Abstract
Background
Substantial variation exists when performing a minimally invasive right hemicolectomy (MIRH) due to disparities in training, expertise and differences in implementation of innovations. This study aimed to achieve national consensus on an optimal and standardized MIRH technique for colon cancer and to develop and validate a video-based competency assessment tool (CAT) for MIRH.
Method
Statements covering all elements of MIRH were formulated. Subsequently, the Delphi technique was used to reach consensus on a standardized MIRH among 76 colorectal surgeons from 43 different centres. A CAT was developed based on the Delphi results. Nine surgeons assessed the same 12 unedited full-length videos using the CAT, allowing evaluation of the intraclass correlation coefficient (ICC).
Results
After three Delphi rounds, consensus (≥80% agreement) was achieved on 23 of the 24 statements. Consensus statements included the use of low intra-abdominal pressure, detailed anatomical outline how to perform complete mesocolic excision with central vascular ligation, the creation of an intracorporeal anastomosis, and specimen extraction through a Pfannenstiel incision using a wound protector. The CAT included seven consecutive steps to measure competency of the MIRH and showed high consistency among surgeons with an overall ICC of 0.923.
Conclusion
Nationwide consensus on a standardized and optimized technique of MIRH was reached. The CAT developed showed excellent interrater reliability. These achievements are crucial steps to an ongoing nationwide quality improvement project (the Right study).
The Delphi technique was employed to achieve a broad national consensus on 23 statements among 76 colorectal surgeons, defining a standardized MIRH. This standard includes complete mesocolic excision, intracorporeal anastomosis and Pfannenstiel incision using a wound protector. Building upon this consensus, a video-based competency assessment tool was developed, exhibiting excellent reliability.
Introduction
Surgical procedures are prone to variations in execution and outcome, influenced by differences in training, experience and variances in implementation of innovations, along with patient and tumour-related parameters1,2. Although of different aetiologies, these variations might be associated with a negative impact on patient outcomes including morbidity, mortality and cancer recurrence3–6. In both colon and rectal cancer, it has been shown that the quality and extent of the surgical resection is directly related to cancer-specific outcomes4,6,7.
Minimally invasive right hemicolectomy (MIRH) is the mainstay of curative treatment for patients with right-sided colon cancer and is one of the most frequently performed colorectal procedures worldwide. Surgical research has focused on refining the procedure through innovations such as low intra-abdominal pressure (IAP), complete mesocolic excision (CME), central vascular ligation (CVL), intracorporeal anastomosis and avoidance of midline extractions by using a Pfannenstiel incision. Each of these developments can benefit short- and/or long-term outcomes8–10. Despite published evidence and guidelines, a significant variability in the implementation of these innovations persists among centres and surgeons.
To reduce unwarranted variations in practice and thereby improve clinical outcomes for a large patient population, there is a need to implement optimized and standardized surgical techniques for resecting right-sided colon cancer, which will hopefully thereafter be widely endorsed by the surgical community11,12. Standardization not only facilitates more efficient training, but can also result in optimized learning curves13. Furthermore, incorporation of a validated competency assessment tool (CAT) enables objective and quantifiable surgical quality assessment (SQA) with detailed feedback for individual surgeons, potentially leading to improved clinical outcomes14.
In response to these challenges, a national large-scale quality improvement program for MIRH was recently launched in the Netherlands (the Right study)15. As part of this project, the objectives of the current study are to standardize and optimize MIRH by reaching consensus regarding all the surgical key elements using the Delphi method, and to develop and validate a video-based CAT to quantify surgical performance and facilitate implementation of the standardized MIRH.
Methods
The study adhered to the reporting guidelines outlined in the Standards for Quality Improvement Reporting Excellence (SQUIRE).
Standardization of minimally invasive right hemicolectomy (Delphi method)
Delphi methodology was used as a structured process that combines the knowledge gathered through several rounds of expert meetings in order to reach consensus on a standardized MIRH16. Based on literature review, published guidelines and expert opinion, statements regarding the key consecutive surgical steps in performing MIRH were formulated by the Right study team (A.A.J.G., B.R.T., P.J.T. and J.B.T.). Two colorectal surgeons from each of the 43 Dutch centres participating in the Right study were invited for a three-round Delphi consensus process. During rounds one and two, panel members voted anonymously on whether they agreed or disagreed with statements using an electronic survey (Google Forms). In case of disagreement, each surgeon had the opportunity to provide specific reasons for their dissent through free-text comments. Panel members who missed one round were invited to participate in subsequent rounds. The statements were refined based on the level of agreement and comments on the statements by the participants during an interactive workshop. For the purpose of the present study that aimed to reach broad support at a national level, consensus was defined as at least 80% agreement. Statements with less than 80% agreement were revised and/or supplementary explanations based on existing literature were provided by the research team in the next round.
