Key Points
Question
Among patients with large vessel occlusion (LVO) stroke, does thrombectomy with the pRESET device restore flow and reduce disability similarly to the Solitaire device?
Findings
In this randomized clinical trial including 340 patients, noninferiority was observed in terms of functional independence (pRESET, 54.9%; Solitaire, 57.5%) and overall disability (shift in modified Rankin Scale score) at 90 days as well as the degree of reperfusion (Expanded Treatment in Cerebral Infarction score of 2b50 or better) within 3 passes and safety.
Meaning
This study provides evidence that among patients treated with thrombectomy for LVO stroke, the pRESET stent retriever was noninferior to the Solitaire stent retriever.
This randomized clinical trial evaluates whether thrombectomy for large vessel occlusion stroke with the pRESET stent retriever is noninferior to treatment with the Solitaire stent retriever.
Abstract
Importance
Stent retriever–based thrombectomy is highly beneficial in large vessel occlusion (LVO) strokes. Many stent retriever designs are currently available, but comparison of these technologies in well-conducted studies is lacking.
Objective
To determine whether thrombectomy for LVO stroke with the pRESET stent retriever is noninferior to treatment with the Solitaire stent retriever.
Design, Setting, and Participants
This study was a multicenter, prospective, randomized, controlled, open-label, adaptive, noninferiority trial with blinded primary end point evaluation. Between October 2019 and February 2022, multicenter participation occurred across 19 research hospitals and/or universities in the US and 5 in Germany. Patients with LVO stroke were enrolled and included up to 8 hours after symptom onset.
Interventions
Patients underwent 1:1 randomization to thrombectomy with the pRESET or Solitaire stent retriever.
Main Outcomes and Measures
The primary outcome was the difference in the rate of 90-day functional independence across the 2 devices, using a −12.5% noninferiority margin for the lower bound of the 1-sided 95% CI of the difference between pRESET and Solitaire retrievers.
Results
Of 340 randomized patients, 170 (50.0%) were female, and the median (IQR) age was 73.0 (64.0-82.0) years. The study procedure was completed in 322 of the 340 randomized patients. The primary end point of 90-day functional independence was achieved by 95 patients (54.9%; 95% CI, 48.7-61.1) in the pRESET group and in 96 (57.5%; 95% CI, 51.2-63.8) in the Solitaire group (absolute difference, −2.57%; 95% CI, −11.42 to 6.28). As the lower bound of the 95% CI was greater than −12.5%, the pRESET retriever was deemed noninferior to the Solitaire retriever. The noninferiority of pRESET over Solitaire was also observed in the secondary clinical end point (90-day shift in modified Rankin Scale score) and in both angiographic end points (Expanded Treatment in Cerebral Infarction [eTICI] score of 2b50 or greater within 3 passes: 146 of 173 [84.4%] vs 149 of 167 [89.2%]; absolute difference, −4.83%; 95% CI, −10.84 to 1.19; eTICI of 2c or greater following the first pass: 76 of 173 [43.7%] vs 74 of 167 [44.3%]; absolute difference, −0.63%; 95% CI, −9.48 to 8.21). Symptomatic intracranial hemorrhage occurred in 0 patients in the pRESET group and 2 (1.2%) in the Solitaire group. Mortality occurred in 25 (14.5%) in the pRESET group and in 24 (14.4%) in the Solitaire group at 90 days. Findings of the per-protocol and as-treated analyses were in concordance with findings of the intention-to-treat analysis.
Conclusions and Relevance
In this study, among patients with LVO stroke, thrombectomy with the pRESET stent retriever was noninferior to thrombectomy with the Solitaire stent retriever. Findings suggest that pRESET offers a safe and effective option for flow restoration and disability reduction in patients with LVO stroke.
Introduction
Mechanical thrombectomy (MT) for large vessel occlusion (LVO) strokes has been the subject of multiple randomized clinical trials, which have invariably demonstrated its overwhelming efficacy.1,2,3,4,5,6,7,8 Many technical and technological advancements have taken place over the last decade. Two early randomized clinical trials of MT demonstrated that stent retrievers were superior to first-generation helical-shaped retrievers in achieving better angiographic and clinical outcomes.9,10 However, as the development of new MT technologies occurred in the intervening years, clinical research has typically consisted of single-arm studies using objective performance criteria derived from pooled studies of predicate devices rather than a randomized trial with a contemporaneous control group.11,12 To date, to our knowledge, there has not been a prospective, randomized clinical study comparing standard with newer-designed stent retriever devices.
