Abstract
The zoonotic pathogen Wohlfahrtiimonas chitiniclastica can cause several diseases in humans, including sepsis and bacteremia. Although the pathogenesis is not fully understood, the bacterium is thought to enter traumatic skin lesions via fly larvae, resulting in severe myiasis and/or wound contamination. Infections are typically associated with, but not limited to, infestation of an open wound by fly larvae, poor sanitary conditions, cardiovascular disease, substance abuse, and osteomyelitis. W. chitiniclastica is generally sensitive to a broad spectrum of antibiotics with the exception of fosfomycin. However, increasing drug resistance has been observed and its development should be monitored with caution. In this review, we summarize the currently available knowledge and evaluate it from both a clinical and a genomic perspective.
Keywords: Wohlfahrtiimonas chitiniclastica, Antibiotic resistance, Arsenic resistance, “One health” approach
Background
Wohlfahrtiimonas chitiniclastica was first isolated from the larvae of Wohlfahrtia magnifica [1], an obligate parasitic fly that causes myiasis by depositing eggs and larvae in wounds of both mammals and humans [2]. W. magnifica (Diptera:Sarcophagidae) was first described by Schiner in 1962 [3]. Cells of W. chitiniclastica are Gram-negative, strictly aerobic, non-motile rods. The G + C content of the DNA of the type strain DSM 18708T is 44.3 mol% and the major fatty acids are C18:1 and C14:0 [1]. Both catalase and oxidase reaction are positive, while biochemical tests for urease, indole and H2S are negative [1]. A key feature is the strong chitinase activity, which may be an indicator of a symbiotic relationship with its host fly and also plays an important role in metamorphosis [1, 4, 5]. To date, several case reports have been published suggesting that W. chitiniclastica can cause various diseases in humans as a zoonotic pathogen [6]. Although the pathogenesis of W. chitiniclastica has not been fully elucidated, the bacterium is expected to invade traumatic skin lesions through fly larvae, resulting in severe myiasis and/or wound contamination [2, 5–7]. Myiasis is defined as the infestation of living humans and vertebrates with dipteran larvae (maggots) that feed, at least for some time, on dead or living tissues, liquid body substances, or ingested food of the host [2]. In this review, we summarize the currently available knowledge on W. chitiniclastica and evaluate it from a clinical and genomic perspective. Since elucidating the significance of W. chitiniclastica as a human pathogen is a major focus of our research, we also refer in this review to previously published data from our own scientific work [6, 8]. The aim of this manuscript is therefore to point out gaps in our knowledge on W. chitiniclastica by summarizing the currently available data and thus to lay the foundation for further research on this species.
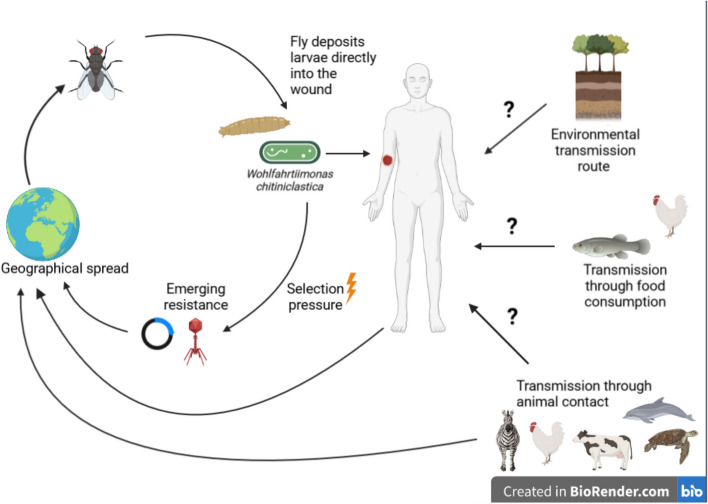
Recently, it could be shown that W. chitiniclastica is of importance for both veterinary and human medicine [8]. Although insects currently appear to be responsible for the main transmission, other transmission routes (e.g. through the contact with soil) may be possible as well [6, 9] (Fig. 2). However, these potential routes still need to be clearly elucidated in future studies. The fact that W. chitiniclastica harbours a resistance to heavy metals (especially arsenic) could assure this species a survival advantage [9]. In addition, it would also make sense to further clarify if other insects (not only flies) are associated with W. chitiniclastica infections. This is of particular interest since W. chitiniclastica occurs worldwide and does not seem to be restricted to a particular climate zone [6]. In addition, it can be assumed that W. chitiniclastica was not detected in the past due to incorrect identification. According to recent studies, a secure identification of W. chitiniclastica is possible using MALDI TOF MS or sequencing of the 16S rRNA gene [6]. With the increasing use of MALDI TOF MS in routine laboratories worldwide, it can be assumed that the number of clinical case reports will increase. This will allow risk factors such as poor hygienic conditions, chronic wounds or diabetes mellitus to be more clearly defined and contribute to a better epidemiological understanding. As there is currently no surveillance system for rare human pathogenic bacteria, clinical case reports play a crucial role in further understanding the epidemiology of W. chitiniclastica infections. Since this bacterium is usually part of a polymicrobial infection, future studies need to be conducted to elucidate pathogenicity by exclusively investigating W. chitiniclastica isolates. For instance, such studies could address the interaction of W. chitiniclastica with the host organism, both in vitro and in vivo (e.g., elucidation of the infection route, interaction with the human skin or the immune system) but also with other microorganisms. As far as sensitivity to antimicrobial agents is concerned, there is a pronounced (possibly primary) resistance to fosfomycin, the genetic basis of which is still unclear. Nevertheless, most isolates seem to be sensitive to quinolones and trimetoprim/sulfamethoxazole. Although W. chitiniclastica is currently regarded as a rare pathogen, it is likely that due to the growth of the world’s population (and thus closer contact between humans and animals), the number of zoonotic infections such as the ones caused by W. chitiniclastica, will increase [10].
Fig. 2.
Schematic representation of the possible transmission routes of W. chitiniclastica. This figure was created with BioRender.com
Members of the genus Wohlfahrtiimonas
The genus Wohlfahrtiimonas of the class Gammaproteobacteria was first established by Tóth et al. in 2008 [1], and it currently consists of three species [11, 12]. These include W. chitiniclastica, Wohlfahrtiimonas populi [13] and Wohlfahrtiimonas larvae [14] (Fig. 1). Both, W. chitiniclastica and W. larvae were first isolated from the larvae of dipteran flies [1, 14], whereas W. populi was isolated from the bark tissue of the Canadian poplar (Populus canadensis) [13]. To our knowledge, neither W. larvae nor W. populi have been associated with infections in animals or humans. Noteworthy, the genus Wohlfahrtiimonas belongs to a distinct lineage close to Ignatzschineria larvae which was isolated from first and second larval stages of same fly W. magnifica [15, 16]. Like W. chitiniclastica, I. larvae are considered emerging human and animal pathogens, and have been linked to infections caused by maggot infestation of open wounds [11].
Fig. 1.
Neighbor-joining phylogenetic tree of partial 16S rRNA gene sequences. Sequences of the type strains were retrieved from GenBank: W. chitiniclastica (accession number AM397063), W. populi (accession number KT988034), W. larvae (accession number JN873914), I. larvae (accession number AJ252143), I. ureiclastica (accession number EU008089), I. indica (accession number EU008088|), and I. cameli (accession number LC377575). Phylogenetic tree construction was completed using NGPhylogeny [151] and visualized with iTOL [152]
Zoonotic transmission routes of W. chitiniclastica
W. chitiniclastica has been described as part of the physiological flora of several fly species such as W. magnifica [1], Lucilia sericata (Meigen, 1826) (Diptera: Calliphoridae) [17–19], Lucilia illustris (Meigen, 1826) (Diptera: Calliphoridae) [20, 21], Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) [22, 23], Hermetia illucens (Linnaeus, 1758) (Diptera: Stratiomyidae) [14, 24, 25] and Musca domestica (Linnaeus, 1758) (Diptera:Muscidae) [26, 27]. To the best of our knowledge, flies mainly ensure the spread of W. chitiniclastica by depositing larvae in wounds and ulcers of vertebrates, also referred to as myiasis [2, 5]. Of note, the potential application of I. larvae and W. chitiniclastica in forensic microbiology was recently investigated from necrophagous insect species [20]. The study showed that W. chitiniclastica was detectable in all developmental stages of L. illustris, with the highest abundances observed in the second and third larval stages [20]. Although further investigations targeting these bacterial species are required to confirm their role as colonization biomarkers in forensic investigations this report highlights the applicative potential of W. chitiniclastica in forensic sciences [20].
In addition to the various fly species, other habitats and zoonotic transmission routes are also conceivable (Fig. 2). W. chitiniclastica has also been detected in arsenic-contaminated soil [9], in the pancreas of a zebra [28], in frozen chicken meat [29], poultry chickens in the Noakhali region of Bangladesh [30], in aquatic plants from Egypt [31] and in the human gut microbiome of deceased individuals [32]. In addition to transfer by insects, transmission by contact with the environment or consumption of food also appears to be possible. For example, W. chitiniclastica was found abundant in fermented animal and fish-based foods [33] and in chicken meat samples obtained from retail markets [34]. Finally, and more importantly, recent studies indicate that W. chitiniclastica may be the cause of several diseases in different organisms. These include marine fish [35, 36], turtles [37], various mammals [7, 38–40] and humans [5, 41], making the bacterium a previously underestimated veterinarian and human pathogen [5, 8]. Initially, W. chitiniclastica was described as strictly aerobic by Tóth et al. [1], whereas both W. populi and W. larvae were described as facultatively anaerobic [13, 14]. Recently, two case studies reported for the first time that the W. chitiniclastica strain found in each case also grew under anaerobic conditions [42, 43]. The fact that W. chitiniclastica can colonize different species under aerobic and anaerobic conditions should be considered an advantage for the bacterium [8]. The facultative anaerobic lifestyle allows to utilize electron acceptors that are byproducts of host inflammation, thereby increasing its prevalence within the community [44]. On the other hand, it also poses an increased risk for zoonotic transmission, whose dynamic interactions between humans, animals, and pathogens should be considered in the context of the “One Health” approach [8, 45, 46].
Human infections reported in association with W. chitiniclastica
As of June 2023, 43 cases of human infection associated with W. chitiniclastica have been published (Table 1). In addition, several case reports relevant to veterinary medicine have also been reported. These include a fatal infection in a deer [7], a hoof infection in a cow [38], a fulminant fish sepsis from India [35], septicemia in a soft-shelled turtle (Pelodiscus sinensis; Testudines, Trionychida) [37], interdigital dermatitis in dairy cows [40], evidence of endocarditis infection in a dolphin [39], and preliminary animal infection experiments by Qi et al. suggest that W. chitiniclastica is pathogenic to mice [38]. However, since the focus of this review is on the human pathogenic aspect, these reports are mentioned only for completeness. Remarkably, myiasis was not detected in any of these veterinary case reports, supporting the hypothesis that the organism colonizes other ecological niches besides the maggot flora [41].
Table 1.
