Abstract
Background
Arsenic is a naturally occurring element that poses a significant threat to human health due to its widespread presence in the environment, affecting millions worldwide. Sources of arsenic exposure are diverse, stemming from mining activities, manufacturing processes, and natural geological formations. Arsenic manifests in both organic and inorganic forms, with trivalent meta-arsenite (As3+) and pentavalent arsenate (As5+) being the most common inorganic forms. The trivalent state, in particular, holds toxicological significance due to its potent interactions with sulfur-containing proteins.
Objective
The primary objective of this review is to consolidate current knowledge on arsenic toxicity, addressing its sources, chemical forms, and the diverse pathways through which it affects human health. It also focuses on the impact of arsenic toxicity on various organs and systems, as well as potential molecular and cellular mechanisms involved in arsenic-induced pathogenesis.
Methods
A systematic literature review was conducted, encompassing studies from diverse fields such as environmental science, toxicology, and epidemiology. Key databases like PubMed, Scopus, Google Scholar, and Science Direct were searched using predetermined criteria to select relevant articles, with a focus on recent research and comprehensive reviews to unravel the toxicological manifestations of arsenic, employing various animal models to discern the underlying mechanisms of arsenic toxicity.
Results
The review outlines the multifaceted aspects of arsenic toxicity, including its association with chronic diseases such as cancer, cardiovascular disorders, and neurotoxicity. The emphasis is placed on elucidating the role of oxidative stress, genotoxicity, and epigenetic modifications in arsenic-induced cellular damage. Additionally, the impact of arsenic on vulnerable populations and potential interventions are discussed.
Conclusions
Arsenic toxicity represents a complex and pervasive public health issue with far-reaching implications. Understanding the diverse pathways through which arsenic exerts its toxic effects is crucial to developing effective mitigation strategies and interventions. Further research is needed to fill gaps in our understanding of arsenic toxicity and to inform public health policies aimed at minimising exposure.
Arsenic toxicity is a crucial public health problem influencing millions of people around the world. The possible sources of arsenic toxicity includes mining, manufacturing processes and natural geological sources. Arsenic exists in organic as well as in inorganic forms. Trivalent meta-arsenite (As3+) and pentavalent arsenate (As5+) are two most common inorganic forms of arsenic. Trivalent oxidation state is toxicologically more potent due to its potential to interact with sulfur containing proteins. Humans are exposed to arsenic in many ways such as environment and consumption of arsenic containing foods. Drinking of arsenic-contaminated groundwater is an unavoidable source of poisoning, especially in India, Bangladesh, China, and some Central and South American countries. Plenty of research has been carried out on toxicological manifestation of arsenic in different animal models to identify the actual mechanism of aresenic toxicity. Therefore, we have made an effort to summarize the toxicology of arsenic, its pathophysiological impacts on various organs and its molecular mechanism of action.
Keywords: methylation, oxidative stress, mitochondrial dysfunctioning, signal transduction, arsenic toxicity
1. Introduction
Arsenic is a natural element having ubiquitous distribution and exists in both organic and inorganic forms in the environment. It is one of the main contaminants involved in the environmental pollution and is known to be a causative agent for extreme global health risks.1 Arsenate and arsenite derivatives of inorganic form are known to be very toxic to humans. Arsenic contamination and its associated problems such as ground water pollution are threatening factors in different countries such as Bangladesh, Cambodia, China, India, Laos, Myanmar, Nepal, Pakistan, Taiwan, Vietnam, India, Mexico, China, Chile, Argentina, and USA. Arsenic contamination affects about 150 million people in the entire world.2 Because of their reliance on As-contaminated water for drinking and irrigation purposes, over 110 million people living in these areas are suffering from serious health risks.3 Arsenic derived from arsenical pesticides and natural mineral deposits or arsenical chemicals that are improperly disposed off polluting the drinking water. It has been reported that 27 million people are consuming arsenic contaminated water, in which arsenic level is more than 50 ppb, especially exposure rate is very high in India and Bangladesh.4 According to WHO recommendation, arsenic level in drinking water must be less than 10 ppb.5 Chronic exposure to arsenic has resulted in huge mass poisoning of the human population, affected more than 100 million individuals and causes cancer and other arsenic-related diseases.6 Epidemiological reports have shown that arsenic contamination affects wide geographical area, with soil, water, air and even food emissions spread across continents.7 Arsenic pollution is spread through natural processes including volcanic eruptions, weathering, and biological activity. Anthropogenic practices also intensify the exposure of arsenic to human settlements, like mine smelting, mining, well drilling and fossil fuel combustion and arsenic-related illnesses. Because of its higher toxic nature it has become a risk not only to humans but also to the other orgasnisms.8 In current era, various arsenic containing compounds are used in different forms such as pesticides and herbicides. However, due to the growing evidence of arsenic toxicity, the use of arsenic containing compound in consumer goods was banned in the US and the European Union in 2004 and 2020, respectively. Nevertheless, arsenic containing compounds are still used in the form of sprays or pesticides.9
In the Western world arsenic was being used for agricultural practices since 2013, but the U.S. Agency for Toxic Substances and Disease Registry has confirmed it as a hazardous substance and has been enlisted in the priority list of 2019.10 Organic arsenic (As) and inorganic arsenic (As) compounds are listed as Group 1 carcinogen, to humans by the International Organization for Cancer Research (IARC).11
In human health, the consequences of chronic arsenic toxicity is known as arsenicosis, which was first coined by12 and later on World Health Organization13 reported that excessive exposure of arsenic to humans causes chronic diseases. The disease was historically characterized as arseniasis, arsenism, arsenicism, etc. Arsenic is a recognized neurotoxicant to the peripheral nervous system and symptoms can last for many years or even whole life, leading to the symmetrical peripheral neuropathy characterized by damage in sensory nerves because they are more vulnerable to arsenic effects than motor nerves and large axon neurons that are impacted than small axon neurons.14 However, acute arsenic exposure cause damage in Central Nervous System such as learning, memory, and attention.15 Arsenic contamination remains a critical global issue affecting millions of people. Efforts to mitigate its impact include regulatory measures, bans on certain arsenic-containing compounds, and ongoing research to better understand its health effects and develop strategies to protect human health and the environment from this pervasive and toxic element. In a nutshell, this review article serves as an overview of the extensive implications of arsenic contamination on a global scale. It highlights the widespread distribution of arsenic in various forms and its role as a significant environmental contaminant, leading to severe health risks for millions of people, particularly through tainted drinking water. This article underscores the toxic nature of arsenic, the extensive geographical reach of contamination, the sources of pollution, and regulatory actions taken in response. It also outlines the health consequences, both on the peripheral and central nervous systems, caused by chronic arsenic exposure. In essence, it lays the foundation for a comprehensive discussion of the multifaceted challenges and implications associated with arsenic contamination.
2. Ways of exposure and absorption
Arsenic is a natural compound present on the earth’s crust and is extensively distributed in the air, water and land in the environment. Arsenic poisoning can occur from a natural sources like erosion and leaching from geological formations or from anthropogenic (industrial, or self-administered) sources.16 The other sources of Arsenic poisoning include arsenic containing insecticides, herbicide, or rodenticide, contaminated drinking and cooking water, tobacco smoking, stored and irrigated food crops, dietary food and feed additives. But, exposure to these sources is usually lower in comparison with exposure to polluted groundwater. Since, Ground water is the major source of drinking water, increased level of arsenic (> 10 μg/L) in the ground causes arsenicosis, a common name generally used for health-related problems such as skin disorders, skin cancers, internal cancers (bladder, kidney, and lung), diseases of the blood vessels of the legs and feet, diabetes, increased blood pressure, and reproductive disorders.17 Increased concentration of arsenic in ground water have been evidenced in Chile, Mexico, China, Argentina, USA, and Hungary as well as in the Indian State of West Bengal, Bangladesh, and Vietnam.5,18
Arsenic, enters the human body predominantly through two routes: inhalation and ingestion. But, to some extent dermal absorption of trivalent arsenic oxide also occurs as it is more lipid-soluble than the pentavalent form. The available research suggests that non-occupational exposure is predominantly through food and water intake, while inhalation playing a minor role.19 The majority of ingested and inhaled arsenic is quickly absorbed into the bloodstream via the gastrointestinal system and lungs. Near around 95% percent of the consumed trivalent arsenic is absorbed by the gastrointestinal tract. After absorption, 95% to 99% of the arsenic is located in erythrocytes, bound to the globin of hemoglobin and is then transported to the other organs of the body like lungs, liver, kidney, and skin. Most arsenic absorbed into the body is converted by the liver to less toxic methylated form that is efficiently excreted in the urine.20
3. Arsenic metabolism and biotransformation
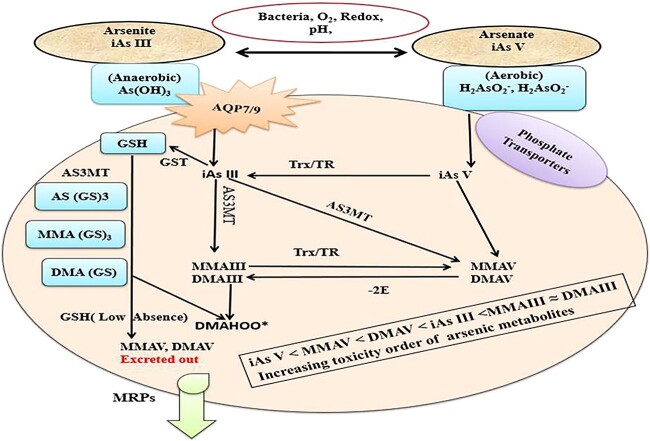
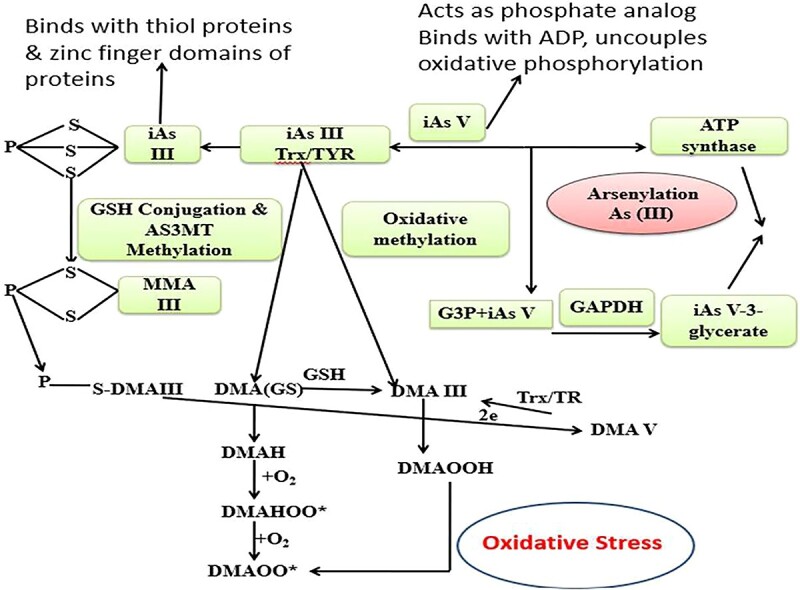
Arsenic is mostly found in the form of inorganic As (iAs), and it has two biologically significant oxidation states: Pentavalent (As V) arsenate in aerobic conditions and Trivalent (As III) arsenite in anaerobic conditions. At neutral pH, As V is found as oxy anions, namely H2AsO2− and H2As2O22−. Whereas As III is generally found as a neutral species (As (OH)3 at physiological pH. Both forms are carried by the same mechanism that transports other metabolically important chemicals.21 Arsenates (V) are taken up via phosphate transporters as it resembles phosphate and is a competitive inhibitor of many phosphate-utilizing enzymes.22 Whereas arsenite (III) are taken up via aquaglyceroporins (AQP) which transport water and glycerin and have a great affinity for thiol (-SH) groups in proteins (Figs 1 and 2).
Fig. 1.
Arsenate (iAs V) enters cells via phosphate transporters, whereas arsenite (iAs III) enters cells by aqua (glycerol) porins (AQP) and glucose transporter (GLUT). It shows how glutathione (GSH) conjugation and thioredoxin reductase work to reduce iAs III and iAs V. Following that, the arsenite methyltransferase (AS3MT) produces a variety of arsenic metabolites that are exported by multidrug resistance proteins (MRP1, MRP2 or MRP4).
Fig. 2.
Arsenic induced mitochondrial dysfunction due to the uncoupling of As V induced oxidative phosphorylation and ATP formation. iAs III binds to protein thiols (coenzyme A, lipoic acid, and proteins with zinc-finger domains like metallothionein [MT]) and is methylated while still conjugated to these proteins. These peroxyl radicals causes lipid peroxidation and protein carbonylation.
