Abstract
GATA family proteins Gln3p, Gat1p, Dal80p, and Deh1p mediate the regulation of nitrogen catabolite repression (NCR)-sensitive gene expression in Saccharomyces cerevisiae. Thus far, Gln3p, Dal80p, and Deh1p have been shown to bind to GATA sequences in NCR-sensitive promoters, in some cases to exactly the same GATA sequences. A minimal Gln3p binding site consists of a single GATA sequence, whereas a Dal80p binding site consists of two GATA sequences in specific orientation, 15 to 35 bp apart, suggesting that Dal80p may bind to DNA as a dimer. Additionally, both Dal80p and Deh1p are predicted to contain a leucine zipper motif near their C termini. Therefore, we tested whether they could form homo- and/or heterodimers in two-hybrid assays. We show that Dal80p-Dal80p, Dal80p-Dal80pLZ (leucine zipper), Dal80pLZ-Dal80pLZ, Dal80p-Deh1pLZ, Dal80pLZ-Deh1pLZ, and Deh1pLZ-Deh1pLZ complexes can form. Dal80p-Dal80p and Dal80pLZ-Dal80pLZ complexes yield 5- to 10-fold stronger signals than the other possible dimers. If Dal80p and Deh1p bind to DNA only after dimerization, then the difference in ability to form complexes could significantly affect their affinity for binding DNA and thus the degree of regulation exerted by each of the two factors.
The control network for nitrogen catabolic enzymes and transport systems of Saccharomyces cerevisiae is regulated in response to the global nitrogen supply in general and to pathway-specific nitrogen sources in particular. The first and dominant mode of regulation is a global transcriptional response to the level of available nitrogen, i.e., nitrogen catabolite repression (NCR) (10). NCR is mediated by the dodecanucleotide element UASNTR, which was first discovered upstream of DAL5 and contains a GATAA sequence at its core (5, 13, 30, 32). Additionally, four transcription factors, two acting positively (Gln3p and Gat1p) (4, 7, 8, 12, 18, 19, 24–26, 37) and two acting negatively (Dal80p and Deh1/Gzf3p) (1, 6, 8, 14–17, 19, 33, 41, 42) are responsible for NCR-sensitive transcription (11). A broad survey to determine the uniformity of Dal80p and Gln3p regulation across the spectrum of nitrogen catabolic genes concluded that these proteins function in opposition to one another in the regulation of most, but not all, NCR-sensitive genes (19). This model has now been extended to include the transcription factors Gat1p and Deh1p (1, 8, 9, 11, 33, 34, 36–38). All four proteins are members of the GATA-binding super family of DNA binding proteins, and three have already been shown to bind to GATA sequences (2, 27). Gln3p and Dal80p not only bind to GATA sequences but, in some cases, they bind to the same GATA sequences (17). This suggested that Dal80p and Gln3p antagonize one another’s operation by competing for the same GATA binding sites upstream of the genes they regulate, i.e., Dal80p behaves like a competitive transcriptional repressor (6, 15, 19); this hypothesis has received an increasing amount of experimental support (1, 9, 34, 36, 42). The concept that Dal80p acts as a competitive repressor of Gln3p transcriptional activation leaves open, however, the question of whether or not it is capable of transcriptional repression by mechanisms similar to those of other repressors such as Sin3p, the Tup1p-Ssn6p complex, or Mot1p (39).
Dal80p does not regulate all of the genes whose transcription is Gln3p dependent. For example, DAL5 and GLN1 expression is highly Gln3p dependent but is not highly regulated by Dal80p (12, 19, 24–26, 31, 34), while DAL3 expression is both Gln3p dependent and Dal80p regulated (16, 17, 19). Explanation of these observations was provided by studies of the Gln3p and Dal80p DNA binding sites. By using electrophoretic mobility shift assays (EMSAs), Gln3p was shown to bind to a single DAL3, GLN1, PUT1, UGA4, CAR1, or GDH2 GATA sequence (4, 29, 35, 40). A Dal80p binding site, on the other hand, more restrictively consists of two GATA sequences, oriented tail-to-tail or head-to-tail, 15 to 35 bp apart (16).
Dal80p is a relatively small protein (269 amino acids [aa]) that possesses two structural features associated with some eucaryal transcription factors: a GATA-type Zn finger motif (residues 31 to 76) and a leucine zipper-coiled coil (residues 229 to 257) (14, 15). Deh1p (Dal Eighty Homologue protein) shares these two features with Dal80p but is twice as large (8, 9, 33, 36). Dal80p, Gln3p, and Gat1p, in contrast, share little homology beyond the highly conserved GATA-type Zn finger motif (14, 15, 18, 24). In particular, Gln3p and Gat1p lack the C-terminal leucine zipper motif required for the normal operation of Dal80p.
There is ample evidence demonstrating that leucine zipper coiled coils can participate in the formation of homo- and heterodimeric or higher-order protein complexes (22, 28). Therefore, we determined whether the leucine zipper motifs of Dal80p and Deh1p could function in this way. Here, we demonstrate that both Dal80p and Deh1p are capable of homodimer formation as measured by the two-hybrid assay and that their leucine zipper motifs are capable of forming heterodimeric complexes as well. The strength of the two-hybrid signals obtained raise the possibility that the Dal80p homodimer may be more stable than a similar Deh1p homodimer or the Dal80p-Deh1p heterodimer.