In the final round, the remaining statements with less than 80% agreement were discussed with the panel members during a physical meeting. Following this discussion, all participating surgeons could vote anonymously to agree or disagree with the remaining statements. The study team guided the entire Delphi process, which included correspondence with the participating surgeons, development and maintenance of the electronic surveys, managing the final physical round and data collection.
Development and validation of video-based competency assessment tool
Based on the results of the Delphi consensus process, the initial version of the video-based procedure-specific CAT was drafted by A.A.J.G. Subsequently, the Right study team provided feedback through multiple rounds, resulting in the first version of the CAT. An existing format of a CAT, assessing surgical procedures step-by-step for exposure, execution, adverse events and end-product quality, was utilized as a template13,17. Each component received a score ranging from 1 to 4, representing risky to skilled execution.
Content validation in this study was conducted through multiple rounds of assessment using four full-length unedited MIRH videos sourced from the Right study’s database, with one video being assessed in every iteration. Following each assessment round, the Right proctor group discussed the evaluation of each step of the CAT, and modifications to the CAT were made as necessary until full consensus was reached. Before the final validity assessment of the CAT, a training session was organized with the entire Right proctor group. This group consisted of 11 colorectal surgeons (J.B.T., P.J.T., B.R.T., E.H.J.B., E.J.T.B., P.v.D., C.H., R.H., A.B.S., A.W.H.v.d.V. and H.L.v.W.) who have had a train-the-trainer session and were assigned as proctors for the future implementation phase of the Right study. For the interrater validity assessment, 12 full-length unedited MIRH videos from the Right study’s database were assessed by the Right proctor group. The first five videos were non-CME procedures and the last seven videos were CME-based procedures. Step 1 (setup and exposure of operating field) and the extracorporeal anastomosis were not included in the analyses as these steps were often not captured in the videos. All videos were assessed on an online platform specifically designed for the Right study (https://asc.amsterdam/sqa-amsterdam/).
Statistical analysis
Google Forms Survey and Microsoft Excel 2016 were used to display data. IBM’s SPSS statistics 28 was used for all statistical analyses. The intraclass correlation coefficient (ICC), with a two-way random, absolute and average measures effects model, on a two-tailed significance level of P < 0.05, was used to evaluate the interobserver reliability for the overall score as well as for the scores of the individual steps of the CAT. An ICC value of <0.4 was considered to be poor reliability, whereas values of ICC 0.4–0.6, 0.6–0.8 and >0.8 were considered as fair, good and excellent reliability, respectively18.
Results
Standardization of minimally invasive right hemicolectomy (Delphi method)
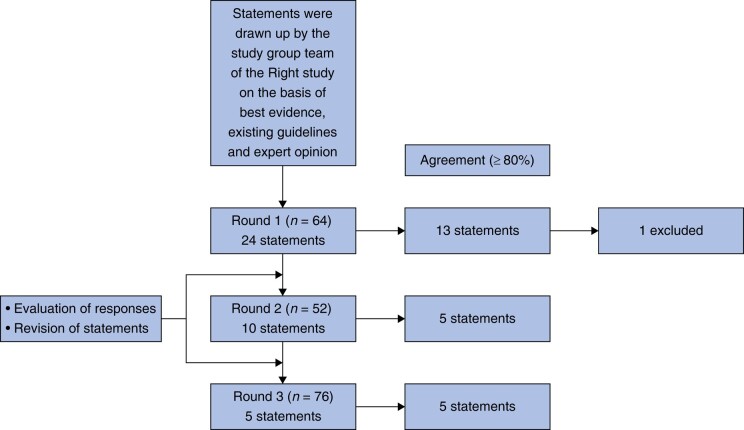
Figure 1 provides an overview of the Delphi process, involving 76 participating surgeons. After the three rounds, consensus (≥80% agreement) had been achieved on 23 of the 24 statements. Statement 18 (‘It is recommended to use clips for vessel ligation or to place stitches at least on the specimen site (for orientation of the specimen)’) was removed after the first round, because it was deemed outside the scope of this consensus process. It was felt not to improve the surgical procedure, but rather improves orientation for the pathologist. Table 1 presents the results of the Delphi study, including all final statements that reached consensus, along with the adjustments made after round one. Files S1–S3 present each statement of every round, with attached pictures to some statements, accompanied by substantiations and relevant literature for the second round.
Fig. 1.
Flowchart of the Delphi process
Table 1.
