Abstract
Background
Severe pneumonia frequently causes irreversible sequelae and represents a major health burden for children under the age of 5. Matrix Metallopeptidase 9 (MMP9) is a zinc-dependent endopeptidase that is involved in various cellular processes. The correlation between MMP9 and the risk of severe childhood pneumonia remains unclear.
Methods
Here we assemble a case–control cohort to study the association of genetic variants in MMP9 gene with severe childhood pneumonia susceptibility in a Southern Chinese population (1034 cases and 8426 controls).
Results
Our results indicate that the allele G in rs3918262 SNP was significantly associated with an increased risk of severe pneumonia. Bioinformatic analyses by expression quantitative trait loci (eQTL), RegulomeDB and FORGEdb database analysis showed that rs3918262 SNP has potential regulatory effect on translational efficiency and protein level of MMP9 gene. Furthermore, MMP9 concentrations were significantly up-regulated in the bronchoalveolar lavages (BALs) of children with severe pneumonia.
Conclusion
In summary, our findings suggest that MMP9 is a novel predisposing gene for childhood pneumonia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08931-4.
Keywords: Pneumonia, MMP9, Single nucleotide polymorphism
Background
Community-acquired pneumonia (CAP) is an infectious lung disease and particularly affects children under 5 years of age [1–3]. Severe CAP is also a leading cause of childhood morbidity and mortality worldwide, with an estimated 120 million cases per year, of which about 1.3 million cases cause mortality [4]. Children with severe pneumonia often develop various sequelae, including chronic obstructive pulmonary diseases (COPD), bronchiolitis obliterans (BO), pulmonary fibrosis, bronchiectasis, and asthma [5–7]. The cause of childhood pneumonia is complicated. Viral and bacterial infections were the primary environmental cause of severe pneumonia [8].
The matrix metallopeptidase 9 (MMP9) is a member of the matrix metalloproteinase (MMP) enzyme family, which is defined by the zinc ion in the catalytic domain [9]. The MMP enzyme family plays an essential role in the degradation and regulation of extracellular matrix (ECM) proteins [10]. MMP9 belongs to the gelatinase subtype of MMP [11], and is involved in multiple biological processes including proteolytic ECM degradation [12], cell migration and invasion [13], cell adhesion [14], cell interaction [15], apoptosis [16], and angiogenesis [17]. Notably, circulating MMP9 level has been reported as a biomarker for severe CAP [18], COVID-19 [19], ventilator-associated pneumonia [20] and acute respiratory distress syndrome (ARDS) [21]. Mechanistically, MMP9 may contribute to inflammation by promoting the infiltration of leukocyte and proinflammatory macrophages, as demonstrated in experimental glomerulonephritis [22]. A recent study showed that the skin lesion infiltrating neutrophils in psoriasis overexpressed the MMP9 gene and secreted MMP9 protein. MMP9 activates vascular endothelial cells through MAPK signaling pathways and enhances CD4+T cell transmigration in psoriatic pathogenesis [23].
It has been shown that polymorphism in MMP9 were associated with breast cancer risk [24] and chronic venous disease [25]. In addition, MMP9 polymorphism has been suggested to play protective roles in diabetic microvascular complications and pressure ulcers [26, 27]. In summary, MMP9 is associated with inflammatory diseases and is a biomarker for severe pneumonia. However, the associations between MMP9 polymorphism and severe childhood pneumonia need further elucidation.
In order to further investigate the association between MMP9 polymorphism and CAP susceptibility, we conducted a case-control study including a South Chinese population with 1034 cases and 8624 controls to verify the effects of selected SNP. Our results suggest epistatic association of MMP9 rs3918262 SNPs and severe childhood pneumonia.
Methods
Study subjects
We recruited 1034 cases and 8624 controls from Guangzhou Women and Children’s Medical Center. Remnant venous blood samples after clinical examination were collected from the patients. The study was approved by the Medical Ethics Committees of the recruiting hospitals (BF2022-257–01, 2016111853, KY-Q-2021–165-02). Information on demographic characteristics, disease severity, and pathogens were retrieved from each patient’s electronic medical record (EMR).
Inclusion and exclusion criteria of the study subjects
The inclusion criteria for cases were as follows: (1) Age < 16 years old; (2) Patients who were diagnosed with severe pneumonia or non-severe pneumonia according to a published criteria [28]: (a) invasive mechanical ventilation; (b) fluid refractory shock; (c) acute need for noninvasive positive-pressure ventilation; and (d) hypoxemia requiring a fraction of inspired oxygen (FiO2) > inspired concentration or flow feasible in the general-care area.
The exclusion criteria were as follows: (1) known or suspected active tuberculosis; (2) primary immunodeficiency; (3) acquired immunodeficiency syndrome (AIDS) and immunosuppressive medications taken before admission; (4) lack of eligible data or blood samples. Control subjects were recruited from the physical examination centers without a medical history of pneumonia, respiratory diseases, immunodeficiency, and autoimmune disease.
Severe pneumonia was further categorized into two subtypes: Diagnosis as primary pneumonia was defined as primary pneumonia caused by pathogens such as viruses, bacteria, fungus, or mycoplasma. Diagnosis as secondary severe pneumonia was defined as secondary pneumonia caused by other diseases such as cardiovascular diseases or injury.
DNA extraction and genotyping
Genomic DNA from the venous blood samples were extract by TIANamp Blood DNA Kits (Tiangen Biothch, DP335-02, Beijing, China) kit according to the manufacturer’s instructions. The purity and concentrations of DNA were examined by a NanoPhotometer ® N50 (Implen GmbH., Munich, Germany). Then extracted DNA was amplified by an ABI-7900 real-time quantitative PCR instrument (Applied Biosystems, Foster City, CA, USA).