Numerous stent retriever devices with varying degrees of radial force, cell size and configuration, diameters, and lengths are now available. In contrast to the Solitaire device (Medtronic), originally designed to treat broad-necked brain aneurysms, the pRESET thrombectomy device (phenox) is a self-expanding laser-cut nitinol stent specifically designed to retrieve thrombi and restore blood flow. Both the Solitaire and the pRESET devices have a longitudinal slit that allows for the device to fold on itself and better configure to the different ranges of vessel diameters encountered during the clot retrieval process. However, pRESET uses a helical rather than a linear slit configuration to better maintain the cell shape integrity independent of expansion diameter. The pRESET device also has a closed proximal ring designed to ensure stable opening and constant wall apposition during retrieval as well as a dual closed-cell design with larger cells to promote deeper clot integration intercalating with smaller cells for better flexibility in tortuous anatomies. In-vitro studies suggest that these design features may result in better thrombus penetration, retention, and complete retrieval compared with other stent retriever devices.13
The aim of the pRESET for Occlusive Stroke Treatment (PROST) randomized clinical trial was to study the safety and efficacy of pRESET and to demonstrate that it is no worse compared with the Solitaire thrombectomy device in the treatment of LVO stroke.
Methods
Study Design
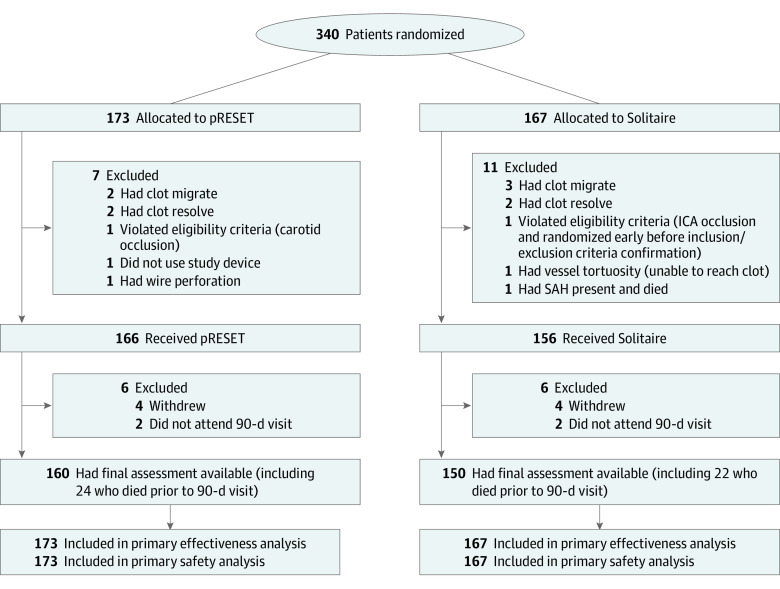
The PROST trial was a multicenter, prospective, randomized, controlled, open-label, adaptive, noninferiority trial with blinded primary end point ascertainment. Patients were randomly assigned in a 1:1 ratio to one of 2 treatment devices (Figure 1). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Figure 1. Participant Flow.
ICA indicates internal carotid artery; SAH, subarachnoid hemorrhage.
The PROST trial was conducted under the guidance and oversight of the US Food and Drug Administration, with the objective of obtaining enough scientific evidence to grant device clearance with a claim of improvement in clinical outcomes rather than the more commonly used thrombectomy device claim of clot removal/restoration of blood flow only. The trial protocol was approved by the institutional review board at each participating site. Enrolled patients or their legally authorized representatives provided written informed consent. The trial was designed and conducted by a steering committee comprised of independent academic investigators and a statistician, in collaboration with the study sponsor (phenox). All study outcome assessments were performed by a blinded team consisting of a stroke neurologist and a study coordinator. A Contract Research Organization, appointed by the sponsor, supported all clinical investigational activities in accordance with the Clinical Investigation Protocol and Good Clinical Practices. An independent data safety monitoring board (DSMB) provided additional oversight of the study conduct. A clinical events committee blinded to treatment assignment adjudicated all neurological events and all serious adverse events. All imaging was uploaded to a core laboratory for blinded review by 2 independent expert readers on consensus. The trial protocol can be found in Supplement 1, and the statistical analysis plan can be found in Supplement 2.
Patient Population and Participating Centers
Patient recruitment occurred across 19 centers in the US and 5 in Germany (eTable 2 in Supplement 3). Inclusion criteria consisted of the following: age of 18 years or older; a score of 6 or higher on the National Institutes of Health Stroke Scale (NIHSS; range of 0 to 42, with higher values indicating more severe deficit), small to moderate infarct (Alberta Stroke Program Early Computed Tomography Score [ASPECTS] of 6 or higher; range of 0 to 10, with higher values indicating less infarct burden) on noncontrast computed tomography (CT) or diffusion-weighted magnetic resonance imaging (DWI) or 50 mL or less on CT perfusion or diffusion-weighted imaging (if automated software was used); prestroke score of 0 or 1 on the modified Rankin scale (mRS; range of 0 [no symptoms] to 6 [death]); and occlusion involving the intracranial internal carotid artery, the first and/or second segments of the middle cerebral artery (M1 or M2), or the vertebrobasilar system that could be treated within 8 hours after symptom onset (defined as the last time the patient was last known well). If eligible, intravenous tissue-type plasminogen activator initiation had to occur within 3 hours from onset. Detailed study inclusion and exclusion criteria are provided in eTable 1 in Supplement 3.
