Key Points
Question
How does a package of multidomain interventions addressing health, nutrition, psychosocial care and support, and environmental hygiene delivered during preconception, pregnancy, and early childhood affect child neurodevelopment at 24 months?
Findings
In this second report of a randomized trial in India assessing interventions during preconception, pregnancy, and early childhood on childhood preterm births and childhood growth as primary outcomes, the secondary outcome was neurodevelopment at 24 months. Among 1712 children assessed, preconception, pregnancy, and early childhood interventions were associated with modest improvements in scores and lower incidence of moderate to severe neurodevelopmental delay in the cognitive, language, and socioemotional domains.
Meaning
Multidomain interventions in the preconception period, along with those in pregnancy and early childhood, may be beneficial for child neurodevelopment.
Abstract
Importance
Multidomain interventions in pregnancy and early childhood have improved child neurodevelopment, but little is known about the effects of additional preconception interventions.
Objective
To evaluate the effect of a multifaceted approach including health; nutrition; water, sanitation, and hygiene (WASH); and psychosocial support interventions delivered during the preconception period and/or during pregnancy and early childhood on child neurodevelopment.
Design, Setting, and Participants
In this randomized trial involving low- and middle-income neighborhoods in Delhi, India, 13 500 participants were assigned to preconception interventions or routine care for the primary outcome of preterm births and childhood growth. Participants who became pregnant were randomized to pregnancy and early childhood interventions or routine care. Neurodevelopmental assessments, the trial’s secondary outcome reported herein, were conducted in a subsample of children at age 24 months, including 509 with preconception, pregnancy, and early childhood interventions; 473 with preconception interventions alone; 380 with pregnancy and early childhood interventions alone; and 350 with routine care. This study was conducted from November 1, 2000, through February 25, 2022.
Interventions
Health, nutrition, psychosocial care and support, and WASH interventions delivered during preconception, pregnancy, and early childhood periods.
Main Outcomes and Measures
Cognitive, motor, language, and socioemotional performance at age 24 months, assessed using the Bayley Scales of Infant and Toddler Development 3 tool.
Results
The mean age of participants at enrollment was 23.8 years (SD, 3.0 years). Compared with the controls at age 24 months, children in the preconception intervention groups had higher cognitive scores (mean difference [MD], 1.16; 98.3% CI, 0.18-2.13) but had similar language, motor, and socioemotional scores as controls. Those receiving pregnancy and early childhood interventions had higher cognitive (MD, 1.48; 98.3% CI, 0.49-2.46), language (MD, 2.29; 98.3% CI, 1.07-3.50), motor (MD, 1.53; 98.3% CI, 0.65-2.42), and socioemotional scores (MD, 4.15; 98.3% CI, 2.18-6.13) than did controls. The pregnancy and early childhood group also had lower incidence rate ratios (RRs) of moderate to severe delay in cognitive (incidence RR, 0.62; 98.3% CI, 0.40-0.96), language (incidence RR, 0.73; 98.3% CI, 0.57-0.93), and socioemotional (incidence RR, 0.49; 98.3% CI, 0.24-0.97) development than did those in the control group. Children in the preconception, pregnancy, and early childhood intervention group had higher cognitive (MD, 2.60; 98.3% CI, 1.08-4.12), language (MD, 3.46; 98.3% CI, 1.65-5.27), motor (MD, 2.31; 98.3% CI, 0.93-3.69), and socioemotional (MD, 5.55; 98.3% CI, 2.66-8.43) scores than did those in the control group.
Conclusions and Relevance
Multidomain interventions during preconception, pregnancy and early childhood led to modest improvements in child neurodevelopment at 24 months. Such interventions for enhancing children’s development warrant further evaluation.
Trial Registration
Clinical Trials Registry–India CTRI/2017/06/008908
This randomized trial assesses interventions that span preconception through early childhood compared with usual care on neurodevelopment among children at age 24 months in low- and middle-income neighborhoods in India.
Introduction
More than 200 million children younger than 5 years in low- and middle-income countries do not reach their full developmental potential due to poor nutrition among multiple other factors.1 Substantial brain development occurs from gestation through the first 2 to 3 years of childhood.2 Stand-alone nutritional and nonnutritional interventions aimed at mothers and/or offspring have shown relatively small effects on child neurodevelopment.3,4,5,6,7,8,9 These interventions have encompassed a variety of approaches, including promotion of breastfeeding, supplementation with macronutrients and micronutrients, improving water and sanitation, interventions for promotion of child play and responsive stimulation, and parenting interventions.3,4,5,6,7,8,9
The effects of interventions during the preconception period have received comparatively less attention than interventions during pregnancy or early childhood. To our knowledge, no previous study has attempted to deliver evidence-based interventions to improve child growth and neurodevelopment throughout the continuum from preconception to pregnancy through a child’s first 2 years of life. This study aimed to evaluate the effect of an integrated package of health; nutrition; psychosocial support; and the water, sanitation, and hygiene (WASH) interventions delivered during preconception, pregnancy, and early childhood periods on child neurodevelopmental outcomes at 24 months of age.
Methods
This study was part of the Women and Infants Integrated Interventions for Growth Study (WINGS), a randomized trial whose primary outcomes were preterm birth and child growth outcomes at birth and 24 months, reported separately.10,11 Neurodevelopment at ages 12 and 24 months were prespecified secondary outcomes.11
Ethical approvals were obtained from the Ethics Review Committee of the Society for Applied Studies (SAS/ERC/LG/2017), New Delhi, India; Vardhman Medical College and Safdarjung Hospital, New Delhi, India (IEC/SJH/VMMC/PROJECT-2017/694), and the World Health Organization, Geneva, Switzerland (ERC.0002934).
Study Design and Participants
This individually randomized trial with a factorial design was conducted in urban low- and middle-income populations in Delhi, India.10,11 Participants were 18- through 30-year-old married women identified via house-to-house survey who had 0 or 1 child and who intended to have more children. Detailed methods are described in the protocol (Supplement 1) and a publication that reports on the primary outcomes on growth.10,11
Because neurodevelopment was a secondary outcome in this trial, assessments for the current study were conducted in a subsample of children. All children who turned 24 months of age between November 1, 2020, and February 25, 2022, were eligible. Within this cohort, all families were approached consecutively for assessment as soon as their child attained 24 months of age, until the intended sample size was achieved.