Current overview of cases of human infection and colonization with W. chitiniclastica as of March 2023. In some cases, antibiotic treatment information was not provided, so there were marked NP (not provided)
| Case | Year | Age | Gender | Region | Underlying disease(s)/reason for hospital admission | Polymicrobial infection | Insect larvae/infested wounds | Antibiotic treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2009 | 60 | Female | Marseille, France | Neutroepenia, thrombocytopenia, fatigue | – | yes | Ceftriaxone | Survived | [71] |
| 2 | 2011 | 70 | Male | Buenos Aires, Argentina | Occlusive peripheral arteriopathy of the lower limbs/sensory impairment | – | – | Ciprofloxacin, ampicillin, ceftazidime, amikacin | Fatal | [61] |
| 3 | 2015 | 82 | Female | Guildford, UK | Recurrent falls, hypertension, chronic kidney disease, ischemic heart disease, hypercholesteraemia, osteoarthritis/found unconscious | yes | yes | Cefuroxime, metronidazole, clarithromycin, Flucoxacillin | Survived | [72] |
| 4 | 2015 | 26 | Male | Salt Lake City, USA | Morbid obesity, lymphoedema, cellulitis/progressive gangrenous changes | yes | – | Cefpodoxime | Survived | [56] |
| 5 | 2015 | 64 | Male | Tartu, Estonia | Gangrene in distal parts of the legs and amputation of the feet/admission due to an accident | yes | – | Amoxicillin/clavulanate | Survived | [31] |
| 6 | 2015 | 47 | Female | Kubang Kerian, Malaysia | Metastatic colorectal adenocarcinoma, immunosuppressed | – | – | Cefoperazone | Survived | [47] |
| 7 | 2015 | 43 | Male | Trivandrum, India | Diabetes, deep ulcer, cellulitis, gangrene/progressing gangrenous changes | – | – | Cefoperaxone/sulbactam, cefpodoxime | Survived | [73] |
| 8 | 2016 | 17 | Male | Cape Town, South Africa | Soft-tissue infection due to an accident | – | – | Ceftriaxone | Survived | [81] |
| 9 | 2016 | 72 | Male | Honolulu, Hawaii, USA | Stroke, found unconscious | yes | yes | Piperacillin/tazobactam, clindamycin, vancomycin | Fatal | [42] |
| 10 | 2016 | 69 | Female | Honolulu, Hawaii, USA | Ruptured cerebral aneurysm and right hemiparesis/sacral pain and painful urination | yes | – | Ceftaroline fosamil, meropenem | Survived | [42] |
| 11 | 2017 | 41 | Female | Columbus, Ohio, USA | Abdominal pain, stage IV right ischial decubitus ulcer, bilateral leg lymphedem, congenital lumbar myelomeningocele causing paraplegiastatus post spinal fixation, extensive sacral decubitus ulcers, obesity, severe lower extremity lymphedema, Arnold Chiari malformation type II with remote ventriculoperitoneal shunt placement and neurogenic bladder with chronic indwelling Foley catheter | yes | – | Vancomycin, cefepime, metronidazol | Fatal | [43] |
| 12 | 2017 | 43 | Male | Dresden, Germany | Diabetic foot, MRSA | yes | – | NP | NP | [5] |
| 13 | 2017 | 78 | Male | Dresden, Germany | Diabetes, coronary heart disease, chronic renal failure, venous insufficiency/progressive ulceral disease | yes | – | NP | NP | [5] |
| 14 | 2017 | 78 | Male | Dresden, Germany | Diabetic foot, ulcus cruris, severe obesity, chronic venous insufficiency, arterial hypertension, chronic heart failure NYHA II, progressive ulceral disease | yes | – | NP | NP | [5] |
| 15 | 2017 | 72 | Male | Dresden, Germany | Diabetic foot, adiposity, thrombosis, thrombophlebitis, anticoagulation, speech disorder as consequence of a tablet, and alcohol intoxication in suicidal intent | yes | – | NP | NP | [5] |
| 16 | 2018 | 57 | Male | Washington, USA | Right ankle wet gangrene, chronic cirrhosis, lung atelectasis | – | yes | NP | NP | [54] |
| 17 | 2018 | 75 | Male | Tokyo, Japan | Squamous cell carcinoma, chronic wounds with maggots | yes | yes | Cefepime, metronidazole | Survived | [48] |
| 18 | 2018 | 37 | Male | Indianapolis, Indiana, USA | Chronic lymphedema and ulcers of the lower left extremity presented with myiasis of the left foot and leg, myasis | yes | yes | Vancomycin, clindamycin, piperacillin/tazobactam | Survived | [17] |
| 19 | 2019 | 54 | Male | Melbourne, Australia | Unconscious collapse at home, chronic inflammatory demyelinating polyneuropathy with severe sensory and motor neuropathy, alcohol dependence, and hereditary hemochromatosis | yes | yes | Piperacillin/tazobactam, meropenem, ciprofloxacin | Survived | [74] |
| 20 | 2019 | 63 | Male | Lexington, Kentucky, USA | Cardiac arrest, anoxic brain injury, foot ucer containing maggots, cirrhosis | yes | yes | Vancomycin, Piperacillin/tazobactam | Fatal | [41] |
| 21 | 2019 | 87 | Female | Lexington, Kentucky, USA | NP | – | yes | NP | NP | [41] |
| 22 | 2020 | 82 | Male | Harrisburg, Pennsylvania, USA | Fall at home with asscociated confusion, mitral valve replacement due to mitral stenosis, pe vascular diseases | yes | yes | Vancomycin, cefepime, daptomycin | Survived | [4] |
| 23 | 2021 | 70 | Male | Fargo, North Dakota, USA | B cell non-Hodgkin lymphoma, chronic left temporal wound | yes | yes | Levofloxacin | Survived | [49] |
| 24 | 2021 | 79 | Female | Gmunden, Austria | End-stage lung cancer, maltnutrition | – | – | Ampicillin/sulbactam | Survived | [50] |
| 25 | 2021 | 63 | Male | Baltimore, Maryland, USA | Deep vein thrombosis, chronic venous insufficiency | – | yes | Ceftriaxone, levofloxacin | Survived | [79] |
| 26 | 2021 | 63 | Male | Brno, Czech Republic | Burn (5%), pediculosis capitis, hepatitis | yes | yes | Amoxicillin, clavulanic acid, metronidazole | Survived | [55] |
| 27 | 2016 | 90 | Male | Dresden, Germany | Tumorous skin formation (head, neck) | yes | – | NP | NP | [6] |
| 28 | 2016 | 82 | Female | Dresden, Germany | Renal failure, ulcus cruris | yes | – | NP | NP | [6] |
| 29 | 2017 | 79 | Male | Dresden, Germany | Diabetic foot, MRSA screening | yes | – | Cefuroxime, levofloxacin | Survived | [6] |
| 30 | 2017 | 43 | Male | Dresden, Germany | Diabetic foot, ulcus cruris | yes | – | No antibiotic treatment | Survived | [6] |
| 31 | 2017 | 78 | Female | Dresden, Germany | Diabetic foot | yes | – | No antibiotic treatment | Survived | [6] |
| 32 | 2017 | 71 | Male | Dresden, Germany | Diabetic foot | yes | – | No antibiotic treatment | Survived | [6] |
| 33 | 2017 | 60 | Male | Dresden, Germany | Diabetic foot | yes | – | NP | NP | [6] |
| 34 | 2018 | 65 | Male | Dresden, Germany | Diabetic foot | yes | – | NP | NP | [6] |
| 35 | 2019 | 75 | Male | Dresden, Germany | Diabetes type 2 | yes | – | NP | NP | [6] |
| 36 | 2019 | 43 | Male | Dresden, Germany | NP | yes | – | NP | NP | [6] |
| 37 | 2021 | 50 | Female | Stanford, California, USA | Basal cell carcinoma, ulcers, cellulitis | – | yes | Ciprofloxacin, amoxicillin/ clavulanic acid | Survived | [51] |
| 38 | 2022 | 48 | Male | Baltimore, Maryland, USA | Diabetes type 2, right-foot plantar surface wound, Osteomyelitis | yes | yes | Vancomycin, piperacillin-tazobactam, linezolid, ciprofloxacin | Survived | [80] |
| 39 | 2022 | 76 | Male | Seoul, Korea | Arterial hypertension, diabetes, diabetic gangrene on distal leg, amputation of three toes | yes | – | Cephamycin, Ampicillin/sulbactam | Survived | [52] |
| 40 | 2022 | 53 | Male | Mechelen, Belgium | Incomplete spinal injury, chronic wound at the right heel, osteomyelitis | yes | – | Amoxillin/clavulanic acid | Survived | [70] |
| 41 | 2022 | 57 | Male | Ankara, Turkey | Soft-tissue infection, osteomyelitis, rheumatoid arthritis | yes | – | Cefepime, cefpodoxime | Survived | [75] |
| 42 | 2022 | 37 | Male | Tulsa, Oklahoma, USA | Diabetes, chronic foot wound, osteomyelitis | yes | – | Piperacillin/tazobactam, ertapenem, daptomycin | Survived | [53] |
| 43 | 2023 | 60 | Female | Royal Oak, Michigan, USA | Liver cirrhosis, chronic venous insufficiency, malnutrion, chronic wound | yes | Vancomycin, cefepim, cefazolin | Survived | [150] |
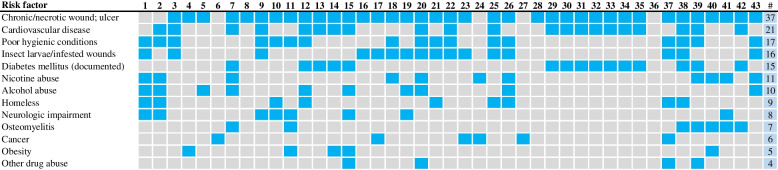
Human patients with W. chitiniclastica infection share some common similarities and risk factors for infection [5, 41]. In particular, patients with chronic/necrotic wounds, cardiovascular disease and poor hygienic conditions were strongly represented, whereas myasis or maggots were only detected in one-third of the patients (Fig. 3). In addition, an association with a history of diabetes, different drug abuse, neurological impairment and osteomyelitis was observed (Fig. 3). It is also worth mentioning that five cases had carcinoma disease as an underlying condition [6, 47–51]. Three of these case studies were part of a polymicrobial infection [6, 48, 49] suggesting that this bacterium may be an opportunistic human pathogen in immunocompromised patients.
Fig. 3.
Comorbidities and risk factors for W. chitiniclastica infection described in the case report listed in Table 1. Heatmap visualizing the presence/absence of comorbidities in each case. Blue color indicates presence and gray indicates absence. # refers to the total number
The average age of onset of the patients listed in Table 1 was 62 years. The youngest patient was 17 years old and the oldest patient was 90 years old. Thirty-two patients were male and 11 were female. In general, W. chitiniclastica was isolated either from the bloodstream or from wound swabs (Fig. 4). In three cases it was a bone sample [31, 52, 53] and once directly from a fly larva [54]. In most cases, patients received antimicrobial treatment with β-lactam antibiotics, and the vast majority survived the infection (Table 1). Strikingly, W. chitiniclastica was often part of a polymicrobial infection in which the bacterium was isolated together with other sepsis-causing pathogens [6, 55]. For example, the bacterium has been described together with Klebsiella pneumoniae, Acinetobacter lwoffii, and Staphylococcus aureus as a possible source of infection [56]. In other case reports, polymicrobial infection with S. aureus, Aeromonas spp., Staphylococcus simulans, and Bacteroides fragilis [42] or with Escherichia coli [6] was observed. Accordingly, it remains unclear whether W. chitiniclastica was the disease-causing pathogen or part of a polymicrobial infection or colonization. Although only rudimentary information is available on the associated microbial community of the W. chitiniclastica case studies, similarities with the microbiome described for diabetic foot syndrome can be identified [6]. In particular, the genera Staphylococcus, Pseudomonas, as well as Streptococcus have recently been described as dominant taxa in chronic diabetic foot ulcers [57–59], while Proteus spp. could not be detected in all patients [59]. Interestingly, W. chitiniclastica was recently described as a non-dominant part of a microbiome from chronic diabetic ulcers in India [60]. However, there is currently no further evidence of a possible key role in relation to diabetes mellitus and diabetic comorbidities.
Fig. 4.
