Abstract
Background:
Early life gut microbiota are affected by several factors that make identification of microbial-adiposity relationships challenging. This review evaluates studies that have investigated the gut microbiota composition associated with adiposity in infants, children, and adolescents and provides evidence-based nutrition recommendations that address microbiota-adiposity links.
Methods:
Electronic databases were systematically searched through January 2020. Eligible studies were published in English and analyzed gut microbiota and adiposity among individuals aged birth to 18 y. Abstracts and full-text articles were reviewed by three independent reviewers.
Results:
Of 45 full-text articles reviewed, 33 were included. No difference in abundance was found for Bacteroidetes (n=7/15 articles), Firmicutes (n=10/17) Actinobacteria (n=8/12), Proteobacteria (n=8/12), Tenericutes (n=4/5), and Verrucomicrobia (n=4/6) with adiposity. Lower abundance of Christensenellaceae (n=3/5) and Rikenellaceae (n=6/8) but higher abundance of F. prausnitzii (n=3/5) and Prevotella (n=5/7) were associated with adiposity.
Conclusions:
A lack of consensus exists for gut microbial composition associations with adiposity. A healthy gut microbiota is associated with a diet rich in fruits and vegetables with moderate consumption of animal fat and protein. Future research should use more robust sequencing technologies to identify all bacterial taxa associated with adiposity and evaluate how diet effects these adiposity-associated microbes.
Keywords: adolescence, childhood, adiposity, gut microbiota
INTRODUCTION
Pediatric obesity is a public health burden associated with metabolic syndrome,1–3 type 2 diabetes,4–6 and cardiovascular disease.1 Data indicate that obesity during childhood and adolescence trends into adulthood,7 increasing the risk of obesity-related diseases across the lifespan. Obesity rates vary by age, affecting 13.9%, 18.4% and 20.6% of youth aged two to five, six to 11, and 12 to 19 years old, respectively.8,9 Lifestyle modifications are the cornerstone approach to obesity prevention;10–13 however, the gut microbiome (microorganisms that inhabit the human intestine) may also play a role. While each human has a unique microbiome composition that emerges as early as infancy,14 specific microbes have been shown to associate with specific health conditions.15 Therefore, it is imperative to understand how these novel differences in microbial community structure contribute to the development of adiposity across the lifespan to potentially target the modulation of the human gut microbiome in obesity prevention strategies.
Infancy is a period of rapid growth where microbial colonization of the gut begins through complex interactions between an individual’s genetics, environment, and behaviors.16 Microbiome disruption during infancy may lead to altered metabolic processes that continue throughout life.17,18 The diet of an infant is an important modifiable factor that may result in microbial dysbiosis or aid in the development of a healthy gut microbiome. Breastfed infants exhibit a higher proportion of Bifidobacterium, bacterial species that ferment the oligosaccharides in human breast milk.19 Whereas, formula-fed infants have significantly higher proportions of bacteria from the phyla Firmicutes and Bacteroidetes, which are associated with overweight and obesity.14 Furthermore, infancy coincides with the transition from liquid to solid foods which results in a reduction of Bifidobacterium and an increase in Firmicutes.20 The cessation of breastfeeding and the introduction of solid foods contribute to the functional maturation of the infant gut microbiome.21 If timed improperly, these important feeding events may promote microbial dysbiosis and a higher risk of obesity later in life.
The gut microbiome continues to develop during childhood and begins to stabilize around five years of age.22,23 The dominant bacterial genera found in the healthy gut microbiome of children include Bacteroides, Prevotella and Bifidobacterium.24 Childhood is a critical period of development when the gut microbiome begins to resemble that of an adult.24 Dietary choices made during this time continue to shape the evolving microbiome.25 Western-style diets have been shown to decrease Bifidobacterium and increase Eubacterium in the gut microbiome,26 associating with a greater prevalence of overweight or obesity.27,28 Differences in the gut microbiome structure have been observed where children with obesity exhibit a higher ratio of Firmicutes-to-Bacteroidetes (F:B ratio), higher amounts of Lactobacillus spp., and lower abundance of Bacteroides vulgatus as compared to lean children.29 This provides further evidence that microbial dysbiosis may contribute to the occurrence of childhood obesity.
Adolescence is characterized by a secondary period of rapid growth but also marks a period of maturation and transition to greater independence. Previous studies have found that the adolescent gut microbiome has significantly higher abundance of Bifidobacterium and Clostridium when compared to the healthy adult gut microbiome.30 Typically, Bifidobacterium is in higher abundance during infancy and Clostridium is more abundant during adulthood;30 however, the simultaneous presence of these bacteria may either be reflective of the important contribution of this developmental stage to the structure of the gut microbiome community or the functional need for microbial metabolites during continued growth and development. Among adolescents with obesity, greater Actinomyces abundance has been observed.31 Shifts in bacterial composition during adolescence to a more stable, adult composition is important for disease prevention as microbial changes could have a lasting effect on health into adulthood.
With such a wealth of microbes in the gut microbiome and the dynamic nature of growth and development, it is challenging to identify microbes that co-exist and potentially contribute to obesity in early life. The purpose of this systematic review was to evaluate studies that have investigated gut bacteria that are associated with adiposity in infants, children, and adolescents. Additionally, this review provides evidence-based dietary approaches that have been shown to impact the obesity-associated gut microbes. Additionally, we provide best practices for future research evaluating microbial relationships with adiposity during these formative years.
METHODS
The protocol was registered through PROSPERO (Registration #: CRD42018108054) and data extraction followed the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.32
Search Strategy
Studies were identified using a standardized search strategy to evaluate articles that assessed differences in the gut microbiome among lean infants, children, and adolescents as compared to those with overweight or obesity. Articles that assessed dietary components associated with microbial composition were identified to provide nutritional recommendations that have shown beneficial effects on microbial composition. The electronic databases (PubMed, CINAHL, and Web of Science) were searched from inception to January 2020 using a combination of the following Medical Subject Headings terms: pediatric obesity, obesity, adiposity, gastrointestinal microbiome, microbiota, child, child preschool, and/or adolescent.
Inclusion and Exclusion Criteria
Inclusion criteria included: (1) randomized or non-randomized trials, crossover, cross-sectional, parallel design, and prospective observational studies; (2) collection and analyses of gut microbiome through fecal samples; (3) at least one adiposity measure; (4) participants aged 0–18 years old; and (5) published in English. Exclusion criteria included all genetic diseases and conditions associated with overweight or obesity.
Data Extraction
A standardized data form (Microsoft Excel, Microsoft, 2010) was used for extraction and tabulation. Two independent reviewers (K.B.V and C.P.O) evaluated the titles and abstracts to identify eligible articles. Full texts of selected articles were reviewed for inclusion and exclusion criteria with reference lists evaluated for additional eligible articles. Disagreements were resolved with a third reviewer (C.M.W) prior to final inclusion decision.
Assessment of Quality
The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies developed by the National Heart, Lung, and Blood Institute33 was used to assess article quality. This 14-item tool evaluates the internal validity and potential flaws in methodology and implementation of cohort and cross-sectional studies. A quality rating of “good” (yes to >7 items), “fair” (yes to 4–6 items), or “poor” (yes to <3 items) was assigned to each article. For items where “no” was selected, reviewers identified the category of potential risk. One independent reviewer (K.B.V) provided responses for all 14 items and an overall quality rating. A secondary reviewer (S.N.T.) reviewed responses and overall quality ratings and any discrepancies were resolved by a third reviewer (C.M.W.).
Assessment of Potential Bias
Identification of bias was based on the Research Triangle Institute (RTI) 29-item bank created by RTI International for evaluation of risk of bias and precision in observational studies.34 One independent reviewer (K.B.V) identified potential sources of bias based on the answers to item bank questions that were applicable to each study design. A secondary reviewer (C.P.O) assessed the responses provided by the first reviewer and any discrepancies were resolved by a third reviewer (C.M.W.).
RESULTS
Study Selection
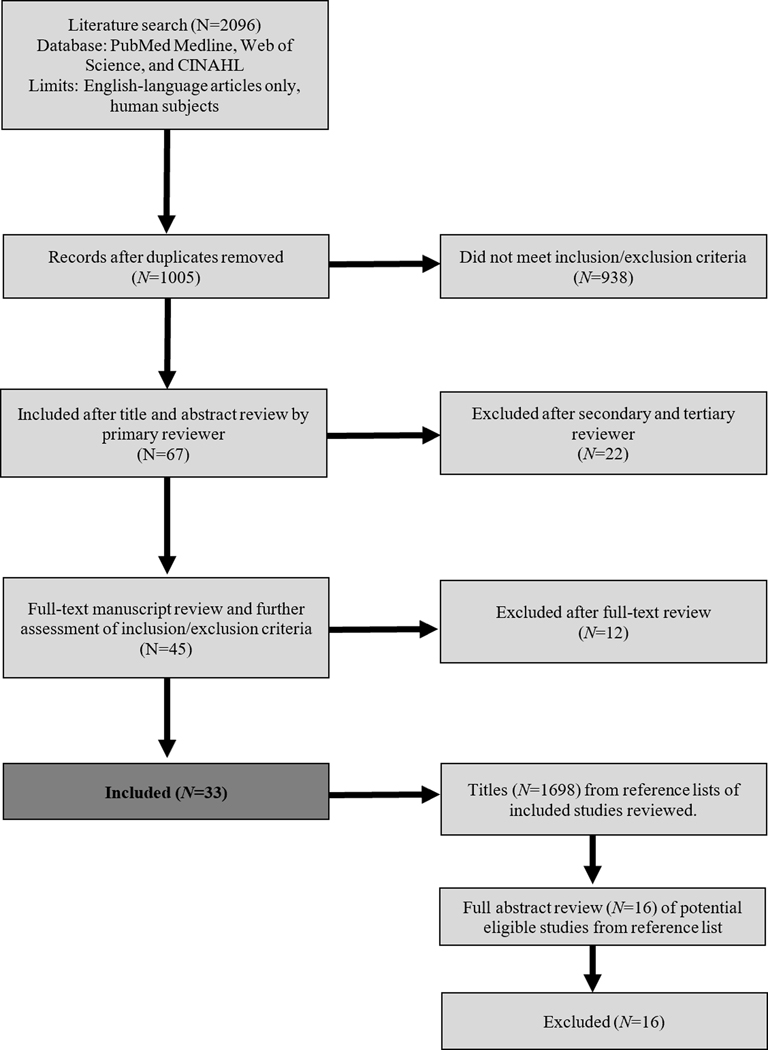
During the search, 2096 studies were identified, of which 1091 were duplicate articles across search strings and electronic databases. Of the 1696 additional titles screened from reference lists, none were eligible for inclusion. Figure 1 shows the total number of screened, excluded, and included full-text articles.
Figure 1.
PRISMA Flow Diagram
Study Characteristics
Supplemental Table 1 summarizes the main characteristics and conclusions of the included articles. Among the included studies, 82% (n=27) were cross-sectional studies29,31,35–59 with a mean sample size of 316. The location of the research also varied with seven from Mexico,35,36,38–40,55,59 six from the USA,42,45,50,51,60,61 four from China,46,52,53,62 three from Italy,31,49,63 and one each from Belgium,29 Brazil,56 Canada,64 Egypt,48 Germany,44 India,43 Iran,37 Korea,47 New Zealand,58 Malawi,65 Sweden,57 Switzerland,41 and Trinidad.54 Forty-two percent (n=14)35,36,39,42,45,46,53,54,58–61,64,65 of the studies used 16S rRNA sequencing alone for microbial analysis with five40,43,52,55,62 using quantitative PCR alone and nine37,38,47–51,56,57 using 16S rRNA sequencing with quantitative PCR. The remaining five29,31,41,44,63 studies used a combination of other methods to asses microbial differences. Various methods of categorizing participants by weight status were used with the most common method (n=12) being body mass index (BMI) percentiles for determination of categorical cut-offs.31,36,38,39,42,43,45,51,52,54,59,61 There was one study that did not provide a definition used for the classification of children with obesity vs. healthy children.46 Fifty-three percent (n=3622/6816) of the included study cohorts were male. Four studies38,46,48,65 did not provide the sex breakdown for their cohort and were not included in the overall percent. Only two studies36,60 reported the gestational age at delivery for their cohorts with both being approximately 39 weeks.
