Abstract
Background
Toxoplasmosis is a serious or life-threatening disease in immunosuppressed patients and pregnant women. This study examined the likely association between Toxoplasma gondii infection and COVID-19 patients with moderate illness.
Methods
Seventy blood samples were collected from patients at the Health Reference Laboratory of Tabriz, Northwest Iran from April 2021 to September 2021. In addition, 70 healthy subjects of the same age (37 ± 15 years) and sex distribution were ethnically matched. Sera samples were examined for the detection of anti-Toxoplasma antibodies using ELISA. Nested-PCR targets were amplified based on the B1 and GRA6 genes. GRA6 amplicons were subjected to sequencing and phylogenetic analysis.
Results
The seroprevalence of toxoplasmosis based on IgG titer was 35.7% in the COVID‑19 patients and 27.1% in the control group, representing not to be associated with the Toxoplasma seropositivity in COVID‑19 patients (P = 0.18) compared to healthy subjects. Anti-T. gondii IgM was not found in any of the patients and healthy individuals. According to PCR amplification of the B1 and GRA6 genes, the frequency of T. gondii in COVID-19 patients was 14.2% (10/70). However, no T. gondii infection was detected in the healthy group. The CD4+T cell count was relatively lower in toxoplasmosis-infected patients (430–450 cells/mm3) than in control group (500–1500 cells/mm3). High genetic diversity (Hd: 0.710) of the type I strain of T. gondii was characterized in the patients. Present results showed that consumption of raw vegetables and close contact with stray cats can increase the transmission of T. gondii to COVID-19 patients (P < 0.01).
Conclusions
The current study revealed that T. gondii type I infection is unequivocally circulating among the COVID-19 patients in Tabriz; However, no significant association was observed between the occurrence of Toxoplasma and the severity of COVID-19. To make more accurate health decisions, multicenter investigations with a larger sample size of different ethnic groups of the Iranian population are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-023-08964-9.
Keywords: Toxoplasmosis, COVID‑19 patients, Sequencing, Serology, Northwest Iran
Introduction
Toxoplasma gondii as an intracellular parasite can infect approximately one-third of the residents worldwide [1]. Nearly, 39.3% of the population of Iran and 35.1-41% of people in the Northwest Iran are infected with toxoplasmosis [2, 3]. Latent toxoplasmosis has long been considered asymptomatic in immunocompetent people, but it can result in serious or life-threatening disease in patients with haematologic malignancy, immunocompromised cancer patients, organ transplants and pregnant women [1, 4–6]. Toxoplasmosis is also associated with various autoimmune [7] and inflammatory diseases [8] as well as some cancers [9, 10].
The first cases of coronavirus disease 19 (COVID-19) with an unknown source were reported in Wuhan, China [11]. SARS-CoV-2 has been shown to impair immune responses and uncontrolled inflammatory responses “cytokine storm” in severe and critical patients with COVID-19. Based on the clinical presentation (from asymptomatic to severe) of mixed infections of COVID-19 with other pathogens, including influenza A [12], Streptococcus pneumoniae [13] fungal [14] and T. gondii [15], indicating these infections could increase the severity of COVID-19. The co-occurrence of COVID-19 and toxoplasmosis as emerging intracellular infections in immunosuppressed individuals may lead to a higher incidence of mental and physical health problems [15–17].
Conflicting results regarding the association between T. gondii and COVID-19 have been discussed by several researchers. Some evidence also suggests that COVID-19 can reactivate latent T. gondii [18, 19]. However, other researchers claim that latent T. gondii infection can trigger suppressor effects and reduce the severity of COVID-19 through overexpression of interferons (I and II) [20, 21]. Therefore, due to the potential impact of toxoplasmosis on COVID-19 mortality, further studies are needed to clarify these apparent contradictions.
In terms of clinical significance, T. gondii infection can progress from the latent phase to the active phase through a decrease in CD8+ and CD4+ cells, which may lead to dangerous complications in COVID-19 patients [15, 22]. The possible association between latent toxoplasmosis and COVID-19 patients has been characterized worldwide [16, 20, 21, 23]. However, there is no case-control study on the genetic diversity of Toxoplasma co-infection in Iranian COVID-19 patients. The current investigation aimed to examine the probable association between toxoplasmosis and COVID-19 using serological and molecular methods in northwest Iran.
