Abstract
Background
The repercussions of the syphilis epidemic differ according to populations. Identifying and acknowledging the differences and specificities of populations is fundamental in the design and implementation of policies aimed at assisting the groups most vulnerable to syphilis.
Objective
To estimate the prevalence of antibodies against Treponema pallidum and associated vulnerability factors among riverside populations of a capital city in the Brazilian Amazon.
Methods
Cross-sectional study was conducted among residents of the periurban islands in Belém, northern Brazil, from August 2020 to January 2021. The inclusion criterion was being a resident of the riverside communities of the Combú Environmental Protection Area, aged 18 years or over. The participants responded to questionnaire and were tested for syphilis using rapid test. Data were analyzed using multiple logistic regression by Minitab version 20® software.
Results
Overall, a total of 325 riverine were included. Age varied from 18 to 91 years (average 40 years). Prevalence of markers for syphilis was 5.9% (95% CI: 3.3%-8.4%). The multiple regression showed that as age increases, the chances of having syphilis also increase (p = 0.001; aOR: 1.04) and riverside dwellers with more than one sexual partner in the last 6 months had more than four chances of having syphilis compared to people who had only one sexual partner (p = 0.007; aOR: 4.20).
Conclusion
Syphilis circulates among traditional populations in the Amazon and is associated with factors of social and individual vulnerability.
Keywords: Primary health care, Sexually transmitted infections, Syphilis, Prevalence
Background
Globally, syphilis cases was 30.91 million in 1990 and 49.71 million in 2019, with an increase of 60.83% in this period. Some regions and populations are disproportionately affected by the infection, mainly males and regions with low sociodemographic indices, such as in countries on the African continent, Latin America and the Caribbean [1]. In Brazil, there was an increase in the detection rate of acquired syphilis until 2018, with stability in 2019 and a decline in 2020 associated with the covid-19 pandemic. In 2022, 213,129 cases of acquired syphilis were reported (99,2 cases/100,000 inhabitants). The infection is also more prevalent in men and people with low education [2, 3].
Studies have shown that the syphilis epidemic and its repercussions differ according to populations [4–10]. Identifying and recognizing the differences and specificities of this process becomes essential in the design and implementation of policies aimed at assisting groups most vulnerable to syphilis [11–13]. In rural communities, the prevalence of syphilis in adults ranged from 0.2% among women in rural Nepal [5] to 16% among men living with HIV in rural Uganda [8]. Among populations living in seven fishing communities on Lake Victoria, in northwest Tanzania, the prevalence was 15.6% [9].
These are vulnerable populations in the social context and access to health services. There is a great lack of knowledge about Sexually Transmitted Infections (STIs), due to the low level of education and the absence of educational actions in health by the primary health care teams, which makes them very vulnerable to STIs [14, 15]. The riverside people, including people who live on the banks of rivers or on islands, have a way of life in which the geographic and environmental context of the place of residence alone is a structural barrier for them to have access to health services. To reduce this barrier, in the Brazilian Amazon, there are models of specific teams that work in Primary Health Care, riverside and river teams [15].
The prevention, diagnosis and treatment of syphilis actions are provided directly by these teams, without the need for referral to reference centers [16]. Due to the difficulty in accessing these communities, there are few studies in Brazil with riverine people [10, 17], of which only one was carried out in the Amazon, in the Marajó Archipelago, but also included populations urban areas of the cities that make up the Archipelago [17, 18]. This lack of studies makes it difficult to assess the population's access to actions aimed at syphilis that are offered by Primary Health Care. This is also due in part to the large number of compulsory syphilis notification forms that are not completely filled out by professionals [19]. In the Brazilian census, riverside dwellers are classified as rural populations [20]. Thus, making it difficult to design and evaluate public policies for these vulnerable populations. Given this scenario, this study aims to estimate the prevalence of antibodies against Treponema pallidum and associated vulnerability factors among riverside populations of a capital city in the Brazilian Amazon.
Methods
Study design
A cross-sectional study was conducted among residents of the periurban islands in Belém, northern Brazil, from August 2020 to January 2021.
Setting
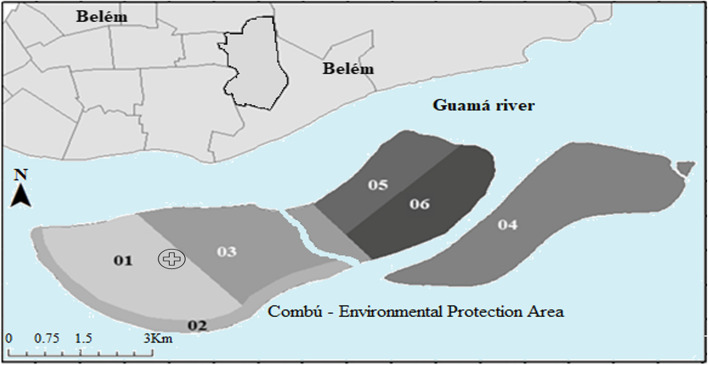
Belém is the capital of the state of Pará, in the brazilian Amazonian. A total of 11,294 people inhabit 39 islands in this city, one percent (1%) of the total population of the Belém. Combú Environmental Protection Area are periurban islands with about 2,200 inhabitants and area 14,770.000 square meters (Fig. 1). The riverine populations had precarious social and health indicators. There are public facilities provided by the government as one elementary school, one speedboat for school transport and healthcare, one basic health unit with one family health strategy team [15, 20–23].