Summary of Existing Literature
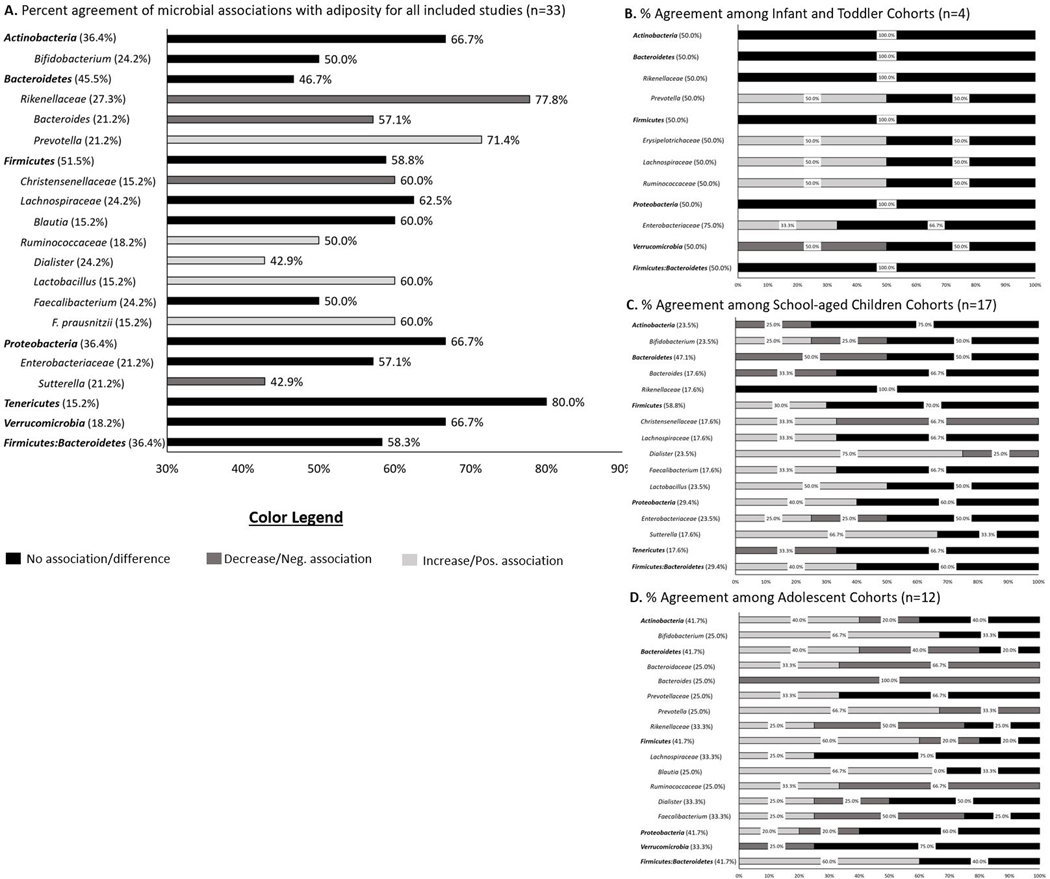
The main findings regarding microbial relationships with adiposity among the included studies are summarized below and in Table 1. This table includes the 26 microbes that were reported in four or more of the included articles, the association with adiposity, age group of each study cohort, and the references for the articles. Supplemental Table 2 includes all 70 microbes that were reported among the included studies. Figure 2A summarizes the 21 microbes that were reported in five or more of the included studies and the percent agreement with the indicated adiposity relationship. The proceeding summary is grouped by developmental period (i.e. infants/toddlers, school-aged children, and adolescents). There were several studies that had age ranges that spanned several stages of development.29,35–42,46,48,49,52–56,59,62,63 For those studies, the stage of development was determined using the mean age of the study population.
Table 1.
Microbial Relationships with Adiposity Reported among Four or More of the Included Studies
| Microbe | Association with Adiposity | Age Group | Reference | ||
|---|---|---|---|---|---|
| % | Relationship | ||||
| Phylum | Actinobacteria | 16.7% | Increase/Positive correlation | Adolescents | Del Chierico et al, Frontiers in Microbiology. 2018;9:1–12 (31). Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). |
| 16.7% | Decrease/Negative correlation | School-aged children | Chen et al, PeerJ. 2020;8:e8317 (53) | ||
| Adolescents | Yuan et al. J of Gastroenterology and Hepatology. 2014;29(6):1292–1298 (51). | ||||
| 66.7% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). Karvonen et al. International J of Obesity. 2019;43:713–723 (45). |
||
| School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). Méndez-Salazar et al, Frontiers in Microbiology. 2018;9(Oct):1–11 (35). Murugesan et al. Euro J of Clin Micro & Infect Diseases. 2015;34(7):1337–1346 (38). Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). |
||||
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| Bacteroidetes | 13.3% | Increase/Positive correlation | Adolescents | Del Chierico et al, Frontiers in Microbiology. 2018;9:1–12 (31). Yuan et al. J of Gastroenterology and Hepatology. 2014;29(6):1292–1298 (51). |
|
| 40.0% | Decrease/Negative correlation | School-aged children | Chen et al, PeerJ. 2020;8:e8317 (53) Méndez-Salazar et al, Frontiers in Microbiology. 2018;9(Oct):1–11 (35). Orbe-Orihuela et al. Salud Publica de Mexico. 2018;60(1):5–11 (40). Xu et al. BMC Microbiology. 2012;12(1):283–289 (62). |
||
| Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| 46.7% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). Karvonen et al. International J of Obesity. 2019;43:713–723 (45). |
||
| School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). Mousavi et al. Iranian Red Crescent Med Journal. 2018;20(4):1–6 (37). Murugesan et al. Euro J of Clin Micro & Infect Diseases. 2015;34(7):1337–1346 (38). Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). |
||||
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). | ||||
| Firmicutes | 35.3% | Increase/Positive correlation | School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). Mousavi et al. Iranian Red Crescent Med Journal. 2018;20(4):1–6 (37). Orbe-Orihuela et al. Salud Publica de Mexico. 2018;60(1):5–11 (40). |
|
| Adolescents | Del Chierico et al, Frontiers in Microbiology. 2018;9:1–12 (31). Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| 5.9% | Decrease/Negative correlation | Adolescents | Yuan et al. J of Gastroenterology and Hepatology. 2014;29(6):1292–1298 (51). | ||
| 58.8% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). Karvonen et al. International J of Obesity. 2019;43:713–723 (45). |
||
| School-aged children | Chen et al, PeerJ. 2020;8:e8317 (53) López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). Méndez-Salazar et al, Frontiers in Microbiology. 2018;9(Oct):1–11 (35). Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Payne et al. Nutrition & Diabetes. 2011;1(7):e12 (41). Xu et al. BMC Microbiology. 2012;12(1):283–289 (62). Murugesan et al. Euro J of Clin Micro & Infect Diseases. 2015;34(7):1337–1346 (38). |
||||
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). | ||||
| Firmicutes:Bacteroidetes | 41.7% | Increase/Positive correlation | School-aged children | Mousavi et al. Iranian Red Crescent Med Journal. 2018;20(4):1–6 (37). Xu et al. BMC Microbiology. 2012;12(1):283–289 (62). |
|
| Adolescents | Bervoets et al, Gut Pathogens. 2013;5:1–10 (29) Hou et al, BioMed Research International. 2017;7585989:1–8 (46). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| 58.3% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). Craig SJC et al. Scientific Reports. 2018;8:14030 (60). |
||
| School-aged children | Hollister et al, Childhood Obesity. 2018;14(2):122–130 (61). López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). Chen et al, PeerJ. 2020;8:e8317 (53) |
||||
| Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). Whisner et al, BMC Microbiology. 2018;18(1)1:11 (50). |
||||
| Proteobacteria | 25.0% | Increase/Positive correlation | School-aged children | Chen et al, PeerJ. 2020;8:e8317 (53) Méndez-Salazar et al, Frontiers in Microbiology. 2018;9(Oct):1–11 (35). |
|
| Adolescents | Yuan et al. J of Gastroenterology and Hepatology. 2014;29(6):1292–1298 (51). | ||||
| 8.3% | Decrease/Negative correlation | Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). | ||
| 66.7% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). Karvonen et al. International J of Obesity. 2019;43:713–723 (45). |
||
| School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Murugesan et al. Euro J of Clin Micro & Infect Diseases. 2015;34(7):1337–1346 (38). |
||||
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Del Chierico et al, Frontiers in Microbiology. 2018;9:1–12 (31). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| Tenericutes | 20.0% | Decrease/Negative correlation | School-aged children | Chen et al, PeerJ. 2020;8:e8317 (53) | |
| 80.0% | No difference/No association | School-aged children | Méndez-Salazar et al, Frontiers in Microbiology. 2018;9(Oct):1–11 (35). Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). |
||
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Yuan et al. J of Gastroenterology and Hepatology. 2014;29(6):1292–1298 (51). |
||||
| Verrucomicrobia | 33.3% | Decrease/Negative correlation | Infant/Toddler | Karvonen et al. International J of Obesity. 2019;43:713–723 (45). | |
| Adolescents | Hou et al, BioMed Research International. 2017;7585989:1–8 (46). | ||||
| 66.7% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | ||
| Adolescents | Del Chierico et al, Frontiers in Microbiology. 2018;9:1–12 (31). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). Yuan et al. J of Gastroenterology and Hepatology. 2014;29(6):1292–1298 (51). |
||||
| Family | Bacteroidaceae | 25.0% | Increase/Positive correlation | Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). |
| 50.0% | Decrease/Negative correlation | Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). | ||
| Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||||
| 25.0% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | ||
| Christensenellaceae | 20.0% | Increase/Positive correlation | School-aged children | Leong et al. Am J of Clinical Nutrition. 2020’111(1):70–78 (58). | |
| 60.0% | Decrease/Negative correlation | School-aged children | Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). |
||
| Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). | ||||
| 20.0% | No difference/No association | Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||
| Coriobacteriaceae | 75.0% | Increase/Positive correlation | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | |
| School-aged children | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). | ||||
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). | ||||
| 25.0% | No difference/No association | School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). | ||
| Enterobacteriaceae | 28.6% | Increase/Positive correlation | Infant/Toddler | Kamng’ona et al. Scientific Reports;2019;9:12893 (65). | |
| School-aged children | Karlsson et al, Obesity. 2012;20(11):2257–61 (57). | ||||
| 14.3% | Decrease/Negative correlation | School-aged children | López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). | ||
| 57.1% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | ||
| School-aged children | Borgo F et al, Childhood Obesity. 2017;13(1):78–84 (63). Payne et al. Nutrition & Diabetes. 2011;1(7):e12 (41). |
||||
| Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). | ||||
| Lachnospiraceae | 37.5% | Increase/Positive correlation | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | |
| School-aged children | Murugesan et al. Euro J of Clin Micro & Infect Diseases. 2015;34(7):1337–1346 (38). | ||||
| Adolescents | Del Chierico et al, Frontiers in Microbiology. 2018;9:1–12 (31). | ||||
| 62.5% | No difference/No association | School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). |
||
| Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). Hu et al, PLoS ONE. 2015;10(7):1–14 (47). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| Prevotellaceae | 25.0% | Increase/Positive correlation | Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). | |
| 75.0% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | ||
| Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| Rikenellaceae | 77.8% | Decrease/Negative correlation | School-aged children | Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). Chen et al, PeerJ. 2020;8:e8317 (53) López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). |
|
| Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). Hu et al, PLoS ONE. 2015;10(7):1–14 (47). Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). |
||||
| 22.2% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | ||
| Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||||
| Ruminococcaceae | 50.0% | Increase/Positive correlation | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | |
| School-aged children | Leong et al. Am J of Clinical Nutrition. 2020’111(1):70–78 (58). | ||||
| Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||||
| 33.3% | Decrease/Negative correlation | Adolescents | Ferrer et al, Environmental Microbiology. 2013;15:211–26 (44). Hu et al, PLoS ONE. 2015;10(7):1–14 (47). |
||
| 16.7% | No difference/No association | School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). | ||
| Veillonellaceae | 50.0% | Increase/Positive correlation | School-aged children | Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). | |
| Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). | ||||
| 50.0% | No difference/No association | Infant/Toddler | Forbes et al, JAMA Pediatrics. 2018;172:e181161 (64). | ||
| Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||||
| Genus | Bacteroides | 57.1% | Decrease/Negative correlation | School-aged children | Leong et al. Am J of Clinical Nutrition. 2020’111(1):70–78 (58). |
| Adolescents | Hou et al, BioMed Research International. 2017;7585989:1–8 (46). Hu et al, PLoS ONE. 2015;10(7):1–14 (47). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||||
| 42.9% | No difference/No association | Infant/Toddler | Karvonen et al. International J of Obesity. 2019;43:713–723 (45). | ||
| School-aged children | Payne et al. Nutrition & Diabetes. 2011;1(7):e12 (41). Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). |
||||
| Bifidobacterium | 37.5% | Increase/Positive correlation | School-aged children | Leong et al. Am J of Clinical Nutrition. 2020’111(1):70–78 (58). | |
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). |
||||
| 12.5% | Decrease/Negative correlation | School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). | ||
| 50.0% | No difference/No association | Infant/Toddler | Karvonen et al. International J of Obesity. 2019;43:713–723 (45). | ||
| School-aged children | Karlsson et al, Obesity. 2012;20(11):2257–61 (57). Payne et al. Nutrition & Diabetes. 2011;1(7):e12 (41). |
||||
| Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||||
| Blautia | 40.0% | Increase/Positive correlation | Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Hou et al, BioMed Research International. 2017;7585989:1–8 (46). |
|
| 60.0% | No difference/No association | Infant/Toddler | Karvonen et al. International J of Obesity. 2019;43:713–723 (45). | ||
| School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). | ||||
| Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||||
| Dialister | 37.5% | Increase/Positive correlation | School-aged children | Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). |
|
| Adolescents | Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). | ||||
| 25.0% | Decrease/Negative correlation | School-aged children | Chen et al, PeerJ. 2020;8:e8317 (53) | ||
| Adolescents | Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). | ||||
| 37.5% | No difference/No association | Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). |
||
| Faecalibacterium | 25.0% | Increase/Positive correlation | School-aged children | Chen et al, PeerJ. 2020;8:e8317 (53) | |
| Adolescents | Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). | ||||
| 25.0% | Decrease/Negative correlation | Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). |
||
| 50.0% | No difference/No association | Infant/Toddler | Karvonen et al. International J of Obesity. 2019;43:713–723 (45). | ||
| School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). Leong et al. Am J of Clinical Nutrition. 2020’111(1):70–78 (58). |
||||
| Adolescents | Riva et al. Environmental Microbiology. 2017;19(1):95–105 (49). | ||||
