Abstract
Background and aim
Interventional cardiologists face challenges in managing chronic total occlusion (CTO) lesions, with conflicting results when comparing rotational atherectomy (RA) to conventional PCI. This meta-analysis aims to provide a critical evaluation of the safety and feasibility of RA in CTO lesions.
Methods
PubMed, Scopus, Web of Science, Ovid, and Cochrane central library until April 2023 were searched for relevant studies. MACE was our primary outcomes, other outcomes were all cause of death, cardiac death, MI, and TVR. Also, we reported angiographic outcomes as technical success, procedural success, and procedural complications in a random effect model. The pooled data was analyzed using odds ratio (OR) with its 95% CI using STATA 17 MP.
Results
Seven studies comprising 5494 patients with a mean follow-up of 43.1 months were included in this meta-analysis. Our pooled analysis showed that RA was comparable to PCI to decrease the incidence of MACE (OR = 0.98, 95% CI [0.74 to 1.3], p = 0.9). Moreover, there was no significant difference between RA and conventional PCI in terms of other clinical or angiographic outcomes.
Conclusion
Our study showed that RA had comparable clinical and angiographic outcomes as conventional PCI in CTO lesions, which offer interventional cardiologists an expanded perspective when addressing calcified lesions.
PROSPERO registration
CRD42023417362.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-023-03673-2.
Keywords: RA, CTO lesions, Non-CTO lesions, PCI
Introduction
A chronic total occlusion (CTO) of coronary vessel is defined a complete blockage of the vessel, with no blood flow through the blocked segment according to the Thrombolysis in Myocardial Infarction (TIMI) grading system, and an estimated occlusion duration of ≥ 3 months [1–3]. CTOs are commonly seen in practice, accounting for approximately 20% of all patients referred for coronary angiography [4, 5]. Between 30 and 50% of patients with established coronary artery disease (CAD) have CTOs [5], and their prevalence is higher in patients who have undergone coronary artery bypass graft (CABG) surgery [4–6].
In general, percutaneous coronary intervention (PCI) for patients with CTO is a challenging procedure and requires not only patience during manipulation, but also requires experience with various instruments and interventional techniques [7, 8]. With the advent of hybrid treatment strategies, the recent success rate for treating CTO using conventional procedures has reached 60–92% when performed by skilled interventionists [9, 10]. However, heavily calcified stenoses present a challenge due to their refractory plaque burden and uneven lesion surface. This can be a contributing factor in the failure of stent delivery or insufficient stent expansion [11–14]. During CTO PCI, the most common retrograde CTO crossing technique is reverse controlled antegrade and retrograde subintimal tracking (reverse CART) [15]. When performing retrograde CTO-PCI in severely calcified lesions, the use of retrograde crossing techniques, in particular reverse CART, has been thought to carry a relatively high risk of dissection and perforation after rotational atherectomy (RA) in these lesions [16].
Rotational atherectomy has been considered to be the most promising intervention in the treatment of CTO lesions and might improve the procedure success rate and it is increasingly applied for the preparation of certain calcified CTOs [17, 18]. Rotational atherectomy is a process that involves cutting a portion of the obstructive atheroma to enable balloon dilatation, plaque fracture, stent delivery, and expansion [19, 20]. It is utilized for complete lesion preparation prior to stenting as well as device passage [21]. However, despite the potential for short-term results of CTO PCI, it has been observed that it might be consistent with a higher possibility of peri-procedural complications [22]. Moreover, the long-term clinical outcome of CTO lesions treated by RA-assisted PCI remains unclear.
At present, the optimal treatment for CTO lesions is still debatable due to conflicting results from the randomized controlled trials (RCTs) comparing RA to non-RA strategies in managing CTO lesions. To date, no systematic review and meta-analysis has compared the preparation of calcified coronary lesions with an atherectomy-based strategy to non-RA PCI in the treatment of CTO lesions. Therefore, we conducted a systematic review and meta-analysis to compare the outcomes and efficacy of these approaches.
Methods
We followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement guidelines when performing this systematic review and meta-analysis [23]. The method was carried out in accordance with the Cochrane handbook of systematic reviews and meta-analysis of interventions (version 5.1.0).
Eligibility criteria
We considered all relevant trials that reported RA as the interventional group and conventional PCI method as the comparable group in patients with chronic total occlusion and reported our primary and secondary outcomes of interest. We excluded irrelevant papers of animal studies, non-English studies, data of unpublished studies, or data from conference abstracts.
Primary and secondary outcomes
The primary outcome of interest was the incidence of major adverse cardiac events (MACE) as a clinical outcome of interest. Other secondary outcomes were all cause of death, cardiac death, myocardial infarction (MI), and target vessel revascularization (TVR). Also, we reported angiographic outcomes as technical success, procedural success, and procedural complications. The definitions of these outcomes were based on the descriptions provided by the authors of the individual studies.
Literature search
We performed a comprehensive literature search on PubMed, Scopus, Web of Science, and Cochrane Library, from inception until April 2023, using this search query: (“rotational atherectomy” OR RA) AND (“coronary artery disease” OR “chronic total occlusion” OR CTO OR “CTO lesions” OR “CTO-PCI”) AND (“non-RA” OR “without RA” OR “stenting without RA” OR “conventional PCI” OR “standard PCI”) AND (“percutaneous coronary intervention” OR PCI OR “drug-eluting stent*” OR DES). All duplicates were removed by EndNote and manual backward citation analysis was done for all the references of the included studies.
Screening of the literature search results
The literature search results were screened in a two-step process. Initially, the titles and abstracts of all articles were assessed for eligibility. Subsequently, full-text screening was conducted for the studies that met the eligibility criteria.
Data extraction
Data from the included studies was extracted and recorded in a standardized data extraction sheet. The extracted data encompassed four main categories: (1) Characteristics of the included studies, (2) Characteristics of the study population, (3) Risk of bias domains, and (4) Outcome measures, which included MACE, All-Cause Death, TVR, MI, stent thrombosis, and cardiac death.