Fig. 1.

Combú Environmental Protection Area. Source: macroproject database
The basic family health unit is responsible for six micro areas and is located in micro area 1, requiring the team to travel by boat to carry out care in the houses (Fig. 2).
Fig. 2.
Geographic location of the island investigates in Belém, state of Pará, Brazilian Amazon. Legend: Micro areas: 1,2,3,4,5,6. symbol: location Combú's basic health unit. Source: Prepared by the authors, in the laboratory of the macroproject research group
Participants
The inclusion criterion was being a resident of the riverside communities of the Combú Environmental Protection Area, aged 18 years or over. Those who were under the influence of psychotropic drugs or alcohol were excluded.
Variables
The main hypothesis of the study was to analyze whether there is an association between vulnerability factors and a reactive result in the treponemal test (antibodies against T. pallidum). The dependent variable to test this hypothesis was the rapid test result for syphilis (treponemal test). This variable was treated as reactive and non-reactive, whereas the response event selected was reactive result (presence of antibodies against T. pallidum in the rapid test).
The independent variables were a) social: sex, age group, marital status, level of education, family income, skin color, number of people living in the house, beneficiaries of the social programs. B) individual: currently have a sexual partner, history of sexually transmitted infections in the last six months, sex with more than one partner in the last 6 months, condom use during recent sexual intercourse (last time), condom broken during sexual intercourse (at any time during life), frequency of condom use (last 3 months). C) programmatic: performed rapid test for Sexually Transmitted Infections—ever in life, Performed rapid test for Sexually Transmitted Infections (last 12 months), free access to condoms in the last 12 months, free access to female condom in the last 12 months, Know Post-Exposure Prophylaxis, Do you know female condom?
Study size
The required sample size was calculated using a confidence interval of 97%; an expected prevalence of syphilis of 8.5% based on a previous study [17], a total of 320 participants. This total sample size was proportionally allocated for six micro-areas of the family health strategy team.
The simple random sampling technique wasn't employed to select the study participants due to the geographic conditions of the Combú island, with flooded areas accessible only by small boats. Thus, sampling was for convenience, at different times and days of the week to recruit people who work. Participants were recruited in the micro-area where they lived with the help of the community health agent responsible for the territory. It took 18 boat expeditions to reach the sample size.
Data sources
The participants were asked to sign an informed consent if they were interested in the study and responded to questionnaire collected by trained researchers in individual and private face-to-face interviews. The instrument used was adapted from structured questionnaire “knowledge, attitudes and practices in the Brazilian population” [24]. This instrument was applied in a pre-test, with seven participants (not included in the study).
To assess prevalence of syphilis, after the interview, the participants were tested for syphilis using rapid test. They were invited to collect blood from the finger pulp of the hand for qualitative determination of total antibodies (IgG, IgM and IgA) against-Treponema pallidum (Kit Syphilis Bio—Bioclin, Minas Gerais, Brazil) [25]. In post-counseling, a trained nurse delivered the results to the participants.
Reactive results were complemented by Rapid Plasm Reagin test (RPR)—RPR Brás -Laborclin [26] performed from blood collected by venipuncture. For RPR, blood samples were processed by Virus Laboratory of the Institute for Biological Sciences, Federal University of Pará. The results were forwarded to the nurse, at the basic health unit on the island, for patient care and treatment when applicable.
Statistical methods
Data were entered in the EPI Info version 7.2.2.16 (developed by the Centers for Diseases Control and Prevention in Atlanta, GA, USA) and exported to Microsoft Excel ®. For categorical variable if more than 20% of values are missed in one variable, we discard the variables. Descriptive statistics were made for categorical variables using absolute and relative frequencies. The result was presented using texts and tables.
The estimate of the prevalence of anti-T. pallidum and its confidence interval were calculated by estimating the proportion in the Bioestat 5.3® program. The main hypothesis of the study was tested using multiple logistic regression using Minitab software version 20®. Simple binomial regression was used to assess the association between each independent variable and the dependent variable. Variables with p-value < 0.20 (age group, Marital status, Level of education, Do you know female condom?, currently have a sexual partner, Sex with more than one partner in the past 6 months) were entered into a multiple logistic regression and the backward elimination method was applied. Age was analyzed in the regression as a continuous variable, being presented in the table by mean and by age group.
In which all variables are inserted into the regression and only the significant ones remain. For the interpretation of the results, adjusted Odd Ratios (aOR) with their respective p values and confidence interval (CI) were considered. The significance level adopted was 0.05.
Results
Overall, a total of 325 riverine living in the Combú Environmental Protection Area were included. Age varied from 18 to 91 years (average 40 years). The majority of participant were in a relationship (Married/stable union/dating) at the time of data collection (70.1%; 228/325); had up to elementary education (56.6%; 184/325); with a monthly family income of less than one minimum wage (70.7%; 222/325) and are beneficiaries of social programs (66.4%; 216/325) (Table 1).