Results of the Delphi study with final statements (including adjustments compared to the statement in round 1) and percentage of agreement per round
| # | Final statement (including the adjustments compared to the statement in round 1) | Agreement round 1–2–3 (%) |
|---|---|---|
| 1 | Preoperatively, it is (mandatory) recommended to review the CT scan for central vascular ligation as part of a D2 lymph node dissection (colonic vascular anatomy.At least the ileocolic vessel configuration in relation to the superior mesenteric vein (SMV), presence of a right colic artery and configurations of Gastrocolic Trunk and middle colic vessels need to be assessed) | 73.4–78.8–98.7 |
| 2 | Preoperatively, it is mandatory to assess the CT scan for suspicious central (D3) lymph nodes and location of the tumour. | 96.9 |
| 3 | In case of a (hepatic flexure tumour or) proximal transverse colon tumour, it is recommended to plan a procedure including central ligation of the middle colic vessels (extended right hemicolectomy). | 56.3–57.7–93.4 |
| 4 | In the presence of highly suspicious central (D3) positive lymph nodes on the CT scan, it is mandatory to perform a formal D3 resection. In case of lack of relevant experience, then (or to) refer the patient to a centre of expertise. | 78.1–90.4 |
| 5 | It is recommended to use the French position (supine split-leg position) allowing both options to operate from the left side or from the position between the legs facilitating central lymphadenectomy (is recommendedusing the conventional laparoscopic approach). | 29.7–46.2–90.8 |
| 6 | It is recommended to use 4 trocars, optional 5 trocars. | 89.1 |
| 7 | It is recommended to place the trocars with the aim to optimally expose the SMV for the purpose of central vascular ligation (D2 lymphadenectomy). | 71.9–86.5 |
| 8 | It is recommended to insufflate the abdomen using a (low-pressure) pneumoperitoneum of 8–12(8-10) mmHg, unless intraoperative conditions require another pressure level. | 68.8–80.8 |
| 9 | It is mandatory to inspect the peritoneum and liver surface for metastases and try to identify the location of the primary tumour before the start of the dissection. | 98.4 |
| 10 | It is mandatory to identify the avascular plane between the visceral peritoneum of the mesentery and the retroperitoneum either through the ileal mesentery or by a subileal approach. | 96.9 |
| 11 | It is mandatory to dissect and expose the descending part of the duodenum and the pancreatic head before moving onto the dissection of the SMV. | 81.3 |
| 12 | It is mandatory to continue the dissection in the avascular plane between the visceral peritoneum of the mesentery and the retroperitoneum as far as possible underneath the ascending and transverse colon. | 93.8 |
| 13 | It is mandatory to visualize and dissect the anterior aspect of the SMV both proximal and distal to the origin of the ileocolic vessels before ligation (critical view of safety). | 50.0–57.7–98.7 |
| 14 | It is mandatory to perform a central ligation of the ileocolic vessels at the level of the right lateral border of the SMV. | 82.8 |
| 15 | It is mandatory to continue dissection in a cranial direction on the anterolateral (anterior) side of the SMV (and identify the trunk of Henle), after ligation of the ileocolic vessels to include all mesenteric tissue lateral from the SMV to facilitate complete mesocolic excision (CME). | 45.3–59.6–94.7 |
| 16 | It is recommended to preserve the gastroepiploic vein and pancreaticoduodenal superior anterior vein during dissection of Henle’s trunk. | 87.5 |
| 17 | It is mandatory to dissect and perform a central ligation of the right colic vessels and right branches (black) or the common trunk (white) of middle colic vessels according to the location of the tumour. | 89.1 |
| 18 | It is recommended to use clips for vessel ligation or to place stitches at least on the specimen site (for orientation of the specimen). | 62.5 |
| 19 | It is recommended to separate the omentum and mesogastrium from the transverse colon in order to facilitate the transection of the transverse mesentery. | 95.3 |
| 20 | It is mandatory to ensure enough mobility of the terminal ileum to perform a tension-free anastomosis. | 100 |
| 21 | It is recommended to have a longitudinal colonic resection margin of at least 5 cm. | 90.6 |
| 22 | It is mandatory to perform an intracorporeal anastomosis. | 62.5–84.6 |
| 23 | It is mandatory to extract the specimen through the Pfannenstiel incision. | 65.6–80.8 |
| 24 | It is mandatory to use a wound protector during the extraction of the specimen. | 100 |
The italic text in parantheses is text removed compared to the text in round 1 and the underlined text is added text compared to the same text in round 1.
SMV, superior mesenteric vein; CME, complete mesocolic excision.
The statements that reached consensus together constitute a document that describes the optimized and standardized MIRH. This procedure includes low IAP, a comprehensive anatomical delineation of the CME technique with CVL and lymphadenectomy described with multiple steps, an intracorporeal anastomosis and extraction of the specimen via a Pfannenstiel incision, while utilizing a wound protector.