MATERIALS AND METHODS
The yeast strains used in this work were EGY48 (MATα 3lexAop::leu2 ura3 trp1 his3) and InvScl (MATα, his3-1 leu2 trp1-289 ura3-52). The latter strain was obtained from Invitrogen.
Construction of the full-length and truncated LexA- and B42-Dal80p fusions in plasmids pEG202 and pJG4-5.
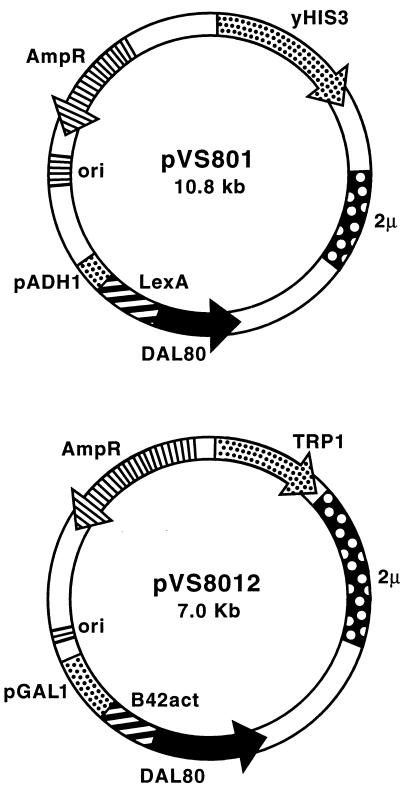
A full-length LexA-Dal80p fusion plasmid was constructed by subcloning the 0.8-kb NdeI-BamHI fragment from plasmid pTSC416 (16) into plasmid pEG202 to yield plasmid pVS801 (Fig. 1).In order to create an activation domain-tagged Dal80p, the 0.8-kb EcoRI-XhoI fragment of plasmid pVS801, containing the full-length DAL80 gene, was subcloned into EcoRI- and XhoI-digested plasmid pJG4-5 yielding plasmid pVS8012. We also constructed pEG202- and pJG4-5-based plasmids containing LexAp fusions to the N-terminal (containing the Zn finger motif), the C-terminal (containing the leucine zipper motif), or the middle portions of Dal80p with PCR primers specifying amplification of DAL80 gene fragments (aa 1 to 135, 132 to 269, and 90 to 210, respectively). Recognition sites for EcoRI and XhoI were added to the upper and lower PCR primer sequences, respectively, to allow directional cloning of the amplification products; plasmid pTSC317 (16) was used as template. Following digestion with EcoRI and XhoI, each PCR product was ligated into both plasmids pEG202 and pJG4-5 to yield plasmids pVS80ZE (LexA-Dal80 ZnFPCR), pVS80ME (LexA-Dal80 MidPCR), pVS80CE (LexA-Dal80 CTermPCR), pVS80ZJ (B42-Dal80 ZnPCR), pVS80MJ (B42-Dal80 MidPCR) and pVS80CJ (B42-Dal80 CTermPCR).
FIG. 1.
Essential features of the LexA and B42 fusion-expressing plasmids used in the activation, repression (transcriptional interference), and two-hybrid assays. GAL1 and ADH1, galactose-inducible and -constitutive promoters, respectively, were used to mediate expression of the test fusion proteins in yeast. TRP1 and URA3 were used to complement trp1 and ura3 mutations of the transformation recipients. LexA and B42 designate the coding sequences for the expression of the LexA (DNA binding) and B42 (acidic transcriptional activator) domains.
Cloning of the Dal80p- and Deh1p-derived leucine zipper motifs, Deh1p- and Gln3p-derived Zn finger motifs, and Put3p-derived Zn-binding cluster into plasmids pEG202 and pJG4-5.
Fragments of the DAL80 and DEH1 genes, encoding their respective leucine zipper motifs, were amplified from yeast genomic DNA (strain TCY1) by using a high-fidelity PCR protocol (Strategene PFU DNA polymerase). The amplified regions were as follows: Dal80p leucine zipper, aa 216 to 269; Deh1p leucine zipper, aa 469 to 551; Deh1p Zn finger region, aa 99 to 197; Gln3p Zn finger motif, aa 293 to 366; and Put3p Zn-binding cluster, aa 2 to 72. PCR products were purified, and following digestion with EcoRI and XhoI, each was ligated into plasmids pEG202 and pJG4-5, which had been digested with EcoRI and XhoI. Dal80p-derived leucine zipper fusions in the pEG202 and pJG4-5 vectors were designated pVS80ZIPE and pVS80ZIPJ, respectively; similarly, the Deh1p-derived leucine zipper fusions were designated pVS1ZIPE and pVS1ZIPJ. Fusions of the Deh1p- and Gln3p-derived Zn fingers were designated pVS1ZNE and pVS1ZNJ and pVS3ZNE and pVS3ZNJ, respectively. The Put3p derivatives were designated pVS3CYSE and pVS3CYSJ. The sequences of all recombinant clones used in this study were verified by dideoxy sequence analysis before they were used (3).
Transcriptional activation, transcription interference, and two-hybrid assay conditions.
