Abstract
Spectral analysis indicated the presence of a cytochrome cbb3 oxidase under microaerobic conditions in Azospirillum brasilense Sp7 cells. The corresponding genes (cytNOQP) were isolated by using PCR. These genes are organized in an operon, preceded by a putative anaerobox. The phenotype of an A. brasilense cytN mutant was analyzed. Under aerobic conditions, the specific growth rate during exponential phase (μe) of the A. brasilense cytN mutant was comparable to the wild-type specific growth rate (μe of approximately 0.2 h−1). In microaerobic NH4+-supplemented conditions, the low respiration of the A. brasilense cytN mutant affected its specific growth rate (μe of approximately 0.02 h−1) compared to the wild-type specific growth rate (μe of approximately 0.2 h−1). Under nitrogen-fixing conditions, both the growth rates and respiration of the wild type were significantly diminished in comparison to those under NH4+-supplemented conditions. Differences in growth rates and respiration between the wild type and the A. brasilense cytN mutant were less pronounced under these nitrogen-fixing conditions (μe of approximately 0.03 h−1 for the wild type and 0.02 h−1 for the A. brasilense cytN mutant). The nitrogen-fixing capacity of the A. brasilense cytN mutant was still approximately 80% of that determined for the wild-type strain. This leads to the conclusion that the A. brasilense cytochrome cbb3 oxidase is required under microaerobic conditions, when a high respiration rate is needed, but that under nitrogen-fixing conditions the respiration rate does not seem to be a growth-limiting factor.
Azospirillum brasilense is a gram-negative soil bacterium that lives in the rhizospheres of various plants, such as maize, wheat, and rice. When combined nitrogen is available, this bacterium is able to grow in anaerobic, microaerobic, or aerobic conditions. Under anaerobic conditions, when NO3− is available, denitrification provides the energy for growth (28, 29). Under microaerobic conditions, A. brasilense can reduce molecular N2 in the absence of combined nitrogen. In aerobic or microaerobic conditions, O2 is used as terminal electron acceptor (17). Like many other bacteria, A. brasilense has a branched respiratory chain. The presence of a respiratory chain that efficiently couples electron transfer with proton pumping at low oxygen concentrations is inferred from the attraction of A. brasilense to low oxygen concentrations. Under these conditions, a maximal proton motive force is generated (3, 53). The existence of a high-affinity terminal oxidase and a second oxidase with a significantly lower affinity in A. brasilense Sp7 was previously noted (4). Moreover, depending on the O2 status of the culture, A. brasilense Sp7 and Cd showed marked differences in cytochrome content (6, 21, 31, 34). For both strains spectral analysis revealed evidence for the presence of cytochrome b (α peak at 560 nm in the reduced-minus-oxidized difference spectrum), cytochrome c (α peak at 552 nm in the reduced-minus-oxidized difference spectrum), and a CO-binding o-like cytochrome (α peak at 558 nm in the reduced-minus-oxidized difference spectrum and a trough at 560 nm in the CO-reduced-minus-reduced difference spectrum) (6, 21, 34). The amounts of cytochromes b and c increased as the O2 concentration was lowered (6, 21, 31, 34). In contrast to the case for A. brasilense Sp7, a cytochrome d (peak at 628 nm in the reduced-minus-oxidized difference spectrum) was found in A. brasilense Cd (34). A cytochrome a (α peak at 603 to 605 nm in the reduced-minus-oxidized difference spectrum), observed under high aeration, was present in A. brasilense Cd (31, 34), but in A. brasilense Sp7 spectral evidence for this oxidase seemed to be less clear and even contradictory (6, 21).
The cytochrome cbb3 cytochrome c oxidase, encoded by the fixNOQP operon in rhizobial species (18, 23, 32, 38, 50) or by a similar cco(cyt)NOQP operon in other bacteria (7, 39, 43, 45), appears to be a cytochrome c terminal oxidase belonging to the heme-copper oxidase superfamily (14). In most rhizobial species this oxidase is essential for nitrogen-fixing endosymbiosis (18, 32, 50) and is characterized by an extremely high O2 affinity (16, 33). In the bacteria Magnetospirillum magnetoaceticum and Agrobacterium tumefaciens, and in Azorhizobium caulinodans growing nonsymbiotically, the cbb3-type cytochrome c terminal oxidase seems to be at least partially responsible for the microaerobic respiration (23, 39, 43). In Rhodobacter capsulatus, however, this oxidase drives aerobic respiration and does not function as the obligate oxidase during microaerobic nitrogen fixation (45). Proton pumping activity of the cytochrome cbb3 oxidase was demonstrated in Paracoccus denitrificans (7).
The purpose of this study was the characterization of the terminal oxidase active during microaerobic growth in A. brasilense. In particular, we were interested in assessing the role of this oxidase during nitrogen fixation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains used and plasmids described in this work are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani medium at 37°C. To grow Azospirillum, minimal medium (MMAB) was used (49). The nitrogen-free medium used for nitrogen fixation was the MMAB medium, devoid of NH4Cl. Solid medium contained 15 g of agar per liter. For conjugation YEP medium (containing 10 g of Bacto Peptone, 5 g of NaCl, and 10 g of yeast extract per liter) was used, and transconjugants of Azospirillum were selected on MMAB medium. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 25 μg/ml; and tetracycline, 10 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Properties | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | hsdR17 endA1 thi-1 gyrA96 relA1 recA1 supE44 ΔlacU169 (φ80lacZ ΔM15) | Gibco-BRL |
| S17-1 | thi endA recA hsdR with RP4-2-Tc::Mu-Km::Tn7 integrated in chromosome | 41 |
| A. brasilense | ||
| Sp7 | Wild type; ATCC 29145 | 44 |
| FAJ851 | cytN mutant; Kmr (plus direction) | This work |
| FAJ852 | cytN mutant; Kmr (minus direction) | This work |
| Plasmids | ||
| pEMBL8 | Cloning vector; Apr | 8 |
| pFAJ853 | pLAFR1 clone from genome bank of A. brasilense Sp7, containing cytNOQP; Tcr | This work |
| pFAJ856 | pSUP202 with the EcoRI/XbaI fragment from pFAJ863 blunt inserted in the PstI site (minus direction); Tcr Kmr Apr | This work |
| pFAJ857 | pSUP202 with the EcoRI/XbaI fragment from pFAJ862 blunt inserted in the PstI site (plus direction); Tcr Kmr Apr | This work |