Development of video-based competency assessment tool
The results of the Delphi study were incorporated into the initial version of the video-based CAT. A.A.J.G. created a preliminary version, and members of the Right study team (A.A.J.G., J.B.T., P.J.T., and B.R.T.) provided feedback in five different rounds, resulting in the first version of the CAT. Subsequent assessments of four videos (one per round) by the Right proctor group, with feedback provided after each round, eventually led to the final CAT (Fig. 2 and File S4). The CAT comprises seven steps: (1) setup and exposure of operating field, (2a) submesenteric dissection through the ileal mesentery (medial-to-lateral) or (2b) subileal mesenteric dissection (caudal-to-cranial), (3) superior mesenteric vein (SMV) dissection and ligation of ileocolic vessels, (4) proximal dissection of the SMV and dissection of Gastrocolic Trunk (GCT) of Henle, (5a) ligation of right branches of middle colic vessels or (5b) ligation of middle colic vessels in cases of a transverse colon tumour, (6) hepatic flexure mobilization and colon and ileum transection and (7) anastomosis. In both steps 2 and 5, either option a or b must be selected. Step 5a applies when the tumour is located in the caecum, ascending colon or hepatic flexure, while step 5b is relevant for tumours located in the transverse colon. Detailed explanation in each step is given concerning how the procedural step has to be evaluated with a score (1–4).
Fig. 2.
Video-based competency assessment tool of minimally invasive right hemicolectomy, step 3
Validation of video-based competency assessment tool
Nine expert colorectal surgeons assessed the 12 videos on four items (exposure, execution, adverse events and end-product quality) within each of the six steps. Because an extracorporeal anastomosis was performed in one of the 12 videos, a total of 2 556 scores were obtained (9×6×4×11 + 9×5×4 = 2 376 + 180).
Table 2 displays the total ICC and the ICC scores for each step of the CAT. The total ICC score for all ratings by the nine proctors of all videos was 0.923, indicating excellent interrater reliability. Steps 2, 3 and 4 achieved excellent reliability, with respective scores of 0.891, 0.957 and 0.953. Steps 5, 6 and 7 showed good reliability based on scores of 0.796, 0.734 and 0.661, respectively.
Table 2.
The total ICC and the ICC per step of the video-based CAT
| Step | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 2–7 (total) |
|---|---|---|---|---|---|---|---|---|
| ICC | NA | 0.891 | 0.957 | 0.953 | 0.796 | 0.734 | 0.661 | 0.923 |
ICC, intraclass correlation coefficient; CAT, competency assessment tool; NA, not applicable.
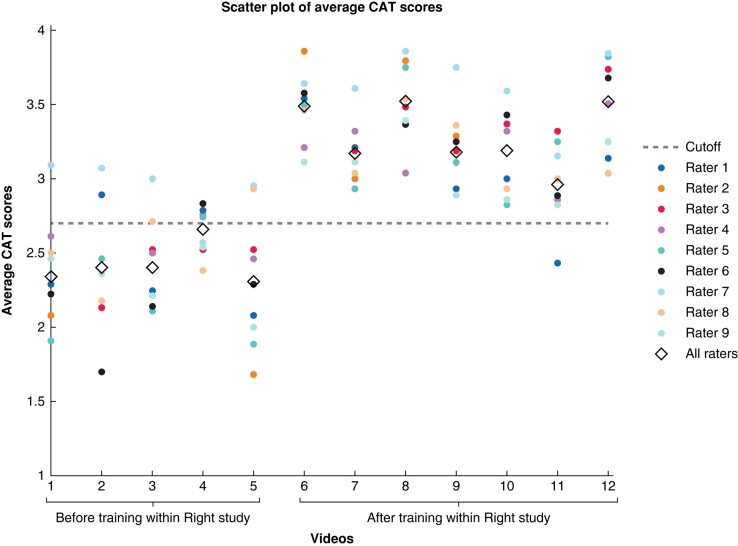
Figure 3 presents the average CAT scores per video per rater and the combined average score for all raters. A horizontal line was added to the figure at the performance-related score of 2.7, which is recognized in the literature as the established cutoff point indicative of a qualitatively well-executed operation13.
Fig. 3.
Scatterplot of the average competency assessment tool (CAT) scores per rater and average score of all raters per video with the cutoff value of 2.7
Discussion
MIRH to treat patients with right-sided colon cancer has been optimized and standardized into 23 detailed steps. Standardization was achieved through a Delphi process, utilizing best evidence and broad national expert consensus. Key elements of this standardized MIRH include low IAP (8-12 mmHg), detailed definition and execution of steps to achieve CME with CVL (in which both the medial-to-lateral and caudal-to-cranial approaches can be used), an intracorporeal anastomosis and specimen extraction through a Pfannenstiel incision while using a wound protector. The consensus-based description of MIRH was the basis for the development of a video-based procedure-specific SQA tool, of which the content and interrater reliability were validated. The tool reached excellent reliability based on an ICC > 0.8, and will be used during further execution of the Right project to evaluate the implementation of the standardized MIRH and as a quantitative measure to assess surgical quality, which will ultimately be correlated with clinical outcomes.