Synthesis of results
For outcomes that involved dichotomous data, the frequency of events and the total number of patients in each group were combined to calculate the odds ratio (OR) with its 95% confidence interval (CI) using the DerSimonian-Laird random-effect model, moreover, a P value less than 0.05 was considered as a significant result. In cases where studies reported data at multiple time points, the last endpoint was considered for the primary analysis. All statistical analyses were conducted using StataMP version 17 for Mac.
Assessment of Heterogeneity
Statistical heterogeneity among studies was evaluated by the Chi-square test (Cochrane Q test). Next, the chi-square statistic, Cochrane Q, was used to calculate the I-squared according to the equation: I2=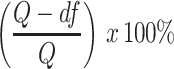 . A Chi-square P value less than 0.1 was considered as significant heterogeneity. I-square values ≥ 50% were considered to indicate high heterogeneity. In scenarios of significant heterogeneity, we employed the leave-one-out sensitivity analysis model to address the reported heterogeneity. Moreover, we used the Galbraith plot to detect any heterogeneity across studies.
. A Chi-square P value less than 0.1 was considered as significant heterogeneity. I-square values ≥ 50% were considered to indicate high heterogeneity. In scenarios of significant heterogeneity, we employed the leave-one-out sensitivity analysis model to address the reported heterogeneity. Moreover, we used the Galbraith plot to detect any heterogeneity across studies.
Quality assessment
To assess the quality of the clinical trials included in our analysis, we utilized the Cochrane Risk of Bias 2 (ROB-2) tool for randomized controlled trials (RCTs) [24]. This tool evaluates the risk of bias in five domains, namely, selection bias, performance bias, detection bias, attrition bias, and reporting bias. The authors’ decisions were categorized as ‘High risk of bias’, ‘Some concerns’, or ‘Low risk of bias’. Observational studies were assessed using the Newcastle–Ottawa Scale (NOS) [25] which involves three domains (selection, comparability, and outcome).
To investigate publication bias across studies, we employed the DOI plot model to analyze the relationship between effect size and standard error [26]. We assessed evidence of publication bias using Egger’s regression test [27].
Results
Literature search
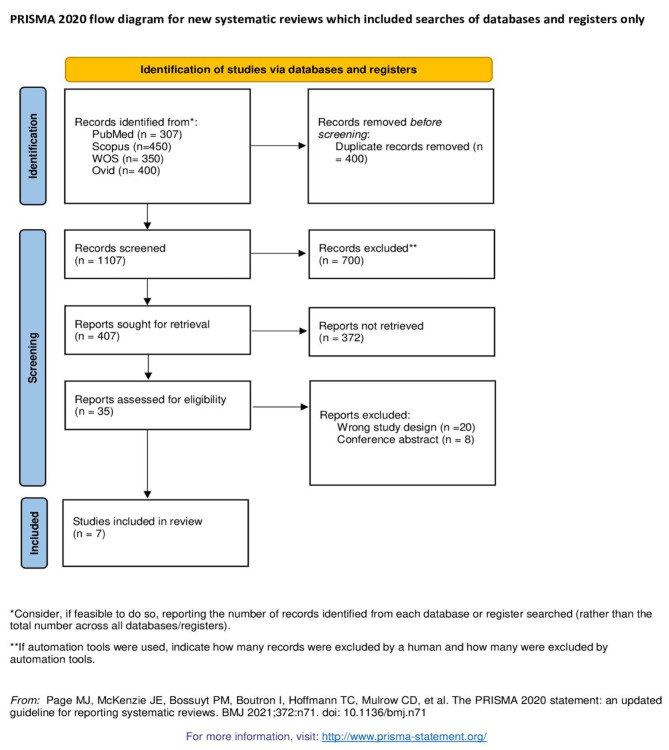
Our electronic search on four databases yielded 1507 articles of which seven studies were finally included in this meta-analysis after title, abstract, and full text screening. The flow chart of included studies is illustrated in PRISMA flow diagram, as shown in Fig. 1.
Fig. 1.
PRISMA flow diagram
Characteristics of included studies
Our meta-analysis included seven studies with a total of 5494 patients. Of the included studies, 5 were observational studies [8, 28]– [31], and only two were randomized controlled trials [13, 14]. The baseline summary and characteristics of all included studies is summarized in Table 1.
Table 1.