Yeast strains EGY48 and InvScl were transformed with reporter plasmid pSH18-34 or pJK101 for transcriptional activation (two-hybrid) and repression (transcription interference) assays, respectively. For activation and repression assays they were additionally transformed with pEG202-based plasmids carrying the desired LexA fusion gene or gene fragment. Two-hybrid assays were carried out by using yeast transformed with LexA gene (pEG202-based) and B42 gene (pJG4-5-based) fusion plasmids. For each assay single colony transformants were inoculated into individual 125-ml flasks containing 25 ml of complete minimal drop-out (CM) medium, supplemented for auxotrophies, containing 0.5% ammonium sulfate and 2% sugars (glucose or raffinose:galactose [2:1]) as nitrogen and carbon sources, respectively (unless indicated otherwise). Cultures were grown to cell densities of A600 = 0.5 to 0.8. Culture samples (10 ml) to which 200 μl of 1% cycloheximide was added were harvested by centrifugation, resuspended in 1 ml of Z buffer (40 mM NAH2PO4, 30 mM Na2HPO4, 10 mM Kcl, 1 mM MgSO4, 50 mM β-mercaptoethanol [pH 7.0]), and permeabilized by vortexing (20 s) in the presence of 50 μl each of 0.1% sodium dodecyl sulfate and chloroform. The permeabilized cell suspension was diluted 10-fold with Z buffer and equilibrated at 30°C. The β-galactosidase reaction was initiated by addition of 0.2 ml of o-nitrophenyl-β-galactopyranoside (4 mg/ml in H2O) and terminated by addition of 0.5 ml of 1 M Na2CO3. Product quantification and enzyme unit conversions were performed as described by Miller (23).
RESULTS
Is Dal80p able to repress transcription supported by a heterologous UAS?
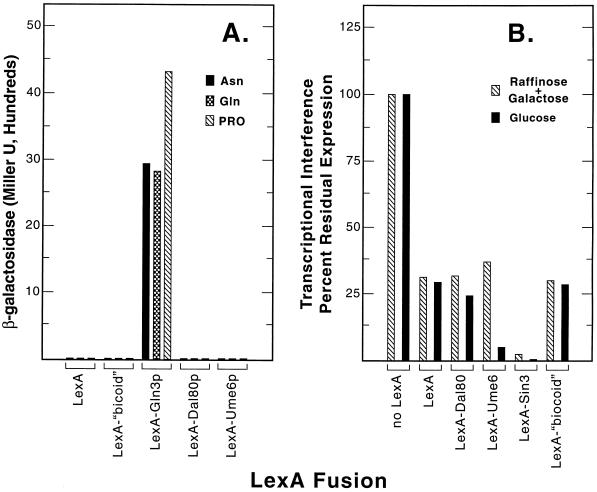
Negative transcriptional regulation by Dal80p could conceptually result from its operation as (i) an active repressor, such as occurs with Sin3p (43), (ii) a competitive repressor by inhibiting Gln3p and Gat1p binding to DNA (11, 19), or (iii) a combination of both modes. As a first step toward evaluating the transcriptional repression potential of Dal80p, we expressed a full-length LexA-Dal80p protein (Fig. 1) and several other LexA fusion proteins in yeast and assayed their abilities to support transcriptional activation (13). Consistent with their roles as negative transcriptional regulators, neither Sin3p, Ume6p, nor Dal80p possessed transactivation potential when targeted to a UAS-less promoter regardless of the nitrogen source provided (Fig. 2A). The results were no different than those observed with LexAp alone or LexAp fused to a transcriptionally “neutral” fragment of bicoid protein (20, 21). These data contrast sharply with those yielded when Gln3p was used in this assay as a positive control (Fig. 2A); strong transcriptional activation was observed.
FIG. 2.
Activation and repression (transcriptional interference) assays of the yeast nitrogen regulators. (A) Transactivation potentials of LexA alone (plasmid pEG202) and the test fusion proteins LexA-bicoid (plasmid pRFHM1), LexA-Gln3 (plasmid pVS32), LexA-Dal80 (plasmid pVS801), and LexA-Ume6 (plasmid pBS62) in strain InvSc1 transformed with plasmid pSH18-34 (20, 21). Cells were provided with 0.1% of one of the following as the sole nitrogen source: asparagine (Asn), glutamine (Gln), or proline (Pro). (B) Repression (transcriptional interference) assay of Dal80p and other yeast negative regulators at different levels of expression of the heterologous reporter gene. The plasmids used were as described above for panel A with the addition of plasmid pLEXASIN3 for the production of LexA-Sin3p. Plasmids were assayed in strain EGY48 transformed with plasmid pJK101 (20, 21). Cells were grown in the presence of an inducing (1.5% raffinose n–0.5% galactose) or repressing (2% glucose) carbon source.
A competitive repressor, which acts exclusively by antagonizing recruitment of the transcriptional activator(s) Gln3p and/or Gat1p through competitive binding, would not be expected to repress transcriptional activation mediated by a heterologous transcriptional activator. To test this expectation, Dal80p, Ume6p, and Sin3p fused to LexAp were recruited to a LexA operator site situated between the heterologous UASGAL1 and the TATA sequence of a transcriptional interference assay system. LexA-Dal80p was incapable of repressing activation directed by UASGAL1 (Fig. 2B). Residual expression of the lacZ reporter gene in the presence of LexA-Dal80p was no less than that typically observed as a result of the steric hindrance which occurs when a neutral protein (LexA alone or LexA-“bicoid” fusion) is positioned between the TATA sequence and the activator-binding site upstream of it (Fig. 2B) (see Materials and Methods). LexA-Dal80p behaved the same way whether transcription, measured by the assay, occurred at a high level, as when cells were grown in minimal galactose plus raffinose medium (Fig. 2B, cross-hatched bars), or a low one, i.e., when glucose was provided in place of galactose (Fig. 2B, filled bars). These experiments demonstrate, however, that even though Dal80p does not actively repress heterologous transcriptional activation, LexA-Dal80p is produced and binds to the LexA sites upstream of the reporter gene.