| pFAJ860 | pUC18 with a 6-kb KpnI fragment of pFAJ853; Apr | This work |
| pFAJ861 | pUC18 with a 1.8-kb BamHI fragment of pFAJ853; Apr | This work |
| pFAJ862 | pFAJ861 with the Kmr cassette from pHP45Ω-Km blunt ligated in the ApaI site (plus direction); Apr Kmr | This work |
| pFAJ863 | pFAJ861 with the Kmr cassette from pHP45Ω-Km blunt ligated in the ApaI site (minus direction); Apr Kmr | This work |
| pHP45Ω-Km | Apr Kmr | 10 |
| pLAFR1 | IncP broad-host-range cosmid; Tcr | 13 |
| pSUP202 | Mobilizable plasmid, suicide vector for A. brasilense; Cmr Tcr Apr | 41 |
| pUC18 | Cloning vector; Apr | 51 |
A. brasilense was grown in a chemostat of 1.8-liter capacity (Applitek). The parameters of fermentation (pH, temperature, dissolved oxygen [DO], and air flow) were controlled by the ML-4100 fermentor control system (New Brunswick). All data from the ML-4100 system were transmitted into a computer loaded with the ASF 2.0 software (New Brunswick). DO levels were monitored with an autoclavable O2 electrode (Ingold). During aerobic growth, the airflow rate was set at 1.8 liters/min. According to the optimal values indicated in the literature (30, 46, 48), a DO concentration of 2.5 μM (2.5 μM DO at 30°C in sterile medium = a pO2 of 0.006 atm) was used for growth under nitrogen-fixing conditions. In order to maintain the DO at a constant level of 2.5 μM (microaerobic growth), the fermentor was sparged with a gas mixture of N2 and air. The N2 flow rate was set to 1.27 liters/min. The airflow rate was controlled by the ML-4100 system through a mass flow controller and automatically adapted according to the DO concentration values. The culture was stirred at a constant rate of 400 rpm. The growth temperature during fermentation was 30°C. The pH was maintained at 6.8 and adjusted with an H3PO4 (1 M) solution during fermentation according to the pH values measured by a pH probe (Ingold). A preculture of 100 ml, used to inoculate the fermentor, was grown in a flask of 250 ml of MMAB with NH4+ at 200 rpm and 30°C until an optical density at 578 nm (OD578) of 1.5 was reached. If cells were intended for growth in nitrogen-fixing conditions, the preculture was washed to remove residual NH4+. Samples of 5 to 10 ml, used for analyzing turbidity, protein concentration in the cells, and residual malate and NH4+ in the supernatant, were withdrawn automatically by a Biosampler (New Brunswick) during fermentation. Samples for acetylene reduction activity were taken anaerobically at the late exponential phase and transferred into gas-tight flasks which had been flushed with the headspace gas of the fermentor to adjust the DO concentration and with 10% (vol/vol) of C2H2 added. After an initial incubation of 30 min at 30°C and 200 rpm, the amount of ethylene produced was measured as previously described (48). Values for the specific nitrogenase activity presented in Results are the averages from at least three independent samples, each assayed at least five times. Data were analyzed by analysis of variance.
Analyses of cells and growth medium during fermentation.
Protein concentrations were determined with the bicinchoninic acid assay (42) with bovine serum albumin as a standard. Protein values are the averages from two independent samples, each measured twice. Cell density was monitored by measuring turbidity (OD578) on an LKB 4057 UV-visible spectrophotometer. The specific growth rate was defined as μ = ln(x2/x1)/(t2 − t1), where x is OD578, t is elapsed fermentation time (EFT), and subscripts 1 and 2 indicate different sampling times. The values for μe (hours−1) mentioned in Results are the average values of μ during exponential growth phase. l-Malate and NH4+ concentrations in the supernatant were determined with test kits from Boehringer Mannheim (27). The O2 concentration in the medium was measured by the Winkler method (Aquamerck oxygen test combination; Merck) (24).
Isolation of membranes.
Bacterial cultures grown in an oxystat under aerobic, microaerobic NH4+-supplemented, and nitrogen-fixing conditions were harvested at the beginning of the stationary phase (OD578 of approximately 1.2). Cells were subsequently centrifuged and suspended in 3 ml of 25 mM TES [N-tris(hydroxymethyl)methyl)methyl-2-aminoethanesulfonic acid]-KOH–5 mM MgCl2 buffer (pH 6.8) containing 10 μg of RNase per ml, 10 μg of DNase I per ml, and 1 mM phenylmethylsulfonyl fluoride. Membrane vesicles were prepared as described by Haaker et al. (16).
Visible difference absorbance spectra.
Visible light spectra were recorded on a dual-wavelength scanning spectrophotometer (Aminco DW2). Scanning was performed from 400 to 700 nm with a 3-nm bandwidth and from 500 to 700 nm with a 1-nm bandwidth at a scan speed of 1 nm/s. For reduced-minus-oxidized spectra, the membranes were reduced with dithionite. For the CO plus dithionite-reduced-minus-dithionite-reduced difference spectra, dithionite-reduced membranes were sparged for 5 min with 100% CO. Measurements were taken after 15 min.
Recombinant DNA techniques.
Standard protocols were used for cloning, restriction mapping, plasmid isolation, transformation, Southern blotting, and hybridization (36). Genomic DNA was isolated as described previously (2). PCR was performed on single colonies from A. brasilense Sp7. The primers used for the amplification of the cytN gene were cytplus (5′-TAGAATTCARTGGTGGTAYGGNCAYAAYGC-3′) and cytminus (5′-CAGAATTCCRTTRATCATNCCSCCCCA-3′). Both primers were provided with EcoRI recognition sites (boldface) to facilitate cloning procedures. The PCR was carried out in a TRIO-thermoblock (Biometra) with 0.2 mM deoxynucleoside triphosphates, 1 μM each primer, and 0.025 U of Taq DNA polymerase (Boehringer) per μl. The following PCR protocol was used: a denaturation period of 6 min at 94°C; followed by 35 cycles of 1 min at 94°C, 1 min at 52°C, and 1 min at 72°C; followed by an extension reaction of 7 min at 72°C.
A 300-bp PCR fragment was cloned in the EcoRI site of the vector pEMBL18, and it revealed an open reading frame (ORF) whose deduced product had similarity to known fixN gene products. This 300-bp EcoRI insert was used as probe to screen a previously constructed genomic library of A. brasilense Sp7 in pLAFR1 (25). One hybridizing clone (pFAJ853) with an insert of approximately 16 kb was digested with KpnI, and the 6-kb fragment hybridizing with the probe was subcloned in pUC18, resulting in pFAJ860 containing the entire cytNOQP operon.