Source of isolation of W. chitiniclastica described in the case report listed in Table 1. The red color indicates the evidence shown. # refers to the total number
Although only a few clinical case reports are available (Table 1), there seem to be no correlation between polymicrobial infection and fatal outcome. In one case, it was reported that monomicrobial sepsis with W. chitiniclastica resulted in the patient’s death [61], whereas in another infection, the patient survived [55]. Primarily, the initial health status of the patient on admission to the hospital appears to have an influence on the outcome [5]. Nevertheless, the trend should be further monitored. Especially considering that antibiotic treatment of chronic wounds such as diabetic ulcer does not significantly alter the composition of the microbiome but leads to the selection of resistant pathogens [62, 63]. The presence of multiple resistance genes in different species colonizing an ecological niche in close proximity to each other provides an ideal starting point to promote the formation of multidrug resistance [63]. With respect to W. chitiniclastica, this means that the organism can quickly develop drug resistance and may become a serious threat.
Methods of identification - what works well, less well and why?
Based on the current literature, biochemical approaches such as API (bioMérieux), BD Phoenix Gram Negative Panel (BD Biosystems) or VITEK 2 (bioMérieux) lead to false and misleading results for the identification of W. chitiniclastica [5, 6, 31, 41, 61]. Almuzara et al. used the API 20 NE system (bioMérieux, France) which resulted in the identification as Oligella urethralis (with 88.5% accuracy) [61]. Similar results were obtained by de Dios et al., where W. chitinclastica was identified as Acinetobacter lwoffii and Brevundimonas diminuta with 98.1 and 88.5% probability, respectively [56]. The BD Phoenix Gram Negative Panel (BD Biosystems, Sparks, MD) lead to a misidentification as Moraxella sp. with a low confidence score of 90% [41]. The VITEK 2 system, used by many laboratories worldwide in microbial routine diagnosis [64], also proved to be ineffective in identifying W. chitiniclastica isolates [6]. In particular, incorrect identification as A. lwoffii [6, 31, 38] or Comamonas testosteroni [56] occurred. Noteworthy, most results were above 96%, representing excellent species identification; nevertheless, misidentification was evident in all strains [6, 31, 38, 56]. To the best of our knowledge, the reaction profile of W. chitiniclastica is not currently included in the VITEK 2 database [6]. Of note, A. lwoffii has been described as part of the physiological skin flora of humans [65, 66] but can also cause severe infections in humans [67]. Therefore, correct identification, including at the best the resistance profile, is crucial to limit the emergence and spread of multidrug-resistant species.
In contrast, MALDI TOF MS, 16S rRNA gene sequencing or rpoB analysis have been shown to be safe and reliable identification methods [6, 8]. However, since the rpoB approach is not widely established in clinical routine diagnostics yet, MALDI TOF MS and 16S rRNA-based identification most likely remains by far the most frequently used method. It must be assumed that W. chitiniclastica was often not detected in the past due to misidentification and that its prevalence in the hospital may have been significantly underestimated [6, 31].
Geographical distribution and epidemiological aspects
W. chitiniclastica has been detected as a zoonotic pathogen in a variety of geographic locations [6]. Initially, the infection was thought to occur only in countries with warm climates [7], but additional human cases have since been reported from a variety of geographic and climatic regions (Table 1). These include 20 cases from Europe, 15 from the United States, 5 from Asia and one each from Africa and Australia (Table 1). Recently, a study surprised with a newly discovered subspecies of W. chitiniclastica [6]. It was originally thought to be an adaptation to the human environment and geographic location [6], but recent follow-up studies rather suggests a broad host and environmental range [8, 68]. Considering that W. chitiniclastica may not be as rare as originally thought, the host and geographic range might be even wider. This underscores the importance of correctly identifying clinically relevant bacteria to monitor the global spread of infectious diseases and their potential geographic changes [69].
Antibiotic susceptibility of W. chitiniclastica
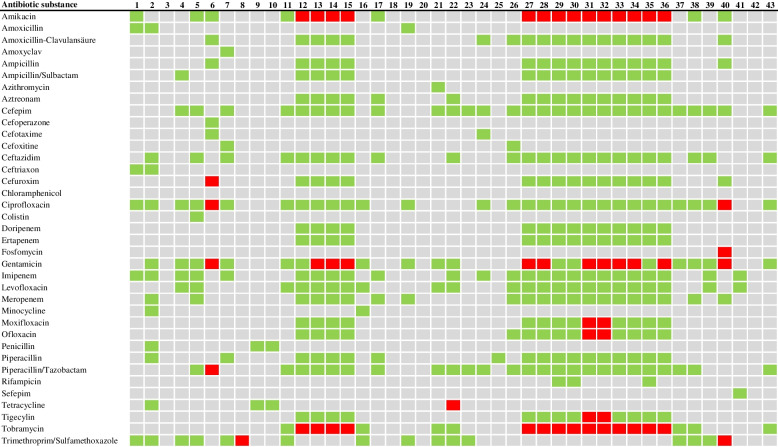
There have been several case reports on the antibiotic susceptibility testing of W. chitiniclastica indicating that the bacterium is generally susceptible to a wide range of antibiotics with the exception of fosfomycin [5, 6, 29, 70]. Figure 5 provides an overview of the resistance profiles of all strains in the 43 cases where human infection was reported. In particular, W. chitiniclastica was found to be sensitive to the majority of beta-lactam antibiotics such as penicillin, cephalosporins, and carbapenems. This is consistent with recent case reports in which infections were successfully treated with cephalosporins [4, 49, 71–73] and carbapenems [42, 74, 75]. Furthermore, no specific resistance genes were detected by previous in silico genomic analyses [6, 8]. Of note, one case reported a strain resistant to piperacillin/tazobactam and cefuroxime [47], and a blaVEB-1 gene cassette [68, 76] and blaOXA-1 gene cassette [77] were detected in a rudimentary genomic report of two different W. chitiniclastica isolates (Table 2), conferring resistance to ceftazidime, ampicillin, and extended-spectrum β-lactamases (ESBL) resistance to different antibiotic classes [78].
Fig. 5.
This heatmap presents the antibiotic resistance profiles for the W. chitiniclastica strains described in the case reports listed in Table 1. Susceptible isolates are highlighted in green color, and resistant isolates in red. Grey color is shown, if no information is available or no testing has been performed
Table 2.
Overview of all publicly available W. chitiniclastica genomes as of March 2023. In addition to information on the host, isolation source, and location, this table provides an overview of the respective genome size and antibiotic resistance genes detected
| Strain | Host | Isolation source | Location | Reference | Genome size (bp) | # CDSs | Antibiotic resistance genes |
|---|---|---|---|---|---|---|---|
| DSM 100374 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,079,313 | 1961 | macA, macB, tehB |
| DSM 100375 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,103,638 | 1932 | macA, macB, tehB |
| DSM 100676 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,139,975 | 1953 | macA, macB, tehB, tet(H), tet(B) |
| DSM 100917 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,144,768 | 1955 | macA, macB, tehB, tet(H), tet(B) |
| DSM 105708 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,084,087 | 1969 | macA, macB, tehB, tet(H), tet(B) |
| DSM 105712 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,133,608 | 1960 | macA, macB, tehB |
| DSM 105838 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,069,521 | 1910 | macA, macB, tehB |
| DSM 105839 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,123,437 | 1966 | macA, macB, tehB |
| DSM 105984 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,120,278 | 1965 | macA, macB, tehB |
| DSM 106597 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,131,555 | 1966 | macA, macB, tehB |
| DSM 108045 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,090,370 | 1950 | macA, macB, tehB |
| DSM 108048 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,074,016 | 1952 | macA, macB, tehB, abaF |
| DSM 110179 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,119,644 | 1965 | macA, macB, tehB |
| DSM 110473 | Homo sapiens | Wound swap | Dresden, Germany | [8] | 2,126,147 | 1970 | macA, macB, tehB |
| DSM 18708 | Wohlfahrtia magnitica | 3rd stage larvae of fly | Mezöfalva, Hungary | [1] | 1,991,020 | 1849 | macA, macB, tehB |
| SH04 | Chrysomya megacephala | – | Pudong, China | [22] | 2,181,980 | 2132 | macA, macB, tehB |
| BM-Y | Zebra | Pancreas | Shenzhen, China | [28] | 2,180,519 | 2029 | macA, macB, tehB, tet(H), tet(D), ant(2″)-Ia, aac(6′)-Ia, ant(3″)-Ib, blaVEB-1 |
| Strain_20 | Chicken | Chicken carcass | Rio de Janeiro, Brazil | [29] | 2,123,239 | 1958 | macA, macB, tehB |
| ATCC 51249 | Homo sapiens | Arm | New York, USA | CDC, Atlanta, USA | 2,136,105 | 1973 | macA, macB, tehB |
| F6512 | Homo sapiens | Foot | New York, USA | CDC, Atlanta, USA | 2,120,698 | 1968 | macA, macB, tehB, tet(H) |
| F6513 | Homo sapiens | Leg | New York, USA | CDC, Atlanta, USA | 2,115,422 | 1975 | macA, macB, tehB, tet(H), aph(3″)-Ib, APH [6]-Id. sul2, strA |
| F6514 | Homo sapiens | Oral lesion | New York, USA | CDC, Atlanta, USA | 2,112,239 | 1974 | macA, macB, tehB, tet(H), aph(3″)-Ib, APH [6]-Id. sul2, strA |
| F6515 | Homo sapiens | Ankle | New York, USA | CDC, Atlanta, USA | 2,134,718 | 2011 | macA, macB, tehB |
| F6516 | Homo sapiens | Arm | New York, USA | CDC, Atlanta, USA | 2,071,321 | 1892 | macA, macB, tehB |
| F9188 | Homo sapiens | Leg wound | Indiana, USA | CDC, Atlanta, USA | 2,127,263 | 1987 | macA, macB, tehB, tet(B), aph(3′)-Ib, aph(3″)-Ib, APH [6]-Id, sul2, strA |
| G9145 | Homo sapiens | Wound | Colorado, USA | CDC, Atlanta, USA | 2,182,988 | 2017 | macA, macB, tehB, tet(B), aph(3′)-Ib, aph(3″)-Ib, sul2, strA, cat3 |
| MUWRP0946 | Homo sapiens | Wound swap | Kampala, Uganda | [77] | 2,080,419 | 1942 | tet(H), sul2, dfrA1, blaOXA-1, aph(3″)-Ib, aac(6′)-Ib-cr |
The majority of strains showed sensitivity to fluoroquinolones (Fig. 5). This is consistent with recent case studies in which infection caused by W. chitiniclastica was successfully treated with levofloxacin [5, 49, 79]. The in silico genomic analysis performed also failed to detect resistance genes specific for fluoroquinolones [6, 8]. Noteworthy, some case reports show resistance to moxifloxacin, ofloxacin [6], and ciprofloxacin [47, 70]. Therefore, when in doubt, levofloxacin should be preferred for planned treatment with fluoroquinolones.
Aminoglycosides such as amikacin, gentamicin, and tobramycin are among the broad-spectrum antibiotics. Several studies have reported that W. chitiniclastica is resistant (Fig. 5) [5, 6, 47, 70], and resistance genes have been detected in different genomic studies [8, 77] (Table 2). Consequently, aminoglycosides are not recommended as first-line therapy.
With respect to tetracycline, W. chitiniclastica tends to exhibit a diverse antibiotic susceptibility profile (Fig. 5). This observation is also reflected in recent case and research studies. While the majority still appears to be susceptible [6, 31, 41–43, 52, 56, 61, 74, 75, 80], increasing incidence can be observed [4, 6, 47, 70]. Comparative genomic analysis reflected this picture and supported the hypothesis of a rather diverse distribution of tetracycline resistance genes (Table 2) [6, 8, 68, 77]. Noteworthy, these included transposon-encoded tetR and tetC [8] as well as a plasmid carrying tetA(H) [68]. This underscored the hypothesis that the majority of resistance genes in W. chitinclastica genomes arose by horizontal gene transfer [8].