Summary and baseline of all included studies
| Characteristics of All included Studies | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Type of Study | Country | Patients (n) (comp/control) | Age (mean) (comp/control) | male, n (comp/control) | mean follow up | stent length, mean (comp/control) | Number of stents, mean (comp/control) | Reference vessel diameter, mean (comp/control) | Lesion length, mean (comp/control) | Diameter stenosis, mean (comp/control) | Medical conditions, n (comp/control) | Target vessel, n (comp/control) | Procedural Characteristics, n (comp/control) | |||||||||||||||
| Previous MI | Previous CABG | Previous PCI | DM | HTN | Dyslipidaemia | Current smoker | PAD | LM | LCX | RCA | LAD | Ostial location | Bifurcation | Moderate/severe tortuosity | Severe calcification | B2/C lesion | 7 F guiding catheter | ||||||||||||
| Wang 2022 | Retrospective study | China | 16/313 | (60.87/59.70) | (16/284) | 30.22 month | (105.5/90.69) | (3.14/2.50) | NA | NA | NA | (4/79) | (0/15) | (10/206) | (8/95) | (14/184) | (5/89) | (3/70) | NA | (0/1) | (0/7) | (12/185) | (4/120) | NA | NA | (14/184) | (16/157) | NA | NA |
| Pagnotta 2010 | Retrospective study | Italy | 45/603 | (70/63) | (29/506) | NA | NA | NA | (2.75/ 2.79) | (26/22) | NA | (24/341) | (18/87) | NA | (20/250) | (34/512) | NA | (13/126) | NA | NA | (8/134) | (18/241) | (13/158) | (0/56) | NA | NA | (14/141) | NA | NA |
| Abdel-Wahab 2013 | randomized active-controlled superiority trial | Germany | 120/120 | 70.5/71.8 | 86/96 | 75.3 months | 27.7/25.2 | 1./1.3 | 3.1/3.1 | 20.6 /18.5 | 81.5/80 | 38/29 | 9/15 | 44/39 | 33/32 | 106/95 | 91/87 | 24/16 | NR | 3/2 | 7/22 | 35/41 | 101/111 | 27/31 | 72/82 | 67/83 | 65/86 | 137/152 | 122/50 |
| Abdel-Wahab 2018 | randomized trial | Germany | 100/100 | 75/74.8 | 75/77 | 77.1 months | 35.41/35.63 | 1.71/1.52 | 3.31/3.25 | 30.07/29.81 | 83.54/83.02 | 22/21 | 13/6 | 55/47 | 34/33 | 93/93 | 69/68 | 9/15 | NR | 20/15 | 16/16 | 40/32 | 61/78 | 35/40 | 61/55 | 44/49 | 100/104 | 129/137 | 111/130 |
| Azzalini 2017 | Retrospective study | Spain, Canada, Italy | 35/968 | 68.9/64.6 | 31/841 | 2 years | 72.8/68.4 | 2/2.2 | NR | NR | NR | NR | NR | NR | 19/351 | 27/711 | 32/765 | 9/205 | NR | NR | 3/180 | 17/509 | 15/275 | NR | NR | NR | 30/413 | NR | NR |
| Huang 2018 | Retrospective study | Taiwan | 26/259 | 71.1/66.4 | 23/215 | 3.4 years | 40.7/34.2 | NR | NR | NR | NR | NR | NR | NR | 13/158 | 19/223 | NR | 4/11 | NR | 1/2 | 1/10 | 14/137 | 11/110 | NR | NR | NR | NR | NR | NR |
| Ayoub 2023 | Retrospective study | Germany | 193/2596 | 70.33/65.73 | 156/2143 | 1 year | 50.55/54.18 | 2.19/1.73 | NR | NR | NR | 66/995 | 65/417 | NR | 84/775 | 178/2229 | 178/2333 | 21/519 | NR | NR | 37/554 | NR | 40/699 | 14/174 | NR | 46/538 | 166/942 | NR | 117/889 |
Risk of bias assessment
The Five observational studies were truly representative of the patients included. The non-exposed group was selected from the same community and the ascertainment of exposure was confirmed by secure records. Furthermore, the two groups included in all the studies were comparable. They also showed adequate periods of follow-up except for Pagnotta 2010. However, the overall quality of all the studies is good, as shown in Table 2.
Table 2.
NOS scale for observational studies
| Cohort studies | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Selection | Comparability | Outcome | Quality Score | |||||||||||
| Study Title | First Author | Year | Study Design (Prospective or retrospective) | mean follow up | Sample (n) (comp/control) | Age at baseline mean (Year) (comp/control) | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | |
| Ayoub 2023 | Mohamed Ayoub | 2023 | Retrospective study | 1 year | (193/2596) | (70.33/65.73) | * | * | * | * | ** | * | * | * | Good |
| Wang 2022 | Jing Wang | 2022 | Retrospective study | 30.22 months | (16/313) | (60.87/59.70) | * | * | * | * | * | * | * | * | good |
| Pagnotta 2010 | Paolo Pagnotta | 2010 | Retrospective study | Not applicable | (45/603) | (70/63) | * | * | * | * | * | * | * | * | good |
| Azzalini 2017 | Lorenzo Azzalini | 2017 | Retrospective study | 2 years | 35/968 | 68.9/64.6 | * | * | * | * | * | * | * | * | good |
| Huang2018 | Wei-Chieh Huang | 2018 | Retrospective study | 3.4 years | 26/259 | 71.1/66.4 | * | * | * | * | * | * | * | * | good |
The two RCTs Abdel-Wahab 2013 and Abdel-Wahab 2018 showed low risk of bias in almost all the five domains of Rob2. However, Abdel-Wahab 2013 showed protocol deviation in 3 patients. They demonstrated adequate randomization process, allocation concealment, appropriate analysis, appropriate outcome measurement, and no baseline difference between the two groups, as shown in Fig. 2.
Fig. 2.
Risk of bias assessment tool-2 (ROB-2) for RCTs
Clinical outcomes
MACE
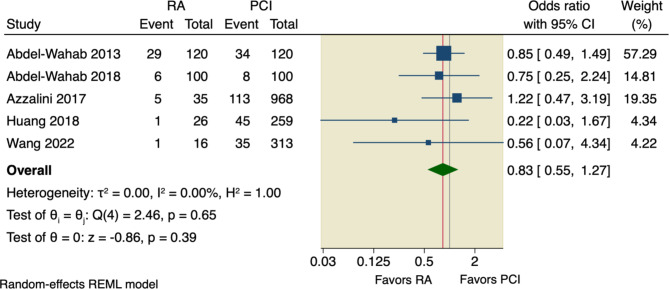
Six studies assessed our primary outcome, MACE, with an incidence rate of 15.91% (78 of 490) in RA group compared to 15.36% (669 of 4356) in the conventional PCI group. The pooled OR did not detect any significant difference between the two studied groups regarding MACE (OR = 0.98, 95% CI [0.74 to 1.3], p = 0.9); the pooled studies were homogenous (I2 = 0.00%, p = 0.62), as shown in Fig. 3.
Fig. 3.