The KpnI fragment of pFAJ860 was further subcloned into pUC18 or pUCBM20 to obtain the overlapping fragments covering the entire cytNOQP operon (approximately 4 kb). All subclones were sequenced on both strands by the chain termination dideoxynucleoside triphosphate method (37) with the AutoRead Sequencing Kit (Pharmacia-LKB) on an automated sequencer (ALF; Pharmacia-LKB), using fluorescein-labeled universal and synthetic oligonucleotide primers. Sequence data were assembled and analyzed with the DNA-analyzing program PC-Gene (Intelligenetics). Sequence data banks were screened for similarities by using the BLAST program (1).
Mutant construction.
To construct cytN insertion mutants, a 1.8-kb BamHI fragment was subcloned into pUC18, resulting in plasmid pFAJ861. A 2.5-kb aphII cassette (encoding Kanamycin resistance [Kmr]) of pHP45Ω-Km was blunt ligated in the ApaI site of pFAJ861, resulting in plasmid pFAJ862 (Kmr cassette in the same orientation as cytN [plus direction]) and pFAJ863 (Kmr cassette in the orientation opposite that of cytN [minus direction]). The resulting fragment was subsequently cloned as an EcoRI/XbaI fragment into the PstI site of the suicide plasmid pSUP202 after blunting all sticky ends. These resulting plasmids, named pFAJ857 (plus direction) and pFAJ856 (minus direction) were subsequently mobilized from E. coli S17-1 into A. brasilense Sp7 by conjugation. Kmr A. brasilense exconjugants were screened for the loss of the recombinant plasmid and for double homologous recombination by replica plating on the appropriate antibiotics as Kmr and tetracycline-sensitive (Tcs) clones. Recombination at the correct location was verified by Southern hybridization with DNA fragments from the cytN gene and the Kmr cassette as probes. The orientation of the cassette was verified by Southern hybridization. In FAJ851, transcription of the Kmr cassette is in the same orientation as that of the downstream genes cytO and cytP, while in FAJ852, transcription of the Kmr gene is opposite to the transcription orientation of the downstream genes.
SDS-PAGE and heme staining.
Membrane proteins, isolated as described above, were subsequently dissolved in denaturing equilibration buffer (60 mM Tris-HCl [pH 6.8], 2% [wt/vol] sodium dodecyl sulfate [SDS], 10% [wt/vol] glycerol, 28 μM bromophenol blue, 5% [vol/vol] β-mercaptoethanol) and separated by polyacrylamide gel electrophoresis (PAGE) in SDS–15% (wt/vol) polyacrylamide gels (20). Protein samples were not heated before electrophoresis. The resulting gels were stained for covalently bound heme with o-dianisidine (12) before being stained with Coomassie blue.
Nucleotide sequence accession number.
The sequence of the cytNOQP operon has been submitted to the GenBank/EMBL database under accession no. AF054871.
RESULTS
Spectral analysis of A. brasilense membranes.
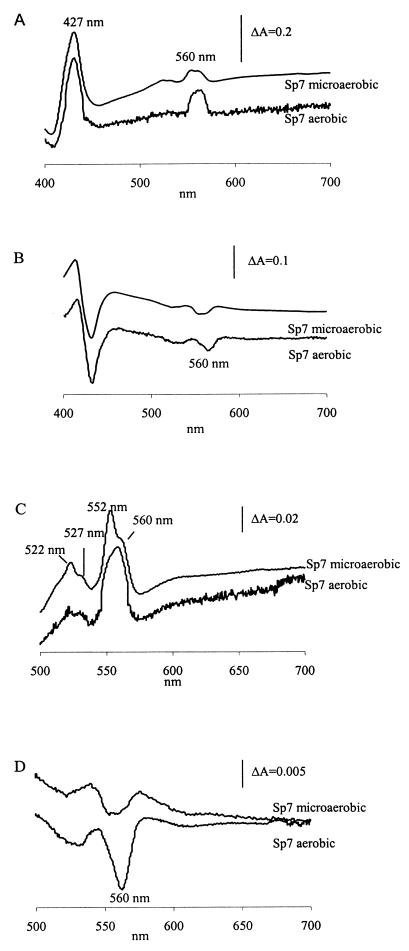
Membranes were isolated from cells as described in Materials and Methods. In the reduced-minus-oxidized spectra of membranes isolated from aerobically and microaerobically grown cells (Fig. 1A and C), the α peak at 552 nm and the β peak at 522 nm are attributable to c-type cytochromes and the 560-nm (β-peak) and 527-nm (β-peak) shoulders are attributable to cytochromes b. In membranes of microaerobically grown cells, the cytochrome c peak at 552 nm was clearly more pronounced than the cytochrome b shoulder at 560 nm (Fig. 1C), suggesting a relatively high cytochrome c/cytochrome b ratio. This high level of cytochrome c was also evident from the β band (522 nm), which showed asymmetry at shorter wavelengths (Fig. 1C). In the CO-binding spectrum reaction of CO with the high-spin heme is responsible for the inverted shoulder at 560 nm (Fig. 1B and D). Spectral analysis revealed that the terminal oxidases expressed in Azospirillum cells were similar in microaerobic conditions, whether or not combined nitrogen was available in the growth medium (data not shown). In aerobic conditions (Fig. 1C) the reduced-minus-oxidized spectrum showed a pronounced peak at 560 nm and a decreased peak at 552 nm. The CO-reduced-minus-reduced difference spectrum showed a clear inverted shoulder at 560 nm (Fig. 1D). These observations indicate a smaller amount of cytochrome c than of cytochrome b and suggest the presence of a second cytochrome b-containing oxidase present in aerobic conditions.
FIG. 1.
Difference spectroscopy of membrane proteins isolated from microaerobically and aerobically grown A. brasilense Sp7. (A and C) Dithionite-reduced-minus-air-oxidized spectra between 400 and 700 nm (5 mg of protein/ml) (A) and between 500 and 700 nm (3 mg of protein/ml) (C). (B and D) CO plus dithionite-reduced-minus-dithionite-reduced difference spectra between 400 and 700 nm (2 mg of protein/ml) (B) and between 500 and 700 nm (2 mg of protein/ml) (D).
Analysis of the DNA sequence and the deduced amino acid sequences.
Identification of the genes encoding this potential cytochrome cbb3 oxidase was done by a PCR-based cloning procedure as described in Materials and Methods. The identified DNA fragment subcloned in pFAJ860 contained all of the genes of A. brasilense corresponding to known fix(cyt,cco)NOQP genes of other bacteria.