Materials and methods
Ethical approval, inclusion and exclusion criteria
All COVID-19 patients signed an informed consent and completed a questionnaire that included demographic data and their behavioral characteristics. This study was approved by the ethical committee of Tabriz University of Medical Sciences and followed the Helsinki’s Declaration (approval number: IR.TBZMED.REC.1400.537). In this study, patients with respiratory distress whose COVID-19 test was negative were excluded from the study. A positive COVID-19 PCR was the inclusion criteria in the current study. The control group consisted of people who had not been infected with the COVID-19 virus or had any immunodeficiency diseases.
Study area, participants and blood sampling
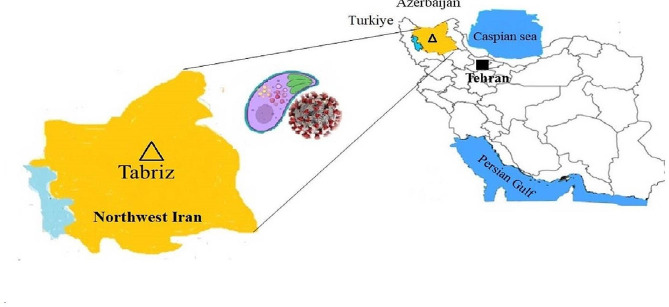
Seventy blood samples were collected from patients with a real-time PCR-positive test for SARS-CoV-2 referred to Health Reference Laboratory of Tabriz, Northwest Iran from April 2021 to September 2021 (Fig. 1). As well, 70 healthy subjects of the same age and sex distribution were ethnically matched. Five ml blood samples were collected from each case and control groups into k2-EDTA (dipotassium ethylenediaminetetraacetic acid as anticoagulant) vials and collected plasma was separated into sterile plain tubes. Then, buffy coat layer and plasma were extracted and stored at -20 °C and transferred to the Health Reference Laboratory for further examination. Omicron was the predominant strain of COVID-19 in the region. None of the patients and healthy people had received the COVID-19 vaccination, corticosteroid therapy or anti-coronavirus drugs in the past.
Fig. 1.
Map of Iran representing the study location (Tabriz, Northwest Iran)
Serological test
Sera samples obtained from all participants (case and control groups) were detected for both anti-T. gondii IgG and IgM antibodies using an ELISA kit (Pishtazteb Co). IgG titers > 1.1 were considered positive.
Nested-PCR targeting T. gondii B1 and GRA6 genes
The genomic DNA of T. gondii was extracted from 140 buffy coat samples (DNA extraction Kit, Yekta Tajhiz Azma, Iran). The B1 gene of T. gondii was amplified by nested-PCR. The fragments of 287 bp and 194 bp were amplified using a pair of external and internal primers, respectively [24]. The PCR thermal cycling conditions reported previously [25]. In addition, T. gondii GRA6 gene (product size: 791 bp) was identified by PCR method. PCR primer sequences and thermal cycling conditions for GRA6 gene were previously described [26].
Sequencing and phylogenetic analysis
PCR products of T. gondii GRA6 gene (n: 6) were successfully sequenced (Codon Genetic Group, Iran) to detect the genotype of T. gondii. The chromatograms were trimmed and edited based on RefSeq (Accession number: KX78158) by the Sequencher 5.4.6. To display codon substitutions, a multiple sequence alignment (MSA) was drawn by BioEdit software. To validate taxonomic position of T. gondii genotypes, a phylogenetic tree based on the Maximum Likelihood algorithm was constructed using MEGA 5.0 software. The topology of the phylogenetic tree was validated by bootstrap values higher than 60%. Hammondia hammondi was addressed as an out-group index. Haplotype (genetic) diversity (Hd) and nucleotide diversity (Nd; π) were estimated using DnaSP software [27]. In this study, GRA6 sequences for the T. gondii isolates were deposited in the GenBank database under Accession nos; OR193704–OR193706.
Statistical analysis
Fisher’s exact test was used to compare frequencies between groups and evaluate the correlation between characteristics of toxoplasmosis in COVID-19 patients. A multiple multinomial logistic regression model was used to assess the correlation between COVID-19 severity and anti-T. gondii IgG findings.
Results
Sociodemographic, clinical findings and risk factors
In the present study, 70 PCR-positive COVID-19 patients with moderate illness and 70 healthy individuals were examined in the Health Reference Laboratory of Tabriz. Among the patients, 80% were female and 20% were male. The mean age of patients was 36 ± 15 years. In the healthy group, 78.5% were female and 21.5% were male. The mean age of healthy group was 37 ± 15 years.