Forest plot of MACE. RA: rotational atherectomy, PCI: percutaneous coronary intervention, CI: confidence interval
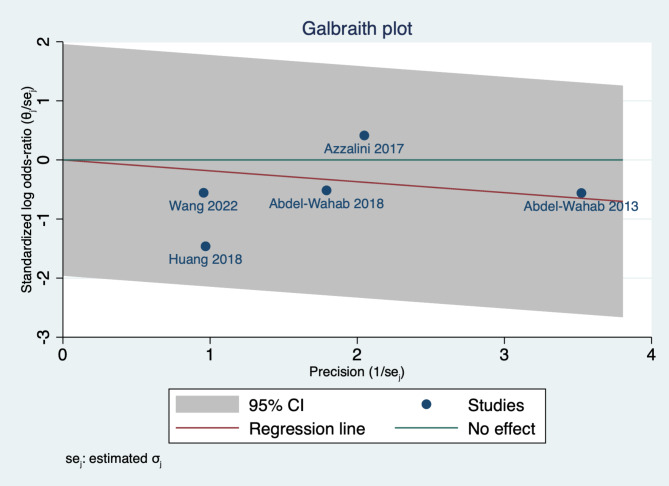
We assessed the statistical heterogeneity using Galbraith plot, and by inspection, all studies were within the 95% CI of the precision area, indicating that there no heterogeneity across studies, as shown in Fig. 4.
Fig. 4.
Galbraith plot assessing heterogeneity across studies assessed MACE
We used DOI plot to detect possible publication bias, and by inspection, there was a major asymmetry with a LFK of -4.7, indicating possible publication bias and further studies are needed to achieve stability, as shown in Fig. 5.
Fig. 5.
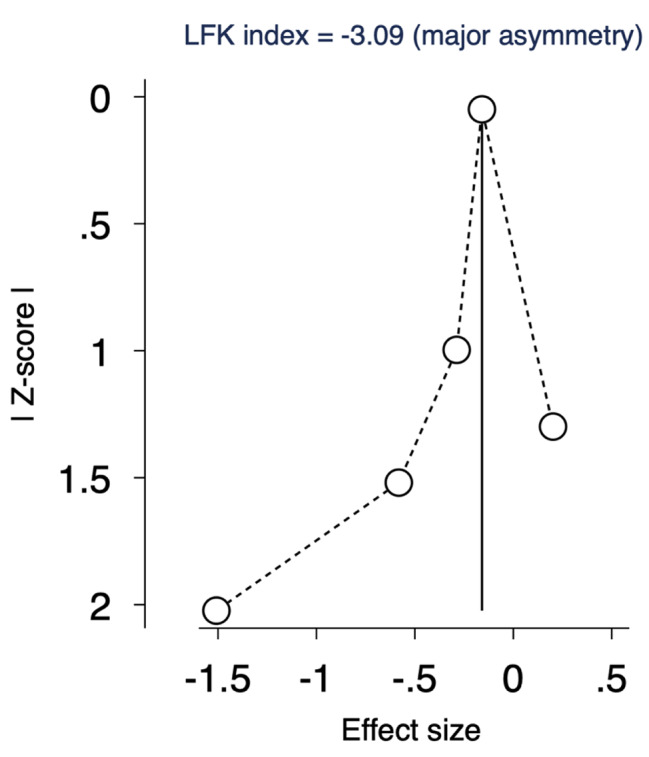
DOI plot assessing publication bias of MACE. LFK= -3.09, indicating major asymmetry
Secondary outcomes
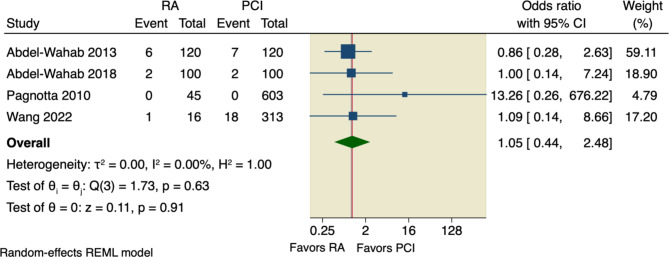
Our pooled analysis showed no significant difference between RA and conventional PCI according to all-cause death (OR = 1.34, 95% CI [0.8 to 2.25], p = 0.26), or cardiac death (OR = 1.46, 95% CI [0.54 to 3.95], p = 0.46); the pooled studies were homogenous with the following values, respectively (I2 = 0.00%, p = 0.69; and I2 = 0.00%, p = 0.43), as shown in Figs. 6 and 7.
Fig. 6.
Forest plot of all-cause death. CI: Confidence interval, RA: rotational atherectomy, PCI: percutaneous coronary intervention
Fig. 7.
Forest plot of cardiac death. CI: Confidence interval, RA: rotational atherectomy, PCI: percutaneous coronary intervention
Sensitivity analysis was done by excluding Pagnotta et al., in which the pooled studies did not favor RA over conventional PCI regarding either all-cause death (OR = 1.29, 95% CI [0.77 to 2.17], p = 0.33) or cardiac death (OR = 1.25, 95% CI [0.45 to 3.51], p = 0.67), as shown in Supplementary Figs. 1, 2.
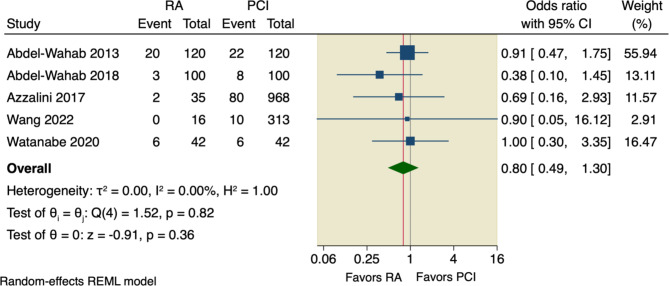
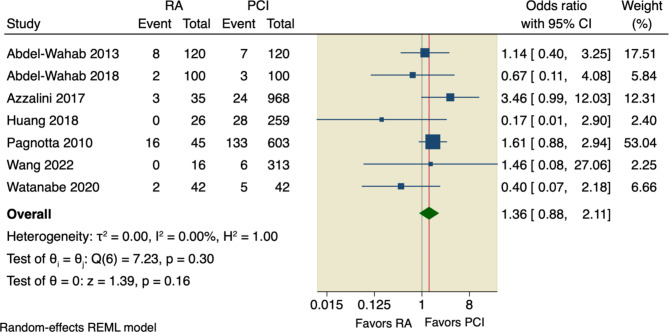
Moreover, our pooled analysis showed no superior effect of RA compared to conventional PCI regarding the incidence of TVR (OR = 1.01, 95% CI [0.74 to 1.36], p = 0.97), or incidence of MI (OR = 1.42, 95% CI [0.92 to 2.18], p = 0.11); the pooled studies were homogenous with the following values, respectively (I2 = 0.00%, p = 0.58; and I2 = 0.00%, p = 0.47), as shown in Figs. 8 and 9.