The data and safety monitoring committee for this trial met in May 2021 after study enrollment had been completed. Based on clear evidence that the interventions yielded benefit for the primary growth-related outcomes at 24 months, the committee recommended halting further assessment of these outcomes. However, the committee recommended that interventions continue for children scheduled to undergo neurodevelopmental assessments at age 24 months; these were completed in early 2022.
Randomization and Masking
After providing written informed consent, eligible participants were randomized to receive either preconception interventions or routine care. Participants who became pregnant were randomized a second time either to pregnancy and early childhood interventions or to routine care (Figure). During both randomization phases, participants were allocated to groups in a 1:1 ratio using permuted block sizes of 8 and 16. There were 4 groups resulting from the 2-step randomization: preconception, pregnancy, and early childhood interventions (group A), preconception interventions only (group B), pregnancy and early childhood interventions only (group C), and no preconception interventions and routine pregnancy and early childhood care (group D).
Figure. Screening, Enrollment, Randomization, and Follow-Up of Participants in the WINGS Trial.
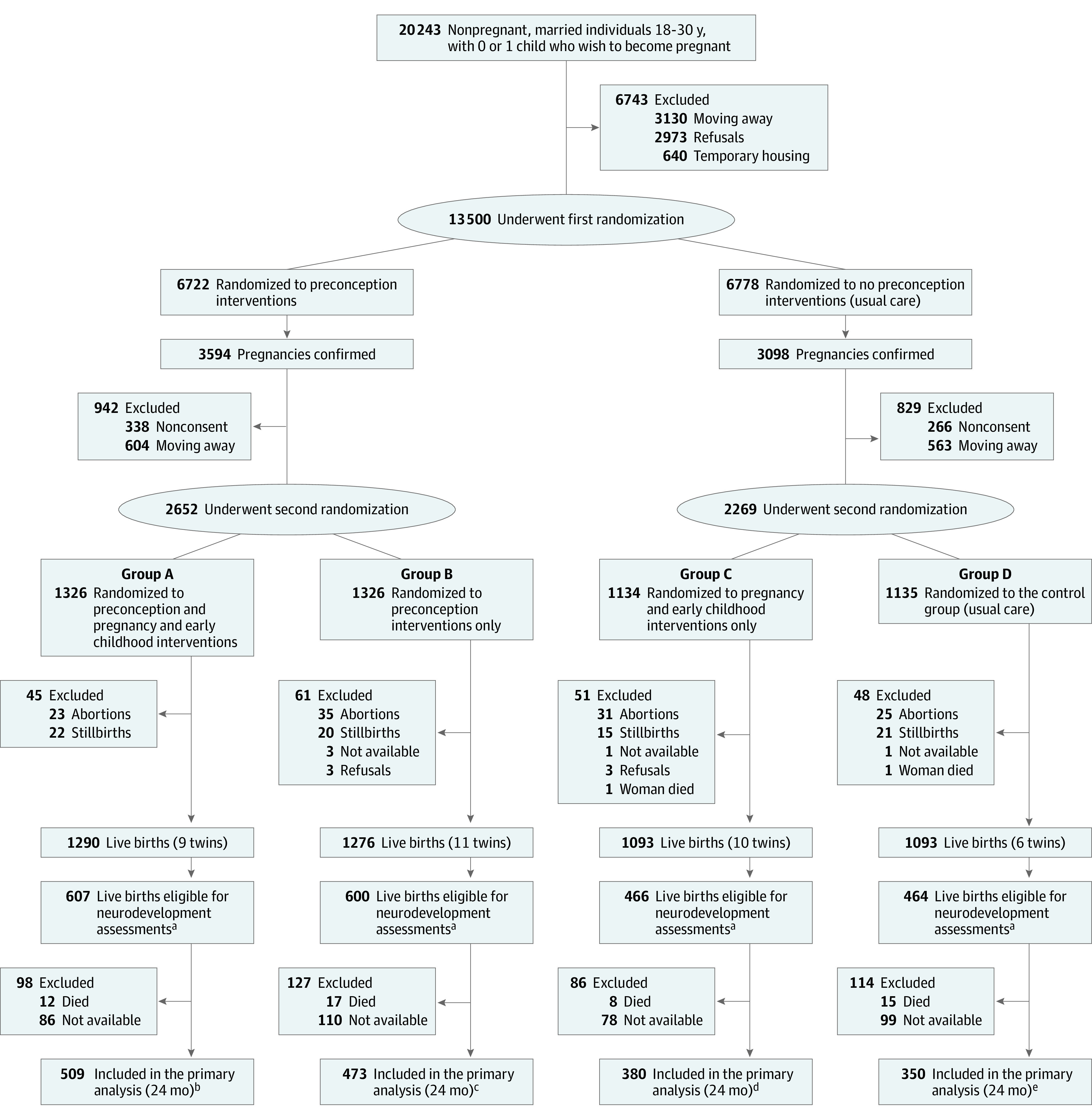
aNeurodevelopment assessments done for a subsample of children born between November 1, 2018, and February 25, 2020, as the secondary outcome of the previously published WINGS trial.11 All families were approached consecutively for assessment soon after their child attained 24 months of age, until the required sample size was achieved. There were no refusals; however, some children (and their families) had moved out of the study area at the time of their scheduled assessment (referred to as “not available”).
bTwo twins.
cTwo twins.
dThree twins.
eTwo twins.
Procedures
The preconception, pregnancy, and early childhood interventions have been described in detail elsewhere.10,11
Preconception
Participants in the control group received preconception care available through a government program (eTable 1 in Supplement 2). Interventions in the preconception period (eTable 2 in Supplement 2) included screening and treatment of participants for infections, undernutrition, anemia, and medical illnesses (eg, thyroid disorders) using standard management protocols.10,11 Participants received micronutrients, iron-folic acid along with dietary counseling, and counseling on menstrual and hand hygiene. Counseling on positive thinking and problem-solving skills was provided using an adaptation of the World Health Organization (WHO) Thinking Healthy manual.12 The Patient Health Questionnaire (PHQ-9) presented in the local language (Hindi) was used to screen participants at 3-month intervals; those with a PHQ-9 score of 10 or higher received facilitated referral to a psychologist or psychiatrist.11,13
Pregnancy
Participants in the control group were advised to register at a health facility for antenatal care to ensure structured medical support, have at least 4 antenatal care checkups; maintain daily consumption of iron folic acid, calcium, and cholecalciferol (vitamin D) throughout pregnancy; obtain supplementary foods available through the Integrated Child Development Services scheme (https://wcd.nic.in/integrated-child-development-services-icds-scheme); and plan to deliver at a health care facility (eTable 1 in Supplement 2).
