Abstract
Background
Immunization, as a preventive strategy against infectious diseases, has consolidated its position as a fundamental pillar in the field of public health. Therefore, the present study aimed to determine the prevalence of the intention to receive the monkeypox (Mpox) vaccine.
Methods
A systematic review and meta-analysis of the available evidence was performed using five databases (PubMed, Scopus, Web of Science, Embase, and ScienceDirect) with a search strategy until July 24, 2023. Data analysis was performed in R software version 4.2.3. The quality of the included cross-sectional studies was assessed using the “JBI-MAStARI”. In addition, a subgroup analysis by population and continent was developed.
Results
Twenty-nine cross-sectional articles with a total sample of 52 658 participants were included. The pooled prevalence of intention to vaccinate against Mpox was 61% (95% CI: 53–69%; 52,658 participants; 29 studies; I2 = 100%). In the subgroup analysis, the intention to be vaccinated against Mpox according to continents was 64% (95% CI: 53–74%; 13,883 participants; 17 studies; I2 = 99%) in Asian countries, 43% (95% CI: 39–47%; 1538 participants; 3 studies; I2 = 53%) in African countries, 62% (95% CI: 45–78%; 35,811 participants; 6 studies; I2 = 99%) in European countries, and 63% (95% CI: 32–89%; 1426 participants; 3 studies; I2 = 99%) in American countries. In the subgroup analysis on the intention to be vaccinated against Mpox, according to study subjects, it was 54% (95% CI: 45–62%; 10,296 participants; 11 studies; I2 = 99%) in the general population, 57% (95% CI: 33–79%; 3333 participants; 10 studies; I2 = 99%) in health care workers, and 76% (95% CI: 70–82%; 39,029 participants; 8 studies; I2 = 98%) in the lesbian, gay, bisexual, transgender, and intersex (LGBTI) community. In addition, as a secondary outcome, a prevalence of refusal of Mpox vaccination was found to be 22% (95% CI: 16–30%; 45,577 participants; 21 studies; I2 = 99%).
Conclusion
The study highlights the importance of recognizing regional and subgroup disparities in Mpox vaccine willingness and refusal. It emphasizes the importance of employing strategies to achieve widespread vaccination coverage and safeguard public health worldwide.
Terms used
Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI), Prospective International Registry of Systematic Reviews (PROSPERO), and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-17473-y.
Keywords: Monkeypox, Vaccine, Vaccine hesitancy, Vaccine intentions, Mpox
Introduction
Within the current public health scenario, the prevention and control of emerging infectious diseases have acquired a fundamental role in the contemporary scientific and medical agenda [1, 2]. In response to these challenges, various strategies have been devised to address them; however, immunization has proven to be an invaluable tool to attenuate the spread of pathogens and safeguard the health of communities [3, 4]. In this context, the focus of the present research is directed towards an infectious agent of growing interest: the monkeypox virus [5].
Monkeypox (Mpox), caused by the monkeypox virus, is a viral disease belonging to the family Poxviridae [6]. Although once considered a rare disease of limited scope, the rapid spread of cases in a number of nations, both endemic and non-endemic, has triggered a global public health emergency [7]. The ability of the Mpox virus to induce death in humans ranges from 1 to 10%, highlighting the importance of assessing the population’s intention to vaccinate against this pathogen [5, 8].
The prevention of infectious diseases through immunization has been consolidated as a fundamental pillar of public health, having achieved the successful eradication of smallpox and a drastic decrease in the incidence of numerous vaccine-preventable diseases [9, 10]. However, to achieve optimal levels of community protection and prevent disease re-emergence, it is essential to understand the factors that influence vaccine acceptance [11, 12]. Intention to receive a vaccine is influenced by a complex interplay of sociodemographic, cultural, psychological, and risk perception variables [13, 14], highlighting the need for detailed research on population intention toward Mpox vaccination.
Therefore, the objective of the present investigation is to determine the prevalence of the intention to receive the Mpox vaccine. These findings could contribute to the development of more effective communication strategies and public health policies, guiding the prevention of Mpox and providing relevant information to strengthen preparedness and response to possible future outbreaks [13].
Materials and methods
Protocol and registration
The process of this research has been duly recorded in PROSPERO (CRD42023 447,971), ensuring transparency and thoroughness in the protocol. The systematic review and meta-analysis adhered to the PRISMA checklist guidelines during its conduct (Table S1).
Eligibility criteria
Inclusion criteria
All cross-sectional studies addressing the prevalence of the intention to vaccinate against Mpox were included. No limitations were applied regarding language, time period, or geographic location. However, only those studies that were fully available, included sample size details, and presented relevant data on any aspect related to the intention of vaccination against Mpox were incorporated.
Exclusion criteria
The studies whose research topics did not align with the objectives of our investigation were excluded, as were those that employed a different design than a cross-sectional study. Likewise, incomplete articles were rejected, either due to insufficient data or a lack of information on the desired results. Finally, an attempt was made to establish contact with the corresponding author via email; however, unfortunately, it was not possible.
Information sources and search strategy
Two researchers conducted thorough searches in various renowned databases, including PubMed, Scopus, Embase, Web of Science, and ScienceDirect. To optimize the search, they used key terms such as “monkeypox”, “Mpox”, “vaccine”, and “attitude”. The specific search strategies employed for each database are detailed in Table S2. The initial search was conducted on July 1, 2023, and was updated on July 24, 2023.
Study selection
The authors used the Rayyan tool to store and manage the results obtained from the search strategy. After removing duplicate articles, a preliminary selection of the remaining ones was carried out by reading titles and abstracts, following pre-established criteria. Subsequently, a comprehensive review of the full reports was conducted to determine their compliance with the inclusion criteria. Any discrepancies were resolved through discussions and consultations with a researcher.
