Abstract
Objective:
Addressing both the initial treatment response and subsequent paclitaxel resistance is a pivotal concern. Nano drug delivery, an emerging approach, presents a cutting-edge alternative to conventional chemotherapy.
Methods:
This investigation synthesized PEGylated nanoparticles (NPs) via the Reverse Phase Evaporation technique for liposomal NPs. Characteristics such as zeta potential, size, drug release and polydispersity index (PDI) were subjected to evaluation. Subsequently, cytotoxicity assays were conducted on gastric cancer cells (AGS) following 24 and 48-hour incubation periods.
Results:
In this study, the liposomal NPs had a zeta potential of -22 mV and a particle size of 285 nm. The Entrapment efficiency was determined as 41% that occurred physically. Additionally, the liposomal NPs demonstrated a high drug retention rate (39% remained after 72 hours), and they exhibited significantly increased cytotoxicity compared to the free drug, confirming their effectiveness as a suitable carrier for paclitaxel during both incubation periods (P<0.05).
Conclusion:
These findings collectively advocate the potential of liposomal NPs as promising contenders for effective nano-drug application in propelling chemotherapy forward.
Key Words: Paclitaxel, nano drug delivery, gastric cancer, liposomal nanoparticle
Introduction
Gastric cancer ranks among the most widespread malignancies, characterized by a substantial mortality rate, and stands as a foremost contributor to global mortality (Afaq et al., 2002). Cancer has always been a big problem in the world. Alzheimer’s disease, microbial diseases such as Mycobacterium tuberculosis and infectious diseases, especially respiratory diseases are also very important in the world (Farnia, 2014; Torkaman et al., 2014, 2015; Derakhshan et al., 2016; Allahyartorkaman et al., 2019; Mahmoudreza Moghimhanjani, 2020; Hatami N, 2022; Taghavirashidizadeh et al., 2022; Khajeh et al., 2023). Currently, the standard treatments consist of surgery and chemotherapy. Nonetheless, patients frequently encounter local recurrence and distant metastasis, contributing to a challenging issue in this disease characterized by low patient survival rates (Ebrahimifar, Hasanzadegan Roudsari, et al., 2017). Recent investigations have unveiled alterations in 300 to 500 genes within each cancer subtype. While cancers stem from intricate aberrations in cellular signaling pathways, numerous contemporary anti-cancer interventions focus on this predicament. This narrow approach yields limited efficacy, poses safety concerns, and escalates the financial burden on patients (Ebrahimifar, Hasanzadegan Roudsari, et al., 2017). Consequently, extensive research endeavors are directed toward the development and utilization of compounds and multi-targeted medications capable of affecting cancer cells. Notably, some herbal products exhibit multi-targeted activity, often exceeding the potential of synthetic drugs. Moreover, these herbal alternatives involve reduced processing costs and exhibit fewer side effects compared to synthetic counterparts (Aggeli et al., 2013). In recent decades, there has been significant attention directed toward the development of drug delivery systems aimed at controlling and managing the distribution of drugs within the body. An approach includes using nanoliposomes, which can navigate through the gaps between endothelial cells in developing tumor blood vessels and accumulate inside the tumor. By encapsulating anticancer drugs, these nanoliposomes make use of these natural properties to increase the concentration of therapeutic agents specifically within the tumor, thereby reducing their harm to healthy tissues(Zhang et al., 2011). Nanomaterials like liposomal NPs present alternative strategies for addressing these challenges and have garnered significant interest. Liposomal NPs have demonstrated their capacity to enhance the effectiveness of chemotherapy medications while concurrently mitigating their adverse effects (Trendowski, 2015). Paclitaxel, a novel anti-microtubule medication, finds widespread use in treating head and neck cancer, breast cancer, gastric cancer, non-small cell lung cancer, and various other malignancies. The US National Comprehensive Cancer Network (NCCN) guidelines advocate Taxanes, either as standalone agents or in conjunction with other drugs, as preferred first-line therapeutics in advanced gastric cancer. Liposomal-paclitaxel (L-PTX), a liposomal variant of paclitaxel, offers heightened effectiveness and diminished adverse effects in comparison to conventional paclitaxel. This formulation is considered relatively safe for individuals with advanced gastric cancer who have a poor performance status (PS) (Ajani et al., 2016). Despite previous endeavors to use various carriers for delivering Paclitaxel, there have been challenges in developing the perfect nanoliposome formulation for this drug. In this study, we fine-tuned the formulation of Paclitaxel nanoliposomes and evaluated their impact on the toxicity of gastric cancer cell lines.