A LexA-Sin3 fusion protein behaved quite differently when subjected to the transcription interference assay. As expected (43), it strongly reduced heterologous GAL1-lacZ expression irrespective of whether the cells were provided with galactose or glucose as the carbon source (Fig. 2B). The outcome with Ume6p was somewhat unexpected; this negative regulator strongly interfered with reporter gene expression only when glucose was used as carbon source, i.e., a condition of low-level transcription (Fig. 2B).
Is Dal80p capable of dimerization?
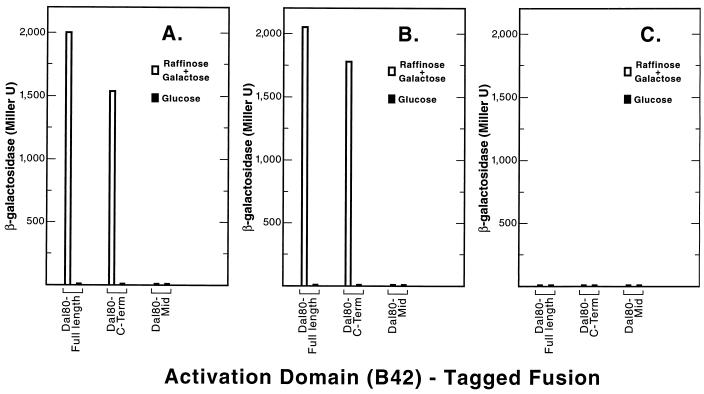
The presence of a C-terminal leucine zipper motif (essential for Dal80p function) (14) and the requirement of two specifically oriented and spaced GATAA sequences for Dal80p binding to DNA raise the possibility that the Dal80p might dimerize (15–17). The lack of measurable activation or repression potential for Dal80p (Fig. 2) made it possible to use a two-hybrid assay (21) to assess this possibility. Plasmids expressing full-length Dal80p (aa 1 to 269) fused to LexA (the “bait”) or the activation domain B42 (the “prey”) were used in the two-hybrid assay. Full-length Dal80p plasmid supported approximately 2,000 U of β-galactosidase production, a result indicative of a strong Dal80p-Dal80p interaction (Fig. 3A, Dal80-Full length, open bar). Furthermore, this high level of β-galactosidase production depended upon expression of full-length DAL80-B42 (prey) from the GAL promoter to which it was fused, i.e., β-galactosidase production was not observed when glucose was used as the carbon source (Fig. 3A, filled bars).
FIG. 3.
Two-hybrid assays of Dal80-derived B42-tagged fusion proteins (prey). The B42-tagged fusions assayed were full-length Dal80p (plasmid pVS8012) and protein fragments Dal80Mid (plasmid pVS80MJ) and Dal80 C-Term (plasmid pVS80CJ) (aa 90 to 210 and 132 to 269, respectively). These plasmids were individually transformed into strain EGY48 with reporter plasmid pSH18-34 (20, 21) and were assayed in cells provided with 0.5% ammonium sulfate as the sole nitrogen source and containing the bait LexA fusion protein full-length Dal80p (plasmid pVS801) (A), Dal80p fragment (plasmid pVS80CE, (B) or Dal80p fragment aa 90 to 210 (plasmid pVS80MEO (C).
To determine the portion of the Dal80p required for interaction, the molecule was divided into three segments: the N-terminal GATA-zinc finger portion (aa 1 to 735), the center portion (Mid) (aa 90 to 210), and the C-terminal leucine zipper portion (aa 132 to 269), respectively. The N-terminal zinc finger region of the protein will be discussed later. When the full-length Dal80p was used as bait and the C-terminal portion of Dal80p was used as prey, reporter gene expression was 75% of that observed when both bait and prey contained full-length Dal80p (Fig. 3A, Dal80-C-Term, open bars). Note that this activity was also dependent upon expression of the prey (Fig. 3A, filled bars). On the other hand, when the center portion of Dal80p was used as prey, no signal was observed (Fig. 3A, Dal80-Mid). When the C-terminal portion of Dal80p rather than full-length protein was used as bait, results nearly identical to those in Fig. 3A were observed (Fig. 3B). In contrast, when the center portion of Dal80p was used as bait no reporter gene expression was observed regardless of the nature of the prey plasmid (Fig. 3C). These data suggest that the C-terminal portion of Dal80p molecules can interact with one another.
Is the Dal80p leucine zipper coiled coil capable of dimerization?