Main and secondary results of the study
This study addresses two fundamental variables: the main one, focused on the intention to be vaccinated against Mpox, and the secondary one, related to the refusal to be vaccinated against this disease. Both were delineated from the following question: Do you plan to be vaccinated against Mpox?
Intention to vaccinate against Mpox
The definition of this primary variable was based on responses related to willingness or likelihood to be vaccinated against Mpox. Participants’ decisions regarding vaccination against this disease highlight the importance of immunization, either as a preventive measure or in response to vaccine availability.
Refusal of the Mpox vaccination
The definition of this secondary variable was based on responses indicating the likelihood of not being vaccinated or refusing the Mpox vaccine.
Quality assessment
Two independent researchers conducted the evaluation of the quality of the included cross-sectional studies using the “JBI-MAStARI” method. In the event of any discrepancies in the assessments, a third investigator was involved to resolve them. The studies were classified based on their quality scores as high (≥ 7 points), moderate (4 to 6 points), or low (< 4 points) [15] (Table S3).
Data collection process and data items
Two expert researchers collected the relevant data from the selected articles. Then, they extracted the following details and recorded them in an Excel spreadsheet: the name of the primary author, publication year, country, sample size, study population, gender (male and female), prevalence of intent to vaccinate against Mpox, number of cases of intent to vaccinate against Mpox, prevalence of refusal to vaccinate against Mpox, number of cases of refusal to vaccinate against Mpox, type of survey, and date of data collection. Finally, a third researcher verified the extracted data to ensure its accuracy and eliminate any incorrect information.
Data analysis
Firstly, the selected articles were entered into a Microsoft Excel spreadsheet for further analysis using R, version 4.2.3. The results were presented using narrative tables and graphs. The estimation of the joint prevalence of Mpox vaccination intent was conducted using the random-effects model with inverse variance weighting. To assess heterogeneity among the studies, the Cochrane Q statistic was used, and its quantification was performed using the I2 index. Values of 25%, 50%, and 75% were considered indicators of low, moderate, and high heterogeneity, respectively. In order to examine publication bias, funnel-shaped graphs were employed, and Egger’s regression test was applied. The presence of potential publication bias was considered when the p-value was less than 0.05.
Additionally, subgroup analyses were conducted based on the study population and continent. The presentation of the pooled prevalence of Mpox vaccination intent was done using a forest plot format, which included 95% confidence intervals.
Results
Study selection
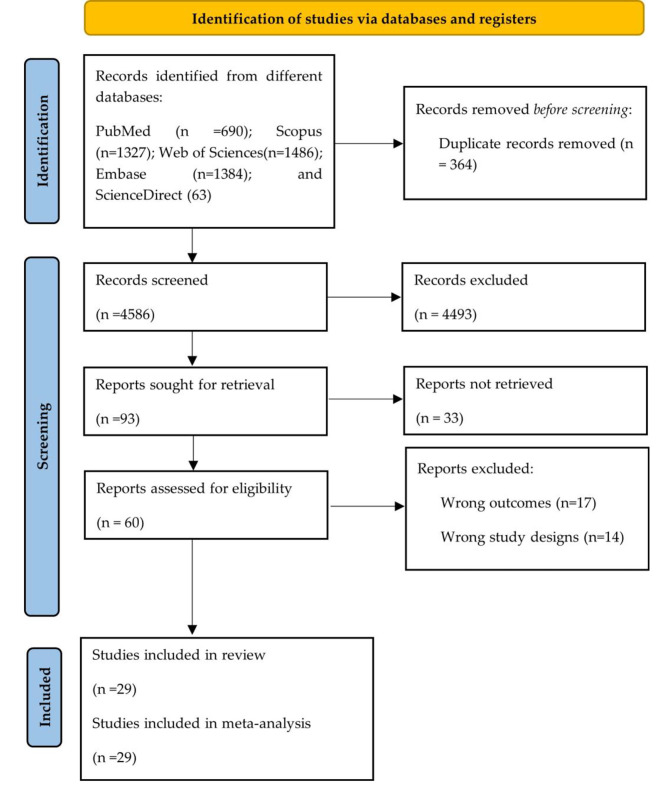
A total of 4950 articles were identified through systematic searches in five databases. After removing 364 duplicate records, 4586 articles were left for review. Subsequently, a thorough evaluation of the full texts (n = 60) was conducted, of which 29 studies fully met the eligibility criteria [16–44]. To visualize the study selection process, the detailed flow diagram in Fig. 1 is presented.
Fig. 1.
Study selection process based on the PRISMA flowchart
Characteristics of the included studie
Table 1 summarizes the characteristics of the included studies [16–44]. This study encompassed 29 cross-sectional research articles, involving a total of 52,658 individuals from 19 countries, published between 2020 and 2023. Of the participant pool, 84.59% (n = 44,543) were men, while 15.26% (n = 8,036) were women. The questionnaires used for data collection were exclusively administered through online surveys, specifically tailored for diverse populations, including the general population, healthcare professionals, and the lesbian, gay, bisexual, transgender, and intersex (LGBTI) community [16–44].
Table 1.