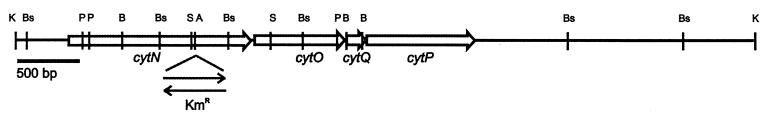
Four ORFs (orf1, orf2, orf3, and orf4) of, respectively, 1,494, 765, 159, and 885 bp were detected (Fig. 2). Each of these ORFs was preceded by a putative Shine-Dalgarno sequence upstream of the ATG start codon. orf1 was preceded by a potential anaerobox (TTGA-N5-ATCAA) 189 bp upstream of the ATG codon (9). At 60 bp downstream of orf4, a sequence with interrupted dyad symmetry (ΔG [25°C] = −25 kcal), followed by a T-rich region, suggests the presence of a Rho-independent transcription terminator (35). The amino acid sequences deduced from these ORFs showed high similarity with those of known genes, i.e., fixN (cyt,ccoN), -O, -Q, and -P. The identified ORFs were therefore designated cytN (orf1), cytO (orf2), cytQ (orf3), and cytP (orf4).
FIG. 2.
Physical map of the 6-kb KpnI fragment of pFAJ860. The 4-kb part containing the cytNOQP operon was completely sequenced on both strands. The region downstream of this 4-kb fragment was only partially sequenced. Both arrows indicate insertion of the Kmr cassette. Abbreviations: A, ApaI; B, BamHI; Bs, BssHII; K, KpnI; P, PstI; S, SalI.
The cytN gene encodes a protein of 498 amino acids (predicted molecular mass of 56 kDa for the apoprotein). CytN of A. brasilense showed identities ranging from 68 to 70% with CytN-like proteins of Rhodobacter sphaeroides (accession no. U58092), Sinorhizobium meliloti (18) (accession no. X15079), P. denitrificans (7), A. caulinodans (23), Bradyrhizobium japonicum (32), and R. capsulatus (45).
cytO encodes an apoprotein of 246 amino acids with a predicted molecular mass of 27.7 kDa. An N-terminal transmembrane helix and a highly conserved heme c-binding site (CYNCH) at position 71 could be identified. CytO showed 62 to 68% identity with the aligned CytO-like proteins of R. sphaeroides (accession no. U58092), S. meliloti (18) (accession no. X15079), P. denitrificans (7), A. caulinodans (23), B. japonicum (32), and R. capsulatus (45).
cytQ encodes a small protein of 53 amino acids with a predicted molecular mass of 6 kDa. It exhibited identities of only 34% with the FixQ protein of B. japonicum (32), 40% with FixQ of S. meliloti (18) (accession no. X15079), 38% with CytQ of A. caulinodans (23), 34% with CcoQ of R. capsulatus (45), 30% with CcoQ of R. sphaeroides (accession no. U58092), and 20% with CcoQ of P. denitrificans (7). In B. japonicum FixQ is not involved in the assembly of the oxidase complex and seems not to be an essential subunit of the complex (55).
cytP codes for an apoprotein of 295 amino acids with a predicted molecular mass of 31.8 kDa. A hydrophobic stretch is located at positions 34 to 50. The protein exhibited two heme-binding motifs (CAACH at position 121 and CAACH at position 220). CytP showed an identity of 42 to 53% with the CytP-like proteins of R. sphaeroides (accession no. U58092), S. meliloti (18) (accession no. X15079), P. denitrificans (7), A. caulinodans (23), B. japonicum (32), and R. capsulatus (45).
Construction and phenotypic analysis of a cytN mutant.
A Kmr insertion mutant was constructed as described in Materials and Methods. The Kmr cassette was inserted in both orientations (Fig. 2).
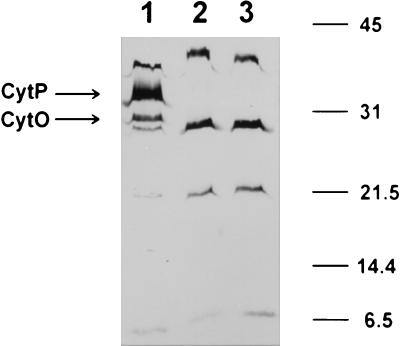
Membranes isolated from both cytN mutants and wild-type cells grown in microaerobic conditions were tested for the presence of covalently bound heme by SDS-PAGE followed by heme staining (Fig. 3). Six heme c-containing proteins, of approximately 6, 21, 27, 28, 32, and 40 kDa, were present in the wild type. In the cytN mutants FAJ851 and FAJ852, the 28- and 32-kDa heme-containing proteins, with molecular masses similar to the predicted molecular masses of A. brasilense CytP (31.8 kDa) and CytO (27.7 kDa), were absent. The other staining bands in both the wild type and cytN mutants represented other, yet-uncharacterized heme-containing proteins, such as NO reductase or bc1 complex proteins present in A. brasilense cells grown under the tested conditions (17).
FIG. 3.
Analysis for covalently bound heme in A. brasilense membrane proteins. Membranes were isolated from microaerobically grown wild-type A. brasilense (Sp7) (lane 1) and cytN mutants (FAJ851 [lane 2] and FAJ852 [lane 3]). Equal amounts of proteins (approximately 200 μg) were loaded. The positions of molecular size markers (Bio-Rad) are indicated by horizontal lines (in kilodaltons).
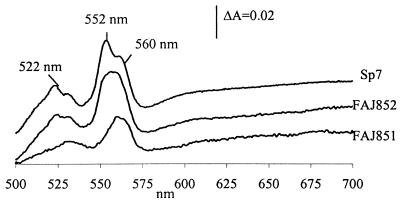
Reduced-minus-oxidized absorbance spectra of membranes isolated from the wild-type Sp7 and from mutants FAJ851 and FAJ852, grown to the beginning of the stationary phase in microaerobic batch cultures, are shown in Fig. 4. The relative high cytochrome c552/cytochrome b560 ratio, characteristic of the presence of a cytochrome cbb3 terminal oxidase, was lowered in the mutants, as shown by the decrease in the cytochrome c peak at 552 nm. Furthermore, these spectra of membranes isolated from A. brasilense cytN mutants in microaerobic conditions showed a high similarity with spectra of membranes isolated from A. brasilense wild-type cells grown in aerobic conditions (Fig. 1C).
FIG. 4.
Dithionite-reduced-minus-air-oxidized spectra, between 500 and 700 nm, of membrane proteins isolated from microaerobically grown cells of A. brasilense Sp7 (wild type) and FAJ851 and FAJ852 (cytN mutants). Equal amounts of protein (approximately 2.6 mg/ml) were analyzed.
