Abstract
Background
Osteoarthritis and hidradenitis suppurativa (HS) share a common inflammatory pathway. However, whether patients with HS have higher risk developing osteoarthritis remained unclear.
Methods
A retrospective cohort design was adopted in this study. Electronic medical records had been retrieved from the US collaborative network in the TriNetX research network. A propensity score matching of 1:1 was performed to match for covariates. In total, 50,931 patients with HS and the same amount of non-HS controls were identified for analyses. Hazard ratio (HR) of osteoarthritis in patient with HS was calculated.
Results
Risk of patients with HS developing osteoarthritis was 1.37-fold higher than that of non-HS controls [95% confidence interval (CI), 1.21–1.55] when followed up for 1 year. The significance remained when the follow-up periods were extended to 3 years and 5 years. When osteoarthritis was stratified on occurring sites, the HR of knee osteoarthritis was 1.19 (95% CI, 1.09–1.29) and the HR of hip osteoarthritis was 1.17 (95% CI, 1.01–1.35) in the 5-year follow-up. The 5-year risk of osteoarthritis remained significant in sensitivity models.
Conclusion
Patients with HS were of high risk of developing osteoarthritis compared with people without HS. The clinical association was recommended to be considered while approaching patients with HS.
Keywords: hidradenitis suppurativa, osteoarthritis, cohort, epidemiology, electronic medical records
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease that leads to the dysfunction of keratinocytes and causes pruritus and pain in patients’ skin lesions (1). According to a recent meta-analysis, the worldwide prevalence of HS was estimated to be 0.4% (2). Comorbidities of HS were found to be involved in various organ systems (3–5). Because proinflammatory cytokines play a critical role in the pathogenesis pathway of HS, it has been reported to be associated with many inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis (6, 7).
Osteoarthritis could have a substantial impact on the quality of life for patients (8), leading to the gradual breakdown of joint cartilage and causing pain, stiffness, and functional impairment (9). Incident osteoarthritis spans across different age groups, affecting both men and women. A recent global analysis indicated that women presented a higher global prevalence of osteoarthritis than men, and the prevalence increased with age (10). Although aging is regarded as one of the critical risk factors for osteoarthritis, this disease is not solely age-dependent. Risk factors, including inflammation status, obesity, and a history of joint injury, play crucial roles in influencing its onset (11, 12).
The association between HS and inflammatory arthritis has been a subject of prolonged discussion (7). HS has been reported to pose more than a three-fold risk of developing inflammatory arthritis compared to non-HS individuals (6). However, to the best of our knowledge, there is a lack of evidence describing the real-world association between HS and osteoarthritis, despite the existence of overlap in immunological pathways between the two diseases (13, 14). Therefore, we conducted a retrospective cohort analysis to provide further information regarding the risk of osteoarthritis in patients with HS.
Methods and materials
A retrospective cohort design was adopted for this study, with analyses conducted using datasets from the TriNetX research network. TriNetX is a global platform that provides access to electronic medical records from collaborative health care organizations (HCOs), comprising over 120 institutions. The platform is widely used to explore connections between different exposures and their outcomes (15–17).
In this research, our focus was on utilizing the US collaborative network, relying on records from HCOs within the United States. The dataset for extraction included data from 60 HCOs across the nation, comprising a prospectively updating dataset of approximately 88 million patient records for further analysis. Within the TriNetX platform, diseases were characterized using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes, and medications were categorized using Anatomical Therapeutic Chemical and RxNorm coding systems. Details of the applied codes in this study are presented in Table S1 .