| Lactobacillus | 60.0% | Increase/Positive correlation | School-aged children | Da Silva et al, Childhood Obesity. 2020;16(3):204–210 (54). Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). |
|
| Adolescents | Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). | ||||
| 40.0% | No difference/No association | School-aged children | Karlsson et al, Obesity. 2012;20(11):2257–61 (57). Payne et al. Nutrition & Diabetes. 2011;1(7):e12 (41). |
||
| Prevotella | 71.4% | Increase/Positive correlation | Infant/Toddler | Kamng’ona et al. Scientific Reports;2019;9:12893 (65). | |
| School-aged children | Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). |
||||
| Adolescents | Nirmalkar et al, Nutrients. 2018;10(2009):1–23 (39). Hu et al, PLoS ONE. 2015;10(7):1–14 (47). |
||||
| 14.3% | Decrease/Negative correlation | Adolescents | Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). | ||
| 14.3% | No difference/No association | Infant/Toddler | Karvonen et al. International J of Obesity. 2019;43:713–723 (45). | ||
| Sutterella | 50.0% | Increase/Positive correlation | School-aged children | Moran-Ramos et al, Gut Microbes. 2020; Jan 23:1–18 (36). Chen et al, PeerJ. 2020;8:e8317 (53) |
|
| Adolescents | Hou et al, BioMed Research International. 2017;7585989:1–8 (46). | ||||
| 33.3% | Decrease/Negative correlation | School-aged children | López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). | ||
| Adolescents | Bai et al. Pediatric Obesity. 2019;14(4):e12480 (42). | ||||
| 16.7% | No difference/No association | Adolescents | Hu et al, PLoS ONE. 2015;10(7):1–14 (47). | ||
| Species | B. ovatus | 25.0% | Increase/Positive correlation | School-aged children | López-Contreras et al. Pediatric Obesity. 2017;13(6):381–388 (59). |
| 25.0% | Decrease/Negative correlation | Adolescents | Mansour et al. Research J of Pharm, Biol, and Chem Sci. 2016;7(5):436–445 (48). | ||
| 50.0% | No difference/No association | School-aged children | Ignacio et al, Clin Microbial Infect. 2016;22:258.e1–258.e8 (56). | ||
| Adolescents | Bervoets et al, Gut Pathogens. 2013;5:1–10 (29) | ||||
| F. prausnitzii | 60.0% | Increase/Positive correlation | Adolescents | Del Chierico et al, Frontiers in Microbiology. 2018;9:1–12 (31). Balamurugan et al, British J of Nutrition. 2010;103:335–338 (43) |
|
| Infant/Toddler | Kamng’ona et al. Scientific Reports;2019;9:12893 (65). | ||||
| 20.0% | Decrease/Negative correlation | School-aged children | Borgo F et al, Childhood Obesity. 2017;13(1):78–84 (63). | ||
| 20.0% | No difference/No association | School-aged children | Payne et al. Nutrition & Diabetes. 2011;1(7):e12 (41). | ||
Microbes are only included in the above table if a relationship was reported with adiposity in two or more of the included articles. The percent column is the percentage of articles in agreement for each indicated association with adiposity by age group. The denominator used to calculate each percentage is the number of articles that reported a relationship with adiposity for that specific microbe. Note: if a study included data for two age groups, then that study was included twice in the denominator for a specific microbe (e.g. the denominator for Actinobacteria is 12)
Figure 2.
Percent agreement of microbial associations with adiposity outcomes among the articles included in the current review by specific microbe for (A) entire cohort regardless of age group, (B) infants and toddlers, (C) school-aged children, and (D) adolescents. Black indicates no association/difference, dark gray indicates a significantly lower abundance or negative association, and light gray indicates significantly higher abundance or positive association. Microbial names are indented to show related taxa at lower taxonomic levels. Bolded microbial names are phyla-level bacteria. Percentage next to microbial name indicates the percent of the included articles that reported this specific microbe. Panel A: Only microbes that were reported in five or more of the included articles. Panels B-D: Only microbes reported in two or more of the included articles for the indicated developmental period.
Infancy/Toddlerhood (Birth to < 4 years).
Of the included studies, only 12% (n=4) evaluated gut microbial composition during infancy and toddlerhood in association with adiposity. Three of these studies used longitudinal, observational60,64,65 study designs with one study using a cross-sectional45 design. The first three studies60,64,65 were of good quality with the last study being of fair study quality;45 however, all studies adjusted for multiple comparisons and controlled for several covariates. Figure 2B provides a summary of the key microbial relationships with adiposity for the four included articles that had infant/toddler-aged populations.
The first study found that the child’s gut microbiota was not associated with weight gain during the first two years of life.60 However, there were distinct clusters of both Bacteroidetes and Firmicutes that were positively associated with growth, whereas Proteobacteria was negatively associated with growth.60 This study also found a discriminant group within the Actinobacteria phyla that was more abundant in children without rapid weight gain.60 Additional findings from this study suggested that microbial obesity signatures were more evident in the oral microbiome (vs. gut microbiome) during infancy and toddlerhood and that obesity-associated gut microbiota shifts may become more pronounced in later stages of life.60
The second study found that the composition of the gut microbiota partially explained a strong, inverse relationship between breastfeeding and risk of overweight.64 It was reported that infants partially breastfed or exclusively formula-fed at 3 months of age had a 63% and 102% greater risk, respectively, of being overweight at 12 months of age when compared to exclusively breastfed infants.64 This study found significantly higher relative abundance of Coriobacteriaceae, Erysipalotrichaceae, Lachnospiraceae, and Ruminococcaceae at 3–4 months of age among infants who became overweight by 12 months.64 Subsequently, the study determined that increasing exclusivity of breastfeeding (formula-fed, partial, exclusive) was associated with decreased relative abundance of Lachnospiraceae and Ruminococcaceae.64 This study provides support that breastfeeding may be protective of overweight via the early gut microbiome.64
The third study found that gut microbiome maturity and diversity at 6 months was associated with weight but not linear growth between 6–12 months of age; however, this relationship did not persist at 12–18 months of age.65 Overall, this study found that Proteobacteria and Bacteroidetes were positively associated whereas Actinobacteria were negatively associated with weight gain.65 It was reported that weight-for-age z-score was positively associated with Prevotella, Campylobacter, Enterobacter ludwigii, Klebsiella, and Salmonella enterica and negatively associated with Actinomyces, Atopobium parvulum, Streptococcus, and C. difficile.65 This provides evidence that specific microbes may play a key role in weight gain during infancy.
Lastly, the cross-sectional study found significantly lower microbial abundance of Verrucomicrobia, Ruminococcus, Akkermansia, Parabacteroidetes but significantly higher abundance of Dorea among three-year old toddlers with greater adiposity.45 This is similar to studies in older children and adolescents44,46,47 that are described in later paragraphs and suggests that early life changes in the gut microbiome may predispose an individual to obesity later in life.
School-aged Children (>4 but < 11 years).
Of the included studies, 52% (n=17) evaluated the microbial composition associated with adiposity among school-aged children. The majority (82%, n=14) were cross-sectional with the remaining studies using case-control (n=2) and longitudinal (n=1) study designs. Ninety-three percent (n=13)35–41,53,54,56–59 of the cross-sectional studies were of fair study quality with the remaining cross-sectional study being of good study quality.55 Six36,40,55,56,58,59 of these studies controlled for potential confounding variables and seven35,36,39,41,55,56,59 adjusted for multiple comparisons. Both of the case-control studies were of fair study quality and controlled for multiple comparisons.62,63 Figure 2C provides a summary of the key microbial relationships with adiposity for the 17 included articles among school-aged children.
There were major differences in the results among the cross-sectional studies. Four studies reported that the relative abundance of Bacteroidetes was significantly lower in children with overweight/obesity,35,40,53,62 whereas four studies found no difference in Bacteroidetes abundance by weight status.37–39,54 Similarly, there were mixed results regarding the abundance of Firmicutes with six studies reporting no difference35,38,39,41,53,59,62 and three studies reporting significantly higher abundance37,40,54 in children with overweight/obesity. These findings led to variability regarding F:B ratio, with three studies reporting no difference53,59,61 and two studies finding a significantly higher ratio37,62 among children with obesity compared to normal-weight controls. Furthermore, there were four studies that found no differences35,38,39,54 and only one study53 that found significantly lower levels of Actinobacteria abundance among children with greater adiposity. Lastly, three studies found no difference38,39,54 in Proteobacteria whereas two studies35,53 reported significantly greater abundance among children with overweight/obesity compared to normal-weight children.
At the class level, two studies reported significantly higher abundance of Betaproteobacteria with greater adiposity.36,53 At the family level, three studies reported conflicting results for Enterobacteriaceae41,57,59 and Christensenellaceae.36,58,59 Three studies found significantly lower levels of Oscillospira.36,38,53 Further evaluation at the genus level revealed a lack of consensus for Bifidobacterium,41,54,56,57 Lactobacillus,39,41,54,56,57 and Faecalibacterium,38,53,54,58 with two studies reporting that children with obesity had elevated Clostridium.39,56 At the species level, Bacteroides fragilis56,57 and ovatus56,59 abundance were inconclusive but two studies reported a significantly lower abundance of Bacteroides plebeius.59,61
One of the case-control studies found a lower Bacteroidetes abundance but significantly higher F:B ratio62 in children with obesity, which was similar to two of the cross-sectional studies.37,40 The other case-control study evaluated microbial abundance at the species level finding significantly lower levels of Akkermansia muciniphila and Faecalibacterium prausnitzii among children with obesity.63
Lastly, the only longitudinal, observational study among school-aged children found minor differences in the microbial communities and no differences in diversity between normal weight controls and children with obesity.61 Bacteroides massiliensis and Bacteroides plebeius were enriched in the stool of children with obesity.61 This study61 was of good study quality and controlled for multiple comparisons. Despite some similarities in microbial taxa across the studies, there is a lack of consensus on specific gut microbes that are unique to school-aged children with overweight or obesity.
Adolescence (> 11 but < 18 years).
Of the included studies, 36% (n=12) evaluated associations between gut microbial composition and adiposity among adolescents. All studies were cross-sectional with substantial variation in results. Figure 2D provides a summary of the key microbial relationships with adiposity for the 12 included articles that focused on adolescents.
Three29,46,49 of the five studies reporting on F:B ratio found a significantly higher ratio in adolescents with overweight/obesity compared to normal-weight controls, while two reported no difference.47,50 There was similar consensus regarding an increased abundance of Firmicutes with greater adiposity31,44,49 with one study51 reporting significantly lower Firmicutes abundance. The majority (60%) of these studies were of fair study quality.29,47,49 In contrast, results were more mixed regarding the abundance of Bacteroidetes,31,44,49,51 Proteobacteria31,44,49,51 and Actinobacteria31,44,49,51 when comparing adolescents with obesity to healthy weight controls. The only phyla with consistent reports was Verrucomicrobia31,49,51 which did not differ by weight status.
Several of the studies reported key differences in microbial composition at the family taxonomic level. Similar to the results among school-aged children, Clostridiaceae abundance was significantly higher among adolescents with obesity.31,44 Ferrer et al. found higher quantities of Streptococcaceae among adolescents with obesity but this was the only study that reported on this family which was of fair study quality.44 Mixed results were observed for other reported families including Bacteroidaceae,44,47,49 Lachnospiraceae,46,47,49 Rikenellaceae,42,44,47 Ruminococcaceae,44,47,49 and Prevotellaceae.44,47,49
Evaluation of differences in bacterial composition at the genus level among adolescents also yielded conflicting results. Sutterella,42,46 Prevotella,42,47 and Faecalibacterium42,47 abundance varied among studies. Three studies46,47,49 reported a significantly lower Bacteroides abundance among adolescents with obesity. Interestingly, Bai et al. found greater Dialister among adolescents with obesity but abundance of this genus was lower among overweight adolescents.42 This study had high study quality and controlled for potential covariates and adjusted for multiple comparisons. Overall, these findings demonstrate that microbes from specific genera may not only be associated with adiposity but also with the degree of adiposity.