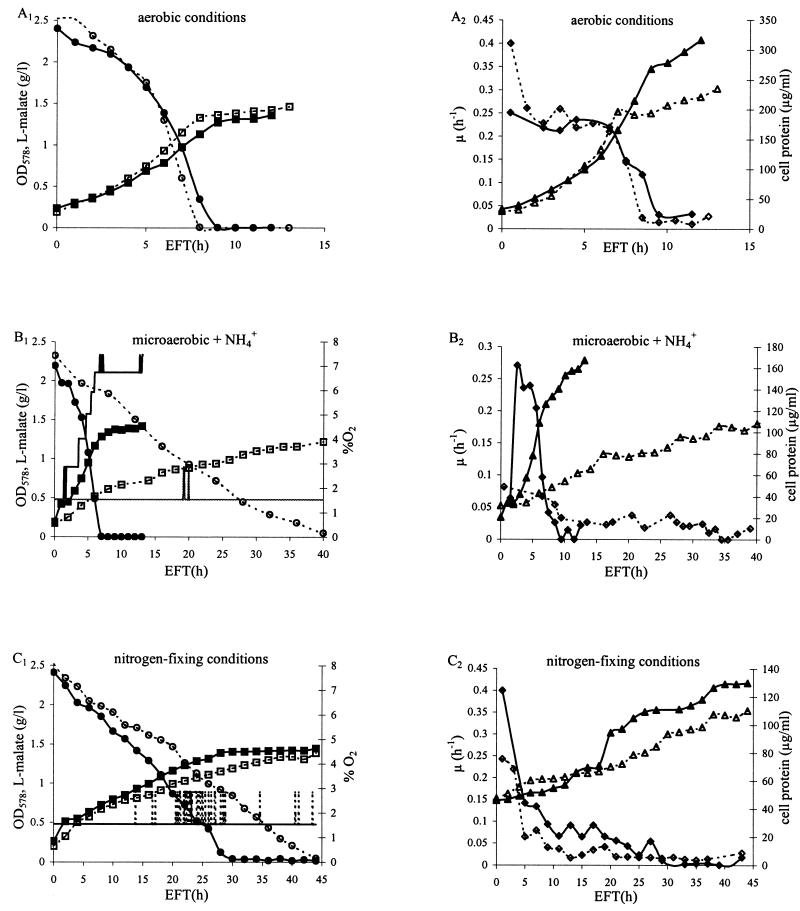
Growth of the cytN mutant and the wild type was compared under different conditions (Fig. 5). Specific growth rates during exponential phase (μe) were calculated as described in Materials and Methods. The A. brasilense cytN mutant and the wild type showed similar growth patterns, illustrated by the increase in OD578 (Fig. 5A1) and protein concentrations (Fig. 5A2), in aerobic conditions, although a higher protein concentration was obtained in wild-type cells. After an EFT of approximately 8 h, when the carbon source malate becomes limiting, cells entered the stationary phase (Fig. 5A1). The specific growth rates of A. brasilense Sp7 and the cytN mutant during exponential phase were similar (μe of approximately 0.2 h−1 [Fig. 5A2]).
FIG. 5.
Comparison of fermentation parameters of the wild-type A. brasilense Sp7 and the A. brasilense cytN mutant FAJ851 under aerobic (A), microaerobic and NH4+-supplemented (B), and nitrogen-fixing (C) conditions. (A1, B1, and C1) OD578 values and malate concentrations. Panels B1 and C1 also show the percentage of O2 present in the incoming airflow during fermentation in microaerobic conditions. Note that in panel C1 the lines for percent O2 during fermentation of the wild-type Sp7 and the cytN mutant FAJ851 coincide. (A2, B2, and C2) Protein concentrations and specific growth rates (μ). Values for protein concentration and l-malate are the averages of at least four different measurements. (A1, B1, and C1) ■, OD578 for Sp7; □, OD578 for FAJ851; •, l-malate for Sp7; ○, l-malate for FAJ851; —, percent O2 for Sp7; - - - , percent O2 for FAJ851. (A2, B2, and C2) ▴, protein for Sp7; ▵, protein for FAJ851; ⧫, μ for Sp7; ◊, μ for FAJ851.
In microaerobic conditions in a medium supplemented with NH4+, the low DO concentration had no influence on the growth behavior of the A. brasilense wild type compared to that in aerobic conditions (Fig. 5A and B). Within a few hours, the OD578 increased drastically (Fig. 5B1) (μe of approximately 0.2 h−1 [Fig. 5B2]), and the stationary phase was reached after an EFT similar to that for aerobically grown cells. In contrast, the specific growth rate of the A. brasilense cytN mutant during exponential phase was considerably affected (μe of approximately 0.02 h−1 [Fig. 5B2]). Only after an EFT of 40 h did the cells reach an OD578 similar to the OD578 obtained for the wild type at the beginning of the stationary phase (Fig. 5B1). The carbon source malate was still not entirely consumed. Respiratory behavior can be judged by the changes in the percentage of O2 present in the incoming gas flow during fermentation. An increase in the percentage of O2 is due to an increase in the airflow rate. The higher O2 concentration in the incoming gas flow together with the simultaneously increased total gas flow through the fermentor causes a higher O2 transfer rate, reflecting the higher O2 consumption by the growing cells. Figure 5B1 shows that when the wild-type cells were grown in microaerobic NH4+-supplemented conditions, the percentage of O2 in the gas flow, automatically adjusted to maintain a constant DO concentration of 2.5 μM, was increased at regular time intervals in order to cope with the high O2 demand of the fast-growing cells. For the A. brasilense cytN mutant grown in similar conditions, a low constant percentage of O2 in the gas flow was sufficient to maintain the DO concentration at 2.5 μM.
The specific growth rate of the wild type during exponential phase under nitrogen-fixing conditions (μe of approximately 0.03 h−1 [Fig. 5C2]) was decreased compared to specific growth rates obtained under the same conditions but in the presence of an NH4+ source (μe of approximately 0.2 h−1 [Fig. 5B2]). The cells needed approximately 30 h to reach stationary phase, and the final cell protein concentration was significantly lower than during NH4+-supplemented growth (Fig. 5C1 and C2). The percentage of O2 in the gas flow during fermentation remained relatively constant, indicating a low O2 demand (Fig. 5C1). In these nitrogen-fixing conditions, the growth and respiratory behaviors of the A. brasilense cytN mutant did not differ drastically from those of the wild type (Fig. 5C1 and C2). A lower rate of consumption of the carbon source malate, a slightly lower specific growth rate during exponential phase (μe of approximately 0.02 h−1), and a reduction of the specific nitrogenase activity of the A. brasilense cytN mutant (13.5 ± 0.99 nmol of ethylene/mg of protein/h) to approximately 80% of the wild-type activity (16.52 ± 1.25 nmol of ethylene/mg of protein/h) were observed for the A. brasilense cytN mutant compared to the wild-type strain. The high specific growth rate observed for both the A. brasilense cytN mutant and the wild type at the start of nitrogen-fixing growth is probably due to the presence of internal NH4+ in the inoculated cells (Fig. 5C2).