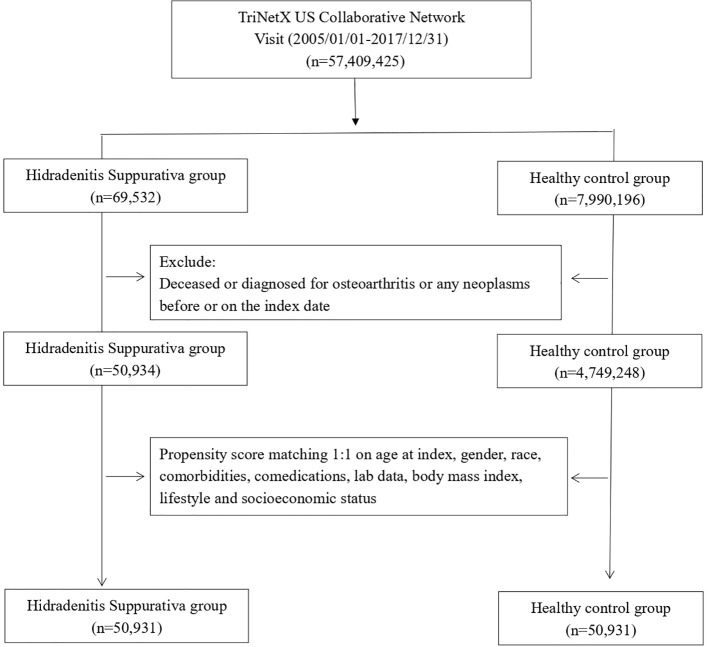
We enrolled patients who had documented visits and received an HS diagnosis within the study time frame, spanning from 1 January 2005 to 31 December 2017. Since TriNetX was prospectively updated, this study period ensured that every included patient underwent a follow-up for a minimum of 5 years. The index date for the HS cohort was set as the diagnosis date of HS. Individuals under 18 years old, those who died before the index date, and those with a history of cancer before the index date were excluded from this study. Healthy controls were identified on the basis of the records of underwent health examinations. To create a proper control group, we implemented propensity score matching. Covariates, including age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia, fracture, and osteoporosis), status of comedication use (glucocorticoids), status of smoking, alcoholism and substance use, medical utilization status, lab data C-reactive protein (CRP), and socioeconomic status (problems related to housing and economic circumstances, and persons with potential health hazards related to socioeconomic and psychosocial circumstances), were set as variables for matching. The end point of this study was defined as incident osteoarthritis. In this study, 1:1 propensity score matching was performed, and 50,323 patients with HS and the same number of non-HS controls were identified for analyses (Figure 1).
Figure 1.
Flowchart of participant selection.
We calculated the hazard ratio of osteoarthritis in the HS group. In stratification analyses, we assessed the risk of osteoarthritis in different age and sex subgroups. In addition, sensitivity analyses were conducted using diverse analytical models. This included the use of various claim-based algorithms with a high positive predictive value (18, 19), consideration of different matching covariates, and implementation of varying washout periods. These approaches were employed to mitigate potential biases.
The framework of the TriNetX research network system was utilized for statistical analyses in this study, conducted between 20 November 2023 and 22 November 2023. Standardized difference (SD) calculations were performed, and a value exceeding 0.1 indicated a statistically meaningful differentiation between the two compared groups. Propensity score matching was executed using a greedy nearest neighbor matching technique with a specific caliper of 0.1. Hazard ratios (HRs) were assessed, and their corresponding 95% confidence intervals (CIs) were calculated whenever applicable.
Statement of ethics
“The dataset within the TriNetX research network underwent de-identification following the guidelines outlined in Section §164.514(a) of the HIPAA Privacy Rule. This de-identification process was certified by a qualified expert, as outlined in Section §164.514(b)(1) of the HIPAA Privacy Rule. All analyses conducted in this study did not involve any intervention or direct engagement with human participants. Consequently, studies utilizing the TriNetX research network were deemed exempt from requiring permission from an Institutional Review Board (IRB) (20)”.
Results
Table 1 presented the baseline characteristics of participants. Patients in the HS group were 32.8 years old on average. Of the 50,931 patients with HS, 37,612 (73.8%) were women. Before matching, the baseline characteristics of age, sex, and race between the HS group and the control group were significantly different. After matching, baseline characteristics become similar between the two groups.
Table 1.
Baseline characteristics of study subjects (before and after propensity score matching).