Fig. 8.
Forest plot of TVR. CI: Confidence interval, RA: rotational atherectomy, PCI: percutaneous coronary intervention
Fig. 9.
Forest plot of MI. CI: Confidence interval, RA: rotational atherectomy, PCI: percutaneous coronary intervention
Angiographic outcomes
Four studies assessed technical success in which the pooled analysis did not favor RA over conventional PCI (OR = 1.06, 95% CI [0.89 to 1.25], p = 0.53); the pooled studies were homogenous (I2 = 0.00%, p = 0.87), as shown Supplementary Fig. 3. Regarding procedural success, the pooled analysis of six studies showed no superior effect of RA over conventional PCI (OR = 1.08, 95% CI [0.94 to 1.24], p = 0.29); the pooled results were homogenous (I2 = 0.00%, p = 0.98), as shown in Supplementary Fig. 4.
The incidence rate of procedural complications was 5.05% (15 of 297) in the RA group compared to 5% (88 of 1760) in the conventional PCI group. The pooled OR did not show any superiority of RA over conventional PCI (OR = 1.72, 95% CI [0.64 to 4.61], p = 0.28); the pooled studies were homogenous (I2 = 18.04%, p = 0.11), as shown in Supplementary Fig. 5.
A sensitivity analysis model was performed, and upon excluding Huang et al., the pooled analysis, yet, did not detect any difference between the two studied groups (OR = 1.34, 95% CI [0.55 to 3.28], p = 0.52), as shown Supplementary Fig. 6.
Discussion
We conducted this meta-analysis to evaluate the safety and feasibility of RA in patients with CTO lesions compared to conventional CTO PCI. Our findings revealed that there was no significant difference between the two procedures in clinical outcomes, including MACE, all-cause death, cardiac death, incidence of TVR, and the incidence of MI. Similarly, we found no significant difference in angiographic outcomes, including technical success, procedural success, and complications.
Revascularization of CTO lesions can enhance long-term survival rates and quality of life, especially in patients with CTO in the left anterior descending territory and extensive ischemic areas [32]. Multiple studies have demonstrated the clinical benefits of successful canalization of CTO lesions in improving anginal pain, survival rate, and left ventricular systolic function [33, 34]. Given the numerous advantages of revascularization, it is essential to properly recanalize resistant CTO lesions.
CTO interventions represent around 25% of patients undergoing coronary angiography, and many interventional cardiologists are committed and enthusiastic about performing CTO PCIs. With well-trained physicians and technical advancements, the success rate for CTO PCIs has increased to over 80% after 15 years of follow-up. However, the mortality rate within 30 days of CTO PCI was 1.3%, and perforation occurred in 4.8% of cases [35, 36]. Another study with a 20-year follow-up found that successful CTO-PCI approaches were associated with a 10-year survival benefit [37].
Despite improvements in PCI technology and techniques, some lesions remain uncrossable or undilatable by balloon [38]. According to a previous multicenter CTO PCI registry, 9% of all lesions are considered uncrossable and are characterized by moderate to severe coronary calcification, moderate to severe coronary tortuosity, and higher J-CTO scores. Additionally, 12% of CTO lesions are undilatable, with most of them associated with a history of CAD, heart failure, diabetes, and higher J-CTO scores [39]. Uncrossable CTO lesions are associated with a lower success rate and more complications. Prolonged procedures also increase the amount of fluoroscopy time required [40].
The uncrossable CTO lesions can be successfully managed by special approaches such as deep seating of the guiding catheter, anchoring balloon technique, buddy wire technique, manual rotation of the Tornus catheter, and child-in-mother guiding catheters [41, 42]. Additionally, higher ballooning pressure, excimer laser, and RA showed a high success rate [38].
One of the most effective managements for resistant CTOs and heavy calcific lesions is RA [43]. Practice guidelines recommend using RA to prepare highly fibrous or calcified lesions that cannot be properly crossed by a balloon or dilated before stenting [36]. Fang et al. conducted a study to compare the feasibility and safety of RA versus a penetrating catheter (Tornus catheter) for treating heavy calcific lesions and it was found that RA was superior to the Tornus catheter in terms of in-hospital and 30-day follow-up outcomes. RA was associated with a higher device and angiographic success rate and a shorter procedural duration. However, both procedures showed similar results regarding major and minor complications [44]. Another study by Pagnotta et al. reported that the RA was superior to the penetrating catheter (Tornus catheter) with a predicted success rate of over 45%. In addition, the RA with a bail-out approach can increase the predicted success rate to 91% in CTO patients [45].
Previous randomized controlled trials reported that there were no significant differences between CTO-PCI and RA, which is in line with our results [13, 14, 46, 47]. The randomized ROTAXUS trial assessed the effect of the RA technique on heavily calcified lesions and fixation of a drug-eluting stent (DES), and they found that RA was not superior to other PCI procedures in terms of in-hospital outcomes and after 9 months of follow-up [13]. At 2-year clinical follow-up, there was no difference in MACE rates between patients with complicated calcified lesions undergoing RA and those undergoing standard PCI before DES implantation. However, the rate of MACE increased to one-third of total patients, which may be associated with the time-dependent occurrence of MACE [46].
The Randomized PREPARE-CALC trial showed similar rates of in-hospital and 9-month lumen loss when modified balloons or RA were used with a heavily calcific lesion [14]. Recently, a post hoc analysis of this trial showed that RA-based lesion preparation had a higher success rate than the modified balloon strategy-based lesion preparation in heavy calcific lesions in the left anterior descending artery. The major cause of this discrepancy was a greater rate of crossover and stent failure in the modified balloon group [47].
