (F) Apparently long villi without fusion or denudation.
Concerning the examination of the ileum (Figure 4), the control group (a) showed normal villi rich in goblet cells and free lumen. However, the second group (b) showed marked shortening and thickness of intestinal villi; enterocyte proliferation, goblet cell hyperplasia, and inflammatory cell infiltrations. While the ileum section from the third group (c) showed hyperplasia of gut association lymphocytic follicles (GALF) or Pyre's patches with nearly tall villi in other sections besides the free lumen. Ileum from the fourth group (d) quails revealed normal villi and other structures. Conversely, the examined sections from the ileum in the fifth group (e) showed mild to moderate thickening of villi due to enterocyte proliferation with goblet cell hyperplasia. In addition, ileum sections from the sixth group (f) showed healthy villi with GALF hyperplasia in some sections.
Figure 4.
Representative photomicrograph of the quail ileum of (A) negative control, (B) 0.5 g colistin antibiotic, (C) 0.5 mL ginger oil/kg diet, (D) 1 mL ginger oil/kg diet, (E) 0.5 mL cinnamon oil/kg diet, and (F) 1 mL cinnamon oil/kg diet (H&E stain; x100) showing: (A) normal intestinal villi with plenty of goblet cells. (B) Short and thick intestinal villi (2-sided arrow). (C and D) Apparently normal villi. (E) Free lumen, healthy villi without denudation or desquamation. (F) Great GALF hyperplasia (star).
DISCUSSION
In recent years, efforts have been made to replace antibiotics with natural feed additives to improve bird performance and increase disease resistance (El-Shall et al., 2022; Dosoky et al., 2023; Youssef et al., 2023b,c). Cabuk et al. (2003,2006) recommend that GO or CO dietary supplementation has a beneficial effect on the digestion of nutrients. It may also have antioxidant and antimicrobial properties in the intestine, which benefits the gastrointestinal system (Ertas et al., 2005; Meligy et al., 2023).
Results indicated that adding herbal oil significantly (P < 0.05) decreased AST levels compared to the control and antibiotic groups. This indicates that these additions successfully enhanced liver function and protected the liver. The hepatoprotective effects of herbal oil supplementation may be attributed to the high level of antioxidant and anti-inflammatory compounds in the supplemented oils (Oladokun et al., 2021; Elewa et al., 2023). Endogenous interferon induction also impacts this hepatoprotective effect (Olgun, 2016). The improvement in liver function may also be due to high levels of phospholipids in herbal oils, which are a major component of cell membrane integrity and contain 2 hydrophobic long-chain fatty acids (LCFA) (Poorghasemi et al., 2015). The same findings were reported by Mahgoub et al. (2018), who found that rosemary-derived herbal oils dramatically decreased blood AST levels in broilers. This finding suggests that the oil has enhanced liver functions and general health in broiler chickens (Polat et al., 2011) and in laying hens (Torki et al., 2018).
Regarding the kidney's performance, the results revealed a significant (P < 0.05) drop in blood urea level in response to adding herbal oils to the quail diet. Blood urea levels were lowest (P < 0.05) within the group receiving a 1.0 mL CO/kg diet. Nevertheless, the group receiving the control and antibiotic diets provided the highest value. Similar results were reported by Mahgoub et al. (2018), who found that blood creatinine and urea levels in broilers were considerably reduced by rosemary oil. Additionally, tea tree or lemongrass essential oils supplementation in broilers reared under different stocking densities significantly decreased urea levels compared to control birds (Abo Ghanima et al., 2021). This finding suggests that herbal oils can enhance renal function and the overall health of the quails.