The current consensus-based description of MIRH includes all established elements that could potentially contribute to better clinical outcomes and extends beyond solely focusing on CME. In addition, its broad support among the majority of Dutch centres will allow for nationwide implementation of the standardized MIRH. This standardization has focused on both laparoscopic and robot-assisted right hemicolectomy. While certain aspects of the standardized MIRH were already incorporated in Dutch colorectal cancer guideline recommendations, the reality shows a lack of implementation of these recommendations. The first phase of the Right study that preceded the Delphi consensus process clearly demonstrated a lack of implementation, especially the CME technique, despite recommendation in the colorectal cancer national guidelines (data to be published). The explanation for failed implementation of CME probably relate to controversy in the literature surrounding the terms CME, CVL and D2/D3 dissection, but also to a large extent due to a lack of surgical training and proctoring programmes for consultant surgeons who have to adapt their technique19. The Right study has incorporated all essential elements to successfully change surgical practice at a national level that should lead to large-scale quality improvement that can be trained in a standardized way and subsequently assessed in a quantitative way using the CAT.
A study by Bertelsen et al. has shown that CME leads to improved long-term oncological outcomes for stage I–III colon cancer patients without increasing morbidity20. In the current standardization, the necessary steps to achieve a specimen with an intact duodenal mesenteric window including the surgical trunk with D2 lymph nodes (Benz type 0 specimen) have been described in detail21. Despite this, controversy about the superiority of CME exists, especially because Benz et al. have shown that CME was not associated with better survival in a prospective multicentre cohort study22. However, standardization and robust surgical quality control and/or competency assessment were missing in this surgical trial. Other procedure-specific steps were incorporated in our standardized MIRH besides CME; a recent RCT has also shown that low IAP (8 mmHg), compared with normal IAP (12 mmHg), showed lower acute pain scores, reduction in 30-day infectious complications, reduced surgical site hypoxia and inflammations markers, lower postoperative cytokine production and a higher quality of recovery score23. Additionally, the intracorporeal anastomosis has been associated with improved postoperative outcomes compared to the extracorporeal anastomosis8,24–30. Furthermore, extracting the specimen through a Pfannenstiel incision offers a lower risk of incisional hernia and other complications compared to both the transverse and midline extraction10,31–33. The authors anticipate that the current optimized and standardized MIRH description including all these elements can improve outcomes if properly implemented in daily practice. Therefore, after having completed phase 1 of the Right study (control cohort in which surgeons performed MIRH as they always did), phase 2 (the Delphi consensus process to define a standardized MIRH) and phase 3 (training of the standardized MIRH), phase 4 of the Right study has been reached: the guided implementation of the optimized and standardized MIRH with proctoring34. After phase 5 (a consolidation phase in which patients are still registered but without proctoring), clinical outcomes will be evaluated and compared to the different phases of the study.
Standardization in surgery has been shown to improve clinical outcomes and to accelerate learning curves11,35,36. The surgical standardization enhances patient safety by highlighting potential risks and allows more focused discussion and subsequent motivated change in the procedure. It also supports training and surgical education with a more consistent stepwise training programme that can be evaluated11. Overall, surgical standardization might play a vital role in improving patient outcomes and advancing surgical practice. Additionally, standardization of the surgical procedure is crucial to enable reliable surgical comparative research when combined with a CAT, enabling evaluation of the quality of the surgical intervention(s)37,38. The literature consistently demonstrates the profound influence of surgical quality on clinical outcomes3,4,6,35,39,40. A national training programme for specialist colorectal surgeons in England (Lapco), using competency-based supervised clinical training, showed reduced mortality and morbidity11. By receiving feedback using SQA scores after MIRH, surgeons can identify areas of strength and weakness in their surgical care processes that could potentially lead to higher competency levels faster, which is expected to translate into improved clinical outcomes.
The feasibility, content validity and reliability of the CAT have been examined in this study. Ultimately, clinical validation of competency assessment will be evaluated in the Right study, involving more than 1000 patients, with the potential to demonstrate that higher CAT scores are associated with better clinical outcomes. Currently, the training sessions of the Right study have been completed, and the implementation phase with proctoring is underway. Subsequently, the construct validity of the CAT can be evaluated by determining whether the scores of the trained surgeons have actually improved after this training and proctoring.