SNP based association analysis
Common SNPs within ± 5 kb flanking of MMP9 were retrieved from “dbSnp153Common” database by using UCSC hgTable, and filtered by the minor allele frequency (MAF > 0.05) in the East Asian (EAS) population. The pairwise linkage disequilibrium (LD) between SNPs were accessed in 1000 Genome EAS population and visualized as LD heatmap by using R package “LDheatmap” [29]. A R2 > 0.8 was viewed as high LD. Tag-SNPs were selected as the minimal set of independent SNPs that represent all SNPs in each LD block.
SNP genotyping and quality control
A Common MMP9 variants were selected using the GTEx portal website (http://www.gtexportal.org/home/) to predict potential associations between the SNP and MMP9 expression levels. For the 1034 severe childhood pneumonia cases and 8624 controls, SNPs were genotyped using a MassARRAY iPLEX Gold system (Sequenom). Hardy–Weinberg equilibrium tests were performed.
Statistical analysis
The χ2 test was applied to test the SNP genotypes for Hardy–Weinberg equilibrium in the control population. The allelic association test examined the difference in allelic frequency distribution between cases and controls. Univariate and multivariate logistic regression models were used to examine the association between genotypes and phenotypes under multiple genetic models, such as additive, codominant, and dominant models. Age and sex were adjusted in the multivariate logistic regression. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated as the effect sizes. P < 0.05 was deemed statistically significant. All analyses were performed by using PLINK (v1.9b).
Bioinformatic analysis
Furthermore, several in silico analyses were conducted to investigate the functional implication of the MMP9 polymorphism. GTEx portal was used to assess the expression quantitative trait locus (eQTL) effects of the SNP (https://gtexportal.org/home/). The selection was made according to the RegulomeDB and FORGEdb database. We kept SNPs with high probability to be regulatory variants (RegulomeDB score higher than 2; https://regulomedb.org/ and FORGEdb score higher than 5; https://forge2.altiusinstitute.org/files/forgedb.html).
MMP9 levels measured by ELISA
Criteria for pneumonia severity and the collection of bronchoalveolar lavage samples were described in our previous study [30]. Bronchoalveolar lavage samples were collected from patients who diagnosed as primary pneumonia and grouped as severe and non-severe patients according to the criteria above. BAL samples were centrifuged at 12,000 rpm for 5 min, and the supernatant was stored at -40 °C until subsequent measurement in ELISA. The expression level of MMP9 was detected according to the manufacturer’s instructions. Briefly, 100 μl of BAL supernatant was added to an antigen-coated plate and incubated with biotinylated detection antibodies and HRP-conjugated molecules. After a 20-min incubation with substrate reagents, the absorbance was measured at 450 nm using an enzyme-linked immunosorbent assay reader (Thermo), and the grouping criteria were the same as before. Non-parametric Kruskal–Wallis test was utilized to compare the differences among the control group, mild pneumonia group, and severe pneumonia group, and p-value < 0.05 was considered statistically significant.
Results
Study subjects
In this study, we assembled a cohort including 1034 severe childhood pneumonia cases and 8426 healthy controls. The demographic features of the participants are shown in Table 1. Patients were aged between 2 days to 15 years, of whom 61.2% were male. Viral (69.7%), bacterial (60.4%) and fungus (10.4%) infections were detected among most of the patients. The percentages of mixed viral-bacterial, viral-fungus, bacterial-fungus, and viral-bacterial-fungus co-infections were 41.1%, 7.4%, 8.0%, and 5.4%, respectively. 75.0% of the patients had received mechanical ventilation, and 94.6% had comorbid conditions. 719 (69.5%) patients had the primary diagnosis as severe pneumonia while others (315, 30.5%) were secondary pneumonia.
Table 1.
Demographics of patients
| Severe pneumonia (n = 1034) | Controls (n = 8426) | |
|---|---|---|
| Age, median (range), years | 0.6 (2 days–15 years) | 6 (1 month–20 years) |
| Gender, n (%) | ||
| Male | 660 (62.1) | 4735 (56.2) |
| Female | 374 (37.9) | 3691 (43.8) |
| Identifier pathogens, n (%) | ||
| Viral | 721 (69.7) | N/A |
| Bacterial | 625 (60.4) | N/A |
| Fugus | 108 (10.4) | N/A |
| Viral + Bacteria | 425 (41.1) | N/A |
| Viral + Fugus | 77 (7.4) | N/A |
| Bacteria + fugus | 83 (8.0) | N/A |
| Viral + Bacteria + Fugus | 56 (5.4) | N/A |
| Comorbid conditions, n (%) | 978 (94.6) | N/A |
| Mechanical ventilation | 776 (75.0) | N/A |
| Diagnosis as primary severe pneumonia | 719 (69.5) | N/A |
| Diagnosis as secondary severe pneumonia | 315 (30.5) | N/A |
N/A Unavailable data
Screening of potential regulatory SNPs in MMP9
We first screened the common SNPs within 5 kb upstream and downstream region of MMP9 gene via UCSC platform. 16 candidate SNPs were further analyzed using linkage disequilibrium (LD) patterns with R2 > 0.8 as a cut-off, and 6 tagSNPs (rs3918251, rs3918254, rs2250889, rs17577, rs3918262, rs9509) in MMP9 gene were selected for genotyping (Fig. 1 and Table S1).
Fig. 1.