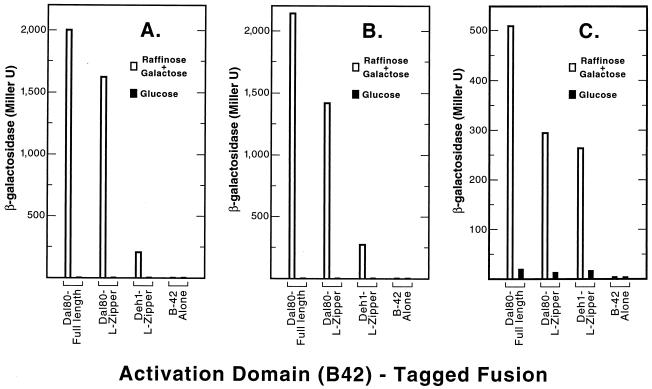
Since a leucine zipper coiled-coil motif is predicted to occur in the C-terminal portion of Dal80p, we assessed whether it alone was sufficient to interact with full-length Dal80p. LexA-full-length Dal80p was used as bait and either full-length Dal80-B42p or the Dal80p leucine zipper motif (aa 216 to 269)-B42p was used as prey in a two-hybrid assay. The leucine zipper motif supported a level of β-galactosidase production which was similar to that observed for the C-terminal portion of the protein (compare Fig. 4A and 3A). We then repeated the experiment using only the leucine zipper motif (aa 216 to 269) as bait. It was able to interact with a full-length Dal80p prey (Fig. 4B). Further, the leucine zipper motif was able to interact with itself, although the β-galactosidase levels were slightly lower than when full-length protein was used (Fig. 4B, Dal80-L-Zipper). Together these data suggest that the leucine zipper coiled coils of two Dal80p molecules are able to mediate formation of a dimer. This interaction appears to be quite strong if the high levels of reporter gene expression are taken as an indicator.
FIG. 4.
Two-hybrid assays of Dal80p- and Deh1p-derived LZ fusions. The B42-tagged fusions (prey) assayed were full-length Dal80p (plasmid pVS8012), Dal80p-derived LZ (plasmid pVS80ZIPJ), and Deh1p-derived LZ (plasmid pVS1ZIPJ). The LexA fusion proteins (baits) were full-length Dal80p (plasmid pVS801) (A), Dal80p-derived LZ (plasmid pVS80ZIPE) (B), and Deh1p-derived LZ (plasmid pVS1ZIPE) (C). The control plasmid used for the expression of B42 alone was pJG4-5. The transformations and assays were as described in the legend for in Fig. 3.
Can Dal80p and Deh1p interact?
We mentioned above that Deh1p shares significant homology with Dal80p (8, 9, 33, 36). This homology and the Dal80p-Dal80p interaction data prompted us to inquire whether Dal80p was able to interact with the Deh1p leucine zipper motif. Plasmids containing full-length Dal80p or the Dal80p leucine zipper region were used as baits and were assayed with a plasmid containing the Deh1p leucine zipper (aa 469 to 551) as prey. The leucine zipper-containing fragment of Deh1p was able to interact with either full-length Dal80p or the Dal80p leucine zipper domain (Fig. 4A and B). The β-galactosidase activity observed for this interaction, however, was six- to eightfold less than that observed for Dal80p-Dal80p interactions.
The potential weak interaction observed between the Dal80p leucine zipper and that of Deh1p might derive from their somewhat different sequences resulting in a less than optimum “fit” of the helices. To test this possibility, a Deh1p leucine zipper-containing fragment was used in place of the Dal80p fragment as bait. The level of β-galactosidase production observed with full-length Dal80p as prey (Fig. 4C) was within two- to threefold of that observed earlier (Fig. 4A). More important, β-galactosidase production derived from interactions of the Deh1p leucine zipper with the Dal80p leucine zipper was nearly identical to that observed when a Deh1p leucine zipper-Deh1p leucine zipper interaction was assayed (Fig. 4C).
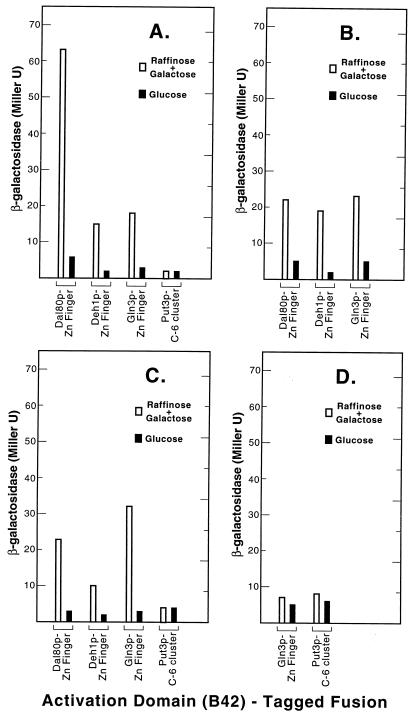
Do the yeast GATA family protein zinc fingers interact?