The ARTIST trial evaluated the efficacy and safety of RA followed by balloon angioplasty with balloon percutaneous transluminal coronary angioplasty, and no significant difference was found in the short-term success rate compared to other procedures. However, RA was associated with minimal luminal diameter after 6 months, considering that the operators used low-pressure inflations [48].
The wide range of atherectomy application rates during CTO PCI, ranging from 3.5 to 9%, is most likely due to variations in equipment accessibility and operator expertise [8, 31]. A previous retrospective study conducted in Europe and Russia on 3540 patients found that the currently used RA procedure, which is mostly utilized for complex lesions, has similar technical success rate compared to CTO-PCI, but carries a higher risk of donor vascular damage and tamponade, requiring pericardiocentesis. Additionally, after excluding failed crossing instances and those where the successful crossing technique was unclear. However, procedural success (94% vs. 96%, p = 0.358) and MACE (5% vs. 3%, p = 0.195) rates were similar [49]. Another study reported the similarity in success rate and MACE between both procedures in-hospital and after 1 year. Nevertheless, this benefit was lost when the multivariate Cox regression analysis was adjusted for confounding factors (HR 1.25, 95% CI, 0.33–1.94, p = 0.242) [8].
A previous study assessed the risk of RA and found that RA was associated with more dissections; however, there were no significant differences between CTO and non-CTO groups [50]. Additionally, the CTO-PCI has a higher rate of dissection, especially with the retrograde method compared with the antegrade method [51].
Moreover, a recent meta-analysis addressed the clinical outcomes of PCI in CTO lesions reported that PCI in CTO lesions was associated with higher odds of vessel perforations and cardiac tamponade during in-hospital stay, while high rates of MACE was noted during long-term outcomes highlighting the challenges and adverse endpoints related to PCI in CTO lesions [52].
In terms of clinical implications, our findings suggest that RA is an important procedure for managing uncrossable or undilatable lesions with a high success rate and less fluoroscopy time. However, CTO-PCI has comparable success rates and complications. Therefore, while RA may not be the preferred first choice for CTO lesions, it remains a practical and viable alternative for difficult-to-treat lesions.
The study has several strengths. It is the first meta-analysis to compare the effectiveness of RA to CTO-PCI in heavy and complex CTO lesions, and we obtained consistent results by combining data from studies with similar characteristics. Our findings have important clinical implications for the management of CTO lesions. However, the study also has some limitations. Firstly, most of the included studies were observational, which may have introduced bias. Secondly, the number of included studies was relatively small, and there was evidence of publication bias. Therefore, we suggest that more prospective clinical trials with larger sample sizes are needed to evaluate the short-term and long-term outcomes associated with RA and CTO-PCI, as well as to compare the different procedures within CTO-PCI and RA.
In conclusion, our results demonstrate that patients who underwent RA and CTO-PCI experienced comparable in-hospital outcomes. In cases where the lesion was heavily calcified and challenging to treat using retrograde CTO-PCI, RA can be a viable option for experienced operators.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
I want to give warm thanks to Tamim Ahmed Abdelaziz, my son, for his kind love and support and I wish him a lot of good luck.
Author contributions
Ahmed Abdelaziz: Conceptualization, Supervision, data collection revision, statistical analysis, writing – original draft and editing. Hanaa Elsayed: screening, data collection, original draft writing. Aboalmagd Hamdaalah: data collection, data validation. Karim Atta: Full-text screening, data collection, data validation. Mohamed Abdelaziz: screening, data collection, revision of data collection, quality assessment. Hallas Kadhim: data collection, quality assessment. Ahmed Mechi: data collection, data validation, Final version. Ahmed Elaraby: quality assessment, full-text screening, data collection, writing – revision. Mahmoud Ezzat: screening, data collection, data revision for analysis. Aya Moustafa Aboutaleb: summary and characteristics, quality assessment, writing – revision. Fayed Mohamed Rzk: quality assessment, manuscript drafting and writing. Mahmoud Eid: screening, data collection, quality assessment. Abdelrahman H. Hafez: summary and characteristics, quality assessment, writing – revision. Mohamed Hatem Ellabban: data collection, manuscript drafting and writing. Hadeer Elsaeed Aboelfarh: data collection, quality assessment. Rahma Abdelfattah: data collection, quality assessment, summary and characteristics. Shaimaa Fadel: data collection, quality assessment, summary and characteristics. Hazem S. Ghaith: Conceptualization, screening, writing – original draft. Emad Addin Zawaneh: quality assessment, TSA analysis, summary and characteristics. Mustafa Suppah: writing – original draft, data revision, Manuscript revision.
Funding
This work was not supported by any funding options.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
Declarations
Ethical approval
Not applicable.