Linkage disequilibrium (LD) heatmap for SNPs with high regulatory potential in MMP9. R2 was used to quantify the scale of LD
We then examined the possible regulatory functions of MMP9 SNPs using RegulomeDB and FORGEdb database. RegulomeDB scores of 1–6 indicate most to least likely to affect binding and expression of target gene were assigned for each of the SNPs. We identified 2 SNPs in MMP9, rs3918262 and rs17577, which showed evidence of strong regulatory potential with a score of 2b (Table 2). The other 4 SNPs in MMP9, rs3918251, rs2250889, rs3918254 and rs9509, exhibited less likely regulatory potential (RegulomeDB score = 4–6). Next we used FORGEdb database to predict the possibility of the MMP9 SNPs to be a regulatory variant. FORGEdb scores of from 0 to 10 and higher scores indicate greater likelihood that the SNP is a regulatory variant. Consistent with RegulomeDB results, rs3918262 and rs17577 had a high FORGEdb score of 9 and 10. Thus, we selected rs3918262 and rs17577 as prioritized for further evaluation in this study.
Table 2.
Regulation potentials of risk alleles of MMP9 gene SNP
| RS_Number | Position | Alleles | MAF | Distance | Dprime | R2 | Correlated_Alleles | FORGEdba | RegulomeDBb | Function |
|---|---|---|---|---|---|---|---|---|---|---|
| rs3918262 | chr20:44643770 | (A/G) | 0.370 | 4989 | 1.000 | 0.332 | A = A, G = G | 8 | 2b | NA |
| rs17577 | chr20:44643111 | (G/A) | 0.168 | 4330 | 0.166 | 0.010 | NA | 10 | 2b | missense |
| rs3918251 | chr20:44638781 | (A/G) | 0.361 | 0 | 1.000 | 1.000 | A = A, G = G | 8 | 4 | NA |
| rs2250889 | chr20:44642406 | (G/C) | 0.250 | 3625 | 1.000 | 0.591 | G = A, C = G | 8 | 4 | missense |
| rs3918254 | chr20:44640391 | (C/T) | 0.188 | 1610 | 0.929 | 0.112 | C = A, T = G | 9 | 4 | NA |
| rs9509 | chr20:44645153 | (T/C) | 0.221 | 6372 | 0.478 | 0.037 | NA | 8 | 6 | NA |
5 SNPs with high regulatory potentials were selected by securing FORGEdb and RegulomeDB. SNPs selected for subsequent analyses were marked in bold
Abbreviations: MAF Minor Allele Frequency, MAF was presented as (MAF in cases)/(MAF in controls), Dprime SNP haplotype frequency, R2 SNP correlation, Correlated alleles refer to alleles that are correlated if linkage disequilibrium is present (R2 > 0.1). rs3918251 was used as reference SNP
aFORGEdb scores indicated the regulatory potentials for SNPs (https://forge2.altiusinstitute.org/files/forgedb.html). FORGEdb scores of from 0 to 10 and higher scores indicate greater likelihood that the SNP is a regulatory variant
bRegulomeDB scores indicated the regulatory potentials for SNPs (https://regulomedb.org/regulome-search). RegulomeDB scores of 1–6 indicate most to least likely to affect binding and expression of target gene. A score 2b denotes that the SNP was within a potential regulatory region supported by TF binding, binding motif, DNase Foorprint, and DNase peak data
Associations between MMP9 polymorphism and the risk of severe pneumonia
Compared with the control subjects, we found that rs3918251, rs2250889 and rs3918262 showed statistical significance after Bonferroni correction for multiple testing. The minor A allele frequency of rs3918251 (0.26 vs. 0.30, OR = 0.83, 95% CI = 0.75–0.92, P = 0.0006), G allele frequency of rs2250889 (0.19 vs. 0.22, OR = 0.84, 95% CI = 0.74–0.94, P = 0.0023) and A allele frequency of rs2250889 (0.11 vs. 0.13, OR = 0.84, 95% CI = 0.73–0.97, P = 0.0198) was significantly associated with decreased risk of severe pneumonia. In contrast, the minor G allele frequency of rs3918262 (0.45 vs. 0.42, OR = 1.16, 95% CI = 1.05–1.27, P = 0.0024) and rs3918254 (0.22 vs. 0.20, OR = 1.13, 95% CI = 1.01–1.27, P = 0.0279) were significantly associated with increased risk of severe pneumonia (Table 3). Moreover, rs3918251 and rs3918262 SNPs have prominent effects on homozygous children compared with that on heterozygous children, whereas rs17577 affects heterozygous individuals (p < 0.0042) (Table 3).
Table 3.
Risk allele frequency of MMP9 gene SNPs in the case-control data set of children with severe pneumonia
| CHR | BP | SNP | Major allele | Minor allele | Minor Allele Frequency in Cases | Minor Allele Frequency in Controls | Test | OR (95% CI) | P |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 44638781 | rs3918251 | G | A | 0.265 | 0.302 | Allelic | 0.832 (0.748–0.925) | 0.0006 |
| Heterozygous | 0.867 (0.755–0.996) | 0.0442 | |||||||
| Homozygous | 0.651 (0.496–0.854) | 0.0019 | |||||||
| 20 | 44642406 | rs2250889 | C | G | 0.191 | 0.220 | Allelic | 0.843 (0.744–0.938) | 0.0023 |
| Heterozygous | 0.841 (0.730–0.969) | 0.0165 | |||||||
| Homozygous | 0.698 (0.496–0.983) | 0.0392 | |||||||
| 20 | 44643770 | rs3918262 | A | G | 0.453 | 0.418 | Allelic | 1.156 (1.052–1.269) | 0.0024 |
| Heterozygous | 1.019 (0.877–1.184) | 0.8066 | |||||||
| Homozygous | 1.374 (1.143–1.652) | 0.0007 | |||||||
| 20 | 44643111 | rs17577 | G | A | 0.114 | 0.132 | Allelic | 0.835 (0.731–0.973) | 0.0198 |
| Heterozygous | 0.782 (0.664–0.921) | 0.0032 | |||||||
| Homozygous | 1.082 (0.666–1.759) | 0.7496 | |||||||
| 20 | 44640391 | rs3918254 | C | T | 0.218 | 0.198 | Allelic | 1.135 (1.014–1.271) | 0.0279 |
| Heterozygous | 1.068 (0.927–1.232) | 0.3619 | |||||||
| Homozygous | 1.474 (1.091–1.991) | 0.0114 | |||||||
| 20 | 44645153 | rs9509 | T | C | 0.232 | 0.219 | Allelic | 1.082 (0.970–1.207) | 0.1588 |
| Heterozygous | 1.016 (0.883–1.168) | 0.8280 | |||||||
| Homozygous | 1.326 (1.001–1.756) | 0.0491 |
P value adjusted by gender and age. The P-values were corrected by Bonferroni’s method with a threshold of 0.0042. Calculation of the OR was also based on the minor allele of each SNP
Abbreviations: CHR Chromosome, BP Base pair (where the SNP is located), SNP Single-nucleotide polymorphism, OR Odds ratio, CI Confidence interval
Nominal significance of MMP9 SNPs with severe pneumonia subtype
To explore the association between MMP9 polymorphism and the severe pneumonia subtype, we further divided our cohort into two subgroups: the primary diagnosis as severe pneumonia (n = 719) and the secondary diagnosis as severe pneumonia (n = 315). Results showed that the minor G allele of rs3918262 SNP showed evidence of a trend towards significance (P = 0.08) between those subgroups (Table 4). None of the other 5 SNPs (rs3918251, rs3918254, rs2250889, rs17577, rs9509) of MMP9 gene were significantly different between two group (Table 4).