Table 1.
Social factors associated with results for Syphilis among riverine, in the Brazilian Amazon. 2020–2021
| Social | Rapid test – T. pallidum | Total n (%) | Binary Regression | Multiple regression | |||
|---|---|---|---|---|---|---|---|
| Non-reactive n (%) | Reactive n (%) | OR (95% CI) | p | aOR (95% CI) | p | ||
| Sex | |||||||
| Male | 178(93.7) | 12(6.3) | 190(58.5) | Ref | |||
| Female | 128(94.8) | 7(5.2) | 135(41.5) | 0.81 (0.31; 2.11) | 0.85 | ||
| Age | |||||||
| Mean (age) | 39.3 | 52.1 | 40.0 | 1.04 (1.01; 1.07) | 0.00 | 1.04 (1.01; 1.07) | 0.00 |
| Age group—18–41 | 187(96.9) | 6(3.1) | 193(60.5) | ||||
| Age group—42–65 | 71(93.4) | 5(6.6) | 76(23.8) | ||||
| Age group -Equal to or greater than 66 | 43(86.0) | 7(14.0) | 50(15.7) | ||||
| NIa | 5 | 1 | 6 | ||||
| Skin color | |||||||
| Black | 69(90.8) | 7(9.2) | 76(23.8) | 2.13 (0.79; 5.72) | 0.20 | ||
| White/brown/yellow | 232(95.5) | 11(4.5) | 243(76.2) | Ref | |||
| NIa | 5 | 1 | 6 | ||||
| Marital status | |||||||
| Married/Stable union/Dating | 218(95.6) | 10(4.4) | 228(70.2) | Ref | |||
| Single/Divorced/Widowed | 88(90.7) | 9(9.3) | 97(29.8) | 2.22 (0.87;5.67) | 0.09 | 0.19 | |
| Level of education | |||||||
| High school/University | 138(97.9) | 3(2.1) | 141(43.4) | Ref | |||
| Never attended school/Elementary | 168(91.3) | 16(8.7) | 184(56.6) | 4.38 (1.25; 15.3) | 0.02 | 0.29 | |
| Family income (minimum wage)b | |||||||
| Up to one | 211(95.0) | 11(5.0) | 222(70.7) | 0.54 (0.21; 1.40) | 0.31 | ||
| Equal to or greater than one | 84(91.3) | 8(8.7) | 92(29.3) | Ref | |||
| NIa | 11 | 0 | 11 | ||||
| Number of people living in the house | |||||||
| Up to two people | 80(96.4) | 3(3.6) | 83(25.5) | 1.88 (0.53; 6.65) | 0.46 | ||
| Equal to or greater than three | 226(93.4) | 16(6.6) | 242(74.5) | Ref | |||
| Participate in social programs (beneficiaries of the social programs) | |||||||
| No | 100(91.7) | 9(8.3) | 109(33.5) | 0.53 (0.21; 1.36) | 0.28 | ||
| Yes | 206(95.4) | 10(4.6) | 216(66.5) | Ref | |||
OR odds ratio, CI confidence intervals, Ref. reference, aOR adjusted odds ratio
aNI: not informed/do not want/do not know—not considered for statistical calculation
bBrazilian monthly minimum wage 2020—BRL 1,045.00 per month
In the rapid test, the prevalence of antibodies against T. pallidum was 5.9% (19/325; 95% CI 3.3%-8.4%) in this study. After all samples were tested in the rapid test for T. pallidum, the reactive samples were tested in the RPR. One (0.3%; 1/325) participant had title equal to 1:8, confirmed diagnosis of syphilis using RPR. Titers less than or equal to 1: 4 were found in 3.4% (11/325) participants.
The social aspects associated with antibodies against T. pallidum among riverine are shown in Table 1. The analysis of the association of age in years (continuous variable) demonstrated that the chances of having a reactive result for syphilis increase with age (OR: 1.04; p = 0.002). Participants with primary education/never attended school are four times more likely to have reactive syphilis (OR: 4.38; p = 0.02). In addition to these two variables, also, the variable marital status with p < 0.20 was selected for the multiple regression.
Social factors are shown in Table 2. Among access to health services factors, only one variable was selected for multiple regression (p < 0.20): knowledge about the female condom.
Table 2.