Competing interests
There was no conflict of interest among all authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koelbl CO, Nedeljkovic ZS, Jacobs AK. Coronary chronic total occlusion (CTO): a review. Rev Cardiovasc Med. Mar. 2018;19(1):38–44. 10.31083/j.rcm.2018.01.896 [DOI] [PubMed]
- 2.Sianos G, et al. European experience with the retrograde approach for the recanalisation of coronary artery chronic total occlusions. A report on behalf of the EuroCTO club. EuroIntervention. May 2008;4(1):84–92. 10.4244/EIJV4I1A15 [DOI] [PubMed]
- 3.Stone GW, et al. Percutaneous recanalization of chronically occluded coronary arteries. Circulation. Oct. 2005;112(15):2364–72. 10.1161/CIRCULATIONAHA.104.481283 [DOI] [PubMed]
- 4.Fefer P, et al. Current perspectives on coronary chronic total occlusions. J Am Coll Cardiol. Mar. 2012;59(11):991–7. 10.1016/j.jacc.2011.12.007 [DOI] [PubMed]
- 5.Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total Coronary Occlusion on treatment strategy. Am J Cardiol. May 2005;95(9):1088–91. 10.1016/j.amjcard.2004.12.065 [DOI] [PubMed]
- 6.Jeroudi OM et al. Oct., Prevalence and management of coronary chronic total occlusions in a tertiary veterans affairs hospital, Catheterization and Cardiovascular Interventions, vol. 84, no. 4, pp. 637–643, 2014, 10.1002/ccd.25264 [DOI] [PMC free article] [PubMed]
- 7.Surmely J-F et al. Jul., New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: the CART technique., J Invasive Cardiol, vol. 18, no. 7, pp. 334–8, 2006. [PubMed]
- 8.Huang W-C, Teng H-I, Chan W-L, Lu T-M. Short-term and long-term clinical outcomes of rotational atherectomy in resistant chronic total occlusion, J Interv Cardiol, vol. 31, no. 4, pp. 458–464, Aug. 2018, 10.1111/joic.12489 [DOI] [PubMed]
- 9.Williams DO et al. Dec., Percutaneous Coronary Intervention in the Current Era Compared With 1985–1986, Circulation, vol. 102, no. 24, pp. 2945–2951, 2000, 10.1161/01.CIR.102.24.2945 [DOI] [PubMed]
- 10.Hoye A, van Domburg RT, Sonnenschein K, Serruys PW. Percutaneous coronary intervention for chronic total occlusions: the Thoraxcenter experience 1992–2002, Eur Heart J, vol. 26, no. 24, pp. 2630–2636, Dec. 2005, 10.1093/eurheartj/ehi498 [DOI] [PubMed]
- 11.Levine GN et al. Dec., 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, Circulation, vol. 124, no. 23, 2011, 10.1161/CIR.0b013e31823ba622
- 12.Moussa I, et al. Coronary stenting after rotational atherectomy in calcified and complex lesions. Circulation. Jul. 1997;96(1):128–36. 10.1161/01.CIR.96.1.128 [DOI] [PubMed]
- 13.Abdel-Wahab M, et al. High-speed rotational atherectomy before Paclitaxel-Eluting Stent Implantation in Complex Calcified Coronary lesions. JACC Cardiovasc Interv. Jan. 2013;6(1):10–9. 10.1016/j.jcin.2012.07.017 [DOI] [PubMed]
- 14.Abdel-Wahab M, et al. High-speed rotational atherectomy Versus Modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. Oct. 2018;11(10). 10.1161/CIRCINTERVENTIONS.118.007415 [DOI] [PubMed]
- 15.Christopoulos G et al. Nov., Application and outcomes of a hybrid approach to chronic total occlusion percutaneous coronary intervention in a contemporary multicenter US registry, Int J Cardiol, vol. 198, pp. 222–228, 2015, 10.1016/j.ijcard.2015.06.093 [DOI] [PMC free article] [PubMed]
- 16.Azzalini L et al. Mar., Subadventitial techniques for chronic total occlusion percutaneous coronary intervention: The concept of ‘vessel architecture,’ Catheterization and Cardiovascular Interventions, vol. 91, no. 4, pp. 725–734, 2018, 10.1002/ccd.27025 [DOI] [PubMed]
- 17.Gruberg L, et al. Effect of plaque debulking and stenting on short- and long-term outcomes after revascularization of chronic total occlusions. J Am Coll Cardiol. Jan. 2000;35(1):151–6. 10.1016/S0735-1097(99)00491-X [DOI] [PubMed]
- 18.Moliterno DJ. Rotational atherectomy for resistant chronic total occlusions: Another spin for tough old problems, Catheterization and Cardiovascular Interventions, vol. 76, no. 3, pp. 372–373, Sep. 2010, 10.1002/ccd.22752 [DOI] [PubMed]
- 19.Barbato E, et al. European expert consensus on rotational atherectomy. EuroIntervention. May 2015;11(1):30–6. 10.4244/EIJV11I1A6 [DOI] [PubMed]
- 20.Maejima N, et al. Relationship between thickness of Calcium on Optical Coherence Tomography and Crack formation after balloon dilatation in calcified plaque requiring rotational atherectomy. Circ J. 2016;80(6):1413–9. doi: 10.1253/circj.CJ-15-1059. [DOI] [PubMed] [Google Scholar]
- 21.Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses – from debulking to plaque modification, a 40-year-long journey, EuroIntervention, vol. 13, no. 6, pp. 696–705, Aug. 2017, 10.4244/EIJ-D-17-00473 [DOI] [PubMed]
- 22.Cavusoglu E, Kini AS, Marmur JD, Sharma SK. Current status of rotational atherectomy, Catheterization and Cardiovascular Interventions, vol. 62, no. 4, pp. 485–498, Aug. 2004, 10.1002/ccd.20081 [DOI] [PubMed]
- 23.Liberati A et al. Dec., The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration, BMJ, vol. 339, no. jul21 1, pp. b2700–b2700, 2009, 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed]
- 24.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16(4):195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74(3):785–94. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayoub M, Corpataux N, Behnes M, et al. Safety and Efficiency of Rotational Atherectomy in chronic total coronary occlusion-one-year clinical outcomes of an Observational Registry. J Clin Med. 2023;12(10):3510. doi: 10.3390/jcm12103510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, et al. Safety and Feasibility of Rotational Atherectomy for Retrograde recanalization of chronically occluded coronary arteries. Front Cardiovasc Med. Jun. 2022;9. 10.3389/fcvm.2022.854757 [DOI] [PMC free article] [PubMed]