Signs and symptoms of those suffering from COVID-19 included fever > 38 °C, cough, shortness of breath, body pain, diarrhea, headache and moderate respiratory distress (means oxygen saturation in the resting state ≥ 93%). Clinical manifestations due to acquired toxoplasmosis (chorioretinitis and lymphadenopathy) were also not observed in COVID-19 patients. In addition, there was no history of death in toxoplasmosis-infected Covid-19 patients. No significant association was found between Toxoplasma IgG results with COVID-19 severity.
Logistic analysis showed that T. gondii was associated with the consumption of raw vegetables and close contact with stray cats in COVID-19 patients (P = 0.001–0.008) (Table 1). However, the incidence of T. gondii infection in COVID-19 patients was not associated with age, gender, education and consumption of raw/undercooked meat (P > 0.05).
Table 1.
Behavioral characteristics and Toxoplasma gondii infection in COVID-19 patients and control group
| Characteristic | Prevalence of T. gondii infection in COVID-19 patients | Prevalence of T. gondii infection in control group | COVID-19 patients vs. Control group | ||||
|---|---|---|---|---|---|---|---|
| No. of tested | IgG Positive No (%) | No. of tested | IgG Positive No (%) |
P | Confidence interval (CI) | OR | |
| Close contact with stray cats | 70 | 19(86.3) | 70 | 2(128.5) | 0.001 | 0.006–0.082 | 0.022 |
| Consumption of raw vegetable | 70 | 23(50) | 70 | 10(27) | 0.008 | 0.000-0.001 | 0.001 |
| Prevalence of IgG | 70 | 25(35 0.7) | 70 | 19(27.1) | 0.18 | 0.00–1.00 | 0.01 |
Serological findings
The seroprevalence of toxoplasmosis based on IgG titer was 35.7% (25/70) in the COVID‑19 patients and 27.1% (19/70) in the control group, representing not to be associated with the Toxoplasma seropositivity in COVID‑19 patients (P = 0.18, CI: 0.00–1.00, OR: 0.01) compared to healthy subjects (Table 1). Anti-T. gondii IgM was not found in any of the patients and healthy individuals.
Phylogenetic analysis and alignment
In this study, ten T. gondii isolates were successfully amplified using the GRA6 and B1 genes in COVID-19 patients, in which no sera samples were positive for both anti-Toxoplasma IgG and IgM antibodies and three sera samples had a high titer of positive IgG. In this study, flow cytometry results showed that the CD4+T cell count in toxoplasmosis-infected COVID-19 patients was partially lower (430–450 cells/mm3) than in healthy subjects (500–1500 cells/mm3). According to PCR amplification of the B1 (194 bp) and GRA6 (791 bp) genes (Fig. 2), the frequency of T. gondii in COVID-19 patients was 14.2% (10/70). However, T. gondii infection in the healthy group was not detected by PCR.
Fig. 2.