| Before matching | After matching a | |||||
|---|---|---|---|---|---|---|
| HS cohort (n = 50,934) |
Control cohort (n = 4,749,248) |
Std diff |
HS cohort (n = 50,931) |
Control cohort (n = 50,931) |
Std diff |
|
| Age at index | ||||||
| Mean ± SD | 32.7 ± 13.7 | 36.4 ± 20.2 | 0.21 | 32.7 ± 13.7 | 33.2 ± 14.2 | 0.03 |
| Sex | ||||||
| Male | 12,728 (25.0) | 2,054,663 (43.3) | 0.39 | 12,728 (25.0) | 12,665 (24.9) | 0.00 |
| Female | 37,615 (73.9) | 2,568,755 (54.1) | 0.42 | 37,612 (73.8) | 37,621 (73.9) | 0.00 |
| Race, n (%) | ||||||
| White | 22,501 (44.2) | 2,802,552 (59.0) | 0.30 | 22,501 (44.2) | 22,367 (43.9) | 0.01 |
| Black or African American | 17,715 (34.8) | 709,601 (14.9) | 0.47 | 17,712 (34.8) | 17,987 (35.3) | 0.01 |
| Asian | 842 (1.7) | 168,173 (3.5) | 0.12 | 842 (1.7) | 1,619 (3.2) | 0.10 |
| American Indian or Alaska Native | 228 (0.4) | 15730 (0.3) | 0.02 | 228 (0.4) | 183 (0.4) | 0.01 |
| Socioeconomic status | ||||||
| Socioeconomic/psychosocial circumstances problem | 959 (1.9) | 31,870 (0.7) | 0.11 | 958 (1.9) | 852 (1.7) | 0.02 |
| Lifestyle | ||||||
| Alcohol dependence, smoking and substance use | 5,590 (11.0) | 138,867 (2.9) | 0.32 | 5,587 (11.0) | 5,668 (11.1) | 0.01 |
| Comorbidities | ||||||
| Hypertension | 5,487 (10.8) | 384,694 (8.1) | 0.09 | 5,486 (10.8) | 5,509 (10.8) | 0.00 |
| Diabetes mellitus | 3,301 (6.5) | 165,703 (3.5) | 0.14 | 3,299 (6.5) | 3,223 (6.3) | 0.01 |
| Hyperlipidemia | 2,844 (5.6) | 250,222 (5.3) | 0.01 | 2,844 (5.6) | 2,730 (5.4) | 0.01 |
| Osteoporosis | 173 (0.3) | 30,227 (0.6) | 0.04 | 173 (0.3) | 134 (0.3) | 0.01 |
| Fracture of shoulder and upper arm | 288 (0.6) | 23,525 (0.5) | 0.01 | 288 (0.6) | 217 (0.4) | 0.02 |
| Fracture of femur | 90 (0.2) | 7,070 (0.1) | 0.01 | 90 (0.2) | 67 (0.1) | 0.01 |
| Fracture of lower leg | 460 (0.9) | 24,218 (0.5) | 0.05 | 460 (0.9) | 376 (0.7) | 0.02 |
| Medications | ||||||
| Glucocorticoids | 9,580 (18.8) | 418,016 (8.8) | 0.29 | 9577 (18.8) | 9,474 (18.6) | 0.01 |
| Medical Utilization Status | ||||||
| Ambulatory visit | 28,403 (55.8) | 2140,433 (45.1) | 0.22 | 28,402 (55.8) | 28,480 (55.9) | 0.00 |
| Inpatient visit | 8,032 (15.8) | 476,128 (10.0) | 0.17 | 8,032 (15.8) | 8,062 (15.8) | 0.00 |
| Laboratory data | ||||||
| BMI, n (%) | ||||||
| ≧ 25 (kg/m2) | 7,800 (15.3) | 329,274 (6.9) | 0.27 | 7,797 (15.3) | 7,818 (15.4) | 0.00 |
| C reactive protein, n (%) | ||||||
| ≧ 3 (mg/L) | 1,778 (3.5) | 55,486 (1.2) | 0.15 | 1,775 (3.5) | 1,690 (3.3) | 0.01 |
If the patient is less or equal to 10, then the results show the count as 10.
Bold font represents that a standardized difference was more than 0.1.
HS, hidradenitis suppurativa.
Propensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia, fracture, and osteoporosis), status of comedication use (glucocorticoids), status of smoking, alcoholism and substance use, medical utilization status, lab data (CRP), and social economic status (problems related to housing and economic circumstances, and persons with potential health hazards related to socioeconomic and psychosocial circumstances).
Osteoarthritis risk in patients with HS was presented in Table 2 . Risk of patients with HS developing osteoarthritis was 1.37-fold higher than that of non-HS controls (95% CI, 1.21–1.55) when followed up for 1 year. The significance remained when the follow-up periods were extended to 3 years (HR, 1.34; 95% CI, 1.25, 1.44) and 5 years (HR, 1.34; 95% CI, 1.27–1.41). When osteoarthritis was stratified on occurring sites, the HR of knee osteoarthritis was 1.19 (95% CI, 1.09–1.29) and the HR of hip osteoarthritis was 1.17 (95% CI, 1.01–1.35) in the 5-year follow-up ( Table 3 ). The 5-year risk of osteoarthritis remained significant in sensitivity models applying different matching algorithms ( Table S2 ) and different definition of HS based on ICD-10-CM-codes ( Table S3 ). In the sensitivity analyses applying various washout periods, significance was also observed ( Table S4 ). When excluding the incident osteoarthritis diagnosed within 36 months after index date, the HR for patients with HS having osteoarthritis in future 5 years was 1.31 (95% CI, 1.22–1.41).
Table 2.
Risk of osteoarthritis under different follow-up time a .
| Outcomes | Hazard ratio (95% Confidence interval) b | ||
|---|---|---|---|
| 1 year | 3 years | 5 years | |
| Osteoarthritis | 1.37 (1.21, 1.55) | 1.34 (1.25, 1.44) | 1.34 (1.27, 1.41) |
HS, hidradenitis suppurativa.