In many species, the majority of arsenic absorbed into the body is metabolized through two processes: (1) oxidation/reduction reactions that interconvert arsenite and arsenate, and (2) methylation reactions in which mostly trivalent forms of arsenic are sequentially methylated in the liver to form mono-, di-, and trimethylated products. In case of human and animal cells, with respect to23 view, AsV is rapidly reduced to AsIII by thioredoxin (Trx)/trx reductase (TR) system, followed by oxidative methylation by Arsenite Methyltransferase (AS3MT) by using S-adenosylmethionine (SAM) as a co-substrate. iAsIII is methylated to monomethylarsonic acid MMAIII or arsenate (MMAV), then reduced to monomethylarsonic acid (MMAIII), then methylated to dimethylarsonic acid (MMAV) and (DMAV) (Fig. 1).24,25
Another method entails methylating iAs without oxidizing them from trivalent to pentavalent states. The creation of glutathione (GSH) conjugated complexes such as As triglutathione [(As (GS)3] is involved in these schemes, which are also known as sequential methylation schemes. This conjugation occurs both enzymatically as well as non-enzymatically by using glutathione-S-transferase.26 The As (GS)3 complexes are methylated by AS3MT to form monomethyl arsenic diglutathione [MMA (GS)2] and produces again dimethylarsinic GSH [DMA (GS)]. In the absence of GSH, the conjugates are hydrolyzed and then oxidized to MMAV and DMAV, which is the main metabolite and is excreted from cells. It has been reported that humans acquire some arsenic as iAs and MMA (MMAIII and MMAV) in their skin, hair, nails, muscle, bones, and teeth during metabolism, which can cause harmful consequences in many tissues and organs later in life (Fig. 1).27,28
One of the striking features of arsenic metabolism is that there are extreme qualitative and quantitative interspecies differences in methylation to the extent that some species do not appear to methylate arsenic at all.29
However, it is well-established fact that arsenite AsIII is more toxic than arsenate AsV, with inorganic As being more toxic than organic As Since, different organic As species represent different degrees of toxicity. For instance, monomethylarsonic acid (MMAV) and dimethylarsinic acid (DMAV) as final As metabolites are less toxic than inorganic arsenic, whereas the degrees of toxicity of intermediate metabolites such as monomethylarsonous acid (MMAIII) and dimethylarsinous acid (DMAIII) are much more higher than inorganic arsenic.30 Consequently, toxicity of various arsenic species increases in the order of AsV < MMAV < DMAV < AsIII <MMAIII ≈ DMAIII (Figs 1 and 2).31 In summary, arsenic metabolism is a complex interplay of chemical reactions and enzymatic processes. The toxicity of arsenic varies depending on its chemical form, with inorganic arsenic being more toxic than organic forms. Understanding these metabolic pathways and toxicity profiles is crucial for assessing the health risks associated with arsenic exposure and implementing effective mitigation strategies.
4. Arsenic metabolism and toxicity: a complex interplay
4.1
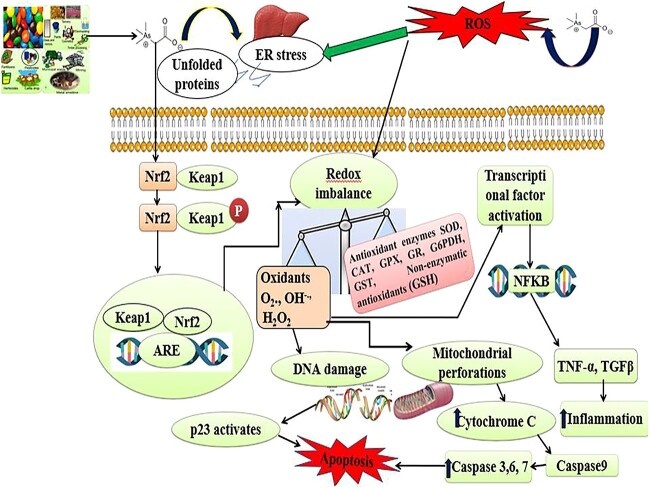
ROS-Mediated Oxidative Stress in Arsenic Toxicity: Oxidative stress mediated by reactive oxygen species (ROS) is one of the central mechanisms for the indication of arsenic toxicity. In biological systems ROS is formed, during the reduction of molecular oxygen and includes the superoxide radical anion (O2−•), hydrogen peroxide (H2O2), hydroxyl radical (•OH), hydroperoxyl radical (HOO•) and peroxyl radical (ROO•). When an imbalance occurs between the production of ROS and their metabolism of detoxification, ROS accumulates and leads to oxidative stress.32 Due to the surplus production of ROS, different signaling pathways get altered and can lead to oxidative modifications of biomolecules associated with loss in function of proteins, organelle damage and even death of cells (Fig. 3).33 Since, the toxicity mechanisms of arsenic are complex and not fully understood. The most probable mechanisms responsible for arsenic poisoning are proposed as follows:
Fig. 3.
Arsenic crosses the membrane lipid bilayer of liver cells, induces oxidative stress due to the production of free radicals such as O2, OH and H2O2 by declining the activities of CAT, SOD, GPX, GR, G6PDH and non-enzymatic antioxidant (GSH), results in DNA damage, protein misfolding, inflammation mitochondrial membrane dysfunctions, release of cytochrome C and finally leads to initiation of caspase dependent apoptosis.
4.1.1 Mitochondrial source: Energy disruption and ROS generation
Usually under controlled and normal physiological conditions, antioxidant system maintains the ROS level within the mitochondria, however ROS generation increases in weakened or aged mitochondria.34 Arsenic toxicity inhibits the cellular respiration and mitochondrial energy production by deactivation of important enzymes of Kreb’s cycle or by inhibition of nicotinamide adenine dinucleotide reduction leading to ROS production.35 As inhibits mitochondrial enzyme complexes I and III in electron transport chain and becomes responsible for the production of O2−•. Arsenic also inhibits succinic dehydrogenase activity and uncoupling oxidative phosphorylation with production of O2−•, which gives rise to other forms of ROS. As III and its metabolite (MMA, DMA) accumulation, decreases the production of succinyl coenzyme-A, condenses with ATP and replaces phosphate, and results in the generation of an unstable arsenate ester bond that gets hydrolyzed quickly.36 The result of this oxidation process inhibits the phosphorylation and decreases the production of ATP and finally decreases the energy supply in the cells. The consequence of arsenic toxicity depletes the energy currency of cells by a process known as Arsenolysis. Likewise, the pyruvate oxidase complex causes pyruvate (end product of glycolysis) to be oxidatively decarboxylated to acetyl coenzyme A and carbon dioxide before entering the Tricarboxylic Acid Cycle (TCA). This enzyme complex is made up of many enzymes and cofactors, one of which contains lipoic acid. The presence of two sulfhydryl (-SH) or thiol groups in a single lipoic acid improves its effectiveness. When trivalent arsenic (Arsenite) is exposed, two hydrogens are lost from the thiol portion, and arsenic binds to the sulfur molecule, forming the dihydrolipoyl-arsenite chelate complex. This compound disables the essential rate limiting enzymes by inhibiting the reoxidation of dihydrolipoyl groups, which is necessary for continued enzymatic activity.31 This toxic impact of arsenic raises the concentration of pyruvate in the blood, lowers energy generation, and eventually destroys the cells. Hence, arsenic toxicity disrupts the energy producing processes and leads to the decrease in the energy level in cells and also increase ROS production, eventually leads to premature cell death.37
4.1.2 NOX and NOS: ROS and RNS production
(NOX) and NO synthases (NOS) are the sources from which ROS/reactive nitrogen species (RNS) are produced. It also maintains enzymatic activities including xanthine oxidases, cyclooxygenases (COX), cytochrome p450 enzymes, lipoxygenases, myeloperoxidases and misfolding of proteins in the endoplasmic reticulum.38 Arsenic toxicity leads to the deposition of intracellular H2O2 through flavoproteins-dependent superoxide, producing enzymes such as NADPH oxidase.39 An interesting route to H2O2 production has been proposed to involve the oxidation of As (III) to As(V) which, under physiological conditions, results in the formation of H2O2. Hydrogen peroxide is not a free radical species; however, when an organism is overloaded by iron (as in the conditions of haemochromatosis, hemolytic anemias and hemodialysis), the high cellular concentrations of “free available iron” may have deleterious effects, as is demonstrated by the participation of Fe (II) in the decomposition of hydrogen peroxide, generating highly reactive hydroxyl radicals (Fenton reaction).40 The formation of •OH in the vicinity of DNA might lead to this radical reacting with DNA bases or with the deoxyribose backbone of DNA to produce modified (damaged) bases or strand breaks. In addition to reactive oxygen species, it has been reported that arsenic exposure can initiate the generation of reactive nitrogen species (RNS) as well.41 However, several contradictory results describing arsenic-induced production of NO• have been reported, one of which concluded that there was no arsenic-induced increase in NO• generation in human liver cells, which inhibited inducible NO synthase gene expression.42 In another study, arsenite was found to inhibit inducible NO synthase gene expression in rat pulmonary artery smooth muscle cells as a result of which there was no increase in NO• generation.43
4.1.3 Redox imbalance: Antioxidant Defense suppression
Arsenic also induces oxidative stress due to redox imbalance i.e. production of excessive oxidants (O2, OH and H2O2) and decrease in the activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione reductase (GR), glucose-6-phosphate dehydrogenase and non-enzymatic antioxidant such as reduced glutathione (GSH). Oxidative stress causes DNA damage, mitochondrial membrane perforations and release of cytochrome C and initiates apoptosis by activation of caspases 9, 3, 6 and 7 (Fig. 3)44. It has been reported that arsenic at a dose of 25 ppm for ten weeks administered to Wistar rats induces chronic damage, decreases blood δ-aminolaevulinic acid dehydrogenase activity (ALAD) and decreases the GSH level. These alterations are related to a significant decrease in red blood cells (RBC), hemoglobin, hematocrit, and SOD activity in blood. In liver and kidney increase in thiobarbituric acid reactive substances, a decrease in GSH: GSSG ratio is associated with a considerable rise in metallothionein in hepatocytes.45
4.2 Signal alteration: dysregulation of cellular signaling pathways
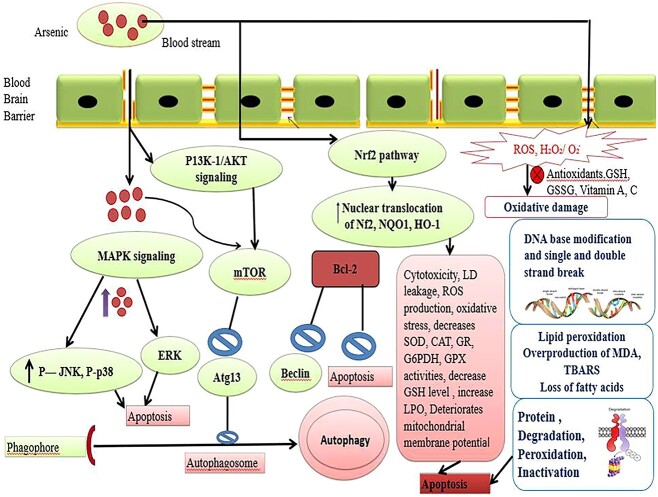
Signal transduction pathways transmit extracellular signals from series intracellular signaling molecules into genes.46 Arsenic being permeable to membrane lipid bilayer and alters various signal transduction pathways via ROS alteration or reversible oxidation of −SH group in proteins, which leads to activation or inhibition of various transcription factors and regulates gene transcription (Fig. 3). As activates the Mitogen-activated protein kinase (MAPK) signaling pathway and helps in expressing a lot of genes for cellular proliferation, differentiation, transformation and apoptosis. The most important members of this family are c Jun N-terminal Kinase (JNK), p38, and ERKs (extra-cellular signal regulated kinases). Among these, JNK and p38 are generally activated by stress responses and involved in apoptosis and growth arrest, whereas ERK signaling transduction generally aids in proliferation, differentiation, and transformation in a time and dose dependent manner. It has been reported that low levels of arsenite stimulate the ERK pathway for further proliferation, whereas high levels induce apoptosis via JNK signaling.47 Furthermore, Arsenic penetration in the cell stimulates the phosphatidylinositol 3-kinase/protein kinase B (P13K-1/AKT) signaling pathways activates Mammalian target of rapamycin (mTOR) and leads to the inhibition of Autophagy-related protein 13 (Atg13) (Fig. 4).48
Fig. 4.
Arsenic (As) cross the blood brain barrier (BBB) and stimulates down regulatory signaling pathways such as mitogen-activated protein kinases (MAPK)/extracellular signal-regulated Kinases1/2 (ERK1/2) and activates P-Jun N-terminal kinase (P-JUNK), p38 and increases reactive oxygen species (ROS) production, lipid peroxidation (LPO) rate and also decreases the activities of antioxidant enzymes (SOD, CAT, GR, G6PDH, GPX), declines reduced glutathione (GSH) level results in the apoptosis.
4.3 Arsenic induced inflammation
Arsenic (Sodium arsenite) induced ROS production activates an inflammatory response, which in turn initiates the pathological marker pathways such as the nuclear factor-κB (NFκB) by increasing phosphorylation and degradation of the inhibitor IkB (Fig. 3). This further increases the TNF-α level.49
4.3.1 Tumor necrosis factor-alpha (TNF-α) pathway
Tumor necrosis factor-alpha (TNF-α) plays an important role in stimulating HSC cells, the principal cells associated with the formation of type I collagen. In addition, arsenic exposure provokes oxidative stress in the liver, CD14 expression becomes up-regulated, enhances the resistance of Kupffer cells or promotes its metabolites. Moreover, the Kupffer cells most probably up-regulate the formation of TNF-α. The activated Kupffer cells release cytokines and stimulate HSCs by initiating the synthesis of collagen.50 The results of arsenic toxicity is liver oxidative stress by NADPH leading to stimulation of Kupffer cells and following TNF-α signaling mechanism, which leads to the apoptosis of hepatocytes, HSC activation, and hepatic fibrosis. An increase in TNF-α production in the liver is associated with up-regulation of a cluster of differentiation 14 (CD14) and toll-like receptor 4 (LTR-4). However, the mechanism through which arsenic activates Kupffer cells leads to the activation of CD14 and TLR-4.
TNF-α presumes a considerable role in the stimulation and upkeep of the inflammatory reaction and is related to the activation of HSCs. TNF-α contributes to the production of steatosis in hepatic tissue. Stimulation of Kupffer cells is significant initiation, leads to the production of TNF-α. Increased TNF-α and oxidative stress incite apoptosis in hepatocytes. Because stimulation of TNF-α activates the release of cytochrome c from mitochondria and the activation of caspases, the ultimate and most essential pathway in the apoptotic cascade. Arsenic toxicity produces oxidative stress by the NADPH oxidase pathway with ROS production and increased Kupffer cells and TNF-α. The consequences of TNF-α production cause apoptosis by releasing the cytochrome c mitochondrial pathway by activation of HSC.51 In addition to TNF-α, IL-6 and IL-1β are also released in response to arsenic-induced NF-κB activation. IL-6 is involved in immune regulation and can stimulate the production of acute-phase proteins. IL-1β is a pro-inflammatory cytokine that contributes to the amplification of the inflammatory response.52,53
Overall, arsenic exposure can lead to oxidative stress and the activation of inflammatory pathways, contributing to inflammation and potentially increasing the risk of various health conditions. Reducing exposure to arsenic, especially in contaminated drinking water and occupational settings, is crucial for mitigating its inflammatory effects on health. In conclusion, arsenic toxicity represents a multifaceted challenge, with a web of interconnected mechanisms at play. These mechanisms include the disruption of mitochondrial function, the generation of reactive oxygen and nitrogen species (ROS and RNS), the disturbance of redox balance, and the alteration of crucial cellular signaling pathways. Collectively, these intricate processes contribute to the detrimental effects of arsenic exposure on living organisms. Understanding these mechanisms is vital for developing effective strategies to mitigate and manage the health risks associated with arsenic contamination in various environmental settings.
4.4 Epigenetics
Epigenetics is a fascinating field of research that explores how various factors can influence heritable changes in gene expression without altering the underlying DNA sequence. These changes result in phenotypic variations without corresponding changes in the genotype. Epigenetic mechanisms play a crucial role in controlling gene expression during an organism’s development and can be influenced by a range of factors, including age, lifestyle, environment, exposure to pollutants, and pathological conditions. There are several key mechanisms responsible for epigenetic changes, including histone modifications, noncoding RNAs (ncRNAs), and DNA methylation.
4.4.1 Histone modifications
Histones are proteins that package and organize DNA into a compact structure called chromatin. Post-translational modifications of histones can either restrict or enhance gene expression by altering chromatin structure and the accessibility of DNA to transcription machinery. For example, histone methylation generally inhibits gene expression, while histone acetylation promotes it. Arsenic alters pyruvate dehydrogenase (PDH) activity, which is predominantly responsible for the formation of acetyl-CoA through the oxidation of pyruvate. Acetyl-CoA is a crucial substrate for acetylation, thus arsenic exposure significantly impact histone acetylation.54 Prenatal exposure to arsenic in mice causes hypo-acetylation at H3K9, which results in impaired episodic and spatial memory. In contrast, developmental exposure to arsenic can cause gender-specific regulation of H3K9 acetylation and methylation.55
Chervona et al. and Arita et al. have investigated the impact of chronic inorganic arsenic (iAs) exposure on epigenetic modifications, specifically focusing on H3K9 (histone H3 lysine 9) acetylation (H3K9ac). Both Chervona et al. and Arita et al. independently reported consistent findings regarding global H3K9ac levels in response to iAs exposure, regardless of gender. Found that global H3K9ac levels were negatively associated with chronic iAs exposure. This suggests that chronic exposure to iAs may lead to a reduction in H3K9ac, which is an epigenetic modification associated with the activation of gene expression. The decrease in H3K9ac levels could potentially result in altered gene regulation and contribute to the biological effects of iAs exposure.56,57
The acetylation of histone H4 at lysine 8 (H4K8ac) appears to be a less commonly studied histone modification in the context of inorganic arsenic (iAs) exposure. Ge et al.58 reported a decrease in H4K8ac levels in a Chinese population exposed to iAs through drinking water. This discrepancy highlights the need for further research to better understand the effects of iAs on histone modifications like H4K8ac and to determine whether these effects are consistent across different populations and experimental contexts. Overall, the influence of iAs on histone acetylation underscores the complexity of its epigenetic effects and highlights the importance of ongoing research to elucidate the mechanisms underlying these changes and their potential impact on human health. Multiple studies have investigated the impact of chronic inorganic arsenic (iAs) exposure on various histone modifications (methylation), shedding light on the complex relationship between iAs exposure and epigenetic changes. These studies have provided diverse findings, including gender-specific associations and discrepancies between different histone modifications.
H3K4me2 and H3K4me3, two histone modifications associated with gene activation, have shown mixed results in response to iAs exposure. Cantone et al.59 reported a positive association between iAs exposure and H3K4me2, while Chervona et al.55 found a positive association with H3K4me3, but this association was gender-specific. However, a study in mice contradicted the human results from Chervona et al., showing opposite associations by gender. These discrepancies highlight the complexity of iAs-induced epigenetic changes.55 H3K9me2, another histone modification, has displayed inconsistent results across studies. Some studies reported increased H3K9me2 levels in response to iAs exposure, while others reported decreased levels. Interestingly, the differences in exposure measures, such as urinary arsenic (uAs) and hair arsenic (hAs), may contribute to these discrepancies. H3K9me3 and H3K27me3, which are associated with gene repression, also showed varying results. Pournara et al.60 reported a negative association between iAs exposure and H3K9me3 in females, while H3K27me3 showed gender-specific associations in Chervona et al.’s study, being positively associated in females and negatively in males.55,60 H3K36me2 and H3K36me3, associated with gene activation, demonstrated mixed results between studies, with both positive and negative associations with iAs exposure reported. These discrepancies may be influenced by factors such as the source of iAs exposure (water vs. air and food) and the specific study population. H3K79me1, associated with gene activation, showed upregulation in response to iAs exposure, particularly in individuals with arsenic-induced skin lesions. Notably, H3K79me1 levels increased linearly with increasing arsenic dose. H4K20me2 and H4K20me3, associated with various cellular processes, displayed decrease in response to iAs exposure in certain contexts. Li et al.61 reported lower levels of H4K20me2 in individuals with arsenicosis, and Bhattacharjee et al., observed a significant decrease in H4K20me3 in arsenic-induced tumor tissue compared to nontumor tissue.61,62 In conclusion, the impact of iAs exposure on histone modifications is complex and can vary depending on the specific modification, gender, exposure source, and study population. These findings emphasize the need for further research to elucidate the mechanisms underlying iAs-induced epigenetic changes and their potential implications for health outcomes.
4.5 DNA methylation
DNA methylation is a fundamental epigenetic process involving the addition of methyl groups to cytosine residues in DNA, typically occurring at CpG dinucleotides situated within gene promoter regions. This biochemical modification plays a pivotal role in gene regulation by effectively silencing genes, primarily through the prevention of transcription factor binding to DNA. Central to the DNA methylation process, the principal methyl donor molecule, S-adenosylmethionine (SAM), which is actively engaged in arsenic metabolism, When excess arsenic is present, SAM is utilized by AS3MT [arsenic (III)methyltransferase] for methylation of arsenic leading to depletion of SAM and S-adenosylhomocysteine (SAH). Depletion of SAM by extensive arsenic metabolism might impose cofactor limitations on the activities of DNMTs.63 Arsenic exposure, notably prevalent in contaminated aquatic ecosystems, has garnered substantial attention in scientific research. Mirbahai et al.64 conducted research on fish residing in arsenic-contaminated aquatic systems and made noteworthy observations. They reported that fish from such environments displayed hepatic carcinogenicity, and this phenomenon was linked to variations in global DNA methylation and disruptions in one-carbon metabolism. Interestingly, these effects were associated with a decrease in choline levels and a reduction in DNA methyltransferases activity, possibly due to an increase in the DNA methyltransferases inhibitor S-adenosyl homocysteine.65 Several studies have reported significant alterations in the methylation status of various genes in response to exposure to inorganic arsenic (iAs). Among the numerous genes studied, only three have consistently shown significant changes across multiple investigations. These genes are the apoptosis regulator death-associated protein kinase (DAPK), the cell cycle inhibitor p16 gene, and the tumor suppressor p53 gene. In all the studies where these genes were examined, they consistently exhibited the same direction of association. Specifically, DAPK consistently showed increased methylation levels in response to iAs exposure, p16 also consistently exhibited increased methylation66,67 and similarly, p53 consistently displayed increased methylation.68 Hossain et al.69 reported iAs exposure was shown to be adversely correlated with p16 expression however thi conclusion was only marginally significant (P = 0.066). Moreover, Banerjee and his co-workers showed that DAPK expression was down by 3.4 times and p16 expression was down 2.2 times in patients compared to controls. In addition,70 found that 75% of methylated DAPK genes had lower levels of DAPK expression than 41% of routinely methylated DAPK genes (P = 0.037).69 Arsenic exposure has been shown to induce hypomethylation of the ERCC2 promoter. ERCC2 is a gene involved in DNA repair mechanisms. The hypomethylation of its promoter, as observed both in vivo and in vitro, leads to overexpression of ERCC2. This overexpression can inhibit the release of the Cdk-activating kinase complex and decrease the phosphorylation of p53 at serine 392. These molecular changes suggest that arsenic exposure may disrupt DNA repair mechanisms, potentially contributing to increased DNA damage observed in individuals exposed to arsenic.71 These findings suggest that iAs exposure may have a consistent impact on the methylation patterns of these genes, potentially contributing to their regulatory functions and implications for health outcomes. In a study conducted by Valles et al.72, the researchers investigated the potential transgenerational effects of arsenic exposure in zebrafish. These effects were not limited to the F0 generation but were also transmitted to the F2 generation, indicating transgenerational impacts. A particularly intriguing finding was the reduction in the expression of brain-derived neurotropic factor (BDNF) at the mRNA level in both the F0 and F2 adult zebrafish. BDNF plays a crucial role in neurodevelopment, including processes like synaptic plasticity and regeneration, which are essential for cognition and memory. The study also revealed an increase in histone H3K4me3 methylation, suggesting an epigenetic mechanism underlying these effects. This epigenetic alteration likely contributed to the observed hypermethylation of the BDNF gene promoter region in both generations, ultimately leading to decreased BDNF expression. These molecular changes had significant behavioral consequences in the F2 generation, including spontaneous tail coiling, anxiety-like behavior, and reduced motor activity and exploratory behavior (Velles et al. 2020).
4.5.1 Noncoding RNAs (ncRNAs)
Constitute a diverse group of RNA molecules that don’t encode proteins but hold pivotal regulatory roles in gene expression. Within this category, microRNAs (miRNAs) have garnered substantial attention due to their involvement in epigenetic regulation, including the modulation of DNA methylation and histone modifications. Exposure to inorganic arsenic (iAs) has been associated with alterations in miRNA (microRNA) expression patterns in various studies, highlighting its potential impact on gene regulation and health outcomes. Among the miRNAs altered in response to iAs exposure, miR-21 emerged as a consistently reported miRNA associated with iAs exposure across multiple studies73,74,75 In these studies, urinary levels of arsenic were used as a measure of exposure, and they consistently reported a positive association between iAs exposure and increased levels of miR-21. Banerjee et al.74 also delved into the downstream effects of miR-21 by evaluating the levels of proteins known to be regulated by miR-21. Their findings indicated an inverse association between miR-21 levels and the levels of these proteins, suggesting that miR-21 may play a role in post-transcriptional regulation of these target genes.74 Luo et al.76 investigated the mechanism of arsenic-induced lung carcinogenesis. They proposed that exposure to arsenic triggers the secretion of interleukin-6 (IL-6). IL-6, in turn, activates the STAT3 signaling pathway. This activation of STAT3 leads to the up-regulation of a specific miRNA called miR-21 in an autocrine (self-stimulating) manner. This up-regulation of miR-21 contributes to Epithelial-Mesenchymal Transition (EMT), which is a process involved in cancer progression. Another miRNA implicated in arsenic-induced cancer is miR-301a. Studies have found that miR-301a is overexpressed in human lung epithelial cells (BEAS-2B cells) exposed to arsenic. This overexpression is mediated through IL-6/STAT3 signaling, similar to miR-21. miR-21 is upregulated through the ROS-mediated activation of the ERK/NF-κB pathway in arsenic-exposed human embryo lung fibroblast cells, contributing to malignant transformation.77 Conversely, various miRNAs, including miR-200b and miR-31, are downregulated in arsenic-transformed cells, impairing their tumor-suppressive functions. For instance, miR-200b’s role in inhibiting cell migration is associated with its targeting of PKCα and involvement in the Wnt5b-PKCα positive feedback loop. Similarly, miR-31 downregulation leads to the overexpression of SATB2 and malignant cell transformation.71 MiR-143, typically downregulated in cancer stem cells, exhibits anti-cancer effects when restored, attributed to its inhibition of LIMK1 protein and cofilin phosphorylation.78 These miRNA alterations offer potential biomarkers for health effects related to arsenic exposure and promising therapeutic targets, although further research, especially population-based studies, is needed to uncover specific miRNA subsets and their applications in early detection and intervention against arsenic-induced health issues.
Furthermore,79 conducted a case-control study in West Bengal, India, focusing on individuals exposed to elevated levels of arsenic in drinking water. They reported upregulation of six senescence-related miRNAs (miR-34a, miR-29a, miR-126, miR-141, and miR-424) in the iAs-exposed group compared to the unexposed control group.80 Conducted a study in Mexico involving individuals exposed to iAs in drinking water. Their findings revealed an increase in circulating plasma miRNA, specifically miR-30c-15p, in subjects exposed to higher levels of iAs.81 examined the effects of iAs exposure on gestational age and birth weight among pregnant women and infants in a Bangladeshi birth cohort. They observed a significant association between low birth weight and high iAs exposure, specifically linked to elevated levels of miR-1290. Ruiz-Vera et al.82 conducted a cross-sectional study in an iAs-exposed region in Mexico, focusing on cardiovascular effects related to miRNAs. They reported an increase in serum miR-155 levels in subjects exposed to iAs compared to unexposed groups, which aligns with similar findings reported by83. These studies collectively provide valuable insights into the complex relationship between iAs exposure and miRNA dysregulation, with implications for various health outcomes and pathways of interest.
In conclusion, epigenetics is a complex and dynamic field of study that sheds light on how environmental factors, including arsenic exposure, can influence gene expression and potentially have long-lasting effects on both individuals and their descendants through epigenetic mechanisms. Understanding these processes is essential for assessing the health risks associated with environmental toxins and developing strategies to mitigate their impact.
5. Arsenic induced neurotoxicity
The brain is highly susceptible to oxidative stress-induced damage as a result of increased oxygen consumption, lower antioxidant defense content, and increased amounts of lipids and fatty acids.84 It has been reported that arsenic rapidly passes the blood-brain barrier and causes oxidative stress in glial cells, neuronal culture, and multiple brain areas.85,86 Since mitochondria is the major target of iAs, a persistent exposure to it damages mitochondrial complexes like I, II, and IV of rat brain, leading to an increase in production of ROS, protein carbonylation, and lipid peroxidation.87 In addition, the levels of mitochondrial transcriptional factor A (TFAM) and peroxisome proliferator-activated gamma receptor co-activator 1-alpha (PGC-1 alpha) decreased as a result of exposure to iAs, reduces biogenesis of mitochondria (Fig. 5).88 iAs toxicity results in the production of dimethylarsine (DMAH) and peroxyl radicals, which then interfere with lipid peroxidation and cause a build-up of oxidized substances. Moreover, the MMAIII and DMAIII metabolites are liable to be more potent toxicants due to their potential to produce free radicals.89
Fig. 5.
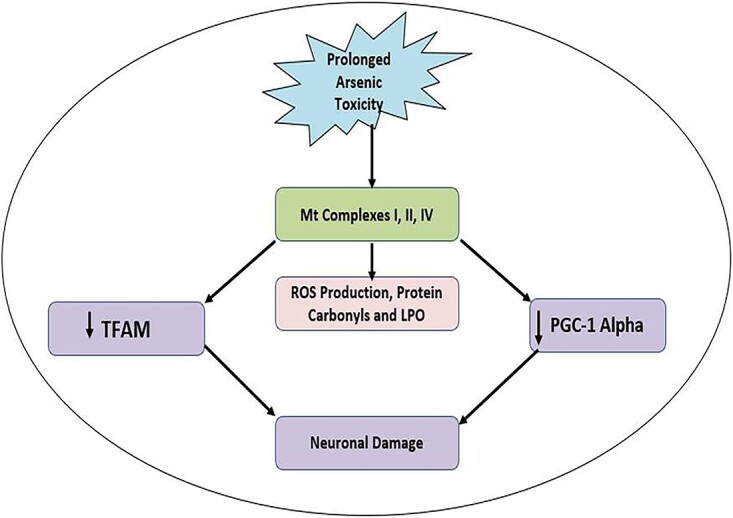
Arsenic induced generation of free radicals like reactive oxygen species (ROS) increased production of reactive oxygen species (ROS) on the different organ systems resulting in mitochondrial dysfunction. This leads to mitochondrial perforations and cytochrome c gets released from mitochondria and finally leads to the cellular apoptosis and neuronal damage.
In neurons and neuroblastoma cells, iAs and its methylated metabolites like MMAIII and DMAIII induce caspases-dependent apoptosis, which involves extracellular signal-regulated kinase 2 (ERK2), p38 or c-jun N-terminal kinases (JNK), through the mitogen-activated protein kinase (MAPK) signaling pathways.90 Under in vivo conditions, Ca2+ facilitates iAs-induced apoptosis in the cerebral cortex and antagonism to neurotrophic signaling, which is thought to be the cause of the spread of iAs-induced death in hippocampal neurons by.91 Manthari et al.92 claim that exposure to iAs during development inhibits the signaling pathways PI3K/Akt/mTOR/mechanistic target of rapamycin (mTOR) and leads to autophagy in the mouse brain (Fig. 4). Since it has been demonstrated that the inhibition of autophagy limits the negative effects of iAs on glioma cell lines.39
Nevertheless, Arsenic poisoning produces thiamine (vitamin B1) shortage, reduces pyruvate decarboxylase action, raises blood pyruvate levels, and finally results in encephalopathy.93 Arsenic poisoning causes DNA damage and oxidative stress in the brain, which results in cell death and accelerates the degradation of dopaminergic neurons, causing clinical manifestations similar to Parkinson’s disease.94,95
Further, arsenic also produces neurotoxicity by upsetting and dismantling the cytoskeletal structure, ultimately leading to axon deterioration and diminishes the nerve conduction velocity in the peripheral nerves to cause peripheral neuropathy (Vahidnia et al. 2008).96 The arsenic exposure and its metabolites like monomethylarsonic acid and monomethylarsonous acid inhibit the NMDA receptors in the hippocampus. All of these influence synaptic plasticity, learning, and memory, which all result in cognitive dysfunction and neuro-behavioral disorders.97,98 Chronic exposure to arsenic modifies the striatum’s axons and nerve fibers’ morphology, which alters the architecture of the central nervous system.99
According to,100 exposure to arsenic alters the metabolism of many neurotransmitters, including glutamate, acetylcholine, gamma-aminobutyric acid, monoamines, and glutamate. It has been reported that considerable decrease in monoamines such as adrenaline, nor-adrenaline, dopamine, and serotonin have been shown in the corpus striatum, frontal cortex, and hippocampus regions of the brain on prolonged arsenic exposure.101 Arsenic induced acute poisoning reduces acetylcholinesterase activity, resulting in a state similar to a cholinergic emergency such as weakness and skewed thinking that can be related to peripheral neuropathy, neuropsychiatric abnormalities, and extrapyramidal diseases.102,103
As a result, exposure to arsenic leads to a variety of neurological deficits, including encephalopathy, Parkinson’s disease, verbal comprehension, Guillain-Barre-like neuropathy, memory problems, and peripheral neuropathy (Vahidnia et al. 2008).93,96,104–106 Hence, it appears that arsenic-induced oxidative stress, induction of thiamine deficiency, suppression of pyruvate decarboxylase, acetylcholinesterase, decrease in biogenic monoamines, and decline in the activities of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), glucose-6-phosphatase dehydrogenase (G6PDH) and glutathione-S-Transferase (GST) seems to play a crucial role in the arsenic-induced neurotoxicity (Fig. 4).107 In conclusion, arsenic exposure poses a significant threat to brain health. It triggers oxidative stress, mitochondrial damage, and disruptions in signaling pathways, leading to a range of neurological issues, including encephalopathy, Parkinson’s-like symptoms, memory problems, and neuropathy. Additionally, arsenic affects neurotransmitter levels and synaptic function, contributing to cognitive dysfunction. These neurological deficits are driven by a complex interplay of factors, including oxidative stress, thiamine deficiency, and enzyme disruption. Addressing and minimizing arsenic exposure is crucial for safeguarding neurological well-being.
6. Arsenic induced hepatotoxicity
Arsenic poisoning mostly affects the liver. Arsenic metabolism in the body results in ROS generation, oxidative stress, and liver damage. Arsenic toxicity in the liver produces increased production of reactive oxygen species (ROS). ROS directly damages cellular macromolecules such lipids, proteins, and DNA, which causes cell death (apoptosis).108,109 Arsenic (III) activates caspase cascade signaling by altering the functional proteins of mitochondria such as B-cell lymphoma 2 (Bcl-2), Cytochrome c, Bcl2 associated X apoptosis regulator (Bax), and Mitochondria Permeability Transition (MPT) (Figs 3 and 4). Furthermore, elevated caspase activity causes activation of stress pathways in the endoplasmic reticulum (ER) of hepatocytes. A significant peak in the liberation of lactate dehydrogenase (LDH) and apoptosis have been observed during arsenic stress. Arsenic-induced stress increases various stress markers such as nitric oxide, lipid peroxidation, ROS and hepatic enzymes such as ALT, AST, ALP, and calcium levels inside the cells.110 An increase in the ranks of AST mainly damages hepatocytes by overcoming the integrity at the functional levels of the hepatic membrane.111,112
According to a study, Arsenic induces liver toxicity by the induction of oxidative stress in mitochondria and decreases the biogenesis in the liver. In the liver, long—lasting arsenic exposure (sodium arsenite treatment) at a concentration of 25 ppm for 12 weeks causes a decrease in the activity of the enzyme mitochondrial superoxide dismutase (MnSOD).113 The decrease in MnSOD’s mRNA and protein expression was the actual target of arsenic at transcription level, along with the rise in oxidative damage at the mitochondrial level. Additionally, exposure to arsenic results in the down-regulation of the mitochondrial and nuclear-encoded subunits of complexes (ND1 and ND2) as well as the COX1 and COX2 cyclooxygenase complexes.114 Arsenic has been shown to cause chronic damage, lower blood levels of GSH, and decrease blood-aminolaevulinic acid dehydrogenase activity (ALAD) when given to Wistar rats for ten weeks at a dose of 25 ppm. These changes are linked to a substantial decline in the blood’s red blood cells (RBC), hemoglobin, hematocrit, and SOD activity. A drop in the GSH: GSSG ratio is linked to a significant increase in metallothionein in hepatocytes when thiobarbituric acid reactive compounds in the liver and kidney are increased.115 Arsenic’s interaction with a cell’s antioxidant system lowers glutathione levels, disrupts the DNA repair mechanism, causes oxidative damage to cells, and ultimately leads to cellular death.116
Arsenic exposed rats showed a number of pathological alterations in their livers, including subtle inflammation, localized illness, cell infiltration, and the growth of giant cells. According to histopathological investigations, arsenic exposure causes a variety of pathological changes in the liver, including the localized necrosis and vacuolization of cytoplasm of cells.117 Accumulation of neutrophils and lymphocytes leads to deterioration of hepatic tissues and necrosis of the central vein. Also, the infusion of arsenic causes necrosis, hepatic dysfunction, and alterations in the bile pigment formation, which raises the level of bilirubin.118,119. In addition, exposure to arsenic increases hepatic injury caused by lipopolysaccharides LPS, raising the size of necro-inflammatory foci and increases the level/activity of oxidative stress indicators. Hence, arsenic intoxication induces oxidative stress, inflammation, reduction in the activities of antioxidant enzymes, elevates protein carbonyls, and lipid peroxides, decrease glutathione level in the liver and increases hepatic damage and leads to necrosis in hepatocytes.120 In summary, arsenic poisoning has a profound impact on the liver. Arsenic metabolism generates reactive oxygen species (ROS), causing oxidative stress and direct cellular damage, leading to apoptosis. It disrupts mitochondrial function, decreases antioxidant enzyme activity, and impairs blood parameters. Arsenic exposure induces liver inflammation, necrosis, and the development of giant cells, while exacerbating hepatic injury when combined with lipopolysaccharides. This comprehensive understanding highlights the severe and multifaceted liver toxicity resulting from arsenic exposure.
7. Arsenic induced nephrotoxicity
Several proteins, including ATP-binding cassette transporter proteins, multidrug resistance protein 1 (MRP1/ABCC1), and the related protein MRP2 (ABCC2), are involved in detoxification of arsenic by the efflux of arsenic-GSH conjugates to the bile. Additionally, the MRP-2 transporter is found in proximal tubule cells (PTCs), where it aids in the efflux of As from the body through urine.121 The presence of MRP-2 transporter in the PTCs enhances the efflux of As and leads to increased ROS generation and other free radicals, inflammation and apoptosis (Lu et al. 2014).122 Arsenic induced toxic effect on PTC is stimulated by reduction of the intracellular GSH stores, stimulation of the caspases-3 and -9 signaling pathway, up-regulates the expression of interleukin-6 and interleukin-8 and initiation of the p-53 apoptotic pathways.123
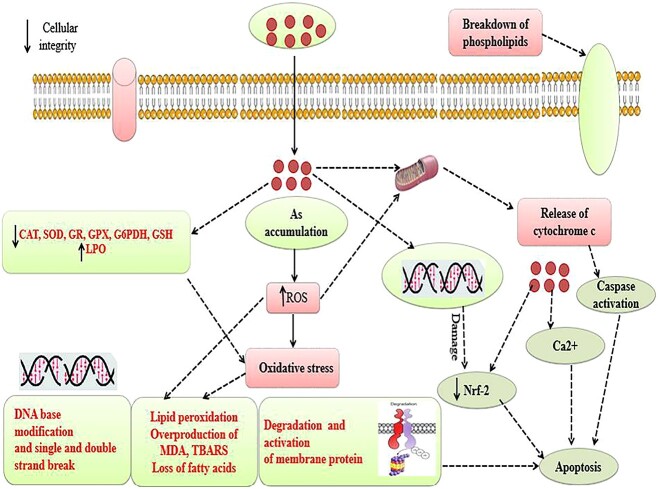
Arsenic uncouples oxidative phosphorylation, reduces the amount of sodium, phosphate, and glucose in the mitochondria, a condition known as Fanconi syndrome (phosphaturia, glucosuria and low-molecular-weight proteinuria).124 Arsenic prompted albuminuria (nephrotoxicity) is related with direct podocyte damage125 or by mechanism linked to dysfunction of endothelial cells as revealed by As up-regulates the expression of vascular cell adhesion molecule-1 (VCAM-1) and the Angiotensin type I receptor.126 Since, serum urea and creatinine are the useful clinical markers of renal injury,127 A group of researchers have studied the effect of Arsenic on renal functioning of male rats and it was found that the levels of both serum urea and serum creatinine were significantly increased which clearly indicated the renal damage.128 Additionally, administration of sodium arsenate at lesser dosages (0.7 mg/kg) results in a modest degradation and vacuolation of the thick ascending region of the nephron but has no effect on the rate of glomerular filtration or the fractional reabsorption of sodium, potassium, and chloride. However, higher dosages (14.6 mg/kg) result in severe acute tubular necrosis and profound glomerular sclerosis in each and every area of the nephron.124 Previous studies reported that arsenic administration via drinking water (22.5 mg/L) elevates the N-acetyl-β-D-glucosaminidase (NAG) level in urine, but it does not excrete albumin in the urine (UAlb). The urinary enzyme N-acetyl-β-D-glucosaminidase (NAG) exists in the lysosomes of proximal tubule epithelial cells. Increased NAG excretion in urine is caused exclusively by proximal tubular cell injury. Hence, in kidney accumulation of arsenic leads to the production of reactive oxygen species (ROS), inhibition of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPX) and also decrease reduced glutathione (GSH) content, however rate of lipid peroxidation (LPO) increases. This ROS production causes oxidative stress induced base modification in DNA and breaks in double stranded DNA, peroxidation of membrane lipid and loss of membrane lipids. Increases in rate of lipid oxidation cause degradation and inactivation of proteins.33 In addition to this, arsenic also damages the phospholipids and damages mitochondrial membrane by making perforations and release of cytochrome C and finally could lead to apoptosis (Fig. 6). In summary, arsenic exposure leads to a range of adverse outcomes, including the induction of oxidative stress, inflammation, and apoptosis, as well as damage to various cellular components, such as DNA and mitochondria. The presence of specific transport proteins, like MRP-2, plays a crucial role in arsenic detoxification but also contributes to adverse consequences. Furthermore, the paragraph emphasizes the various renal consequences of arsenic exposure, from proximal tubular cell damage and Fanconi syndrome to albuminuria and glomerular sclerosis. These findings underscore the significant health risks associated with arsenic exposure and the intricate mechanisms through which it affects kidney function and overall well-being.
Fig. 6.
In kidney accumulation of arsenic leads to the production of reactive oxygen species (ROS), inhibition of antioxidant enzymes such as CAT, SOD, GR, GPX and also decrease & GSH content, however rate of lipid peroxidation (LPO) increases. This ROS production causes oxidative stress induced base modification in DNA and breaks in double stranded DNA, peroxidation of membrane lipid and loss of membrane lipids.
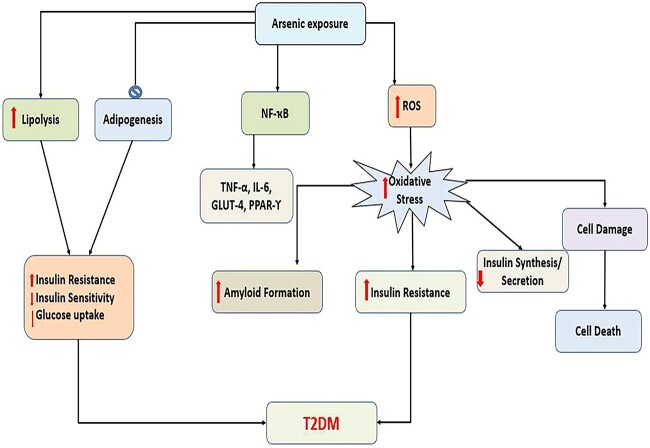
8. Arsenic exposure and diabetes mellitus
Arsenic affects the normal function of the liver, pancreatic islets, adipose tissues, and muscle. Arsenic impairs glucose transport and metabolism in the liver and reduces energy production through gluconeogenesis. Arsenic increases amyloid formation, insulin resistance, decreases insulin synthesis and secretion, and causes -cell damage, dysfunction, and death by inducing oxidative stress, all of which lead to Diabetes mellitus (Fig. 7).129 Insulin resistance, which is typically associated with abnormal insulin secretion, is the major pathophysiological event contributing to the development of Type 2 diabetes (T2DM). Arsenic inhibits adipogenesis and increases lipolysis in white adipose tissue in peripheral tissues such as adipocytes and muscle cells. It also surges insulin resistance, declines insulin sensitivity and glucose uptake by the alteration of the expression and/or activity of different key genes/proteins such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), tumor necrosis factor-alpha (TNF)-α, interleukin (IL)-6, glucose transporter type 4 (GLUT4) and PPARγ. Arsenic also alters some important signaling pathways including Ras–MAP Kinase–AP-1 cascade and PI(3)K–Akt pathway.19 Enhanced oxidative stress, phosphorus substitution, high affinity for sulfhydryl groups, and alteration of gene/protein expression, dysregulation of microRNAs (miRNAs) are all probable pathways for arsenic induced T2D. However, evidence suggested that long-term arsenic exposure has been linked to hyperglycemia, inflammation, and oxidative stress, all of which may lead to the development of diabetes.130 In summary, arsenic exposure has diverse and damaging effects on the body. It disrupts detoxification mechanisms, causing ROS generation and inflammation in kidney cells, leading to kidney issues like Fanconi syndrome and nephrotoxicity. Arsenic also disrupts glucose metabolism, insulin function, and energy production, contributing to the development of Type 2 diabetes. These findings emphasize the profound health risks associated with chronic arsenic exposure, underscoring the importance of mitigating this exposure to safeguard public health.
Fig. 7.
Arsenic exposure leading to increased ROS and increased oxidative stress which further surges insulin resistance, declines insulin sensitivity and glucose uptake, alteration of the expression of (NF-kB), (IL)-6, (GLUT4) and PPARγ resulting in T2DM.
9. Arsenic exposure and hypertension
The most commonly proposed mechanisms of arsenic-induced hypertension are oxidative stress and disruption of NO signaling. It is well established that arsenic exposure creates oxidative stress by generating excessive ROS which further disrupts the antioxidant defense system and interacts with biological macromolecules (i.e. lipids, proteins, and DNA) resulting in both structural and functional alteration.19 The release of NO, which promotes vascular relaxation by increasing guanosine 3′, 5′-cyclic monophosphate (cGMP) levels in the smooth muscle, is required to maintain normal blood pressure. NO formation and bioavailability were decreased by arsenic exposure, resulting in vasoconstriction and hypertension.131 As shown in Fig. 8, Arsenic-induced NO signaling disruption impairs vascular muscle calcium (Ca2+) signaling and increases blood pressure by enhancing myosin light chain phosphorylation-mediated vasoconstriction in blood vessels. Arsenic exposure is also involved in peripheral vascular resistance, upregulation of inflammatory mediators; atherosclerosis-related genes such as IL-6, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) and causes hypertension. Arsenic also has an influential role in the RAS which plays the most important role in blood pressure regulation. Arsenic exposure resulted in a concentration-dependent augmentation of the angiotensin (Ang) II-induced aortic contractile response as well as a contemporaneous concentration-related rise in plasma Ang II and angiotensin-converting enzyme (ACE) concentrations, implying that higher Ang II levels and exacerbation of Ang II signaling may contribute to vascular remodeling, which could be a key molecular basis for arsenic-induced hypertension.19 In conclusion, arsenic-induced hypertension is primarily driven by oxidative stress and disruption of nitric oxide (NO) signaling. Arsenic exposure leads to excess reactive oxygen species (ROS), causing oxidative damage and interfering with NO-mediated vascular relaxation. This results in vasoconstriction and elevated blood pressure. Arsenic also affects inflammation, atherosclerosis-related genes, and the renin-angiotensin system (RAS), further contributing to hypertension. The intricate mechanisms involved emphasize the need to address and reduce arsenic-related health risks associated with high blood pressure.
Fig. 8.
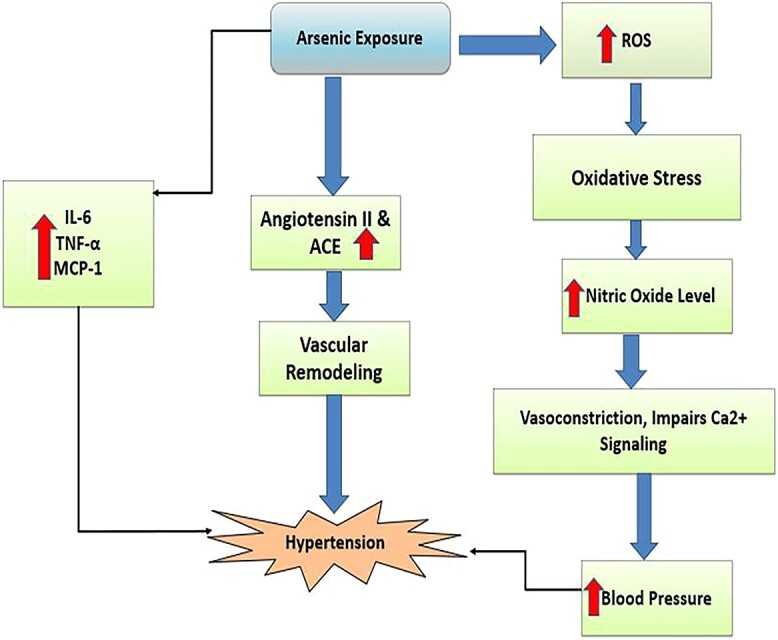
Arsenic exposure resulting in increased generation of ROS leading to oxidative stress, augmentation of the angiotensin (Ang) II- and angiotensin-converting enzyme (ACE), NO concentrations which could be a key molecular basis for arsenic-induced hypertension.
10. Arsenic and skin diseases
It has been found that Six months to two years or more of continuous arsenic exposure is the average time period before the appearance of cutaneous manifestations. Pigmentary changes are the earliest cutaneous changes of chronic arsenic toxicity. The various patterns described are diffuse melanosis, spotted melanosis or freckled raindrop pattern, leukomelanosis, dyschromia, and mucosal pigmentation. The common sites affected are the nipples, axilla, groin, palms, soles, and pressure points, but may later extend to other parts of the body.132
10.1 Diffuse melanosis and spotted melanosis or raindrop pigmentation
Diffuse hyperpigmentation is usually seen in the palms and soles. It can involve any part of the body, but is most common over the sun protected sites. It usually appears on the chest, back of trunk, and limbs as spotty hyperpigmentation like that of “rain drops on a dusty road”. In a study conducted in Kolkata in 1994, raindrop pigmentation was the most common cutaneous change seen in 71% of total 110 patients with suspected arsenicosis.132
10.2 Leukomelanosis
These are depigmented macules appearing on the normal skin or on a hyperpigmented background and are seen in about 1/3rd of the patients. It appears in the advanced stage of the disease or in those who have stopped drinking arsenic-contaminated water, but had presented with spotted melanosis in the early stages. This was first observed by Saha et al.133 who attributed it to destruction of melanocytes in the later stages.
10.3 Keratosis
Arsenic keratosis is a premalignant condition, which develops as a result of chronic arsenic exposure. It is the most sensitive early marker of arsenic toxicity. The lesions appear as multiple, firm, punctate, symmetric corn-like papules which may coalesce to form scaly, erythematous, hyperpigmented, or verrucous plaques. They are commonly located on sites prone to friction or trauma like palms and soles, but can also be present on the dorsum of extremities (dorsal keratosis), trunk, genitalia, and eyelids.132
10.4 Cutaneous malignancies
International Agency for Research on Cancer has classified arsenic as a Class I human carcinogen. The skin is thought to be the most vulnerable site for arsenic-induced cancers. They are usually multiple and can appear on both keratotic lesions and uninvolved skin (mainly sun-protected areas). In situ and invasive Squamous Cell Carcinoma (SCC), Basal Cell Carcinoma (BCC), and, on rare occasions, Merkel cell carcinoma and malignant melanoma are among the malignancies. On average, the time elapsed between first arsenic exposure and cutaneous malignancy is 17.8 years for Bowen’s disease and superficial BCC and 19.7 years for invasive SCC.132 In brief, long-term arsenic exposure leads to various skin changes, starting with pigmentary alterations like melanosis and progressing to leukomelanosis and premalignant arsenic keratosis. These effects underscore the serious health risks associated with chronic arsenic exposure, emphasizing the need for prevention and regular monitoring in affected populations.
11. Arsenic exposure and reproductive disorders
Infertility affects millions of people of reproductive age worldwide. Exposure to toxic heavy metals is one of the potential causes of human infertility. Oxidative stress is one of the most important factors in infertility. According to recent research, excessive production of reactive oxygen species (ROS) or reactive nitrogen species (RNS) can harm the male and female reproductive systems, resulting in infertility. Several epidemiological studies have evaluated blood levels of arsenic in infertile women; among them, a notable investigation was conducted in China by Hsiao-Ling Lei et al., and the results showed that blood levels of arsenic were significantly higher in infertile women compared to pregnant women.134 Several urinary biomarkers, including acylcarnitines, uridine (a stimulant of energy expenditure and apoptosis), and methyl xanthine, have been linked to arsenic-induced oxidative stress and male infertility. Numerous animal and human studies have shown that arsenic exposure causes infertility by altering levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone, as well as causing damage to the spermatogenesis and oogenesis processes. Arsenic is known to harm the male reproductive system by causing difficulties in egg-sperm fusion, abnormalities in sperm shape, disturbances in sperm capacitation, impairments in the glycolysis pathway, and disruptions in lipid and amino acid metabolism.135 In conclusion, Arsenic exposure is associated with infertility, primarily due to oxidative stress and disruption of the male and female reproductive systems. Studies have demonstrated elevated levels of arsenic in infertile women, emphasizing its role in infertility. Arsenic exposure leads to changes in hormone levels, damage to spermatogenesis and oogenesis, and disruptions in various reproductive processes, affecting both egg-sperm fusion and sperm health. These findings underscore the significant impact of heavy metal exposure on human infertility, highlighting the importance of understanding and mitigating this health concern.
12. Arsenic and carcinogenicity
It is well known that arsenic causes multiple human cancers like liver, lung, urinary bladder, skin, kidney etc. and the carcinogenicity of some most common types of cancers caused due to arsenic exposure are briefly discussed below:
12.1 Carcinogenic effects of arsenic on lungs
Lung cancer has long been linked to chronic arsenic exposure. There have been numerous reports of AKT and mTOR activation in human bronchial epithelial cells following arsenic exposure. Regardless of dosage (0–20 mM), arsenic compound type (NaAsO2, AsCl3), or length of contact (4 h to 26 weeks), all conditions of arsenic exposure result in increased activity of these pathways. Arsenic-exposed cells exhibit increased proliferation, survival, and anchorage-independent growth, in addition to activated AKT and mTOR activity, all of which are distinct hallmarks of malignant cell transformation. Thus, arsenic can probably activate the PI3K/AKT/mTOR pathway and foment cell proliferation in normal cells.136
12.2 Carcinogenic effects of arsenic on urinary system
The major effects of arsenic in urinary tract are kidney and bladder cancer, the latter being the ninth most common cancer worldwide.137 Epidemiological surveys of populations exposed to elevated levels of inorganic arsenic in drinking water have shown a strong and dose-response correlation with bladder cancer.138 Despite the discovery of numerous tumor markers, arsenic-induced bladder cancer continues to be prevalent.139 This situation led the WHO to lower the recommended limit for the concentration of arsenic in drinking water from 25 μg/L to 10 μg/L. However, many developing countries continue to endorse the earlier upper limit. The likely cause of the correlation between a contaminated water supply and bladder cancer is quite simple: arsenic, once consumed, is mainly excreted through urine (Palma-Lara et al. 2019).140
12.3 Carcinogenic effects of arsenic on skin
Significant associations have been reported between dermatological lesions and the risk of skin cancer. Tseng et al discovered a dose-response relationship between drinking water arsenic levels and skin cancers. Bowen’s disease, basal cell carcinoma, and squamous cell carcinoma are the most common arsenic-induced skin cancers. Bowen’s disease is a precancerous skin carcinoma in situ that has been well documented as a result of arsenical exposure. Clinically, Arsenic-induced Bowen’s Disease (BD) can be distinguished from non-arsenical Bowen’s Disease by its occurrence loci on sun-protected areas of the body and its multiple and recrudescent lesions. Abnormal cellular proliferation and dysplasia are observed in the epidermal lesion of BD with significant apoptotic and dyskeratotic keratinocytes. Most of non-arsenical BD showed complete remission after surgical operation, however, many of As-BD may recur after surgery. Furthermore, it has been reported that As-BD lesion is able to transform into invasive SCC, BCC and combined forms of the skin cancer. Arsenic-induced cancers of other internal organs are reported to associate with As-BD lesions. Individuals with documented As-BD are subjected to more aggressive screening for long-term complications, particularly the development of lung and urinary bladder cancers. Following suspected arsenic exposure, As-BD began within 10 years, invasive skin cancer after 20–30 years, and pulmonary cancer after 30 years. As a result, the pathological and clinical characteristics of As-BD may provide evidence of arsenic-induced cellular responses in the early stages of chemical carcinogenesis.141 In brief, arsenic exposure is linked to various cancers, including lung, kidney, and bladder cancers, as well as skin cancers like Bowen’s disease. The activation of specific cellular pathways, such as PI3K/AKT/mTOR, plays a role in lung cancer development. Urinary system cancers are strongly associated with high arsenic levels in drinking water. Skin lesions, like Bowen’s disease, are early indicators, with potential to progress into invasive cancers in other organs, highlighting the need for early recognition and intervention.
13. Conclusion
The Arsenic exposure through various routes may account for different health intimidation. Arsenic induced toxicity leads to the generation of ROS and accelerates oxidative stress, upregulates proinflammatory cytokines and inflammatory mediators and inactivates endothelial NOS (eNOS), Chronic arsenic exposure causes enormous toxicological manifestations in different organs such as liver, kidney, brain, reproductive system, cardiovascular abnormalities, diabetes mellitus through oxidative stress, suppression of pyruvate decarboxylate and acetyl cholinesterase. In addition to this arsenic toxicity may cause cancer by increasing oxidative stress induced DNA damage and chromosomal alterations and also by hindering the signaling cascades in cells. Targeting and modulation of these signaling pathways may develop a novel pharmacological approach to suppress the arsenic exposure related toxic manifestations. Moreover, new methods are required to evaluate the effect of arsenic and how people can combat with the socio-economic consequences of the diseases. Hence, considering the global burden of arsenic toxicity more research is required to cope with this serious public health burden.
14. Further recommendations
The foremost action that shall be taken to reduce the arsenic toxicity includes decrease the chances of arsenic exposure supply of safe quality drinking water, manufacturing food stuffs. Therefore, removal/reduction of arsenic from drinking water can be better option to prevent the arsenic toxicity. This can be carried out through different ways such as alternative arsenic safe water source and treatment of arsenic contaminated water. In addition to this various arsenic-resistance microbes (ARMs) are able to reduce the As (III) to get rid of extreme arsenic exposure.142 There are other chemical remediation methods such as adsorption by specific media, immobilization, and modified coagulation along with filtration, precipitation, immobilization, transgenic arsenic bioremediators and complexation reactions. Considering these facts, there is great need to identify the arsenic effected regions, sources of arsenic, to use approaches to reduce the arsenic induced toxic manifestation, and development of some pharmacological remedy to combat the pathogenesis of arsenic induced toxicity.
Hence, future studies are required to explore the molecular mechanism of arsenic ions remediation used for the other arsenic bio-mediators, researchers can also formulate proposal on transgenic form of the bio-mediators having higher potential, better growth, adaptability and higher production of biomass for increased bioaccumulation capacity. Therefore, research should be based on the identification these mechanisms and evaluate the genes associated with accumulation of arsenic in different organisms. Some ferns can be used for the remediation of arsenic from soil due to the potency to accumulate arsenic further, studies shall be focused on the assessment of capacity and ability of organism (microorganisms, plants and fungi) to remediate arsenic ions. We hope that this review article will lead to the new pathways for the development of therapeutic drug against Arsenic toxicity.
Acknowledgments
Authors are thankful to central library and departmental library (Department of Zoology) of Baba Ghulam Shah Badshah University, Rajouri, Jammu and Kashmir, India for providing access to different journals and books to this article. Authors are also thankful to Indian Council of Medical Research (GOI), Govt. of India (F.No. 59/7/2020/TRM/BMS, dated 25.03.2021) and University Grants Commission (UGC), Govt. of India (F.30-441/2018 (BSR), dated 07-06-2018) for financial Assistance.
Contributor Information
Shahid Yousuf Ganie, Toxicology and Pharmacology Laboratory, Department of Zoology, School of Biosciences and Biotechnology, Baba Ghulam Shah Badshah University, Rajouri, Jammu and Kashmir 185234, India.
Darakhshan Javaid, Toxicology and Pharmacology Laboratory, Department of Zoology, School of Biosciences and Biotechnology, Baba Ghulam Shah Badshah University, Rajouri, Jammu and Kashmir 185234, India.
Younis Ahmad Hajam, Department of Life Sciences and Allied Health Sciences, Sant Baba Bhag Singh University, Jalandhar, Punjab 144030, India.
Mohd Salim Reshi, Toxicology and Pharmacology Laboratory, Department of Zoology, School of Biosciences and Biotechnology, Baba Ghulam Shah Badshah University, Rajouri, Jammu and Kashmir 185234, India.
Author contributions
Shahid Yousuf (Writing, editing, Diagrams, Final Draft preparation), Darakhshan Javaid (Writing, Tables and Figures, Final Draft preparation, Formal analysis), Younus Ahamad Hajam (Writing, Tables and Figures, Final Draft preparation), and Mohd. Salim Reshi (Conceptualization, Supervision, Project Administration, Resources, Writing—review, editing and Final Draft Preparation)
Funding
No financial support was available for this study.
Conflict of interest statement. The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1. Sturchio E, Zanellato M, Minoia C, Bemporad E. Arsenic: environmental contamination and exposure. In: Masotti A, editor. Sources, environmental impact, toxicity and human health-amedical geology perspective; New York: Nova publisher; 2013. p. 1.
- 2. Ozturk M, Metin M, Altay V, Bhat RA, Ejaz M, Gul A, Unal BT, Hasanuzzaman M, Nibir L, Nahar K, et al. Arsenic and human health: genotoxicity, epigenomic effects, and cancer signaling. Biol Trace Elem Res. 2021:200(3):988–1001. [DOI] [PubMed] [Google Scholar]
- 3. Stroud JL, Norton GJ, Islam MR, Dasgupta T, White RP, Price AH, Meharg AA, McGrath SP, Zhao FJ. The dynamics of arsenic in four paddy fields in the Bengal delta. Environ Pollut. 2011:159(4):947–953. [DOI] [PubMed] [Google Scholar]
- 4. Wang JS, Wai CM. Arsenic in drinking water—a global environmental problem. J Chem Educ. 2004:81(2):207. [Google Scholar]
- 5. Yunus FM, Khan S, Chowdhury P, Milton AH, Hussain S, Rahman M. A review of groundwater arsenic contamination in Bangladesh: the millennium development goal era and beyond. Int J Environ Res Public Health. 2016:13(2):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hussain A, Raveendran VA, Kundu S, Samanta T, Shunmugam R, Pal D, Sarma JD. Mechanisms of arsenic-induced toxicity with special emphasis on arsenic-binding proteins. Arsenic-Anal Toxicol Studies. 2018:10(2):57–75. [Google Scholar]
- 7. Teixeira MC, Santos AC, Fernandes CS, Ng JC. Arsenic contamination assessment in Brazil–Past, present and future concerns: A historical and critical review. Science of the total environment. 2020:730:138217. [DOI] [PubMed] [Google Scholar]
- 8. Hu L, Zhang D, Qian Y, Nie Z, Long Y, Shen D, Fang C, Yao J. Microbes drive changes in arsenic species distribution during the landfill process. Environ Pollut. 2022:292(Pt A):118322. [DOI] [PubMed] [Google Scholar]
- 9. Nurchi VM, Buha Djordjevic A, Crisponi G, Alexander J, Bjørklund G, Aaseth J. Arsenic toxicity: molecular targets and therapeutic agents. Biomol Ther. 2020:10(2):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CDCP (Centers for Disease Control and Prevention), Agency for Toxic Substances and Disease Registry, Strategic Plan for Public Health Workforce Development . Toward a life-long learning system for public health practitioners. Washington, DC: US Department of Health and Human Services; 2001. [Google Scholar]
- 11. Smith AH, Ercumen A, Yuan Y, Steinmaus CM. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J Expo Sci Environ Epidemiol. 2009:19(4):343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mazumder DG. Health effects chronic arsenic toxicity. In Handbook of arsenic toxicology 2015; (pp. 137–177). Academic Press. [Google Scholar]
- 13. World Health Organization (WHO) . A field guide for detection, management and surveillance of arsenicosis cases. In: Caussy D, editors. Technical publication no. 30. New Delhi: WHO, SEARO; 2015. pp. 5–18. [Google Scholar]
- 14. Costa LG, Manzo L. Biomarkers occupational neurotoxicology. CRC Press; 2020, Boca Raton:United States; pp. 75–100. [Google Scholar]
- 15. Rodrıguez VM, Jiménez-Capdeville ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003:145(1):1–18. [DOI] [PubMed] [Google Scholar]
- 16. Raju NJ. Arsenic in the geo-environment: a review of sources, geochemical processes, toxicity and removal technologies. Environ Res. 2022:203:111782. [DOI] [PubMed] [Google Scholar]
- 17. Joshi S. Arsenic toxicity of groundwater remediation for drinking water. In: Khan T, Khan AR, Raza S, Azad I, Lawrence AJ, editors. Medicinal and environmental chemistry: experimental advances and simulations (part II); 2021. Netherlands: Bentham Publishers, p. 58.
- 18. Shankar S, Shanker U, Shikha. Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Sci World J. 2014:2014:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rehman MU, Khan R, Khan A, Qamar W, Arafah A, Ahmad A, Ahmad A, Akhter R, Rinklebe J, Ahmad P. Fate of arsenic in living systems: implications for sustainable and safe food chains. J Hazard Mater. 2021:417:126050. [DOI] [PubMed] [Google Scholar]
- 20. Stýblo M, Venkatratnam A, Fry RC, Thomas DJ. Origins, fate, and actions of methylated trivalent metabolites of inorganic arsenic: progress and prospects. Arch Toxicol. 2021:95(5):1547–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahaman MS, Rahman MM, Mise N, Sikder MT, Ichihara G, Uddin MK, Kurasaki M, Ichihara S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ Pollut. 2021:289:117940. [DOI] [PubMed] [Google Scholar]
- 22. Braun R, Schönberger N, Vinke S, Lederer F, Kalinowski J, Pollmann K. Application of next generation sequencing (NGS) in phage displayed peptide selection to support the identification of arsenic-binding motifs. Viruses. 2020:12(12):1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Challenger F. Biological methylation. Chemical Reviews 1945:36(3):315–361. [Google Scholar]
- 24. Friedman SL. Cytokines and fibrogenesis. In Seminars in liver disease 1999; (Vol. 19, No. 02, pp. 129–140). © 1999 by Thieme Medical Publishers, Inc. [DOI] [PubMed] [Google Scholar]
- 25. Anushree and Ahsan J. Effects of arsenic: neurological and cellular perspective. In: Kumar N. editor. Arsenic toxicity: challenges and solutions. Singapore: Springer; 2021. pp. 127–151. [Google Scholar]
- 26. Hirano S. Biotransformation of arsenic and toxicological implication of arsenic metabolites. Arch Toxicol. 2020:94(8):2587–2601. [DOI] [PubMed] [Google Scholar]
- 27. Roy NK, Murphy A, Costa M. Arsenic methyltransferase and methylation of inorganic arsenic. Biomol Ther. 2020:10(9):1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Limmer MA, Seyfferth AL. Altering the localization and toxicity of arsenic in rice grain. Sci Rep. 2022:12(1):5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanyal T, Bhattacharjee P, Paul S, Bhattacharjee P. Recent advances in arsenic research: significance of differential susceptibility and sustainable strategies for mitigation. Front Public Health. 2020:8:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moustafa DA. Arsenic speciation within the mammalian metabolism. [Doctoral dissertation]. USA: Johns Hopkins University; 2020. [Google Scholar]
- 31. Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005:79(4):183–191. [DOI] [PubMed] [Google Scholar]
- 32. Hamza M, Alam S, Rizwan M, Naz A. Ahmed T, Hashmi MZ. editors. Hazardous environmental micro-pollutants, health impacts and allied treatment technologies; 2022. Switzerland: Springer.. pp. 241–288.
- 33. Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, Pramodh S, Alsulimani A, Alkhanani MF, Harakeh S, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cell. 2022:11(3):552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021:22(9):4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cantoni O, Zito E, Guidarelli A, Fiorani M, Ghezzi P. Mitochondrial ROS, ER stress, and Nrf2 crosstalk in the regulation of mitochondrial apoptosis induced by arsenite. Antioxidants (Basel). 2022:11(5):1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fatoki JO, Badmus JA. Arsenic as an environmental and human health antagonist: a review of its toxicity and disease initiation. J Hazard Mater Adv. 2022:5:100052. [Google Scholar]
- 37. Hosseini MJ, Shaki F, Ghazi-Khansari M, Pourahmad J. Toxicity of arsenic (III) on isolated liver mitochondria: a new mechanistic approach. Iran J Pharm Res. 2013:12(Suppl):121–138. [PMC free article] [PubMed] [Google Scholar]
- 38. Prakash S, Verma AK. Arsenic: it's toxicity and impact on human health. Int J Biol Inno. 2021:3(1):38–47. [Google Scholar]
- 39. Garza-Lombó C, Pappa A, Panayiotidis MI, Gonsebatt ME, Franco R. Arsenic-induced neurotoxicity: a mechanistic appraisal. J Biol Inorg Chem. 2019:24(8):1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu Y, Li J, Lou B, Wu R, Wang G, Lu C, Wang H, Pi J, Xu Y. The role of reactive oxygen species in arsenic toxicity. Biomol Ther. 2020:10(2):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang Y, Gao M, Deng Y, Khan ZH, Liu X, Song Z, Qiu W. Efficient oxidation and adsorption of As (III) and As (V) in water using a Fenton-like reagent, (ferrihydrite)-loaded biochar. Sci Total Environ. 2020:715:136957. [DOI] [PubMed] [Google Scholar]
- 42. Drzeżdżon J, Jacewicz D, Chmurzyński L. The impact of environmental contamination on the generation of reactive oxygen and nitrogen species—consequences for plants and humans. Environ Int. 2018:119:133–151. [DOI] [PubMed] [Google Scholar]
- 43. Sun HJ, Ding S, Guan DX, Ma LQ. Nrf2/Keap1 pathway in countering arsenic-induced oxidative stress in mice after chronic exposure at environmentally-relevant concentrations. Chemosphere. 2022:303(Pt 3):135256. [DOI] [PubMed] [Google Scholar]
- 44. Zeng Q, Yi H, Huang L, An Q, Wang H. Long-term arsenite exposure induces testicular toxicity by redox imbalance, G2/M cell arrest and apoptosis in mice. Toxicology. 2019:411:122–132. [DOI] [PubMed] [Google Scholar]
- 45. Fu Z, Xi S. The effects of heavy metals on human metabolism. Toxicol Mech Methods. 2020:30(3):167–176. [DOI] [PubMed] [Google Scholar]
- 46. Garla R, Sharma N, Shamli, Kaushal N, Garg ML. Effect of zinc on hepatic and renal tissues of chronically arsenic exposed rats: a biochemical and histopathological study. Biol Trace Elem Res. 2021:199(11):4237–4250. [DOI] [PubMed] [Google Scholar]
- 47. Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020:19(3):1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Medda N, De SK, Maiti S. Different mechanisms of arsenic related signaling in cellular proliferation, apoptosis and neo-plastic transformation. Ecotoxicol Environ Saf. 2021:208:111752. [DOI] [PubMed] [Google Scholar]
- 49. Carpenter RL, Jiang BH. Roles of EGFR, PI3K, AKT, and mTOR in heavy metal-induced cancer. Curr Cancer Drug Targets. 2013:13(3):252–266. [DOI] [PubMed] [Google Scholar]
- 50. Bhattacharjee P, Chatterjee D, Singh KK, Giri AK. Systems biology approaches to evaluate arsenic toxicity and carcinogenicity: An overview. International journal of hygiene and environmental health, 2013:216(5):574–586. [DOI] [PubMed] [Google Scholar]
- 51. Choudhury S, Ghosh S, Mukherjee S, Gupta P, Bhattacharya S, Adhikary A, Chattopadhyay S. Pomegranate protects against arsenic-induced p53-dependent ROS-mediated inflammation and apoptosis in liver cells. J Nutr Biochem. 2016:38:25–40. [DOI] [PubMed] [Google Scholar]
- 52. Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol Appl Pharmacol. 2011:251(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao D, Yi H, Sang N. Arsenic intake-induced gastric toxicity is blocked by grape skin extract by modulating inflammation and oxidative stress in a mouse model. Ecotoxicol Environ Saf. 2022:233:113305. [DOI] [PubMed] [Google Scholar]
- 54. Chakraborty A, Ghosh S, Biswas B, Pramanik S, Nriagu J, Bhowmick S. Epigenetic modifications from arsenic exposure: a comprehensive review. Sci Total Environ. 2022:810:151218. [DOI] [PubMed] [Google Scholar]
- 55. Martinez VD, Lam WL. Health effects associated with pre- and perinatal exposure to arsenic. Front Genet. 2021:12:664717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Arita A, Shamy MY, Chervona Y, Clancy HA, Sun H, Hall MN, Qu Q, Gamble MV, Costa M. The effect of exposure to carcinogenic metals on histone tail modifications and gene expression in human subjects. J Trace Elem Med Biol. 2012:26(2–3):174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chervona Y, Hall MN, Arita A, Wu F, Sun H, Tseng HC, Ali E, Uddin MN, Liu X, Zoroddu MA, et al. Associations between arsenic exposure and global posttranslational histone modifications among adults in Bangladesh. Cancer Epidemiol Biomark Prev. 2012:21(12):2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ge Y, Zhu J, Wang X, Zheng N, Tu C, Qu J, Ren X. Mapping dynamic histone modification patterns during arsenic-induced malignant transformation of human bladder cells. Toxicol Appl Pharmacol. 2018:355:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cantone L, Nordio F, Hou L, Apostoli P, Bonzini M, Tarantini L, Angelici L, Bollati V, Zanobetti A, Schwartz J, et al. Inhalable metal-rich air particles and histone h3k4 dimethylation and h3k9 acetylation in a cross-sectional study of steel workers. Environ Health Perspect. 2011:119(7):964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pournara A, Kippler M, Holmlund T, Ceder R, Grafström R, Vahter M, Broberg K, Wallberg AE. Arsenic alters global histone modifications in lymphocytes in vitro and in vivo. Cell Biol Toxicol. 2016:32(4):275–284. [DOI] [PubMed] [Google Scholar]
- 61. Li J, Ma L, Wang X, Li D, Zeng Q, Xing X, Li C, Xie L, Chen L, Chen W, et al. Modifications of h3k9me2, h3k36me3 and h4k20me2 may be involved in arsenic-induced genetic damage. Toxicol Res (Camb). 2016:5(5):1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bhattacharjee P, Paul S, Bhattacharjee P. Understanding the mechanistic insight of arsenic exposure and decoding the histone cipher. Toxicology. 2020:430:152340. [DOI] [PubMed] [Google Scholar]
- 63. Clare CE, Brassington AH, Kwong WY, Sinclair KD. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu Rev Anim Biosci. 2019:7(1):263–287. [DOI] [PubMed] [Google Scholar]
- 64. Mirbahai L, Southam AD, Sommer U, Williams TD, Bignell JP, Lyons BP, Viant MR, Chipman JK. Disruption of DNA methylation via S-adenosylhomocysteine is a key process in high incidence liver carcinogenesis in fish. J Proteome Res. 2013:12(6):2895–2904. [DOI] [PubMed] [Google Scholar]
- 65. Samikkannu T, Chen C-H, Yih L-H, Wang ASS, Lin S-Y, Chen T-C, Jan K-Y. Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chem Res Toxicol. 2003:16(3):409–414. [DOI] [PubMed] [Google Scholar]
- 66. Banerjee N, Paul S, Sau TJ, Das JK, Bandyopadhyay A, Banerjee S, Giri AK. Epigenetic modifications of DAPK and p16 genes contribute to arsenic-induced skin lesions and nondermatological health effects. Toxicol Sci. 2013:135(2):300–308. [DOI] [PubMed] [Google Scholar]
- 67. Intarasunanont P, Navasumrit P, Waraprasit S, Chaisatra K, Suk WA, Mahidol C, Ruchirawat M. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environ Health. 2012:11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hossain MB, Vahter M, Concha G, Broberg K. Environmental arsenic exposure and DNA methylation of the tumor suppressor gene p16 and the DNA repair gene mlh1: effect of arsenic metabolism and genotype. Metallomics. 2012:4(11):1167–1175. [DOI] [PubMed] [Google Scholar]
- 69. Chen QY, Li J, Sun H, Wu F, Zhu Y, Kluz T, Jordan A, DesMarais T, Zhang X, Murphy A, et al. Role of miR-31 and SATB2 in arsenic-induced malignant BEAS-2B cell transformation. Mol Carcinog. 2018:57(8):968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Paul S, Banerjee N, Chatterjee A, Sau TJ, das JK, Mishra PK, Chakrabarti P, Bandyopadhyay A, Giri AK. Arsenic-induced promoter hypomethylation and over-expression of ERCC2 reduces DNA repair capacity in humans by non-disjunction of the ERCC2-Cdk7 complex. Metallomics. 2014:6(4):864–873. [DOI] [PubMed] [Google Scholar]
- 71. Valles S, Hernández-Sánchez J, Dipp VR, Huerta-González D, Olivares-Bañuelos TN, González-Fraga J, Bardullas U. Exposure to low doses of inorganic arsenic induces transgenerational changes on behavioral and epigenetic markers in zebrafish (Danio rerio). Toxicol Appl Pharmacol. 2020:396:115002. [DOI] [PubMed] [Google Scholar]
- 72. Banerjee N, Bandyopadhyay AK, Dutta S, das JK, Roy Chowdhury T, Bandyopadhyay A, Giri AK. Increased microRNA 21 expression contributes to arsenic induced skin lesions, skin cancers and respiratory distress in chronically exposed individuals. Toxicology. 2017:378:10–16. [DOI] [PubMed] [Google Scholar]
- 73. Kong AP, Xiao K, Choi KC, Wang G, Chan MH, Ho CS, Chan I, Wong CK, Chan JCN, Szeto CC. Associations between microRNA (miR-21, 126, 155 and 221), albuminuria and heavy metals in Hong Kong Chinese adolescents. Clin Chim Acta. 2012:413(13–14):1053–1057. [DOI] [PubMed] [Google Scholar]
- 74. Luo F, Xu Y, Ling M, Zhao Y, Xu W, Liang X, Jiang R, Wang B, Bian Q, Liu Q. Arsenite evokes IL-6 secretion, autocrine regulation of STAT3 signaling, and miR-21 expression, processes involved in the EMT and malignant transformation of human bronchial epithelial cells. Toxicol Appl Pharmacol. 2013:273(1):27–34. [DOI] [PubMed] [Google Scholar]
- 75. Maiti S. Arsenic-induced mutagenesis and carcinogenesis: a possible mechanism. In: Singh SJ, editor. Handbook of arsenic toxicology. Cambridge: Academic Press; 2023. pp. 253–301. [Google Scholar]
- 76. Ngalame NN, Makia NL, Waalkes MP, Tokar EJ. Mitigation of arsenic-induced acquired cancer phenotype in prostate cancer stem cells by miR-143 restoration. Toxicol Appl Pharmacol. 2016:312:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chatterjee D, Bandyopadhyay A, Sarma N, Basu S, Roychowdhury T, Roy SS, Giri AK. Role of microRNAs in senescence and its contribution to peripheral neuropathy in the arsenic exposed population of West Bengal, India. Environ Pollut. 2018:233:596–603. [DOI] [PubMed] [Google Scholar]
- 78. Beck R, Bommarito P, Douillet C, Kanke M, Del Razo LM, García-Vargas G, Stýblo, M. Circulating miRNAs associated with arsenic exposure. Environmental science & technology, 2018:52(24):14487–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rahman ML, Liang L, Valeri L, Su L, Zhu Z, Gao S, Mostofa G, Qamruzzaman Q, Hauser R, Baccarelli A, et al. Regulation of birthweight by placenta-derived miRNAs: evidence from an arsenic-exposed birth cohort in Bangladesh. Epigenetics. 2018:13(6):573–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ruíz-Vera T, Ochoa-Martínez ÁC, Zarazúa S, Carrizales-Yáñez L, Pérez-Maldonado IN. Circulating miRNA-126, -145 and -155 levels in Mexican women exposed to inorganic arsenic via drinking water. Environ Toxicol Pharmacol. 2019:67:79–86. [DOI] [PubMed] [Google Scholar]
- 81. Shrivastava S, Nirala SK, Reshi MS, Shukla S, Sharma A, Uthra C. Protective efficacy of vitamin F against acrylamide induced toxicity: studies on oxidative stress biomarkers. Open Biomark J. 2019:9(1):62–69. [Google Scholar]
- 82. Zhang Y, Duan X, Li J, Zhao S, Li W, Zhao L, Li B. Inorganic arsenic induces NRF2-regulated antioxidant defenses in both cerebral cortex and hippocampus in vivo. Neurochemical research, 2016:41:2119–2128. [DOI] [PubMed] [Google Scholar]
- 83. Hashim A, Ahmed MG, Manikoth S. Mechanism behind arsenic induced neurotoxicity with emphasis on neural-protein expression. J Indian Soc Toxicol. 2019:15(1):34–38. [Google Scholar]
- 84. Thakur M, Rachamalla M, Niyogi S, Datusalia AK, Flora SJS. Molecular mechanism of arsenic-induced neurotoxicity including neuronal dysfunctions. Int J Mol Sci. 2021:22(18):10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ameer SS, Xu Y, Engström K, Li H, Tallving P, Nermell B, Boemo A, Parada LA, Peñaloza LG, Concha G, et al. Exposure to inorganic arsenic is associated with increased mitochondrial DNA copy number and longer telomere length in peripheral blood. Front Cell Dev Biol. 2016:4:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zamora PL, Rockenbauer A, Villamena FA. Radical model of arsenic (III) toxicity: theoretical and EPR spin trapping studies. Chem Res Toxicol. 2014:27(5):765–774. [DOI] [PubMed] [Google Scholar]