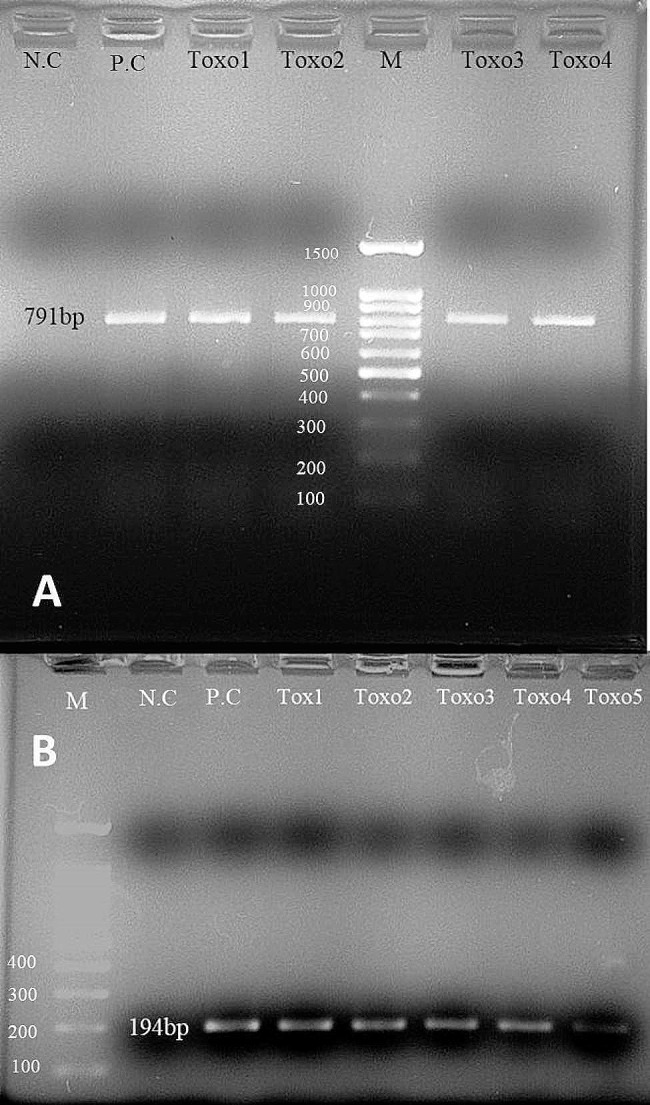
Single round-PCR assay targeting T. gondii GRA6 (A: Toxo1-Toxo4: 791 bp) and nested-PCR assay targeting T. gondii B1 (B: Toxo1-Toxo5: 194 bp). M; Ladder marker (size marker: 100 bp), P.C; positive control, N.C; negative control
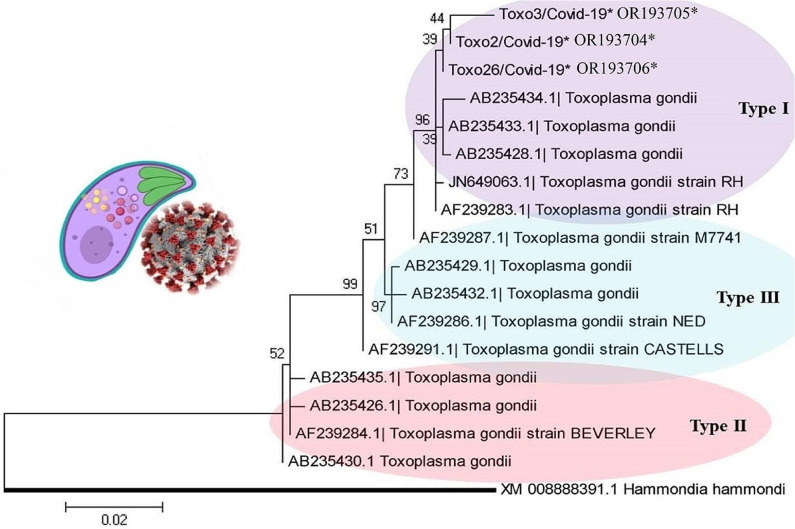
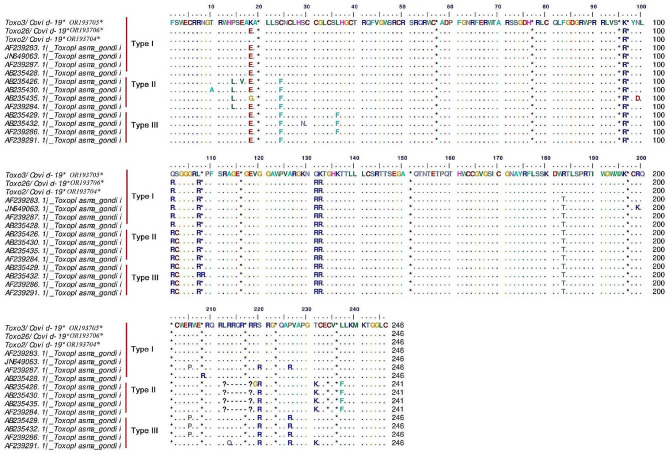
The phylogenetic tree revealed the type I genotype of Toxoplasma (Toxo2/3/26-COVID-19; Accession nos; OR193704–OR193706) obtained from COVID-19 patients, placed in their specific clades (Fig. 3). The genomic analysis of the GRA6 sequences of T. gondii showed genetic diversity (Hd: 0.710) including three haplotypes; However, the Nd value was low (0.00631) (Table 2). Based on multiple alignment analyses of the GRA6 gene, five amino acid substitutions (non-synonymous) of T. gondii (codon positions; 19 (lysine (K) instead of glutamic acid (E)), 96 (K instead of arginine (R)), 101(R instead of glutamine (Q)), 107 (leucine (L) instead of R) and 132 (Q instead of R) were observed in the COVID-19 sequences (Toxo2/3/26-COVID-19*) (Fig. 4).