Reports of GATA-type Zn finger motifs mediating protein-protein interactions in vertebrates prompted us to investigate potential interactions between the yeast metal-binding clusters. LexA- and B42-tagged fusions of the Dal80p-, Deh1p-, and Gln3p-derived GATA-type Zn fingers as well as the unrelated Put3p-derived binuclear cluster domains were constructed and assayed by using the two-hybrid assay. A Dal80p Zn Finger-LexAp bait mediated an interaction with a Dal80p Zn Finger-LexAp prey that was three- to fourfold higher than interactions with Deh1p Zn Finger-B42p or Gln3p Zn Finger-B42p prey (Fig. 5A). In all three cases the interaction was highly dependent upon expression of the prey protein in raffinose plus glucose minimal medium. When the experiment was performed with a Deh1p Zn Finger-LexA bait, β-galactosidase production supported by each of the three interactions was roughly the same and was about one-third the level observed with the Dal80p Zn Finger-Dal80p Zn Finger interaction (Fig. 5B). Finally, when Gln3p Zn Finger-LexAp was used as bait, the highest signal was observed with a Gln3p Zn Finger-B42p prey followed closely by the Dal80p Zn Finger-B42p prey (Fig. 5C). β-Galactosidase production with Deh1p Zn Finger-B42p as prey was only one-half to one-third of that observed with the Dal80p and Gln3p prey plasmids (Fig. 5C). With a Put3p C6 cluster-containing fragment serving as either bait (Fig. 5D) or prey (Fig. 5A), the signal was never higher than the background level observed in the absence of the prey being expressed, i.e., in cells growing in minimal glucose medium. These results are consistent with the suggestion that some interaction between the Zn finger motifs of the Dal80, Deh1, and Gln3 proteins can occur, but those interactions are minor compared to those mediated by the leucine zipper motifs of the Dal80 and Deh1 proteins.
FIG. 5.
Two-hybrid assays of the DNA binding domains derived from yeast regulators of nitrogen metabolism. The B42-tagged fusions assayed were Dal80p-derived Zn finger (plasmid pVS80ZNJ), Deh1p-derived Zn finger (plasmid pVS1ZNJ), Gln3p-derived Zn finger (plasmid pVS3ZNJ), and Put3p-derived C6 binuclear cluster (plasmid pVS3CYCJ). The LexA-tagged bait fusions were Dal80p-derived Zn finger (plasmid pVS80ZNE) (A), Deh1p-derived Zn finger (plasmid pVS1ZNE) (B), Gln3p-derived Zn finger (plasmid pVS3ZNE) (C), and Put3p-derived C6 binuclear Zn cluster (plasmid pVSP3CYCE) (D). The transformations and assays were as described in the legend for in Fig. 3.
DISCUSSION
Data derived from two-hybrid assays indicate that Dal80p molecules possess considerable potential to self-associate, which is mediated by the C-terminal leucine zipper coiled-coil domain. These findings support the hypothesis that Dal80p’s leucine zipper coiled coil is the domain of the protein through which dimerization is mediated (14–17). These observations correlate well with the observed stringent requirements of two GATA-containing sequences for Dal80p binding to a DNA target (16) and the requirement of the leucine zipper motif for wild-type Dal80p activity (14). Our data raise the possibility that Deh1p is similarly capable of self-association and hence predict that the Deh1p binding site will possess characteristics similar, though not identical, to those of Dal80p.
An interesting conjecture is raised by the two-hybrid assay results, i.e., that Dal80p and Deh1p appear capable of interacting with one another, in vivo. If the two-hybrid assay accurately represents the capabilities of Dal80p and Deh1p to interact, it raises the possibility that cells may contain Dal80p and Deh1p homo- and heterodimers. If such heterodimers can form, it is interesting to query whether three types of negative sites may exist upstream of Dal80p- and/or Deh1p-regulated genes, i.e., those associated with Dal80p-Dal80p, Deh1p-Deh1p homodimers, and Dal80p-Deh1p heterodimers.
Interpretation of two-hybrid assay results can be compromised by many factors deriving from the assay system itself, for example, the need to fuse LexA or a Gal4 DNA binding site to the bait protein and a B-42 transcriptional activation domain to the prey protein. However, under the best of conditions, the relative stabilities of interactions can be reflected in the data obtained; all else being equal, more stable interactions would a priori be expected to yield higher β-galactosidase production than unstable ones. Therefore, to the extent that the β-galactosidase values we observe are reflective of the stability of the protein-protein interactions underlying them, an interesting question is raised. Are the actual differences between the self-association potentials of Dal80p and Deh1p molecules as great as the β-galactosidase activities we see, and if so, do they have biological implications? They might, for example, if only the dimeric forms of Dal80p and Deh1p bind to DNA.
Finally, protein-protein interaction between the zinc finger motifs of the GATA factor proteins we studied can be demonstrated to occur in the two-hybrid assay. However, again to the extent that β-galactosidase is reflective of protein-protein stability, these interactions would appear to be quite small relative to those mediated by the leucine zipper motifs. This, in turn, argues that any physiological significance that might be envisioned for such zinc finger-zinc finger interactions should be viewed cautiously.
ACKNOWLEDGMENTS
We thank Roger Brent and his colleagues for plasmids and strains used in the interaction trap assays as well as for helpful advice. We thank David Stillman (pLexA-Sin3p), Tom Cunningham (pTSC317 and pTSC416), and William Smart (pBS62) for plasmids they provided and members of the UT Yeast Group who read this manuscript and offered suggestions for improvement. Oligonucleotides were prepared by the UT Molecular Resource Center. This work was supported by Public Health Service grant GM-35642.