Randomization
On confirmation of eligibility, patients were randomly allocated 1:1 using the Oracle Clinical One web-based site blocked dynamic stratified randomization. A fixed, nonadaptive, randomization scheme was used. Stratification was completed by age (younger than 65 vs 65 years and older), occlusion site (internal carotid artery vs middle cerebral artery vs basilar artery), baseline NIHSS score (less than 17 vs 17 or greater), intravenous tissue-type plasminogen activator usage (yes vs no), time from symptom onset (less than 4 hours vs 4 or more hours), and study center.
Interventions and Thrombectomy Procedure
Arterial puncture was performed within 90 minutes of the mandatory screening CT or magnetic resonance imaging. Decisions regarding the type of anesthesia, use of balloon-guided catheters, and use of intermediate catheters were at the discretion of the operators. Required use of the assigned device for the first 3 passes was mandatory, per protocol. If MT with the assigned device failed to restore adequate cerebral blood flow (grade 2b to 3 on the Expanded Treatment in Cerebral Infarction [eTICI] scale; range of 0 to 3, with higher grades indicating increased reperfusion [grade 2b indicates reperfusion of ≥50% of the occluded territory])14 after 3 passes, the treating physician could decide what treatment therapy (if any) was deemed appropriate for the patient. This was recorded as rescue treatment and considered a device failure and a failure to the primary efficacy end point. If an alternative treatment was used prior to 3 passes and successful reperfusion had not been achieved, this was also deemed as rescue treatment and assigned device failure. If an additional treatment was used after the achievement of successful reperfusion within 3 passes of the assigned device, this was considered as adjunctive therapy and not a failure of the assigned device. Following the index procedure, each patient was subjected to follow-up assessment at 24 hours, 7 days, 30 days, and 90 days (eTable 3 in Supplement 3).
Outcomes
The primary analysis was performed based on the intention-to-treat (ITT) principle. Patients who discontinued prematurely, were noncompliant to the study treatment, or received the wrong study treatment were included in the primary analysis within the respective treatment group they had been randomized to. Secondary analyses were performed on both the per-protocol and as-treated populations.
Efficacy End Points
The primary efficacy end point assessed global disability by the proportion of patients with an mRS score 2 or less at 90 days postprocedure. Secondary efficacy end points included: (1) distribution of 90-day mRS scores across the entire spectrum of disability (ordinal shift); (2) successful reperfusion based on the proportion of patients with eTICI grade of 2b50 flow or greater in the target vessel with 3 or fewer passes of the assigned device; and (3) proportion of patients achieving near complete or complete reperfusion (eTICI grade of 2c or greater) following the first pass of the assigned device.
Safety Outcomes
The primary safety end point was the rate of symptomatic intracerebral hemorrhage (sICH) within 24 hours (range of 16 to 36 hours) postprocedure according to the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria (local or remote parenchymal hemorrhage type 2 on the posttreatment imaging combined with a neurologic deterioration of 4 points or more compared with baseline or the lowest posttreatment NIHSS value or death).15 The overall 90-day mortality constituted the key secondary safety end point. An additional 9 tertiary (exploratory) end points were also prespecified (eTable 10 in Supplement 3).
Statistical Analysis
The initial target sample size for the study was calculated at 214 patients, assuming 46% of the Solitaire patients and 50.5% of the pRESET patients would have an mRS score of 0 to 2 at 90 days. The maximum sample size was 340 total patients, based on 49.5% and 50.5% of the patients randomized to Solitaire and pRESET, respectively, achieving an mRS score of 0 to 2 at 90 days (type I error rate, 5%; power, 80%). Due to the lack of good contemporary data, a prespecified interim assessment based on conditional power was performed by the independent DSMB after 50% of the minimum target sample size (107 patients) had completed the 90-day evaluation or prematurely discontinued from the study. The conditional power was in the promising zone, allowing the target sample size to be increased beyond 214 patients. This DSMB assessment led to a recommendation to increase enrollment to the maximum target sample size of 340 patients.
The noninferiority margin was based on a comprehensive review of the results from the published literature from clinical trials in a similar patient population that reported the mRS score at 90 days postprocedure. Results from the publications were summarized using the inverse standard error weighting method of Fleiss.16 The inverse variance weighting meta-analysis weights the percentages by the inverse of the standard error of the rate and results with a weighted average that is adjusted if the rates from the published literature are heterogeneous. The analysis of the primary and secondary end point analyses was based on the observed data, with multiple imputation for missing end point data to complete the ITT population.
A −12.5% noninferiority margin was used for the primary effectiveness end point (90-day mRS score of 2 or less). If the lower bound of the 1-sided 95% CI of the treatment difference (pRESET minus Solitaire) was more than −12.5%, pRESET was considered noninferior to Solitaire. A 5% noninferiority margin was used for the primary safety end point (sICH). If the upper bound of the 1-sided 95% Cl of the treatment difference was less than 5%, pRESET was considered noninferior to Solitaire. The first and second secondary end points were based on the best eTICI result within 3 or fewer passes and after the first pass, respectively. If the lower bounds of the 1-sided 95% CI of the treatment difference were greater than −12.1%, pRESET was considered noninferior to Solitaire. The third secondary end point was based on 90-day mortality. If the upper bound of the 1-sided 95% CI of the treatment difference was less than 10%, pRESET was considered noninferior to Solitaire. The fourth secondary end point was based on the 90-day mRS score shift. Mantel-Haenszel standardized risk estimate was calculated using the individual randomization factors and the 90-day mRS score as strata. If the 95% CI of the upper bound was greater than 0.875, suggesting the shift toward better outcomes across the entire spectrum of disability is not more than 12.5% better with Solitaire compared with pRESET, noninferiority was established.