Data presented here were the value of follow-up from 90 days after index date to the respective following-up years.
Propensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia, fracture, and osteoporosis), status of comedication use (glucocorticoids), status of smoking, alcoholism and substance use, medical utilization status, lab data (CRP), and social economic status (problems related to housing and economic circumstances, and persons with potential health hazards related to socioeconomic and psychosocial circumstances).
Bold values indicates that those values were of statistical significance.
Table 3.
Risk of site of osteoarthritis in the 5-year follow-up a .
| Outcomes | Hazard ratio (95% confidence interval) b |
|---|---|
| Knee osteoarthritis | 1.19 (1.09, 1.29) |
| Hip osteoarthritis | 1.17 (1.01, 1.35) |
HS, hidradenitis suppurativa.
Data presented here were the value of follow-up from 90 days after index date to the respective following-up years.
Propensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia, fracture, and osteoporosis), status of comedication use (glucocorticoids), status of smoking, alcoholism and substance use, medical utilization status, lab data (CRP), and socioeconomic status (problems related to housing and economic circumstances, and persons with potential health hazards related to socioeconomic and psychosocial circumstances).
Bold values indicates that those values were of statistical significance.
Results of stratification analyses were presented in Table 4 . The 5-year risk of developing future osteoarthritis in male patients with HS was 1.20-fold higher than that of non-HS male population (95% CI, 1.07–1.33). For female patients with HS, the HR was 1.31 (95% CI, 1.23–1.40). In patients with HS ranged from 18 to 64 years old, the HR of developing osteoarthritis was 1.19 (95% CI, 1.07–1.33), whereas, in patients with HS greater than 65 years old, the HR was 1.38 (95% CI, 1.24–1.54), compared with non-HS controls in respective age subgroups.
Table 4.
Stratification analysis of osteoarthritis risk in patients with HS.
| Cases occurring new-onset osteoarthritis | |||
|---|---|---|---|
| Subgroups | HS cohort (no. of event/HS patient amount in each subgroup) |
Control cohort (no. of event/non-HS patient amount in each subgroup) |
HR (95% CI) a |
| Gender | |||
| Male | 707/12,376 | 597/12,376 | 1.20 (1.07, 1.33) |
| Female | 2,084/36,498 | 1,588/36,498 | 1.31 (1.23, 1.40) |
| Age at index date | |||
| 18–64 years old | 745/12,728 | 625/12,728 | 1.19 (1.07, 1.33) |
| ≥ 65 years old | 722/3,950 | 555/3,950 | 1.38 (1.24, 1.54) |
Propensity score matching was performed on age at index, sex, race, body mass index, status of comorbidities (including diabetes mellitus, hypertension, hyperlipidemia, fracture, and osteoporosis), status of comedication use (glucocorticoids), status of smoking, alcoholism and substance use, medical utilization status, lab data (CRP), and socioeconomic status (problems related to housing and economic circumstances, and persons with potential health hazards related to socioeconomic and psychosocial circumstances).
In order to protect the privacy of participants, the TriNetX system was not able to present the exact number of participant if the number was less than 10. Hence, for these stratification groups, we were not able to calculate the hazard ratio.
Bold values indicates that those values were of statistical significance.
Discussion
According to the results of the current study, patients with HS were associated with a higher risk of future osteoarthritis compared to non-HS controls, with an HR of 1.37 (95% CI, 1.21–1.55) within the first year after the index date. This elevated risk remained statistically significant in different sex and age subgroups.
Pathogenesis of HS involved the elevation of proinflammatory cytokines that could trigger subsequent immunological cascades (21). Although the pathogenesis of HS has not been fully understood, it was pointed out that sustained inflammatory reaction within dermatological diseases had the potential to trigger systemic immunological involvement, consequently fostering the emergence of comorbidities in various organ systems (22–24). Given these conditions, the issue of HS comorbidities had long been discussed and was acknowledged as intricate, encompassing several different functional problems including allergic status (25), gastrointestinal dysfunctions (26, 27), and renal diseases (28). Among the comorbidities of HS, integrated pieces of evidence had suggested that spondyloarthritis and rheumatoid arthritis were of high prevalence and incidence in the HS population (6, 29). A recent small-scale case-control study stated that some of the bone function indicators, such as trabecular bone score and hip bone mineral density, were low in patients with HS compared with that in controls (30). Although previous meta-analyses stated that HS could be associated with other inflammatory arthritis (6), the association between HS and osteoarthritis has not been clearly clarified because of insufficient data in observational studies. Thereby, to fulfill the knowledge gap, we provided real-world information regarding the risk of osteoarthritis in patients with HS after considering the influence of various confounders.