REFERENCES
- 1.Andre B, Talibi D, Boudekou S S, Hein C, Vissers S, Coornaert D. Two mutually exclusive regulatory systems inhibit UASGATA, a cluster of 5′GAT(A/T)A-3′ upstream from the UGA4 gene of Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:558–564. doi: 10.1093/nar/23.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arceci R J, King A A, Simon M C, Orkin S H, Wilson D B. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J S, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1994. [Google Scholar]
- 4.Blinder D, Magasanik B. Recognition of nitrogen-responsive upstream activation sequences of Saccharomyces cerevisiae by the product of the GLN3 gene. J Bacteriol. 1995;177:4190–4193. doi: 10.1128/jb.177.14.4190-4193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bysani N, Daugherty J R, Cooper T G. Saturation mutagenesis of the UASNTR (GATAA) responsible for nitrogen catabolite repression-sensitive transcriptional activation of the allantoin pathway genes in Saccharomyces cerevisiae. J Bacteriol. 1991;173:4977–4982. doi: 10.1128/jb.173.16.4977-4982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chisholm G, Cooper T G. Isolation and characterization of mutations that produce the allantoin-degrading enzymes constitutively in Saccharomyces cerevisiae. Mol Cell Biol. 1982;2:1088–1095. doi: 10.1128/mcb.2.9.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman J A, Rai R, Cooper T G. Genetic evidence for Gln3p-independent, nitrogen catabolite repression-sensitive gene expression in Saccharomyces cerevisiae. J Bacteriol. 1995;177:6910–6918. doi: 10.1128/jb.177.23.6910-6918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffman J A, Rai R, Cunningham T, Svetlov V, Cooper T G. Gat1p, a GATA family protein whose production is sensitive to nitrogen catabolite repression, participates in transcription activation of nitrogen catabolic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:847–858. doi: 10.1128/mcb.16.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffman J A, Rai R, Loprete D M, Cunningham T, Svetlov V, Cooper T G. Cross regulation of four GATA factors that control nitrogen catabolic gene expression in Saccharomyces cerevisiae. J Bacteriol. 1997;179:3416–3429. doi: 10.1128/jb.179.11.3416-3429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper T G. Nitrogen metabolism in Saccharomyces cerevisiae. In: Strathern J N, Jones E W, Broach J, editors. The molecular biology of the yeast Saccharomyces: metabolism and gene expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. pp. 39–99. [Google Scholar]
- 11.Cooper T G. Allantion degradative system—an integrated transcriptional response to multiple signals. In: Marzluf G, Bambrl R, editors. Mycota. III. Berlin, Germany: Springer Verlag; 1996. pp. 139–169. [Google Scholar]
- 12.Cooper T G, Ferguson D, Rai R, Bysani N. The GLN3 gene product is required for transcriptional activation of allantoin system gene expression in Saccharomyces cerevisiae. J Bacteriol. 1990;172:1014–1018. doi: 10.1128/jb.172.2.1014-1018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper T G, Rai R, Yoo H S. Requirement of upstream activation sequences for nitrogen catabolite repression of the allantoin system genes in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:5440–5444. doi: 10.1128/mcb.9.12.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coornaert D, Vissers S, Andre B, Grenson M. The UGA43 negative regulatory gene of Saccharomyces cerevisiae contains both a GATA-1 type zinc finger and a putative leucine zipper. Curr Genet. 1992;21:301–307. doi: 10.1007/BF00351687. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham T S, Cooper T G. Expression of the DAL80 gene, whose product is homologous to the GATA factors and is a negative regulator of multiple nitrogen catabolic genes in Saccharomyces cerevisiae, is sensitive to nitrogen catabolite repression. Mol Cell Biol. 1991;11:6205–6215. doi: 10.1128/mcb.11.12.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham T S, Cooper T G. The Saccharomyces cerevisiae DAL80 repressor protein binds to multiple copies of GATAA-containing sequences (URSGATA) J Bacteriol. 1993;175:5851–5861. doi: 10.1128/jb.175.18.5851-5861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham T S, Dorrington R A, Cooper T G. The UGA4 UASNTR site required for GLN3-dependent transcriptional activation also mediates DAL80-responsive regulation and DAL80 protein binding in Saccharomyces cerevisiae. J Bacteriol. 1994;176:4718–4725. doi: 10.1128/jb.176.15.4718-4725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunningham T S, Svetlov V, Rai R, Cooper T G. Saccharomyces cerevisiae Gln3p binds to UASNTR elements and activates transcription of nitrogen catabolite repression-sensitive genes. J Bacteriol. 1995;178:3470–3479. doi: 10.1128/jb.178.12.3470-3479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daugherty J R, Rai R, El Berry H M, Cooper T G. Regulatory circuit for responses of nitrogen catabolic gene expression to the GLN3 and DAL80 proteins and nitrogen catabolite repression in Saccharomyces cerevisiae. J Bacteriol. 1993;175:64–73. doi: 10.1128/jb.175.1.64-73.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estojak J R, Brent R, Golemis E A. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995;15:5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golemis E A, Gyurius J, Brent R. Two hybrid systems/interaction traps. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J S, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1994. pp. 13.14.1–13.14.17. [Google Scholar]