DISCUSSION
The similarity between spectra shown in this work and those reported for the purified cbb3-type cytochrome c oxidase complexes from B. japonicum (33), R. capsulatus (15), and M. magnetoacticum (43) suggests that an analogous cytochrome cbb3 oxidase is present in microaerobically grown Azospirillum cells. Accordingly, and consistent with previous results, a relative increase in the level of cytochrome c versus cytochrome b was observed during a shift from aerobic to microaerobic conditions (6, 21).
Genetic evidence of a cytNOQP operon in A. brasilense supports this biochemical analysis. The A. brasilense cytNOQP operon is preceded by a putative anaerobox. So far, no direct evidence for the existence of an FNR-like protein in Azospirillum is available (47).
cytN of A. brasilense encodes subunit I of the cbb3-type terminal oxidase. The highly conserved histidine residues shown to be involved in the binding of the high-spin b/CuB reaction center (5, 26) are conserved at positions 362, 274, 275, and 224 in the A. brasilense CytN. The histidine residues assumed to be the axial ligands for the low-spin heme b (22, 56) are located at positions 74 and 364. The histidine residue implicated in Mg2+ and Mn2+ binding in B. japonicum FixN (54) is present at position 354. The histidine residue suggested to bind and release protons in B. japonicum FixN (54) is at position 260. Based on a structural comparison between subunit I of conventional cytochrome c oxidases, containing 12 transmembrane helices, and the cytochrome cbb3 oxidases, usually characterized by 14 potential transmembrane helices, Zufferey et al. (56) hypothesized that the first 2 of these 14 transmembrane helices of CytN-like proteins should be cytoplasmic. This hypothesis was supported by studies with fusion proteins (56). Interestingly the A. brasilense CytN protein seems to be truncated and lacks these two first transmembrane helices encountered in other sequenced CytN-like proteins.
To investigate the role of the cytochrome cbb3 oxidase, a Kmr insertion mutant of A. brasilense cytN was constructed. Results from heme-stained SDS-PAGE gels and spectral analysis of membranes from both A. brasilense wild-type and A. brasilense cytN mutant cells led us to conclude that the A. brasilense cytN mutant lacks a functional cytochrome cbb3 terminal oxidase.
Subsequently, growth analysis was performed. In microaerobic conditions a high respiration rate potentially supporting efficient energy production allows the A. brasilense wild-type cells to grow at rates similar to those obtained in highly aerated cultures, despite the low DO concentration. As the A. brasilense cytN mutant was not able to sustain such growth, the cytochrome cbb3 oxidase seems to be responsible for the high respiration rates observed at low DO concentrations. During nitrogen fixation, the specific growth rate of the wild type was considerably lower than in NH4+-supplemented conditions. As nitrogen fixation is a very energy-consuming process, a shortage of ATP seems a plausible cause for growth limitation. This seems to be the case in symbiotic microorganisms such as Rhizobium or Bradyrhizobium species, where nitrogen fixation takes place in nodules. These nodules create the optimal low O2 concentration to prevent O2 damage to the nitrogenase and function simultaneously as an O2 delivery system (40) to a high-affinity cytochrome cbb3 terminal oxidase. This oxidase allows high respiration rates and generation of a proton motive force at nanomolar concentrations of O2 (33). Cytochrome cbb3 mutants are completely (32) or at least partially (19, 23) unable to fix N2, indicating the importance of energy as a limiting factor. Assuming that energy limitation explains the lower specific growth rates of the wild-type A. brasilense during nitrogen fixation, the A. brasilense cytN mutant affected in its cytochrome cbb3 terminal oxidase would be expected to show an even more pronounced energy-limited growth. However, only minor differences were observed between the specific growth rates of the A. brasilense cytN mutant and the wild type in these conditions, and the nitrogenase of the A. brasilense cytN mutant still retained approximately 80% of its activity. Therefore, a more likely explanation of growth limitation in nitrogen-fixing conditions seems to be the shortage of NH4+. Possibly the nitrogenase cannot produce sufficient NH4+ to cope with the high NH4+ consumption by fast-growing cells. Alternatively, the strict regulation of the nitrogen-fixing process cannot be excluded as a growth-limiting factor. If cells fix nitrogen at high rates, the internal NH4+ accumulating in the cells could switch off the system (52). Nitrogen fixation and thus the NH4+ concentration consequently decrease, which in turn allows the system to resume nitrogen fixation. Conceivably, there can never be an accumulation of sufficient NH4+ to allow fast growth and subsequent energy limitation.
Similar to the observations made for A. caulinodans (19, 23), an unknown alternative oxidase can partially overcome the absence of the cytochrome cbb3 terminal oxidase in microaerobic conditions, either in presence or absence of NH4+, since A. brasilense cytN mutants could still grow. Similar to previous results for A. brasilense Sp7 (6), but in contrast to those for A. brasilense Cd (34), no terminal oxidase containing cytochrome d seems to be present. No indications could be found for the presence of heme a, not even in membranes of aerobically grown cells. Although the concentrations of heme a discovered before in A. brasilense Sp7 were barely detectable (21), it cannot be ruled out that under certain conditions this cytochrome c oxidase is expressed. Comparison of the reduced-minus-oxidized spectra of membranes from the A. brasilense cytN mutant and the wild type points in the direction of an additional heme b-containing terminal oxidase such as, e.g., a bo-quinol oxidase during microaerobic growth. This oxidase also seems to be present in fully aerated membranes of the wild type. A conclusive interpretation of the CO-reduced-minus-reduced spectra (data not shown) was hampered by interference of the absorption maxima of this potential bo-quinol complex by the absorption maxima of other proteins putatively present in the membranes, such as the bc1 complex, denitrifying complexes, or even other alternative oxidases (11). However, the presence of a bo-quinol oxidase seems to be consistent with earlier reports on A. brasilense Sp7, which indicate the presence of particulate cytochrome b (6, 21) and a CO-binding cytochrome o (6, 21) in aerobic conditions. Likewise, spectral analysis suggested the presence of a cytochrome o-containing terminal oxidase in A. brasilense Cd, expressed in aerobic but also in microaerobic conditions (34). This terminal oxidase, however, seemed to function after the antimycin A inhibition site (after the cytochrome c reductase complex). In addition it was shown that an alternative oxidase, other than cytochrome caa3-type cytochrome c oxidase and less sensitive to KCN, could accept electrons from TMPD (N,N,N′-tetramethyl-p-phenylenediamine) plus ascorbate, indicating the presence of another cytochrome c-type terminal oxidase (34). We suggest that, given the spectral similarities between cytochrome o-containing and cbb3-type terminal oxidases, the cytochrome o-like cytochrome c oxidase identified previously is identical to the cytochrome cbb3 cytochrome c terminal oxidase of A. brasilense Sp7 characterized in this study. The presence of such a cbb3-type cytochrome c oxidase might have accounted for the residual reduction of ascorbate in the presence of a low concentration KCN, which is known to inhibit the cytochrome caa3-type cytochrome c oxidase.