The findings of this study have several important implications for future practice. If the Right study shows improvement in quality of care after implementing the standardized MIRH, it will underscore the importance of robust national educational programmes and the potential for eliminating unwarranted variations in oncological procedures. Assuming feasibility, this concept can be extrapolated to many other surgical procedures, in which a Delphi consensus process based on best evidence and expert opinion and its translation into a CAT are used to determine and train a standardized surgical technique supported by SQA.
Collaborators
Sanne van Aalten, Frits Aarts, Gabor S.A. Abis, Caroline S. Andeweg, Astrid H. Baan, Coen I. M. Baeten, Okan Bastian, Juliette Blauw, Marjolein Blussé van Oud-Alblas, Frank C. den Boer, Evert-Jan G. Boerma, Matthijs D. M. Bolmers, Robbert J. I. Bosker, Steve M. M. de Castro, Ivan M. Cherepanin, Stefan H. E. M. Clermonts, Usha K. Coblijn, Ahmet Demirkiran, Yassmina Derraze, Robert Dijkstra, Youssef El-Massoudi, Jeroen A. van Essen, Danny J. Evers, Hans F. J. Fabry, Sofie Fransen, Hauwy Goei, Jan Gooszen, Johannes Govaert, Frederike A. B. Grimme, Brechtje Grotenhuis, Anne den Hartog, Tjarda van Heek, Jeroen Heemskerk, Bob H. M. Heijnen, Cas D. P. van ‘t Hullenaar, Gabie M. de Jong, Frederik H. W. Jonker, Martin R. Ketting, Jordy J. S. Kiewiet, Joop L. M. Konsten, Sietze A. Koopal, Robert T. J. Kortekaas, Emmanuel Lagae, Bas Lamme, Tanja Lettinga, Harold E. Lont, Tim Lubbers, Hendrik A. Marsman, Dietrich J. L. de Mey, Daan E. Moes, Peter A. Neijenhuis, Lindsey C. F. de Nes, Joost Nonner, Jikke M. T. Omloo, Steven J. Oosterling, Bas Polle, Apollo Pronk, Rutger-Jan Renger, Marnix A. J. de Roos, Jeroen E. Rütter, Arjan P. Schouten van der Velden, Ernst J. Spillenaar Bilgen, Ernst J. A. Steller, Hein B. A. C. Stockmann, Jan H. M. B. Stoot, Yuk K. Sze, Koen Talsma, Sanne C. Veltkamp, Tim Verhagen, Paul M. Verheijen, Maarten Vermaas, Wouter J. Vles, Robert J. de Vos tot Nederveen Cappel, Dareczka K. Wasowicz, Marinke Westerterp, Kevin P. Wevers, Carlijn D. M. Witjes, Frans T. W. E. van Workum, Ronald J. Zijlstra, David D. E. Zimmerman.
Supplementary Material
Contributor Information
Alexander A J Grüter, Department of Surgery, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Treatment and Quality of Life, Cancer Center Amsterdam, Amsterdam, The Netherlands.
Boudewijn R Toorenvliet, Department of Surgery, Ikazia Hospital, Rotterdam, The Netherlands.
Eric H J Belgers, Department of Surgery, Zuyderland Medisch Centrum, Heerlen, The Netherlands.
Eric J T Belt, Department of Surgery, Albert Schweitzer Ziekenhuis, Dordrecht, The Netherlands.
Peter van Duijvendijk, Department of Surgery, Gelre Hospitals, Apeldoorn, The Netherlands.
Christiaan Hoff, Department of Surgery, Medisch Centrum Leeuwarden, Leeuwarden, The Netherlands.
Roel Hompes, Department of Surgery, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands.
Anke B Smits, Department of Surgery, St.Antonius Ziekenhuis, Nieuwegein, The Netherlands.
Anthony W H van de Ven, Department of Surgery, Flevoziekenhuis, Almere, The Netherlands.
Henderik L van Westreenen, Department of Surgery, Isala, Zwolle, The Netherlands.
Hendrik J Bonjer, Department of Surgery, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Pieter J Tanis, Department of Surgery, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands; Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus MC, Rotterdam, The Netherlands.