Participants in the intervention groups were encouraged and facilitated by the study team to register at a health care facility. Antenatal visits included weight measurement and screening for medical conditions. Micronutrient supplements, iron folic acid, calcium, and vitamin D were provided. Nutritional supplementation in the form of locally prepared snacks and milk were provided to all intervention participants. Other interventions included installation of water filters, provision of bottles for water storage, and setting up of handwashing stations with the provision of soap and disinfectants. Positive thinking and problem-solving skills were promoted, as well as screening for and management of depressive symptoms (eTable 2 in Supplement 2).
Postnatal Period
Participants in the control group were advised to arrange postnatal health checkups and to access services available through a government program (eTable 1 in Supplement 2). Postnatal visits for participants in the intervention group were facilitated by the study team (details in eTable 2 in Supplement 2). The participants were supplemented for 6 months with locally prepared snacks and milk that provided 600 kcal of energy and 20 g of protein. In addition, micronutrient supplements were given. Exclusive breastfeeding until 6 months was promoted with support from trained lactation counselors. Counseling on positive thinking and problem-solving skills and screening and management of depressive symptoms were continued. Counseling for complementary feeding in addition to breastmilk was started at 6 months of age. Infants were given a micronutrient-enriched milk-cereal mix to provide 40% to 60% of daily requirements, and iron supplements were recommended. The Care for Child Development participants manual, developed by the WHO and the United Nations Children’s Fund (UNICEF) was adapted and used to promote child development.14 Details about the early child play and stimulation interventions used in our study along with details on the training on the psychosocial interventions are presented in eTable 2 in Supplement 2. Adherence with interventions (health, nutrition, psychosocial support, and WASH) was assessed using key indicators by study workers either through observation and documented records or by interviewing the adult participants during home visits. The study observed high adherence with the interventions delivered.10 Details on the adherence indicators are available at https://www.bmj.com/content/bmj/suppl/2022/10/26/bmj-2022-072046.DC1/tans072046.ww.pdf.
Outcomes
Neurodevelopmental outcomes at 12 and 24 months of age were prespecified secondary outcomes in this trial (Supplement 1) and were assessed by an independent team of trained personnel who were not aware of the group allocation using standardized measures. These outcomes were scores on the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) at 24 months of age.15,16 (Composite scores for each domain range from 40-160 with a mean of 100 [SD, 15], higher scores indicate better development.) The BSID-III was adapted to make the items culturally relevant and to improve understanding of the language items. The assessment was conducted in a study clinic by trained and standardized psychologists. Cognitive, motor, language, and socioemotional performance was assessed at 24 months of age.15,16 It was decided at the time of the sample size calculation that a change of approximately 2 points on BSID-III would qualify as a clinically significant difference. The Ages and Stages Questionnaire (ASQ-3) was used to assess 5 domains of development (ie, communication, gross motor, fine motor, problem solving, and personal social; range, 0-60; higher scores indicate better development).17 The ASQ-3 is a parent-reported assessment and was conducted at each child’s home at ages 12 and 24 months by trained and standardized study team workers.
The quality of stimulation, support, and opportunities for learning available for children at home was assessed using the Home Observation for Measurement of the Environment (HOME) inventory, a 45-item tool, a higher overall score indicates greater home stimulation.18 Data were collected by trained independent outcome team members based on observation or caregiver interviews in home visits. An adapted version of the Observation of Mother Child Interaction (OMCI) tool developed by Rasheed and Yousafzai19 was used to collect data on mother-child interaction at 6, 12, and 18 months of child age. The data collected were based on the observation of how the mother interacted with the child. Higher scores reflected more positive and responsive mother-child interactions.
Statistical Analysis
In the primary analysis, participants were counted with the group to which they were originally randomized. Three prespecified comparisons evaluated the effect of preconception interventions (groups A and B vs C and D), pregnancy and early childhood interventions (groups A and C vs B and D), and combined preconception, pregnancy and early childhood interventions (group A vs D). A 98.3% CI was used to account for multiple comparisons (3 comparisons) (significance level, .05/3 or .017). Interaction between preconception interventions and pregnancy and early childhood interventions was assessed.
Sample size was calculated for a power of 80% with a 2-sided α of .05, assuming that pregnancy and early childhood interventions (groups A and C) would lead to an increase in the SD of 0.15 (2.25 points) in BSID-III scores compared with routine care (groups B and D). Calculations also assumed that combined preconception, pregnancy and early childhood interventions (group A) would lead to an increase in the SD of 0.20 (3.0 points) in the neurodevelopment scores compared with the control group (group D). The final sample size was 1712 children, assuming approximately 10% refusals.
Generalized linear models were used to calculate the mean difference (MD) for continuous outcomes and incidence risk ratio (RR) for categorical outcomes. For moderate to severe neurodevelopmental impairment at 24 months of age, the absolute risk reduction was also reported. Moderate to severe neurodevelopmental impairment was defined as a composite score of less than 85 on the BSID-III.20
The baseline characteristics of the randomization groups were compared, and the analysis plan prespecified that characteristics with differences of more than 10% between groups would be adjusted in the final model.11 This was important because not all enrolled and randomized participants became pregnant and had live births during the study period. The final models were adjusted for maternal height, maternal body mass index (BMI) at the time of pregnancy detection, paternal years of schooling, family income, below-poverty-line status, and place of birth and whether the birth was a twin or multiple. Analyses were conducted in Stata version 16.0 (StataCorp).
Results
The trial resulted in 2137 live births eligible for neurodevelopmental assessment (Figure). Of these, 1712 children (80%) were assessed at 24 months of age (509 in group A, 473 in group B, 380 in group C, and 350 in group D (Figure). The baseline characteristics of participants in these groups were similar except for maternal height and BMI at the time of pregnancy detection, father’s years of schooling, annual family income, families with below-poverty-line status, and location of delivery (Table 1). Most characteristics of participants whose children had neurodevelopmental assessment were similar to those who did not (eTable 3 in Supplement 2).