Access to health services factors associated with results for Syphilis among riverine, in the Brazilian Amazon. 2020–2021
| Access to health services | Rapid test – T. pallidum | Total n (%) | Binary Regression | Multiple regression | |||
|---|---|---|---|---|---|---|---|
| Non-reactive n (%) | Reactive n (%) | OR (95% CI) | p | aOR (95% CI) | p | ||
| Performed rapid test for STI (ever in life) | |||||||
| No | 140(95.9) | 6(4.1) | 146(46.9) | 0.60 (0.21; 1.66) | 0.45 | ||
| Yes | 154(93.3) | 11(6.7) | 165(53.1) | Ref | |||
| NIa | 12 | 2 | 14 | ||||
| Performed rapid test for STI (last 12 months) | |||||||
| No | 215(95.1) | 11(4.9) | 226(72.9) | 0.66 (0.23; 1.85) | 0.61 | ||
| Yes | 78(92.9) | 6(7.1) | 84(27.1) | Ref | |||
| NIa | 13 | 2 | 15 | ||||
| Access to condoms (12 months) | |||||||
| No | 133 (93.7) | 9 (6.3) | 142 (44.5) | 1.37 (0.47; 3.97) | 0.74 | ||
| Yes, I bought it in a commercial establishment | 45 (91.8) | 4 (8.2) | 49 (15.4) | 1.80 (0.48; 6.70) | 0.59 | ||
| Yes, free in actions and in the health service | 122(95.3) | 6(4.7) | 128 (40.1) | Ref | |||
| NIa | 5 | 0 | 6 | ||||
| Access to female condom (last 6 months) | |||||||
| No | 271 (93.8) | 18 (6.2) | 289 (88.9) | 2.32 (0.30; 17.9) | 0.64 | ||
| Yes, free in the health service | 35(97.2) | 1(2.8) | 36 (11.1) | Ref | |||
| Know Post-Exposure Prophylaxis | |||||||
| No | 280(94.3) | 17(5.7) | 297(91.4) | 0.78 (0.17; 3.60) | 0.90 | ||
| Yes | 26(92.9) | 2(7.1) | 28(8.6) | Ref | |||
| Do you know female condom? | |||||||
| No | 77(89.5) | 9(10.5) | 86(26.8) | 3.44 (1.03; 11.6) | 0.06 | 0.24 | |
| Yes, professionals and/or the media | 225 | 10 | 235 | Ref | |||
| NIa | 4 | 0 | 4 | ||||
STI Sexually Transmitted Infections, OR odds ratio, CI confidence intervals, Ref. reference, aOR adjusted odds ratio
a NI: not informed/do not want/do not know—not considered for statistical calculation
Among the individual factors (Table 3), the following variables were selected for multiple regression (p < 0.20): Have currently have a sexual partner (p = 0.05) and sex with more than one partner in the past 6 months (OR: 2.94; p = 0.03).
Table 3.
Individual factors associated with results for Syphilis among riverine, in the Brazilian Amazon. 2020–2021
| Individual | Rapid test – T. pallidum | Total n (%) | Binary Regression | Multiple regression | |||
|---|---|---|---|---|---|---|---|
| Non-reactive Reactive n (%) | OR (95% CI) | p | aOR (95% CI) | p | |||
| Currently have a sexual partner | |||||||
| No | 62(88.6) | 8(11.4) | 70(21.5) | 2.86 (1.10; 7.41) | 0.05 | 0.59 | |
| Yes | 244(95.7) | 11(4.3) | 255(78.5) | Ref | |||
| History of STI (last 6 months) | |||||||
| No | 29(96.7) | 1(3.3) | 30(9.2) | Ref | |||
| Yes | 277(93.9) | 18(6.1) | 295(90.8) | 1.88 (0.24; 14.6) | 0.83 | ||
| Sex with more than one partner in the past 6 months | |||||||
| No | 252(95.5) | 12(4.5) | 264(82.2) | Ref | |||
| Yes | 50(87.7) | 7(12.3) | 57(17.8) | 2.94 (1.10; 7.83) | 0.03 | 4.20 (1.46; 12.05) | 0.007 |
| NIa | 4 | 4 | |||||
| Frequency of condom use (last 3 months) | |||||||
| Never | 164(94.3) | 10(5.7) | 174(57.4) | 0.79 (0.20; 3.01) | 0.98 | ||
| Sometimes | 82(94.3) | 5(5.7) | 87(28.7) | 0.76 (0.17; 3.36) | 0.97 | ||
| Everytime | 39(92.9) | 3(7.1) | 42(13.9) | Ref | |||
| NIa | 21 | 1 | 22 | ||||
| Condom use during recent sexual intercourse (last time) | |||||||
| No | 207(94.5) | 12(5.5) | 219(68.7) | 0.77 (0.29; 2.01) | 0.78 | ||
| Yes | 93(93.0) | 7(7.0) | 100(31.3) | Ref | |||
| NIa | 6 | 0 | 6 | ||||
| Broken condom (at any time during life) | |||||||
| No | 218(94.0) | 14(6.0) | 232(73.7) | Ref | |||
| Yes | 78(94.0) | 5(6.0) | 83(26.3) | 0.99 (0.34; 2.86) | 0.79 | ||
| NIa | 10 | 0 | 10 | ||||
STI Sexually Transmitted Infections, OR odds ratio, CI confidence intervals, Ref. reference, aOR adjusted odds ratio
a NI: not informed/do not want/do not know—not considered for statistical calculation
Variables with p-value < 0.20 (age group, Marital status, Level of education, Do you know female condom?, currently have a sexual partner, sex with more than one partner in the past 6 months) were entered into a multiple logistic regression and the backward elimination method was applied.