Concerning the impacts of different levels of herbal oils and antibiotics in diets of growing quails (Table 3), quails fed GO and CO had lower significant cholesterol values than control birds with no effects on TG, LDL, and HDL. The present findings are supported by the results obtained by Polat et al. (2011), who reported that herbal oils had a hypocholesterolemic impact on broilers. According to Ghasemi and Taherpour (2015), serum cholesterol values were also lower in the diet supplemented with mannan-oligosaccharide and ginger essential oils (100 mg/kg). Additionally, Abd El-khalek and Elnaggar (2016) concluded that adding several medicinal herbs, whether singly or in combination, at any level significantly lowered the plasma total cholesterol levels in Gimmizah chicks.
Furthermore, herbal oils were reported to reduce TG and cholesterol while affecting laying quails’ lipid levels (Torki et al., 2018). Contrarily, Tekeli et al., 2006 reported that both Z. officinale and S. aromaticum food treatments increased blood levels of TG and cholesterol in broilers. The significant hypocholesterolemic effect of CO supplementation may be attributed to its inhibition impact on 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (El-Sabrout et al., 2023). This coenzyme is critical in cholesterol synthesis (Wang et al., 2023). Furthermore, the hypocholesterolemic effect of GO may be due to interfering with intestinal sterol absorption (Laka et al., 2022). Also, some ginger compounds reduce intestinal reabsorption of biliary cholesterol in laying hens, which modulates whole-body cholesterol in favor of lowering plasma cholesterol content (Akbarian et al., 2011).
Table 4 represents the effects of diet GO and CO dietary supplements on the blood antioxidant health of quails. Compared to the control and antibiotic groups, all herbal oil groups had greater values for TAC, SOD, GPX, and GSR (P < 0.05). In contrast, the herbal oil groups recorded the lower (P < 0.05) concentrations of MDA. It can be claimed that providing developing Japanese quails with a diet supplemented with GO or CO may enhance their serum oxidative status, which has a good impact on the bird's health. Our findings are consistent with those of Cetin Babaoglu et al. (2017) and Galobart et al. (2001), who found that herbal oils improve oxidative stress markers in Pharaoh quails (Coturnix coturnix Pharaoh).
Additionally, Türk et al. (2016) demonstrated the anti-oxidative properties of rosemary oil against lipid peroxidation caused by heat stress in quail. Furthermore, Bulbul (2012) reported the antioxidative properties of rosemary and GO supplementation in quails. However, An et al. (2019) found that ginger extraction only altered the SOD feature and reduced the birds’ MDA value, not the activity of GSH-PX or TAOC. The high values and activities of TAC, SOD, CAT, GP, and GSH and the suppression of MDA level of quail received diets supplemented with GO and CO indicate their ability in free radicals scavenging and render them a potent natural antioxidant (Reda et al., 2020). The antioxidant characteristics of essential oils could be enhanced by regulating and inducing the activity of antioxidant enzymes (Hsu and Liu, 2004). In addition, using essential oils phenolic mixtures enhances CAT's antioxidant capability and detoxifies the hydrogen peroxide to convert lipid hydroperoxides to none or less toxic components (Fki et al., 2005).
Regarding histopathologic examination, the jejunum displayed a nearly empty lumen, a few fusions, and mild goblet cell metaplasia. In addition to the free lumen, other areas of the ileum displayed hyperplasia of GALF or Pyre's regions with almost tall villi. The fowl in group 4 had multifocal intestinal lymphocytic clumping encircled by mild to severe hepatocyte senescence. On the other hand, the duodenum looked tall and had a few fusions of villi and remnants of removal in its lumina. Additionally, the jejunum displayed visibly tall, slender villi with hardly denuded villus tips. In addition, various structures, including villi in the ileum inspected portions, appeared normal. Our findings agree with those of Mahmoud et al. (2014), who investigated the effects of ginger and cinnamon on the gut and liver of intestines attacked by parasites.