ACKNOWLEDGMENTS
K.M. is a recipient of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. This work was supported by grants (to J.V.) from the Flemish Government (GOA) and the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
We thank the Laboratory of Industrial Microbiology, KULeuven, Heverlee, Belgium, for kindly providing the fermentation equipment.
REFERENCES
- 1.Altschul S F, Gish W, Milles W, Myersand E W, Lipman D J. Basic local alignment tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1991. pp. 4.8.1–4.8.5. [Google Scholar]
- 3.Barak R, Nur I, Okon Y, Henis Y. Aerotactic response of Azospirillum brasilense. J Bacteriol. 1982;152:643–649. doi: 10.1128/jb.152.2.643-649.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergersen F J, Turner G L. Properties of terminal oxidase systems of bacteroids from root nodules of soybean and cowpea and of N2-fixing bacteria grown in continuous culture. J Gen Microbiol. 1980;118:235–252. [Google Scholar]
- 5.Calhoun M W, Thomas J W, Hill J J, Hosler J P, Shapleigh J P, Tecklenburg M M J, Ferguson-Miller S, Babcock G T, Alben J O, Gennis R B. Identity of the axial ligand of the high-spin heme in cytochrome oxidase: spectroscopic characterization of mutants in the bo-type oxidase of Escherichia coli and the aa3-type oxidase of Rhodobacter sphaeroides. Biochemistry. 1993;32:10905–10911. doi: 10.1021/bi00091a046. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S K, Mishra A K, Chakrabartty P K. Cytochromes in Azospirillum brasilense. Curr Microbiol. 1984;11:343–348. [Google Scholar]
- 7.de Gier J-W L, Schepper M, Reijnders W N M, van Dyck S J, Slotboom D J, Warne A, Saraste M, Krab K, Finel M, Stouthamer A H, van Spanning R J M, van der Oost J. Structural and functional analysis of aa3-type and cbb3-type cytochrome c oxidases of Paracoccus denitrificans reveals significant differences in proton-pump design. Mol Microbiol. 1996;20:1247–1260. doi: 10.1111/j.1365-2958.1996.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 8.Dente L, Cesareni G, Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983;11:1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiglmeier K, Honoré N, Iuchi S, Lin E C C, Cole S T. Molecular genetic analysis of FNR-dependent promoters. Mol Microbiol. 1989;3:869–878. doi: 10.1111/j.1365-2958.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 10.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson S J. Denitrification and its control. Antonie Leeuwenhoek. 1994;66:89–110. doi: 10.1007/BF00871634. [DOI] [PubMed] [Google Scholar]
- 12.Francis R T, Becker R R. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem. 1984;136:509–514. doi: 10.1016/0003-2697(84)90253-7. [DOI] [PubMed] [Google Scholar]
- 13.Friedman A M, Long S R, Brown S E, Buikema S E, Ausubel F M. Construction of a broad host range cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Horsman J A, Barquera B, Rumbley J, Ma J, Gennis R B. The superfamily of heme-copper respiratory oxidases. J Bacteriol. 1994;176:5587–5600. doi: 10.1128/jb.176.18.5587-5600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray K A, Grooms M, Myllykallio H, Moomaw C, Slaughter C, Daldal F. Rhodobacter capsulatus contains a novel cb-type cytochrome c oxidase without a CuA center. Biochemistry. 1994;33:3120–3127. doi: 10.1021/bi00176a047. [DOI] [PubMed] [Google Scholar]
- 16.Haaker H, Szafran M, Wassink H, Klerk H, Appels M. Respiratory control determines respiration and nitrogenase activity of Rhizobium leguminosarum bacteroids. J Bacteriol. 1996;178:4555–4562. doi: 10.1128/jb.178.15.4555-4562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann A, Zimmer W. Physiology of Azospirillum. In: Okon Y, editor. Azospirillum/plant associations. Boca Raton, Fla: CRC Press; 1994. pp. 15–39. [Google Scholar]
- 18.Kahn D, Batut J, Daveran M L, Fourment J. Structure and regulation of the fixNOQP operon from Rhizobium meliloti. In: Palacios R, Mora J, Newton W E, editors. New horizons in nitrogen-fixation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. p. 474. [Google Scholar]
- 19.Kaminski P A, Kitts C L, Zimmerman Z, Ludwig R A. Azorhizobium caulinodans uses both cytochrome bd (quinol) and cytochrome cbb3 (cytochrome c) terminal oxidases for symbiotic N2 fixation. J Bacteriol. 1996;178:5989–5994. doi: 10.1128/jb.178.20.5989-5994.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lalande R, Knowles R. Cytochrome content in Azospirillum brasilense Sp7 grown under aerobic and denitrifying conditions. Can J Microbiol. 1986;33:151–156. [Google Scholar]
- 22.Lemieux L J, Calhoun M W, Thomas J W, Ingledew W J, Gennis R B. Determination of the ligands of the low spin heme of the cytochrome o ubiquinol oxidase complex using site-directed mutagenesis. J Biol Chem. 1992;267:2105–2113. [PubMed] [Google Scholar]
- 23.Mandon K, Kaminski P A, Elmerich C. Functional analysis of the fixNOQP region of Azorhizobium caulinodans. J Bacteriol. 1994;176:2560–2568. doi: 10.1128/jb.176.9.2560-2568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniel L E. Measurement of dissolved oxygen. In: Laskin A I, Lechevalier H H, editors. CRC handbook of microbiology. Vol. 6. Boca Raton, Fla: CRC Press; 1984. p. 339. [Google Scholar]
- 25.Michiels C. Genetic determinants involved in Azospirillum brasilense surface polysaccharide production. PhD thesis. Leuven, Belgium: Katholieke Universiteit Leuven; 1989. [Google Scholar]
- 26.Minagawa J, Mogi T, Gennis R B, Anraku Y. Identification of heme and copper ligands in subunit I of the cytochrome bo complex in Escherichia coli. J Biol Chem. 1992;267:2096–2104. [PubMed] [Google Scholar]
- 27.Möllering H. l-(−)-Malate: determination with malate dehydrogenase and aspartate aminotransferase. In: Bergmeyer H U, editor. Methods of enzymatic analysis 3rd ed. VII. Weinheim, Germany: Verlag Chemie; 1985. pp. 39–47. [Google Scholar]