The multiple regression showed that as age increases the chances of having syphilis also increase (p = 0.001; OR: 1.04) and riverside dwellers with more than one sexual partner in the last 6 months had more than four chances of having syphilis compared to people who had only one sexual partner (p = 0.007; OR: 4.20).
Discussion
The estimated prevalence of antibodies against T.pallidum among the riverside population of a capital in the Brazilian Amazon, Belém, was 5.9%, with social and individual vulnerability factors associated with the presence of infection markers. Active syphilis had a low frequency among participants. In this community, which has a family health strategy team, no association was found between vulnerability factors related to access to health services and syphilis.
The North region, Brazilian Amazon, had the second lowest detection rate of acquired syphilis in 2022, with 86.3 cases per 100,000 inhabitants (16,518 cases), behind only the Northeast region with 55.4 cases per 100,000 (32,084 cases) [2]. Studies carried out in Brazil found a lower prevalence than the present study, such as among quilombola women (another traditional population) where the prevalence was 4.3% by rapid test [27] and among sugarcane cutters sugar, serological markers of lifelong syphilis were detected in 2.5% (by rapid treponemic test) and active syphilis in 1.2% [4]. However, a higher prevalence than the present study was found among riverside dwellers who do not inhabit islands, in the state of Paraíba in the Northeast region, where the prevalence was 11.6% by rapid treponemic test [10] and among the inhabitants of the largest river island in the country Marajó Island, in Pará, the prevalence was 8.5% by immunoenzymatic assay, ELISA type [17].
In the present study, the final logistic regression model demonstrated that the factors associated with antibodies against T. pallidum were having sex with more than one partner in the last six months and older age. Among riverside dwellers in Paraíba, the number of partners was a factor associated with syphilis (more than two sexual partners in the last 12 months, p = 0.005), along with two other factors, previous history of STIs (p < 0.001) and history of imprisonment (p = 0.010) [10].
This result is related to the type of antibodies detected in the rapid test used, as it detects antibodies from past infection. Therefore, older age allows for a longer period of exposure to bacteria when prevention methods against infection are not used. In Brazil, in basic primary care health units in the single health system, reverse testing is used to diagnose syphilis, which consists of performing a rapid treponemal test to detect antibodies against T. pallidum of the IgG, IgA and IgM classes. The reagent results are subjected to a second test to detect active infection [2, 16].
Among women in rural areas of China, multiple analysis of sociodemographic factors identified that older age is associated with a reactive result for syphilis, along with other factors such as low education (elementary or lower), being from ethnic minorities and of specific provinces. In the multiple analysis of obstetric and sexual history, an association was demonstrated with markers for T. pallidum, having a history of pregnancy and STI or gynecological disease, women who never used condoms and those with husbands who tested positive for syphilis [28]. The number of partners and age were not factors associated with syphilis among people in rural areas of Ghana. In this population, significant factors associated with syphilis infection included sub-district of residence, and history of coerced sexual intercourse [29].
In studies carried out in the general population, the number of sexual partners was among the factors identified in the multiple regression [30, 31]. The number of sexual partners in the last year (three or more) was also shown to be a factor for gestational syphilis among women in maternity wards in a city in the Northeast region of Brazil [30]. Among blood donors in Chengdu, China, having two or more partners was associated with syphilis along with factors other than age [31].
These studies demonstrate that, along with other factors, the number of partners is an important factor in increasing exposure to T. pallidum [10, 28–31]. The recognition of the profile or vulnerability index of a population makes it possible to target specific strategies for each population [27–36]. Brazil has a national health system with universal access to all levels of health care that has been able to improve the health indicators of the most vulnerable populations, despite the great social inequality that still persists. More recently, the COVID-19 pandemic has demonstrated this importance and the need for public policies aimed at reducing social inequalities [32, 37].
In Brazil, the rapid test for syphilis should be offered in all basic health units, being an important screening strategy for remote populations, such as in the Amazon. The nurse is legally able to request the test, perform it and issue the report, but there is still uncertainty when delivering a reactive result [12]. For the riverside population studied, having a family health strategy team facilitates access to diagnosis and treatment, as well as actions aimed at prevention. Techno-assistance models riverine and river family health teams were strategies that are present in Brazil's National Primary Care Policy that led to the inclusion of a population that is dispersed over large areas of the municipalities' territory, consequently, they can expand access to HIV screening. syphilis and start treatment to interrupt the chain of transmission [16, 38].
Health professionals who work in these teams receive free training aimed at reducing syphilis. In Brazil, between February 2019 and September 2020, free training and lifelong learning strategies were offered to health professionals aimed at reducing syphilis, with about 22,000 students participating [39]. A previous study has already shown that the increase in the number of nurses was significantly associated with chronic diseases [40]. In this way, it may be able to minimize the vulnerability of access to health services, as observed in the study.
Among the limitations of the study is the sample calculation that was carried out using the number of inhabitants of the island aged 18 or over as a population parameter, as well as the sampling method was non-probabilistic. Another limitation was the cross-sectional observational design, which cannot establish a cause and effect relationship between the factors and syphilis, since a cohort was not carried out. The study did not address other (behavioral) variables that could be associated with syphilis. Generalizations must be made considering the techniques used in the analysis, in the detection of infection markers and in the population studied.