Fig. 3.
Phylogenetic tree drawn by various types (I-III) of T. gondii using the GRA6 gene based on the Maximum Likelihood algorithm with a Kimura 2-parameter model. The distance scale was estimated 0.02. T. gondii (Toxo2/3/26-COVID-19*; Accession numbers; OR193704–OR193706*) obtained from COVID-19 patients marked by an asterisk (*). Hammondia hammondi was considered as an out-group branch. Bootstrap values of higher than 60% were supported the topology on each branch
Table 2.
Diversity indices of T. gondii obtained from COVID-19 patients in Tabriz, Northwest Iran.
| Area | City | N | Hn | Hd ± SD | Number of segregating sites | Nd (π) |
|---|---|---|---|---|---|---|
| Northwest Iran | Tabriz | 6 | 3 | 0.710 ± 0.272 | 7 | 0.00631 |
N: number of isolates; Hn: number of haplotype; Hd: haplotype (gene) diversity and Nd: nucleotide diversity
Fig. 4.
Multiple alignments of T. gondii GRA6 sequences. The non-synonymous (amino acid) substitutions of T. gondii sequences (Toxo2/3/26-COVID-19*) including five codon positions; 19, 96, 101, 107 and 132 were observed in the COVID-19 patients
Discussion
Bi-functional effects in the relationship between T. gondii and COVID-19 patients have been stated by several studies [18–21]. Current results showed a relatively high seroprevalence of anti-Toxoplasma IgG antibody (35.7%) among the COVID-19 patients in Tabriz; however, no meaningful association was observed between Toxoplasma seropositivity in COVID‑19 patients compared to healthy subjects.
A study reported that overall mortality in moderate or severe COVID-19 disease was associated with a positive anti-Toxoplasma’s IgG titer in Mazandaran, Northern Iran. However, no significant association was found between T. gondii infection and COVID-19 severity [17]. Geraili et al. (2022) have also shown that acute and latent toxoplasmosis infections circulate among COVID-19 patients in Golestan Province, Northern Iran. Furthermore, the prevalence of anti-T. gondii IgM and IgG antibodies were 5.0% and 26.1%, respectively, in COVID-19 patients. However, no significant associations have been found between T. gondii infection and COVID-19 severity [28].
On the other hand, Sharaf-El-Deen (2021) showed that toxoplasmosis through over-expression of lymphocytic PD-1 can be considered an independent risk factor for the severity of COVID-19 [15]. Roe (2021) pointed out that SARS-CoV-2 in synergy with active toxoplasmosis, intensifies the clinical symptoms in COVID-19 patients and leads to a challenging public health concern in all around the world [29].
Roe (2021) reported the association between T. gondii infection and higher mortality in COVID-19 patients with schizophrenia in Germany [30]. Nevertheless, another study did not confirm a strong association between T. gondii infection and susceptibility to COVID‑19 [21]. In a recent study in the Mexican population, the prevalence of IgG and IgM anti-Toxoplasma antibodies was demonstrated in 27.34% and 13.6% of COVID-19 patients, respectively [31]. A study conducted on the Czech and Slovak populations showed that toxoplasmosis is not considered a risk factor for COVID-19 and Toxoplasma-infected patients [16].
The current results showed that risk factors such as close contact with stray cats and consumption of raw vegetables can increase the transmission of T. gondii infection to COVID-19 patients. Hence, educational policies such as thoroughly washing contaminated vegetables to remove oocysts and feeding cooked food to stray cats should be applied to COVID-19 patients with toxoplasmosis.
In this study, despite the low number of CD4+T cells, no severe clinical symptoms of active toxoplasmosis (lymphadenopathy, chorioretinitis and cerebral/pulmonary manifestations) were detected in COVID-19 patients infected with high titer of T. gondii IgG. However, Erol et al. (2022) reported secondary toxoplasmosis chorioretinitis with retinal detachment that developed shortly after COVID-19 infection [23].