Table 1. Baseline Characteristics of Participants Whose Children Had Neurodevelopmental Assessments.
| Characteristics at enrollment | No. (%) of participants | |||||
|---|---|---|---|---|---|---|
| First randomization preconception interventions | Second randomization | |||||
| Yes (n = 982) | No (n = 730) | Preconception, pregnancy and early childhood interventions, group A (n = 509) | Preconception interventions only, group B (n = 473) | Pregnancy and early childhood interventions only, group C (n = 380) | Control, group D (n = 350) | |
| Age, mean (SD), y | 23.8 (3.0) | 23.8 (3.1) | 24.4 (3.5) | 24.6 (3.0) | 24.4 (3.2) | 24.6 (3.0) |
| Height, mean (SD), cm | 152.5 (5.8) | 152.3 (5.5) | 152.6 (6.0) | 152.3 (5.7) | 152.6 (5.6) | 152 (5.3) |
| <150 cm | 320 (32.6) | 252 (34.5) | 160 (31.4) | 160 (33.8) | 132 (34.7) | 120 (34.3) |
| BMI | ||||||
| ≥25 | 224 (22.8) | 168 (23.0) | 117 (23.0) | 114 (24.1) | 85 (22.4) | 87 (24.9) |
| 18.5 to 24.99 | 591 (60.2) | 445 (61.0) | 306 (60.1) | 297 (62.8) | 235 (61.8) | 208 (59.4) |
| <18.5 | 167 (17.0) | 117 (16.0) | 86 (16.9) | 62 (13.1) | 60 (15.8) | 55 (15.7) |
| Joint or extended familya | 686 (69.9) | 502 (68.8) | 353 (69.4) | 333 (70.4) | 264 (69.5) | 238 (68.0) |
| Education ≥12 y | ||||||
| Participant | 475 (48.4) | 355 (48.6) | 241 (47.4) | 234 (49.5) | 189 (49.7) | 166 (47.4) |
| Spouse | 499 (50.8) | 356 (48.8) | 254 (49.9) | 245 (51.8) | 195 (51.3) | 161 (46.0) |
| Homemaker | 931 (94.8) | 698 (95.6) | 482 (94.7) | 449 (94.9) | 365 (96.1) | 333 (95.1) |
| Annual family income, median (IQR), US$ | 3000 (1923-3590) | 2307 (1692-3333) | 3077 (1923-3589) | 2821 (1923-3590) | 2307 (1731-3589) | 2307 (1692-3205) |
| Family below poverty lineb | 15 (1.5) | 41 (5.6) | 9 (1.8) | 6 (1.3) | 18 (4.7) | 23 (6.6) |
| Family member covered by health insurance scheme | 55 (5.6) | 115 (15.8) | 28 (5.5) | 27 (5.7) | 57 (15.0) | 58 (16.6) |
| Place of birth | ||||||
| Large hospitals | 412 (81.0) | 274 (57.9) | 321 (84.5) | 213 (60.8) | ||
| Small hospitals or birthing centers | 86 (16.9) | 162 (34.3) | 47 (12.4) | 107 (30.6) | ||
| Home birth | 11 (2.1) | 37 (7.8) | 12 (3.1) | 30 (8.6) | ||
Abbreviation: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared.
Joint or extended family includes adult relatives other than the enrolled parents and siblings living together in a household.
Families having an annual income of less than 24 000 Indian rupee (INR; US $360; 1 INR equivalent to US $0.015).
Effect of the Interventions on Neurodevelopmental Outcomes
The interaction between preconception interventions and pregnancy and early childhood interventions for neurodevelopmental outcomes based on BSID-III and ASQ-3 at 24 months of age were assessed. There was no evidence of interaction, and the comparisons of individual groups have been presented (eTable 4 in Supplement 2). There were no evident harms or unintended effects due to the study interventions in any of the groups.
Preconception Interventions Alone
Compared with the control groups (C and D) at 24 months of age, children in the preconception intervention groups (A and B) had higher BSID-III cognitive scores (MD, 1.16; 98.3% CI, 0.18 to 2.13) but similar language, motor and socioemotional scores (Table 2). On the ASQ-3 at 12 and 24 months of age, mean scores on communication, gross and fine motor, problem solving, and personal social domains did not differ between the preconception intervention and control groups (Table 2 and Table 3). The OMCI mother-child bonding score at 6, 12, and 18 months as well as the HOME score at 24 months did not differ between the preconception intervention and routine care groups (Table 3). Except for the socioemotional domain with an incidence RR of 0.52 (98.3% CI, 0.28 to 0.98) and an absolute risk reduction of −2.0% (98.3% CI, −4.0% to 0%), the proportion of children with moderate to severe delay in all other domains considered (ie, cognition, language, and motor) were not different between the preconception intervention and control groups (Table 4).
Table 2. Effect on Neurodevelopment Outcomes Assessed at Age 24 Months.