Conclusions
The results of the study demonstrate that the bacteria that causes syphilis circulates among the riverside population of the Brazilian Amazon. Social and individual factors associated with infection markers made it possible to understand the vulnerability profile of this population, which is important for directing specific care, such as health education on the transmission and prevention methods of syphilis for the young and adult population. In this context, it is important to detect the cultural aspects and lifestyle habits of the population.
Acknowledgements
We acknowledge the participants of this study and Primary Health Care teams.
Authors’ contributions
CCP, JJSG: contributed to acquisition and analysis of data, and manuscript drafting. CLFC, RARS, CYUPA, AMPCR: Designed the proposal, writes the first draft of the manuscript. EPB, GRONF*: Designed the proposal, analysis of data, interpretation of data, writes the first draft of the manuscript. All authors read and approved the manuscript.
Funding
This research was funded by Programa Nacional de Cooperação Acadêmica (PROCAD) Amazônia 2018/CAPES, grant number Processo no. 1699/2018 /88881.200527/2018–01 and The APC was funded by Pró-reitoria de Pesquisa e Pós- graduação of the Federal University of Para (PAPQ-2023).
Availability of data and materials
The datasets used during this current study are also available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the research ethics committee of the Institute of Health Sciences, Federal University of Pará, approved under protocol 3,331,577. All participants were informed about the objectives of the macroproject and the study. Data from participants who agreed to participate in the study and signed an informed consent form were included.
The study was organized by the Research Ethics Committee under authorization from the Municipal Health Department of Belém, as the study was carried out with the collaboration of the Combú family health team, whose community health agents who accompanied the researchers are residents of the area. Also, the test results were forwarded to the team nurse. All participants who agreed to participate in the study signed the free and informed consent form. The study was only started after approval by the Research Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen T, Wan B, Wang M, Lin S, Wu Y, Huang J. Evaluating the global, regional, and national impact of syphilis: results from the global burden of disease study 2019. Sci Rep. 2023;13(1):11386. doi: 10.1038/s41598-023-38294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil. Ministério da Saúde. Boletim epidemiológico 2023. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2023/boletim-epidemiologico-de-sifilis-numero-especial-out.2023. Accessed 02 Nov 2023.
- 3.Seara-Morais GJ, Pousada BF, Escaleira FF, Doi AM, Welter EAR, Avelino-Silva VI. Mobility restrictions during the COVID-19 pandemic and reduced outpatient HIV and syphilis testing in Brazil. Braz J Infect Dis. 2023;27(3):102771. doi: 10.1016/j.bjid.2023.102771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Castro Rocha DFN, da Cunha Rosa LR, de Almeida Silva C, de Oliveira BR, Martins TLS, Martins RMB, de Matos MA, Dos Santos Carneiro MA, Soares JP, de Oliveira E Silva AC, de Souza MM, Cook RL, Caetano KAA, Teles SA. Epidemiology of HIV, syphilis, and hepatitis B and C among manual cane cutters in low-income regions of Brazil. BMC Infect Dis. 2018;18(1):546. 10.1186/s12879-018-3439-4. [DOI] [PMC free article] [PubMed]
- 5.Shakya S, Thingulstad S, Syversen U, Nordbø SA, Madhup S, Vaidya K, Karmacharya BM, Åsvold BO, Afset JE. Prevalence of sexually transmitted infections among married women in Rural Nepal. Infect Dis Obstet Gynecol. 2018;2018:4980396. doi: 10.1155/2018/4980396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Yang Y, Wang Y, Yang D, Yang Y, Dong S, Li C, Chen Y, Jiang Q, Zhou Y. Prevalence and associated factors of Treponema pallidum infection in a rural area of southwestern China. BMC Public Health. 2020;20(1):824. doi: 10.1186/s12889-020-08952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza RL, Dos Santos Madeira LDP, Pereira MVS, da Silva RM, de Luna Sales JB, Azevedo VN, Feitosa RNM, Monteiro JC, de Oliveira Guimarães Ishak M, Ishak R, Ribeiro ALR, Oliveira-Filho AB, Machado LFA. Prevalence of syphilis in female sex workers in three countryside cities of the state of Pará, Brazilian Amazon. BMC Infect Dis. 2020;20(1):129. 10.1186/s12879-020-4850-1. [DOI] [PMC free article] [PubMed]