T. gondii can cause latent toxoplasmosis in the brain, central nervous system (CNS) and muscles [32]. TH1-associated pro-inflammatory cytokines (cell-mediated immunity) are the main immunity responses against T. gondii infection [33]. According to this fact, a chronic inflammatory response against toxoplasmosis could exacerbate the severity of COVID-19.
Recent evidence shows that CD4 T cell and CD8 T cell depletion occurs during co-infection of coronavirus disease and toxoplasmosis, called “polyspecific T cell exhaustion” leading to overproduction of TH2 cytokines [34]. Subsequently, switching the TH1 response to TH2 could reactivate latent toxoplasmosis in COVID-19 patients.
CD4 T cell depletion increases CD8 T cell depletion because the lack of interleukin-21 signals secreted by CD4 T cells directly increases CD8 T cell depletion [35]. Consequently, loss of CD4 T cell functions due to CD4 T cell depletion leads to a reduction in interferon-γ levels, a crucial cytokine required to control both chronic and acute toxoplasmosis [34]. On the other hand, Lucas et al. (2020) demonstrated that helminths can alter the severity of COVID-19. Indeed, during helminth infections, host immune responses shift toward TH2 polarization with a controlled inflammatory component that reduces mortality/morbidity in COVID-19 patients [36].
In this study, high genetic diversity of T. gondii GRA6 sequences (including five codon substitutions) was found in COVID-19 patients. The emergence of haplotype diversity in latent toxoplasmosis should be considered in view of the emergence of treatment-resistant alleles and/or the development of pathogenesis, particularly in symptomatic COVID-19 patients with high disease severity.
In this study, we were unable to determine the level of T. gondii genetic diversity in COVID-19 patients infected with high titer of T. gondii IgM antibody. Furthermore, we could not authentically compare the haplotype substitutions of the GRA6 sequences of T. gondii between latent and active toxoplasmosis. One of the limitations of the present study was that the number of T. gondii sequences obtained from COVID-19 patients was small to infer large-scale genetic diversity.
The present study revealed that T. gondii type I infections are unequivocally circulating among COVID-19 patients in Tabriz, Northwest Iran. This study found an insignificant correlation between Toxoplasma infection and COVID-19 patients. The detection of T. gondii in COVID-19 patients will help to develop an epidemiological understanding of toxoplasmosis and implement preventive programs in the region. To make more accurate health decisions, multicenter investigations with a larger sample size of different ethnic groups of the Iranian population are needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Nested-PCR assay by targeting T. gondii B1
Supplementary Material 2: Nested-PCR assay by targeting T. gondii GRA6
Acknowledgements
This is a report of a database from the thesis of Mr Mehdi Hasanzadeh registered in Tabriz University of Medical Sciences. We would like to thank the patients & staff of COVID-19 Reference Laboratory in Tabriz in the northwest Iran due to the collaboration in this study.
Author contributions
M.H. (MH) & A.S. (AS) & M.M.-O. (MMO) & E.A. (EA): contributed to the acquisition of data, carried out the molecular genetic studies and have been involved in drafting the manuscript. M.P. (MP) & N.S. (NS) & S.M. (SM): participated in the design of the study, contributed to data collection and helped to draft the manuscript. A.S. & MMO: participated in carrying out the molecular genetic studies. All authors read and approved the final version of the manuscript.
Funding
This study was financially supported by Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (Grant no.67743).
Data availability
The datasets generated and/or analyzed during the current study are available in the NCBI database, [Accession numbers: OR193704–OR193706].