Jurriaan B Tuynman, Department of Surgery, Amsterdam UMC location Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
the Right collaborators group:
Sanne van Aalten, Frits Aarts, Gabor S A Abis, Caroline S Andeweg, Astrid H Baan, Coen I M Baeten, Okan Bastian, Juliette Blauw, Marjolein Blussé van Oud-Alblas, Frank C den Boer, Evert-Jan G Boerma, Matthijs D M Bolmers, Robbert J I Bosker, Steve M M de Castro, Ivan M Cherepanin, Stefan H E M Clermonts, Usha K Coblijn, Ahmet Demirkiran, Yassmina Derraze, Robert Dijkstra, Youssef El-Massoudi, Jeroen A van Essen, Danny J Evers, Hans F J Fabry, Sofie Fransen, Hauwy Goei, Jan Gooszen, Johannes Govaert, Frederike A B Grimme, Brechtje Grotenhuis, Anne den Hartog, Tjarda van Heek, Jeroen Heemskerk, Bob H M Heijnen, Cas D P van ‘t Hullenaar, Gabie M de Jong, Frederik H W Jonker, Martin R Ketting, Jordy J S Kiewiet, Joop L M Konsten, Sietze A Koopal, Robert T J Kortekaas, Emmanuel Lagae, Bas Lamme, Tanja Lettinga, Harold E Lont, Tim Lubbers, Hendrik A Marsman, Dietrich J L de Mey, Daan E Moes, Peter A Neijenhuis, Lindsey C F de Nes, Joost Nonner, Jikke M T Omloo, Steven J Oosterling, Bas Polle, Apollo Pronk, Rutger-Jan Renger, Marnix A J de Roos, Jeroen E Rütter, Arjan P Schouten van der Velden, Ernst J Spillenaar Bilgen, Ernst J A Steller, Hein B A C Stockmann, Jan H M B Stoot, Yuk K Sze, Koen Talsma, Sanne C Veltkamp, Tim Verhagen, Paul M Verheijen, Maarten Vermaas, Wouter J Vles, Robert J de Vos tot Nederveen Cappel, Dareczka K Wasowicz, Marinke Westerterp, Kevin P Wevers, Carlijn D M Witjes, Frans T W E van Workum, Ronald J Zijlstra, and David D E Zimmerman
Author contributions
Alexander Grüter (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing—original draft, Writing—review & editing), Boudewijn Toorenvliet (Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing), Eric H. Belgers (Investigation, Resources, Writing—review & editing), Eric Belt (Investigation, Resources, Writing—review & editing), Peter Van Duyvendijk (Investigation, Resources, Writing—review & editing), Christiaan Hoff (Investigation, Resources, Writing—review & editing), Roel Hompes (Investigation, Resources, Writing—review & editing), Anke Smits (Investigation, Resources, Writing—review & editing), Anthony van de Ven (Investigation, Resources, Writing—review & editing), H van Westreenen (Investigation, Resources, Writing—review & editing), H. Jaap Bonjer (Project administration, Supervision, Writing—review & editing), Pieter Tanis (Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing), and Jurriaan B. Tuynman (Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing)
Funding
We have not received funding for this work. However, Medtronic and Ethicon have provided financial support for the hands-on training and proctoring of the standardized technique. It is important to note that their support was provided without exerting any influence over the standardized procedure.
Disclosure
The authors declare there are no conflicts of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
This study data are not openly available. The authors are willing to share the data upon reasonable request.
References
- 1. Roberts DJ, Zygun DA, Ball CG, Kirkpatrick AW, Faris PD, James MTet al. Challenges and potential solutions to the evaluation, monitoring, and regulation of surgical innovations. BMC Surg 2019;19:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet 2013;382:1121–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Birkmeyer JD, Finks JF, O’Reilly A, Oerline M, Carlin AM, Nunn ARet al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013;369:1434–1442 [DOI] [PubMed] [Google Scholar]
- 4. Curtis NJ, Foster JD, Miskovic D, Brown CSB, Hewett PJ, Abbott Set al. Association of surgical skill assessment with clinical outcomes in cancer surgery. JAMA Surg 2020;155:590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heiden BT, Eaton DB, Chang S-H, Yan Y, Baumann AA, Schoen MWet al. Association between surgical quality metric adherence and overall survival among US veterans with early-stage non-small cell lung cancer. JAMA Surg 2023;158:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stulberg JJ, Huang R, Kreutzer L, Ban K, Champagne BJ, Steele SRet al. Association between surgeon technical skills and patient outcomes. JAMA Surg 2020;155:960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brajcich BC, Stulberg JJ, Palis BE, Chung JW, Huang R, Nelson Het al. Association between surgical technical skill and long-term survival for colon cancer. JAMA Oncol 2021;7:127–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Oostendorp S, Elfrink A, Borstlap W, Schoonmade L, Sietses C, Meijerink Jet al. Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: a systematic review and meta-analysis. Surg Endosc 2017;31:64–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferri V, Vicente E, Quijano Y, Duran H, Diaz E, Fabra Iet al. Right-side colectomy with complete mesocolic excision vs conventional right-side colectomy in the treatment of colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2021;36:1885–1904. (CME) [DOI] [PubMed] [Google Scholar]