Table 4.
Risk allele frequency of MMP9 gene SNPs between severe pneumonia subgroups
| CHR | BP | SNP | Major allele | Minor allele | Minor Allele Frequency in primary pneumonia | Minor Allele Frequency in secondary pneumonia | OR (95% CI) | P |
|---|---|---|---|---|---|---|---|---|
| 20 | 44643770 | rs3918262 | A | G | 0.440 | 0.484 | 0.846 (0.699–1.026) | 0.0888 |
| 20 | 44643111 | rs17577 | G | A | 0.120 | 0.010 | 1.212 (0.889–1.654) | 0.2231 |
| 20 | 44638781 | rs3918251 | G | A | 0.270 | 0.250 | 1.118 (0.888–1.409) | 0.3420 |
| 20 | 44642406 | rs2250889 | C | G | 0.196 | 0.181 | 1.110 (0.865–1.426) | 0.4114 |
| 20 | 44640391 | rs3918254 | C | T | 0.222 | 0.209 | 1.074 (0.846–1.363) | 0.5565 |
| 20 | 44645153 | rs9509 | T | C | 0.233 | 0.229 | 1.020 (0.812–1.282) | 0.8628 |
The P-values were corrected by Bonferroni’s method with a threshold of 0.0042. Calculation of the OR was also based on the minor allele of each SNP
Abbreviations: CHR Chromosome, BP Base pair (where the SNP is located), SNP Single-nucleotide polymorphism, OR Odds ratio, CI Confidence interval, P P-value adjusted by gender and age
Expression quantitative trait loci (eQTLs) of MMP9 SNPs
To identify functional effects of MMP9 variants, we conducted expression quantitative trait loci (eQTL) analysis on 6 SNPs of MMP9. As shown in Table 5, eQTL analysis indicated that the minor alleles of rs3918251, rs17577 and rs3918262 were not only associated with the increased mRNA expression (Effect size > 0) of MMP9, and but also its vicinity gene CD40 (Fig. 1). From GTEx database, those SNPs affected MMP9 and CD40 mRNA expression within subcutaneous adipose, skin and testis, whereas the minor allele of rs3918251 may downregulate the mRNA expression of MMP9 in cultured fibroblasts (Table 5).
Table 5.
eQTL results curated from GTEx database for SNPs on of risk alleles of MMP9 gene
| RS ID | R2 | Gene Symbol | Tissue | Effect Size | P |
|---|---|---|---|---|---|
| rs3918251 | 1 | MMP9 | Cells - Cultured fibroblasts | -0.203 | 8.55E-08 |
| rs3918251 | 1 | CD40 | Adipose - Subcutaneous | 0.156 | 1.51E-05 |
| rs3918251 | 1 | MMP9 | Skin - Not Sun Exposed (Suprapubic) | 0.259 | 2.46E-07 |
| rs3918251 | 1 | MMP9 | Skin - Sun Exposed (Lower leg) | 0.182 | 4.12E-05 |
| rs3918251 | 1 | CD40 | Skin - Sun Exposed (Lower leg) | 0.136 | 1.20E-04 |
| rs17577 | 1 | MMP9 | Testis | 0.348 | 8.02E-05 |
| rs3918261 | 1 | MMP9 | Testis | 0.340 | 1.16E-04 |
| rs3918262 | 1 | CD40 | Adipose - Subcutaneous | 0.181 | 2.65E-05 |
| rs3918262 | 1 | CD40 | Skin - Sun Exposed (Lower leg) | 0.177 | 4.05E-05 |
Functional relevance of SNP on gene expression in GTEx database. Significant eQTL results of MMP9 SNPs on the nearby genes were summarized
eQTL Expression quantitative trait loci, Effect size The degree to which an SNP influences the expression of genes in a particular tissue
Levels of MMP9 in the bronchoalveolar lavages (BALs) of children with severe pneumonia
In order to validate the levels of MMP9 proteins in the lung of children with severe pneumonia, we measured MMP9 concentrations by ELISA within the BAL samples freshly collected from children with primary pneumonia, with control samples taken from patients without a concurrent infection but requiring surgical removal of inhaled foreign objects. Patients were further grouped as severe and non-severe patients according to the criteria in Methods. We found that compared with control and non-severe subjects, the MMP9 levels were significantly increased in severe pneumonia patients, whereas no significant difference was found between the control and non-severe groups (Fig. 2). We further evaluated the effects of the MMP9 SNPs on the measured MMP9 protein levels from ELISA in the severe patient group. BAL MMP9 levels were significantly increased in patients with rs3918251 AG/AA genotype when compared with GG genotype (P < 0.05), whereas the remaining SNPs show no significant association with the expression of MMP9 protein (P value ranged from 0.124 to 0.815) (sFig. 1).