Declarations
Ethics approval and consent to participate
All COVID-19 patients signed an informed consent and fulfilled a questionnaire that included demographic data and their behavioral features. Ethical approval was granted by the ethical committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1400.537).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dubey J, Jones J. Toxoplasma Gondii Infection in humans and animals in the United States. Int J Parasitol. 2008;38(11):1257–78. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, Rahimi M-T, Sharif M. Seroprevalence of Toxoplasma Gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. 2014;137:185–94. doi: 10.1016/j.actatropica.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Webster JP. Review of Toxoplasmosis of animals and humans by JP Dubey. Parasites & Vectors. 2010;3:112. doi: 10.1186/1756-3305-3-112. [DOI] [Google Scholar]
- 4.Ali MI, Abd El Wahab WM, Hamdy DA, Hassan A. Toxoplasma Gondii in cancer patients receiving chemotherapy: seroprevalence and interferon gamma level. J Parasitic Dis. 2019;43:464–71. doi: 10.1007/s12639-019-01111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shams A-DE, Awad AHH. Seroprevalence of Toxoplasma Gondii in immunocompromised cancer patients in Basrah Provence, Southern Iraq. J Basrah Res(Sci) 2022;48(2):57–64. [Google Scholar]
- 6.Shaw T, El-Taweel H, Gammal M, Khali S, Ibrahim H. Toxoplasmosis in adult patients with haematologic malignancy: seroprevalence of anti-toxoplasma antibodies and molecular diagnosis. Parasitologists United Journal. 2023;16(1):79–86. doi: 10.21608/puj.2023.203903.1209. [DOI] [Google Scholar]
- 7.Shapira Y, Agmon-Levin N, Selmi C, Petríková J, Barzilai O, Ram M, Bizzaro N, Valentini G, Matucci-Cerinic M, Anaya J-M. Prevalence of anti-toxoplasma antibodies in patients with autoimmune Diseases. J Autoimmun. 2012;39(1–2):112–6. doi: 10.1016/j.jaut.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Lidar M, Langevitz P, Shoenfeld Y. The role of Infection in inflammatory bowel Disease: initiation, exacerbation and protection. Isr Med Association Journal: IMAJ. 2009;11(9):558–63. [PubMed] [Google Scholar]
- 9.Vittecoq M, Elguero E, Lafferty KD, Roche B, Brodeur J, Gauthier-Clerc M, Missé D, Thomas F. Brain cancer mortality rates increase with Toxoplasma Gondii seroprevalence in France. Infect Genet Evol. 2012;12(2):496–8. doi: 10.1016/j.meegid.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, Missé D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma Gondii is common. Biol Lett. 2012;8(1):101–3. doi: 10.1098/rsbl.2011.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus Pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konala VM, Adapa S, Gayam V, Naramala S, Daggubati SR, Kammari CB, Chenna A. Co-infection with Influenza A and COVID-19. Eur J case Rep Intern Med 2020, 7(5). [DOI] [PMC free article] [PubMed]
- 13.Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, Zhu F, Zhu B, Cui L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pemán J, Ruiz-Gaitán A, García-Vidal C, Salavert M, Ramírez P, Puchades F, García-Hita M, Alastruey-Izquierdo A, Quindós G. Fungal co-infection in COVID-19 patients: should we be concerned? Revista Iberoamericana De Micologia. 2020;37(2):41–6. doi: 10.1016/j.riam.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharaf-El-Deen SA. Toxoplasma Gondii as a possible risk factor for COVID-19 severity: a case-control study. Egypt J Med Microbiol. 2021;30(2):125–32. doi: 10.51429/EJMM30217. [DOI] [Google Scholar]
- 16.Flegr J. Toxoplasmosis is a risk factor for acquiring SARS-CoV-2 Infection and a severe course of COVID-19 in the Czech and Slovak population: a preregistered exploratory internet cross-sectional study. Parasites & Vectors. 2021;14(1):1–11. doi: 10.1186/s13071-021-05021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montazeri M, Nakhaei M, Fakhar M, Pazoki H, Pagheh AS, Nazar E, Zakariaei Z, Mirzaeian H, Sharifpour A, Banimostafavi ES. Exploring the Association between Latent Toxoplasma Gondii Infection and COVID-19 in hospitalized patients: First Registry-based study. Acta Parasitol 2022:1–8. [DOI] [PMC free article] [PubMed]
- 18.Roe K. A role for T-cell exhaustion in Long COVID‐19 and severe outcomes for several categories of COVID‐19 patients. J Neurosci Res. 2021;99(10):2367–76. doi: 10.1002/jnr.24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021:1494. [DOI] [PMC free article] [PubMed]