| Outcomes | Group, mean (SD) scorea | Mean difference (98.3% CI)b | |||||
|---|---|---|---|---|---|---|---|
| A (n = 509) | B (n = 473) | C (n = 380) | D (n = 350) | Preconception intervention (A+B) vs none (C+D) | Pregnancy intervention (A+C) vs none (B+D) | Preconception and pregnancy intervention (A) vs none (D) | |
| Bayley Scales of Infant and Toddler Development 3 composite scorec | |||||||
| Cognitive | 95.0 (7.9) | 93.7 (8.1) | 93.9 (7.5) | 92.4 (9.5) | 1.16 (0.18 to 2.13) | 1.48 (0.49 to 2.46) | 2.60 (1.08 to 4.12) |
| Language | 93.5 (9.8) | 91.3 (10.1) | 92.2 (9.7) | 90.3 (11.7) | 1.12 (−0.10 to 2.34) | 2.29 (1.07 to 3.50) | 3.46 (1.65 to 5.27) |
| Motor | 97.4 (7.1) | 95.9 (7.2) | 96.4 (6.4) | 95.2 (8.8) | 0.87 (−0.01 to 1.75) | 1.53 (0.65 to 2.42) | 2.31 (0.93 to 3.69) |
| Socioemotional | 117.0 (15.4) | 113.3 (17.0) | 115.9 (16.1) | 111.5 (17.9) | 1.50 (−0.45 to 3.45) | 4.15 (2.18 to 6.13) | 5.55 (2.66 to 8.43) |
| Ages and Stages Questionnaire 3 score d | |||||||
| Communication | 52.0 (10.3) | 48.8 (13.3) | 51.1 (11.5) | 47.5 (13.8) | 1.05 (−0.41 to 2.52) | 3.43 (1.95 to 4.91) | 4.66 (2.49 to 6.82) |
| Gross motor | 52.8 (8.0) | 51.3 (9.0) | 52.6 (8.4) | 50.8 (9.9) | 0.25 (−0.80 to 1.30) | 1.68 (0.63 to 2.74) | 2.06 (0.47 to 3.65) |
| Fine motor | 47.0 (9.1) | 44.6 (10.4) | 45.8 (9.2) | 44.6 (10.3) | 0.50 (−0.65 to 1.65) | 1.93 (0.76 to 3.09) | 2.44 (0.76 to 4.12) |
| Problem solving | 52.0 (10.2) | 49.4 (11.5) | 50.6 (11.8) | 49.2 (11.5) | 0.71 (−0.64 to 2.05) | 2.06 (0.72 to 3.40) | 2.56 (0.66 to 4.47) |
| Personal social | 49.2 (10.4) | 47.2 (10.5) | 48.8 (10.6) | 46.3 (11.7) | 0.57 (−0.71 to 1.86) | 2.10 (0.81 to 3.38) | 2.86 (0.94 to 4.79) |
The numbers denote children who were assessed for their neurodevelopment, the secondary outcome of the previously published WINGS trial,11 at 24 months of age. Group A represents the preconception, pregnancy, and early childhood intervention; group B, only the preconception intervention; group C, only the pregnancy and early childhood intervention; and group D, control (usual care).
Adjusted for clustering for twin birth, maternal height, maternal body mass index at the time of pregnancy detection, paternal years of schooling, family income, below-the-poverty-line status, and place of delivery.
Composite scores for each domain range from 40 to 160 with a mean of 100 (SD, 15; higher scores indicate better development).
Scores in each domain range from 0 to 60 (higher scores indicate better development).
Table 3. Neurodevelopmental Outcomes at 12 Months .
| Outcomes | Group, No. (%)a | Mean difference (98.3% CI)b | |||||
|---|---|---|---|---|---|---|---|
| A (n = 403) | B (n = 380) | C (n = 323) | D (n = 320) | Preconception intervention (A+B) vs none (C+D) | Pregnancy intervention (A+C) vs none (B+D) | Preconception and pregnancy intervention (A) vs none (D) | |
| Ages and Stages Questionnaire 3 c | |||||||
| Communication | 51.1 (10.5) | 48.5 (11.7) | 50.8 (10.2) | 48.2 (12.2) | 0.20 (−1.22 to 1.62) | 2.55 (1.08 to 4.02) | 2.89 (0.77 to 5.02) |
| Gross motor | 52.1 (10.8) | 49.7 (12.1) | 52.5 (10.3) | 48.4 (15.2) | 0.35 (−1.23 to 1.92) | 3.46 (1.84 to 5.09) | 3.98 (1.43 to 6.54) |
| Fine motor | 47.6 (12.3) | 44.9 (13.2) | 48.1 (10.5) | 42.8 (13.8) | 0.83 (−0.80 to 2.45) | 3.85 (2.22 to 5.49) | 4.79 (2.39 to 7.19) |
| Problem solving | 45.5 (12.2) | 45.1 (12.9) | 47.2 (11.5) | 43.5 (13.3) | −0.19 (−1.79 to 1.42) | 1.82 (0.14 to 3.50) | 1.90 (0.51 to 4.31) |
| Personal social | 42.7 (13.2) | 40.7 (13.1) | 42.2 (13.7) | 39.8 (14.7) | 0.73 (−1.04 to 2.50) | 2.29 (0.48 to 4.09) | 3.18 (0.59 to 5.77) |
| Observation of Mother-Child Interaction d | |||||||
| At 6 mo | 31.6 (4.9) | 30.7 (4.5) | 31.4 (4.7) | 30.5 (4.3) | 0.26 (−0.49 to 1.01) | 0.84 (−0.08 to 1.60) | 1.17 (−0.13 to 2.22) |
| At 12 mo | 35.6 (6.5) | 35.2 (6.2) | 36.2 (6.0) | 35.2 (6.5) | −0.29 (−1.13 to 0.55) | 0.73 (−0.12 to 1.58) | 0.34 (−0.93 to 1.60) |
| At 18 mo | 38.0 (5.8) | 38.2 (5.6) | 38.6 (5.5) | 37.6 (6.3) | −0.04 (−0.80 to 0.72) | 0.33 (−0.44 to 1.10) | 0.26 (−0.88 to 1.40) |
| Home Observation for Measurement of the Environmente | |||||||
| 24 mo | 42.8 (3.5) | 42.7 (3.4) | 43.1 (3.5) | 42.9 (3.3) | −0.23 (−0.75 to 0.30) | 0.12 (−0.41 to 0.65) | −0.05 (−0.82 to 0.73) |
Abbreviations: ASQ-3, Ages and Stages Questionnaire, Third Edition; HOME, Home Observation for Measurement of the Environment.
Denotes the number (%) of children who were assessed for neurodevelopment, the secondary outcome of the previously published WINGS trial,11 using ASQ-3 tool. See footnote a in Table 2 for the group definitions.
Adjusted for clustering for twin births, maternal height, maternal body mass index at the time of pregnancy detection, paternal years of schooling, family income, below-the-poverty-line status, and place of delivery.
Scores range from 0 to 60 (higher scores indicate better development).
Assessments were conducted for a subsample of children: 6 months: 254 in group A, 223 in group B, 205 in group C, and 202 in group D; 12 months: 373 in group A, 348 in group B, 301 in group C, and 295 in group D; and 18 months: 398 in group A, 373 in group B, 313 in group C, and 313 in group D. The tool consists of a total of 19 items (score range, 0-3, with a maximum 57; higher scores indicate a more responsive interaction).
HOME assessments were conducted in a subsample (296 in groups A and B each, 213 in group C, and 192 in group D). Consists of 45 items with responses coded at either 0 (not observed or reported during visit) and 1 (observed or reported during visit). The maximum score possible is 45. Higher scores indicate better stimulation and learning opportunities at home.