Fig. 2.
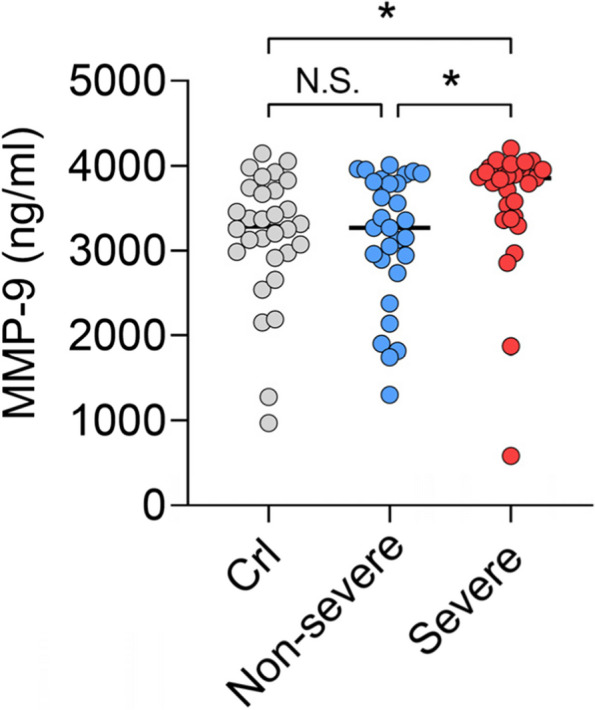
MMP9 concentrations in the BALs of children in control group (n = 30), non-severe pneumonia group (n = 30) and severe pneumonia group (n = 29). (*:P < 0.05)
Discussion
Community-acquired pneumonia is a common cause of morbidity and mortality in children under 5 years [31]. Although most of the cases are self-limiting disease, around 13% of severe pneumonia cases in PICU unfortunately pass away [32]. Serious sequelae, such as bronchiolitis obliterans (BO), pulmonary fibrosis and bronchiectasis [5–7], are another burden for those who recover from severe pneumonia. Risk factors of severe pneumonia are numerous, including age, immunodeficiencies, malnutrition, chronic lung diseases, and cystic congenital thoracic malformations [33]. Previous research has documented the existence of gender disparities in community-acquired pneumonia [34]. Consistent with these findings, our cohort also exhibited a gender distinction, as over 50% of the patients were male.
The matrix metalloproteinase (MMP) family plays essential roles in lung organogenesis and the pathogenesis of inflammatory diseases [35]. Dysregulation of MMP contributes to a series of lung tissue damage and disorders such as asthma [36], idiopathic pulmonary fibrosis (IPF) [37], emphysema [38], ARDS [39] and COPD [40]. MMP are also involved in the remodeling of lung after inflammation [41, 42]. Among MMP family members, matrix metalloproteinase MMP9 is enriched in lungs of asthma, IPF and COPD, and also promotes lung remodeling [43]. Associations are also found for MMP9 polymorphisms with lung cancer [44], COPD [45] and asthma [46].
In this study, we first screened the common SNP within MMP9 gene and located 6 candidate SNPs (Table S1). Then we identified rs3918262 and rs17577 as priority SNP by RegulomeDB and FORGEdb analysis (Table 2). Notably, our study showed that the G allele frequency of rs3918262 was significantly associated with increased risk of severe pneumonia (Table 3). No previous study has mentioned the function of this allele. Moreover, rs3918262 showed evidence of a trend towards significance between patients primary or secondary diagnosed as severe or pneumonia (Table 4). Secondary pneumonia was defined as pneumonia caused by non-respiratory reasons [47]. This finding suggests that the minor allele of G of rs3918262 is potentially associated with infections.
From expression quantitative trait loci (eQTL) analysis, we found SNP rs17477 and rs3918262 of MMP9 gene potential regulatory effect on MMP9 or CD40 expression. As CD40 gene is located downstream of the MMP9 gene and in its vicinity, these SNPs may influence the expression of the CD40 gene. In addition, CD40 has been reported to be associated with inflammatory immune diseases such as Kawasaki disease [48], suggesting its potential relevance in regulating pneumonia as well. Most of the alleles exhibit up-regulated effects as the effect sizes were > 0. However, no significant difference was detected in lung tissue. Nevertheless, results from other organs suggest that these SNPs may up-regulate the protein expression of MMP9 or CD40.
Additionally, eQTL, RegulomeDB and FORGEdb results suggest that rs17577, which is located in the coding region of MMP9 gene, had strong regulatory potential on gene expression. Rs17577 was reported to be associated with pediatric asthma [49, 50], breast cancer [51], and ischemic stroke [52] etc. Although the P-value (P = 0.0198) of rs17577 SNP between control and pneumonia group did not meet the threshold (0.00083) of Bonferroni’s correction, this SNP may have important impact on MMP9 expression and potentially was a risk factor of severe pneumonia. It should be noted that no previous study reported about the regulatory functions of the minor allele of G of rs3918262.
Finally, we measure the MMP9 protein levels in the BAL of children with severe or non-severe pneumonia. Results showed that the MMP9 concentrations were up-regulated in severe patients (Fig. 2). In the literature, MMP9 is produced by activated alveolar macrophages and neutrophils, catalyzes the proteolysis of the extracellular matrix and plays a role in leukocyte migration [53, 54]. Taken together with our regulatory results, MMP9 SNPs may contribute to severe pneumonia susceptibility through regulatory properties which may impact MMP9 translational efficiency and protein level. However, these remain to be investigated experimentally in the future. Finally, we demonstrated that protein levels of MMP9 were significantly increased in the BAL of patient with severe pneumonia, suggesting that MMP9 polymorphism may associated with the expression levels of MMP9 in severe pneumonia.