- 28.Nelson L M, Knowles R. Effect of oxygen and nitrate on nitrogen-fixation and denitrification by Azospirillum brasilense grown in continuous cultures. Can J Microbiol. 1978;24:1395–1403. doi: 10.1139/m78-223. [DOI] [PubMed] [Google Scholar]
- 29.Neyra C, Döbereiner J, Lalande R, Knowles R. Denitrification by N2-fixing Spirillum lipoferum. Can J Microbiol. 1977;23:300–305. doi: 10.1139/m77-044. [DOI] [PubMed] [Google Scholar]
- 30.Okon Y, Houchins J P, Albrecht S L, Burris R H. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol. 1977;98:87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- 31.Okon Y, Nur I, Henis Y. Effect of oxygen concentration on electron transport components and microaerobic properties of Azospirillum brasilense. In: Klingmüller W, editor. Azospirillum. II. Genetics, physiology, ecology. Basel, Switzerland: Birkhäuser Verlag; 1983. pp. 115–126. [Google Scholar]
- 32.Preisig O, Anthamatten D, Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci USA. 1993;90:3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preisig O, Zufferey R, Thöny-Meyer L, Appleby C A, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiner O, Okon Y. Oxygen recognition in aerotactic behavior of Azospirillum brasilense Cd. Can J Microbiol. 1986;32:829–834. [Google Scholar]
- 35.Rosenberg M, Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlüter A, Patschowski T, Quandt J, Selinger L B, Weider S, Krämer M, Zhou L, Heynes M F, Priefer U B. Functional and regulatory analysis of the two copies of the fixNOQP operon in Rhizobium leguminosarum strain VF39. Mol Plant-Microbe Interact. 1997;10:605–616. doi: 10.1094/MPMI.1997.10.5.605. [DOI] [PubMed] [Google Scholar]
- 39.Schlüter A, Rüberg S, Krämer M, Weidner S, Priefer U B. A homolog of the Rhizobium meliloti nitrogen-fixation gene fixN is involved in the production of a microaerobically induced oxidase activity in the phytopathogenic bacterium Agrobacterium tumefaciens. Mol Gen Genet. 1995;247:206–215. doi: 10.1007/BF00705651. [DOI] [PubMed] [Google Scholar]
- 40.Sheehy J E, Webb J. Oxygen diffusion pathways and nitrogen-fixation in legume root nodules. Ann Bot. 1991;67:85–92. [Google Scholar]
- 41.Simon R, Priefer U, Pühler A. A broad host range mobilisation system for in vivo genetic engineering transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 42.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujitomo E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 43.Tamegai H, Fukumori Y. Purification, and some molecular and enzymatic features of a novel ccb-type cytochrome c oxidase from a microaerobic denitrifier, Magnetospirillum magnetoaceticum. FEBS Lett. 1994;347:22–26. doi: 10.1016/0014-5793(94)00500-1. [DOI] [PubMed] [Google Scholar]
- 44.Tarrand J J, Krieg N R, Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with description of a new genus, Azospirillum gen. nov. and two species Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978;24:967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- 45.Thöny-Meyer L, Beck C, Preisig O, Hennecke H. The ccoNOQP gene cluster codes for a cb-type cytochrome oxidase that functions in aerobic respiration of Rhodobacter capsulatus. Mol Microbiol. 1994;14:705–716. doi: 10.1111/j.1365-2958.1994.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 46.Tibelius K H, Knowles R. Effect of hydrogen and oxygen on uptake-hydrogenase activity in nitrogen-fixing and ammonium-grown Azospirillum brasilense. Can J Microbiol. 1983;29:1119–1125. [Google Scholar]
- 47.Tripathi A K. Existence of fixJ- and fixK-like genes in Azospirillum brasilense. Indian J Exp Biol. 1993;31:559–561. [Google Scholar]
- 48.Vande Broek A, Keijers V, Vanderleyden J. Effect of oxygen on the free-living nitrogen-fixation activity and expression of the Azospirillum brasilense nifH gene in various plant-associated diazothophs. Symbiosis. 1996;21:25–40. [Google Scholar]
- 49.Vanstockem M, Michiels K, Vanderleyden J, Van Gool A. Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum: physical analysis of Tn5 and Tn5-mob insertion mutants. Appl Environm Microbiol. 1987;53:410–415. doi: 10.1128/aem.53.2.410-415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vargas C, Wu G, Delgado M-J, Poole R K, Downie J A. Identification of symbiosis-specific c-type cytochromes and a putative oxidase in bacteroids of Rhizobium leguminosarum biovar viciae. Microbiology. 1996;142:41–46. doi: 10.1099/13500872-142-1-41. [DOI] [PubMed] [Google Scholar]
- 51.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Burris R H, Ludden P W, Roberts G P. Posttranslational regulation of nitrogenase activity by anaerobiosis and ammonium in Azospirillum brasilense. J Bacteriol. 1993;175:6781–6788. doi: 10.1128/jb.175.21.6781-6788.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhulin I B, Bespalov V A, Johnson M S, Taylor B L. Oxygen taxis and proton motive force in Azospirillum brasilense. J Bacteriol. 1996;178:5199–5204. doi: 10.1128/jb.178.17.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zufferey R, Arslan E, Thöny-Meyer L, Hennecke H. How replacements of the 12 conserved histidines of subunit I affect assembly, cofactor binding and enzymatic activity of the Bradyrhizobium japonicum cbb3-type oxidase. J Biol Chem. 1998;273:6452–6459. doi: 10.1074/jbc.273.11.6452. [DOI] [PubMed] [Google Scholar]
- 55.Zufferey R, Preisig O, Hennecke H, Thöny-Meyer L. Assembly and function of the cytochrome cbb3 oxidase subunits in Bradyrhizobium japonicum. J Biol Chem. 1996;271:9114–9119. doi: 10.1074/jbc.271.15.9114. [DOI] [PubMed] [Google Scholar]
- 56.Zufferey R, Thöny-Meyer L, Hennecke H. Histidine 131, not histidine 43, of the Bradyrhizobium japonicum FixN protein is exposed towards the periplasm and essential for the function of the cbb3-type cytochrome oxidase. FEBS Lett. 1996;394:349–352. doi: 10.1016/0014-5793(96)00982-9. [DOI] [PubMed] [Google Scholar]