Comparative results were considered significant if the 2-tail P value was less than .05. A 2-tailed Fisher exact test was used to compare proportions. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Randomization and Baseline Characteristics
Between October 2019 and February 2022, a total of 340 patients were randomized across 24 sites; of these, 170 (50.0%) were female, and the median (IQR) age was 73.0 (64.0-82.0) years. A total of 173 patients (50.9%) were randomized to the pRESET arm and 167 (49.1%) to the Solitaire arm. The study procedure was completed on 322 patients (94.7%). Of the 18 patients that did not complete the study procedure, 7 were from the pRESET arm and 11 were from the Solitaire arm (Figure 1).
Baseline demographic, clinical, and imaging characteristics were similar in the 2 trial groups (Table 1). The median (IQR) baseline NIHSS score was 16 (12.0-20.0) and the median (IQR) ASPECTS was 9 (8.0-10.0). A total of 110 patients (32.4%) received intravenous thrombolysis. Most patients had occlusion of the proximal middle cerebral artery M1 segment (177 of 340 [52.1%]) or M2 segment (74 of 340 [21.8%]). The median (IQR) time from stroke onset to arterial puncture was 190.5 (134.0-262.0) minutes.
Table 1. Baseline Patient and Procedural Characteristics in the Intention-to-Treat Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total population (N = 340) | pRESET (n = 173) | Solitaire (n = 167) | |
| Age, median (IQR), y | 73.0 (64.0-82.0) | 73.0 (64.0-81.0)) | 75.0 (63.0-83.0) |
| Sex | |||
| Female | 170 (50.0) | 83 (48.0) | 87 (52.1) |
| Male | 170 (50.0) | 90 (52.0) | 80 (47.9) |
| NIHSS score | |||
| Total, No. | 339 | 173 | 166 |
| Median (IQR) | 16.0 (12.0-20.0) | 16.0 (12.0-20.0) | 16.0 (12.0-21.0) |
| ASPECTS, median (IQR) | |||
| Total, No. | 336 | 171 | 165 |
| Median (IQR) | 9.0 (8.0-10.0) | 9.0 (8.0-10.0) | 9.0 (8.0-10.0) |
| Intravenous tPA use | 110 (32.4) | 57 (32.9) | 53 (31.7) |
| Glucose, median (IQR), mg/dL | |||
| Total, No. | 264 | 138 | 126 |
| Median (IQR) | 116.5 (97.5-141.0) | 114 (97.0-139.0) | 118 (100.0-142.0) |
| Medical history | |||
| Hypertension | 269 (79.1) | 137 (79.2) | 132 (79.0) |
| Atrial fibrillation | 146 (42.9) | 77 (44.5) | 69 (41.3) |
| Diabetes | 91 (26.8) | 43 (24.9) | 48 (28.7) |
| Current smoking | 77 (22.6) | 42 (24.3) | 35 (21.0) |
| Dyslipidemia | 148 (43.5) | 82 (47.4) | 66 (39.5) |
| Previous stroke or transient ischemic attack | 68 (20.0) | 33 (19.1) | 35 (21.0) |
| Previous myocardial infarction | 80 (23.5) | 38 (22.0) | 42 (25.1) |
| Systolic blood pressure, mm Hg | |||
| Total, No. | 337 | 171 | 166 |
| Median (IQR) | 148 (132.0-163.0) | 150 (132.0-165.0) | 146 (132.0-161.0) |
| Prestroke mRS score | |||
| 0 | 251 (73.8) | 130 (75.1) | 121 (72.5) |
| 1 | 84 (24.7) | 41 (23.7) | 43 (25.7) |
| 2 | 3 (0.9) | 1 (0.6) | 2 (1.2) |
| Not reported | 2 (0.6) | 1 (0.6) | 1 (0.6) |
| Site of occlusion | |||
| Intracranial ICA/carotid T | 47 (13.9) | 23 (13.3) | 24 (14.4) |
| MCA - M1 | 177 (52.2) | 89 (51.5) | 88 (52.8) |
| MCA - M2 | 74 (21.8) | 43 (24.9) | 31 (18.6) |
| Basilar artery | 17 (5.1) | 9 (5.2) | 8 (4.8) |
| Cervical ICA (tandem) | 3 (0.9) | 2 (1.2) | 1 (0.6) |
| Other | 6 (1.8) | 2 (1.2) | 4 (2.4) |
| Side of occlusion, left | 146 (42.9) | 78 (45.1) | 68 (40.7) |
| Time from symptom to intravenous tPA, median (IQR), min | 89.5 (71.0-116.0) | 94 (72.0-116.0) | 86 (71.0-115.0) |
| General anesthesia | 126 (37.1) | 64 (37.0) | 62 (37.1) |
| Time from symptom to puncture, min | |||
| Total, No. | 310 | 161 | 149 |
| Median (IQR) | 190.5 (134-262) | 183 (133-252) | 192 (137-285) |
| Time from symptom to deployment, min | |||
| Total, No. | 304 | 156 | 148 |
| Median (IQR) | 216 (150-282) | 204 (150-276) | 216 (150-306) |
| Time from symptom to revascularization (eTICI grade of 2b50 or greater), min | |||
| Total, No. | 249 | 125 | 124 |
| Median (IQR) | 216 (156-288) | 204 (156-276) | 222 (162-312) |
| Time from puncture to revascularization (eTICI grade of 2b50 or greater), min | |||
| Total, No. | 261 | 130 | 131 |
| Median (IQR) | 27 (19-37) | 27 (19-38) | 26 (19-37) |
| BGC only | 61 (17.9) | 28 (16.2) | 33 (19.8) |
| IC only | 130 (38.2) | 67 (38.7) | 63 (37.7) |
| BGC with IC | 91 (26.8) | 52 (30.1) | 39 (23.4) |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; BGC, balloon-guided catheter; eTICI, Expanded Treatment in Cerebral Infarction; IC, intermediate catheter; ICA, internal carotid artery; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue-type plasminogen activator.