Many W. chitiniclastica strains are susceptible to trimethoprim/sulfamethoxazole [4, 43, 48, 49, 74]. However, initial resistant strains have been reported from South Africa [81] and Belgium [70], as well as some sulfonamide resistance gene-containing genomes (Table 2) [8, 77]. A plasmid-encoded alteration in dihydrofolate reductase leading to insensitivity to trimethoprim/sulfamethoxazole is particularly common in bacterial pathogens [82] with pronounced geographic differences [83]. For example, trimethoprim/sulfamethoxazole has been shown to be particularly effective against enterotoxin-producing E. coli and Shigella species in Guadalajara, Mexico [84], whereas resistance levels of > 90% have been observed in Thailand [85]. In the case of the resistant W. chitiniclastica isolates [70, 81], it would be conceivable that the organism has expanded its resistance profile through the uptake of a resistance plasmid. In addition, trimethoprim/sulfamethoxazole is a relatively inexpensive drug. Consequently, it has been widely used worldwide for many different infections, which in turn has further promoted the development of resistance [86].
Macrolides, such as azithroymycin, clarithromycin and erythromycin, are antibiotics with bacteriostatic activity. As of June 2023, only Fenwick et al. have reported an azithroymycin-resistant strain [41]. Another research study performed a comprehensive in vitro resistance analysis, and demonstrated relatively high MIC (minimal inhibitory concentrations) values for clarithromycin and erythromycin [6]. However, PK/PD (non-species-related) breakpoints based on the EUCAST (European Committee on Antimicrobial Susceptibility Testing) were not available at that time. Consequently, an evaluation according to the criteria published by EUCAST was not possible, so that no final statement on resistance or susceptibility could be made [6]. In a follow-up study, it was shown that the macA and macB genes could be detected in all W. chitiniclastica genomes that were publicly available at that time (Table 2) [8]. The genes macA and macB encode for macrolide-specific efflux pumps [87, 88]. Therefore, primary resistance to macrolides is feasible. In addition, W. chitiniclastica appears to be resistant to tellurite (Table 2) [8]. This aspect is not surprising since potassium tellurite has been used extensively as an antimicrobial agent in the past and, as a result, many Gram-positive and Gram-negative bacteria have developed resistance [89].
Last but not least, W. chitiniclastica is known for its pronounced fosfomycin resistance [5, 6, 29, 70]. Surprisingly, this is not reflected in previous genome studies [6, 8, 22, 28, 29, 77]. Only one genome reveals the presence of the transporter gene abaF (Table 2) [8], which confers resistance to fosfomycin [90]. Other well-described fosfomycin resistance genes, such as fosA, fosC, or fomB [91], do not appear to play a role. It is therefore suggested that W. chitiniclastica has an as yet undescribed resistance mechanism to fosfomycin that remains to be discovered [8].
Compared to other pathogens such as A. lwoffii, the antibiotic resistance profile of W. chitiniclastica is still relatively narrow [92]. Nevertheless, increasing drug resistance has been observed, and its development should be followed with caution (Fig. 5) [8]. For W. chitiniclastica infection, levofloxacin and cephalosporins, such as cefepime, appear to be suitable options. However, it should be noted that sensitivity to antibiotics may vary depending on the strain and the specific conditions of infection. Because exposure to many antibiotics leads to tremendous selection pressure, including the spread of resistance [93], it is recommended that clinical isolates be tested for antibiotic susceptibility in order to select the most appropriate antibiotic treatment.
Striking genomic features of W. chitiniclastica and their relevance to adaptation to environmental change
As of June 2023, NCBI lists 28 genomes of W. chitiniclastica strains (Table 2), 24 of which have been isolated in the course of human disease [8, 77]. The remaining genomes were isolated from an animal source [22, 28, 29]. A recent comparative genomic study has shed light on various aspects such as virulence factors, mobile genomic elements and pangenomic features [8]. The composition of the pangenome revealed a core genome size of 43%, which is highly conserved compared to other species such as Clostridium perfringens (12.6%) [94], Pseudomonas aeruginosa (26%) [95], and K. pneumoniae (26%) [96], to name a few. Bacteria with a comparatively large core genome often lack a diverse repertoire of virulence and resistance factors and are less able to adapt flexibly and rapidly to changing environmental conditions [95, 97]. This is consistent with recent observations on W. chitiniclastica, which are susceptible to most known antibiotics except fosfomycin [5, 6, 29, 70], supporting the notion that members of this species are metabolically conserved compared with others [8]. However, this could change over time. Recent studies have shown that genome-encoded transposons, bacteriophages and plasmids are ubiquitous in W. chitiniclastica genomes [8, 29, 68], which could be a key element for the acquisition of new resistance genes [8]. Surprisingly, tetracycline resistance genes in particular were found to be associated with mobile genetic elements such as the tetA(H)-carrying plasmid [68] and tetR and tetC encoded by Tn10 [8]. Tetracycline is a broad-spectrum antibiotic and is widely used in human and veterinary medicine to treat bacterial infections due to its low price and limited side effects [98–100]. Moreover it has been added to animal feed as a growth promoter [98, 99]. A recent systematic review showed that there is still continuous contamination with tetracyclines in both aquatic and terrestrial animals, leading to selection pressure on antibiotic-resistant bacteria [98] and by that to an alarming rise in antibiotic resistance to tetracycline [101]. As stated above, there is an increasing incidence of drug resistance within the W. chitiniclastica clade, most likely acquired through horizontal gene transfer. Noteworthy, all genomes studied to date contain CRISPR-Cas elements and so-called anti-CRISPR proteins (Acr) [8, 29, 77]. The acronym CRISPR stands for “Clustered Regularly Interspaced Short Palindromic Repeats” and is part of the adaptive immune system that enables prokaryotes to recognize and destroy invading foreign DNA [102]. Therefore, in theory, W. chitiniclastica should be well equipped against invasive genetic elements including bacteriophages, plasmids, and transposons [103]. On the other hand, Anti-CRISPR (Acr) proteins represent the regulatory counterpart and are thought to be able to inhibit CRISPR-Cas actions [104]. Recent studies have shown that numerous acr genes are present in the genomes of various prokaryotes such as Moraxella bovoculi or Pseudomonas spp. to name a few [105, 106]. Indeed, more than 30% of P. aeruginosa strains contain both acr and CRISPR-Cas genes [107]. Moreover, a positive correlation between the presence of antimicrobial restiance genes and acr genes has been demonstrated [108]. Currently, the interplay between CRISPR-Cas immunity and ACR activities is thought to be a key element in the adaptation of W. chitiniclastica to new environmental conditions [8]. Although the exact function has not been conclusively determined [104], Acr proteins can be expected to slow down the adaptive immune system in W. chitiniclastica when needed and enable the uptake of additional resistance genes through genetic mutations and/or horizontal gene transfer between the same or different species to maintain their survivability under the disturbed environmental conditions.
First insights into potential virulence traits
To date, knowledge about potential virulence traits of W. chitiniclastica is limited. Although much research remains to be done, the first interesting findings have recently been published [8]. The ubiquitous genome-encoded presence of several “multidrug efflux systems” and type II secretion systems (TS2) suggests a central role in the pathogenesis of W. chitiniclastica. In general, proteins secreted by T2S systems are associated with the destruction of various tissues, cellular damage, and disease. These include proteases, cellulases, pectinases, phospholipases, lipases, and toxins, but secretion of other substances is also feasible [109]. In Vibrio cholerae, for example, the T2S system supports secretion not only of cholera toxins and hemagglutinin proteases but also of chitinases [109–111]. W. chitiniclastica is known for its distinct chitinase activity [1], which is probably an indicator of a symbiotic relationship with its host fly while also playing an important role in metamorphosis [1, 4, 5]. Thus, involvement of the T2S system in its secretion seems possible [8].
Some W. chitiniclastica strains harbor the toxin-encoding gene relG, which is known to inhibit mycobacterial growth when expressed independently [112]. Moreover, the ubiquitous presence of the conserved virulence factor B (cvfB) suggests a central role in the virulence of W. chitiniclastica. Recent studies showed that deletion of CvfB results in reduced virulence in S. aureus and decreased production of hemolysin, DNase, and protease [113]. Apart from that, other exotoxin encoding genes appear to be missing or are yet unknown suggesting an alternative virulence profile [8]. Undoubtedly, identifying pathogenesis and toxin encoding genes of W. chitiniclastica and its interaction with the host should be further investigated as it could serve as novel targets for drug development. However, there is still a long way to go.
Toxin-antitoxin (TA) modules are ubiquitous in bacteria and are thought to be involved in various physiological processes including virulence [114]. Recently, a W. chitiniclastica isolate from China was studied with a novel blaVEB-1 carrying plasmid [68], which, in addition to antibiotic resistance, also encodes the TA modules RelBE and YefM/YoeB [68]. TA systems that are localized on plasmids are associated with plasmid stabilization and have been shown to increase plasmid maintenance [115, 116]. In contrast, the role of chromosomally encoded TA systems in bacterial physiology has not yet been conclusively elucidated [117]. It is assumed that they have a decisive influence on adaptation to new environmental conditions, improved stress resistance and the stabilization of chromosomal regions [114], which gives the bacteria a considerable fitness advantage [118].
TA modules are also present in several W. chitiniclastica genomes [8]. These include the type II TA system YefM-YoeB and PasTI [119]. Previous studies have shown that YefM-YoeB is involved in the colonization of new niches, survival in the host and general stress tolerance [119]. It is therefore conceivable that the TA system is involved in the invasion of new habitats and provides W. chitiniclastica with a decisive fitness advantage in the course of a polymicrobial infection. PasTI enables cell formation in the presence of antibiotics and increases the pathogen’s resistance to nutrient limitation as well as oxidative and nitrosative stress [119]. It is worth noting that the function of the pasT gene has recently been reannotated based on new experimental evidence [120]. While PasT increases the antibiotic tolerance of pathogens, the function of PasTI as a TA system could not be confirmed [120]. Instead, the putative toxin PasT corresponds to a bacterial homolog of the mitochondrial protein Coq10, which plays a central role in respiratory electron transport as an important cofactor in the ubiquinone-dependent electron transport chain [120]. Therefore, it can currently only be speculated whether the pasT gene of W. chitiniclastica is primarily involved in virulence and/or energy production. Overall, the role of chromosomally or plasmid-encoded TA systems in bacterial physiology has not yet been conclusively clarified.
Arsenic resistance genes and their impact on the spread of antibiotic resistance
Arsenic is a natural component of both aquatic and terrestrial habitats. In general, arsenic contamination is relatively low, but the high toxicity of arsenic derivatives is a serious public health concern worldwide [121]. On the other hand, various arsenic compounds have been successfully used as antimicrobial agents in the past and have been used to treat trichomoniasis, malaria, ulcers, and syphilis, as well as a variety of other diseases [122, 123]. This further favored the spread of arsenic resistance genes [121]. Recently, there has also been renewed interest in arsenic as a cancer drug for the treatment of acute promyelocytic leukemia [123, 124]. However, agriculture and industry have primarily contributed to arsenic spread and contamination [125]. In agriculture, animal farming and industrial sectors, arsenic-containing compounds have been used extensively as pesticides and as feed additives, especially in the poultry and swine industries [126, 127]. Roxarsone, for example, was used exclusively in animal farming, particularly in poultry, to promote growth and prevent gastrointestinal infections [125, 128]. Although many arsenic compounds are no longer used, their residues from previous activities are still present, especially in agricultural soils [129] leading to constant selection pressure on bacteria with a tolerance to arsenic [130].
W. chitiniclastica was detected in arsenic-contaminated soil in Bangladesh [9]. Recently, a comprehensive genomic study of W. chitiniclastica demonstrated the presence of arsenic resistance family genes in all genomes [8]. However, there were some discrepancies with respect to the classical arsRDABC operon, and it is possible that W. chitiniclastica has a previously unknown or alternative regulatory and/or arsenic tolerance mechanism [8]. In addition to the well-known arsRDABC operon, there is an alternative chromosomal arsenic resistance mechanism that has been demonstrated, for example, in Alcaligenes faecalis [131]. Here, arsenic is used as a terminal electron acceptor in the absence of oxygen during anaerobic heterotrophic growth [123, 132]. This raises the question of whether the arsenic resistance families encoded in the genome allow growth under anaerobic conditions in the presence of arsenic. Interestingly, W. chitiniclastica was described as strictly aerobic when it was first described [1], while both W. populi and W. larvae were described as facultatively anaerobic [13, 14]. Recently two case studies reported that the respective W. chitiniclastica strain also grew under anaerobic conditions [42, 43]. This allows initial speculation about further metabolic properties of W. chitiniclastica that have not yet been described, according to which strains of this species can be characterized mainly as facultative anaerobes. However, further experimental studies in combination with in silico genome analyses, at best including targeted genetic manipulations, are required to confirm this hypothesis.