There are some limitations for this study. First, only children were included in our cohort which may reduce the statistical power of the study. Second, the regulatory effects of the SNPs should be validated by subsequent experiments. Finally, only Chinese Han subjects from Southern China were enrolled in this study, and the conclusions should be extrapolated to other ethnic groups with caution.
Conclusions
Nevertheless, this study indicates that the polymorphisms in MMP9 are associated with the susceptibility to severe pneumonia in Southern Chinese children. Future study may elucidate mechanisms of action and reveal whether MMP9 may serve as a potential biomarker or therapeutic target for severe childhood pneumonia.
Supplementary Information
Additional file 1: Table S1. Final SNP Clip.
Additional file 2: Supplemental figure 1. BAL MMP9 levels of severe pneumonia patients with rs3918251 GG of AG/AA genotype (*:P < 0.05).
Acknowledgements
We thank the patients and their guardians for participating in this work and the Clinical Biological Resource Bank for assistance with cohort recruitment.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- ARDS
Acute respiratory distress syndrome
- BALs
Bronchoalveolar lavages
- BO
Bronchiolitis obliterans
- BP
Base pair
- CAP
Community-acquired pneumonia
- CD
Cluster of differentiation
- CHR
Chromosome
- CIs
Confidence intervals
- COPD
Chronic obstructive pulmonary
- COVID-19
Coronavirus disease 2019
- DNA
Deoxyribonucleic acid
- EAS
East Asian
- ECM
Extracellular matrix
- ELISA
Enzyme linked immunosorbent assay
- EMR
Electronic medical record
- eQTL
Expression quantitative trait loci
- FiO2
Fraction of inspired oxygen
- HRP
Horseradish peroxidase
- HWE
Hardy-Weinberg equilibrium
- IPF
Idiopathic pulmonary fibrosis
- LD
Linkage disequilibrium
- MAF
Minor Allele Frequency
- MAPK
Mitogen-activated protein kinase
- MMP9
Matrix Metallopeptidase 9
- MMP
Matrix metalloproteinase
- mRNA
Messenger RNA
- OR
Odds ratios
- PCR
Polymerase chain reaction
- PICU
Pediatric intensive care unit
- RNA
Ribonucleic acid
- REF
Major allele
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SNP
Single nucleotide polymorphism
Authors’ contributions
B.L. conceived the ideas and supervised the project. B.L., L.C. and L.M. assembled the pneumonia cohort. L.C., F.X. and B.L. performed the experiments. X.Z. performed bioinformatics analysis. B.L. wrote the manuscript with significant input from F.X., Y.Z. and X.Z.. All authors discussed and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82001676 to B.L.).
Availability of data and materials
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Human research was approved by the Medical Ethics Committees of the Guangdong Provincial Hospital of Traditional Chinese Medicine (KY-Q-2021-165-02), Guangzhou Women and Children’s Medical Center (2016111853) and Guangdong Provincial People’s Hospital (KY-Q-2021-165-02). Written informed consent to participate was taken from the legal guardians of all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Cai and Xiaoyu Zuo contributed equally to this work.
Contributor Information
Falin Xu, Email: xufalin72@126.com.
Bingtai Lu, Email: lubingtaip@163.com.
References
- 1.Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, Feikin DR, Mackenzie GA, Moisi JC, Roca A, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381(9875):1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86(5):408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tramper-Stranders GA. Childhood community-acquired pneumonia: a review of etiology- and antimicrobial treatment studies. Paediatr Respir Rev. 2018;26:41–48. doi: 10.1016/j.prrv.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmond K, Scott S, Korczak V, Ward C, Sanderson C, Theodoratou E, Clark A, Griffiths U, Rudan I, Campbell H. Long term sequelae from childhood pneumonia; systematic review and meta-analysis. PLoS One. 2012;7(2):e31239. doi: 10.1371/journal.pone.0031239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brightling C, Greening N. Airway inflammation in COPD: progress to precision medicine. Eur Respir J. 2019;54(2):1900651. doi: 10.1183/13993003.00651-2019. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Wang D, Wang L, Wang S, Roden AC, Zhao H, Li X, Prakash YS, Matteson EL, Tschumperlin DJ, et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L487–L497. doi: 10.1152/ajplung.00301.2018. [DOI] [PubMed] [Google Scholar]
- 8.Shah SN, Bachur RG, Simel DL, Neuman MI. Childhood pneumonia. JAMA. 2017;318(5):490. doi: 10.1001/jama.2017.9428. [DOI] [PubMed] [Google Scholar]
- 9.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoor C, Vaidya S, Wadhwan V, Hitesh, Kaur K, Pathak A. Seesaw of matrix metalloproteinases (MMPs) J Cancer Res Ther. 2016;12(1):28–35. doi: 10.4103/0973-1482.157337. [DOI] [PubMed] [Google Scholar]
- 11.Choe G, Park JK, Jouben-Steele L, Kremen TJ, Liau LM, Vinters HV, Cloughesy TF, Mischel PS. Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin Cancer Res. 2002;8(9):2894–2901. [PubMed] [Google Scholar]