- 22.Hu J C, O’Shea E K, Kim P S, Sauer R T. Sequence requirements for coiled-coils: analysis with lambda repressor-GCN4 leucine zipper fusions. Science. 1990;250:1400–1403. doi: 10.1126/science.2147779. [DOI] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. p. 403. [Google Scholar]
- 24.Minehart P L, Magasanik B. Sequence and expression of GLN3, a positive nitrogen regulatory gene of Saccharomyces cerevisiae encoding a protein with a putative zinc finger DNA-binding domain. Mol Cell Biol. 1991;11:6216–6228. doi: 10.1128/mcb.11.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minehart P L, Magasanik B. Sequence of the GLN1 gene of Saccharomyces cerevisiae: role of the upstream region in regulation of glutamine synthetase expression. J Bacteriol. 1992;174:1828–1836. doi: 10.1128/jb.174.6.1828-1836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell A P, Magasanik B. Regulation of glutamine-repressible gene products by the GLN3 function in Saccharomyces cerevisiae. Mol Cell Biol. 1984;4:2758–2766. doi: 10.1128/mcb.4.12.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omichinski J G, Close G M, Schaad O, Felsenfeld G, Trainor C, Appella E, Stahl S J, Gronenborn A M. NMR structure of a specific DNA complex of Zn-containing DNA binding domain of GATA-1. Science. 1993;261:438–446. doi: 10.1126/science.8332909. [DOI] [PubMed] [Google Scholar]
- 28.Pu W T, Struhl K. Dimerization of leucine zippers analyzed by random selection. Nucleic Acids Res. 1993;21:4348–4355. doi: 10.1093/nar/21.18.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rai R, Daugherty J R, Cooper T G. UASNTR functioning in combination with other UAS elements underlies exceptional patterns of nitrogen regulation in Saccharomyces cerevisiae. Yeast. 1995;11:247–260. doi: 10.1002/yea.320110307. [DOI] [PubMed] [Google Scholar]
- 30.Rai R, Genbauffe F S, Cooper T G. Transcriptional regulation of the DAL5 gene in Saccharomyces cerevisiae. J Bacteriol. 1987;169:3521–3524. doi: 10.1128/jb.169.8.3521-3524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rai R, Genbauffe F S, Cooper T G. Structure and transcription of the allantoate permease gene (DAL5) from Saccharomyces cerevisiae. J Bacteriol. 1987;170:266–271. doi: 10.1128/jb.170.1.266-271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rai R, Genbauffe F S, Sumrada R A, Cooper T G. Identification of sequences responsible for transcriptional activation of the allantoate permease gene in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:602–608. doi: 10.1128/mcb.9.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasmussen S W. A 37.5 kb region of yeast chromosome X includes the SME1, MEF2, GSH1, and CSD3 genes, a TCP-1 related gene, an open reading frame similar to the DAL80 gene and a tRNA-A. Yeast. 1995;11:873–883. doi: 10.1002/yea.320110909. [DOI] [PubMed] [Google Scholar]
- 34.Rowen D W, Esiobu N, Magasanik B. Role of GATA factor Nil2p in nitrogen regulation of gene expression in Saccharomyces cerevisiae. J Bacteriol. 1997;179:3761–3766. doi: 10.1128/jb.179.11.3761-3766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smart W C, Coffman J A, Cooper T G. Combinatorial regulation of the Saccharomyces cerevisiae CAR1 (arginase) promoter in response to multiple environmental signals. Mol Cell Biol. 1996;16:5876–5887. doi: 10.1128/mcb.16.10.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soussi-Boudekou S, Vissers S, Urrestarazu A, Jauniaux J-C, Andre B. Gzf3p, a fourth GATA factor involved in nitrogen-regulated transcription in Saccharomyces cerevisiae. Mol Microbiol. 1997;23:1157–1168. doi: 10.1046/j.1365-2958.1997.3021665.x. [DOI] [PubMed] [Google Scholar]
- 37.Stanbrough M, Magasanik B. Two transcription factors, Gln3p and Nil1p, use the same GATAAG sites to activate the expression of GAP1 of Saccharomyces cerevisiae. J Bacteriol. 1996;178:2465–2468. doi: 10.1128/jb.178.8.2465-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanbrough M, Magasanik B. Role of the GATA factors Gln3p and Nil1p of Saccharomyces cerevisiae in the expression of nitrogen-regulated genes. Proc Natl Acad Sci USA. 1995;92:9450–9454. doi: 10.1073/pnas.92.21.9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svetlov V, Cooper T G. Review: compilation and characteristics of dedicated transcription factors in Saccharomyces cerevisiae. Yeast. 1995;11:1439–1484. doi: 10.1002/yea.320111502. [DOI] [PubMed] [Google Scholar]
- 40.Svetlov V, Cooper T G. The minimal transactivation region of Saccharomyces cerevisiae is localized to 13 amino acids. J Bacteriol. 1997;179:7644–7652. doi: 10.1128/jb.179.24.7644-7652.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talibi D, Grenson M, Andre B. Cis- and trans-activating elements determining induction of the genes of the γ-aminobutyrate (GABA) utilization pathway in Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:550–557. doi: 10.1093/nar/23.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vissers S, Andre B, Muyldermans F, Grenson M. Positive and negative regulatory elements control the expression of the UGA gene coding for the inducible 4-aminobutyric-acid-specific-permease in Saccharomyces cerevisiae. Eur J Biochem. 1989;181:357–361. doi: 10.1111/j.1432-1033.1989.tb14732.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Stillman D J. Transcriptional repression in Saccharomyces cerevisiae by a Sin3-LexA fusion protein. Mol Cell Biol. 1993;13:1805–1814. doi: 10.1128/mcb.13.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]