Clinically, patients with syndromic HS were often observed to present with articular involvement, and the association between synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome has long been discussed (31, 32). In the involved pathways of HS and osteoarthritis, some of the involved key cytokines were in common. For instance, in patients with HS, because of the chronic inflammation status in follicles, the proinflammatory cytokines, such as interleukin 1b (IL-1b) and tumor necrosis factor-alpha (TNF-alpha), would be elevated and lead to systemic inflammation (1, 21). In patients with osteoarthritis, these cytokines could also result in the impairment of tissues in the musculoskeletal system, wherein IL-1b could play a critical role in cartilage damage and TNF-alpha was associated with triggering further inflammatory reactions (33). Moreover, the influence of IL-17 was also a possible attribution of the HS–osteoarthritis association. The activation of IL-17 cytokine was regarded as one of the critical process contributing to the dysfunction of keratinocytes and systemic inflammation (14), and the concentration of IL-17 was found to be high in patients with HS (34). IL-17 contributes to the pathogenesis of HS by stimulating the proliferation of keratinocytes. This, in turn, prompts keratinocytes to produce inflammatory mediators such as antimicrobial peptides and cytokines, which could drive inflammation and recruit immune cells (14, 35). Studies revealed that the concentration of IL-17 in patients with osteoarthritis was higher than those without osteoarthritis (36). IL-17 was proposed to play a potential role in the promotion of chondrocyte senescence and apoptosis in patients with osteoarthritis (37). It stimulates the production of reactive oxygen species, chemokines, and inflammatory factors like IL-1β in chondrocytes. This results in premature chondrocyte senescence, cell cycle dysfunction, and apoptosis, contributing to cartilage breakdown (38). Moreover, in the recent in vitro and in vivo studies, IL-17 was reported to promote the occurrence of cartilage deformation (13, 39, 40). However, in the current study design, we were not able to monitor the patients’ inflammatory status and clarify the actual cytokine reaction pathway. Therefore, future clinical studies were needed to elucidate the role of the aforementioned cytokines in the observed HS–osteoarthritis association.
There is currently a great interest surrounding the emerging systemic therapeutics for HS, including potential combination regimens, which may not only improve HS symptoms but beneficially impact comorbidities like inflammatory arthritis (41). These treatments could not only alleviate HS symptoms but also positively affect related conditions such as inflammatory comorbidities. Clinical trials have demonstrated the effectiveness of anti–IL-17 biologics like secukinumab and bimekizumab in reducing the severity of HS (42, 43). Pieces of evidence also suggest that Janus kinase inhibitors such as baricitinib and tofacitinib could be beneficial in HS management (44). These systemic therapies could potentially reduce chronic inflammation associated with common comorbidities; however, current pieces of evidence regarding the head-to-head comparison evaluating whether biologics use could be effective in reducing the inflammatory comorbidity status in patients with HS are lacking. It is important to carefully monitor the response of related conditions like arthritis to these promising HS treatments.
In our study, we found that the risk of patients with HS developing osteoarthritis was slightly higher within a 1-year follow-up period (HR, 1.37; 95% CI, 1.21–1.55) compared with the 3-year (HR, 1.34; 95% CI, 1.25–1.44) and 5-year (HR, 1.34; 95% CI, 1.27–1.41) follow-up periods. The marginally increased risk of an outcome event in the short-term follow-up period could potentially be due to the effect of reversed causation bias (45), which is common in real-world studies (46). Under the same washout periods, incident osteoarthritis events could inadvertently include those who already have underlying osteoarthritis but are diagnosed shortly after an HS diagnosis. To address this issue of reverse causality, we applied different washout periods in sensitivity models, and the association between HS and osteoarthritis remained significant in each model.
Limitation of this study should be considered while interpreting the results. First, we were only able to provide follow-up time up to 5 years after the index date in the current study design. Hence, we were not able to evaluate the long-term association between HS and osteoarthritis. Second, although we have tried our best to address the confounding bias caused by potential confounders via propensity score matching method, some unknown confounders could still influence the evaluation of association between the two diseases. Third, although we have applied validated coding algorithms in the definition of HS (18, 19), as in other studies utilizing database to perform secondary analyses (47, 48), misclassification bias due to the coding algorithm could still lead to potential imprecise results and should not be neglected while interpreting the clinical implication of the current study.