- 10. Lee L, Abou-Khalil M, Liberman S, Boutros M, Fried GM, Feldman LS. Incidence of incisional hernia in the specimen extraction site for laparoscopic colorectal surgery: systematic review and meta-analysis. Surg Endosc 2017;31:5083–5093 [DOI] [PubMed] [Google Scholar]
- 11. Hanna GB, Mackenzie H, Miskovic D, Ni M, Wyles S, Aylin Pet al. Laparoscopic colorectal surgery outcomes improved after national training program (LAPCO) for specialists in England. Ann Surg 2022;275:1149–1155 [DOI] [PubMed] [Google Scholar]
- 12. Wyles SM, Miskovic D, Ni Z, Darzi AW, Valori RM, Coleman MGet al. Development and implementation of the Structured Training Trainer Assessment Report (STTAR) in the English National Training Programme for laparoscopic colorectal surgery. Surg Endosc 2016;30:993–1003 [DOI] [PubMed] [Google Scholar]
- 13. Miskovic D, Ni M, Wyles SM, Kennedy RH, Francis NK, Parvaiz Aet al. Is competency assessment at the specialist level achievable? A study for the national training programme in laparoscopic colorectal surgery in England. Ann Surg 2013;257:476–482 [DOI] [PubMed] [Google Scholar]
- 14. Gruter AAJ., Van Lieshout AS, van Oostendorp SE, Henckens SPG, Ket JCF, Gisbertz SSet al. Video-based tools for surgical quality assessment of technical skills in laparoscopic procedures: a systematic review. Surg Endosc 2023;37:4279–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gruter AAJ, Coblijn UK, Toorenvliet BR, Tanis PJ, Tuynman JB, Aselmann Het al. National implementation of an optimal standardised technique for right-sided colon cancer: protocol of an interventional sequential cohort study (Right study). Tech Coloproctol 2023;27:1083–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One 2011;6:e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsai AY, Mavroveli S, Miskovic D, van Oostendorp S, Adamina M, Hompes Ret al. Surgical quality assurance in COLOR III: standardization and competency assessment in a randomized controlled trial. Ann Surg 2019;270:768–774 [DOI] [PubMed] [Google Scholar]
- 18. Groenier M, Brummer L, Bunting BP, Gallagher AG. Reliability of observational assessment methods for outcome-based assessment of surgical skill: systematic review and meta-analyses. J Surg Educ 2020;77:189–201 [DOI] [PubMed] [Google Scholar]
- 19. Sica GS, Vinci D, Siragusa L, Sensi B, Guida AM, Bellato Vet al. Definition and reporting of lymphadenectomy and complete mesocolic excision for radical right colectomy: a systematic review. Surg Endosc 2023;37:846–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bertelsen CA, Neuenschwander AU, Jansen JE, Tenma JR, Wilhelmsen M, Kirkegaard-Klitbo Aet al. 5-Year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol 2019;20:1556–1565 [DOI] [PubMed] [Google Scholar]
- 21. Benz S, Tannapfel A, Tam Y, Grünenwald A, Vollmer S, Stricker I. Proposal of a new classification system for complete mesocolic excison in right-sided colon cancer. Tech Coloproctol 2019;23:251–257 [DOI] [PubMed] [Google Scholar]
- 22. Benz SR, Feder IS, Vollmer S, Tam Y, Reinacher-Schick A, Denz Ret al. Complete mesocolic excision for right colonic cancer: prospective multicentre study. Br J Surg 2022;110:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albers KI, Polat F, Helder L, Panhuizen IF, Snoeck MMJ, Polle SWet al. Quality of recovery and innate immune homeostasis in patients undergoing low-pressure versus standard-pressure pneumoperitoneum during laparoscopic colorectal surgery (RECOVER): a randomized controlled trial. Ann Surg 2022;276:e664–e673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aiolfi A, Bona D, Guerrazzi G, Bonitta G, Rausa E, Panizzo Vet al. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: an updated systematic review and cumulative meta-analysis. J Laparoendosc Adv Surg Tech A 2020;30:402–412 [DOI] [PubMed] [Google Scholar]
- 25. Brown RF, Cleary RK. Intracorporeal anastomosis versus extracorporeal anastomosis for minimally invasive colectomy. J Gastrointest Oncol 2020;11:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carnuccio P, Jimeno J, Pares D. Laparoscopic right colectomy: a systematic review and meta-analysis of observational studies comparing two types of anastomosis. Tech Coloproctol 2014;18:5–12 [DOI] [PubMed] [Google Scholar]
- 27. Cirocchi R, Trastulli S, Farinella E, Guarino S, Desiderio J, Boselli Cet al. Intracorporeal versus extracorporeal anastomosis during laparoscopic right hemicolectomy—systematic review and meta-analysis. