Abstract
In Asia, especially Vietnam, AF is a common arrhythmia and is linked to a higher risk of stroke and systemic embolism. Anticoagulation therapy for stroke prevention in AF patients can result in bleeding complications. To effectively manage AF, adopting appropriate anticoagulation and addressing modifiable risk factors are crucial. Vietnamese clinicians are particularly interested in non-vitamin K antagonist oral anticoagulants (NOACs), a recent development in AF treatment. However, the lack of head-to-head trials comparing NOACs makes selecting a specific NOAC challenging. This review aims to provide a comprehensive overview of the available clinical evidence on NOACs for stroke prevention in AF to assist clinicians in making informed decisions and improving treatment outcomes in patients with AF. The first part of this review will present the current landscape of AF in Vietnam, focusing on AF prevalence and highlighting gaps in clinical practice. Furthermore, this part extensively discusses the anticoagulation strategy for both primary and secondary stroke prevention in AF.
Keywords: Atrial fibrillation, vitamin K antagonists, non-vitamin K antagonist oral anticoagulant, primary stroke prevention, secondary stroke prevention
AF, the most common arrhythmia, affects approximately 60 million people worldwide and contributes to over 8 million disability-adjusted life years.[1] Based on published data from Asia, the prevalence of AF is in the range 0.49–1.9% across different regions.[2] Due to an ageing population, the incidence and prevalence of AF are expected to increase, with prevalence projected to reach 4.01% in Asia by 2050.[3] The burden of AF is due not only to its high prevalence, but also its adverse clinical outcomes. AF increases the risk of stroke by approximately fivefold and is associated with poor prognosis.[4,5] Appropriate anticoagulation, combined with effective symptom management and mitigation of modifiable risk factors, is crucial for the comprehensive management of AF.[6]
For many years, vitamin K antagonists (VKAs) were the only option for stroke prevention in patients with AF, reducing the stroke risk by approximately 60%.[7] However, the use of and adherence to VKAs can be challenging because of several disadvantages, such as those related to pharmacokinetics, pharmacodynamics, food and drug interactions and a narrow therapeutic range.[8,9] The introduction of non-vitamin K antagonist oral anticoagulants (NOACs), namely rivaroxaban, edoxaban, dabigatran and apixaban, marked a significant milestone in preventing stroke in patients with AF. In a pooled analysis of Phase III trials, NOACs not only overcame the disadvantages of VKAs, but also demonstrated a more favourable benefit–risk profile than VKAs.[10] Since their introduction, the use of NOACs has become widespread, and this has resulted in improved clinical outcomes, such as ischaemic stroke and mortality rates, in patients with AF.[11,12] In the absence of any contraindications, NOACs are now being considered the preferred first-line treatment for preventing stroke in patients with AF.[13,14] As the era of NOACs draws to a close, the introduction of newer anticoagulants, such as Factor XIa inhibitors, has initiated Phase III trials. However, despite advances, several unmet clinical needs persist that NOACs are unable to satisfactorily address. A meta-analysis revealed that NOACs are less effective than VKAs in preventing thrombotic events in antiphospholipid syndrome.[15] Furthermore, rivaroxaban, apixaban (PROACT Xa study; NCT04142658) and dabigatran have all proven inadequate as replacements for VKAs in patients with mechanical valves or rheumatic AF.[16,17] Certain populations, including patients with thrombocytopenia, end-stage renal disease and those undergoing dialysis, were intentionally excluded from pivotal trials. As a result, the decision to use NOACs in these populations relies heavily on clinical experience or data from non-randomised controlled trials.
The availability of four NOACs offers physicians a range of options for stroke prevention in patients with AF. However, this also presents a challenge for clinicians who are trying to determine the best NOAC to choose in a given clinical context. This decision is made more difficult by the lack of randomised head-to-head trials comparing the four available NOACs and the limited clinical guidelines available to help in their selection. The objectives of this article are to provide a comprehensive review of the available clinical evidence on NOACs in the literature and make recommendations based on available evidence and expert opinion, while also taking into consideration specific contexts in Vietnam.
Methods
A literature search was conducted using PubMed and the electronic library of local medical universities using the keywords ‘Atrial Fibrillation’, ‘Atrial Fibrillation’ AND ‘Vitamin K antagonists’ and ‘Atrial Fibrillation’ AND ‘Non-Vitamin K antagonists’, ‘Atrial Fibrillation’ AND ‘Stroke prevention’. Relevant articles published in English and Vietnamese focusing on stroke prevention in patients with AF in the Asian region, including Vietnam, as well as global data, were selected. The selected articles were reviewed to understand the treatment options and available clinical evidence for stroke prevention in patients with AF. Specific clinical conditions encountered by practising physicians in Vietnam were given careful consideration.
AF and the Treatment Landscape in Vietnam
Cardiovascular diseases are the leading cause of death in Vietnam, responsible for 31% of all-cause mortality.[18] Hypertension and diabetes, which are prevalent non-communicable diseases in Vietnam (prevalence 25.1% and 4.1%, respectively), are also well-known risk factors for AF.[19,20] Data on AF prevalence in the Vietnamese population are limited, but AF prevalence is expected to increase due to the ageing population. A population-based study found the AF prevalence in the general population to be approximately 1%, with 81.67% being non-valvular AF.[21] This prevalence rate is comparable with rates reported in several Asian countries and is lower than the prevalence observed in the white population.[2] However, due to a high population burden and the increasing number of elderly individuals, it is projected that Asia will have the highest number of patients affected by AF in the future. A few other studies in Vietnam reported that the AF prevalence was approximately 9% among individuals aged over 80 years and 3.9% among those aged over 60 years.[21,22] In the of Nguyen et al., AF was diagnosed using a 12-lead ECG, and thus subclinical or paroxysmal AF may not have been detected.[22] Therefore, the prevalence of AF in Vietnam may be underestimated.
Most of the patients with AF in Vietnam are elderly and female, with an average age over 65 years (Table 1).
Table 1: Epidemiological Data on Vietnamese Patients with AF.
| Authors and year | Age of patients with AF (years), mean ± SD | Population aged ≥65 years (%) | Women (%) |
|---|---|---|---|
| Lien et al. 2013[21] | NR | 70 | NR |
| Tu et al. 2016[22] | 79.1 ± 8.9 | 88.9 | 61.1 |
| Chi et al. 20017[23] | 67.93 ± 28.44 | 58.9 | 53.4 |
| Lien 2019[24] | 66.71 ± 11.41 | NR | 47.8 |
| Binh and Toan 2022[25] | NR | 55 | 61 |
| Khôi and Nga 2022[26] | 72.64 ± 11.91 | 78.2 | 56.4 |
NR = not reported.
Published studies showed that the Vietnamese population with AF has a high stroke risk, with the percentage of patients with a CHA2DS2-VASc score ≥2 ranging from 74.5% to 100%.[22,23,25–27] A population-based study found that 74.5% of patients with AF were at high risk of stroke, whereas an earlier study reported that up to 90% of patients with AF were at a high risk.[25,27] Generally, Asians have a higher risk of stroke than the white population, particularly those with low-risk stratification on the CHA2DS2-VASc score.[28] According to two independent studies, Asians with a CHA2DS2-VASc score of 0–1 have an annual stroke risk approximately 10-fold higher than that of the white population.[29,30] There is a possibility that the widely used CHA2DS2-VASc score may not be the optimal tool for accurately stratifying the low-risk Asian population. This highlights the need for the development of stroke risk stratification scores specific to the Asian AF population. Such scores would enable better classification and, subsequently, the initiation of more appropriate interventions.
The multifactorial aetiology of AF is often associated with multiple comorbidities in patients, including cardiovascular conditions. Hypertension is the most common comorbidity among Vietnamese AF patients, with a prevalence range of 56.9–80.2% reported in studies.[22–25,27] The prevalence of AF in hypertensive patients in Vietnam was observed to be 11.9%.[21] There is a well-established pathophysiological relationship between hypertension and AF.[31] With over one-quarter of the Vietnamese population having hypertension, its co-occurrence with AF creates an additional burden on both patients and the healthcare system.[19] Heart failure and diabetes are also common comorbidities in Vietnamese patients with AF, but the prevalence varies greatly between studies, ranging from 17.6% to 50% for heart failure and from 15.2% to 44.4% for diabetes.[22–25,27] Figure 1 shows the clinical characteristics of Vietnamese AF patients.
Figure 1: Clinical Characteristics of Vietnamese AF Patients.
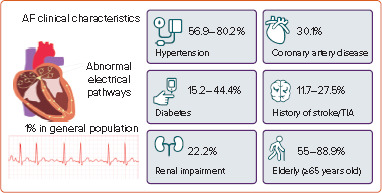
TIA = transient ischaemic attack.
During the pre-NOACs era, VKAs were the mainstay of stroke prevention in patients with AF in Vietnam. However, given their inherent disadvantages, poor adherence to treatment, a lack of patient knowledge and the inability to maintain the international normalised ratio (INR) within the therapeutic range, the use of VKAs has declined. In one study, the proportion of Vietnamese non-valvular AF (NVAF) patients with an INR between 2.0 and 3.0 with acenocoumarol was 27.8%.[27] This rate increased slightly, reaching 32.6%, when the treating physician was advised to monitor the INR more closely.[27] In a recently published study of 211 Vietnamese patients with AF, the proportion of patients achieving INR 2.0–3.0 was 43.1%, whereas the proportion of patients adhering to VKAs was 38.4%.[32] Only 16.6% and 14.2% of patients were compliant with prescription adherence and the minimisation of adverse events when using VKAs, respectively.[33] The management of INR levels in Asian patients is more challenging compared with that in the White population. Furthermore, Asian patients using VKAs face an elevated risk of major bleeding and intracranial haemorrhage (ICH) than their White counterparts.[34] This can be explained by the observation that Asian populations exhibit specific polymorphisms in genes involved in the metabolism of and response to VKAs. In Asian patients, the prevalence of certain polymorphisms in the vitamin K epoxide reductase complex subunit 1 (VKORC1) gene, which is associated with lower VKA dose requirements, is considerably higher compared with that in other ethnic groups.[35] In addition, Asian patients may be less likely to have cytochrome P450 family 2 subfamily C member 9 (CYP2C9) polymorphisms that result in poor VKA metabolism and lower dose requirements.[34,35] As a result, Asians may be more susceptible to major bleeding events and ICH due to increased VKA dose variations.
Of note, NOACs were introduced in Vietnam approximately 10 years ago, beginning with dabigatran and rivaroxaban, and followed by apixaban and edoxaban in the past few years. These four drugs have revolutionised the treatment landscape. The total days of treatment with NOACs and all oral anticoagulants has increased from 22% to 46% within 5 years, from 2018 to 2022. (IQVIA, personal communication, 2022). The most commonly used agent in clinical practice in Vietnam is rivaroxaban. The change in the treatment pattern over time not only reflects the superiority of NOACs over VKAs, but also demonstrates the effectiveness of medical education programmes in relation to anticoagulation in AF over the past decade. In a recently published guideline on the management of AF, the Vietnam Heart Association recommended NOACs as the preferred choice if there were no contraindications.[36] In Vietnam, NOACs are only approved for patients with NVAF, where AF is defined as without a mechanical valve prosthesis or with moderate-to-severe mitral stenosis.[36] Currently, there are no dedicated randomised control trials (RCTs) specifically focused on the use of NOACs in the Asian population, apart from the J-ROCKET study, which examined rivaroxaban in the Japanese population.[37] Asian participants accounted for varying proportions in Phase III trials, ranging from 6.5% (ROCKET-AF) to 15.4% (RELY).[37] A pooled analysis of Asian subgroups in these trials revealed that the standard dose of NOACs reduced the risk of stroke/systemic embolism (SE) to a greater extent in Asians than in non-Asians compared with VKAs, with an OR of 0.65 (95% CI [0.52–0.83]) versus 0.85 (95% CI [0.77–0.93]; p for interaction=0.045). Similarly, the predominance of NOACs over VKAs in the Asian population was observed in terms of haemorrhagic stroke and major bleeding. In comparing Asian with non-Asian populations, the use of NOACs was associated with a potentially lower risk of ICH among Asians (p for interaction=0.059).[37] This finding has significant implications for clinical practice, particularly in the context of concerns surrounding the ICH risk in Asians receiving anticoagulation. In addition, when comparing NOACs to VKAs, Asians exhibited a lower incidence of gastrointestinal (GI) bleeding than non-Asians, with an OR of 0.79 (95% CI 0.48–1.32; p=0.378) for Asians versus 1.44 (95% CI [1.12–1.85]; p=0.005) for non-Asians (p for interaction=0.041).[37] This observation was further supported by a systematic review meta-analysis of the Asian population, which demonstrated a decreased risk of GI bleeding with all four NOACs compared with VKAs.[38] Although GI bleeding is a significant concern associated with NOACs, interestingly, the findings suggest that Asian race may act as a protective factor against GI bleeding when using NOACs. Notably, the Asian population showed no significant heterogeneity in terms of efficacy and safety outcomes with respect to individual NOACs.[37]
Despite major advances in stroke prevention in patients with AF, there are still gaps in clinical practice in Vietnam. The evidence suggests that the proportion of patients with AF receiving anticoagulants is low. In a study published in 2016, only 22.2% of patients with AF who had a CHA2DS2-VASc score ≥2 were prescribed anticoagulants.[22] The 2017 study of Do and Cao estimated that the rate of anticoagulant use among acute stroke patients with both AF and a CHA2DS2-VASc score ≥2 was only 17.3%.[23] In another study, it was estimated that 66.4% of patients with AF were undertreated with anticoagulants.[27] Fear of bleeding and misconceptions regarding safety concerns while using anticoagulation therapy were suspected to be the main causes of its underuse. Bleeding events are often observed more easily than stroke events. This may contribute to the misconception that safety in the context of anticoagulation therapy is a low rate of bleeding, without considering the severity of bleeding events, such as the rate of fatal bleeding. In a study of more than 15,000 Asian patients, the leading cause of death in patients with AF was cerebral infarction, followed by MI, diabetes and heart failure.[39] That study also showed that bleeding was not among the leading causes of death in patients with AF.[39] Therefore, the concept of safety in anticoagulation therapy should be considered as providing more protection to patients, encompassing the efficient prevention of strokes, the mitigation of bleeding risk and severity and the proper management of underlying comorbidities.
Primary Stroke Prevention
Primary stroke prevention is considered a prophylactic treatment in patients with AF who have no prior history of stroke/transient ischaemic attack (TIA). Most AF patients in Vietnam are subjects for primary stroke prevention.[27] In the 2012 study by Hankey et al., even though all the patients included in the study received anticoagulation treatment for stroke prevention, the risk of stroke in patients with a history of stroke was 1.7-fold higher than in those without a history of stroke.[40] It is important to highlight that AF patients experiencing their first stroke event face a poor prognosis. Findings from the ROCKET-AF and ENGAGE AF-TIMI 48 studies revealed that approximately 30% of these patients would die following the first episode of AF-related stroke.[40,41] Therefore, in primary stroke prevention, the primary objective of anticoagulation is the prevention of stroke rather than bleeding. Adequate anticoagulation to prevent the first stroke event reduces not only the risk of death, but also the burden of secondary stroke prevention.
Data for NOACs from pivotal trials are summarised in Table 2.[40–43] NOACs were found to be as effective as VKAs in preventing primary stroke. Rivaroxaban (HR 0.65; 95% CI [0.47–0.90]) and dabigatran 150 mg twice daily (RR 0.60; 95% CI [0.45–0.78]) were superior to VKAs in stroke and SE prevention.[40,43] In terms of stroke severity, rivaroxaban reduced the risk of a fatal stroke more than VKAs, but there was no statistically significant difference in the risk of fatal stroke between edoxaban and VKAs.[40–43] As for safety outcomes, all four NOACs significantly reduced the risk of ICH compared with VKAs. NOACs were comparable to (rivaroxaban, dabigatran 150 mg twice daily) or significantly reduced (dabigatran 110 mg, edoxaban, apixaban) the risk of major bleeding versus VKAs. Rivaroxaban significantly reduced the risk of fatal bleeding, whereas the effect of edoxaban was not significantly different from VKAs.[40–43] Data on apixaban and dabigatran on the risk of fatal bleeding in primary stroke prevention has not been published yet.[42,43] In the RELY and Aristotle trials, where primary stroke prevention was approximately 80% in the whole population, both apixaban and dabigatran failed to demonstrate a reduced risk of fatal bleeding compared with VKAs.[44–46]
Table 2: Data on Non-vitamin K Antagonist Oral Anticoagulants in Primary Stroke Prevention in AF Patients with No Prior Stroke or Transient Ischaemic Attack – Insights from Pivotal Trials.
| Rivaroxaban, HR (95% CI)[40] | Dabigatran 150 mg, RR [95% CI][43] | Dabigatran 110 mg, RR [95% CI][43] | Apixaban, HR [95% CI][42] | Edoxaban, HR [95% CI][41] | |
|---|---|---|---|---|---|
| Stroke/SE | 0.65 [0.47–0.90]* | 0.60 [0.45–0.78]* | 0.93 [0.73–1.18] | 0.82 [0.65–1.03] | 0.88 [0.72–1.08] |
| Fatal stroke | 0.39 [0.20–0.75]* | NR | NR | NR | 0.97 [0.66–1.44] |
| Disabling or fatal stroke | 0.51 [0.31–0.82]* | 0.62 [0.43–0.89]* | 1.01 [0.73–1.39] | 0.60 [0.41–0.86]* | 1.09 [0.79–1.49] |
| ICH | 0.46 [0.24–0.89]* | 0.43 [0.27–0.68]* | 0.35 [0.21–0.57]* | 0.44 [0.30–0.66]* | 0.41 [0.27–0.61]* |
| Major bleeding | 1.11 [0.92–1.34] | 0.91 [0.77–1.06] | 0.85 [0.72–0.99]* | 0.68 [0.58–0.80]* | 0.79 [0.68–0.91]* |
| Fatal bleeding | 0.46 [0.23–0.90]* | NR | NR | NR | 0.61 [0.37–1.01] |
*Significant difference. ICH = intracranial haemorrhage; NR = not reported; SE = systemic embolism.
A retrospective cohort study involving around 20,000 patients demonstrated that rivaroxaban reduced stroke risk and mortality risk by 19% (HR 0.81; 95% CI [0.73–0.91]) and 24% (HR 0.76; 95% CI [0.61–0.95]), respectively, compared with warfarin for primary stroke prevention.[47] An observational cohort study of 61,678 patients with NVAF, conducted using data extracted from the Danish nationwide database, showed that apixaban, rivaroxaban and dabigatran reduced the risk of stroke/SE/mortality composite events more than warfarin for primary stroke prevention.[48] However, there were differences in a few important safety and efficacy endpoints. Rivaroxaban and dabigatran reduced the risk of ICH more than warfarin; dabigatran and apixaban reduced the risk of major bleeding more than warfarin; and only rivaroxaban reduced the risk of stroke/SE more than warfarin.[48] A non-interventional prospective study in the Japanese AF population showed that the risks of both thrombotic and bleeding events were numerically lower in patients treated with dabigatran 150 mg twice daily compared with dabigatran 110 mg twice daily.[49]
In the absence of randomised head-to-head studies comparing NOACs, the selection of medications should be based on individual patient characteristics. It is challenging to recommend a single drug for the entire population. If the primary goal is to reduce a patient’s stroke risk, rivaroxaban and dabigatran 150 mg twice daily could be considered preferred options. For patients with a history of life-threatening bleeding events, such as ICH, apixaban and edoxaban may be more suitable choices. If the patient requires multiple medications or prefers once-daily dosing, rivaroxaban and edoxaban would be the preferred options, respectively. Furthermore, the selection should also consider comorbidities, which are discussed in Part 2 of the article.[50]
Secondary Stroke Prevention
The prevalence of patients with a history of stroke among those with AF in Vietnam ranges from 11.7% to 27.5%.[23,27,51] A study by Nguyen et al. found that 9.5% of 6,601 Vietnamese patients who had their first stroke episode had AF.[52] In a recent prospective multicentre study conducted by Ton et al., the prevalence of AF among 2,300 acute stroke patients in Vietnam was found to be 5.4%.[53] It is important to note that patients with a history of stroke are prevalent in the Vietnamese AF population and should be given proper attention in medical practice. A study conducted among the Vietnamese population showed that a history of stroke increases the risk of stroke by 2.3-fold versus NVAF without a history of stroke.[24] In addition, the prognosis of AF-related stroke patients in Vietnam is poor. Two separate studies found that 33.8% of AF-related stroke patients died within 90 days, compared with only 10.4% of stroke patients without AF.[23,54]
Approximately 70–80% of the pivotal trial population for NOACs did not have a prior history of stroke, except in the ROCKET-AF trial, which evaluated rivaroxaban. In that trial, approximately 52% of participants had a prior history of stroke.[40,44,45,55,56] Meta-analyses were performed from the subgroup analysis data of the population with a prior stroke obtained from the pivotal trials.[57] The results showed that NOACs significantly reduced the risk of stroke/SE compared with VKAs (OR 0.86; 95% CI [0.77–0.97]). The difference in the risk of ischaemic stroke between NOACs and VKAs was not significant (OR 1.01; 95% CI [0.88–1.16]).[57] NOACs also reduced the risk of major bleeding (OR 0.86; 95% CI [0.75–0.99]) and all-cause mortality (OR 0.90; 95% CI [0.81–1.01]) more than VKAs.[57,58] There are no RCTs currently comparing NOACs in patients with AF who have previously had a stroke. Indirect comparisons of Phase III trials were performed in this population, but most efficacy and safety criteria were similar across NOACs.[59] Therefore, there is no strong evidence to favour one NOAC over the other. It should be noted that the prevalence of dysphagia in stroke patients in Vietnam is 71.6%, of which 21.7% is severe dysphagia.[60] Patients with dysphagia may require a nasogastric tube. In such cases, dabigatran should not be used because opening or crushing capsules may increase the risk of bleeding.
Real-world studies evaluating the effectiveness of NOACs in daily clinical practice have also been published. The REAFFIRM study used the MarketScan claims database from January 2012 to June 2015 to assess the efficacy of rivaroxaban, apixaban and dabigatran compared with warfarin in secondary stroke prevention in patients with AF.[61] In that retrospective analysis, all three NOACs were equivalent or superior to warfarin for the endpoints of ischaemic stroke, ICH and major bleeding.[61] Of the three NOACs, rivaroxaban significantly reduced the risk of the composite outcomes of ischaemic stroke and ICH compared with warfarin.[60] These observations are consistent with results from previous RCTs. A retrospective analysis was conducted among more than 11,000 stroke survivors between October 2011 and December 2014.[61] In that analysis, NOACs significantly reduced the risk of all-cause mortality (a [a] HR 0.88; 95% CI [0.82–0.95]; p<0.001) and haemorrhagic stroke (aHR 0.69; 95% CI [0.50–0.95]; p=0.02) compared with warfarin.[62] However, the risk of embolic stroke was not significantly different between the two treatment groups.[62] A meta-analysis of real-world studies in patients with a history of stroke has recently been published.[63] The results of that analysis showed that NOACs were associated with decreased risks of stroke (HR 0.82; 95% CI [0.69–0.97]), all-cause mortality (HR 0.87; 95% CI [0.81–0.94]), major bleeding (HR 0.77; 95% CI [0.64–0.92]) and ICH (HR 0.54; 95% CI [0.38–0.77).[63]
In patients with acute stroke, the blood–brain barrier is disrupted and they are prone to haemorrhagic transformation with anticoagulants. Conversely, the risk of stroke recurrence in the first 14 days in patients with AF was high.[64] Therefore, the timing of resuming anticoagulant therapy after acute stroke is also a concern. According to expert opinion, international clinical guideline-recommended NOACs should be started in 1–3 days if the patient has a TIA, at ≥3 days if the patient has ischaemic stroke and a mild neurological deficit, at ≥6–8 days if the patient has ischaemic stroke and a moderate neurological deficit, at ≥12–14 days if the patient has ischaemic stroke and a severe neurological deficit and at ≥3–28 days if the patient has a haemorrhagic transformation.[65] (Re-)initiation of NOACs in haemorrhagic stroke can be considered within 4–8 weeks.[65] TIMING is the first published RCT on the timing of resuming anticoagulants after stroke, with the results showing that early resumption of an anticoagulant (≤4 days after stroke onset) was non-inferior to delayed resumption (5–10 days after stroke onset) on the composite of recurrent ischaemic stroke, symptomatic ICH or all-cause mortality at 90 days in AF patients with acute stroke.[66] The rates of ischaemic stroke and death in the early resumption group were numerically lower, but this difference was not statistically significant.[65] The number of patients enrolled in TIMING was lower than expected, which may have reduced the power of the study.[66] Two other RCTs on the timing of restarting anticoagulants after stroke, namely OPTIMAS (NCT03759938) and ELAN (NCT03148457), are ongoing. Until there is sufficient evidence to make strong recommendations, decisions on anticoagulation therapy after stroke must be individualised based on specific clinical contexts.
Meta-analysis revealed that the incidence of dementia 5 years after a stroke episode was 20%; if the patient had an AF-related stroke, the risk of dementia increased by 1.5-fold.[67] The results of another meta-analysis showed that NOACs reduced the risk of dementia in patients with AF compared with warfarin (OR 0.56; 95% CI [0.34–0.94]; p=0.03).[68] However, the result was heterogeneous across NOACs. Rivaroxaban (OR 0.67; 95% CI [0.61–0.75]; p<0.00001) and apixaban (OR 0.58; 95% CI [0.50–0.67]; p<0. 00001) reduced the risk of dementia significantly versus warfarin, but not dabigatran did not (OR 0.97; 95% CI [0.88–1.08]; p=0.61).[68]
In summary, we recommend that NOACs be preferred over VKAs for secondary stroke prevention in AF. No specific NOACs are preferred over others.
Discussion and Expert Opinion
To the best of our knowledge, this is the first publication to present a comprehensive overview of AF in Vietnam. A review of current medical literature indicated a scarcity of data regarding AF and the efficacy and safety of anticoagulants in Vietnamese patients. This dearth of information is a prevalent issue observed in lower-middle-income countries (LMICs).[69] Consequently, there is a pressing imperative for additional epidemiological research initiatives and studies to generate further insights that can inform the development of effective management strategies for AF. In pursuit of this common objective of enhancing the quality of AF management, collaborative research programmes involving LMICs and countries with shared clinical backgrounds warrant consideration because they could foster the creation of augmented resources.
The limited data available suggest that Vietnamese patients with AF have a high risk of stroke with a high prevalence of comorbidities. Regrettably, the rate of anticoagulation use among patients with AF in Vietnam remains exceedingly low. Although underusing anticoagulants is a pressing issue in developed countries, it is even more severe in LMICs, including Vietnam.[70] Despite various initiatives aimed at enhancing access to anticoagulation for patients with AF, such as the WHO’s inclusion of NOACs in the Essential Medicines List and medical educational programmes in AF, underutilising anticoagulation remains a significant unresolved problem.[71] The concept of ‘safety first’ is correct, but clinical practitioners need to expand the concept of safety to prevent more harmful events for the patient and reduce the severity when these events occur. The obsession with bleeding events sometimes distracts clinicians from the primary goal of anticoagulation, which is stroke prevention. On a positive note, the emergence of generic versions of NOACs has improved patient access to more affordable treatments. Nonetheless, recent concerns have arisen regarding the quality of these medications.[72] Consequently, health authorities must implement appropriate measures to ensure the quality management of these drugs.
In the realm of stroke prevention, whether for primary or secondary purposes, the usage of anticoagulation assumes a pivotal role in averting stroke and enhancing prognosis for individuals with AF. It is important to underscore the significance of prioritising primary stroke prevention, because it holds promise for ameliorating clinical outcomes in the future. In both primary and secondary prevention cohorts, NOACs have exhibited a favourable risk–benefit profile compared with VKAs. Independent studies have revealed subtle discrepancies among the NOACs on safety and efficacy data. Nonetheless, the choice of anticoagulant must be evaluated on a case-by-case basis, factoring in not only clinical data, but also financial implications, insurance coverage, patient preferences, treatment adherence and individual tolerability to specific medications. Importantly, when there is an indication, any anticoagulant is better than no anticoagulation.
Conclusion
Part 1 of this review provides an overview of AF in Vietnam, encompassing epidemiological data, clinical features, treatment options and gaps in AF management. Regarding primary and secondary stroke prevention, NOACs demonstrate advantages over VKAs in terms of both efficacy and safety. In primary prevention, the selection of NOAC should be carefully evaluated based on the individual patient’s condition. Various factors, including but not limited to stroke risk, bleeding risk, comorbidities and patient preferences, must be considered when making clinical decisions. In the context of secondary stroke prevention, no significant differences were observed between NOACs.
Acknowledgments
The authors thank the Medical Affairs Department of Bayer, Vietnam, headed by Huong Thi Lan Tran, for providing financial support for medical writing services and article processing charges. The authors also thank Lakshman Puli of IQVIA, India, for his medical writing and editorial support. THQH and MTT contributed equally and share first authorship.
References
- 1.Elliott AD, Middeldorp ME, Van Gelder IC et al. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. 2023;20:404–17. doi: 10.1038/s41569-022-00820-8. [DOI] [PubMed] [Google Scholar]
- 2.Zulkifly H, Lip GYH, Lane DA. Epidemiology of atrial fibrillation. Int J Clin Pract. 2018;72:e13070. doi: 10.1111/ijcp.13070. [DOI] [PubMed] [Google Scholar]
- 3.Chao TF, Liu CJ, Tuan TC et al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest. 2018;153:453–66. doi: 10.1016/j.chest.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 5.Lin HJ, Wolf PA, Kelly-Hayes M et al. Stroke severity in atrial fibrillation. The Framingham study. Stroke. 1996;27:1760–4. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 6.Michaud GF, Stevenson WG. Atrial fibrillation. N Engl J Med. 2021;384:353–61. doi: 10.1056/NEJMcp2023658. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern. Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Piccini JP, Hernandez AF, Zhao X et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009;54:1280–9. doi: 10.1016/j.jacc.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Yim JM, Belew KM et al. Status of antithrombotic therapy for patients with atrial fibrillation in university hospitals. Arch Intern Med. 1996;156:2311–6. doi: 10.1001/archinte.1996.00440190053006. [DOI] [PubMed] [Google Scholar]
- 10.Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 11.Haas S, Camm JA, Harald D et al. GARFIELD-AF: risk profiles, treatment patterns and 2-year outcomes in patients with atrial fibrillation in Germany, Austria and Switzerland (DACH) compared to 32 countries in other regions worldwide. Clin Res Cardiol. 2023;112:759–71. doi: 10.1007/s00392-022-02079-y. [DOI] [PubMed] [Google Scholar]
- 12.Chao TF, Chiang CE, Lin YJ et al. Evolving changes of the use of oral anticoagulants and outcomes in patients with newly diagnosed atrial fibrillation in Taiwan. Circulation. 2018;138:1485–7. doi: 10.1161/CIRCULATIONAHA.118.036046. [DOI] [PubMed] [Google Scholar]
- 13.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–51. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 14.Hindricks G, Potpara T, Dagres N et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Cao S, Yu B, He T. Comparing the efficacy and safety of direct oral anticoagulants versus vitamin K antagonists in patients with antiphospholipid syndrome: a systematic review and meta-analysis. Blood Coagul. Fibrinolysis. 2022;33:389–401. doi: 10.1097/MBC.0000000000001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly SJ, Karthikeyan G, Ntsekhe M et al. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med. 2022;387:978–88. doi: 10.1056/NEJMoa2209051. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Connolly SJ, Brueckmann M et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Cardiovascular diseases (CVD) in Vietnam. https://www.who.int/vietnam/health-topics/cardiovascular-diseases (accessed 17 May 2023)
- 19.Son PT, Quang NN, Viet NL et al. Prevalence, awareness, treatment and control of hypertension in Vietnam – results from a national survey. J Hum Hypertens. 2012;26:268–80. doi: 10.1038/jhh.2011.18. [DOI] [PubMed] [Google Scholar]
- 20.Bao TQ, Hoang VM, Lan VH et al. Risk factors for non-communicable diseases among adults in Vietnam: findings from the Vietnam STEPS survey 2015. J Glob Health Sci. 2020;2:e7. doi: 10.35500/jghs.2020.2.e7. [DOI] [Google Scholar]
- 21.Lien NTB. Prevalence and risk factors of atrial fibrillation. J. Pract Med. 2013;5:72–5. [in Vietnamese] [Google Scholar]
- 22.Nguyen TN, Huyen VT, Nguyen TX et al. Prevalence, risk factors and pharmacological treatment of atrial fibrillation in older hospitalized patients in Vietnam. Int Cardiovasc Forum. 2016;8:79–84. doi: 10.17987/icfj.v8i0.339. [DOI] [Google Scholar]
- 23.Do MC, Cao PP. Predictors of poor outcomes after ischemic stroke in patients with atrial fibrillation. Ho. Chi Minh City Med J. 2015;19:240–5. [in Vietnamese] [Google Scholar]
- 24.Lien N. Clinical and subclinical characteristics and risk factors of stroke in patients with acute cerebral infarction with nonvalvular atrial fibrillation (2019). https://sdh.hmu.edu.vn/news/cID439_dac-diem-lam-sang-can-lam-sang-cac-yeu-to-nguy-co-dot-quy-cua-nhoi-mau-nao-cap-o-benh-nhan-rung-nhi-khong-do-benh-van-tim.html (accessed 19 December 2022)
- 25.Binh V, Toan M. Clinical characteristics, risk factors of patients with non-valvular atrial fibrillation treated at Thái Binh Medical University Hospital. VMJ. 2022;514(2):318–22. doi: 10.51298/vmj.v514i2.2657. [in Vietnamese] [DOI] [Google Scholar]
- 26.Khôi VH, Nga LT. Clinical features and outcome ischemic stroke patients with atrial fibrillation. VMJ. 2022;510(1):235–8. doi: 10.51298/vmj.v514i2.2657. [in Vietnamese]. [DOI] [Google Scholar]
- 27.Mai TTP, Dung HT, Hien PTT et al. Evaluation of pharmacists’ role in rational use of antithrombotic drugs in patients with non-valvular atrial fibrillation. J Pharm Sci. 2016;43:85–90. doi: 10.14456/mujps.2016.10. [DOI] [Google Scholar]
- 28.Chiang CE, Wang KL, Lin SJ. Asian strategy for stroke prevention in atrial fibrillation. Europace. 2015;17((Suppl 2)):ii31–9. doi: 10.1093/europace/euv231. [DOI] [PubMed] [Google Scholar]
- 29.Siu CW, Lip GYH, Lam KF, Tse HF. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm. 2014;11:1401–8. doi: 10.1016/j.hrthm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J. 2012;33:1500–10. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 31.Staerk L, Sherer JA, Ko D et al. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120:1501–17. doi: 10.1161/CIRCRESAHA.117.309732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huong P, Giang T, Dung V, Giang L. Adherence to vitamin K antagonist treatment among patients with atrial fibrillation at Quang Ninh General Hospital. J Nurs Sci. 2020;3:106–115. [Google Scholar]
- 33.Nguyen NQ, Dang VD. Levels of knowledge about using oral anticoagulants of patient with non-valvular atrial fibrillation at military Central Hospital 108. J Nurs Sci. 2022;5:34–43. [Google Scholar]
- 34.Zawawi NA, Abdul Halim Zaki I, Ming LC et al. Anticoagulation control in different ethnic groups receiving vitamin K antagonist for stroke prevention in atrial fibrillation. Front Cardiovasc Med. 2021;8:736143. doi: 10.3389/fcvm.2021.736143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mak M, Lam C, Pineda SJ et al. Pharmacogenetics of warfarin in a diverse patient population. J Cardiovasc Pharmacol Ther. 2019;24:521–33. doi: 10.1177/1074248419843530. [DOI] [PubMed] [Google Scholar]
- 36.Minh TT, Phuoc DV, Viet NL Vietnam Heart Association: guideline diagnosis and treatment of atrial fibrillation. Vietnam National Heart Association; 2022. [In Vietnamese]
- 37.Wang KL, Lip GYH, Lin SJ, Chiang CE. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–61. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang KT, Sun WC, Tsai TJ et al. The risk of gastrointestinal bleeding between non-vitamin K antagonist oral anticoagulants and vitamin K antagonists in the Asian atrial fibrillation patients: a meta-analysis. Int J. Environ Res Public Health. 2021;18:137. doi: 10.3390/ijerph18010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee E, Choi EK, Han KD et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One. 2018;13:e0209687. doi: 10.1371/journal.pone.0209687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hankey GJ, Patel MR, Stevens SR et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11:315–22. doi: 10.1016/S1474-4422(12)70042-X. [DOI] [PubMed] [Google Scholar]
- 41.Rost NS, Giugliano RP, Ruff CT et al. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: findings from ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Stroke. 2016;47:2075–82. doi: 10.1161/STROKEAHA.116.013540. [DOI] [PubMed] [Google Scholar]
- 42.Easton JD, Lopes RD, Bahit MC et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the Aristotle trial. Lancet Neurol. 2012;11:503–11. doi: 10.1016/S1474-4422(12)70092-3. [DOI] [PubMed] [Google Scholar]
- 43.Diener HC, Connolly SJ, Ezekowitz MD et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9:1157–63. doi: 10.1016/S1474-4422(10)70274-X. [DOI] [PubMed] [Google Scholar]
- 44.Granger CB, Alexander JH, McMurray JJV et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 45.Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 46.Skaistis J, Tagami T. Risk of fatal bleeding in episodes of major bleeding with new oral anticoagulants and vitamin K antagonists: a systematic review and meta-analysis. PLoS One. 2015;10:e0137444. doi: 10.1371/journal.pone.0137444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alberts M, Chen YW, Lin JH et al. Risks of stroke and mortality in atrial fibrillation patients treated with rivaroxaban and warfarin. Stroke. 2020;51:549–55. doi: 10.1161/STROKEAHA.119.025554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsen TB, Skjøth F, Nielsen PB et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. doi: 10.1136/bmj.i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasaka M, Uchiyama S, Atarashi H et al. Dabigatran for Japanese patients with atrial fibrillation and prior stroke: a subgroup analysis of the J-Dabigatran surveillance program. J Stroke Cerebrovasc. Dis. 2020;29:104717. doi: 10.1016/j.jstrokecerebrovasdis.2020.104717. [DOI] [PubMed] [Google Scholar]
- 50.Minh TT, Tri HHQ, Lan N et al. Selection of non-vitamin k antagonist oral anticoagulant for stroke prevention in atrial fibrillation based on patient profile: perspectives from Vietnamese experts. Part 2. Eur Cardiol. 2023;18:e62. doi: 10.15420/ecr.2023.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoàng LG, Bình HA. To evaluate of risk factors by CHA2DS2-VASc score in patients are diagnosed stroke with non-valvular atrial fibrillation. Viet J Diabetes Endocrinol. 2021;44:71–81. doi: 10.47122/vjde.2020.44.10. [in Vietnamese] [DOI] [Google Scholar]
- 52.Nguyen TH, Gall S, Cadilhac DA et al. Processes of stroke unit care and outcomes at discharge in Vietnam: findings from the registry of stroke care quality (RES-Q) in a major public hospital. J Stroke Med. 2019;2:119–27. doi: 10.1177/2516608519869132. [DOI] [Google Scholar]
- 53.Ton MD, Dao PV, Nguyen DT et al. Sex disparity in stroke outcomes in a multicenter prospective stroke registry in Vietnam. Int J Stroke. 2023;18:1102–11. doi: 10.1177/17474930231177893. [DOI] [PubMed] [Google Scholar]
- 54.Pham TL, Blizzard L, Srikanth V et al. Case-fatality and functional status three months after first-ever stroke in Vietnam. J Neurol Sci. 2016;365:65–71. doi: 10.1016/j.jns.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 56.Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J. Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 57.Ntaios G, Papavasileiou V, Diener HC et al. Nonvitamin-K-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized controlled trials. Int J Stroke. 2017;12:589–96. doi: 10.1177/1747493017700663. [DOI] [PubMed] [Google Scholar]
- 58.Diener HC, Hankey GJ, Easton JD et al. Non-vitamin K oral anticoagulants for secondary stroke prevention in patients with atrial fibrillation. Eur Heart. J Suppl. 2020;22((Suppl I)):I13–21. doi: 10.1093/eurheartj/suaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasmussen LH, Larsen TB, Graungaard T et al. Primary and secondary prevention with new oral anticoagulant drugs for stroke prevention in atrial fibrillation: indirect comparison analysis. BMJ. 2012;345:e7097. doi: 10.1136/bmj.e7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thu Hien NT, Thong TH, Tung LT et al. Dysphagia and associated factors among patients with acute ischemic stroke in Vietnam. Ann Med. Surg (Lond) 2022;84:104887. doi: 10.1016/j.amsu.2022.104887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coleman CI, Peacock WF, Bunz TJ, Alberts MJ. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and previous stroke or transient ischemic attack. Stroke. 2017;48:2142–9. doi: 10.1161/STROKEAHA.117.017474. [DOI] [PubMed] [Google Scholar]
- 62.Xian Y, Xu H, O’Brien EC et al. Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the patient-centered research into outcomes stroke patients prefer and effectiveness research (PROSPER) study. JAMA Neurol. 2019;76:1192–202. doi: 10.1001/jamaneurol.2019.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Z, Ding X, Ye Z et al. Non-vitamin K antagonist oral anticoagulants versus vitamin K antagonists in atrial fibrillation patients with previous stroke or intracranial hemorrhage: a systematic review and meta-analysis of observational studies. Clin Cardiol. 2021;44:917–24. doi: 10.1002/clc.23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hart RG, Coull BM, Hart D. Early recurrent embolism associated with nonvalvular atrial fibrillation: a retrospective study. Stroke. 1983;14:688–93. doi: 10.1161/01.str.14.5.688. [DOI] [PubMed] [Google Scholar]
- 65.Steffel J, Collins R, Antz M et al. Europace. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation.;2021;23:1612–76. doi: 10.1093/europace/euab065. [DOI] [PubMed] [Google Scholar]
- 66.Oldgren J, Åsberg S, Hijazi Z et al. Early versus delayed non-vitamin K antagonist oral anticoagulant therapy after acute ischemic stroke in atrial fibrillation (TIMING): a registry-based randomized controlled noninferiority study. Circulation. 2022;146:1056–66. doi: 10.1161/CIRCULATIONAHA.122.060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waziry R, Claus JJ, Hofman A. Dementia risk following ischemic stroke: a systematic review and meta-analysis of factors collected at time of stroke diagnosis. J Alzheimers Dis. 2022;90:1535–46. doi: 10.3233/JAD-220317. [DOI] [PubMed] [Google Scholar]
- 68.Lee ZX, Ang E, Lim XT, Arain SJ. Association of risk of dementia with direct oral anticoagulants versus warfarin use in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2021;77:22–31. doi: 10.1097/FJC.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 69.Khurshid R, Awais M, Malik J. Electrophysiology practice in low- and middle-income countries: an updated review on access to care and health delivery. Heart Rhythm O2. 2023;4:69–77. doi: 10.1016/j.hroo.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos IS, Goulart AC, Olmos RD et al. Atrial fibrillation in low- and middle-income countries: a narrative review. Eur Heart J Suppl. 2020;22((Suppl O)):O61–77. doi: 10.1093/eurheartj/suaa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Cesare M, Jarvis JD, Scarlatescu O et al. NOACs added to WHO’s essential medicines list: recommendations for future policy actions. Glob Heart. 2020;15:67. doi: 10.5334/gh.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.US Food and Drug Administration. Ascend Laboratories LLC issues voluntary nationwide recall of dabigatran etexilate capsules, USP 75 mg and 150 mg, due to the detection of N-nitroso-dabigatran (NDAB) impurity. 2023. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/ascend-laboratories-llc-issues-voluntary-nationwide-recall-dabigatran-etexilate-capsules-usp-75-mg (accessed 18 May 2023)