The liver of the quail in group 5 showed extensive fatty changes without any aggregation of inflammatory cells in the examined tissues. Although each duodenal, jejunal, and ileal slice under examination showed long, healthy villi, free lumen, and minimal goblet cell growth, absent denudations were common. The liver of quail from group 6 obtained histological slices that exhibited centro-lobular fatty alterations along with minute localized inflammatory cell aggregations, and the duodenum from group 6 acquired histological parts that demonstrated considerable villi fusion and mild necrosis of its tips. Jejunum portions, however, were tall without any signs of fusion or denudation. Additionally, the quail ileum seemed in good health and was organized with villi with severe GALF hyperplasia in other areas. Krauze et al. (2021b) investigated the impact of a phytobiotic including CO and citric acid on the gut and liver of broiler chickens and reported similar histological findings. The identical histopathology observations were also reported in broiler chicks given turmeric and ginger (Sahoo et al., 2019).
CONCLUSIONS
The results of this study clearly confirmed that feeding of 0.5 mL/kg diet and CO and 0.5 mL/kg diet GO as natural feed additives to growing Japanese quail improved their blood biochemical parameters, electorate oxidative stress, and enhanced intestinal and hepatic histology when compared to control birds. Also, it may be an acceptable substitute for antibiotics (colistin) in the diets of growing Japanese quail.
DATA AVAILABILITY
Data will be available upon request from the corresponding author.
ACKNOWLEDGMENTS
The authors acknowledge Princess Nourah Bint Abdul Rahman University Researchers Supporting Project number (PNURSP2023R5), Princess Nourah Bint Abdul Rahman University, Riyadh, Saudi Arabia.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this study.
REFERENCES
- Abd El-Hack M.E., Salem H.M., Khafaga A.F., Soliman S.M., El-Saadony M.T. Impacts of polyphenols on laying hens’ productivity and egg quality: a review. J. Anim. Physiol. Anim. Nutr. 2022;107:928–947. doi: 10.1111/jpn.13758. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives: a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult.Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-khalek E., ELnaggar A.S. Growth and physiological response of Gimmizah chicks to dietary supplementation with ginger, black seeds, thyme and oregano oil as natural feed additives. Egyptian Poult. Sci. J. 2016;36:1163–1182. [Google Scholar]
- Abo El-Maati M.F., Mahgoub S.A., Labib S.M., Al-Gaby A.M., Ramadan N.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016;8:494–504. [Google Scholar]
- Abo Ghanima M.M., Swelum A.A., Shukry M., Ibrahim S.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Ammari A.A., El-Tarabily K.A., Younis M.E. Impacts of tea tree or lemongrass essential oils supplementation on growth, immunity, carcass traits, and blood biochemical parameters of broilers reared under different stocking densities. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed E.M., Attia A.I., Ibrahem Z.A., El-Hack A. Effect of dietary ginger and cinnamon oils supplementation on growing Japanese quail performance. Zagazig J. Agri. Res. 2019;46:2037–2046. [Google Scholar]
- Akbarian A., Golian A., Ahmadi A.S., Moravej H. Effects of ginger root (Zingiber officinale) on egg yolk cholesterol, antioxidant status and performance of laying hens. J. Appl. Anim. Res. 2011;39:19–21. [Google Scholar]
- An S., Liu G., Guo X., An Y., Wang R. Ginger extract enhances antioxidant ability and immunity of layers. Anim. Nutr.. 2019;5:407–409. doi: 10.1016/j.aninu.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyraz N., Ozcan M. Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. Int. J. Food Microbiol. 2006;107:238–242. doi: 10.1016/j.ijfoodmicro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Bulbul A., Bulbul T., Biricik H., Yesilbag D., Gezen S.S. Effects of various levels of rosemary and oregano volatile oil mixture on oxidative stress parameters in quails. Afr. J. Biotechnol. 2012;11:1800–1805. [Google Scholar]