There were several studies that investigated differences in concentrations of specific bacterial species. Two studies reported greater abundance of F. prausnitzii31,43 while two other studies reported lower abundance of B. vulgatus29,48 among adolescents with obesity. For the remaining bacterial composition differences, there was either a lack of agreement between the studies or only one study that reported on a microbe.
Evaluation of Study Quality
Table 2 provides the overall quality scores and ratings for each study while Supplemental Table 3 provides the responses to each of the 14 items on the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Of the 33 included studies, eight studies37,42,50,55,60,61,64,65 received an overall quality rating of ‘good’ with four of the articles60,64–66 being longitudinal studies. The article that received a response of ‘yes’ to the majority of the 14 items was Kamng’ona et al. which followed infants from birth to 18 months of age.65 The majority (n=23) of the studies had an overall quality rating of ‘fair’,29,31,36–40,42–44,45,48,49,51,53–57,61,62–64, with only two studies46,48 being rated ‘poor’.
Table 2.
Summary of Quality, Potential Bias, Analytical Methods, and Covariate/Multiple comparison Adjustments of the Included Studies
| Source | Quality Assessment | RTI Potential Bias | Statistical Methods Used | Statistical Adjustments | ||
|---|---|---|---|---|---|---|
| Overall Score | Quality Rating | Covariate | Multiple Comparisons | |||
| Bai et al. Pediatric Obesity. 2019;14(4):e12480.42 | 7 | Good | Information, Reporting | PERMANOVA, Linear decomposition model, Multivariate analysis | Age, sex, antibiotic and probiotic use | Benjamini-Hochberg correction using a false discovery rate |
| Balamurugan R et al. British J of Nutrition. 2010;103:335–338.43 | 5 | Fair | Performance | Mann-Whitney U-test | None | None |
| Bervoets L et al. Gut Pathogens. 2013;5:1–10.29 | 5 | Fair | Independent samples t-test, Mann-Whitney U-test, Chi-square test, Multiple linear regression | Age, gender | None | |
| Borgo F et al. Childhood Obesity. 2017;13(1):78–84.63 | 6 | Fair | Performance, Information, Selection bias/confounding, Reporting | Mann Whitney U test, ANOVA | None | None |
| Chen et al. PeerJ. 2020;8:e8317.53 | 6 | Fair | Performance | Independent t-test (two-tailed), Wilcoxon rank-sum test, and LEfSe | None | None |
| Craig SJC et al. Scientific Reports. 2018;8:1–14.60 | 9 | Good | Selection bias/confounding, information | Pearson’s chi-squared test, Function-on-Scalar Linear models; Choi significance measures, Mann-Whitney U and Kruskal-Wallis tests, Multiple linear regression, FLAME, and LEfSe | Gender, antibiotic exposure, medication use, delivery mode, intervention assignment, gestational diabetes, gestational weight gain, maternal smoking, family income, and dietary categories at age 2 years | Bonferroni-corrected p-values |
| Da Silva et al. Childhood Obesity. 2020;16(3):204–210.54 | 6 | Fair | Selection bias/confounding, Information, Performance | Chi-square test, Independent t-test (two-tailed), Wilcoxon rank-sum test | None | None |
| Del Chierico F et al. Frontiers in Microbiology. 2018;9:1–12.31 | 5 | Fair | Information | ANOVA, least significant difference (LSD) test, Mann–Whitney U-test, receiver operating characteristic (ROC), discriminant analysis (DA), principal component analysis (PCA), Spearman’s correlations. LEfSe | None | False discovery rate correction of p-values |
| Ferrer et al. Environmental Microbiology. 2013;15:211–26.44 | 4 | Fair | Precision, Information, Information | NA | None | None |
| Forbes et al. JAMA Pediatrics. 2018;172:e181161.64 | 8 | Good | Selection bias/confounding, Information | Logistic regression, Chi-squared tests, Multivariable logistic regression, Kruskal-Wallis tests, Post-hoc Dunn tests | Birth mode, parity, gestational diabetes, infant sex, birth weight, and maternal/neonatal antibiotic exposure | False discovery rate correction of p-values |
| Hollister et al. Childhood Obesity. 2018;14(2):122–130.61 | 9 | Good | Selection bias/confounding, Information | Independent t-test (two-tailed), Wilcoxon rank-sum test, ANCOVA | Baseline microbiota composition | Benjamini-Hochberg correction |
| Hou et al. BioMed Res Int. 2017;7585989:1–8.46 | 3 | Poor | Selection bias/ confounding, Performance, Information | Principal coordinate analysis using QIIME, Spearman correlation coefficient, PERMANOVA, Wilcoxon’s rank sum test, LDA algorithm using LEfSe | None | QIIME default method for pairwise comparisons to reject null hypothesis; therefore, no adjustments were made |
| Hu et al. PLoS ONE. 2015;10(7):1–14.47 | 6 | Fair | Selection bias/confounding, Performance, Reporting | Chi-square test, Student’s t-test, Pearson partial correlation coefficient, Mann-Whitney U-test, | Age, gender | False discovery rate correction of p-values |
| Huerta-Avila et al. Nutrients. 2019;11(6):1–14.55 | 7 | Good | Chi-square test, one-way ANOVAs with Bonferroni correction, Multiple linear regression with path analysis, Principal components and factor analyses | Age, sex, first-degree family history of obesity, leisure time physical activity | Bonferroni post-test correction | |
| Ignacio et al, Clin Microbial Infect. 2016;22:258.e1–258.e8.56 | 6 | Fair | Selection bias/ confounding | Chi-square tests, Spearman correlation, Kruskal-Wallis test, Multiple and logistic regression | Age and gender | Dunn and Tukey correction |
| Kamng’ona et al. Scientific Reports;2019;9:12893.65 | 10 | Good | Selection bias/ confounding | Multiple linear regression, Logistic regression, Repeated measures ANCOVA | Intervention group; child age on day of stool collection; maternal age, height, body mass index, parity, education, HIV status, and hemoglobin at enrollment; household assets, food security, source of drinking water, residential location and access to sanitary facility; season at time of stool sample collection; mode of delivery (vaginal or cesarean); site of delivery; and child sex | Benjamini-Hochberg correction using a false discovery rate (FDR) of 0.15 |
| Karlsson et al. Obesity. 2012;20(11):2257–61.57 | 5 | Fair | Selection bias/ confounding, Information | Student’s t-test, Mann-Whitney rank sum test, Multivariate data analysis with principal component analysis | None | None |
| Karvonen et al. International J of Obesity. 2019;43:713–723.45 | 6 | Fair | Selection bias/ confounding, Information | Chi-square tests, Mann-Whitney U test, Spearman correlation, Logistic regression | Maternal education Other confounders were examined but did not remain in final models because they did not change the estimates. |
Bonferroni-corrected p-values |
| Leong et al. Am J of Clinical Nutrition. 2020;111(1):70–78.58 | 5 | Fair | Selection bias/confounding | Student’s t-test, Chi-square test, Principal component analysis, Spearman correlation, Multivariate models | Model 1: Parity, household deprivation, mode of delivery. Model 2: breastfeeding, protein, fiver, non-starch polysaccharide, nut/seeds/legumes, meat/fish/poultry, BMI z-score |
None |
| López-Contreras et al. Pediatric Obesity. 2018;13(6):381–388.59 | 6 | Fair | Spearman correlation, Mann-Whitney U-test, Chi-squared test with Haldane’s correction | Age, gender | Benjamini-Hochberg correction using a false discovery rate (FDR) of 0.05, Haldane’s correction | |
| Mansour et al. Res J Pharm, Biol, & Chem Sci. 2016;7:436–445.48 | 3 | Poor | Selection bias/confounding, Information, Performance, Precision | NA | None | None |
| Méndez-Salazar et al. Frontiers in Microbiology. 2018;9(Oct):1–11.35 | 5 | Fair | Precision, Information | Spearman correlation, One-way ANOVA, Kruskal-Wallis test, LefSe, Principal components analysis | None | Bonferroni-corrected p-values; Tukey’s adjusted p-values for multiple comparisons |
| Moran-Ramos et al. Gut Microbes. 2020; Jan 23:1–18.36 | 5 | Fair | Selection bias/confounding | PERMANOVA, Canonical correspondence analysis, Spearman rank correlation, Multivariate logistic regression analysis using stepwise modeling | Age, sex, and monthly income | Benjamini-Hochberg correction using a false discovery rate (FDR) |
| Mousavi et al. Iranian Red Crescent Med J. 2018;20(4):1–6.37 | 7 | Good | Reporting | Student’s t-test, One-way ANOVA, Kruskal-Wallis test, Mann-Whitney U-test, Chi-square test, Spearman’s rank correlation coefficient | None | None |
| Murugesan et al. Euro J Clin Micro & Infect Dis. 2015;34:1337–46.38 | 6 | Fair | Selection bias/confounding, Performance, Information, Overall believability | Chi-square test, ANOVA, Kruskal-Wallis test, Principal components analysis | None | None |
| Nirmalkar et al. Nutrients. 2018;10(2009):1–23.39 | 5 | Fair | Multivariate association with linear models, Pearson correlations, Spearman correlations, One-way ANOVA, Mann-Whitney U-test | None | Benjamini-Hochberg correction using a false discovery rate (FDR), QIIME default corrections; multiple testing was performed using the p.adjust function in R | |
| Orbe-Orihuela et al. Salud Publica de Mexico. 2018;60(1):5–11.40 | 5 | Fair | Kruskal-Wallis test, Logistic regression, Spearman rank correlation coefficient | Age, gender, total energy intake, and heredo-familial history of obesity | None | |
| Payne et al. Nutrition & Diabetes. 2011;1(7):e12.41 | 5 | Fair | Selection bias/confounding, Performance, Precision | One-way ANOVA | None | Tukey–Kramer HSD test |
| Riva et al. Environmental Microbiology. 2017;19(1):95–105.49 | 6 | Fair | Student’s t-test, Chi-square test, Pearson correlation coefficient, Linear regression models, PERMANOVA | None | False discovery rate correction of p-values | |
| Whisner et al. BMC Microbiology. 2018;18(1)1:11.50 | 7 | Good | Wilcoxon-Kruskal Wallis test, Principal coordinate analysis, Linear discriminant analysis using LEfSe | None | False discovery rate correction of p-values | |
| Xu et al. BMC Microbiology. 2012;12(1):283–289.62 | 5 | Fair | Information | Chi-square test, Spearman’s correlation, Kruskal-Wall with Mann-Whitney U-test, One-way ANOVA | None | Bonferroni-corrected p-values |
| Yuan et al. J of Gastro & Hepat. 2014;29:1292–8.51 | 6 | Fair | One-way ANOVA, Kruskal-Wallis test. Fisher’s exact test, Spearman’s rank correlation coefficient | None | Tukey’s correction for pairwise comparisons; Dunn’s multiple comparison tests | |
| Zhao et al. Frontiers in Pediatrics. 2019;7:518.52 | 5 | Fair | Selection bias/confounding, Information, Performance, | Independent samples t-test, Chi-square test, Kruskal-Wallis test, Wilcoxon rank sum test | None | None |
The quality assessment tool for observational cohort and cross-sectional studies by the National Heart, Lung, and Blood Institute was used for quality assessment (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). A quality rating algorithm was created where studies that had an answer of “yes” for more than 7 of the questions received a quality rating of “good”, studies that received an answer of “yes” for 4 to 7 of the questions received a quality rating of “fair” and studies that had an answer of “yes” for 3 or fewer of the questions received a quality rating of “poor”. Types of bias included selection, information, measurement, and confounding.
Evaluation of Potential Bias and Precision
There were 52 potential sources of bias or threats to precision identified among the included studies using the RTI 29-item bank. The most common types of bias were information (30%, n=16) and selection bias/confounding (32%, n=17). Eight studies29,39,40,49–51,55,59 had no potential sources of bias or threats to precision. Of the remaining studies, 21% (n=7) had one,31,36,37,43,53,58,62 27% (n=9) had two,35,42,45,56,57,60,61,64,65 18% (n=6) had three,41,44,46,47,52,54 and 9% (n=3) had four38,48,63 potential sources of bias or threats to precision. Additionally, we report the various statistical methods used and if any multiple comparison or covariate adjustments were made. This information is summarized in Table 2 along with the overall quality assessment data.
Dietary Influence on Adiposity and Gut Microbial Modification
Despite the limited data available in children, there is a growing body of literature pertaining to the role that diet plays in microbial diversity and abundance. Growth of many beneficial microbes are inhibited by a Western-style diet that is high in fat. Conversely, the abundance of beneficial microbes is enhanced with plant-based diets including a variety of grains, fruits, and vegetables. Despite the known role that diet plays in the development of adiposity as well as the impact it has on the gut microbiome, 45.5% (n=15) of the included studies did not assess dietary intake. Among the studies that did evaluate diet (n=18), five used a dietary recall,35,38,39,43,50 six used a food frequency questionnaire36,49,55,59,63 or block screener,61 four used parent report of feeding practices or dietary intake,45,58,60,64 two used non-validated dietary questionnaires,41,42 and one used a five day food record.29 Although almost half of the included studies evaluated diet, only 12% (n=4) of the included studies35,42,45,58 evaluated diet-microbiota associations in relation to adiposity. Therefore, it was challenging to identify direct relationships among dietary factors, the microbiota, and adiposity outcomes. Table 3 provides a summary of the 26 microbes reported in four or more of the included articles, their metabolic function, and evidence-based dietary approaches reported in the included studies and broader literature that may modify their abundance.