Characteristics of included studies on intention to vaccinate against monkeypox
| Authors | Year | Study design | Country | Sample size (n) | Study population | Sex | Prevalence of vaccination intention | Participants with intention to be vaccinated | Survey Type | Refusal of monkeypox vaccination (n;%) | Data Collection Date | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | |||||||||||
| Araoz-Salinas JM, et al. (16) | 2023 | Cross sectional | Peru | 373 | LGBTI community | 317* | 23 | 88.5% | 330 | Online survey | 43 (11.5%) | 1 November 2022–17 January 2023 |
| Mahameed H, et al. (17) | 2023 | Cross sectional | Jordan | 495 | Healthcare Workers | 204 | 291 | 28.9% | 143 | Online survey | 187 (37.8%) | January 2023 |
| Wang B, et al. (18) | 2023 | Cross sectional | China | 2135 | General population | 798 | 1337 | 68.8% | 1468 | Online survey | 667 (31.2%) | 30 August − 15 September 2022 |
| Al-Mustapha AI, et al. (19) | 2023 | Cross sectional | Nigeria | 822 | General population | 472 | 342 | 46.37% | 339 | Online survey | NR | 16–29 August 2022 |
| Fu L, et al. (20) | 2023 | Cross sectional | China | 577 | LGBTI community | 577* | 0 | 56.8% | 328 | Online survey | 249 (43.2%) | 10 August − 9 September 2022 |
| Dukers-Muijrers NHTM, et al. (21) | 2023 | Cross sectional | Netherlands | 1856 | LGBTI community | 1856* | 0 | 81.5% | 1512 | Online survey | 223 (12%) | 22 July − 5 September 2022 |
| Tran BX, et al. (22) | 2023 | Cross sectional | Vietnam | 842 | General population | 239 | 595 | 65.4% | 551 | Online survey | 13 (1.5%) | April - August 2022 |
| Ghazy RM, et al. (23) | 2023 | Cross sectional | Ghana | 605 | General population | 368 | 237 | 46.1% | 279 | Online survey | 326 (53.9%) | 27 November − 6 December 2022 |
| Hong J, et al. (24) | 2023 | Cross sectional | China | 1032 | Healthcare Workers | 266 | 766 | 90.12% | 930 | Online survey | 102 (9.88%) | 30 May – 1 August 2022 |
| Jamaleddine Y, et al. (25) | 2023 | Cross sectional | Lebanon | 493 | General population | 119 | 374 | 56.6% | 279 | Online survey | 88 (17.9%) | 6–20 September 2022 |
| Dong C, et al. (26) | 2023 | Cross sectional | China | 521 | General population | 264 | 257 | 76.40% | 398 | Online survey | 35 (6.7%) | 29 September 2022–5 October 2022 |
| Chen Y, et al. (27) | 2023 | Cross sectional | China | 154 | LGBTI community | 154* | 0 | 63% | 97 | Online survey | 57 (37%) | 1–31 August 2022 |
| Lounis M, et al. (28) | 2023 | Cross sectional | Algeria | 111 | Healthcare Workers | 33 | 78 | 38.7% | 43 | Online survey | NR | 28 June − 18 September 2022 |
| Ahmed SK, et al. (29) | 2023 | Cross sectional | Iraq | 510 | General population | 277 | 233 | 25.9% | 132 | Online survey | 217 (42.5%) | 27–30 July 2022 |
| Riad A, et al. (30) | 2022 | Cross sectional | Czech Republic | 341 | Healthcare Workers | 33 | 303 | 8.8% | 30 | Online survey | 153 (44.9%) | September 2022 |
| Zucman D, et al. (31) | 2022 | Cross sectional | France | 155 | LGBTI community | 155* | 0 | 66.4% | 103 | Online survey | 52 (33.6%) | July - August 2022 |
| Reyes-Urueña J, et al. (32) | 2022 | Cross sectional | Europe | 32,902 | LGBTI community | 32,902* | 0 | 82% | 26,980 | Online survey | 2686 (8.2%) | 30 July–12 August 2022 |
| Bates BR, et al. (33) | 2022 | Cross sectional | United States | 197 | Physicians | 113 | 69 | 48.3% | 96 | Online survey | 101 (51.7%) | 2–11 September 2022 |
| Zheng M, et al. (34) | 2022 | Cross sectional | China | 2618 | LGBTI community | 2618* | 0 | 90.2% | 2362 | Online survey | NR | 1–3 July 2022 |
| Sahin TK, et al. (35) | 2022 | Cross sectional | Turkey | 283 | Physicians | 117 | 166 | 31.4% | 89 | Online survey | 85 (30%) | 20 August–2 September 2022 |
| Wang H, et al. (36) | 2022 | Cross sectional | Netherlands | 394 | LGBTI community | 394* | 0 | 70.01% | 276 | Online survey | NR | July 2022 |
| Salim NA, et al. (37) | 2022 | Cross sectional | Indonesia | 75 | Healthcare workers | 49 | 26 | 77.3% | 58 | Online survey | 2 (2.70%) | 2–5 August 2022 |
| Riccò, M, et al. (38) | 2022 | Cross sectional | Italy | 163 | Physicians | 57 | 106 | 64.4% | 105 | Online survey | NR | 24–31 May 2022 |
| Meo SA et al. (39) | 2022 | Cross sectional | Saudi Arabia | 1020 | General population | 466 | 554 | 43.7% | 446 | Online survey | NR | 15 May 2022–15 July 2022 |
| Temsah MH, et al. (40) | 2022 | Cross sectional | Saudi Arabia | 1546 | General population | 650 | 896 | 50.6% | 782 | Online survey | NR | 27 May 2022–5 June 2022 |
| Kumar N, et al. (41) | 2022 | Cross sectional | Pakistan | 946 | University students | 432 | 514 | 67.7% | 640 | Online survey | 148 (15.6%) | 15–30 October 2022 |
| Lin GSS, et al. (42) | 2022 | Cross sectional | Malaysia | 229 | Dental students | 75 | 154 | 74.24% | 170 | Online survey | 8 (3.5%) |
25 July - 7 August 2022 |
| Winters M, et al. (43) | 2022 | Cross sectional | United States | 856 | General population | 410 | 436 | 46% | 394 | Online survey | 248 (29%) | June 2022 |
| Harapan H, et al. (44) | 2020 | Cross sectional | Indonesia | 407 | Physicians | 128 | 279 | 93.6% | 381 | Online survey | NR | 25 May 2019–25 July 2019 |
M/F: Male/Female; NR: Not reported; *MSM: men who have sex with men; LGBTI: Lesbian, gay, bisexual, transgender, and intersex
Quality of the included studies and publication bias
The included cross-sectional studies were characterized by their high level of quality, which was assessed using the JBI-MAStARI tool [16–44] (Table S3). Egger’s test for the evaluation of publication bias obtained a value of p = 0.0005 (t = -3.99, df = 27), thus rejecting the null hypothesis of symmetry. Thus, it can be shown that the asymmetry in the results and in the image explains the wide differences in the reported prevalence values; however, publication bias cannot be demonstrated (Figure S1).