- 12.Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur J Med Chem. 2020;194:112260. doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 13.Gam DH, Park JH, Kim JH, Beak DH, Kim JW. Effects of Allium sativum stem extract on growth and migration in melanoma cells through inhibition of VEGF, MMP-2, and MMP-9 genes expression. Molecules. 2021;27(1):21. doi: 10.3390/molecules27010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Wang W, Sun S, Xu Y, Fang Z, Cong L. Expression and potential role of MMP-9 in intrauterine adhesion. Mediators Inflamm. 2021;2021:6676510. doi: 10.1155/2021/6676510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulli T, Koivunen E, Hyypia T. Cell-surface interactions of echovirus 22. J Biol Chem. 1997;272(34):21176–21180. doi: 10.1074/jbc.272.34.21176. [DOI] [PubMed] [Google Scholar]
- 16.Yao X, Jiang W, Yu D, Yan Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019;10(2):703–712. doi: 10.1039/C8FO02013B. [DOI] [PubMed] [Google Scholar]
- 17.Bellafiore M, Battaglia G, Bianco A, Farina F, Palma A, Paoli A. The involvement of MMP-2 and MMP-9 in heart exercise-related angiogenesis. J Transl Med. 2013;11:283. doi: 10.1186/1479-5876-11-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang TY, Yu YL, Lin CW, Tsao SM, Yang SF, Yeh CB. The circulating level of MMP-9 and its ratio to TIMP-1 as a predictor of severity in patients with community-acquired pneumonia. Clin Chim Acta. 2013;424:261–266. doi: 10.1016/j.cca.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Carolina D, Couto AES, Campos LCB, Vasconcelos TF, Michelon-Barbosa J, Corsi CAC, Mestriner F, Petroski-Moraes BC, Garbellini-Diab MJ, Couto DMS, et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed Pharmacother. 2021;142:112067. doi: 10.1016/j.biopha.2021.112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng W, Cao X, Sun W, Zheng L, Fan B, Zhou S, Liu H, Wang H, Wang W, Liu X. A functional polymorphism at the miR4915p binding site in the 3’untranslated region of the MMP9 gene increases the risk of developing ventilator associated pneumonia. Int J Mol Med. 2021;48(6):217. doi: 10.3892/ijmm.2021.5050. [DOI] [PubMed] [Google Scholar]
- 21.Lanchou J, Corbel M, Tanguy M, Germain N, Boichot E, Theret N, Clement B, Lagente V, Malledant Y. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit Care Med. 2003;31(2):536–542. doi: 10.1097/01.CCM.0000048626.02184.F8. [DOI] [PubMed] [Google Scholar]
- 22.Kluger MA, Zahner G, Paust HJ, Schaper M, Magnus T, Panzer U, Stahl RA. Leukocyte-derived MMP9 is crucial for the recruitment of proinflammatory macrophages in experimental glomerulonephritis. Kidney Int. 2013;83(5):865–877. doi: 10.1038/ki.2012.483. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Zhu Z, Li Q, Lin Y, Dang E, Meng H, Sha N, Bai H, Wang G, An S, et al. Neutrophils enhance cutaneous vascular dilation and permeability to aggravate psoriasis by releasing matrix metallopeptidase 9. J Invest Dermatol. 2021;141(4):787–799. doi: 10.1016/j.jid.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Felizi RT, Veiga MG, Carelli Filho I, Souto RPD, Fernandes CE, Oliveira E. Association between matrix metallopeptidase 9 polymorphism and breast cancer risk. Rev Bras Ginecol Obstet. 2018;40(10):620–624. doi: 10.1055/s-0038-1673366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slonkova V, Slonkova V, Jr, Vasku A, Vasku V. Genetic predisposition for chronic venous insufficiency in several genes for matrix metalloproteinases (MMP-2, MMP-9, MMP-12) and their inhibitor TIMP-2. J Eur Acad Dermatol Venereol. 2017;31(10):1746–1752. doi: 10.1111/jdv.14447. [DOI] [PubMed] [Google Scholar]
- 26.Latifa K, Sondess S, Hajer G, Manel BH, Souhir K, Nadia B, Abir J, Salima F, Abdelhedi M. Evaluation of physiological risk factors, oxidant-antioxidant imbalance, proteolytic and genetic variations of matrix metalloproteinase-9 in patients with pressure ulcer. Sci Rep. 2016;6:29371. doi: 10.1038/srep29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Wu X, Cai T, Gao W, Zhou X, Zhao J, Yao J, Shang H, Dong J, Liao L. Matrix metalloproteinase 9 gene promoter (rs 3918242) mutation reduces the risk of diabetic microvascular complications. Int J Environ Res Public Health. 2015;12(7):8023–8033. doi: 10.3390/ijerph120708023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M, Lu B, Fan H, Guo X, Du S, Yang D, Xu Y, Li Y, Che D, Liu Y, et al. Heightened local Th17 cell inflammation is associated with severe community-acquired pneumonia in children under the age of 1 year. Mediators Inflamm. 2021;2021:9955168. doi: 10.1155/2021/9955168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin JH, Blay S, McNeney B, Graham J. LDheatmap: an R function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J Stat Softw. 2006;16(4):1–9. [Google Scholar]
- 30.Lu B, Liu M, Wang J, Fan H, Yang D, Zhang L, Gu X, Nie J, Chen Z, Corbett AJ, et al. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol. 2020;13(5):824–835. doi: 10.1038/s41385-020-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun KW, Wallihan R, Juergensen A, Mejias A, Ramilo O. Community-acquired pneumonia in children: myths and facts. Am J Perinatol. 2019;36(S 02):S54–S57. doi: 10.1055/s-0039-1691801. [DOI] [PubMed] [Google Scholar]
- 32.Koh J, Wong JJ, Sultana R, Wong PPC, Mok YH, Lee JH. Risk factors for mortality in children with pneumonia admitted to the pediatric intensive care unit. Pediatr Pulmonol. 2017;52(8):1076–1084. doi: 10.1002/ppul.23702. [DOI] [PubMed] [Google Scholar]