SI conversion factor: To convert glucose to mmol/L, multiply by 0.0555.
Efficacy Outcomes
In the ITT population, the primary efficacy end point of an mRS score of 2 or less at 90 days was achieved by 95 patients (54.9%; 95% CI, 48.7-61.1) in the pRESET group and 96 patients (57.5%; 95% CI, 51.2-63.8) in the Solitaire group (Table 2). The absolute difference in outcome was −2.57% (95% CI, −11.4 to 6.3), suggesting that, across this patient cohort, it is 95% likely that the proportion of patients achieving 90-day independence with the pRESET device was no worse than 11.4% lower and no better than 6.3% higher than with the Solitaire device (eFigure 1 in Supplement 3). As the lower bound of the 1-sided 95% CI of the difference was above the prespecified threshold of −12.5%, the pRESET approach was found to be noninferior to the Solitaire approach. Similar results demonstrating noninferiority of pRESET vs Solitaire were also found among the per-protocol population (82 of 138 [59.4%] vs 71 of 128 [55.5%]) and as-treated population (92 of 166 [55.4%] vs 87 of 156 [56.1%]).
Table 2. Primary and Secondary Efficacy and Safety Outcomes in the Intention-to-Treat Population.
| Characteristic | No. (%) | Difference between Solitaire and pRESET, % (asymptotic 1-sided 95% CI)a | |
|---|---|---|---|
| pRESET (n = 173) | Solitaire (n = 167) | ||
| Primary efficacy outcome | |||
| mRS score <2 at 90 d | 95 (54.91) | 96 (57.49) | −2.57 (−11.42 to 6.28) |
| Secondary efficacy outcomes | |||
| eTICI grade ≥2b50 achieved in ≤3 passes | 146 (84.39) | 149 (89.22) | −4.83 (−10.84 to 1.19) |
| eTICI grade ≥2c achieved after first pass | 76 (43.68) | 74 (44.31) | −0.63 (−9.48 to 8.21) |
| Primary safety outcome | |||
| sICH per SITS-MOST criteria | 0 | 2 (1.2) | −1.20 (−2.58 to 0.19) |
| Secondary safety outcome | |||
| Overall 90-d mortality | 25 (14.5) | 24 (14.4) | 0.08 (−6.19 to 6.35) |
Abbreviations: eTICI, Expanded Treatment in Cerebral Infarction; mRS, modified Rankin Scale; sICH, symptomatic intracerebral hemorrhage; SITS-MOST, Safe Implementation of Thrombolysis in Stroke-Monitoring Study.
The risk difference is derived from the standard Wald asymptotic confidence limits.
In terms of the secondary clinical end point (90-day mRS score shift), the lower bound of the 2-sided 90% CI from the risk estimate from pRESET exceeded the prespecified threshold for each randomization strata, indicating that the stratified ordinal analysis of mRS at 90 days postprocedure was not greater than 12.5% worse compared with Solitaire (Figure 2; eFigure 2 in Supplement 3). The noninferiority of pRESET over Solitaire was also established for both angiographic end points: an eTICI grade of 2b50 flow or greater within 3 or fewer passes of the assigned device (146 of 173 [84.4%] vs 149 of 167 [89.2%]; difference, −4.83%; 95% CI, −10.8 to 1.19) and near complete or complete reperfusion (eTICI grade of 2c or greater) following the first pass of the assigned device (75 of 173 [43.7%] vs 74 of 167 [44.3%]; difference, −0.63%; 95% CI, −9.48 to 8.21). The results of the per-protocol and as-treated analyses were in concordance with the ITT analysis (eTables 4 to 9 in Supplement 3). Details regarding rescue therapy in patients who failed to achieve eTICI grade of 2b50 or better within 3 passes with the assigned device are shown in eTable 11 in Supplement 3.