- 8.Chitneni P, Bwana MB, Muyindike W, Owembabazi M, Kalyebara PK, Byamukama A, Mbalibulha Y, Smith PM, Hsu KK, Haberer JE, Kaida A, Matthews LT. STI prevalence among men living with HIV engaged in safer conception care in rural, southwestern Uganda. PLoS ONE. 2021;16(3):e0246629. doi: 10.1371/journal.pone.0246629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapiga S, Hansen CH, Downs JA, Sichalwe S, Hashim R, Mngara J, van Dam GJ, Corstjens PLAM, Kingery JR, Peck RN, Grosskurth H. The burden of HIV, syphilis and schistosome infection and associated factors among adults in the fishing communities in northwestern Tanzania. Trop Med Int Health. 2021;26(2):204–213. doi: 10.1111/tmi.13520. [DOI] [PubMed] [Google Scholar]
- 10.Pereira Nogueira W, Figueiredo Nogueira M, de Almeida NJ, Freire MEM, Gir E, Silva ACOE. Syphilis in riverine communities: prevalence and associated factors. Rev Esc Enferm USP. 2022;56:e20210258. doi: 10.1590/1980-220X-REEUSP-2021-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS. Syphilis Nat Rev Dis Primers. 2017;3:17073. doi: 10.1038/nrdp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Araújo TCV, de Souza MB. Role of primary health care teams in rapid testing for sexually transmitted infections. Saúde debate. 2021;45(131):1075–1087. doi: 10.1590/0103-1104202113110I. [DOI] [Google Scholar]
- 13.Parmejiani EP, Queiroz ABA, Cunha MPL, Carvalho AL de O, dos Santos GS, Bezerra J da F, et al. Riverside men's knowledge and ways of acting regarding condom use. Texto contexto - enferm. 2022;31:e20220155. doi: 10.1590/1980-265X-TCE-2022-0155en. [DOI] [Google Scholar]
- 14.Ribeiro L, Moreira W, Batista-de-Carvalho A, Pitanga-de-Sousa M, Lopes-Carvalho M, Quezado-de-Castro T. Vulnerabilidades de pescadores de comunidades ribeirinhas às Infecções Sexualmente Transmissíveis. Revista Cubana de Enfermería. 2017; 33 (3) https://revenfermeria.sld.cu/index.php/enf/article/view/1231.
- 15.Garnelo L, Parente RCP, Puchiarelli MLR, Correia PC, Torres MV, Herkrath FJ. Barriers to access and organization of primary health care services for rural riverside populations in the Amazon. Int J Equity Health. 2020;19(1):54. doi: 10.1186/s12939-020-01171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freitas FLS, Benzaken AS, Passos MRL, Coelho ICB, Miranda AE. Brazilian protocol for sexually transmitted infections 2020: acquired syphilis. Rev Soc Bras Med Trop. 2021;54(suppl 1):e2020616. doi: 10.1590/0037-8682-616-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira GRON, Freitas FB, Queiroz MAF, Lima SS, Vallinoto ACR, de O GuimarãesIshak M, Ishak R. Epidemiology and risk factors for chlamydia trachomatis, treponema pallidum, hepatitis B virus and hepatitis C virus in the Marajó Archipelago, Brazilian Amazon. J Community Med Health Educ. 2019;9:643. [Google Scholar]
- 18.Machado LFA, Fonseca RRS, Queiroz MAF, Oliveira-Filho AB, Cayres-Vallinoto IMV, Vallinoto ACR, Ishak MOG, Ishak R. The epidemiological impact of STIs among general and vulnerable populations of the Amazon region of Brazil: 30 years of surveillance. Viruses. 2021;13(5):855. doi: 10.3390/v13050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garbin AJÍ, Martins RJ, Belila NM, Exaltação SM, Garbin CAS. Reemerging diseases in Brazil: sociodemographic and epidemiological characteristics of syphilis and its under-reporting. Rev Soc Bras Med Trop. 2019;52:e20180226. doi: 10.1590/0037-8682-0226-2018. [DOI] [PubMed] [Google Scholar]
- 20.Gama ASM, Fernandes TG, Parente RCP, Secoli SR. Inquérito de saúde em comunidades ribeirinhas do Amazonas, Brasil [A health survey in riverine communities in Amazonas State, Brazil] Cad Saude Publica. 2018;34(2):e00002817. doi: 10.1590/0102-311X00002817. [DOI] [PubMed] [Google Scholar]
- 21.Brasil. National Register of Health Establishments (Cadastro Nacional de Estabelecimento de Saúde, in Portuguese). Combu Family Health Unit. 2023. https://cnes.datasus.gov.br/pages/estabelecimentos/consulta.jsp. Accessed 30 Jan 2023.
- 22.Belém City Hall. https://codem.belem.pa.gov.br/wp-content/uploads/2021/06/Ilha-do-Combu.pdf.
- 23.Galvão JJDS, Cunha CLF, Pinho ECC, Paiva DJDS, de Castro NJC, Nascimento VGC, de Azevedo Junior WS, da Silva RAR, Feitosa RNM, Vallinoto ACR, Botelho EP, Ferreira GRON. Seroprevalence of Chlamydia trachomatis and associated factors among vulnerable riverine in the Brazilian Amazon. Int J Environ Res Public Health. 2022;19(23):15969. doi: 10.3390/ijerph192315969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasil. Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Departamento de DST, Aids e Hepatites Virais. Pesquisa de Conhecimento, atitudes e práticas na população Brasileira. 2013. http://antigo.aids.gov.br/pt-br/pub/2016/pesquisa-de-conhecimentos-atitudes-e-praticas-na-populacao-brasileira-pcap-2013. Accessed 15 July 2019.
- 25.Bioclin. https://quibasa.bioclin.com.br/anexos/INSTRUCOES_SIFILIS.pdf. Accessed 02 Nov 2023.