- 20.Abdel-Hamed EF, Ibrahim MN, Mostafa NE, Moawad HS, Elgammal NE, Darwiesh EM, El-Rafey DS, ElBadawy NE, Al-Khoufi EA, Hindawi SI. Role of interferon gamma in SARS-CoV-2-positive patients with parasitic Infections. Gut Pathogens. 2021;13(1):1–7. doi: 10.1186/s13099-021-00427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jankowiak Ł, Rozsa L, Tryjanowski P, Møller AP. A negative covariation between toxoplasmosis and CoVID-19 with alternative interpretations. Sci Rep. 2020;10(1):1–7. doi: 10.1038/s41598-020-69351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8 + T-cell response via PD-1–PDL-1 blockade. Proc Natl Acad Sci. 2011;108(22):9196–201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erol MK, Bozdogan YC, Suren E, Gedik B. Treatment of a full-thickness macular hole and retinal detachment secondary to toxoplasma chorioretinitis that developed shortly after COVID-19: a case report. J Fr Ophtalmol 2022. [DOI] [PMC free article] [PubMed]
- 24.Lecordier L, Moleon-Borodowsky I, Dubremetz J-F, Tourvieille B, Mercier C, Deslée D, Capron A, Cesbron-Delauw M-F. Characterization of a dense granule antigen of Toxoplasma Gondii (GRA6) associated to the network of the parasitophorous vacuole. Mol Biochem Parasitol. 1995;70(1–2):85–94. doi: 10.1016/0166-6851(95)00010-X. [DOI] [PubMed] [Google Scholar]
- 25.Fallahi S, Rostami A, Birjandi M, Zebardast N, Kheirandish F, Spotin A. Parkinson’s Disease and Toxoplasma Gondii Infection: sero-molecular assess the possible link among patients. Acta Trop. 2017;173:97–101. doi: 10.1016/j.actatropica.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Parsaei M, Spotin A, Matini M, Mahjub H, Aghazadeh M, Ghahremani G, Taherkhani H. Prevalence of toxoplasmosis in patients infected with Tuberculosis; a sero-molecular case-control study in northwest Iran. Comp Immunol Microbiol Infect Dis. 2022;81:101720. doi: 10.1016/j.cimid.2021.101720. [DOI] [PubMed] [Google Scholar]
- 27.Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19(18):2496–7. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- 28.Geraili A, Badirzadeh A, Sadeghi M, Mousavi SM, Mousavi P, Shahmoradi Z, Hosseini S-M, Hejazi SH, Rafiei-Sefiddashti R. Toxoplasmosis and symptoms severity in patients with COVID-19 in referral centers in Northern Iran. J Parasitic Dis. 2023;47(1):185–91. doi: 10.1007/s12639-022-01556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roe K. The symptoms and clinical manifestations observed in COVID-19 patients/long COVID-19 symptoms that parallel Toxoplasma Gondii Infections. J Neuroimmune Pharmacol. 2021;16(3):513–6. doi: 10.1007/s11481-021-09997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roe K. The link between Toxoplasma gondii Infections and higher mortality in COVID-19 patients having schizophrenia. Eur Arch Psychiatry Clin NeuroSci 2021:1–2. [DOI] [PMC free article] [PubMed]
- 31.Galván-Ramírez ML, Salas-Lais AG, Muñoz-Medina JE, Fernandes-Matano L, Pérez LRR. Franco De León K: Association of Toxoplasmosis and COVID-19 in a Mexican Population. Microorganisms. 2023;11(6):1441. doi: 10.3390/microorganisms11061441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao J, Prandovszky E, Kannan G, Pletnikov MV, Dickerson F, Severance EG, Yolken RH. Toxoplasma Gondii: biological parameters of the connection to schizophrenia. Schizophr Bull. 2018;44(5):983–92. doi: 10.1093/schbul/sby082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in Tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor β. Proc Natl Acad Sci. 1997;94(8):3926–31. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe K. The role of polyspecific T-cell exhaustion in severe outcomes for COVID-19 patients having latent pathogen Infections such as Toxoplasma Gondii. Microb Pathog. 2021;161:105299. doi: 10.1016/j.micpath.2021.105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osokine I, Snell LM, Cunningham CR, Yamada DH, Wilson EB, Elsaesser HJ, de la Torre JC, Brooks D. Type I interferon suppresses de novo virus-specific CD4 Th1 immunity during an established persistent viral Infection. Proc Natl Acad Sci. 2014;111(20):7409–14. doi: 10.1073/pnas.1401662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siles-Lucas M, González-Miguel J, Geller R, Sanjuan R, Pérez-Arévalo J, Martínez-Moreno Á. Potential influence of helminth molecules on COVID-19 pathology. Trends Parasitol. 2021;37(1):11–4. doi: 10.1016/j.pt.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Nested-PCR assay by targeting T. gondii B1
Supplementary Material 2: Nested-PCR assay by targeting T. gondii GRA6
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the NCBI database, [Accession numbers: OR193704–OR193706].