At first glance, bacterial arsenic resistance appears to be of little interest to human medicine despite that fact that arsenic resistance genes are widely distributed in human pathogens [123]. Although improper use of antibiotics is known to favor the selection and spread of antibiotic resistance [133], metal contamination can also promote the spread of antibiotic resistance through multifactorial coselection mechanisms [133–135]. It has recently been shown, that the use of heavy metals for growth promotion in poultry farms resulted in the coselection of mobile genetic elements and antimicrobial resistance genes [135]. Often, the corresponding genes are encoded in a common resistance gene cassette on the same mobile genetic element such as transposons or plasmids [123]. For example, the sulfonamide resistance gene sul2 has been detected together with the arsenic resistance genes arsA, arsB, arsC, arsD, and arsR [136]. In fact, arsenic-polluted environments have been described as contributing to the co-selection of antimicrobial resistance genes and mobile genetic elements [125]. These include β-lactamases (blaCMY/ampC), macrolides (erm35), MLSB (erm(F)), tetracyclines (tet(B)), aminoglycosides (aadA/aacC), and transposons (Tn21/Tn22/Tn24/Tn614) [125, 137–139]. This has been demonstrated in numerous human pathogens [123], such as Campylobacter jejuni [140], S. aureus [141], and K. pneumoniae [142], to name a few. In all cases, there is a selection advantage for bacterial survival. Unlike antibiotics, metals do not degrade in the environment and their presence could therefore represent a long-term selection pressure [134]. Although the overuse of antibiotics is one of the main driving force of antibiotic resistance, arsenic-polluted environments have been described to contribute to the co-selection of genes for antimicrobial resistance [125]. For example, the presence of arsenic and other metals in a Chinese poultry production was recently shown to have a stronger impact on the composition of metal tolerance and antibiotic resistance genes than some antibiotics [135]. Interestingly, a positive correlation was found between arsenic concentrations and the resistance genes for aminoglycosides [aac [60]- Ia], macrolides (erm35), bacitracin (bacA) and tetracycline (tet genes) [135]. Another study from rural Bangladesh showed that co-resistance to arsenic and antibiotics in E. coli was more pronounced in areas with high arsenic levels than in areas with low arsenic levels [143].
In the context of the development of antibiotic resistance and its far-reaching consequences, arsenic resistance in W. chitiniclastica is of critical importance and should be considered in the development of strategies to combat antibiotic resistance. Again, it would be useful to seek interdisciplinary collaboration based on the “One Health” concept to rapidly identify environmental conditions with increased risk of metal-induced coselection and to counteract the spread of antibiotic resistance genes [123, 133, 144, 145]. The focus should not only be on the restrictive use of antibiotics. The positive association found between arsenic exposure and antimicrobial resistance in arsenic-contaminated areas is a major public health concern and warrants increased efforts to reduce arsenic exposure [143]. There is an urgent need to develop guidelines on national as well as international level to control the rampant and uncontrolled use of numerous chemical substances including arsenic-containing compounds. In addition, it is particularly necessary to launch a far-reaching awareness-raising campaign for the general public by providing targeted information about the risks of improper and unjustified use of antibiotics and metal-containing compounds, and show what each individual can do to prevent the development of resistant bacteria.
The relevance of genomic studies for understanding infectious diseases
In recent years, more and more studies have been published showing the benefits of investigating bacterial genomes for diagnostic microbiology and how genomic comparisons make it possible to significantly reduce analysis times and increase the accuracy of the results [146]. The most important applications are the investigation of antimicrobial susceptibility, the disclosure of virulence factors, surveillance and the clarification of outbreaks in hospitals, but also the assignment of a clear species affiliation of an isolate [146]. The phenotypic expression of resistance in Enterobacterales for example may indicate the presence of carbapenemase, although it is based on efflux pumps or changes in membrane permeability and therefore has no direct impact on hospital hygiene measures [147, 148]. The relevance of the correlation between phenotypic expression of antimicrobial susceptibility and genomic data can also be illustrated by our own studies on W. chitiniclastica. The postulation of a jet unknown resistance mechanism for fosfomycin was only possible by comparing the (high) MIC values with the genetic databases for resistance genes [6]. In addition, genomic investigations can reveal previously unknown biovars with a potential clinical impact. Antonation et al. for instance were able to show that a certain clade of african Bacillus cereus strains exhibited virulence properties of Bacillus anthracis by harbouring the corresponding virulence plasmids. The authors thus named the new biovar Bacillus cereus biovar anthracis [149]. This is significant because B. cereus, in contrast to B. anthracis, usually causes only transient and mild intoxications or infections. Although this biovar has not yet appeared in a human medical context, this cannot be ruled out in the future due to worldwide travel, but also due to the fact that B. cereus is capable of spore formation. Regardless, the investigations of bacterial genomes will allow us to gain a deeper understanding of the distribution and diversity of rare pathogens and their impact on public health and wildlife populations [149].
Conclusion
This review provides an overview of the current knowledge and perspectives of W. chitiniclastica from a clinical and genomic perspective. This bacterium has recently been described as a rare but potentially emerging human pathogen whose occurrence is associated with, but not limited to, certain flies. However, because conventional biochemical identification tools can be unreliable and misleading in identifying this organism, this species may be even more widespread than previously thought. Cases of W. chitiniclastica infection usually have a number of characteristic underlying conditions. In particular, these include poor hygienic conditions and chronic wounds. In addition, W. chitiniclastica is often found to be part of a polymicrobial infection and is considered an opportunistic pathogen in immunocompromised patients. The presence of multiple resistance genes in different species colonizing an ecological niche in close proximity to each other provides an ideal starting point to promote multidrug resistance formation. Although W. chitiniclastica is generally sensitive to most classes of antimicrobial agents, increasing drug resistance has been observed. This trend should be critically monitored and evaluated in the context of the “One Health” concept. Deciphering virulence systems and pathogenicity will be the next critical step in understanding W. chitiniclastica in order to develop strategies to control its spread.
Authors’ contributions
PS had the idea and the concept for the review, contributed to the revision of the manuscript and approved the present version. AK analyzed the literature, prepared the tables and figures, and wrote the first version of the manuscript. TR contributed to the revision of the manuscript and approved the present version. BB contributed to the revision of the manuscript and approved the present version.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
No original data are analysed in this manuscript. The genome data mentioned in this manuscript are available via NCBI.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tóth EM, Schumann P, Borsodi AK, Kéki Z, Kovács AL, Márialigeti K. Wohlfahrtiimonas chitiniclastica gen. Nov., sp. nov., a new gammaproteobacterium isolated from Wohlfahrtia magnifica (Diptera: Sarcophagidae) Int J Syst Evol Microbiol. 2008;58(4):976–981. doi: 10.1099/ijs.0.65324-0. [DOI] [PubMed] [Google Scholar]
- 2.Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49(10):1092–1098. doi: 10.1111/j.1365-4632.2010.04577.x. [DOI] [PubMed] [Google Scholar]
- 3.Schiner IR. Fauna Austriaca: die Fliegen (Diptera) Nach der analytischen Methode bearb.,mit der Characteristik almmilicher europäischer Gattungen, der Beechraibung aller in Deutschland vorkommenden Arten und der Aufzahlung aller bisher beschriebenen europaischen Arte [Internet]. Vol. T.1 (1862). Wien: C. Gerolds Sohn; 1862. https://www.biodiversitylibrary.org/item/38016.
- 4.Snyder S, Singh P, Goldman J. Emerging pathogens: a case of Wohlfahrtiimonas chitiniclastica and Ignatzschineria indica bacteremia. IDCases. 2020;19:e00723. doi: 10.1016/j.idcr.2020.e00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröttner P, Rudolph WW, Damme U, Lotz C, Jacobs E, Gunzer S. Wohlfahrtiimonas chitiniclastica: current insights into an emerging human pathogen. Epidemiol Infect. 2017;145(7):1292–1303. doi: 10.1017/S0950268816003411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopf A, Bunk B, Coldewey SM, Gunzer F, Riedel T, Schröttner P. Identification and antibiotic profiling of Wohlfahrtiimonas chitiniclastica, an underestimated human pathogen. Front Microbiol. 2021;12 [DOI] [PMC free article] [PubMed]
- 7.Thaiwong T, Kettler NM, Lim A, Dirkse H, Kiupel M. First report of emerging zoonotic pathogen Wohlfahrtiimonas chitiniclastica in the United States. J Clin Microbiol. 2014;52(6):2245–2247. doi: 10.1128/JCM.00382-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopf A, Bunk B, Coldewey SM, Gunzer F, Riedel T, Schröttner P. Comparative genomic Analysis of the human pathogen Wohlfahrtiimonas Chitiniclastica provides insight into the identification of antimicrobial Resistance genotypes and potential virulence traits. Front Cell Infect Microbiol. 2022;12(July):1–14. doi: 10.3389/fcimb.2022.912427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanyal SK, Mou TJ, Chakrabarty RP, Hoque S, Hossain MA, Sultana M. Diversity of arsenite oxidase gene and arsenotrophic bacteria in arsenic affected Bangladesh soils. AMB Express. 2016;6(1). [DOI] [PMC free article] [PubMed]
- 10.Vouga M, Greub G. Emerging bacterial pathogens: the past and beyond. Clin Microbiol Infect. 2016;22(1):12–21. doi: 10.1016/j.cmi.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montecillo JAV. Phylogenomics and comparative genomics analyses support the creation of the novel family Ignatzschineriaceae fam. Nov. comprising the genera Ignatzschineria and Wohlfahrtiimonas within the order Cardiobacteriales. Res Microbiol. 2022;103988. [DOI] [PubMed]
- 12.Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. 2020;70(11):5607–5612. doi: 10.1099/ijsem.0.004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Fang W, Xue H, Yang X-Q, Xie S-J, Wang L-F. Wohlfahrtiimonas populi sp. nov., isolated from symptomatic bark of a Populus × euramericana canker. Int J Syst Evol Microbiol. 2017;67(11):4424–4428. doi: 10.1099/ijsem.0.002307. [DOI] [PubMed] [Google Scholar]
- 14.Lee JK, Lee YY, Park KH, Sim J, Choi Y, Lee S-J. Wohlfahrtiimonas larvae sp. nov., isolated from the larval gut of Hermetia illucens (Diptera: Stratiomyidae) Antonie Van Leeuwenhoek. 2014;105(1):15–21. doi: 10.1007/s10482-013-0048-5. [DOI] [PubMed] [Google Scholar]
- 15.Tóth EM, Borsodi AK, Euzéby JP, Tindall BJ, Márialigeti K. Proposal to replace the illegitimate genus name Schineria Tóth et al. 2001 with the genus name Ignatzschineria gen. Nov. and to replace the illegitimate combination Schineria larvae Tóth et al. 2001 with Ignatschineria larvae comb. nov. Int J Syst Evol Microbiol. 2007;57(1):179–180. doi: 10.1099/ijs.0.64686-0. [DOI] [PubMed] [Google Scholar]
- 16.Tóth E, Kovács G, Schumann P, Kovács AL, Steiner U, Halbritter A, et al. Schineria larvae gen. Nov., sp. nov., isolated from the 1st and 2nd larval stages of Wohlfahrtia magnifica (Diptera: Sarcophagidae) Int J Syst Evol Microbiol. 2001;51(Pt 2):401–407. doi: 10.1099/00207713-51-2-401. [DOI] [PubMed] [Google Scholar]
- 17.Lysaght TB, Wooster ME, Jenkins PC, Koniaris LG. Myiasis-induced sepsis: a rare case report of Wohlfahrtiimonas chitiniclastica and Ignatzschineria indica bacteremia in the continental United States. Medicine (Baltimore) 2018;97(52):e13627. doi: 10.1097/MD.0000000000013627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maleki-Ravasan N, Ahmadi N, Soroushzadeh Z, Raz AA, Zakeri S, Dinparast DN. New insights into Culturable and Unculturable Bacteria across the life history of medicinal maggots Lucilia sericata (Meigen) (Diptera: Calliphoridae) Front Microbiol. 2020;11:1–17. doi: 10.3389/fmicb.2020.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holloway BA. Morphological characters to identify adult Lucilia sericata (Meigen, 1826) and L. cuprina (Wiedemann, 1830) (Diptera: Calliphoridae). New Zeal J Zool [Internet]. 1991 Oct 1;18(4):413–20. 10.1080/03014223.1991.10422847.