To recapitulate, we report that patients with HS were of high risk of developing osteoarthritis compared with people without HS, and the risk was 1.41-fold (95% CI, 1.24–1.60) in the first year after HS diagnosis. The clinical association was recommended to be taken into account in the treatment plan while approaching patients with HS.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Data in this study were retrieved from TriNetX Research Network. All data available in the database were administrated by the TriNetX platform. Detailed information can be retrieved at the official website of the research network (https://trinetx.com). Requests to access these datasets should be directed to TriNetX platform (https://trinetx.com).
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
HC: Conceptualization, Data curation, Investigation, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. CW: Conceptualization, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. WL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Validation, Writing – original draft, Writing – review & editing. SG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. TC: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by research grants to W-CL from the National Science and Technology Council (111-2320-B-005 -009 -MY3), Taiwan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1285560/full#supplementary-material
References
- 1. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol (2020) 82(5):1045–58. doi: 10.1016/j.jaad.2019.08.090 [DOI] [PubMed] [Google Scholar]
- 2. Jfri A, Nassim D, O'Brien E, Gulliver W, Nikolakis G, Zouboulis CC. Prevalence of hidradenitis suppurativa: A systematic review and meta-regression analysis. JAMA Dermatol (2021) 157(8):924–31. doi: 10.1001/jamadermatol.2021.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gau SY, Lee CY, Kuan YH, Ma KS, Wei JC. Hyperthyroidism and hypothyroidism in patients with hidradenitis suppurativa: a systematic review and meta-analysis. Int J Dermatol (2023) 62(3):e116–20. doi: 10.1111/ijd.16484 [DOI] [PubMed] [Google Scholar]
- 4. Phan K, Charlton O, Smith SD. Hidradenitis suppurativa and metabolic syndrome - systematic review and adjusted meta-analysis. Int J Dermatol (2019) 58(10):1112–7. doi: 10.1111/ijd.14500 [DOI] [PubMed] [Google Scholar]
- 5. Varney P, Guo W, Brown M, Usmani H, Marquez J, Ayasse M, et al. A systematic review and meta-analysis of sexual dysfunction in patients with hidradenitis suppurativa. Int J Dermatol (2023) 62(6):737–46. doi: 10.1111/ijd.16328 [DOI] [PubMed] [Google Scholar]
- 6. Almuhanna N, Finstad A, Alhusayen R. Association between hidradenitis suppurativa and inflammatory arthritis: A systematic review and meta-analysis. Dermatology (2021) 237(5):740–7. doi: 10.1159/000514582 [DOI] [PubMed] [Google Scholar]
- 7. Richette P, Molto A, Viguier M, Dawidowicz K, Hayem G, Nassif A, et al. Hidradenitis suppurativa associated with spondyloarthritis – results from a multicenter national prospective study. J Rheumatol (2014) 41(3):490–4. doi: 10.3899/jrheum.130977 [DOI] [PubMed] [Google Scholar]
- 8. Atukorala I, Hunter DJ. A review of quality-of-life in elderly osteoarthritis. Expert Rev Pharmacoecon Outcomes Res (2023) 23(4):365–81. doi: 10.1080/14737167.2023.2181791 [DOI] [PubMed] [Google Scholar]
- 9. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet (2015) 386(9991):376–87. doi: 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 10. Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis (2020) 79(6):819–28. doi: 10.1136/annrheumdis-2019-216515 [DOI] [PubMed] [Google Scholar]
- 11. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med (2016) 59(3):134–8. doi: 10.1016/j.rehab.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 12. Nedunchezhiyan U, Varughese I, Sun AR, Wu X, Crawford R, Prasadam I. Obesity, inflammation, and immune system in osteoarthritis. Front Immunol (2022) 13:907750. doi: 10.3389/fimmu.2022.907750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mimpen JY, Baldwin MJ, Cribbs AP, Philpott M, Carr AJ, Dakin SG, et al. Interleukin-17A causes osteoarthritis-like transcriptional changes in human osteoarthritis-derived chondrocytes and synovial fibroblasts in vitro . Front Immunol (2021) 12:676173. doi: 10.3389/fimmu.2021.676173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher JM, Moran B, Petrasca A, Smith CM. IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin Exp Immunol (2020) 201(2):121–34. doi: 