- 30.Pagnotta P et al. Sep., Rotational atherectomy in resistant chronic total occlusions, Catheterization and Cardiovascular Interventions, vol. 76, no. 3, pp. 366–371, 2010, 10.1002/ccd.22504 [DOI] [PubMed]
- 31.Azzalini L et al. Apr., Long-term outcomes of rotational atherectomy for the percutaneous treatment of chronic total occlusions, Catheterization and Cardiovascular Interventions, vol. 89, no. 5, pp. 820–828, 2017, 10.1002/ccd.26829 [DOI] [PubMed]
- 32.Liao R, Li Z, Wang Q, Lin H, Sun H. Revascularization of chronic total occlusion coronary artery and cardiac regeneration. Front Cardiovasc Med. Aug. 2022;9. 10.3389/fcvm.2022.940808 [DOI] [PMC free article] [PubMed]
- 33.B. DAVE, Recanalization of chronic total occlusion lesions: a critical Appraisal of current devices and techniques. J Clin Diagn Res, 2016, 10.7860/JCDR/2016/21853.8396 [DOI] [PMC free article] [PubMed]
- 34.Guo L, et al. Long-term outcomes of successful recanalization compared with Optimal Medical Therapy for Coronary Chronic Total occlusions in patients with and without left ventricular systolic dysfunction. Front Cardiovasc Med. Apr. 2021;8. 10.3389/fcvm.2021.654730 [DOI] [PMC free article] [PubMed]
- 35.Zimmermann FM et al. Dec., Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial, Eur Heart J, vol. 36, no. 45, pp. 3182–3188, 2015, 10.1093/eurheartj/ehv452 [DOI] [PubMed]
- 36.Lawton JS, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. Jan. 2022;145(3). 10.1161/CIR.0000000000001039 [DOI] [PubMed]
- 37.Suero JA, et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. Aug. 2001;38(2):409–14. 10.1016/S0735-1097(01)01349-3 [DOI] [PubMed]
- 38.McQuillan C, Jackson MWP, Brilakis ES, Egred M. Uncrossable and undilatable lesions—A practical approach to optimizing outcomes in < scp > PCI, Catheterization and Cardiovascular Interventions, vol. 97, no. 1, pp. 121–126, Jan. 2021, 10.1002/ccd.29001 [DOI] [PubMed]
- 39.Karacsonyi J et al. Jul., Prevalence, indications and management of balloon uncrossable chronic total occlusions: Insights from a contemporary multicenter US registry, Catheterization and Cardiovascular Interventions, vol. 90, no. 1, pp. 12–20, 2017, 10.1002/ccd.26780 [DOI] [PubMed]
- 40.Karacsonyi J et al. Mar., Angiographic Features and Clinical Outcomes of Balloon Uncrossable Lesions during Chronic Total Occlusion Percutaneous Coronary Intervention, J Pers Med, vol. 13, no. 3, p. 515, 2023, 10.3390/jpm13030515 [DOI] [PMC free article] [PubMed]
- 41.Reifart N, Enayat D, Giokoglu K. A novel penetration catheter (Tornus) as bail-out device after balloon failure to recanalise long, old calcified chronic occlusions, EuroIntervention, vol. 3, no. 5, pp. 617–621, Mar. 2008, 10.4244/EIJV3I5A110 [DOI] [PubMed]
- 42.Dash D. Interventional Management of ‘Balloon-Uncrossable’ coronary chronic total occlusion: is there any way out? Korean Circ J. 2018;48(4):277. doi: 10.4070/kcj.2017.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaul A, et al. Current treatment modalities for calcified coronary artery Disease: a review article comparing novel intravascular lithotripsy and traditional rotational atherectomy. Cureus Oct. 2020 doi: 10.7759/cureus.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang H-Y, et al. Can a penetration catheter (Tornus) Substitute Traditional Rotational Atherectomy for recanalizing chronic total occlusions? Int Heart J. 2010;51(3):147–52. doi: 10.1536/ihj.51.147. [DOI] [PubMed] [Google Scholar]
- 45.Pagnotta P et al. Sep., Tornus catheter and rotational atherectomy in resistant chronic total occlusions, Int J Cardiol, vol. 167, no. 6, pp. 2653–2656, 2013, 10.1016/j.ijcard.2012.06.124 [DOI] [PubMed]
- 46.de Waha S et al. Mar., Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: Two-year clinical outcome of the randomized ROTAXUS trial, Catheterization and Cardiovascular Interventions, vol. 87, no. 4, pp. 691–700, 2016, 10.1002/ccd.26290 [DOI] [PubMed]
- 47.Rheude T, et al. Outcomes of rotational atherectomy versus modified balloon angioplasty in severely calcified coronary lesions based on target lesion location: a post hoc analysis of the PREPARE-CALC randomised trial. EuroIntervention. Jul. 2020;16(4):e322–4. 10.4244/EIJ-D-19-00488 [DOI] [PubMed]
- 48.Dahl Jvom et al. Feb., Rotational Atherectomy Does Not Reduce Recurrent In-Stent Restenosis, Circulation, vol. 105, no. 5, pp. 583–588, 2002, 10.1161/hc0502.103347 [DOI] [PubMed]
- 49.Xenogiannis I, et al. Usefulness of atherectomy in chronic total occlusion interventions (from the PROGRESS-CTO Registry). Am J Cardiol. May 2019;123(9):1422–8. 10.1016/j.amjcard.2019.01.054 [DOI] [PubMed]
- 50.Brinkmann C, Eitan A, Schwencke C, Mathey DG, Schofer J. Rotational atherectomy in CTO lesions: too risky? Outcome of rotational atherectomy in CTO lesions compared to non-CTO lesions, EuroIntervention, vol. 14, no. 11, pp. e1192–e1198, Dec. 2018, 10.4244/EIJ-D-18-00393 [DOI] [PubMed]
- 51.Xu R et al. Jan., Procedural and In-hospital Outcomes of Rotational Atherectomy in Retrograde Coronary Chronic Total Occlusion Intervention, Angiology, vol. 72, no. 1, pp. 44–49, 2021, 10.1177/0003319720949312 [DOI] [PubMed]
- 52.Abdelaziz A, Elsayed H, Atta K, et al. Short- and long-term outcomes of percutaneous coronary interventions in chronic and non-chronic total occlusions: a Meta-analysis of 690,123 patients. Curr Probl Cardiol Published Online June. 2023;18. 10.1016/j.cpcardiol.2023.101890 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.