Prevalence of intention to vaccinate against Mpox
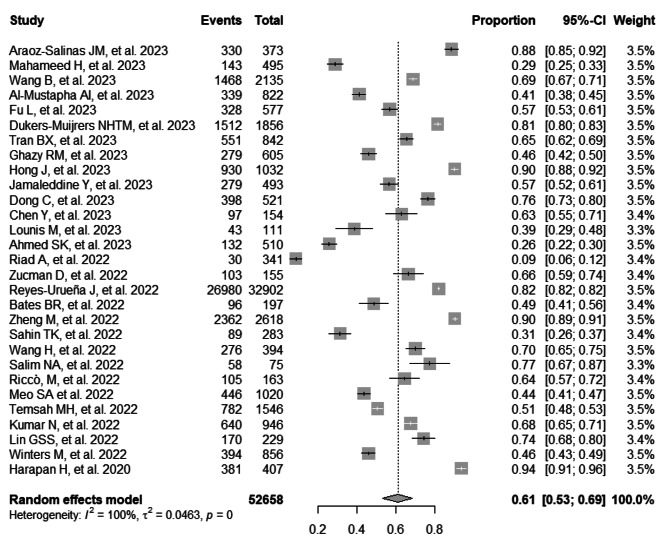
The combined prevalence of the intention to vaccinate against Mpox was 61% (95% CI: 53–69%; 52,658 participants; 29 studies; I2 = 100%) [16–44] (Fig. 2). Figure 3 illustrates the pooled prevalence of the intention to vaccinate against Mpox in different countries, according to the data collected in the studies analyzed. Analyzing the data by continent, the following vaccination intention prevalences were found: In Asian countries, it was 64% (95% CI: 53–74%; 13,883 participants; 17 studies; I2 = 99%) [17, 18, 20, 22, 24–27, 29, 34, 35, 37, 39–42, 44]; in African countries, it was 43% (95% CI: 39–47%; 1538 participants; 3 studies; I2 = 53%) [19, 23, 28]; in European countries, it was 62% (95% CI: 45–78%; 35,811 participants; 6 studies; I2 = 99%) [21, 30–32, 36, 38]; and in American countries, it was 63% (95% CI: 32–89%; 1426 participants; 3 studies; I2 = 99%) [16, 33, 43] (Figure S3). Furthermore, when focusing on the target population of the studies, the following vaccination intention prevalences against Mpox were observed: among the general population, it was 54% (95% CI: 45–62%; 10,296 participants; 11 studies; I2 = 99%) [18, 19, 22, 23, 25, 26, 29, 39–41, 43]; among healthcare workers, it was 57% (95% CI: 33–79%; 3333 participants; 10 studies; I2 = 99%) [17, 24, 28, 30, 33, 35, 37, 38, 42, 44]; and among the LGBTI community, it was 76% (95% CI: 70–82%;39,029 participants; 8 studies; I2 = 98%) [16, 20, 21, 27, 31, 32, 34, 36] (Figure S5).
Fig. 2.
Forest plot illustrating the combined prevalence of intention to vaccinate against monkeypox
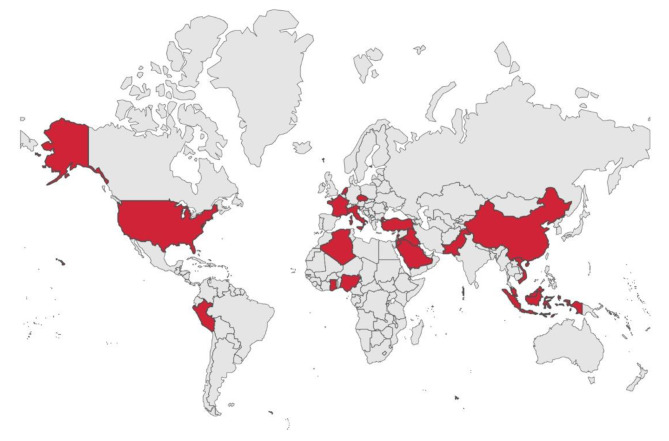
Fig. 3.