10.1111/cei.13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Proietti R, Rivera-Caravaca JM, Lopez-Galvez R, Harrison SL, Marin F, Underhill P, et al. Cerebrovascular, cognitive and cardiac benefits of SGLT2 inhibitors therapy in patients with atrial fibrillation and type 2 diabetes mellitus: results from a global federated health network analysis. J Clin Med (2023) 12(8):2814. doi: 10.3390/jcm12082814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmale IL, Poulakis A, Abend A, Luitje ME, Man LX. Chronic rhinosinusitis with nasal polyposis treated with dupilumab: real-world use and outcomes. J Allergy Clin Immunol Pract (2023) 11(10):3203–3210. doi: 10.1016/j.jaip.2023.07.038 [DOI] [PubMed] [Google Scholar]
- 17. Yong SB, Gau SY, Li CJ, Tseng CW, Wang SI, Wei JCC. Associations between COVID-19 outcomes and asthmatic patients with inhaled corticosteroid. Front Pharmacol (2023) 14:1204297. doi: 10.3389/fphar.2023.1204297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smiley C, Strunk A, Butt M, Kirby J, Garg A. Positive predictive value of diagnosis codes for hidradenitis suppurativa and cutaneous abscess. Dermatology (2023) 239(2):273–6. doi: 10.1159/000528752 [DOI] [PubMed] [Google Scholar]
- 19. Strunk A, Midura M, Papagermanos V, Alloo A, Garg A. Validation of a case-finding algorithm for hidradenitis suppurativa using administrative coding from a clinical database. Dermatology (2017) 233(1):53–7. doi: 10.1159/000468148 [DOI] [PubMed] [Google Scholar]
- 20. Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry (2022) 9(10):815–27. doi: 10.1016/S2215-0366(22)00260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sabat R, Jemec GBE, Matusiak L, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers (2020) 6(1):18. doi: 10.1038/s41572-020-0149-1 [DOI] [PubMed] [Google Scholar]
- 22. Garg A, Malviya N, Strunk A, Wright S, Alavi A, Alhusayen R, et al. et al: Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol (2022) 86(5):1092–101. doi: 10.1016/j.jaad.2021.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gau SY, Hsiao YP, Liao WC, Ma KS, Wu MC. Risk of liver dysfunction and non-alcoholic fatty liver diseases in people with hidradenitis suppurativa: A systematic review and meta-analysis of real-world evidences. Front Immunol (2022) 13:959691. doi: 10.3389/fimmu.2022.959691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gau SY, Preclaro IAC, Wei JC, Lee CY, Kuan YH, Hsiao YP, et al. Risk of psoriasis in people with hidradenitis suppurativa: A systematic review and meta-analysis. Front Immunol (2022) 13:1033844. doi: 10.3389/fimmu.2022.1033844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gau SY, Chan WL, Tsai JD. Risk of atopic diseases in patients with hidradenitis suppurativa: A systematic review and meta-analysis of observational studies. Dermatology (2023) 239(2):314–22. doi: 10.1159/000528920 [DOI] [PubMed] [Google Scholar]
- 26. Chen WT, Chi CC. Association of hidradenitis suppurativa with inflammatory bowel disease: A systematic review and meta-analysis. JAMA Dermatol (2019) 155(9):1022–7. doi: 10.1001/jamadermatol.2019.0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phan K, Tatian A, Woods J, Cains G, Frew JW. Prevalence of inflammatory bowel disease (IBD) in hidradenitis suppurativa (HS): systematic review and adjusted meta-analysis. Int J Dermatol (2020) 59(2):221–8. doi: 10.1111/ijd.14697 [DOI] [PubMed] [Google Scholar]
- 28. Gau SY. Increased risk of renal diseases in people with hidradenitis suppurativa: a systematic review and meta-analysis. Int J Dermatol (2023) 62(1):e4–6. doi: 10.1111/ijd.16423 [DOI] [PubMed] [Google Scholar]
- 29. Hanna N, Silverberg OM, Reaume M, Gladman D, Davis MDP, Piguet V, et al. Incidence, prevalence, and predictors of inflammatory arthritis in patients with hidradenitis suppurativa: a systematic review and meta-analysis. Int J Dermatol (2022) 61(9):1069–79. doi: 10.1111/ijd.15860 [DOI] [PubMed] [Google Scholar]
- 30. Navarro I, Gonzalez-Lopez MA, Sierra I, Olmos JM, Blanco R, Hernandez JL. Bone metabolism in patients with hidradenitis suppurativa: A case-control study. Acta Derm Venereol (2022) 102:adv00825. doi: 10.2340/actadv.v102.3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen W, Ito T, Lin SH, Song Z, Al-Khuzaei S, Jurik AG, et al. Does SAPHO syndrome exist in dermatology? J Eur Acad Dermatol Venereol (2022) 36(9):1501–6. doi: 10.1111/jdv.18172 [DOI] [PubMed] [Google Scholar]