- 87. Mao J, Yang J, Zhang Y, Li T, Wang C, Xu L, Hu Q, Wang X, Jiang S, Nie X, et al. Arsenic trioxide mediates HAPI microglia inflammatory response and subsequent neuron apoptosis through p38/JNK MAPK/STAT3 pathway. Toxicol Appl Pharmacol. 2016:303:79–89. [DOI] [PubMed] [Google Scholar]
- 88. Pandey R, Rai V, Mishra J, Mandrah K, Kumar Roy S, Bandyopadhyay S. From the cover: arsenic induces hippocampal neuronal apoptosis and cognitive impairments via an up-regulated BMP2/Smad-dependent reduced BDNF/TrkB signaling in rats. Toxicol Sci. 2017:159(1):137–158. [DOI] [PubMed] [Google Scholar]
- 89. Manthari RK, Tikka C, Ommati MM, Niu R, Sun Z, Wang J, Zhang J, Wang J. Arsenic induces autophagy in developmental mouse cerebral cortex and hippocampus by inhibiting PI3K/Akt/mTOR signaling pathway: involvement of blood-brain barrier’s tight junction proteins. Arch Toxicol. 2018:92(11):3255–3275. [DOI] [PubMed] [Google Scholar]
- 90. Singh AP, Goel RK, Kaur T. Mechanisms pertaining to arsenic toxicity. Toxicol Int. 2011:18(2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh A, Kukreti R, Saso L, Kukreti S.. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules, 2019:24(8):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Felix K, Manna SK, Wise K, Barr J, Ramesh GT. Low levels of arsenite activates nuclear factor-κB and activator protein-1 in immortalized mesencephalic cells. J Biochem Mol Toxicol. 2005:19(2):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol. 2009:239(2):169–177. [DOI] [PubMed] [Google Scholar]
- 94. Krüger K, Straub H, Hirner AV, Hippler J, Binding N, Muβhoff U. Effects of monomethylarsonic and monomethylarsonous acid on evoked synaptic potentials in hippocampal slices of adult and young rats. Toxicol Appl Pharmacol. 2009:236(1):115–123. [DOI] [PubMed] [Google Scholar]
- 95. Luo JH, Qiu ZQ, Shu WQ, Zhang YY, Zhang L, Chen JA. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol Lett. 2009:184(2):121–125. [DOI] [PubMed] [Google Scholar]
- 96. Ríos R, Zarazúa S, Santoyo ME, Sepúlveda-Saavedra J, Romero-Díaz V, Jiménez V, Pérez-Severiano F, Vidal-Cantú G, Delgado JM, Jiménez-Capdeville ME. Decreased nitric oxide markers and morphological changes in the brain of arsenic-exposed rats. Toxicology. 2009:261(1-2):68–75. [DOI] [PubMed] [Google Scholar]
- 97. Rodrıguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol. 2002:24(6):743–750. [DOI] [PubMed] [Google Scholar]
- 98. Yadav RS, Shukla RK, Sankhwar ML, Patel DK, Ansari RW, Pant AB, Islam F, Khanna VK. Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. Neurotoxicology. 2010:31(5):533–539. [DOI] [PubMed] [Google Scholar]
- 99. Ganie SY, Javaid D, Hajam YA, Reshi MS. Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: a critical appraisal. Toxicology. 2022:472:153181. [DOI] [PubMed] [Google Scholar]
- 100. Patlolla AK, Tchounwou PB. Serum acetyl cholinesterase as a biomarker of arsenic induced neurotoxicity in sprague-dawley rats. Int J Environ Res Public Health. 2005:2(1):80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gopalkrishnan A, Rao MV. Amelioration by vitamin A upon arsenic induced metabolic and neurotoxic effects. J Health Sci. 2006:52(5):568–577. [Google Scholar]
- 102. Piao F, Ma N, Hiraku Y, Murata M, Oikawa S, Cheng F, Zhong L, Yamauchi T, Kawanishi S, Yokoyama K. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J Occup Health. 2005:47(5):445–449. [DOI] [PubMed] [Google Scholar]
- 103. Yip SF, Yeung YM, Tsui EYK. Severe neurotoxicity following arsenic therapy for acute promyelocytic leukemia: potentiation by thiamine deficiency. Blood. 2002:99(9):3481–3482. [DOI] [PubMed] [Google Scholar]
- 104. Ghosh J, Sil PC. Mechanism for arsenic-induced toxic effects. In: Singh SJ. editor. Handbook of arsenic toxicology. Cambridge: Academic Press; 2015. pp. 203–231. [Google Scholar]
- 105. Dua TK, Dewanjee S, Khanra R. Prophylactic role of Enhydra fluctuans against arsenic-induced hepatotoxicity via anti-apoptotic and antioxidant mechanisms. Redox Rep. 2016:21(4):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shafik NM, El Batsh MM. Protective effects of combined selenium and Punica granatum treatment on some inflammatory and oxidative stress markers in arsenic-induced hepatotoxicity in rats. Biol Trace Elem Res. 2016:169(1):121–128. [DOI] [PubMed] [Google Scholar]
- 107. Verma Y, Rana SVS. Liver and kidney function in rats co-treated with fluoride and arsenic for different time intervals. Fluoride. 2022:55(1):90–98. [Google Scholar]
- 108. Abhilash S, Siviyasankar R, Binu P, Arathi P, Harikumaran Nair R. Different administration patterns of docosahexaenoic acid in combating cytotoxic manifestations due to arsenic trioxide (acute promyelocytic leukemia drug) induced redox imbalance in hepatocytes. Prostaglandins Other Lipid Mediat. 2018:136:64–75. [DOI] [PubMed] [Google Scholar]
- 109. Reshi MS, Uthra C, Yadav D, Sharma S, Singh A, Sharma A, Jaswal A, Sinha N, Shrivastava S, Shukla S. Silver nanoparticles protect acetaminophen induced acute hepatotoxicity: a biochemical and histopathological approach. Regul Toxicol Pharmacol. 2017:90:36–41. [DOI] [PubMed] [Google Scholar]
- 110. Ro SH, Bae J, Jang Y, Myers JF, Chung S, Yu J, Natarajan SK, Franco R, Song HS. Arsenic toxicity on metabolism and autophagy in adipose and muscle tissues. Antioxidants (Basel). 2022:11(4):689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Prakash C, Kumar V. Chronic arsenic exposure-induced oxidative stress is mediated by decreased mitochondrial biogenesis in rat liver. Biol Trace Elem Res. 2016:173(1):87–95. [DOI] [PubMed] [Google Scholar]
- 112. Gora RH, Baxla SL, Kerketta P, Patnaik S, Roy BK. Hepatoprotective activity of Tephrosia purpurea against arsenic induced toxicity in rats. Indian J Pharm. 2014:46(2):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Susan A, Rajendran K, Sathyasivam K, Krishnan UM. An overview of plant-based interventions to ameliorate arsenic toxicity. Biomed Pharmacother. 2019:109:838–852. [DOI] [PubMed] [Google Scholar]
- 114. Muthumani M. Tetrahydrocurcumin potentially attenuates arsenic induced oxidative hepatic dysfunction in rats. J Clin Toxicol. 2013:3(4):1–10. [Google Scholar]
- 115. Mershiba SD, Dassprakash MV, Saraswathy SD. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep. 2013:40(5):3681–3691. [DOI] [PubMed] [Google Scholar]
- 116. Ewere EG, Okolie NP, Ndem JI, Eze GI, Oyebadejo SA. Irvingia gabonensis leaf extract scavenges nitric oxide and hydrogen peroxide in vitro and modulates arsenic-induced hepatic oxidative stress in wistar rats. Clin Phytosci. 2022:8(1):1–12. [Google Scholar]
- 117. Renu K, Saravanan A, Elangovan A, Ramesh S, Annamalai S, Namachivayam A, Abel P, Madhyastha H, Madhyastha R, Maruyama M, et al. An appraisal on molecular and biochemical signalling cascades during arsenic-induced hepatotoxicity. Life Sci. 2020:260:118438. [DOI] [PubMed] [Google Scholar]
- 118. Hope JH, Hope BE. A review of the diagnosis and treatment of Ochratoxin A inhalational exposure associated with human illness and kidney disease including focal segmental glomerulosclerosis. J Environ Public Health. 2012:2012:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jimi S, Uchiyama M, Takaki AYA, Suzumiya J, Hara S. Mechanisms of cell death induced by cadmium and arsenic. In: Lee HK, DiMauro S, Tanaka M, Wei YH, editors. Mitochondrial pathogenesis. Berlin, Heidelberg: Springer; 2004. pp. 325–331. [Google Scholar]
- 120. Tokumoto M, Lee JY, Fujiwara Y, Uchiyama M, Satoh M. Inorganic arsenic induces apoptosis through downregulation of Ube2d genes and p53 accumulation in rat proximal tubular cells. J Toxicol Sci. 2013:38(6):815–820. [DOI] [PubMed] [Google Scholar]
- 121. Robles-Osorio ML, Sabath-Silva E, Sabath E. Arsenic-mediated nephrotoxicity. Ren Fail. 2015:37(4):542–547. [DOI] [PubMed] [Google Scholar]
- 122. Li Z, Piao F, Liu S, Wang Y, Qu S. Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp Toxicol Pathol. 2010:62(5):543–547. [DOI] [PubMed] [Google Scholar]
- 123. Hossain E, Ota A, Takahashi M, Karnan S, Damdindorj L, Konishi Y, Konishi H, Hosokawa Y. Arsenic upregulates the expression of angiotensin II type I receptor in mouse aortic endothelial cells. Toxicol Lett. 2013:220(1):70–75. [DOI] [PubMed] [Google Scholar]
- 124. Reshi MS, Yadav D, Uthra C, Shrivastava S, Shukla S. Acetaminophen-induced renal toxicity: preventive effect of silver nanoparticles. Toxicol Res (Camb). 2020:9(4):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jain A, Agrawal S, Flora SJ. Arsenic and nicotine co-exposure lead to some synergistic effects on oxidative stress and apoptotic markers in young rat blood, liver, kidneys and brain. Toxicol Rep. 2015:2:1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Alam F, Asiful Islam M, Hua Gan S, Mohamed M, Haryo Sasongko T. DNA methylation: an epigenetic insight into type 2 diabetes mellitus. Curr Pharm Des. 2016:22(28):4398–4419. [DOI] [PubMed] [Google Scholar]
- 127. Rehman K, Fatima F, Akash MSH. Biochemical investigation of association of arsenic exposure with risk factors of diabetes mellitus in Pakistani population and its validation in animal model. Environ Monit Assess. 2019:191(8):1–15. [DOI] [PubMed] [Google Scholar]
- 128. Cunha Martins A Jr, Carneiro MFH, Grotto D, Adeyemi JA, Barbosa F Jr. Arsenic, cadmium, and mercury-induced hypertension: mechanisms and epidemiological findings. J Toxicol Environ Health B. 2018:21(2):61–82. [DOI] [PubMed] [Google Scholar]
- 129. Rajiv SV, George M, Nandakumar G. Dermatological manifestations of arsenic exposure. J Skin Sex Transm Dis. 2022:5:14–21. [Google Scholar]
- 130. Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. A review of arsenic poisoning and its effects on human health. Crit Rev Environ Sci Technol. 1999:29(3):281–313. [Google Scholar]
- 131. Lei HL, Wei HJ, Ho HY, Liao KW, Chien LC. Relationship between risk factors for infertility in women and lead, cadmium, and arsenic blood levels: a cross-sectional study from Taiwan. BMC Public Health. 2015:15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zargari F, Rahaman MS, KazemPour R, Hajirostamlou M. Arsenic, oxidative stress and reproductive system. J Xenobiot. 2022:12(3):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Palma-Lara I, Martínez-Castillo M, Quintana-Pérez JC, Arellano-Mendoza MG, Tamay-Cach F, Valenzuela-Limón OL, García-Montalvo EA, Hernández-Zavala A. Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmacol. 2020:110:104539. [DOI] [PubMed] [Google Scholar]
- 134. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017:71(1):96–108. [DOI] [PubMed] [Google Scholar]
- 135. Aballay LR, Díaz MDP, Francisca FM, Muñoz SE. Cancer incidence and pattern of arsenic concentration in drinking water wells in Córdoba, Argentina. Int J Environ Health Res. 2012:22(3):220–231. [DOI] [PubMed] [Google Scholar]
- 136. Narayan VM, Adejoro O, Schwartz I, Ziegelmann M, Elliott S, Konety BR. The prevalence and impact of urinary marker testing in patients with bladder cancer. J Urol. 2018:199(1):74–80. [DOI] [PubMed] [Google Scholar]
- 137. Huang HW, Lee CH, Yu HS. Arsenic-induced carcinogenesis and immune dysregulation. Int J Environ Res Public Health. 2019:16(15):2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chen J, Rosen BP. The arsenic methylation cycle: how microbial communities adapted methylarsenicals for use as weapons in the continuing war for dominance. Front Environ Sci. 2020:8:43. [Google Scholar]
- 139. Lu G, Xu H, Chang D, Wu Z, Yao X, Zhang S, Li Z, Bai J, Cai Q, Zhang W. Arsenic exposure is associated with DNA hypermethylation of the tumor suppressor gene p16. J Occup Med Toxicol. 2014:9(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lu Y, Yuan H, Deng S, Wei Q, Guo C, Yi J, Wu J, Li R, Wen L, He Z, et al. Arsanilic acid causes apoptosis and oxidative stress in rat kidney epithelial cells (NRK-52e cells) by the activation of the caspase-9 and -3 signaling pathway. Drug Chem Toxicol. 2014:37(1):55–62. [DOI] [PubMed] [Google Scholar]
- 141. Vahidnia A, Romijn F, Van der Voet GB, De Wolff FA. Arsenic-induced neurotoxicity in relation to toxicokinetics: effects on sciatic nerve proteins. Chem Biol Interact. 2008:176(2-3):188–195. [DOI] [PubMed] [Google Scholar]
- 142. Zeng Q, Zou Z, Wang Q, Sun B, Liu Y, Liang B, Liu Q, Zhang A. Association and risk of five miRNAs with arsenic-induced multiorgan damage. Sci Total Environ. 2019:680:1–9. [DOI] [PubMed] [Google Scholar]