- 20.Iancu L, Necula-Petrareanu G, Purcarea C. Potential bacterial biomarkers for insect colonization in forensic cases: preliminary quantitative data on Wohlfahrtiimonas chitiniclastica and Ignatzschineria indica dynamics. Sci Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-65471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Li L, Wang J, Wang M, Yang L, Tao L, et al. Development of the green bottle fly Lucilia illustris at constant temperatures. Forensic Sci Int. 2016;267:136–144. doi: 10.1016/j.forsciint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Cao XMX-M, Chen T, Xu L-ZLZ, Yao L-SLS, Qi J, Zhang X-LXLX-L, et al. Complete genome sequence of Wohlfahrtiimonas chitiniclastica strain SH04, isolated from Chrysomya megacephala collected from Pudong international airport in China. Genome Announc. 2013;1(2):4–5. doi: 10.1128/genomeA.00119-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabre RM, Adham FK, Chi H. Life table of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae) Acta Oecol. 2005;27(3):179–183. doi: 10.1016/j.actao.2004.12.002. [DOI] [Google Scholar]
- 24.Tanga CM, Waweru JW, Tola YH, Onyoni AA, Khamis FM, Ekesi S, et al. Organic Waste Substrates Induce Important Shifts in Gut Microbiota of Black Soldier Fly (Hermetia illucens L.): Coexistence of Conserved, Variable, and Potential Pathogenic Microbes [Internet]. Vol. 12, Frontiers in Microbiology. 2021. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2021.635881. [DOI] [PMC free article] [PubMed]
- 25.Qi Y, Xu J, Tian X, Bai Y, Gu X. The complete mitochondrial genome of Hermetia illucens (Diptera: Stratiomyidae) Mitochondrial DNA Part B. 2017;2(1):189–190. doi: 10.1080/23802359.2017.1307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta AK, Nayduch D, Verma P, Shah B, Ghate HV, Patole MS, et al. Phylogenetic characterization of bacteria in the gut of house flies (Musca domestica L.) FEMS Microbiol Ecol. 2012;79(3):581–593. doi: 10.1111/j.1574-6941.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 27.Malik A, Singh N, Satya S. House fly (Musca domestica): a review of control strategies for a challenging pest. J Environ Sci Heal Part B. 2007;42(4):453–469. doi: 10.1080/03601230701316481. [DOI] [PubMed] [Google Scholar]
- 28.Zhou W, Li M, Zhu L, Hua F, Ji X, Sun Y, et al. Complete genome sequence of Wohlfahrtiimonas chitiniclastica strain BM-Y, isolated from the pancreas of a zebra in China. Genome Announc. 2016;4(3):2015–2016. doi: 10.1128/genomeA.00643-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matos J, Faria AR, Carvalho Assef APD, de Freitas-Almeida ÂC, Albano RM, Queiroz MLP. Draft genome sequence of a Wohlfahrtiimonas chitiniclastica strain isolated from frozen chicken in Rio De Janeiro, Brazil. Microbiol Resour Announc. 2019;8(49):1–2. doi: 10.1128/MRA.00352-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munim MA, Das SC, Hossain MM, Hami I, Topu MG, Gupta S Das. Unveiling multi-drug resistant (MDR) gram negative pathogenic bacteria from poultry chickens in the Noakhali region of Bangladesh. bioRxiv [Internet]. 2023; Available from: https://www.biorxiv.org/content/early/2023/09/26/2023.09.26.559636. [DOI] [PMC free article] [PubMed]
- 31.Kõljalg S, Telling K, Huik K, Murruste M, Saarevet V, Pauskar M, et al. First report of Wohlfahrtiimonas chitiniclastica from soft tissue and bone infection at an unusually high northern latitude. Folia Microbiol (Praha) 2015;60(2):155–158. doi: 10.1007/s12223-014-0355-x. [DOI] [PubMed] [Google Scholar]
- 32.DeBruyn JM, Hauther KA. Postmortem succession of gut microbial communities in deceased human subjects. Peer J. 2017;12(5):e3437–e3437. doi: 10.7717/peerj.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samil A. The Sudabiome: oral and gut microbiome parameters of the Sudanese population including dietary and cultural [Toombak] metagenomics [Internet] University College Cork; 2022. [Google Scholar]
- 34.Anjaria P, Koringa P, Bhavsar P, Soni M, Desai M, Nayak J, et al. Exploring the hidden Microbial world of market chicken meat: a culture-independent Analysis of surface microbiota. SSRN. 1941:1–34. https://ssrn.com/abstract=4412769.
- 35.Reddy MRK, Mastan SA. Wohlfahrtiimonas chitiniclastica fulminant sepsis in pangasius sutchi-first report. Turk J Fish Aquat Sci. 2013;13(4):753–758. [Google Scholar]
- 36.Naik OA, Shashidhar R, Rath D, Bandekar JR, Rath A. Characterization of multiple antibiotic resistance of culturable microorganisms and metagenomic analysis of total microbial diversity of marine fish sold in retail shops in Mumbai, India. Environ Sci Pollut Res. 2018;25(7):6228–6239. doi: 10.1007/s11356-017-0945-7. [DOI] [PubMed] [Google Scholar]
- 37.Chung TH, Yi SW, Kim BS, Kim WI, Shin GW. Identification and antibiotic resistance profiling of bacterial isolates from septicaemic soft-shelled turtles (Pelodiscus sinensis) Vet Med (Praha) 2017;62(3):169–177. doi: 10.17221/65/2016-VETMED. [DOI] [Google Scholar]
- 38.Qi J, Gao Y, Wang G, Li L, Li L, Zhao X, et al. Identification of Wohlfahrtiimonas chitiniclastica isolated from an infected cow with hoof fetlow, China. Infect Genet Evol [Internet] 2016;41:174–176. doi: 10.1016/j.meegid.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Diaz-Delgado J, Eva S, Isabel VA, Lucas D, Marisa A, Manuel A, et al. Endocarditis associated with wohlfahrtiimonas chitiniclastica in a short-beaked common dolphin (Delphinus delphis) J Wildl Dis. 2015;51(1):283–286. doi: 10.7589/2014-03-072. [DOI] [PubMed] [Google Scholar]
- 40.Takci A, Mogulkoc MN, Sancak T. Determination of the causative agent of periparturient period interdigital dermatitis that adversely affects reproduction and milk production in cows by MALDI-TOF 1. 2023. p. 5150. [Google Scholar]
- 41.Fenwick AJ, Arora V, Ribes JA. Wohlfahrtiimonas chitiniclastica: two clinical cases and a review of the literature. Clin Microbiol Newsl [Internet] 2019;41(4):33–38. doi: 10.1016/j.clinmicnews.2019.01.006. [DOI] [Google Scholar]
- 42.Nogi M, Bankowski MJ, Pien FD. Wohlfahrtiimonas chitiniclastica infections in 2 elderly patients, Hawaii, USA. Emerg Infect Dis. 2016;22(3):567–568. doi: 10.3201/eid2203.151701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavez JA, Alexander AJ, Balada-Llasat JM, Pancholi P. A case of Wohlfahrtiimonas chitiniclastica bacteremia in continental United States. JMM Case Reports. 2017;4(12):10–12. doi: 10.1099/jmmcr.0.005134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winter SE, Lopez CA, Bäumler AJ. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013;14(4):319–327. doi: 10.1038/embor.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch TJ, Fricke WF, McDermott PF, White DG, Rosso M-L, Rasko DA, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One. 2007;2(3):e309. doi: 10.1371/journal.pone.0000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantas L, Suer K. Review: the important bacterial zoonoses in “one health” concept. Front Public Health. 2014;2:1–8. doi: 10.3389/fpubh.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suraiya S, Zuraina N, Ahmad F, Rahman ZA. Fatal Wohlfahrtiimonas chitiniclastica bacteremia in an immunocompromised patient. Clin Microbiol Newsl. 2017;39(21):172–173. doi: 10.1016/j.clinmicnews.2017.07.003. [DOI] [Google Scholar]
- 48.Katanami Y, Kutsuna S, Nagashima M, Takaya S, Yamamoto K, Takeshita N, et al. Wohlfahrtiimonas chitiniclastica bacteremia hospitalized homeless man with squamous cell carcinoma. Emerg Infect Dis. 2018;24(9):1746–1748. doi: 10.3201/eid2409.170080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bueide P, Hunt J, Bande D, Guerrero DM. Maggot wound therapy associated with Wohlfahrtiimonas chitiniclastica blood infection. Cureus. 2021;13(1):10–13. doi: 10.7759/cureus.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dovjak P, Kroißenbrunner M, Iglseder B. Myiasis absent Wohlfahrtiimonas chitiniclastica bacteremia in a lung cancer patient: a case report. Eur J Med Res. 2021;26(1):1–5. doi: 10.1186/s40001-021-00576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leeolou MC, Perrault DP, Sivaraj D, Chang ALS, Chen K, Trotsyuk AA, et al. A rare case of Wohlfahrtiimonas chitiniclastica infection in California. JAAD Case Reports. 2021;17:55–57. doi: 10.1016/j.jdcr.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi WJ, Lee DW, Choi HJ. Wohlfahrtiimonas chitiniclastica infection without Myiasis in South Korea: an extremely rare case report. J Wound Manag Res. 2022;18(1):38–41. doi: 10.22467/jwmr.2021.01669. [DOI] [Google Scholar]
- 53.Yeates AC, State O, Demopoulos GG, State O, Williams CL, State O. A case of Wohlfahrtiimonas Chitiniclastica contributing to Polymicrobial osteomyelitis in the United States. Oklahoma State Univ Cent Heal Sci. 2022;6(1).