Materials and Methods
Materials
Paclitaxel, Lecithin, Cholesterol and MTT were purchased from Zhechem (Hangzhou, China), Acros’ company (Geel, Belgium) and Sigma (St. Louis, USA), respectively. PEG 3350 was provided by KEC Company (Arak, Iran). The AGS gastric cancer cell line was supplied by the Cell Bank of the Pasteur Institute of Iran, located in Tehran, Iran. All utilized materials were of analytical-grade quality.
Preparation of drug-loaded nanoparticles
The method of choice to fabricate pegylated liposomal NPs was the reverse phase evaporation method. In brief, a mixture of cholesterol, lecithin, paclitaxel and PEG 3350 in a molar ratio of 8:12:1:1, respectively, was dissolved in 96% ethanol. The solvent was subsequently removed by subjecting it to vacuum conditions (45°C, 90 rpm) using a rotary evaporator, leading to the formation of a film inside the round-bottom flask. After adding phosphate buffered saline (PBS; pH 7.2), vesicles loaded with the drug were generated. The mixture was agitated for a duration of 7 hours at a temperature of 45 °C. The final concentrations of paclitaxel, lecithin, PEG 3350 and cholesterol were 1.2 mM, 14 mM, 1.3 mM, and 10 mM, respectively. For enhanced size uniformity, the NPs underwent sonication using a bath sonicator device (50 W, Bandelin Sonopuls HD 2070, Germany) for 6 minutes and homogenization (M-110P lab homogenizer; Trident Equipment’s Pvt. Ltd., India) for 10 minutes at 21,420 g. The same procedure was applied to generate blank NPs. Furthermore, standard drug solutions were formulated using identical concentrations of the drug in PBS.
Nanoparticle characterization
The zeta potential and size of the NPs were determined using a Zetasizer instrument (Nano ZS3600, Malvern Instruments, and UK). The nanoparticle suspension was diluted to the appropriate concentration, and the absorbance was assessed at 630 nm. Afterward, the size and zeta potential of suspension was evaluated by Zetasizer instrument.
Entrapment efficiency
The amount of drug encapsulated within the NPs was assessed using a spectrophotometric method. To do this, the nanoparticle pellet was isolated by centrifugation (49,087 g, 30 minutes, and 4°C). The drug concentration in the supernatant was determined by employing a standard curve at 230 nm, and the entrapment efficiency was computed using the formula below:
Entrapment efficiency (%) = Initial concentration of Drug−Concentration of drug in supernatant/ Initial concentration of drug ×100.
Drug release study
The release of the drug from the NPs was investigated using the dialysis bag technique. In brief, a nanoparticle precipitate was obtained and subsequently re-suspended in fresh PBS. This suspension, along with a standard drug solution of equivalent concentration dissolved in 5 mL PBS (0.3 mg/mL), was introduced into separate dialysis bags (with a cut-off of 10,000 Da; Sigma). These bags were then immersed in a PBS solution and subjected to stirring (200 rpm, 100 mL, room temperature). At specific time points, 2 mL of buffer was taken from each bag and replaced with an equivalent amount of fresh buffer. The drug concentration released into the PBS was measured using a spectrophotometric method.
In vitro cytotoxicity
The gastric cancer cell line (AGS) was cultivated at a density of 104 cells in each well of 96-well plates. The cell culture medium consisted of RPMI-1640, which was supplemented with 10% fetal bovine serum (FBS), along with penicillin/streptomycin antibiotics at concentrations of 0.1 mg/mL and 0.06 mg/mL, respectively. The cultures were maintained in a humidified atmosphere containing 5% CO2. Following a 24-hour incubation, the cell culture medium was removed, and new medium containing either paclitaxel-encapsulated NPs or regular paclitaxel at the same concentrations was added to the wells. After incubating for 24 and 48 hours, the cell culture medium was substituted with an MTT solution (0.5 mg/mL) and left to incubate for 3 hours. The formazan crystals obtained were dissolved in pure isopropanol (100%), and their absorbance was quantified at 540 nm using a microplate scanning spectrophotometer (ELISA reader; Organon Teknika, the Netherlands) (Amiri et al., 2016). Cytotoxicity was assessed using the following formula:
Cytotoxicity (%) =1− mean absorbance of drug-treated cells /mean absorbance of negative control ×100,
Viability (%) =100− Cytotoxicity (%).
Additionally, the program Pharm was employed to calculate the half-maximal inhibitory concentration (IC50).