- Cabuk M., Alcicek A., Bozkurt M., Imre N. Antimicrobial properties of the herbal oils isolated from aromatic plants and using possibility as alternative feed additives, II. Nat. Anima. Nutr. Cong. 2003;18:18–20. [Google Scholar]
- Cabuk M., Bozkurt M., Alcicek A., Akbas Y., Kucukyılmaz K. Effect of a herbal essential oil mixture on growth and internal organ weight of broilers from young and old breeder flocks. S. Afr. J. Anim. Sci. 2006;36:135–141. [Google Scholar]
- Cetin Babaoglu H., Bayrak A., Ozdemir N., Ozgun N. Encapsulation of clove essential oil in hydroxypropyl beta-cyclodextrin for characterization, controlled release, and antioxidant activity. J. Food Process. Preserv. 2017;41:e13202. [Google Scholar]
- Chandrakar C., Shakya S., Patyal A., Bhonsle D., Pandey A.K. Detection of antibiotic residues in chicken meat from different agro-climatic zones of Chhattisgarh, India by HPLC-PDA and human exposure assessment and risk characterization. Food Control. 2023;148 [Google Scholar]
- Dosoky W.M., Farag S.A., Almasmoum H.A., Khisheerah N.S., Youssef I.M., Ashour E.A., Mohamed L.A., Moustafa M., Al-Shehri M., Jaremko M., Abd El-Hack M.E. Influences of dietary supplementation of ginger powder and frankincense oil on productive performance, blood biochemical parameters, oxidative status and tissues histomorphology of laying Japanese quail. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragland S., Senoo H., Wake K., Holte K., Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003;133:1286–1290. doi: 10.1093/jn/133.5.1286. [DOI] [PubMed] [Google Scholar]
- Elewa M.S., Abou-Kassem D.E., El-Hindawy M.M., Madkour M., Elsharkawy M.S., Afifi M., Alagawany M. Effect of coconut oil on growth performance, carcass criteria, liver and kidney functions, antioxidants and immunity, and lipid profile of broilers. Sci. Rep. 2023;13:13974. doi: 10.1038/s41598-023-41018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sabrout K., Khalifah A., Mishra B. Application of botanical products as nutraceutical feed additives for improving poultry health and production. Vet. World. 2023;16:369. doi: 10.14202/vetworld.2023.369-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertas O.N., Guler T., Ciftci M., Dalkilic B., Yılmaz O. The effect of a dietary supplement coriander seeds on the fatty acid composition of breast muscle in Japanese Quail. Revue Med. Vet. 2005;156:514–518. [Google Scholar]
- Farag M.R., Alagawany M.M., Dhama K. Antidotal effect of turmeric (Curcuma longa) against endosulfan-induced cytogenotoxicity and immunotoxicity in broiler chicks. Int. J. Pharmacol. 2014;10:429–439. [Google Scholar]
- Fki I., Bouaziz M., Sayadi S. Hypocholesterolemic effects of phenolic-rich extracts of Chemlali olive cultivar in rats fed a cholesterol-rich diet. Bioorg. Med. Chem. 2005;13:5362–5370. doi: 10.1016/j.bmc.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Galobart J., Barroeta A.C., Baucells M.D., Codony R., Ternes W. Effect of dietary supplementation with rosemary extract and α-tocopheryl acetate on lipid oxidation in eggs enriched with ω3-fatty acids. Poult. Sci. 2001;80:460–467. doi: 10.1093/ps/80.4.460. [DOI] [PubMed] [Google Scholar]
- Ghasemi H.A., Taherpour K. Comparison of broiler performance, blood biochemistry, hematology and immune response when feed diets were supplemented with ginger herbal oils or mannan-oligosaccharide. Iran J. Vet. Med. 2015;9:195–205. [Google Scholar]
- Hsu D.Z., Liu M.Y. Sesame oil protects against lipopolysaccharide-stimulated oxidative stress in rats. Crit. Care Med. 2004;32:227–231. doi: 10.1097/01.CCM.0000104947.16669.29. [DOI] [PubMed] [Google Scholar]
- Jammoul A., El Darra N. Evaluation of antibiotics residues in chicken meat samples in Lebanon. Antibiotics. 2019;8:69. doi: 10.3390/antibiotics8020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel C. Tracing modes of action and the roles of plant extracts in non-ruminants. Rec. Adva. Anim Nutr. 2001:135–150. [Google Scholar]
- Krauze M. Vol. 8. IntechOpen Limited 5 Princes Gate Court,London, SW7 2QJ; United Kingdom: 2021. Phytobiotics, a natural growth promoter for poultry. (Advanced Studies in the 21st Century Animal Nutrition). [Google Scholar]
- Krauze M., Cendrowska-Pinkosz M., Matuseviĉius P., Stępniowska A., Jurczak P., Ognik K. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals. 2021;11:399. doi: 10.3390/ani11020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laka K., Makgoo L., Mbita Z. Cholesterol-lowering phytochemicals: targeting the mevalonate pathway for anticancer interventions. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.841639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño M.B.Z., Yunda D.F.U., Osma J.A., Maldonado-Celis M.E. Coffee, ginger and cinnamon: molecular mechanisms of action on human health. Molecular Mechanisms of Functional Food. 2022:404–430. [Google Scholar]
- Mahgoub S.A., El-Hack M.E.A., Saadeldin I.M., Hussein M.A., Swelum A.A., Alagawany M. Impact of Rosmarinus officinalis cold-pressed oil on health, growth performance, intestinal bacterial populations, and immunocompetence of Japanese quail. Poult. Sci. 2018;98:2139–2149. doi: 10.3382/ps/pey568. [DOI] [PubMed] [Google Scholar]
- Mahmoud A., Attia R., Safaa S.A.I.D., Ibraheim Z. Ginger and cinnamon: can this household remedy treat giardiasis? Parasitological and histopathological studies. Iran. J. Parasitol. 2014;9:530. [PMC free article] [PubMed] [Google Scholar]
- Meligy A.M., Abd El-Hamid M.I., Yonis A.E., Elhaddad G.Y., Abdel-Raheem S.M., El-Ghareeb W.R., Mohamed M.H.A., Ismail H., Ibrahim D. Liposomal encapsulated oregano, cinnamon, and clove oils enhanced the performance, bacterial metabolites antioxidant potential, and intestinal microbiota of broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 9th ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Oladokun S., MacIsaac J., Rathgeber B., Adewole D. Essential oil delivery route: effect on broiler chicken's growth performance, blood biochemistry, intestinal morphology, immune, and antioxidant status. Animals. 2021;11:3386. doi: 10.3390/ani11123386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgun O. The effect of dietary essential oil mixture supplementation on performance, egg quality and bone characteristics in laying hens. Ann. Anim. Sci. 2016;16:1115–1125. [Google Scholar]
- Polat U., Yesilbag D., Eren M. Serum biochemical profile of broiler chickens fed diets containing rosemary and rosemary volatile oil. J. Biol. Environ. Sci. 2011;5:23–30. [Google Scholar]
- Poorghasemi M., Seidavi A., Qotbi A., Chambers J., Laudadio V., Tufarelli V. Effect of dietary fat source on humoral immunity response of broiler chickens. Eur. Poult. Sci. 2015;79:1–8. [Google Scholar]
- Reda F.M., Alagawany M., Mahmoud H.K., Mahgoub S.A., Elnesr S.S. Use of red pepper oil in quail diets and its effect on performance, carcass measurements, intestinal microbiota, antioxidant indices, immunity and blood constituents. Animal. 2020;14:1025–1033. doi: 10.1017/S1751731119002891. [DOI] [PubMed] [Google Scholar]
- Sahoo N., Mishra S., Swain R., Acharya A., Pattnaik S., Sethy K., Sahoo L. Effect of turmeric and ginger supplementation on immunity, antioxidant, liver enzyme activity, gut bacterial load and histopathology of broilers. Indian J. Anim. Sci. 2019;9:774–779. [Google Scholar]
- Salem H.M., El-Saadony M.T., Abd El Mageed T.A, Soliman S.M., Khafaga A.F., Saad A.M., Swelum A.A., AbuQamar S.F., El-Tarabily K.A., Abd El-Hack M.E. Promising prospective effects of Withania somnifera on broiler performance and carcass characteristics: a comprehensive review. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.918961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS . SAS Institute Inc.; Cary: 2004. Statistical Analysis Systems, Version 9.1. [Google Scholar]
- Suvarna S.K., Layton C., Bancroft J.D. 7th ed. Churchill Livingstone, Elsevier; London, England: 2013. Bancroft’s Theory and Practice of Histological Techniques. [Google Scholar]
- Tekeli, A., L. Celik, H. R. Kutlu, and M. Gorgulu. 2006. Effect of dietary supplemental plant extracts on performance, carcass characteristics, digestive system development, intestinal microflora and some blood parameters of broiler chicks. Proceedings of 12th European Poultry Conference, 2006, Adana-Turkey, 10-14.