Table 4. Risk of Neurodevelopmental Delay Among Children Aged 24 Months.
| Outcomes | Group, No. (%)a | Preconception intervention, yes vs no, A+B vs C+D | Pregnancy intervention, yes vs no group, A+C vs B+D | Preconception and pregnancy intervention, yes vs no group, A vs D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A (n = 509) | B (n = 473) | C (n = 380) | D (n = 350) | IRR (98.3% CI)b | ARR (%) (98.3% CI)b | IRR (98.3% CI)b | ARR (%) (98.3% CI)b | IRR (98.3% CI)b | ARR (%) (98.3% CI)b | |
| Cognitive | 28 (5.5) | 30 (6.3) | 24 (6.3) | 43 (12.3) | 0.66 (0.44 to 1.01) | −3.0 (−6.0 to 0) | 0.62 (0.40 to 0.96) | −3.0 (−7.0 to 0.00) | 0.44 (0.24 to 0.80) | −7.0 (−12.0 to −2.0) |
| Language | 78 (15.3) | 106 (22.4) | 69 (18.2) | 84 (24.0) | 0.90 (0.92 to 1.14) | −3.0 (−8.0 to 2.0) | 0.73 (0.57 to 0.93) | −6.0 (−11.0 to −1.0) | 0.65 (0.46 to 0.93) | −8.0 (−15.0 to −1.0) |
| Motor | 16 (3.1) | 21 (4.4) | 13 (3.4) | 22 (6.3) | 0.78 (0.44 to 1.37) | −1.0 (−3.0 to 2.0) | 0.65 (0.36 to 1.17) | −2.0 (−4.0 to 1.0) | 0.52 (0.24 to 1.12) | −3.0 (−6.0 to 2.0) |
| Socioemotional | 8 (1.6) | 15 (3.2) | 11 (2.9) | 22 (6.3) | 0.52 (0.28 to 0.98) | −2.0 (−4.0 to 0) | 0.49 (0.24 to 0.97) | −2.0 (−4.0 to 0) | 0.28 (0.10 to 0.79) | −4.0 (−8.0 to −1.0) |
Abbreviations: ARR, absolute risk reduction; IRR, incidence rate ratio.
Denotes the number (%) of children who were assessed for neurodevelopment, the secondary outcome of the previously published WINGS trial,11 using the Bayley Scales of Infant and Toddler Development Third Edition assessment. See footnote a in Table 2 for the group definitions.
Adjusted for clustering for twin birth, maternal height, maternal body mass index at the time of pregnancy detection, paternal years of schooling, family income, below-the-poverty-line status, and place of delivery. Neurodevelopmental delay was defined as composite score of less than 85 on Bayley Scales of Infant and Toddler Development, Third Edition, assessment.
Pregnancy and Early Childhood Interventions
Children in the pregnancy and early childhood intervention groups (A and C) had higher cognitive (MD, 1.48; 98.3% CI, 0.49-2.46), language (MD, 2.29; 98.3% CI, 1.07-3.50), motor (MD, 1.53; 98.3% CI, 0.65-2.42) and socioemotional scores (MD, 4.15; 98.3% CI, 2.18-6.13) than those in the control groups (B and D). Children in the pregnancy and early childhood intervention groups had higher mean scores on all ASQ-3 developmental domains compared with the control groups (Table 2 and Table 3).
The mother-child bonding score and the HOME score showed no significant differences between the pregnancy and early childhood intervention groups and the control groups (Table 3). The pregnancy and early childhood intervention groups compared with the control groups had a lower proportion with moderate to severe delay outcomes with incidence RRs of 0.62 (98.3% CI 0.40 to 0.96) for cognitive, 0.73 (98.3% CI, 0.57 to 0.93) for language, and 0.49 (98.3% CI, 0.24 to 0.97) for socioemotional domains. The absolute risk reductions were −3.0% (98.3% CI, −7.0% to 0.0%) for cognitive, −6.0%, (98.3% CI, −11.0% to −1.0%) for language, and −2.0% (98.3% CI, −4.0% to 0%) for socioemotional domains (Table 4).
Combined Preconception, Pregnancy, and Early Childhood Interventions
Children in the preconception, pregnancy, and early childhood intervention group (A) had significantly higher cognitive (MD, 2.60; 98.3% CI, 1.08 to 4.12), language (MD, 3.46; 98.3% CI, 1.65 to 5.27), motor (MD, 2.31; 98.3% CI, 0.93 to 3.69), and socioemotional scores (MD, 5.55; 98.3% CI, 2.66 to 8.43) than the control group (D; Table 2). On the ASQ-3 based assessments, children in the preconception, pregnancy, and early childhood intervention group had higher mean scores on all developmental domains compared with the control group (Tables 2 and 3). The mother-child bonding score and the HOME score were statistically similar between groups A and D (Table 3).
The preconception, pregnancy, and early childhood intervention group (A) compared with the control group (D) had a lower proportion of moderate to severe delay with incidence RRs of 0.44 (98.3% CI, 0.24 to 0.80) for cognitive; 0.65 (98.3% CI, 0.46 to 0.93) for language, and 0.28 (98.3% CI, 0.10 to 0.79) for socioemotional domains. The absolute risk reductions were −7.0% (98.3% CI, −12.0% to −2.0%) for cognitive, −8.0%, (98.3% CI, −15.0% to −1.0%) for language, and −4.0% (98.3% CI, −8.0% to −1.0%) for socioemotional domains (Table 4).
Interaction Between Interventions
The interaction between preconception interventions and pregnancy and early childhood interventions for neurodevelopmental outcomes based on BSID-III and ASQ-3 scores at 24 months of age was assessed. There was no evidence of interaction (eTable 4 in Supplement 2). No harms or unintended effects due to the study interventions were observed in any of the groups.
Discussion
A set of multidomain preconception, pregnancy, and early childhood interventions resulted in modest but significant improvements in cognitive, language, and socioemotional outcomes in children at 24 months of age compared with routine care. Interventions in pregnancy and early childhood alone also resulted in significant improvement, although the effects were smaller than combined preconception, pregnancy, and early childhood interventions. Preconception interventions alone did not have significant effect on neurodevelopmental outcomes except for small differences in the cognitive and socioemotional domains. Additional studies are required to better understand the intricate dynamics between various interventions and contextual elements and thereby provide granular insights on how best to optimize child neurodevelopmental outcomes.