Statistical analysis
The study data were analyzed with GraphPad Prism version 9, and significance was determined for p-values < 0.05.
Results
Nanoparticles characterization
The size, size distribution, and zeta potential of nanodrug were determined to be 285 nm, 0.44, and -22 mV, respectively. Furthermore, the size and distribution of NPs were reduced following the application of sonication and homogenization procedures (Table 1).
Table 1.
How Sonication and Homogenization Processes Affect Nanoparticle Size and Size Distribution
| Compounds | Size (nm) | Size distribution |
|---|---|---|
| Nanodrug before sonication and homogenization | 440 | 0.64 |
| Nanodrug after sonication and homogenization | 285 | 0.44 |
Entrapment efficiency
According to the loading efficiency results, NPs were linked to 41% of the primary drug that was used. In other words, the percentage of entrapped drug was 41%.
Drug release study
The findings from the drug release study demonstrated a consistent and prolonged release of paclitaxel from the NPs (Table 2). Within the initial hour of the investigation, there was an initial rapid release accounting for 15% of the cumulative discharge. However, by the 72-hour mark, merely 39% of the encapsulated drug had been released (Figure 1). In contrast, nearly the entirety of the standard drug was released from the liposomes within the initial 32 hours.
Table 2.
The Data Illustrates the Cumulative Release of Paclitaxel in Phosphate-Buffered Saline, Presented as a Percentage, for Both the Encapsulated and Standard Drug Formulations
| Cumulative paclitaxel release (%) | ||
|---|---|---|
| Time (h) | Nanodrug | Drug |
| 1 | 7±0.2 | 20±1.1 |
| 3 | 8±0.7 | 29±1.5 |
| 8 | 9.5±0.6 | 38±1.7 |
| 12 | 10±0.9 | 49±2.6 |
| 24 | 15.1±0.4 | 75±3.3 |
| 32 | 18.4±1.1 | 97±4.1 |
| 48 | 25±1.4 | - |
| 60 | 31±1.9 | - |
| 72 | 39±2 | - |
Figure 1.
Progressive Release of Paclitaxel in Both Its Standard and Encapsulated Formulations Over Time. The findings are presented as mean ± 5% values
In vitro cytotoxicity
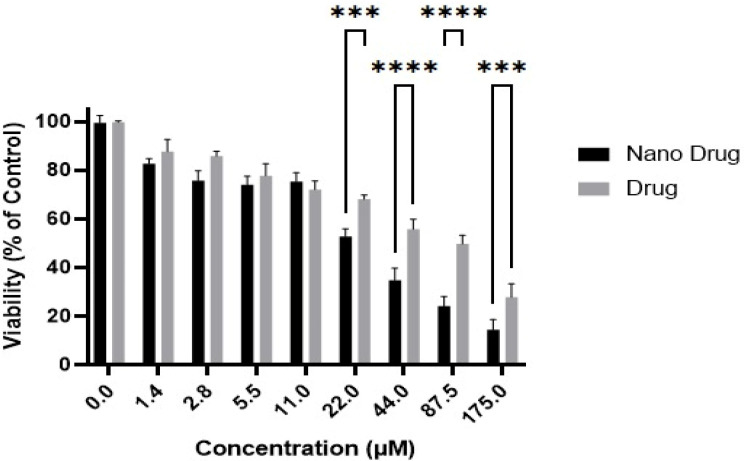
Initially, it was determined that NPs of this kind possess no cytotoxicity. The outcomes indicated notable variations in the cytotoxicity of both the nano drug and the free drug over the course of 24 hours (Figure 2) and 48 hours (Figure 3). Specifically, the IC50 values for the nano drug and the free drug were calculated as 30.7±2.8 µM and 59.0±4.7 µM, respectively, following a 24-hour incubation period. Similarly, during a 48-hour incubation, the IC50 values were estimated as 22.1±1.1 µM for the nano drug and 44.9±3.9 µM for the free drug. Additionally, it is observed that the cytotoxicity of the nano drug displayed a greater increase in response to escalating concentrations compared to the standard drug.
Figure 2.
Cell Viability of the AGS Gastric Cancer Cell Line was Assessed in Response to Different Drug Concentrations, Including Paclitaxel and Paclitaxel-Loaded NPs, Following a 24-hour Incubation Period (Graph represents mean ± SD; n= 3 independent experiments, ****p < 0.0001, ***p = 0.0002, **p = 0.0021, *p =0.0332, two-way ANOVA with Šidák test).
Figure 3.