- 54.Bonwitt JH, Tran M, Dykstra EA, Eckmann K, Bell ME, Leadon M, et al. Fly reservoir associated with wohlfahrtiimonas bacteremia in a human. Emerg Infect Dis. 2018;24(2):370–373. doi: 10.3201/eid2402.170913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hladík M, Lipovy B, Kaloudova Y, Hanslianova M, Vitkova I, Deissova T, et al. Human infections by Wohlfahrtiimonas chitiniclastica: a Mini-review and the first report of a burn wound infection after accidental Myiasis in Central Europe. Microorganisms. 2021;9(9):1–11. doi: 10.3390/microorganisms9091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Dios A, Fisher MA, Dingle TC, Hamula CL, Tayal A, Jacob S. First report of Wohlfahrtiimonas chitiniclastica isolation from a patient with cellulitis in the United States. J Clin Microbiol. 2015;53(12):3942–3944. doi: 10.1128/JCM.01534-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The Neuropathic Diabetic Foot Ulcer Microbiome Is Associated With Clinical Factors. Diabetes. 2013;62(3):923 LP–923930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolcott RD, Hanson JD, Rees EJ, Koenig LD, Phillips CD, Wolcott RA, et al. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen [Internet] 2016;24(1):163–174. doi: 10.1111/wrr.12370. [DOI] [PubMed] [Google Scholar]
- 59.Gardiner M, Vicaretti M, Sparks J, Bansal S, Bush S, Liu M, et al. A longitudinal study of the diabetic skin and wound microbiome. Peer J. 2017;2017(7):3543. doi: 10.7717/peerj.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suryaletha K, John J, Radhakrishnan MP, George S, Thomas S. Metataxonomic approach to decipher the polymicrobial burden in diabetic foot ulcer and its biofilm mode of infection. Int Wound J. 2018;15(3):473–481. doi: 10.1111/iwj.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almuzara MN, Palombarani S, Tuduri A, Figueroa S, Gianecini A, Sabater L, et al. First case of fulminant sepsis due to Wohlfahrtiimonas chitiniclastica. J Clin Microbiol. 2011;49(6):2333–2335. doi: 10.1128/JCM.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loesche M, Gardner SE, Kalan L, Horwinski J, Zheng Q, Hodkinson BP, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol. 2017;137(1):237–244. doi: 10.1016/j.jid.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalan LR, Brennan MB. The role of the microbiome in nonhealing diabetic wounds. Ann N Y Acad Sci. 2019;1435(1):79–92. doi: 10.1111/nyas.13926. [DOI] [PubMed] [Google Scholar]
- 64.O’Hara CM. Manual and automated instrumentation for identification of Enterobacteriaceae and other aerobic gram-negative bacilli. Clin Microbiol Rev. 2005;18(1):147–162. doi: 10.1128/CMR.18.1.147-162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol. 1997;35(11):2819–2825. doi: 10.1128/jcm.35.11.2819-2825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berlau J, Aucken H, Malnick H, Pitt T. Distribution of Acinetobacter species on skin of healthy humans. Eur J Clin Microbiol Infect Dis. 1999;18(3):179–183. doi: 10.1007/s100960050254. [DOI] [PubMed] [Google Scholar]
- 67.Ku SC, Hsueh PR, Yang PC, Luh KT. Clinical and microbiological characteristics of bacteremia caused by Acinetobacter lwoffii. Eur J Clin Microbiol Infect Dis. 2000;19(7):501–505. doi: 10.1007/s100960000315. [DOI] [PubMed] [Google Scholar]
- 68.Guan J, Zhou W, Guo J, Zheng L, Lu G, Hua F, et al. A Wohlfahrtiimonas chitiniclastica with a novel type of blaVEB–1-carrying plasmid isolated from a zebra in China. Front Microbiol. 2023;14 https://www.frontiersin.org/articles/10.3389/fmicb.2023.1276314. [DOI] [PMC free article] [PubMed]
- 69.Riedel T, Bunk B, Schröttner P. Editorial: characterization of rare and recently first described human pathogenic bacteria. Front Cell Infect Microbiol [Internet]. 2023:13. Available from: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1212627. [DOI] [PMC free article] [PubMed]
- 70.De Smet D, Goegebuer T, Ho E, Vandenbroucke M, Lemmens A. First case of Wohlfahrtiimonas chitiniclastica isolation from a patient with a foot ulcer infection in Belgium. Acta Clin Belg. 2022;25:1–3. doi: 10.1080/17843286.2022.2090770. [DOI] [PubMed] [Google Scholar]
- 71.Rebaudet S, Genot S, Renvoise A, Fournier PE, Stein A. Wohlfahrtiimonas chitiniclastica bacteremia in homeless woman. Emerg Infect Dis. 2009;15(6):985–987. doi: 10.3201/eid1506.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campisi L, Mahobia N, Clayton JJ. Wohlfahrtiimonas chitiniclastica bacteremia associated with Myiasis, United Kingdom. Emerg Infect Dis. 2015;21(6):1068–1069. doi: 10.3201/eid2106.140007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suryalatha K, John J, Thomas S. Wohlfahrtiimonas chitiniclastica-associated osteomyelitis: a rare case report. Future Microbiol. 2015;10(7):1107–1109. doi: 10.2217/fmb.15.44. [DOI] [PubMed] [Google Scholar]
- 74.Connelly K, Freeman E, Smibert O, Lin B. Wohlfahrtiimonas chitiniclastica bloodstream infection due to a maggot-infested wound in a 54-year-old male. J Global Infect Dis. 2019;11(3):125–126. doi: 10.4103/jgid.jgid_58_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karaca MO, Gürler M, Afacan M, Terzi MM, Evren E, Çınar Aydın G, et al. Wohlfahrtiimonas chitiniclastica-related soft-tissue infection and osteomyelitis: a rare case report. Turkish J Trauma Emerg Surg. 2022;28(7):1038–1041. doi: 10.14744/tjtes.2022.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S, Cai Y, Meng C, Ding X, Huang J, Luo X, et al. The role of the microbiome in diabetes mellitus. Diabetes Res Clin Pract. 2021;172:108645. doi: 10.1016/j.diabres.2020.108645. [DOI] [PubMed] [Google Scholar]
- 77.Byarugaba DK, Erima B, Wokorach G, Najjuka F, Kiyengo J, Kwak YI, et al. Genome Sequence Analysis of a Wohlfahrtiimonas chitiniclastica Strain Isolated from a Septic Wound of a Hospitalized Patient in Uganda. (Hdrec 087). [DOI] [PMC free article] [PubMed]
- 78.Sugumar M, Kumar KM, Manoharan A, Anbarasu A, Ramaiah S. Detection of OXA-1 β-lactamase gene of Klebsiella pneumoniae from blood stream infections (BSI) by conventional PCR and in-silico analysis to understand the mechanism of OXA mediated resistance. PLoS One. 2014;9(3):1–8. doi: 10.1371/journal.pone.0091800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harfouch O, Luethy PM, Noval M, Baghdadi JD. Wohlfahrtiimonas chitiniclastica Monomicrobial bacteremia in a homeless man. Emerg Infect Dis. 2021;27(12):3195–3197. doi: 10.3201/eid2712.210327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmad Y, Gaston DC, Simner PJ, Gray J, Zhong D, Gudenkauf B, et al. The brief Case : the Fly who cried Wohlf. J Clin Microbiol. 2022;60(6):1–5. doi: 10.1128/jcm.01073-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoffmann R, Fortuin F, Newton-Foot M, Singh S. First report of Wohlfahrtiimonas chitiniclastica bacteraemia in South Africa. SAMJ South African Med J. 2016;106:1062. doi: 10.7196/SAMJ.2016.v106i11.11449. [DOI] [Google Scholar]
- 82.Huovinen P, Sundstrom L, Swedberg G, Skold O. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother. 1995;39(2):279–289. doi: 10.1128/AAC.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N. Trimethoprim-Sulfamethoxazole Revisited. Arch Intern Med. 2003;163(4):402–410. doi: 10.1001/archinte.163.4.402. [DOI] [PubMed] [Google Scholar]
- 84.Bandres JC, Mathewson JJ, Ericsson CD, Dupont HL. Trimethoprim/sulfamethoxazole remains active against Enterotoxigenic Escherichia coli and Shigella species in Guadalajara, Mexico. Am J Med Sci. 1992;303(5):289–291. doi: 10.1097/00000441-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Hoge CW, Gambel JM, Srijan A, Pitarangsi C, Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26(2):341–345. doi: 10.1086/516303. [DOI] [PubMed] [Google Scholar]
- 86.Eliopoulos GM, Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis. 2001;32(11):1608–1614. doi: 10.1086/320532. [DOI] [PubMed] [Google Scholar]
- 87.Kobayashi S, Kuzuyama T, Seto H. Characterization of the fomA and fomB gene products from Streptomyces wedmorensis, which confer fosfomycin resistance on Escherichia coli. Antimicrob Agents Chemother. 2000;44(3):647–650. doi: 10.1128/AAC.44.3.647-650.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yum S, Xu Y, Piao S, Sim S-H, Kim H-M, Jo W-S, et al. Crystal structure of the periplasmic component of a tripartite macrolide-specific efflux pump. J Mol Biol. 2009;387(5):1286–1297. doi: 10.1016/j.jmb.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 89.Valková D, Valkovičová L, Vávrová S, Kováčová E, Mravec J, Turňa J. The contribution of tellurite resistance genes to the fitness of Escherichia coli uropathogenic strains. Cent Eur J Biol. 2007;2(2):182–191. [Google Scholar]
- 90.Sharma A, Sharma R, Bhattacharyya T, Bhando T, Pathania R. Fosfomycin resistance in Acinetobacter baumannii is mediated by efflux through a major facilitator superfamily (MFS) transporter-AbaF. J Antimicrob Chemother. 2017;72(1):68–74. doi: 10.1093/jac/dkw382. [DOI] [PubMed] [Google Scholar]
- 91.Silver LL. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med. 2017;7(2):1–11. doi: 10.1101/cshperspect.a025262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu Y, Zhang W, Liang H, Liu L, Peng G, Pan Y, et al. Whole-genome sequence of a multidrug-resistant clinical isolate of Acinetobacter lwoffii. J Bacteriol. 2011;193(19):5549–5550. doi: 10.1128/JB.05617-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wright GD. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat Rev Microbiol. 2007;5(3):175–186. doi: 10.1038/nrmicro1614. [DOI] [PubMed] [Google Scholar]
- 94.Kiu R, Caim S, Alexander S, Pachori P, Hall LJ. Probing genomic aspects of the multi-host pathogen Clostridium perfringens reveals significant pangenome diversity, and a diverse array of virulence factors. Front Microbiol. 2017;8. [DOI] [PMC free article] [PubMed]
- 95.Costa SS, Guimarães LC, Silva A, Soares SC, Baraúna RA. First steps in the Analysis of prokaryotic Pan-genomes. Bioinform Biol Insights. 2020;14. [DOI] [PMC free article] [PubMed]
- 96.McInerney JO, McNally A, O’Connell MJ. Why prokaryotes have pangenomes. Nat Microbiol. 2017;2. [DOI] [PubMed]
- 97.Rouli L, Merhej V, Fournier PE, Raoult D. The bacterial pangenome as a new tool for analysing pathogenic bacteria. New Microbes New Infect. 2015;7:72–85. doi: 10.1016/j.nmni.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mussa EAM, Alsalahi A, Aljaberi MA, Jasni AS, Desa MNM, Al-Mahdi AYM, et al. Acquired tetracycline resistance genes by transposons and virulence factors in enterococci recovered from overland and aquatic animals: a systematic review. Rev Aquac [Internet] 2022;14(1):399–413. doi: 10.1111/raq.12605. [DOI] [Google Scholar]
- 99.Zahid S, Bin-Asif H, Hasan KA, Rehman M, Ali SA. Prevalence and genetic profiling of tetracycline resistance (Tet-R) genes and transposable element (Tn916) in environmental Enterococcus species. Microb Pathog. 2017;111:252–261. doi: 10.1016/j.micpath.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 100.Pan Y, Zeng J, Li L, Yang J, Tang Z, Xiong W, et al. Coexistence of Antibiotic Resistance Genes and Virulence Factors Deciphered by Large-Scale Complete Genome Analysis. mSystems. 2020;5:3. doi: 10.1128/mSystems.00821-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sheykhsaran E, Baghi HB, Soroush MH, Ghotaslou R. An overview of tetracyclines and related resistance mechanisms. Rev Res Med Microbiol. 2019;30(1) https://journals.lww.com/revmedmicrobiol/fulltext/2019/01000/an_overview_of_tetracyclines_and_related.8.aspx.