- 26.Labroclin. https://www.laborclin.com.br/wp-content/uploads/2023/03/170756.pdf. Accessed 02 Nov 2023.
- 27.Dias JA, Luciano TV, Santos MCLFS, Musso C, Zandonade E, Spano LC, Miranda AE. Infecções sexualmente transmissíveis em mulheres afrodescendentes de comunidades quilombolas no Brasil: prevalência e fatores associados [Sexually transmissible infections in African-descendant women in maroon communities in Brazil: prevalence and associated factors]. Cad Saude Publica. 2021;37(2):e00174919. Portuguese. 10.1590/0102-311X00174919. [DOI] [PubMed]
- 28.Liao KJ, Zhang SK, Liu M, Wang QM, Liu J, Shen HP, Zhang YP. Seroepidemiology of syphilis infection among 2 million reproductive-age women in rural china: a population-based. Cross-Sectional Study Chin Med J (Engl) 2017;130(18):2198–2204. doi: 10.4103/0366-6999.213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banong-le M, Ofosu SK, Anto F. Factors associated with syphilis infection: a cross-sectional survey among outpatients in Asikuma Odoben Brakwa District, Ghana. BMC Infect Dis. 2019;19(1):360. doi: 10.1186/s12879-019-3967-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macêdo VC, Lira PIC, Frias PG, Romaguera LMD, Caires SFF, Ximenes RAA. Risk factors for syphilis in women: case-control study. Rev Saude Publica. 2017;51:78. doi: 10.11606/S1518-8787.2017051007066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Luo L, Xi G, Wan L, Zhong L, Chen X, Gong T, Li S, He Y, Li N. Seroprevalence and risk factors on Syphilis among blood donors in Chengdu, China, from 2005 to 2017. BMC Infect Dis. 2019;19(1):509. doi: 10.1186/s12879-019-4128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zachek CM, Coelho LE, Domingues RMSM, Clark JL, De Boni RB, Luz PM, Friedman RK, de Andrade ÂCV, Veloso VG, Lake JE, Grinsztejn B. The intersection of HIV, social vulnerability, and reproductive health: analysis of women living with HIV in Rio de Janeiro, Brazil from 1996 to 2016. AIDS Behav. 2019;23(6):1541–1551. doi: 10.1007/s10461-019-02395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayres JR, Paiva V, França I, Jr, Gravato N, Lacerda R, Della Negra M, Marques HH, Galano E, Lecussan P, Segurado AC, Silva MH. Vulnerability, human rights, and comprehensive health care needs of young people living with HIV/AIDS. Am J Public Health. 2006;96(6):1001–1006. doi: 10.2105/AJPH.2004.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mah JC, Penwarden JL, Pott H, Theou O, Andrew MK. Social vulnerability indices: a scoping review. BMC Public Health. 2023;23(1):1253. doi: 10.1186/s12889-023-16097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comins CA, Rucinski KB, Baral S, Abebe SA, Mulu A, Schwartz SR. Vulnerability profiles and prevalence of HIV and other sexually transmitted infections among adolescent girls and young women in Ethiopia: a latent class analysis. PLoS ONE. 2020;15(5):e0232598. doi: 10.1371/journal.pone.0232598.Erratum.In:PLoSOne.2020Jul23;15(7):e0236910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dailey AF, Gant Z, Hu X, Johnson Lyons S, Okello A, Satcher JA. Association Between social vulnerability and rates of HIV diagnoses among black adults, by selected characteristics and region of residence - United States, 2018. MMWR Morb Mortal Wkly Rep. 2022;71(5):167–170. doi: 10.15585/mmwr.mm7105a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho AR, Souza LR, Gonçalves SL, Almeida ERF. Social vulnerability and health crisis in Brazil. Cad Saude Publica. 2021;37(9):e00071721. doi: 10.1590/0102-311X00071721. [DOI] [PubMed] [Google Scholar]
- 38.Lima RTS, Fernandes TG, Martins Júnior PJA, Portela CS, Santos Junior JDOD, Schweickardt JC. Health in sight: an analysis of primary health care in riverside and rural Amazon areas. Cien Saude Colet. 2021;26(6):2053–2064. doi: 10.1590/1413-81232021266.02672021. [DOI] [PubMed] [Google Scholar]
- 39.de Moraispinto R, de Medeiros Valentim RA, Fernandes da Silva L, Góis Farias de Moura Santos Lima T, Kumar V, Pereira de Oliveira CA, Martins Gomes de Gusmão C, de Paiva JC, de Andrade I. Analyzing the reach of public health campaigns based on multidimensional aspects: the case of the syphilis epidemic in Brazil. BMC Public Health. 2021;21(1):1632. doi: 10.1186/s12889-021-11588-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kodama S, Uwatoko F, Koriyama C. Relationship between changes in the public health nurses' workforce and the empirical Bayes estimates of standardized mortality ratio: a longitudinal ecological study of municipalities in Japan. BMC Health Serv Res. 2023;23(1):266. doi: 10.1186/s12913-023-09273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during this current study are also available from the corresponding author on reasonable request.