This study was novel in several respects. First, the conventional approach of examining individual interventions was not adopted. Instead, an intervention package was rigorously tested. This package was thoughtfully curated, drawing from a systematic review of contemporary literature. Second, the study included the preconception period, a relatively understudied period in terms of understanding how interventions during this time influence birth outcomes, as well as growth and neurodevelopment outcomes at 24 months of age. Third, the question addressed was novel, evaluating the maximum impact on growth and neurodevelopment when interventions of known benefit were delivered together from preconception until the child was aged 24 months with high quality and rigor. The adoption of a factorial design enabled discernment of the distinct effects of (1) preconception interventions alone; (2) pregnancy and early childhood interventions alone; and (3) combined preconception, pregnancy, and early childhood interventions.
Other strengths include a robust study design with a large sample size, high adherence with the interventions, and the trial’s setting in low- to middle-income populations, enhancing its generalizability to high-risk groups. Outcome assessments were conducted by a team of psychologists trained in standardized procedures. Another novel aspect is that the current study reported on the proportion of children in whom developmental delay was prevented, an outcome not addressed in previous studies. This study observed a 35% to 72% reduction in the risk of moderate to severe delay in cognitive, language, and socioemotional domains for children who received the package of interventions from preconception until 24 months of child age.
This trial found that integrated nutrition and stimulation interventions improved both growth and neurodevelopmental outcomes. A previous article from the current trial reported that the interventions in this study improved growth outcomes at birth and 24 months.10 This trial’s findings on growth contrast with previous trials that tested integrated interventions in children and documented improvements in child development outcomes but were unable to demonstrate impact on growth. A recent meta-analysis by Dulal et al21 included 22 trials involving children younger than 5 years that had integrated interventions consisting of nutritional and child-stimulation components. The review found that the interventions did not improve growth but did improve developmental scores compared with either routine care or stand-alone nutritional interventions.
This study observed a larger effect on both growth and neurodevelopment than previous studies that only evaluated nutritional interventions, directed to either mother and/or child.3,5,22,23,24,25 On the other hand, this study had somewhat smaller effects on neurodevelopment compared with studies focused on provision of learning opportunities in young children, positive parenting, and responsive care interventions alone. Such studies documented significantly higher effect sizes of approximately 0.30 to 0.60 SDs for cognitive, language, motor, and social-emotional development.8,25,26 The higher effect sizes could reflect that such studies focused mostly on optimal delivery of the stimulation interventions through frequent contacts and more intensive delivery mechanisms that may not otherwise be possible with integrated interventions wherein focus tends to be shared among all individual components.
The specific mechanisms by which the current study’s package of interventions led to improved neurodevelopment were not evaluated. However, interventions to improve nutritional status, manage medical morbidities, and enhance psychological health during preconception and pregnancy, along with improved breastfeeding, provision of high-quality complementary food, adequate management of infections, and optimal delivery of early child–stimulation interventions in the postnatal period could have played a critical role.
Limitations
This study had several limitations. First, because the interventions were delivered concurrently, it was not possible to discern the individual effects of the intervention components on child developmental outcomes. Second, complete blinding could not be established due to the nature of interventions. However, an independent team, not involved in the intervention delivery or aware of the group allocation, conducted the assessment of the study outcomes. Third, restrictions due to the COVID-19 pandemic had some effects on intervention delivery, and outcome assessment was delayed for some participants. Fourth, no universally accepted standard cutoff exists for defining moderate to severe neurodevelopmental delay using the BSID-III, and the choice of 85 was based on existing literature. Fifth, the raw scores for each domain in the BSID-III assessment were converted into a composite score based on US norms. When applied to children in India, the resulting scores might not have indicated their actual developmental function. However, this may not have biased the observed findings because of the study’s design as a randomized clinical trial.
Sixth, the study was conducted among low- to mid-socioeconomic populations of urban Delhi, but only 1% to 6% of the families were below the poverty line, a lower percentage than expected. Regardless of whether they were below the poverty line, the families within the study area exhibited similar access to health care facilities, nutritional practices, water and sanitation access, and cultural norms pertaining to childcare. Moreover, the health and nutrition indicators observed in the control group mirrored those found in many other regions across India.10,27 Consequently, the findings can be extrapolated to a wide range of locations within the country, as well as to similar resource-limited settings globally.
Conclusion
Multidomain interventions during preconception, pregnancy, and early childhood led to modest improvements in child neurodevelopment at 24 months. Such interventions for enhancing children’s development warrant further evaluation.
Study Protocol
Statistical Analysis Plan
eTable 1. Control Group: National Programs for women of reproductive age, pregnant women and under-twos
eTable 2. Study interventions
eTable 3. Characteristics of participants whose children had neurodevelopmental evaluations, compared to those were not evaluated
eTable 4. Interaction between the groups that received preconception or pregnancy intervention compared to those who did not receive these interventions, for neurodevelopment outcomes at 24 months of age
Nonauthor Collaborators. Women and Infants Integrated Interventions for Growth (WINGS) Study
Data Sharing Statement
References
- 1.Lu C, Black MM, Richter LM. Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level. Lancet Glob Health. 2016;4(12):e916-e922. doi: 10.1016/S2214-109X(16)30266-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox SE, Levitt P, Nelson CA III. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28-40. doi: 10.1111/j.1467-8624.2009.01380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prado EL, Larson LM, Cox K, Bettencourt K, Kubes JN, Shankar AH. Do effects of early life interventions on linear growth correspond to effects on neurobehavioural development? a systematic review and meta-analysis. Lancet Glob Health. 2019;7(10):e1398-e1413. doi: 10.1016/S2214-109X(19)30361-4 [DOI] [PubMed] [Google Scholar]