- 32. Vekic DA, Woods J, Lin P, Cains GD. SAPHO syndrome associated with hidradenitis suppurativa and pyoderma gangrenosum successfully treated with adalimumab and methotrexate: a case report and review of the literature. Int J Dermatol (2018) 57(1):10–8. doi: 10.1111/ijd.13740 [DOI] [PubMed] [Google Scholar]
- 33. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol (2011) 7(1):33–42. doi: 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 34. Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol (2011) 65(4):790–8. doi: 10.1016/j.jaad.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 35. Scala E, Cacciapuoti S, Garzorz-Stark N, Megna M, Marasca C, Seiringer P, et al. Hidradenitis suppurativa: where we are and where we are going. Cells (2021) 10(8):2094. doi: 10.3390/cells10082094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee YH, Song GG. Association between IL-17 gene polymorphisms and circulating IL-17 levels in osteoarthritis: a meta-analysis. Z Rheumatol (2020) 79(5):482–90. doi: 10.1007/s00393-019-00720-2 [DOI] [PubMed] [Google Scholar]
- 37. Jrad AIS, Trad M, Bzeih W, El Hasbani G, Uthman I. Role of pro-inflammatory interleukins in osteoarthritis: a narrative review. Connect Tissue Res (2023) 64(3):238–47. doi: 10.1080/03008207.2022.2157270 [DOI] [PubMed] [Google Scholar]
- 38. Xiao J, Zhang P, Cai FL, Luo CG, Pu T, Pan XL, et al. IL-17 in osteoarthritis: A narrative review. Open Life Sci (2023) 18(1):20220747. doi: 10.1515/biol-2022-0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Na HS, Park JS, Cho KH, Kwon JY, Choi J, Jhun J, et al. Interleukin-1-interleukin-17 signaling axis induces cartilage destruction and promotes experimental osteoarthritis. Front Immunol (2020) 11:730. doi: 10.3389/fimmu.2020.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Z, Zheng C, Zhong Y, He J, Cao X, Xia H, et al. Interleukin-17 can induce osteoarthritis in rabbit knee joints similar to hulth's method. BioMed Res Int (2017) 2017:2091325. doi: 10.1155/2017/2091325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maronese CA, Ingram JR, Marzano AV. Has the time come to assess small-molecule/biologic drug combinations for the management of moderate-to-severe hidradenitis suppurativa? Br J Dermatol (2023) 189(4):467–8. doi: 10.1093/bjd/ljad224 [DOI] [PubMed] [Google Scholar]
- 42. Glatt S, Jemec GBE, Forman S, Sayed C, Schmieder G, Weisman J, et al. et al: efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: A phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol (2021) 157(11):1279–88. doi: 10.1001/jamadermatol.2021.2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kimball AB, Jemec GBE, Alavi A, Reguiai Z, Gottlieb AB, Bechara FG, et al. et al: Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet (2023) 401(10378):747–61. doi: 10.1016/S0140-6736(23)00022-3 [DOI] [PubMed] [Google Scholar]
- 44. Martora F, Scalvenzi M, Ruggiero A, Potestio L, Battista T, Megna M. Hidradenitis suppurativa and JAK inhibitors: A review of the published literature. Medicina (Kaunas) (2023) 59(4):801. doi: 10.3390/medicina59040801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sattar N, Preiss D. Reverse causality in cardiovascular epidemiological research: more common than imagined? Circulation (2017) 135(24):2369–72. doi: 10.1161/CIRCULATIONAHA.117.028307 [DOI] [PubMed] [Google Scholar]
- 46. Gau SY, Huang JY, Yong SB, Cheng-Chung Wei J. Higher risk of hyperthyroidism in people with asthma: evidence from a nationwide, population-based cohort study. J Allergy Clin Immunol Pract (2022) 10(3):751–758 e751. doi: 10.1016/j.jaip.2021.09.021 [DOI] [PubMed] [Google Scholar]
- 47. Gau SY, Huang KH, Lee CH, Kuan YH, Tsai TH, Lee CY. Bidirectional association between psoriasis and nonalcoholic fatty liver disease: real-world evidence from two longitudinal cohort studies. Front Immunol (2022) 13:840106. doi: 10.3389/fimmu.2022.840106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gau SY, Lai JN, Yip HT, Wu MC, Wei JC. Higher dementia risk in people with gastroesophageal reflux disease: A real-world evidence. Front Aging Neurosci (2022) 14:830729. doi: 10.3389/fnagi.2022.830729 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: Data in this study were retrieved from TriNetX Research Network. All data available in the database were administrated by the TriNetX platform. Detailed information can be retrieved at the official website of the research network (https://trinetx.com). Requests to access these datasets should be directed to TriNetX platform (https://trinetx.com).