Map illustrating the prevalence of the intention to vaccinate against monkeypox in different countries of the world: Peru (88%), Jordan (29%), China (76%), Nigeria (41%), Netherlands (76%), Vietnam (65%), Ghana (46%), Lebanon (57%), Algeria (39%), Iraq (26%), Czech Republic (9%), France (66%), United States (47%), Turkey (31%), Indonesia (87%), Italy (64%), Saudi Arabia (47%), Pakistan (68%), and Malaysia (74%)
Prevalence of refusal of vaccination against Mpox
The aggregated prevalence of vaccination refusal against Mpox was found to be 22% (95% CI: 16–30%; 45,577 participants; 21 studies; I2 = 99%) [16–18, 20–27, 29–33, 35, 37, 41–43] (Figure S2). When analyzing the data by continents, the following prevalence rates of vaccination refusal against Mpox were observed: in Asian countries, 19% (95% CI: 11–28%; 8292 participants; 13 studies; I2 = 99%) [17, 18, 20, 22, 24–27, 29, 35, 37, 41, 42]; in European countries, 23% (95% CI: 12–35%; 35,254 participants; 4 studies; I2 = 99%) [21, 30–32]; and in American countries, 29% (95% CI: 12–50%;1426 participants; 3 studies; I2 = 98%) [16, 33, 43] (Figure S4). Furthermore, a subgroup analysis focused on the target population of the studies was conducted, and the following prevalence rates of vaccination refusal against Mpox were found: among the general population, 22% (95% CI: 11–36%; 6908 participants; 8 studies; I2 = 99%) [18, 22, 23, 25, 26, 29, 41, 43]; among healthcare workers, 23% (95% CI: 10–39%; 2652 participants; 7 studies; I2 = 99%) [17, 24, 30, 33, 35, 37, 42]; and among the LGBTI community, 22% (95% CI: 13–34%;36,017 participants; 6 studies; I2 = 99%) [16, 20, 21, 27, 31, 32] (Figure S6).
Discussion
Improving vaccination is essential for several diseases with available vaccines. In addition to creating safe and effective vaccines, it is necessary to solve logistical challenges, ensure equitable distribution, and promote acceptance in the population to guarantee the demand for vaccines [45].
Monkeypox is gradually becoming a globally relevant public health issue. There are still uncertainties regarding the exact routes of transmission of this disease [8, 46]. Therefore, it is essential to propose sound preventive approaches, such as the implementation of targeted vaccination programs against the Mpox virus, to address this issue efficiently [45].
The present systematic review and meta-analysis determined the prevalence of intention to receive the Mpox vaccine. The combined prevalence of intention to be vaccinated against Mpox was 61%. According to investigations, the prevalence of intention to be vaccinated against Mpox ranged from 8.8 to 93.6% [30, 44]. Riad A et al. showed that 51% of participants were willing to receive the Mpox vaccine if it was offered free, safe, and effective [47]. Another study proposed by Alarifi AM et al. reported that 52.7% of the participants expressed a willingness to receive the Mpox vaccine. The results indicated that the main reasons for this willingness were trust in the Saudi Arabian Ministry of Health (57.7%) and perception of the vaccine as a social responsibility (44.6%) [48]. A systematic review and meta-analysis study proposed by Ulloque-Badaracco JR et al. reported a pooled prevalence of acceptance of the Mpox vaccination of 56% [45].
Globally, vaccination represents a fundamental strategy to mitigate both the spread and severity of contagious viral infections, especially for immunocompromised individuals [49]. Smallpox vaccination provides cross-protection for both smallpox and Mpox, preventing approximately 85% of Mpox virus infection. Two vaccines are available: modified vaccinia Ankara (Jynneos/Imamune/Imvanex, Bavarian Nordic, Hørsholm, Denmark) and ACAM2000 (Emergent BioSolutions, Gaithersburg, MD, USA) [50, 51].
In the subgroup analysis by continents on the intention to be vaccinated against Mpox, the following prevalences were found: Asia (64%), Europe (62%), America (63%), and Africa (43%). Ulloque-Badaracco JR, et al. reported that the prevalence of Mpox vaccine uptake was 50% in Asian countries and 70% in European countries [45]. In addition, in China and Indonesia, they reported the highest prevalence of intention to vaccinate against Mpox, around 90.2% and 93.6%, respectively [34, 44]. This variation could be due to how different countries respond to the severity of a disease and take precautions, which is related to socioeconomic and cultural factors, access to information, and distrust in the health system and government policies.
In the subgroup analysis on the intention to be vaccinated against Mpox, focused on the target population of the studies, the following prevalences were found: general population (54%), health care workers (57%), and the LGBTI community (76%). The study conducted by Alarifi AM et al. revealed that physicians and pharmacists demonstrated a higher willingness to receive the Mpox vaccine, with percentages of 57.5% and 56.1%, respectively, compared to nurses, whose willingness was 46.7% [48]. Ulloque-Badaracco JR et al. reported that the prevalence of vaccine acceptance was 43.0% in the general population, 63.0% in health care workers, and 84.0% in the LGBTI community [45]. In addition, the results may indicate an increased awareness among study subjects of the importance of prevention in different groups that have faced barriers to medical care. The current Mpox outbreak continues to impact primarily men who have sex with men and who have reported having recent sexual encounters with one or more male partners [52]. Therefore, it is crucial to monitor people who have been in contact with the reported cases in order to prevent the spread of this disease.
Another important secondary outcome found by the study was that the pooled prevalence of Mpox vaccination refusal was 22%. Finally, it is worth mentioning that both Americans and healthcare workers exhibited the highest rates of refusal towards Mpox vaccination, with 29% and 23% refusal, respectively. Riad A et al. showed that 30.6% and 18.1% of participants were unsure and refused the Mpox vaccination [47]. Another study proposed by Alarifi AM et al. reported that 47.3% of participants refused the Mpox vaccination [48]. Ulloque-Badaracco JR et al. in their systematic review and meta-analysis, reported a refusal of Mpox vaccination of 24% [45]. One investigation identified insufficient information about the vaccine, fear of unknown adverse reactions, and doubts about the effectiveness and safety of the vaccine as the most reported reasons for unwillingness to receive the Mpox vaccine [48].