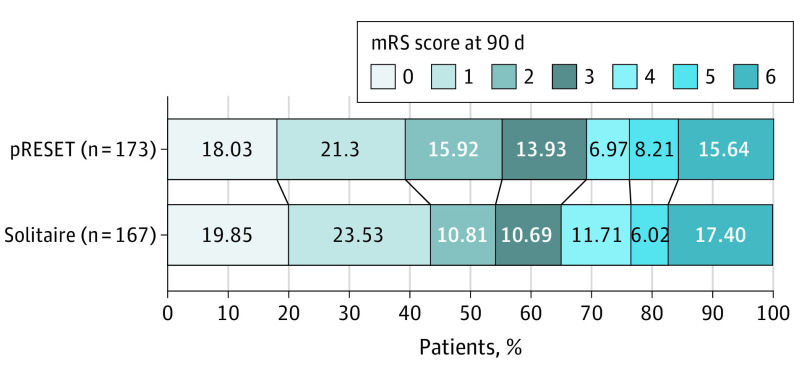
Figure 2. Distribution of Modified Rankin Scale (mRS) Score at 3 Months by Treatment Group.
The mRS score measures degree of disability (score range, 0 [no symptoms] to 6 [death]). The proportion of patients who achieved functional independence at 3 months (mRS score of 0 to 2) was 54.9% (95% CI, 48.7-61.1) in the pRESET group and 57.5% (95% CI, 51.2-63.8) in the Solitaire group. The absolute difference in outcome was −2.57% (95% CI, −11.4 to 6.3), confirming that pRESET was noninferior to Solitaire. The imputation by randomized treatment assignment when the mRS score was dichotomized rendered a slightly different proportion than when the actual mRS scores were imputed for patients with missing 90-day mRS scores.
Safety Outcomes
There were no postprocedure sICHs in the pRESET arm (0 of 173) compared with 2 of 167 (1.2%) in the Solitaire arm. This difference of −1.2% ensured that the lower bound of the 95% CI was above the prespecified threshold of −5%. Mortality at 90 days was 14.5% (25 of 173) for pRESET vs 14.4% (24 of 167) for Solitaire, with the lower bound of the 95% CI being within the prespecified threshold of 10%. Both the per-protocol and as-treated populations demonstrated no difference in the overall results with the noninferiority criteria being met. There were no observed device malfunctions (eTables 6 and 9 in Supplement 3). Results of the exploratory end points are reported in eTable 10 in Supplement 3.
Discussion
To our knowledge, the PROST trial is the first randomized clinical trial aiming to compare a novel vs an established stent retriever technology, establishing a new scientific benchmark for stroke device trials. The trial was designed to demonstrate the safety and efficacy of the pRESET device by studying various clinical, safety, and angiographic end points. These results were compared against a contemporary control arm to demonstrate noninferiority to the predicate device (Solitaire). In all primary and secondary end points and analyses, the prespecified threshold of noninferiority was met.
Despite the rapid growth in the number of randomized clinical trials in the field of MT, most of the existing high-level evidence relates to either the proof of concept or the expansion in the indications for the procedure. Indeed, there is still a paucity of randomized clinical data regarding the performance across different thrombectomy approaches. The SWIFT and TREVO-2 trials, both concluded more than a decade ago, were the first randomized trials to compare different treatment modalities.9,10 Despite their small sample sizes (114 and 178 patients, respectively), these trials established the superiority of the stent retriever technology over the Merci device, serving as the landmark for the modern thrombectomy era. The Penumbra Separator 3D trial, the ASTER trial, and the COMPASS trial were randomized trials that compared contact aspiration vs stent retriever approaches as first-line treatment modalities.17,18,19 While these studies had some methodological differences in terms of the specific devices they included as well as the measurement and analysis of their primary outcomes, they demonstrated the overall equivalence across these 2 technical modalities. More recently, the ASTER 2 trial compared the combined contact aspiration plus stent retriever to the stent retriever alone approaches as first-line treatment modality.20 The trial failed to show any significant differences in terms of the primary end point of near-complete or complete (eTICI grade 2c) reperfusion at the end of the procedure. However, there were some significant findings and trends favoring the combined approach for angiographic measures computed after the use of the assigned intervention alone.