- Torki M., Sedgh-Gooya S., Mohammadi H. Effects of adding essential oils of rosemary, dill and chicory extract to diets on performance, egg quality and some blood parameters of laying hens subjected to heat stress. J. Appl. Anim. Res. 2018;46:1118–1126. [Google Scholar]
- Türk G., Çeribaşı A.O., Şimşek Ü.G., Çeribaşı S., Güvenç M., Kaya Ş.Ö., Yaman M. Dietary rosemary oil alleviates heat stress-induced structural and functional damage through lipid peroxidation in the testes of growing Japanese quail. Anim. Reprod. Sci. 2016;164:133–143. doi: 10.1016/j.anireprosci.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Wang H., Wu K., Mi X., Rajput S.A., Qi D. Effects of 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors on cholesterol metabolism in laying hens. Animals. 2023;13:1868. doi: 10.3390/ani13111868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Liu X., Zhao S., Li S., Zhang J. Research note: preservative effect of compound spices extracts on marinated chicken. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of herbal extracts as feed additives for swine and poultry. J Anim. Sci. 2007;86:140–148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Youssef I.M., Abdel-Ghany A.M., Ahmed E.G., Mahmoud S.M. Mandarah male chicks productive performance and organs weight as affected by using various routes of synbiotics treatments. J. Ani. Poult. Fish Prod. 2018;7:1–8. [Google Scholar]
- Youssef I.M., Khalil H.A., Swelum A.A., Al-Ghadi M.A., Balasubramanian B., Hassan M.S., Abd El Halim H.S., Abd El-Hack M.E., Youssef K.M., Abo-Samra M.A. Influence of dietary chitosan-oligosaccharides supplementation on productive and reproductive performance of laying hens. Ann. Anim. Sci. 2023 doi: 10.2478/aoas-2023-0082. [DOI] [Google Scholar]
- Youssef I.M., Khalil H.A., Shakoori A.M., Bagadood R.M., Alyahyawi A.Y., Alhazzaa R.A., Fakiha K.G., Nasr N., Abo-Samra M.A., Hassan M.S., Abd El Halim H.S., Abd El-Hack M.E., Jaremko M., Al-Nemi R., Youssef K.M. Immune response, hematological traits, biochemical blood parameters and histological status of laying hens influenced by dietary chitosan-oligosaccharides. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef I.M., Khalil H.A., Jaber F.A., Alhazzaa R.A., Alkholy S.O., Almehmadi A.M., Alhassani W.E., Al-Shehri M., Hassan H., Hassan M.S., Abd El Halim H.S., Abd El-Hack M.E., Youssef K.M., Abo-Samra M.A. Influence of dietary mannan-oligosaccharides supplementation on hematological characteristics, blood biochemical parameters, immune response and histological state of laying hens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon request from the corresponding author.