Table 3.
Evidence-Based Dietary Approaches that Promote or Inhibit Obesity-Associated Gut Microbes
| Microbe | Metabolic Function | Diet-Microbe Relationships Reported in Included Studies | Diet-Microbe Relationships Reported in the Literature | ||
|---|---|---|---|---|---|
| Increased Abundance | Decreased Abundance | ||||
| Phylum | Actinobacteria | Degradation and decomposition of organic substances such as cellulose, polysaccharides, protein, fats, and organic acids; Production of bioactive enzymes that degrade starch.95 | Not applicable | High-fat, high animal protein diet;96 Dairy, especially cheese and cheese flavoring;95 Light or low-calorie beer;95 Low-fiber diet26 | Increase dietary fiber intake; reduce consumption of animal protein |
| Bacteroidetes | Fermentation of polysaccharides and indigestible carbohydrates; Production of short-chain fatty acids.97 | Not applicable | Increase fruit and vegetable consumption;98,99 increase dietary fiber intake;98,99 reduce consumption of animal protein | High-fat, high animal protein diet;100 Low-fiber diet100 | |
| Firmicutes | Carbohydrate metabolism101 | Not applicable | Dietary intake higher in fruits, vegetables, and fibers;102,103 High carbohydrate foods96,104 | Low-fat diet | |
| Firmicutes:Bacteroidetes | Not Applicable | Positively associated with vegetable consumption and negatively associated with meat consumption60 | See Firmicutes and Bacteroidetes above. | See Firmicutes and Bacteroidetes above. | |
| Proteobacteria | Anaerobic environment homeostasis;105 Colonization of mucus layer105 | Not applicable | Insoluble fiber,106 plant-based foods (i.e. berries);106 Wild-gamey animal meat;106 Breast milk107 | Mediterranean diet,106 Digestible starch and sugar106 | |
| Tenericutes | Fermentation of sugars;108 starch synthesis and degradation;108 Acetate production;108 Cardiolipin and peptidoglycan synthesis108 | Not applicable | Cereals and starchy foods;109 Fruit and vegetable consumption;109 Meat, honey, berries and tubers;106 Dietary fiber intake50 | Italian-style gluten-free diet110 | |
| Verrucomicrobia | Propionate producing and mucin-degrading;111 Decreased metabolic endotoxemia;102,112 Maintenance of intestinal integrity;113 Modulation of mucus thickness;114 Pro-inflammatory activity115 | Not applicable | Foods high in polyphenols;114 Fermented foods such as unsweetened yogurt;116 Inulin and pomegranate extract;86 Vegetarian and vegan diets117,118 | Western high-fat diet;114 Low FODMAP diets;114 Walnut consumption119 | |
| Family | Bacteroidaceae | Production of lactate and short chain fatty acids;108,109 Utilization of human milk oligosaccharides108,109 | Not applicable | Increase consumption of fruits and vegetables and other plant-based foods;98 High-fiber plant-based foods98 | High-fat diet;98 Diet rich in starchy foods such as potatoes98 |
| Christensenellaceae | Butanoate and propanoate metabolism;122 Butyrate synthesis122 | Not applicable | Mediterranean diet;80 Plant-based foods such as vegetables proteins, dietary fiber, and polyphenols.123 | ||
| Coriobacteriaceae | Lipid metabolism124 | Not applicable | Formula-fed infants;124 Moderate protein and carbohydrate intake125 | High protein, low carbohydrate diets125 | |
| Enterobacteriaceae | Sulfate-reducing126 Lactic acid production75 | Not applicable | Moderate dietary protein restriction;91 breastfed infants;75,126 Animal meat;105,127 High-fat, low-fiber diets;117 Reduction in polysaccharide intake;25 Western-diet98 | Early introduction of solid foods;70 Inulin;105 | |
| Lachnospiraceae | Degrade complex polysaccharides to short-chain fatty acids for energy harvesting.112 | Increased with omnivore diets42 | Diets high in animal sources;93 Omnivore diet;93 | High-fiber diet96,113 | |
| Prevotellaceae | Degradation of dietary carbohydrates and proteins;110 Production of acetate, formate, propionate, and succinate.110 | Not applicable | Animal protein, honey, and berries;106 Cereals and starchy foods;114 Legumes114 | Plant-based and fermented foods;98 | |
| Rikenellaceae | Nitrogen and protein metabolism;91 Butyrate synthesis122 | Not applicable | High-fat diet;96,115 Milk and milk derivatives;114 Fruit juice;114 Fish;81 Refined carbohydrates.81 | Decreased consumption of vegetables;81 Increased intake of legumes, nuts, and seeds.81 | |
| Ruminococcaceae | Short-chain fatty acid production116 | Not applicable | Mediterranean diet,106 Digestible starch and sugar106 | Insoluble fiber,106 plant-based foods (i.e. berries);106 Wild-gamey animal meat;106 | |
| Veillonellaceae | Propionate production;134 Lactate utilization;75 Degradation of fiber;106 Amino acid, carbohydrate, and energy metabolism135 | Not applicable | Breastfed infants;75 Fiber intake;75 Plant-based diets;106 Carbohydrate intake110 | Introduction of complementary foods;107 Gluten-free diet;110,129 Higher protein and energy intake110 | |
| Genus | Bacteroides | Degradation of dietary soluble polysaccharides;119,120 Utilization of amino acids for energy;119 Production of short-chain fatty acids and organic acids.119 | Increased with omnivore diets;42 Positive association with fat and carbohydrate intake35 | Higher consumption of animal protein and saturated fat;25,92 Low dietary intake of carbohydrates.92 | Legumes;98 Iron and fiber from plant-based foods98 |
| Bifidobacterium | Maintenance of gut barrier, prevention of permeability and bacterial translocation;112 Metabolite production such as acetic acid70 | Increased with vegetarian diets;42 Negative association with intake of nuts, seeds, and legumes;58 Positive association with fiber and non-starch polysaccharide58 | Oligofructose;139 Probiotics;28,140 Yogurt consumption;141 Prebiotics;112,142 Breastfeeding;70,143 Whole grain consumption98,144 | Gluten-free diet;145 Walnut consumption;119 Wild/gamey meat and foraging foods such as berries131 | |
| Blautia | Sugar fermentation, flavonoid metabolism, leptin production, de novo lipogenesis and cholesterol genesis, protection against lipid accumulation146 | Not applicable | High protein, low carbohydrate diet;125 Brown rice and whole grain barley;144 Savory snacks;140 Processed meat and milk consumption140 | Reduced duration of exclusive breastfeeding;75 High fat diet;117 Low dietary fiber intake;80 Fish, eggs, and bean consumption140 | |
| Dialister | Glucose metabolism;121 Soluble corn fiber fermentation122 | Not applicable | Whole-grain bread;123 Increased consumption of B vitamins;124 Walnut consumption125 | High protein, low carbohydrate diet126 | |
| Faecalibacterium | Short chain fatty acid production, especially butyrate25,150 | Increased with vegetarian diets;42 Positive association with nuts, seeds, legumes and meat, fish and poultry consumption58 |
High protein, low carbohydrate;125 high intake of folate and B vitamins149 | Walnut consumption;119 Wild/gamey meat and foraging foods such as berries131 | |
| Lactobacillus | Carbohydrate metabolism;127 Formation of lactic acid end products127 | Increased with vegetarian diets42 | Plant-based foods;127,128 Fermented foods (e.g. yogurt, cheese, pickles)127,129 | Rice consumption;128 Fast food;130 Snacks and junk food130 | |
| Prevotella | Degradation of dietary soluble polysaccharides;119,120 Production of acetate, formate, propionate, and succinate;110 | Positively associated with monounsaturated fat and vitamin B135 | Vegetarian diet;68 High-fiber diets;28,68 Mediterranean diet;25,132 High intake of carbohydrates26,133 | Low dietary intake of sugars;26 Gluten-free diet25,134 | |
| Sutterella | Potential pathogenic microbe leading to GI and metabolic dysfunction154,155 | Not applicable | Cereals and starchy foods;109 Vegetables and fruits109 | Soluble corn fiber148 | |
| Species | Bacteroides ovatus | Degradation of plant polysaccharides;138 Utilization of plant and host glycans;138 Degradation of polyphenols102 | Not applicable | Human milk oligosaccharides;121 Pectin;117 Dietary polyphenols102 | Mediterranean diet80 |
| Faecalibacterium prausnitzii | Fermentation of carbohydrates;37 Anti-inflammatory effects;141 | Not applicable | High-fiber intake;92 Vegetarian and/or vegan diet;93 | Low fiber intake;142 High insoluble fiber intake142 | |
DISCUSSION
To the best of our knowledge, the present review is the first to systematically explore and critically evaluate the current state of the science regarding differences in gut microbiome composition among infants, children, and adolescents with overweight/obesity compared to normal-weight counterparts. Additionally, this review provides evidence-based dietary approaches that have been shown to promote or reduce the abundance of adiposity-associated microbes. Further, we provide best practice recommendations for future research evaluating adiposity-associated microbes during these formative years. Taken together, these studies demonstrate differences in early life microbiota composition that are associated with unique growth and dietary patterns that may increase the risk of overweight and obesity later in life. However, conflicting results among the included studies make consensus challenging.
Infancy/Toddlerhood
There is substantial evidence to support the dynamic relationship between feeding practices during infancy and toddlerhood and the early development of the microbiome. The protective effect of breastfeeding against obesity67 may be due to key differences in the abundance of Bacteroidetes, Firmicutes, and Verrucomicrobia,68 when compared to formula-fed infants. Interestingly, a decreased abundance of Verrucomicrobia was shown to be associated with overweight/obesity in toddlers, which was not affected by early life feeding practices (i.e. breastfeeding vs. formula-feeding) despite 31% of infants in this study consuming formula in the first year of life.45 This relationship has been corroborated in adults with obesity.69 The infant gut microbiome of formula-fed infants is more diverse with greater abundance of Staphylococci,70 Bacteroides,71,72 and Atopobium.70,72 In the current review, there was no difference in Bacteroides,45 but Atopobium parvulum65 abundance was reduced among infants/toddlers with greater adiposity. One study found that formula feeding was associated with enrichment of Lachnospiraceae at 3–4 months which was subsequently enriched among overweight infants at 12 months,64 indicating a unique microbial family linked to both early life feeding practices and adiposity. However, Lachnospiraceae enrichment was not associated with overweight/obesity among toddlers in another study.45 Moreover, the quality of the studies included in this section received a higher quality rating of either fair or good with all of the studies controlling for covariates and adjusting for multiple comparisons. Taken together, further investigation of these potential microbe-adiposity relationships with breast vs. formula-feeding is needed.
This early developmental time period is also marked by the transition from semi-solid to solid foods. The introduction of complementary foods results in greater within-sample (alpha) diversity and a shift to Firmicutes and Bacteroidetes as the dominant phyla in the infant microbiota.73 Of the included studies, there were no differences in microbial abundance among infants/toddlers by adiposity status for Bacteroidetes,45,64 Firmicutes,45,64 or F:B ratio.60,64 This might be because the studies spanned birth to 36 months or the low alpha diversity of the infant microbiome.74 The relative abundance of Lachnospiraceae and Ruminococcaceae have been noted to increase with the introduction of solid foods75 and their enrichment at 3–4 months has been associated with greater adiposity at 12 months.64 Lending further support for these microbial families, greater abundance of Dorea (family Lachnopiraceae)45 and Ruminococcus obeum (family Ruminococcaceae)65 have been observed among infants with overweight/obesity. Although studies included in this review did not explore specific feeding practices, Lachnospiraceae abundance may be an important indicator of diet-microbe-weight interactions as this family has been positively associated with protein intake among infants/toddlers.75 Further, many of these findings are supported in adult populations,69,76,77 indicating that microbial abundance of these microbial taxa may serve as early life indicators of obesity that continue into adulthood.
School-aged Children.
Unlike the studies among infants/toddlers, the current review found that school-aged children with overweight/obesity had significantly greater abundance of Clostridium39,56 with lower relative abundance of Akkermansia muciniphila,57,63 Oscillospira,38,53 and Rikenellaceae.53,59 A recent systematic review found that specific bacterial species belonging to the genus Clostridium were associated with adiposity;78 however, studies among school-aged children in the current review did not find a relationship between Clostridium spp. and adiposity. Depletion of Oscillospira and Rikenellaceae have been reported among adults with obesity,79 and associated with high-protein diets,80 and legume consumption.81 There is strong evidence that lower abundance of Akkermansia muciniphila is associated with obesity in adults.82 Weight loss,83,84 caloric restriction,85 and dietary intake of pomegranate extract,86 inulin (i.e. soluble fiber),87 and fermentable oligo-, di-, and mono-saccharides88 have all been reported to increase the relative abundance of Akkermansia muciniphila. Reduced abundance of B. plebeius was reported to be an obesity-associated microbe in two child studies59,61 but dietary factors were not associated.59 Overall, these results provide support for adiposity-associated microbial communities; however, differences in the above-mentioned microbial taxa may be heavily influenced by dietary changes during the formative years.