- 4.Devakumar D, Fall CH, Sachdev HS, et al. Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis. BMC Med. 2016;14:90. doi: 10.1186/s12916-016-0633-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ip P, Ho FKW, Rao N, et al. Impact of nutritional supplements on cognitive development of children in developing countries: a meta-analysis. Sci Rep. 2017;7(1):10611. doi: 10.1038/s41598-017-11023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumwine JK, Nankabirwa V, Diallo HA, et al. Exclusive breastfeeding promotion and neuropsychological outcomes in 5-8 year old children from Uganda and Burkina Faso: results from the PROMISE EBF cluster randomized trial. PLoS One. 2018;13(2):e0191001. doi: 10.1371/journal.pone.0191001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tofail F, Fernald LC, Das KK, et al. Effect of water quality, sanitation, hand washing, and nutritional interventions on child development in rural Bangladesh (WASH Benefits Bangladesh): a cluster-randomised controlled trial. Lancet Child Adolesc Health. 2018;2(4):255-268. doi: 10.1016/S2352-4642(18)30031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong J, Franchett EE, Ramos de Oliveira CV, Rehmani K, Yousafzai AK. Parenting interventions to promote early child development in the first three years of life: a global systematic review and meta-analysis. PLoS Med. 2021;18(5):e1003602. doi: 10.1371/journal.pmed.1003602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yousafzai AK, Obradović J, Rasheed MA, et al. Effects of responsive stimulation and nutrition interventions on children’s development and growth at age 4 years in a disadvantaged population in Pakistan: a longitudinal follow-up of a cluster-randomised factorial effectiveness trial. Lancet Glob Health. 2016;4(8):e548-e558. doi: 10.1016/S2214-109X(16)30100-0 [DOI] [PubMed] [Google Scholar]
- 10.Taneja S, Chowdhury R, Dhabhai N, et al. ; WINGS Study Group . Impact of a package of health, nutrition, psychosocial support, and WaSH interventions delivered during preconception, pregnancy, and early childhood periods on birth outcomes and on linear growth at 24 months of age: factorial, individually randomised controlled trial. BMJ. 2022;379:e072046. doi: 10.1136/bmj-2022-072046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taneja S, Chowdhury R, Dhabhai N, et al. ; Women and Infants Integrated Growth Study (WINGS) Group . Impact of an integrated nutrition, health, water sanitation and hygiene, psychosocial care and support intervention package delivered during the pre- and peri-conception period and/or during pregnancy and early childhood on linear growth of infants in the first two years of life, birth outcomes and nutritional status of mothers: study protocol of a factorial, individually randomized controlled trial in India. Trials. 2020;21(1):127. doi: 10.1186/s13063-020-4059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Thinking Healthy: A Manual for Psychosocial Management of Perinatal Depression. Version 1.0. 2015. World Health Organization. Accessed May 9, 2023. https://iris.who.int/handle/10665/15293
- 13.Patient health questionnaire (PHQ) screeners. Pfizer. Accessed May 18, 2023. https://www.phqscreeners.com/
- 14.United Nations Children’s Fund . Care for Child Development: Improving the Care for Young Children. World Health Organization. 2012 [Google Scholar]
- 15.Bayley N. Bayley Scales of Infant and Toddler Development: Technical Manual. 3rd ed. Harcourt Assessment; 2006. https://journals.sagepub.com/doi/10.1177/0734282906297199 [Google Scholar]
- 16.Bayley N. Bayley Scales of Infant and Toddler Development: Administration Manual. 3rd ed. Harcourt Assessment; 2006. [Google Scholar]
- 17.Squire J, Twombly E, Bricker D, Potter L. ASQ-3: User’s Guide. Paul H. Brookes Publishing Co; 2009. [Google Scholar]
- 18.Caldwell BM, Bradley RH. Home Inventory: Administration Manual Comprehensive Edition. University of Arkansas for Medical Sciences; 2003. [Google Scholar]
- 19.Rasheed MA, Yousafzai AK. The development and reliability of an observational tool for assessing mother-child interactions in field studies—experience from Pakistan. Child Care Health Dev. 2015;41(6):1161-1171. doi: 10.1111/cch.12287 [DOI] [PubMed] [Google Scholar]
- 20.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. 2014;75(5):670-674. doi: 10.1038/pr.2014.10 [DOI] [PubMed] [Google Scholar]
- 21.Dulal S, Prost A, Karki S, Saville N, Merom D. Characteristics and effects of integrated nutrition and stimulation interventions to improve the nutritional status and development of children under 5 years of age: a systematic review and meta-analysis. BMJ Glob Health. 2021;6(7):e003872. doi: 10.1136/bmjgh-2020-003872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudfeld CR, Bliznashka L, Salifou A, et al. Evaluation of multiple micronutrient supplementation and medium-quantity lipid-based nutrient supplementation in pregnancy on child development in rural Niger: a secondary analysis of a cluster randomized controlled trial. PLoS Med. 2022;19(5):e1003984. doi: 10.1371/journal.pmed.1003984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prado EL, Arnold CD, Wessells KR, et al. Small-quantity lipid-based nutrient supplements for children age 6-24 months: a systematic review and individual participant data meta-analysis of effects on developmental outcomes and effect modifiers. Am J Clin Nutr. 2021;114(suppl 1):43S-67S. doi: 10.1093/ajcn/nqab277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewey KG, Wessells KR, Arnold CD, et al. Characteristics that modify the effect of small-quantity lipid-based nutrient supplementation on child growth: an individual participant data meta-analysis of randomized controlled trials. Am J Clin Nutr. 2021;114(suppl 1):15S-42S. doi: 10.1093/ajcn/nqab278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aboud FE, Yousafzai AK. Global health and development in early childhood. Annu Rev Psychol. 2015;66:433-457. doi: 10.1146/annurev-psych-010814-015128 [DOI] [PubMed] [Google Scholar]
- 26.Yousafzai AK, Rasheed MA, Rizvi A, Armstrong R, Bhutta ZA. Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: a cluster-randomised factorial effectiveness trial. Lancet. 2014;384(9950):1282-1293. doi: 10.1016/S0140-6736(14)60455-4 [DOI] [PubMed] [Google Scholar]
- 27.International Institute for Population Sciences . National Family Health Survey (NFHS-5), 2019-21: India. Vol I. International Institute for Population Sciences. 2021. Accessed June 12, 2023. https://dhsprogram.com/pubs/pdf/FR375/FR375.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Patient health questionnaire (PHQ) screeners. Pfizer. Accessed May 18, 2023. https://www.phqscreeners.com/
Supplementary Materials
Study Protocol
Statistical Analysis Plan
eTable 1. Control Group: National Programs for women of reproductive age, pregnant women and under-twos
eTable 2. Study interventions
eTable 3. Characteristics of participants whose children had neurodevelopmental evaluations, compared to those were not evaluated
eTable 4. Interaction between the groups that received preconception or pregnancy intervention compared to those who did not receive these interventions, for neurodevelopment outcomes at 24 months of age
Nonauthor Collaborators. Women and Infants Integrated Interventions for Growth (WINGS) Study
Data Sharing Statement