- 102.Kirchner M, Schneider S. CRISPR-Cas: von einem bakteriellen adaptiven Immunsystem zu einem vielseitigen Werkzeug für die Gentechnik. Angew Chem. 2015;127(46):13710–13716. doi: 10.1002/ange.201504741. [DOI] [Google Scholar]
- 103.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819):1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 104.Landsberger M, Gandon S, Meaden S, Rollie C, Chevallereau A, Chabas H, et al. Anti-CRISPR phages cooperate to overcome CRISPR-Cas immunity. Cell. 2018;174(4):908–916.e12. doi: 10.1016/j.cell.2018.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marino ND, Pinilla-Redondo R, Csörgő B, Bondy-Denomy J. Anti-CRISPR protein applications: natural brakes for CRISPR-Cas technologies. Nat Methods. 2020;17(5):471–479. doi: 10.1038/s41592-020-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bondy-Denomy J, Pawluk A, Maxwell KL, Davidson AR. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature. 2013;493(7432):429–432. doi: 10.1038/nature11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van BA, Soriaga LB, Lafave MC, Akella S, Veyrieras J, Barbu EM, et al. Phylogenetic distribution of CRISPR-Cas Systems in Antibiotic. MBio. 2015;6(6):1–13. doi: 10.1128/mBio.01796-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shehreen S, Chyou TY, Fineran PC, Brown CM. Genome-wide correlation analysis suggests different roles of CRISPR-Cas systems in the acquisition of antibiotic resistance genes in diverse species. Philos Trans R Soc B Biol Sci. 2019;374(1772). [DOI] [PMC free article] [PubMed]
- 109.Sandkvist M. Type II secretion and pathogenesis. Infect Immun. 2001;69(6):3523–3535. doi: 10.1128/IAI.69.6.3523-3535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Connell TD, Metzger DJ, Lynch J, Folster JP. Endochitinase is transported to the extracellular milieu by the eps- encoded general secretory pathway of vibrio cholerae. J Bacteriol. 1998;180(21):5591–5600. doi: 10.1128/JB.180.21.5591-5600.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stager CE, Davis JR. Automated systems for identification of microorganisms. Clin Microbiol Rev [Internet] 1992;5(3):302–327. doi: 10.1128/CMR.5.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korch SB, Malhotra V, Contreras H, Clark-Curtiss JE. The mycobacterium tuberculosis relBE toxin:antitoxin genes are stress-responsive modules that regulate growth through translation inhibition. J Microbiol. 2015;53(11):783–795. doi: 10.1007/s12275-015-5333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matsumoto Y, Kaito C, Morishita D, Kurokawa K, Sekimizu K. Regulation of exoprotein gene expression by the Staphylococcus aureus cvfB gene. Infect Immun. 2007;75(4):1964–1972. doi: 10.1128/IAI.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lobato-Márquez D, Díaz-Orejas R, García-del PF. Toxin-antitoxins and bacterial virulencea. FEMS Microbiol Rev. 2016;40(5):592–609. doi: 10.1093/femsre/fuw022. [DOI] [PubMed] [Google Scholar]
- 115.Gotfredsen M, Gerdes K. The Escherichia coli relBE genes belong to a new toxin–antitoxin gene family. Mol Microbiol. 1998;29(4):1065–1076. doi: 10.1046/j.1365-2958.1998.00993.x. [DOI] [PubMed] [Google Scholar]
- 116.Wei Y, Ye L, Li Y, Yang F, Liu D, Guo X, et al. Functional characterization of RelBE toxin-antitoxin system in probiotic Bifidobacterium longum JDM301. Acta Biochim Biophys Sin Shanghai. 2016;48(8):741–749. doi: 10.1093/abbs/gmw056. [DOI] [PubMed] [Google Scholar]
- 117.Van Melderen L, Saavedra De Bast M. Bacterial Toxin–Antitoxin Systems: More Than Selfish Entities? PLoS Genet. 2009;5(3):e1000437. doi: 10.1371/journal.pgen.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma D, Gu H, Shi Y, Huang H, Sun D, Hu Y. Edwardsiella piscicida YefM-YoeB: a type II toxin-antitoxin system that is related to antibiotic Resistance, biofilm formation, serum survival, and host infection. Front Microbiol. 2021;12:1–15. doi: 10.3389/fmicb.2021.646299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Norton JP, Mulvey MA. Toxin-antitoxin Systems are important for niche-specific colonization and stress Resistance of Uropathogenic Escherichia coli. PLoS Pathog. 2012;8(10):e1002954. doi: 10.1371/journal.ppat.1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fino C, Vestergaard M, Ingmer H, Pierrel F, Gerdes K, Harms A. PasT of Escherichia coli sustains antibiotic tolerance and aerobic respiration as a bacterial homolog of mitochondrial Coq10. Microbiologyopen. 2020;9(8):1–36. doi: 10.1002/mbo3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ben FI, Zhang C, Li YP, Zhao Y, Alwathnani HA, Saquib Q, et al. Distribution of arsenic resistance genes in prokaryotes. Front Microbiol. 2018;9:1–11. doi: 10.3389/fmicb.2018.02473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cai L, Liu G, Rensing C, Wang G. Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC Microbiol. 2009;9:1–11. doi: 10.1186/1471-2180-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hobman JL, Crossman LC. Bacterial antimicrobial metal ion resistance. J Med Microbiol. 2015;64:471–497. doi: 10.1099/jmm.0.023036-0. [DOI] [PubMed] [Google Scholar]
- 124.Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 125.Rebelo A, Almeida A, Peixe L, Antunes P, Novais C. Unraveling the Role of Metals and Organic Acids in Bacterial Antimicrobial Resistance in the Food Chain. Antibiotics. 2023;12. [DOI] [PMC free article] [PubMed]
- 126.Paul NP, Galván AE, Yoshinaga-Sakurai K, Rosen BP, Yoshinaga M. Arsenic in medicine: past, present and future. BioMetals. 2023;36(2):283–301. doi: 10.1007/s10534-022-00371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Argudín MA, Hoefer A, Butaye P. Heavy metal resistance in bacteria from animals. Res Vet Sci. 2019;122:132–147. doi: 10.1016/j.rvsc.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 128.Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. Roxarsone, inorganic arsenic, and other arsenic species in chicken: a U.S.-based market basket sample. Environ Health Perspect. 2013;121(7):818–824. doi: 10.1289/ehp.1206245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tóth G, Hermann T, Da Silva MR, Montanarella L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int. 2016;88:299–309. doi: 10.1016/j.envint.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 130.Mourão J, Rebelo A, Ribeiro S, Peixe L, Novais C, Antunes P. Tolerance to arsenic contaminant among multidrug-resistant and copper-tolerant Salmonella successful clones is associated with diverse ars operons and genetic contexts. Environ Microbiol. 2020;22(7):2829–2842. doi: 10.1111/1462-2920.15016. [DOI] [PubMed] [Google Scholar]
- 131.Silver S, Phung LT. The genes and enzymes of bacterial of inorganic arsenic oxidation and reduction. Appl Environ Microbiol. 2005;71(2):599–608. doi: 10.1128/AEM.71.2.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silver S, Phung LT. A bacterial view of the periodic table: Genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol. 2005;32(11–12):587–605. doi: 10.1007/s10295-005-0019-6. [DOI] [PubMed] [Google Scholar]
- 133.Seiler C, Berendonk TU. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front Microbiol. 2012;3:1–10. doi: 10.3389/fmicb.2012.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baker-Austin C, Wright MS, Stepanauskas R, McArthur JV. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14(4):176–182. doi: 10.1016/j.tim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 135.Mazhar SH, Li X, Rashid A, Su J, Xu J, Brejnrod AD, et al. Co-selection of antibiotic resistance genes, and mobile genetic elements in the presence of heavy metals in poultry farm environments. Sci Total Environ. 2021;755:142702. doi: 10.1016/j.scitotenv.2020.142702. [DOI] [PubMed] [Google Scholar]
- 136.Luo G, Li B, Li L-G, Zhang T, Angelidaki I. Antibiotic Resistance Genes and Correlations with Microbial Community and Metal Resistance Genes in Full-Scale Biogas Reactors As Revealed by Metagenomic Analysis. Environ Sci Technol. 2017;51(7):4069–4080. doi: 10.1021/acs.est.6b05100. [DOI] [PubMed] [Google Scholar]
- 137.Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DGJ. Co-occurrence of resistance genes to antibiotics, biocides and metals reveals novel insights into their co-selection potential. BMC Genomics. 2015;16(1):1–14. doi: 10.1186/s12864-015-2153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao Y, Cocerva T, Cox S, Tardif S, Su J-Q, Zhu Y-G, et al. Evidence for co-selection of antibiotic resistance genes and mobile genetic elements in metal polluted urban soils. Sci Total Environ. 2019;656:512–520. doi: 10.1016/j.scitotenv.2018.11.372. [DOI] [PubMed] [Google Scholar]
- 139.Zhang M, Wan K, Zeng J, Lin W, Ye C, Yu X. Co-selection and stability of bacterial antibiotic resistance by arsenic pollution accidents in source water. Environ Int. 2020;135(2019):105351. doi: 10.1016/j.envint.2019.105351. [DOI] [PubMed] [Google Scholar]
- 140.Fouts DE, Mongodin EF, Mandrell RE, Miller WG, Rasko DA, Ravel J, et al. Major structural differences and novel potential virulence mechanisms from the genomes of multiple campylobacter species. PLoS Biol. 2005;3(1). [DOI] [PMC free article] [PubMed]
- 141.Shore AC, Deasy EC, Slickers P, Brennan G, O’Connell B, Monecke S, et al. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(8):3765–3773. doi: 10.1128/AAC.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sandegren L, Linkevicius M, Lytsy B, Melhus Å, Andersson DI. Transfer of an Escherichia coli ST131 multiresistance cassette has created a Klebsiella pneumoniae-specific plasmid associated with a major nosocomial outbreak. J Antimicrob Chemother. 2012;67(1):74–83. doi: 10.1093/jac/dkr405. [DOI] [PubMed] [Google Scholar]
- 143.Amin MB, Talukdar PK, Asaduzzaman M, Roy S, Flatgard BM, Islam MR, et al. Effects of chronic exposure to arsenic on the fecal carriage of antibiotic-resistant Escherichia coli among people in rural Bangladesh. PLOS Pathog. 2022;18(12):e1010952. doi: 10.1371/journal.ppat.1010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Walsh TR. A one-health approach to antimicrobial resistance. Nat Microbiol [Internet] 2018;3(8):854–855. doi: 10.1038/s41564-018-0208-5. [DOI] [PubMed] [Google Scholar]
- 145.Hernando-Amado S, Coque TM, Baquero F, Martínez JL. Antibiotic Resistance: moving from individual health norms to social norms in one health and Global Health. Front Microbiol. 2020;11:1–20. doi: 10.3389/fmicb.2020.01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Didelot X, Bowden R, Wilson DJ, Peto TEA, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet [Internet] 2012;13(9):601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pecora ND, Li N, Allard M, Li C, Albano E, Delaney M, et al. Genomically informed surveillance for carbapenem-resistant enterobacteriaceae in a health care system. MBio. 2015;6(4). [DOI] [PMC free article] [PubMed]
- 148.Tagini F, Greub G. Bacterial genome sequencing in clinical microbiology: a pathogen-oriented review. Eur J Clin Microbiol Infect Dis. 2017;36(11):2007–2020. doi: 10.1007/s10096-017-3024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Antonation KS, Grützmacher K, Dupke S, Mabon P, Zimmermann F, Lankester F, et al. Bacillus cereus Biovar anthracis causing Anthrax in sub-Saharan Africa—chromosomal Monophyly and broad geographic distribution. PLoS Negl Trop Dis. 2016;10(9):1–14. doi: 10.1371/journal.pntd.0004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kozyk M, Fisher J. Wohlfahrtiimonas chitiniclastica : a rare infection reported in an adult with liver cirrhosis. Clin Case Rep. 2023;11(2):e697. doi: 10.1002/ccr3.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lemoine F, Correia D, Lefort V, Doppelt-Azeroual O, Mareuil F, Cohen-Boulakia S, et al. NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019;47(W1):W260–W265. doi: 10.1093/nar/gkz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Letunic I, Bork P. Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47(W1):256–259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data are analysed in this manuscript. The genome data mentioned in this manuscript are available via NCBI.