Unfortunately, the majority of studies included for this age range did not report on how diet may have contributed to findings regarding gut microbial abundance and measures of adiposity. There was only one study that examined diet-microbe-adiposity interactions. Leong et al found increased breastfeeding and lower BMI z-score were associated with reduced abundance of Christensenellaceae and Ruminococcaceae.58 This study also found that less consumption of nuts, seeds, and legumes was associated with greater Bifidobacterium but reduced Bacteroides abundance. Additionally, it was reported that greater total fiber intake was associated with greater abundance of Faecalibacterium, Eubacterium, and Roseburia.58 Méndez-Salazar and colleagues found that Bacteroides positively correlated with fat and carbohydrate intake, Proteobacteria was positively associated with fat intake, and Lachnospiraceae was negatively associated with total energy intake.35 Unfortunately, this study did not extend its analyses to examine how these diet-microbe associations related to adiposity outcomes.
Adolescents.
Included studies of adolescents found conflicting results regarding differences in Firmicutes31,44,49,51 and Bacteroidetes31,44,49,51 phyla; however, two studies reported significantly higher abundance of Actinobacteria31,44 among adolescents with obesity. This is somewhat surprising as adult studies have reported no difference in Actinobacteria between normal-weight adults and those with obesity.76 Although these two studies did not explore dietary effects, previous research has shown that the abundance of Actinobacteria increases with high-fat diets26 and decreases with vegetarian diets42 providing a potential dietary explanation for this microbe-adiposity relationship. Significantly lower abundance of Christensenellaceae36,44 and Rikenellaceae36,42,44,47 and greater abundance of Clostridiaceae31,44 and Veillonellaceae36,47 were observed in adolescent studies included in this systematic review. Previous research has found similar results among adults with obesity for Christensenellaceae,89 Rikenellaceae,90 and Veillonellaceae.90 None of the 12 studies among adolescent populations examined diet-microbe-adiposity relationships, despite five studies29,42,43,49,50 assessing dietary factors. Two of these studies43,49 evaluated differences in diet between children with and without adiposity. The remaining three studies29,42,50 that evaluated diet-microbe relationships found no relationship between these bacterial families and dietary factors. The remaining studies did not evaluate dietary information which made it difficult to identify direct links between diet-microbe associations and adiposity. Nonetheless, previous research has reported reduced abundance of Rikenellaceae with decreased protein intake91 and consumption of vegetables.81 Similarly, Clostridiaceae abundance was reduced with inulin and polyphenol-rich pomegranate extract consumption.86 Lastly, B. vulgatus29,48 was significantly lower and F. prausnitzii,31,43 was significantly higher among adolescents with obesity, but dietary effects on these microbe-adiposity relationships were not explored. Increased abundance of F. prausnitzii has been reported with diets high in fiber92 or vegetarian/vegan diets93 whereas B. vulgatus abundance was reduced with greater rice consumption.94 Exploration of the effects of dietary practices on adiposity-microbiome relationships are greatly needed in adolescent populations.
Strengths and Limitations
Although this review adds to the current body of literature about gut microbiota associations with adiposity early in life, it is not without limitations. First, most of the included studies received a quality rating of fair and primarily used cross-sectional study designs which only provide a snapshot of explored associations and cannot indicate cause-effect relationships. Furthermore, the heterogeneity among the bacterial communities appraised in the included articles made synthesis across studies challenging. Additionally, the included studies used a variety of methods for both adiposity and microbial analyses and obtained measures from participants living in various geographical locations, all of which may partially explain the inconsistencies in study results. Lastly, while 18 of the included studies collected dietary intake information, only 12% (n=4) of the included studies35,42,45,58 evaluated both microbial abundance and diversity as well as dietary factors in order to assess the effect of diet-microbiota associations on adiposity. Due to a paucity in the literature, the current review referenced other articles that evaluated specific relationships between dietary factors and the gut microbiome to identify potential areas for intervention, but these findings should be confirmed in the age groups presented in this review.
CONCLUSION
Current data suggest the importance of the gut microbiome in the formative years on influencing obesity risk but identifying specific microbes that associate with obesity remains difficult given limitations in evidence. It is important that future research investigates the microbial community at different taxonomic levels, especially specific bacterial species, in relation to overweight and obesity in order to obtain a clear picture of which microbial strains serve as early indicators of obesity in infancy, childhood, and adolescence. This should be done for specific age groups as the microbiome differs across early life and may require unique dietary intervention depending on age. Strong evidence indicates the importance of breastfeeding for infants which impacts both microbiome community structure and weight outcomes. For children and adolescents, a well-balanced diet that consists of high intakes of fruits and vegetables with moderate animal fat and protein consumption seems to be associated with a healthy gut microbiome. This recommended dietary approach is not novel but remains difficult for most of the population considering the prominence of the Western-style diet. Overall, there is great need for longitudinal studies in infancy, childhood, and adolescence to understand how dietary behaviors and the gut microbiome interact to influence adiposity. Table 4 provides a summary of best practices for future research evaluating microbial relationships with adiposity, particularly the need to conduct formal mediation/moderation analyses that assess the influence of other factors (i.e. dietary factors) on microbe-adiposity relationships.
Table 4.
Best Practices for Future Research Evaluating Microbial Relationships with Adiposity
| Area of Research | Best Practice Suggestion |
|---|---|
| Population | Avoid spanning across multiple developmental periods. Instead focus on one age group to capture unique changes in microbes and adiposity risk in relation to that age. |
| If the study includes a wide range of ages, i.e. spans different periods of development, report the microbial relationships separately for different age groups. | |
| Study design | More longitudinal studies are needed particularly when assessing microbe-diet associations and their impact on obesity. |
| Multiple data collection timepoints throughout the various developmental time periods that include fecal, diet, and adiposity data collection are needed. | |
| Microbial Analysis Methodology | Use the same PCR primers as previous research. Primers for the V4 region of the 16S rRNA gene is recommended by the Human Microbiome Project, when performing high-throughput 16S sequencing. |
| Differentiate bacterial taxa at the species level whenever possible. | |
| If possible, consider using metagenomics shotgun sequencing in order to gather a more comprehensive list of microbial strains present in the sample. | |
| Adiposity Measures | Use a variety of measures for assessing adiposity to ensure that there are measurement similarities across studies. |
| Report all adiposity measures as part of the article or in supplemental materials. | |
| Dietary Intake Measures | Evaluate dietary intake using 24-hour dietary recalls. |
| Assess diet on the same day as fecal collection whenever possible. | |
| Control for diet in analyses through mediation/moderation analyses or multivariate models. | |
| Statistical analyses | Control for multiple comparisons by using available programs such as QIIME2. |
| Formal mediation or moderation analyses to examine the effect of other factors (diet, antibiotics, geography) on microbe-adiposity relationships. | |
| Covariates/Confounders | Report key information such as geographic location, time period of the study, season of fecal collection, birth mode, antibiotic use, etc., which may also interact with variables of interest to influence the microbiome. |
These best practices are only suggestions based on the limitations of the current systematic review and those reported in a recent publication (See Reference). We suggest this article as a good reference for developing research projects that study, analyze, and interpret the human gut microbiome. Reference: Allaband C et al. Clinical Gastroenterology and Hepatology. 2019;17(2):218–230.
Supplementary Material
Supplemental Table 1. Study Characteristics and Evaluation of Included Articles
Supplemental Table 2. Microbial Relationships with Adiposity Reported among Two or More of the Included Studies
Supplemental Table 3. Complete Evaluation of Quality and Potential Bias of Included Studies
Acknowledgements:
A portion of time preparing this manuscript was supported by two grants from the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL147931 (Whisner) and National Institute of Diabetes and Digestive and Kidney Disease under Award Number R01DK10757901 (Vander Wyst). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Potential conflicts of interest: The authors have no conflicts of interest relevant to this article to disclose.
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Coutinho PR, Leite N, Lopes WA, et al. Association between adiposity indicators, metabolic parameters and inflammatory markers in a sample of female adolescents. Arch Endocrinol Metab. 2015;59(4):325–334. doi: 10.1590/2359-3997000000070 [DOI] [PubMed] [Google Scholar]
- 2.Johnson WD, Kroon JJM, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of Risk Factors for Metabolic Syndrome in Adolescents. Arch Pediatr Adolesc Med. 2009;163(4):371. doi: 10.1001/archpediatrics.2009.3 [DOI] [PubMed] [Google Scholar]
- 3.Miller JM, Kaylor MB, Johannsson M, Bay C, Churilla JR. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001–2010 national health and nutrition examination survey. Metab Syndr Relat Disord. 2014;12(10):527–532. doi: 10.1089/met.2014.0055 [DOI] [PubMed] [Google Scholar]
- 4.Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J Endocr Soc. 2017;1(5):524–537. doi: 10.1210/js.2017-00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinehr T, Roth CL. Inflammation Markers in Type 2 Diabetes and the Metabolic Syndrome in the Pediatric Population. 2018. doi: 10.1007/s11892-018-1110-5 [DOI] [PubMed] [Google Scholar]
- 6.Lascar N, Brown J, Pattison H, Barnett AH, Bailey C, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):69–80. [DOI] [PubMed] [Google Scholar]
- 7.Buscot M-J, Thomson RJ, Juonala M, et al. BMI Trajectories Associated With Resolution of Elevated Youth BMI and Incident Adult Obesity. Pediatrics. 2018;141(1):e20172003. doi: 10.1542/peds.2017-2003 [DOI] [PubMed] [Google Scholar]
- 8.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief. Hyattsville, MD; 2017. doi: 10.1017/S1368980017000088 [DOI] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA - J Am Med Assoc. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreira E, Novo A, Vaz JA, Pereira A. Dietary program and physical activity impact on biochemical markers in patients with type 2 diabetes: A systematic review. Aten Primaria. 2018;50(10):590–610. doi: 10.1016/j.aprim.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savoye M, Shaw M, Dziura J, et al. Effects of a Weight Management Program on Body Composition and Metabolic Parameters in Overweight Children: A Randomized Controlled Trial. JAMA. 2007;297(24):2697–2704. doi: 10.1001/jama.297.24.2697 [DOI] [PubMed] [Google Scholar]
- 12.Green CA, Yarborough BJH, Leo MC, et al. The STRIDE Weight Loss and Lifestyle Intervention for Individuals Taking Antipsychotic Medications: A Randomized Trial. Am J Psychiatry. 2014;172(1):1–11. doi: 10.1176/appi.ajp.2014.14020173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Look AHEAD Research Group. Long Term Effects of a Lifestyle Intervention on Weight and Cardiovascular Risk Factors in Individuals with Type 2 Diabetes: Four Year Results of the Look AHEAD Trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334.Long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koleva PT, Bridgman SL, Kozyrskyj AL. The Infant Gut Microbiome: Evidence for Obesity Risk and Dietary Intervention. 2015:2237–2260. doi: 10.3390/nu7042237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovel J, Dieleman LA, Kao D, Mason AL, Wine E. The Human Gut Microbiome in Health and Disease. In: Metagenomics: Perspectives, Methods, and Applications. Elsevier Inc.; 2017:197–213. doi: 10.1016/B978-0-08-102268-9.00010-0 [DOI] [Google Scholar]
- 16.Johnson CP, Blasco PA. Infant growth and development. Pediatr Rev. 1997;18(7):224–242. doi: 10.1542/pir.18-7-224 [DOI] [PubMed] [Google Scholar]
- 17.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang I, Corwin EJ, Brennan PA, Jordan S, Murphy JR, Dunlop A. The infant microbiome: Implications for infant health and neurocognitive development. Nurs Res. 2016;65(1):76–88. doi: 10.1097/NNR.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho NT, Li F, Lee-Sarwar KA, et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9(4169):1–13. doi: 10.1038/s41467-018-06473-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Muinck EJ, Trosvik P. Individuality and convergence of the infant gut microbiota during the first year of life. Nat Commun. 2018;9(1):1–8. doi: 10.1038/s41467-018-04641-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Videhult FK, West CE. Nutrition, gut microbiota and child health outcomes. Curr Opin Clin Nutr Metab Care. 2016;19(3):208–213. doi: 10.1097/MCO.0000000000000266 [DOI] [PubMed] [Google Scholar]
- 22.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J, Ringel-Kulka T, Heikamp-De Jong I, et al. Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J. 2016;10(4):1002–1014. doi: 10.1038/ismej.2015.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong H, Penders J, Shi Z, et al. Impact of early events and lifestyle on the gut microbiota and metabolic phenotypes in young school-age children. Microbiome. 2019;7(1):1–14. doi: 10.1186/s40168-018-0608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh RK, Chang H-W, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(73):1–17. doi: 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu GD, Chen J, Hoffmann C, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science (80- ). 2011;334(6052):105–108. doi: 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 28.Million M, Lagier JC, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect. 2013;19(4):305–313. doi: 10.1111/1469-0691.12172 [DOI] [PubMed] [Google Scholar]
- 29.Bervoets L, Van Hoorenbeeck K, Kortleven I, et al. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013;5(1):1–10. doi: 10.1186/1757-4749-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O. Distal Gut Microbiota of Adolescent Children is Different from that of Adults. FEMS Microbiol Ecol. 2011;77(2):404–412. doi: 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Chierico F, Abbatini F, Russo A, et al. Gut microbiota markers in obese adolescent and adult patients: Age-dependent differential patterns. Front Microbiol. 2018;9(JUN):1–12. doi: 10.3389/fmicb.2018.01210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7). doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Heart Lung and Blood Institute. Study Quality Assessment Tools. U.S Department of Health & Human Services. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Published 2019. Accessed August 29, 2019.