- 33.de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet. 2020;396(10253):786–798. doi: 10.1016/S0140-6736(20)31550-6. [DOI] [PubMed] [Google Scholar]
- 34.Corica B, Tartaglia F, D’Amico T, Romiti GF, Cangemi R. Sex and gender differences in community-acquired pneumonia. Intern Emerg Med. 2022;17(6):1575–1588. doi: 10.1007/s11739-022-02999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87(1):69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demedts IK, Brusselle GG, Bracke KR, Vermaelen KY, Pauwels RA. Matrix metalloproteinases in asthma and COPD. Curr Opin Pharmacol. 2005;5(3):257–263. doi: 10.1016/j.coph.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015;53(5):585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gharib SA, Manicone AM, Parks WC. Matrix metalloproteinases in emphysema. Matrix Biol. 2018;73:34–51. doi: 10.1016/j.matbio.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aschner Y, Zemans RL, Yamashita CM, Downey GP. Matrix metalloproteinases and protein tyrosine kinases: potential novel targets in acute lung injury and ARDS. Chest. 2014;146(4):1081–1091. doi: 10.1378/chest.14-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navratilova Z, Kolek V, Petrek M. Matrix metalloproteinases and their inhibitors in chronic obstructive pulmonary disease. Arch Immunol Ther Exp (Warsz) 2016;64(3):177–193. doi: 10.1007/s00005-015-0375-5. [DOI] [PubMed] [Google Scholar]
- 41.Golestani R, Razavian M, Ye Y, Zhang J, Jung JJ, Toczek J, Gona K, Kim HY, Elias JA, Lee CG, et al. Matrix metalloproteinase-targeted imaging of lung inflammation and remodeling. J Nucl Med. 2017;58(1):138–143. doi: 10.2967/jnumed.116.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu G, Philp AM, Corte T, Travis MA, Schilter H, Hansbro NG, Burns CJ, Eapen MS, Sohal SS, Burgess JK, et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther. 2021;225:107839. doi: 10.1016/j.pharmthera.2021.107839. [DOI] [PubMed] [Google Scholar]
- 43.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28(1):12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Jia MX, Wang JH, Lu JL, Deng J, Tang JX, Liu C. Association of MMP9-1562C/T and MMP13-77A/G polymorphisms with non-small cell lung cancer in southern Chinese population. Biomolecules. 2019;9(3):107. doi: 10.3390/biom9030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito I, Nagai S, Handa T, Muro S, Hirai T, Tsukino M, Mishima M. Matrix metalloproteinase-9 promoter polymorphism associated with upper lung dominant emphysema. Am J Respir Crit Care Med. 2005;172(11):1378–1382. doi: 10.1164/rccm.200506-953OC. [DOI] [PubMed] [Google Scholar]
- 46.Nakashima K, Hirota T, Obara K, Shimizu M, Doi S, Fujita K, Shirakawa T, Enomoto T, Yoshihara S, Ebisawa M, et al. A functional polymorphism in MMP-9 is associated with childhood atopic asthma. Biochem Biophys Res Commun. 2006;344(1):300–307. doi: 10.1016/j.bbrc.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 47.Bacharach T, Nelson HS. Secondary pneumonia. JAMA. 1962;181:1135–1136. doi: 10.1001/jama.1962.03050390037012a. [DOI] [PubMed] [Google Scholar]
- 48.Huang FY, Chang TY, Chen MR, Chiu NC, Chi H, Lee HC, Lin SP, Chen CK, Chan HW, Chen WF, et al. Genetic polymorphisms in the CD40 ligand gene and Kawasaki disease. J Clin Immunol. 2008;28(5):405–410. doi: 10.1007/s10875-008-9203-6. [DOI] [PubMed] [Google Scholar]
- 49.Zou F, Zhang J, Xiang G, Jiao H, Gao H. Association of matrix metalloproteinase 9 (MMP-9) polymorphisms with asthma risk: a meta-analysis. Can Respir J. 2019;2019:9260495. doi: 10.1155/2019/9260495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jimenez-Morales S, Martinez-Aguilar N, Gamboa-Becerra R, Jimenez-Ruiz JL, Lopez-Ley D, Lou H, Saldana-Alvarez Y, Dean M, Orozco L. Polymorphisms in metalloproteinase-9 are associated with the risk for asthma in Mexican pediatric patients. Hum Immunol. 2013;74(8):998–1002. doi: 10.1016/j.humimm.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 51.Yan C, Sun C, Lu D, Zhao T, Ding X, Zamir I, Tang M, Shao C, Zhang F. Estimation of associations between MMP9 gene polymorphisms and breast cancer: evidence from a meta-analysis. Int J Biol Markers. 2022;37(1):13–20. doi: 10.1177/17246008221076145. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Zhang L, Huang H, Qin X, Huang Z, Lan J, Xu S, Tang H, Huang C. Relationship between the matrix metalloproteinase-9 gene polymorphisms and ischemic stroke. Int J Clin Exp Pathol. 2019;12(3):949–956. [PMC free article] [PubMed] [Google Scholar]
- 53.Takino T, Koshikawa N, Miyamori H, Tanaka M, Sasaki T, Okada Y, Seiki M, Sato H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene. 2003;22(30):4617–4626. doi: 10.1038/sj.onc.1206542. [DOI] [PubMed] [Google Scholar]
- 54.Zhou M, Huang SG, Wan HY, Li B, Deng WW, Li M. Genetic polymorphism in matrix metalloproteinase-9 and the susceptibility to chronic obstructive pulmonary disease in Han population of south China. Chin Med J (Engl) 2004;117(10):1481–1484. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Final SNP Clip.
Additional file 2: Supplemental figure 1. BAL MMP9 levels of severe pneumonia patients with rs3918251 GG of AG/AA genotype (*:P < 0.05).
Data Availability Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.