In large part due to historical regulatory pathways and financial constraints, most of the recent thrombectomy device trials have opted for a single-arm design. Consequently, these studies have relied on a comparison with past trials, adding a high degree of heterogeneity in terms of the treatment delivered, the population studied, and the adjudication process across the different trials. Patient characteristics and operator/center experience are equally important to device performance in determining clinical outcomes after thrombectomy, significantly hindering outcome comparisons across studies. For instance, patients in the PROST trial were on average 7 years older and less frequently received prethrombectomy intravenous thrombolysis than those in the TIGER trial (two-thirds vs one-third).12 Acknowledging these limitations, it is noteworthy that the 2 key angiographic outcomes in the PROST trial compare favorably with those of other recent trials. Specifically, the rates of eTICI grades of 2b50 or greater within 3 or fewer passes were 84.4% with pRESET in the PROST trial, 80.2% in the ARISE-2 trial, 84.6% in the TIGER trial, 83% in the aspiration and 81% in the stent retriever arms of the COMPASS trial, and 86.2% in the combined group and 72.3% in the stent retriever alone arm of the ASTER-2 trial.11,12,19,20 Likewise, the rates of eTICI grades of 2c or higher after a single pass were 44% with pRESET in the PROST trial, 40% in the ARISE-2 trial, 41.4% in the TIGER trial, and 40.9% in the combined group and 33.7% in the stent retriever alone arm of the ASTER-2 trial. The rates of serious adverse events did not differ significantly between the study groups overall (Table 3). Also, there were no observed device deficiencies that could have led to device-related or procedure-related serious adverse events. Although the definition of sICH varies between studies, the rates recorded in this study were comparable if not favorable to other studies.11,12,21
Table 3. Adverse Events in the Intention-to-Treat Population.
| Adverse event | No. (%) | Risk estimate (90% CI)a | P value | |
|---|---|---|---|---|
| pRESET (n = 173) | Solitaire (n = 167) | |||
| Procedure-related and/or device-related serious adverse event | 36 (20.8) | 37 (22.2) | NA | NA |
| Perforation | 3 (1.7) | 2 (1.2) | NA | NA |
| Dissection | 2 (1.2) | 1 (0.6) | NA | NA |
| Embolization to new territory | 0 | 2 (1.2) | NA | NA |
| Access site complication | 0 | 2 (1.2) | NA | NA |
| Mortality | 0 | 1 (0.6) | NA | NA |
| Evidence of new infarct outside the original at-risk territory | 2 (1.2) | 4 (2.4) | NA | .43 |
| Mass effect or intracranial tumor | 1 (0.6) | 0 | NA | >.99 |
| Evidence of carotid dissection or complete cervical occlusion | 0 | 1 (0.6) | NA | .49 |
| Any intracranial hemorrhage at 24 h | 73 (42.2) | 65 (38.9) | 0.01 (−12.03 to 0.06) | .58 |
| Stroke-related mortality | 7 (4.1) | 6 (3.6) | 0.03 (−2.96 to 3.87) | >.99 |
The probability value is derived from the 2-tailed Fisher exact test. The risk difference is derived from the standard Wald asymptotic confidence limits.
Limitations
Our study has some limitations. There was a solid rationale for the noninferiority margins for the primary and secondary outcomes in our study, which were also similar to the noninferiority margins used in other noninferiority MT randomized clinical trials.9,10,17,19,22 However, previous studies involving stroke and neuroendovascular expert surveys have identified that the minimal clinically important differences for functional independence and substantial reperfusion (eTICI grade of 2b to 3 within 3 passes) across 2 therapies should be set at much lower values, ranging between 1% and 5% and 3.1% and 5%, respectively.23,24 Unfortunately, the sample sizes required to show either superiority or noninferiority with such low margins have made their adoption impractical for device trials. Moreover, the financial and logistical constraints to adhere to such high expectations would deprive many patients from access to new technologies that might be a superior treatment overall, serve as rescue strategy in case of failure to previously existent technology, or lower treatment costs by fostering a more competitive market. These are some of the reasons why the Food and Drug Administration only requires proof of substantial equivalence to devices that are currently on the market.
There was a small number of patients with posterior circulation occlusions. During the enrollment of this study, the COVID-19 pandemic caused widespread lockdowns to occur. Originally, the protocol had called for in person follow-up visits, but due to COVID-19 restrictions, the assessments were conducted by telephone. Nonetheless, multiple studies have shown excellent agreement between in-person and telephone assessments.25,26
Conclusions
Among patients with acute ischemic stroke due to large vessel occlusion, thrombectomy with the pRESET stent retriever was noninferior to thrombectomy with the Solitaire stent retriever, with respect to all prespecified primary and secondary safety and efficacy end point measures in the PROST trial. pRESET alternatively offers a safe and effective option for the restoration of blood flow and for reducing disability in patients experiencing LVO stroke.