- 34.Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65(2):163–178. doi: 10.1016/j.jclinepi.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 35.Méndez-Salazar EO, Ortiz-López MG, Granados-Silvestre MDLÁ, Palacios-González B, Menjivar M. Altered gut microbiota and compositional changes in firmicutes and proteobacteria in mexican undernourished and obese children. Front Microbiol. 2018;9(OCT):1–11. doi: 10.3389/fmicb.2018.02494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran-Ramos S, Lopez-Contreras BE, Villarruel-Vazquez R, et al. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microbes. 2020;Jan 23:1–18. doi: 10.1080/19490976.2020.1712985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mousavi SH, Mehrara S, Barzegari A, Ostadrahimi A. Correlation of Gut Microbiota Profile with Body Mass Index Among School Age Children. Iran Red Crescent Med J. 2018;20(4). doi: 10.5812/ircmj.58049 [DOI] [Google Scholar]
- 38.Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis. 2015;34(7):1337–1346. doi: 10.1007/s10096-015-2355-4 [DOI] [PubMed] [Google Scholar]
- 39.Nirmalkar K, Murugesan S, Pizano-Zárate ML, et al. Gut microbiota and endothelial dysfunction markers in obese Mexican children and adolescents. Nutrients. 2018;10(12). doi: 10.3390/nu10122009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orbe-Orihuela YC, Lagunas-Martínez A, Bahena-Román M, et al. High relative abundance of firmicutes and increased TNF-α levels correlate with obesity in children. Salud Publica Mex. 2018;60(1):5–11. doi: 10.21149/8133 [DOI] [PubMed] [Google Scholar]
- 41.Payne AN, Chassard C, Zimmermann M, Müller P, Stinca S, Lacroix C. The metabolic activity of gut microbiota in obese children is increased compared with normal-weight children and exhibits more exhaustive substrate utilization. Nutr Diabetes. 2011;1(7):e12–e12. doi: 10.1038/nutd.2011.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai J, Hu Y, Bruner DW. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr Obes. 2019;14(4). doi: 10.1111/ijpo.12480 [DOI] [PubMed] [Google Scholar]
- 43.Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AMS, Ramakrishna BS. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr. 2010;103(3):335–338. doi: 10.1017/S0007114509992182 [DOI] [PubMed] [Google Scholar]
- 44.Ferrer M, Ruiz A, Lanza F, et al. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ Microbiol. 2013;15(1):211–226. doi: 10.1111/j.1462-2920.2012.02845.x [DOI] [PubMed] [Google Scholar]
- 45.Karvonen AM, Sordillo JE, Gold DR, et al. Gut microbiota and overweight in 3-year old children. Int J Obes. 2019;43(4):713–723. doi: 10.1038/s41366-018-0290-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou Y-P, He Q-Q, Ouyang H-M, et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. Biomed Res Int. 2017;7585989:1–8. doi: 10.1155/2017/7585989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu HJ, Park SG, Jang HB, et al. Obesity alters the microbial community profile in Korean Adolescents. PLoS One. 2015;10(7):1–14. doi: 10.1371/journal.pone.0134333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansour NM, Ismail NA. Quantitative of intestinal microbial lactobacillus and Bacteroides groups and the prevalence of their selected species among Egyptian obese subjects. Res J Pharm Biol Chem Sci. 2016;7(5):436–445. [Google Scholar]
- 49.Riva A, Borgo F, Lassandro C, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19(1):95–105. doi: 10.1111/1462-2920.13463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whisner CM, Maldonado J, Dente B, Krajmalnik-Brown R, Bruening M. Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: A cross-sectional study. BMC Microbiol. 2018;18(1):1–11. doi: 10.1186/s12866-018-1362-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan J, Baker SS, Liu W, et al. Endotoxemia unrequired in the pathogenesis of pediatric nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2014;29(6):1292–1298. doi: 10.1111/jgh.12510 [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Zhou J, Liu J, Wang Z, Chen M, Zhou S. Metagenome of Gut Microbiota of Children With Nonalcoholic Fatty Liver Disease. Front Pediatr. 2019;7(December):1–8. doi: 10.3389/fped.2019.00518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Sun H, Jiang F, et al. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ. 2020;2020(1):1–26. doi: 10.7717/peerj.8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Da Silva CC, Monteil MA, Davis EM. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child Obes. 2020;16(3):204–210. doi: 10.1089/chi.2019.0280 [DOI] [PubMed] [Google Scholar]
- 55.Huerta-Ávila EE, Ramírez-Silva I, Torres-Sánchez LE, et al. High relative abundance of lactobacillus reuteri and fructose intake are associated with adiposity and cardiometabolic risk factors in children from mexico city. Nutrients. 2019;11(6):1–14. doi: 10.3390/nu11061207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ignacio A, Fernandes MR, Rodrigues VAA, et al. Correlation between body mass index and faecal microbiota from children. Clin Microbiol Infect. 2016;22:258.e1–258e.8. doi: 10.1016/j.cmi.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 57.Karlsson CLJ, Önnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. 2012;20(11):2257–2261. doi: 10.1038/oby.2012.110 [DOI] [PubMed] [Google Scholar]
- 58.Leong C, Haszard JJ, Heath ALM, et al. Using compositional principal component analysis to describe children’s gut microbiota in relation to diet and body composition. Am J Clin Nutr. 2020;111(1):70–78. doi: 10.1093/ajcn/nqz270 [DOI] [PubMed] [Google Scholar]
- 59.López-Contreras BE, Morán-Ramos S, Villarruel-Vázquez R, et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr Obes. 2017;13(6):381–388. doi: 10.1111/ijpo.12262 [DOI] [PubMed] [Google Scholar]
- 60.Craig SJC, Blankenberg D, Parodi ACL, et al. Child Weight Gain Trajectories Linked To Oral Microbiota Composition. Sci Rep. 2018;8(1):1–14. doi: 10.1038/s41598-018-31866-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hollister EB, Foster BA, Dahdouli M, Ramirez J, Lai Z. Characterization of the Stool Microbiome in Hispanic Preschool Children by Weight Status and Time. Child Obes. 2018;14(2):122–130. doi: 10.1089/chi.2017.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu P, Li M, Zhang J, Zhang T. Correlation of intestinal microbiota with overweight and obesity in Kazakh school children. BMC Microbiol. 2012;12(1):1. doi: 10.1186/1471-2180-12-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borgo F, Verduci E, Riva A, et al. Relative Abundance in Bacterial and Fungal Gut Microbes in Obese Children: A Case Control Study. 2017;13(1):78–84. doi: 10.1089/chi.2015.0194 [DOI] [PubMed] [Google Scholar]
- 64.Forbes JD, Azad MB, Vehling L. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018;172(7):704. doi: 10.1001/jamapediatrics.2018.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamng’ona AW, Young R, Arnold CD, et al. The association of gut microbiota characteristics in Malawian infants with growth and inflammation. Sci Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-49274-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollister EB, Riehle K, Luna RA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36. doi: 10.1186/s40168-015-0101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: A meta-analysis. World Rev Nutr Diet. 2016;114:110–111. doi: 10.1159/000441820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan YK, Estaki M, Gibson DL. Clinical consequences of diet-induced dysbiosis. Ann Nutr Metab. 2013. doi: 10.1159/000354902 [DOI] [PubMed] [Google Scholar]
- 69.Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020. doi: 10.1038/s41430-020-0607-6 [DOI] [PubMed] [Google Scholar]
- 70.Martin R, Makino H, Yavuz AC, et al. Early-Life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One. 2016;11(6):1–30. doi: 10.1371/journal.pone.0158498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824 [DOI] [PubMed] [Google Scholar]
- 72.Bezirtzoglou E, Tsiotsias A, Welling GW. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH). Anaerobe. 2011;17(6):478–482. doi: 10.1016/j.anaerobe.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 73.Differding MK, Benjamin-Neelon SE, Hoyo C, Østbye T, Mueller NT. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020;20(1):56. doi: 10.1186/s12866-020-01723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derrien M, Alvarez AS, de Vos WM. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019;27(12):997–1010. doi: 10.1016/j.tim.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 75.Laursen MF, Andersen LBB, Michaelsen KF, et al. Infant Gut Microbiota Development Is Driven by Transition to Family Foods Independent of Maternal Obesity. mSphere. 2016;1(1):1–16. doi: 10.1128/msphere.00069-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nardelli C, Granata I, D’Argenio V, et al. Characterization of the Duodenal Mucosal Microbiome in Obese Adult Subjects by 16S rRNA Sequencing. Microorganisms. 2020;8(4):485. doi: 10.3390/microorganisms8040485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kasai C, Sugimoto K, Moritani I, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15(1):1–10. doi: 10.1186/s12876-015-0330-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Indiani CMDSP Rizzardi KF, Castelo PM Ferraz LFC, Darrieux M Parisotto TM. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child Obes. 2018;14(8):501–509. doi: 10.1089/chi.2018.0040 [DOI] [PubMed] [Google Scholar]
- 79.Peters BA, Shapiro JA, Church TR, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-28126-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. 2018;9(MAY):1–11. doi: 10.3389/fmicb.2018.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berding K, Holscher HD, Arthur AE, Donovan SM. Fecal microbiome composition and stability in 4- to 8-year old children is associated with dietary patterns and nutrient intake. J Nutr Biochem. 2018;56:165–174. doi: 10.1016/j.jnutbio.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 82.Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 83.Kim BS, Song MY, Kim H. The anti-obesity effect of Ephedra sinica through modulation of gut microbiota in obese Korean women. J Ethnopharmacol. 2014;152(3):532–539. doi: 10.1016/j.jep.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 84.Remely M, Tesar I, Hippe B, Gnauer S, Rust P, Haslberger AG. Gut microbiota composition correlates with changes in body fat content due to weight loss. Benef Microbes. 2015;6(4):431–439. doi: 10.3920/BM2014.0104 [DOI] [PubMed] [Google Scholar]
- 85.Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. doi: 10.1136/gutjnl-2014-308778 [DOI] [PubMed] [Google Scholar]
- 86.Zhang S, Yang J, Henning SM, et al. Dietary pomegranate extract and inulin affect gut microbiome differentially in mice fed an obesogenic diet. Anaerobe. 2017;48:184–193. doi: 10.1016/j.anaerobe.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 87.Roshanravan N, Mahdavi R, Alizadeh E, et al. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J Cardiovasc Thorac Res. 2017;9(4):183–190. doi: 10.15171/jcvtr.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. doi: 10.1136/gutjnl-2014-307264 [DOI] [PubMed] [Google Scholar]
- 89.Kaplan RC, Wang Z, Usyk M, et al. Erratum: Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity (Genome Biology (2019) 20 (219) DOI: 10.1186/s13059-019-1831-z). Genome Biol. 2020;21(1):1–21. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2015;14(1):1–14. doi: 10.1186/s12866-014-0311-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan P, Liu P, Song P, Chen X, Ma X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci Rep. 2017;7(2):1–12. doi: 10.1038/srep43412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reyes LM, Vázquez RG, Arroyo SMC, et al. Correlation between diet and gut bacteria in a population of young adults. Int J Food Sci Nutr. 2016;67(4):470–478. doi: 10.3109/09637486.2016.1162770 [DOI] [PubMed] [Google Scholar]
- 93.Matijašić BB, Obermajer T, Lipoglavšek L, Grabnar I, Avguštin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. 2014;53:1051–1064. doi: 10.1007/s00394-013-0607-6 [DOI] [PubMed] [Google Scholar]
- 94.Isolauri E, Salminen S. The impact of early gut microbiota modulation on the risk of child disease: Alert to accuracy in probiotic studies. Benef Microbes. 2015;6(2):167–171. doi: 10.3920/BM2014.0114 [DOI] [PubMed] [Google Scholar]
- 95.Anandan R, Dharumadurai D, Manogaran GP. An introduction to the actinobacteria. Microbiol Today. 2007;34(2):60–62. doi: 10.5772/62329 [DOI] [Google Scholar]
- 96.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1). doi: 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Birg A, Ritz NL, Lin HC. Chapter 20 - The Unknown Effect of Antibiotic-Induced Dysbiosis on the Gut Microbiota. In: Microbiome and Metabolome in Diagnosis, Therapy, and Other Strategic Applications. ; 2019:195–200. [Google Scholar]