Viability of the AGS Gastric Cancer Cell Line was Evaluated against Varying Drug Concentrations, Encompassing of Both Paclitaxel and Paclitaxel-Loaded NPs, Following a 48-hour Incubation Period (Graph represents mean ± SD; n= 3 independent experiments, ****p < 0.0001, ***p = 0.0002, **p = 0.0021, *p =0.0332, two-way ANOVA with Šidák test).
Discussion
The reverse phase evaporation method was identified as an appropriate technique for fabricating Paclitaxel-loaded liposomal NPs. Results from the Zetasizer instrument confirmed the particle formation at a nanoscale level, with the Zeta potential showing a direct correlation with the stability of the nanoparticle suspension (Poy et al., 2016). The observed Zeta potential values validated the favorable stability of the particles. Additionally, the processes of sonication and homogenization not only resulted in a reduction of particle size but also enhanced homogeneity by reducing size variations. Various formulations of liposomal NPs loaded with Anti-cancer have been synthesized, and some are currently being evaluated in clinical trials (Mohammadinezhad et al., 2023). Among these formulations, Lipoplatin, with a size of 285 nm, stands out as a particularly promising. Our formulation, in particular, leverages cost-effective materials. The effectiveness of liposomal NPs as drug carriers is closely tied to the physicochemical attributes of the liposomal membrane, the inherent characteristics of the components used, dimensions, surface charge, and the structural arrangement of lipids (Ebrahimifar, Nili-Ahmadabadi, et al., 2017). Furthermore, the correct choice of liposome preparation method is contingent upon the physicochemical attributes of the substances to be enclosed and the constituents employed in the liposomal formulation ( Mohammadinezhad et al., 2023). In the current investigation, the fabrication of pegylated liposomal NPs was achieved seamlessly through a singular process, encompassing both sonication and homogenization. The outcomes of this study validated the employed protocol, the selected liposomal components, and the molar ratios of the utilized materials, affirming their appropriateness for nanoparticle preparation. An observed entrapment efficiency of 41% was deemed satisfactory. In contrast, the study conducted by Xiao et al., (2004) reported a loading efficiency of 80%. It is worth noting that our research capitalized on more cost-effective materials and simpler techniques. The release of drugs from nanoparticles significantly influences the biological impact of carriers (Poy et al., 2016). In our investigation, we observed a sustained Paclitaxel release profile. The release pattern began with an initial burst of release within the first hour of the study, likely due to the release of drug molecules that were adsorbed on the surface of the nanoparticles. Furthermore, our study confirmed the nanoparticle’s effective capacity for drug retention. The gradual and controlled release of the drug from the NPs could be linked to the inclusion of PEG in the formulation. This phenomenon is also reported in the study by EbrahimiFar et al., (2017). Additionally, PEG plays a role in enhancing the stability of NPs and increasing the likelihood of precise drug delivery to tumor sites, consequently augmenting drug efficacy (Poy et al., 2016). The cytotoxicity results for Paclitaxel NPs demonstrated that the encapsulated form exhibits significantly greater potency when compared to the standard drug. This augmentation was statistically significant. Other studies have also reported an enhancement in anti-cancer effectiveness through liposomal NPs (Roudsari et al., 2016; Mohammadinezhad et al., 2023). In a study by Mohammadinezhad et al., (2023) they formulated a cationic liposome incorporating cisplatin and assessed its impact on cytotoxicity in the breast cancer cell lines. Their findings revealed that the liposomal formulation heightened cisplatin’s cytotoxic effects by 24% when contrasted with the standard drug. Similarly, Roudsari et al., (2016) discovered that the cytotoxic impact of lipoplatin on the carboplatin-resistant lung cancer cell line surpassed that of the standard drug. In our present study, we observed a 2-fold increase in cytotoxicity in comparison to the standard drug. This could potentially be attributed to the sustained release of the drug from the NPs. This indicates that this formulation holds promise as a potent alternative for future chemotherapeutic approaches in the management of Gastric cancer.
Author Contribution Statement
Zahra Abedi Cham Heidari, and Elham Mortazavi Mamaghani performed the experimental tests. Cell culture was carried out by Mohammadreza Allahyartorkaman. Ali Hheidari performed the MTT assay. Elaheh Mozaffari performed the statistical analysis. Elham Saberian and Parizad Ghanbarikondori wrote the manuscript draft.
Acknowledgements
None.
Data availability
Not applicable as we used information from previously published articles.
Approved by any scientific Body
Not applicable as the manuscript is not a part of any student thesis or study.
Ethical issue and approval
Not applicable as we used information from previously published articles.
Consent for publication
All authors have given consent for publication.