This study highlights the importance of recognizing regional and subgroup disparities in willingness to vaccinate and refusal of Mpox vaccination. The findings emphasize the need to implement communication and education strategies tailored to particular contexts in order to enhance vaccination uptake. Additionally, identifying populations with higher refusal rates can guide specific efforts to address concerns and strengthen vaccine confidence within these groups. Ultimately, understanding these factors is essential to achieving optimal levels of vaccination coverage and safeguarding global public health.
The present study has some limitations. First, information about Mpox is constantly evolving. Second, it is crucial to recognize the possibility of bias in the incorporated studies. Third, it is important to keep in mind that the studies addressed in the meta-analysis may cover diverse populations, interventions, and outcomes, thus making it difficult to extrapolate the findings to other populations. In addition, it is crucial to improve the instruments and methods for measuring the intention, acceptance, and refusal of the Mpox vaccination. Several factors, such as confidence in the efficacy and safety of the vaccine, health professionals’ recommendations, government policies, perceptions of disease risk, as well as other social and cultural aspects, may influence these attitudes. It is suggested that future research should focus on assessing the Mpox vaccine acceptance variable, which is defined as a person’s willingness to receive or adopt a specific vaccine, supported by confidence and safety in that vaccine. Regarding its strengths, this current study has a rigorous methodological approach, as it was conducted following the guidelines proposed by the PRISMA guidelines. Furthermore, it constitutes the first systematic review and meta-analysis analyzing the prevalence of the intention to receive the Mpox vaccine. In addition, all the procedures used to select the studies were performed independently by two or more authors.
Conclusions
A combined prevalence of 61% of the intention to vaccinate against Mpox was found, with significant differences across continents and the target population of the studies. Additionally, a considerable prevalence of vaccination refusals against Mpox was identified in different groups and regions, highlighting the importance of implementing appropriate strategies to enhance vaccination acceptance and understanding.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
Conceptualization, J.J.B., D.A.L.F. and M.J.V.G.; methodology, R.S. and J.J.B; soft-ware, D.A.L.F.; validation, A.J.R.M.; formal analysis, D.A.L.F.; investigation, M.J.V.G. and R.S.; resources, D.A.L.F.; data curation, D.A.L.F. and A.J.R.M.; writing—original draft preparation, J.J.B., D.A.L.F., M.J.V.G., R.S., and A.J.R.M.; writing—review and editing, J.J.B., D.A.L.F., M.J.V.G., R.S., and A.J.R.M.; visualization, J.J.B.; supervi-sion, R.S., and A.J.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, et al. Infectious Disease in an era of global change. Nat Rev Microbiol. 2022 doi: 10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker AD, Grantz KH, Hegde ST, Bérubé S, Cummings DAT, Wesolowski A. Development and dissemination of Infectious Disease dynamic transmission models during the COVID-19 pandemic: what can we learn from other pathogens and how can we move forward? Lancet Digit Health. 2021 doi: 10.1016/S2589-7500(20)30268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellwanger JH, da Veiga ABG, Kaminski V, de L, Valverde-Villegas JM, de Freitas AWQ, Chies JAB. Control and prevention of infectious Diseases from a one health perspective. Genet Mol Biol. 2021 doi: 10.1590/1678-4685-GMB-2020-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The unfinished agenda of communicable diseases among Children and adolescents before the COVID-19 pandemic, 1990–2019: a systematic analysis of the global burden of Disease Study 2019. The Lancet. 2023 doi: 10.1016/S0140-6736(23)00860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.León-Figueroa DA, Bonilla-Aldana DK, Pachar M, Romaní L, Saldaña-Cumpa HM, Anchay-Zuloeta C, et al. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.León-Figueroa DA, Barboza JJ, Garcia-Vasquez EA, Bonilla-Aldana DK, Diaz-Torres M, Saldaña-Cumpa HM, et al. Epidemiological Situation of Monkeypox Transmission by possible sexual contact: a systematic review. Trop Med Infect Dis. 2022 doi: 10.3390/tropicalmed7100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farahat RA, Sah R, El-Sakka AA, Benmelouka AY, Kundu M, Labieb F, et al. Human monkeypox Disease (MPX) Infez Med. 2022 doi: 10.53854/liim-3003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.León-Figueroa DA, Barboza JJ, Saldaña-Cumpa HM, Moreno-Ramos E, Bonilla-Aldana DK, Valladares-Garrido MJ, et al. Detection of Monkeypox Virus according to the Collection Site of samples from confirmed cases: a systematic review. Trop Med Infect Dis. 2023 doi: 10.3390/tropicalmed8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014 doi: 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poland GA, Kennedy RB, Tosh PK. Prevention of monkeypox with vaccines: a rapid review. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salalli R, Dange JR, Dhiman S, Sharma T. Vaccines development in India: advances, regulation, and challenges. Clin Exp Vaccine Res. 2023 doi: 10.7774/cevr.2023.12.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues CMC, Plotkin SA. Impact of vaccines; Health, Economic and Social perspectives. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajkhowa P, Dsouza VS, Kharel R, Cauvery K, Mallya BR, Raksha DS, et al. Factors influencing Monkeypox Vaccination: a cue to policy implementation. J Epidemiol Glob Health. 2023 doi: 10.1007/s44197-023-00100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Huang QZ, Zhang H, Liu ZX, Chen XH, Ye LL, et al. The land-scape of immune response to monkeypox virus. eBioMedicine. 2023 doi: 10.1016/j.ebiom.2022.104424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020 doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araoz-Salinas JM, Ortiz-Saavedra B, Ponce-Rosas L, Soriano-Moreno DR, Soriano-Moreno AN, Alave J, et al. Perceptions and intention to get vaccinated against Mpox among the LGBTIQ + community during the 2022 outbreak: a cross-sectional study in Peru. Vaccines. 2023 doi: 10.3390/vaccines11051008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahameed H, Al-Mahzoum K, AlRaie LA, Aburumman R, Al-Naimat H, Alhiary S, et al. Previous vaccination history and psychological factors as significant predictors of willingness to Receive Mpox Vaccination and a favorable attitude towards compulsory vaccination. Vaccines. 2023 doi: 10.3390/vaccines11050897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Peng X, Li Y, Fu L, Tian T, Liang B, et al. Perceptions, precautions, and vaccine acceptance related to monkeypox in the public in China: a cross-sectional survey. J Infect Public Health. 2023 doi: 10.1016/j.jiph.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Mustapha AI, Ogundijo OA, Sikiru NA, Kolawole B, Oyewo M, El-Nadi H, et al. A cross-sectional survey of public knowledge of the monkeypox Disease in Nigeria. BMC Public Health. 2023 doi: 10.1186/s12889-023-15398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu L, Sun Y, Li Y, Wang B, Yang L, Tian T, et al. Perception of and Vaccine Readiness towards Mpox among men who have sex with men living with HIV in China: a cross-sectional study. Vaccines. 2023 doi: 10.3390/vaccines11030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dukers-Muijrers NHTM, Evers Y, Widdershoven V, Davidovich U, Adam PCG, Op de Coul ELM, et al. Mpox vaccination willingness, determinants, and communication needs in gay, bisexual, and other men who have sex with men, in the context of limited vaccine availability in the Netherlands (Dutch Mpox-survey) Front Public Health. 2022 doi: 10.3389/fpubh.2022.1058807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran BX, Anh Do L, Hoang TP, Boyer L, Auquier P, Fond G, et al. Crucial choices in a global health crisis: revealing the demand and willingness to pay for a hypothetical monkeypox vaccine - the PREVENT study. J Glob Health. 2023 doi: 10.7189/jogh.13.04033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazy RM, Yazbek S, Gebreal A, Hussein M, Addai SA, Mensah E, et al. Monkeypox Vaccine Acceptance among ghanaians: a call for action. Vaccines. 2023 doi: 10.3390/vaccines11020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong J, Pan B, Jiang HJ, Zhang QM, Xu XW, Jiang H, et al. The willingness of Chinese healthcare workers to receive monkeypox vaccine and its Independent predictors: a cross-sectional survey. J Med Virol. 2023 doi: 10.1002/jmv.28294. [DOI] [PubMed] [Google Scholar]
- 25.Jamaleddine Y, El Ezz AA, Mahmoud M, Ismail O, Saifan A, Mayta Z, et al. Knowledge and attitude towards monkeypox among the Lebanese population and their attitude towards vaccination. J Prev Med Hyg. 2023 doi: 10.15167/2421-4248/jpmh2023.64.1.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong C, Yu Z, Zhao Y, Ma X. Knowledge and vaccination intention of monkeypox in China’s general population: a cross-sectional online survey. Travel Med Infect Dis. 2023 doi: 10.1016/j.tmaid.2022.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Li Y, Fu L, Zhou X, Wu X, Wang B, et al. Knowledge of human mpox (Monkeypox) and attitude towards Mpox Vaccination among male sex workers in China: a cross-sectional study. Vaccines. 2023 doi: 10.3390/vaccines11020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lounis M, Bencherit D, Abdelhadi S. Knowledge and awareness of Algerian healthcare workers about human monkeypox and their attitude toward its vaccination: an online cross-sectional survey. Vacunas. 2023 doi: 10.1016/j.vacun.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed SK, Abdulqadir SO, Omar RM, Abdullah AJ, Rahman HA, Hussein SH, et al. Knowledge, attitude and worry in the Kurdistan Region of Iraq during the Mpox (Monkeypox) outbreak in 2022: an online cross-sectional study. Vaccines. 2023 doi: 10.3390/vaccines11030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riad A, Drobov A, Rozmarinová J, Drapáčová P, Klugarová J, Dušek L, et al. Monkeypox Knowledge and Vaccine Hesitancy of Czech Healthcare Workers: A Health Belief Model (HBM)-Based study. Vaccines. 2022 doi: 10.3390/vaccines10122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zucman D, Fourn E, Touche P, Majerholc C, Vallée A. Monkeypox Vaccine Hesitancy in French men having sex with men with PrEP or living with HIV in France. Vaccines. 2022 doi: 10.3390/vaccines10101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes-Urueña J, D’Ambrosio A, Croci R, Bluemel B, Cenciarelli O, Pharris A, et al. High monkeypox vaccine acceptance among male users of smartphone-based online gay-dating apps in Europe, 30 July to 12 August 2022. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2022 doi: 10.2807/1560-7917.ES.2022.27.42.2200757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bates BR, Grijalva MJ. Knowledge, attitudes, and practices towards monkeypox during the 2022 outbreak: an online cross-sectional survey among clinicians in Ohio, USA. J Infect Public Health. 2022 doi: 10.1016/j.jiph.2022.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng M, Qin C, Qian X, Yao Y, Liu J, Yuan Z, et al. Knowledge and vaccination acceptance toward the human monkeypox among men who have sex with men in China. Front Public Health. 2022 doi: 10.3389/fpubh.2022.997637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin TK, Erul E, Aksun MS, Sonmezer MC, Unal S, Akova M. Knowledge and attitudes of Turkish Physicians towards Human Monkeypox Disease and related vaccination: a cross-sectional study. Vaccines. 2022 doi: 10.3390/vaccines11010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Paulo KJI, d’Abreu de G, Gültzow T, Zimmermann HML, Jonas KJ. Monkeypox self-diagnosis abilities, determinants of vaccination and self-isolation intention after diagnosis among MSM, the Netherlands, July 2022. Eurosurveillance. 2022 doi: 10.2807/1560-7917.ES.2022.27.33.2200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salim NA, Septadina IS, Permata M, Hudari H. KNOWLEDGE, ATTITUDE, AND PERCEPTION OF ANTICIPATING 2022 GLOBAL HUMAN MONKEYPOX INFECTION AMONG INTERNAL MEDICINE RESIDENTS AT PALEMBANG INDONESIA: AN ONLINE SURVEY. J Kedokt Dan Kesehat Publ Ilm Fak Kedokt Univ Sriwij. 2022 doi: 10.32539/JKK.V9I3.18799. [DOI] [Google Scholar]
- 38.Riccò M, Ferraro P, Camisa V, Satta E, Zaniboni A, Ranzieri S, et al. When a neglected Tropical Disease goes Global: knowledge, attitudes and practices of Italian Physicians towards Monkeypox, preliminary results. Trop Med Infect Dis. 2022 doi: 10.3390/tropicalmed7070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meo SA, Al-Khlaiwi T, Aljofan ZF, Alanazi AI, Meo AS. Public perceptions of the Emerging Human Monkeypox Disease and Vaccination in Riyadh, Saudi Arabia: a cross-sectional study. Vaccines. 2022 doi: 10.3390/vaccines10091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temsah MH, Aljamaan F, Alenezi S, Alhasan K, Saddik B, Al-Barag A, et al. Monkeypox caused less worry than COVID-19 among the general population during the first month of the WHO Monkeypox alert: experience from Saudi Arabia. Travel Med Infect Dis. 2022 doi: 10.1016/j.tmaid.2022.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar N, Ahmed F, Raza MS, Rajpoot PL, Rehman W, Khatri SA et al. Monkeypox Cross-Sectional Survey of Knowledge, Attitudes, Practices, and Willingness to Vaccinate among University Students in Pakistan. Vaccines. 2022. 10.3390/vaccines11010097. [DOI] [PMC free article] [PubMed]
- 42.Lin GSS, Tan WW, Chan DZK, Ooi KS, Hashim H. Monkeypox awareness, knowledge, and attitude among undergraduate preclinical and clinical students at a Malaysian dental school: an emerging outbreak during the COVID-19 era. Asian Pac J Trop Med. 2022 doi: 10.4103/1995-7645.359787. [DOI] [Google Scholar]
- 43.Winters M, Malik AA, Omer SB. Attitudes towards Monkeypox vaccination and predictors of vaccination intentions among the US general public. PLoS ONE. 2022 doi: 10.1371/journal.pone.0278622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harapan H, Setiawan AM, Yufika A, Anwar S, Wahyuni S, Asrizal FW, et al. Physicians’ willingness to be vaccinated with a Smallpox vaccine to prevent monkeypox viral Infection: a cross-sectional study in Indonesia. Clin Epidemiol Glob Health. 2020 doi: 10.1016/j.cegh.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulloque-Badaracco JR, Alarcón-Braga EA, Hernandez-Bustamante EA, Al-kassab-Córdova A, Benites-Zapata VA, Bonilla-Aldana DK, et al. Acceptance towards Monkeypox Vaccination: a systematic review and Meta-analysis. Pathogens. 2022 doi: 10.3390/pathogens11111248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Morales AJ, León-Figueroa DA, Sah R, Villamil-Gomez WE. Arboviral Diseases and monkeypox – an epidemiological overlapping differential diagnosis? Rev Cuerpo Méd Hosp Nac Almanzor Aguinaga Asenjo. 2022. 10.35434/rcmhnaaa.2022.153.1678.
- 47.Riad A, Rybakova N, Dubatouka N, Zankevich I, Klugar M, Koščík M, et al. Belarusian Healthcare professionals’ views on Monkeypox and Vaccine Hesitancy. Vaccines. 2023 doi: 10.3390/vaccines11081368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alarifi AM, Alshahrani NZ, Sah R. Are Saudi Healthcare Workers Willing to receive the Monkeypox Virus Vaccine? Evidence from a descriptive-baseline survey. Trop Med Infect Dis. 2023 doi: 10.3390/tropicalmed8080396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.See KC. Vaccination for the Prevention of Infection among immunocompromised patients: a concise review of recent systematic reviews. Vaccines. 2022 doi: 10.3390/vaccines10050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.See KC. Vaccination for Monkeypox Virus Infection in humans: a review of key considerations. Vaccines. 2022 doi: 10.3390/vaccines10081342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortiz-Saavedra B, León-Figueroa DA, Montes-Madariaga ES, Ricardo-Martínez A, Alva N, Cabanillas-Ramirez C, et al. Antiviral treatment against Monkeypox: a scoping review. Trop Med Infect Dis. 2022 doi: 10.3390/tropicalmed7110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barboza JJ, León-Figueroa DA, Saldaña-Cumpa HM, Valladares-Garrido MJ, Moreno-Ramos E, Sah R, et al. Virus identification for Monkeypox in Human seminal fluid samples: a systematic review. Trop Med Infect Dis. 2023 doi: 10.3390/tropicalmed8030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.