- 98.Jang HB, Choi M-K, Kang JH, Park SI, Lee H-J. Association of dietary patterns with the fecal microbiota in Korean adolescents. BMC Nutr. 2017;3(1):1–11. doi: 10.1186/s40795-016-0125-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson EL, Heaver SL, Walters WA, Ley RE. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J Mol Med. 2017;95(1). doi: 10.1007/s00109-016-1492-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ottman N, Smidt H, de Vos WM, Belzer C. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2(104):1–11. doi: 10.3389/fcimb.2012.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8836–8847. doi: 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–230. doi: 10.1038/ismej.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66(4):515–522. doi: 10.1016/j.alit.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 105.Moon CD, Young W, Maclean PH, Cookson AL, Bermingham EN. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. Microbiologyopen. 2018;7(5):1–20. doi: 10.1002/mbo3.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turroni S, Rampelli S, Centanni M, et al. Enterocyte-associated microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5(3654):1–12. doi: 10.3389/fmicb.2016.00865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davis EC, Dinsmoor AM, Wang M, Donovan SM, Donovan SM. Microbiome Composition in Pediatric Populations from Birth to Adolescence : Impact of Diet and Prebiotic and Probiotic Interventions. Dig Dis Sci. 2020;(0123456789). doi: 10.1007/s10620-020-06092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y, Huang JM, Zhou YL, et al. Phylogenomics of expanding uncultured environmental Tenericutes provides insights into their pathogenicity and evolutionary relationship with Bacilli. BMC Genomics. 2020;21(1):1–12. doi: 10.1186/s12864-020-06807-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Filippo C, Di Paola M, Ramazzotti M, et al. Diet, environments, and gut microbiota. A preliminary investigation in children living in rural and Urban Burkina Faso and Italy. Front Microbiol. 2017;8(OCT):1–14. doi: 10.3389/fmicb.2017.01979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ercolini D, Francavilla R, Vannini L, et al. From an imbalance to a new imbalance: Italian-style gluten-free diet alters the salivary microbiota and metabolome of African celiac children. Sci Rep. 2015. doi: 10.1038/srep18571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia municiphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(5):1469–1476. doi: 10.1099/ijs.0.02873-0 [DOI] [PubMed] [Google Scholar]
- 112.Cani PD, Possemiers S, Van De Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Geerlings S, Kostopoulos I, de Vos W, Belzer C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms. 2018;6(3):75. doi: 10.3390/microorganisms6030075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. 2017;33(2004):194–201. doi: 10.1016/j.jff.2017.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes. 2018;9(4):308–325. doi: 10.1080/19490976.2018.1465157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.González S, Fernández-Navarro T, Arboleya S, De Los Reyes-Gavilán CG, Salazar N, Gueimonde M. Fermented dairy foods: Impact on intestinal microbiota and health-linked biomarkers. Front Microbiol. 2019;10(1046):1–10. doi: 10.3389/fmicb.2019.01046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Senghor B, Sokhna C, Ruimy R, Lagier JC. Gut microbiota diversity according to dietary habits and geographical provenance. Hum Microbiome J. 2018;7–8(June 2017):1–9. doi: 10.1016/j.humic.2018.01.001 [DOI] [Google Scholar]
- 118.Reiss A, Jacobi M, Rusch K, Schwietz A. Association of dietary type with fecal microbiota and short chain fatty acids in vegans and omnivores. J Int Soc Microbiota. 2016;1:1–9. doi: 10.18143/JISM [DOI] [Google Scholar]
- 119.Holscher HD, Guetterman HM, Swanson KS, et al. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: A randomized controlled trial. J Nutr. 2018;148(6):861–867. doi: 10.1093/jn/nxy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev. 2017;18(1):18–31. doi: 10.1111/obr.12484 [DOI] [PubMed] [Google Scholar]
- 121.Marcobal A, Barboza M, Sonnenburg ED, et al. Bacteroides in the Infant Gut Consume Milk Oligosaccharides via Mucus-Utilization Pathways. Cell Host Microbe. 2011;10(5):1–14. doi: 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peters BA, Shapiro JA, Church TR, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8(1):1–13. doi: 10.1038/s41598-018-28126-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.García-Mantrana I, Selma-Royo M, González S, Parra-Llorca A, Martínez-Costa C, Collado MC. Distinct maternal microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes. 2020;00(00):1–17. doi: 10.1080/19490976.2020.1730294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lahti L, Salonen A, Kekkonen RA, et al. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ. 2013;2013(1):1–25. doi: 10.7717/peerj.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hooda S, Vester Boler BM, Kerr KR, Dowd SE, Swanson KS. The gut microbiome of kittens is affected by dietary protein:carbohydrate ratio and associated with blood metabolite and hormone concentrations. Br J Nutr. 2013;109(9):1637–1646. doi: 10.1017/S0007114512003479 [DOI] [PubMed] [Google Scholar]
- 126.Rampelli S, Candela M, Turroni S, et al. Microbiota and lifestyle interactions through the lifespan. Trends Food Sci Technol. 2016;57:265–272. doi: 10.1016/j.tifs.2016.03.003 [DOI] [Google Scholar]
- 127.Qasem W, Azad MB, Hossain Z, et al. Assessment of complementary feeding of Canadian infants: Effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr. 2017;17(1):1–12. doi: 10.1186/s12887-017-0805-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):1–15. doi: 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bonder MJ, Tigchelaar EF, Cai X, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8(1):1–11. doi: 10.1186/s13073-016-0295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sagheddu V, Patrone V, Miragoli F, Puglisi E, Morelli L. Infant early gut colonization by Lachnospiraceae: High frequency of Ruminococcus gnavus. Front Pediatr. 2016;4(JUN):7–12. doi: 10.3389/fped.2016.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schnorr SL, Candela M, Rampelli S, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS One. 2012;7(10). doi: 10.1371/journal.pone.0047713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kang C, Wang B, Kaliannan K, et al. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio. 2017;8(3):1–15. doi: 10.1128/mBio.00470-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rios-Covian D, Salazar N, Gueimonde M, de los Reyes-Gavilan CG. Shaping the metabolism of intestinal Bacteroides population through diet to improve human health. Front Microbiol. 2017;8(MAR):1–6. doi: 10.3389/fmicb.2017.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cerdó T, García-Valdés L, Altmäe S, Ruíz A, Suárez A, Campoy C. Role of microbiota function during early life on child’s neurodevelopment. Trends Food Sci Technol. 2016;57:273–288. doi: 10.1016/j.tifs.2016.08.007 [DOI] [Google Scholar]
- 136.Rosenberg E. The Prokaryotes: Firmicutes and Tenericutes. Fourth. (Delong EF, Lory S, Stackebrandt E, Thompson F, eds.). New York, NY: Springer; 2014. [Google Scholar]
- 137.Sánchez E, Donat E, Ribes-Koninckx C, Fernández-Murga ML, Sanz Y. Duodenal-mucosal bacteria associated with celiac disease in children. Appl Environ Microbiol. 2013;79(18):5472–5479. doi: 10.1128/AEM.00869-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martens EC, Lowe EC, Chiang H, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9(12):e1001221. doi: 10.1371/journal.pbio.1001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martin FPJ, Sprenger N, Yap IKS, et al. Panorganismal gut microbiome-host metabolic crosstalk. J Proteome Res. 2009;8(4):2090–2105. doi: 10.1021/pr801068x [DOI] [PubMed] [Google Scholar]
- 140.Matsuyama M, Morrison M, Cao KAL, et al. Dietary intake influences gut microbiota development of healthy Australian children from the age of one to two years. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-48658-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suzuki Y, Ikeda K, Sakuma K, et al. Association between yogurt consumption and intestinal microbiota in healthy young adults differs by host gender. Front Microbiol. 2017;8(MAY):1–11. doi: 10.3389/fmicb.2017.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oozeer R, Van Limpt K, Ludwig T, et al. Intestinal microbiology in early life: Specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr. 2013;98(2). doi: 10.3945/ajcn.112.038893 [DOI] [PubMed] [Google Scholar]
- 143.Savage JH, Lee-Sarwar KA, Sordillo JE, et al. Diet during Pregnancy and Infancy and the Infant Intestinal Microbiome. J Pediatr. 2018;203:47–54.e4. doi: 10.1016/j.jpeds.2018.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martínez I, Lattimer JM, Hubach KL, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013;7(2):269–280. doi: 10.1038/ismej.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102(8):1154–1160. doi: 10.1017/S0007114509371767 [DOI] [PubMed] [Google Scholar]
- 146.Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The controversial role of human gut lachnospiraceae. Microorganisms. 2020;8(4):1–25. doi: 10.3390/microorganisms8040573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morotomi M, Nagai F, Sakon H, Tanaka R. Dialister succinatiphilus sp. nov. and Barnesiella intestinihominis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2008;58(12):2716–2720. doi: 10.1099/ijs.0.2008/000810-0 [DOI] [PubMed] [Google Scholar]
- 148.Whisner CM, Martin BR, Nakatsu CH, et al. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J Nutr. 2016;146(7):1298–1306. doi: 10.3945/jn.115.227256 [DOI] [PubMed] [Google Scholar]
- 149.Gurwara S, Ajami NJ, Jang A, et al. Dietary nutrients involved in one-carbon metabolism and colonic mucosa-associated gut microbiome in individuals with an endoscopically normal colon. Nutrients. 2019;11(613):1–14. doi: 10.3390/nu11030613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198 [DOI] [PubMed] [Google Scholar]
- 151.Walter J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl Environ Microbiol. 2008;74(16):4985–4996. doi: 10.1128/AEM.00753-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.La-ongkham O, Nakphaichit M, Leelavatcharamas V, Keawsompong S, Nitisinprasert S. Distinct gut microbiota of healthy children from two different geographic regions of Thailand. Arch Microbiol. 2015;197:561–573. doi: 10.1007/s00203-015-1089-0 [DOI] [PubMed] [Google Scholar]
- 153.Mitsou EK, Kakali A, Antonopoulou S, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117:1645–1655. doi: 10.1017/S0007114517001593 [DOI] [PubMed] [Google Scholar]
- 154.Sakon H, Nagai F, Morotomi M, Tanaka R. Sutterella parvirubra sp. nov. and Megamonas funiformis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2008;58(4):970–975. doi: 10.1099/ijs.0.65456-0 [DOI] [PubMed] [Google Scholar]
- 155.Williams BL, Hornig M, Parekh T, Ian Lipkin W. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3(1):1–11. doi: 10.1128/mBio.00261-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gophna U, Konikoff T, Nielsen HB. Oscillospira and related bacteria – From metagenomic species to metabolic features. Environ Microbiol. 2017;19(3):835–841. doi: 10.1111/1462-2920.13658 [DOI] [PubMed] [Google Scholar]
- 157.De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. doi: 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- 158.Knight R. Dietary effects on human gut microbiome diversity. Br J Nutr. 2015;113(2015):S1–S5. doi: 10.1017/S0007114514004127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E. The Prokaryotes - Delta and Epsilon Subclasses. Deeply Rooting Bacteria; 2006. doi: 10.1007/0-387-30747-8 [DOI] [Google Scholar]
- 160.Talarico ST, Santos FE, Brandt KG, Martinez MB, Taddei CR. Anaerobic bacteria in the intestinal microbiota of Brazilian children. Clinics. 2017;72(3):154–160. doi: 10.6061/clinics/2017(03)05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Benus RFJ, Van Der Werf TS, Welling GW, et al. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br J Nutr. 2010;104(5):693–700. doi: 10.1017/S0007114510001030 [DOI] [PubMed] [Google Scholar]
- 162.Compare D, Coccoli P, Rocco A, et al. Gut-liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22(6):471–476. doi: 10.1016/j.numecd.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 163.Yaron S, Shachar D, Abramas L, et al. Effect of high β-palmitate content in infant formula on the intestinal microbiota of term infants. J Pediatr Gastroenterol Nutr. 2013;56(4):376–381. doi: 10.1097/MPG.0b013e31827e1ee2 [DOI] [PubMed] [Google Scholar]
- 164.McAllan L, Skuse P, Cotter PD, et al. Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice. PLoS One. 2014;9(2):e88904. doi: 10.1371/journal.pone.0088904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hosoi T, Ametani A, Kiuchi K, Kaminogawa S. Changes in fecal microflora induced by intubation of mice with Bacillus subtilis (natto) spores are dependent upon dietary components. Can J Microbiol. 1999;45(1):59–66. doi: 10.1139/cjm-45-1-59 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Study Characteristics and Evaluation of Included Articles
Supplemental Table 2. Microbial Relationships with Adiposity Reported among Two or More of the Included Studies
Supplemental Table 3. Complete Evaluation of Quality and Potential Bias of Included Studies