Trial Protocol
Statistical Analysis Plan
eTable 1. Study Inclusion and Exclusion Criteria
eTable 2. Enrollment by Site
eTable 3. Schedule of Assessments
eTable 4. Primary and Secondary Efficacy Outcomes – Per Protocol
eTable 5. Baseline Patient and Procedural Characteristics in the Per-Protocol Population
eTable 6. Adverse Events – Per Protocol
eTable 7. Primary and Secondary Efficacy Outcomes – As Treated
eTable 8. Baseline Patient and Procedural Characteristics in the As-Treated Population
eTable 9. Adverse Events – As Treated
eTable 10. Exploratory End Points – ITT Population
eTable 11. Recue Therapy in Patients Who Failed to Achieve eTIC Grade I≥2b50 Within 3 Passes With the Assigned Device
eFigure 1. Primary End Point in the Intention-to-Treat Population
eFigure 2. Forest Plot of Mantel-Haenszel Standardized Risk Estimates for the Secondary Efficacy End Point of Overall Disability at 90 Days (Ordinal mRS Score Shift)
Data Sharing Statement
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 5.Martins SO, Mont’Alverne F, Rebello LC, et al. ; RESILIENT Investigators . Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. 2020;382(24):2316-2326. doi: 10.1056/NEJMoa2000120 [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 8.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saver JL, Jahan R, Levy EI, et al. ; SWIFT Trialists . Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241-1249. doi: 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 10.Nogueira RG, Lutsep HL, Gupta R, et al. ; TREVO 2 Trialists . Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231-1240. doi: 10.1016/S0140-6736(12)61299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidat OO, Bozorgchami H, Ribó M, et al. Primary results of the multicenter ARISE II study (Analysis of Revascularization in Ischemic Stroke With EmboTrap). Stroke. 2018;49(5):1107-1115. doi: 10.1161/STROKEAHA.117.020125 [DOI] [PubMed] [Google Scholar]
- 12.Gupta R, Saver JL, Levy E, et al. ; TIGER Trial Investigators . New class of radially adjustable stentrievers for acute ischemic stroke: primary results of the multicenter TIGER trial. Stroke. 2021;52(5):1534-1544. doi: 10.1161/STROKEAHA.121.034436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machi P, Jourdan F, Ambard D, et al. Experimental evaluation of stent retrievers’ mechanical properties and effectiveness. J Neurointerv Surg. 2017;9(3):257-263. doi: 10.1136/neurintsurg-2015-012213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liebeskind DS, Bracard S, Guillemin F, et al. ; HERMES Collaborators . eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433-438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 15.Wahlgren N, Ahmed N, Dávalos A, et al. ; SITS-MOST investigators . Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275-282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 16.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121-145. doi: 10.1177/096228029300200202 [DOI] [PubMed] [Google Scholar]
- 17.Nogueira RG, Frei D, Kirmani JF, et al. ; Penumbra Separator 3D Investigators . Safety and efficacy of a 3-dimensional stent retriever with aspiration-based thrombectomy vs aspiration-based thrombectomy alone in acute ischemic stroke intervention: a randomized clinical trial. JAMA Neurol. 2018;75(3):304-311. doi: 10.1001/jamaneurol.2017.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapergue B, Blanc R, Gory B, et al. ; ASTER Trial Investigators . Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318(5):443-452. doi: 10.1001/jama.2017.9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turk AS III, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;393(10175):998-1008. doi: 10.1016/S0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 20.Lapergue B, Blanc R, Costalat V, et al. ; ASTER2 Trial Investigators . Effect of thrombectomy with combined contact aspiration and stent retriever vs stent retriever alone on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER2 randomized clinical trial. JAMA. 2021;326(12):1158-1169. doi: 10.1001/jama.2021.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 22.Fischer U, Kaesmacher J, Strbian D, et al. ; SWIFT DIRECT Collaborators . Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet. 2022;400(10346):104-115. doi: 10.1016/S0140-6736(22)00537-2 [DOI] [PubMed] [Google Scholar]
- 23.Lin CJ, Saver JL. Noninferiority margins in trials of thrombectomy devices for acute ischemic stroke: is the bar being set too low? Stroke. 2019;50(12):3519-3526. doi: 10.1161/STROKEAHA.119.026717 [DOI] [PubMed] [Google Scholar]
- 24.Lin CJ, Saver JL. The minimal clinically important difference for achievement of substantial reperfusion with endovascular thrombectomy devices in acute ischemic stroke treatment. Front Neurol. 2020;11:524220. doi: 10.3389/fneur.2020.524220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savio K, Pietra GL, Oddone E, Reggiani M, Leone MA. Reliability of the modified Rankin Scale applied by telephone. Neurol Int. 2013;5(1):e2. doi: 10.4081/ni.2013.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, Rinkel GJ. Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis. 2010;29(2):137-139. doi: 10.1159/000262309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Study Inclusion and Exclusion Criteria
eTable 2. Enrollment by Site
eTable 3. Schedule of Assessments
eTable 4. Primary and Secondary Efficacy Outcomes – Per Protocol
eTable 5. Baseline Patient and Procedural Characteristics in the Per-Protocol Population
eTable 6. Adverse Events – Per Protocol
eTable 7. Primary and Secondary Efficacy Outcomes – As Treated
eTable 8. Baseline Patient and Procedural Characteristics in the As-Treated Population
eTable 9. Adverse Events – As Treated
eTable 10. Exploratory End Points – ITT Population
eTable 11. Recue Therapy in Patients Who Failed to Achieve eTIC Grade I≥2b50 Within 3 Passes With the Assigned Device
eFigure 1. Primary End Point in the Intention-to-Treat Population
eFigure 2. Forest Plot of Mantel-Haenszel Standardized Risk Estimates for the Secondary Efficacy End Point of Overall Disability at 90 Days (Ordinal mRS Score Shift)
Data Sharing Statement