Conflict of interest
The authors declare no potential conflict of interest.
References
- Afaq F. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Bioscience. 2002;7:d784–92. doi: 10.2741/afaq. [DOI] [PubMed] [Google Scholar]
- Aggeli I-K. Curcumin acts as a pro-oxidant inducing apoptosis via JNKs in the isolated perfused Rana ridibunda heart. J Exp Zoology. 2013;319:328–39. doi: 10.1002/jez.1797. [DOI] [PubMed] [Google Scholar]
- Ajani JA. Gastric Cancer, Version 3 2016, NCCN Clinical Practice Guidelines in Oncology. J Nat Comprehensive Cancer Networ. 2016;14:1286–1312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- Allahyartorkaman M. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: A multicenter surveillance. Sci Rep. 2019 doi: 10.1038/s41598-019-55112-y. doi: 10.1038/s41598-019-55112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri B. Preparation, characterization and cytotoxicity of silibinin- containing nanoniosomes in T47D human breast carcinoma cells. Asian Pac J Cancer Prev. 2016;2016:3833–6. [PubMed] [Google Scholar]
- Ebrahimifar M, Hasanzadegan Roudsari M. Enhancing Effects of Curcumin on Cytotoxicity of Paclitaxel, Methotrexate and Vincristine in Gastric Cancer Cells. Asian Pac J Cancer Prev. 2017;18:65–8. doi: 10.22034/APJCP.2017.18.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimifar M, Nili-Ahmadabadi A. Preparation, Characterization and Cytotoxic Effects of Pegylated Nanoliposomal Containing Carboplatin on Ovarian Cancer Cell Lines. Indian J Clini Biochem. 2017;32:230–4. doi: 10.1007/s12291-016-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnia P. Estimation of Recent Transmission of Mycobacterium Tuberculosis Strains among Iranian and Afghan Immigrants: A Cluster-Based Study. J Clin Diagn Res. 2014 doi: 10.7860/JCDR/2014/8886.4864. doi: 10.7860/jcdr/2014/8886.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatami N, RS K, NZ M SS. The First Case of the Subretinal Abscess After Sars-CoV2 Infection. Acta Med Iran. 2022;60:132–4. [Google Scholar]
- Khajeh S. D-allose: Molecular Pathways and Therapeutic Capacity in Cancer. Curr Mol Pharmacol. 2023;16:801–10. doi: 10.2174/1874467216666221227105011. [DOI] [PubMed] [Google Scholar]
- Mohammadinezhad F. Preparation, Characterization and Cytotoxic Studies of Cisplatin-containing Nanoliposomes on Breast Cancer Cell Lines. Asian Pac J Cancer Biol. 2023;8:155–9. [Google Scholar]
- Poy D. Preparation, characterization, and cytotoxic effects of liposomal nanoparticles containing cisplatin: an in vitro study. Chem Biol Drug Design Engl. 2016;88:568–73. doi: 10.1111/cbdd.12786. [DOI] [PubMed] [Google Scholar]
- Roudsari MH. Investigation of Characteristics and Behavior of Loaded Carboplatin on the, Liposomes Nanoparticles, on the Lung and Ovarian Cancer: An In-Vitro Evaluation. Asian Pac J Cancer Biol. 2016;1:9–13. [Google Scholar]
- Torkaman MRA. Estimation of Recent Transmission of Mycobacterium Tuberculosis Strains among Iranian and Afghan Immigrants: A Cluster-Based Study. J Clin Diagn Res. 2014;8:DC05–8. doi: 10.7860/JCDR/2014/8886.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkaman MRA. Comparison of loop-mediated isothermal amplification and real-time PCR for detecting Bordetella pertussis. J Med Microbiol Engl. 2015:463–5. doi: 10.1099/jmm.0.000021. [DOI] [PubMed] [Google Scholar]
- Trendowski M. Using cytochalasins to improve current chemotherapeutic approaches. Anti-Cancer Agents Med Chem. 2015;15:327–35. doi: 10.2174/1871520614666141016164335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C. Sustained release of cisplatin from multivesicular liposomes: potentiation of antitumor efficacy against S180 murine carcinoma. J Pharm Sci United States. 2004;93:1718–24. doi: 10.1002/jps.20086. [DOI] [PubMed] [Google Scholar]
- Zhang X. In vitro and in vivo study of a nanoliposomal cisplatin as a radiosensitizer. Inte J Nanomedicine. 2011;2011:437–44. doi: 10.2147/IJN.S15997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable as we used information from previously published articles.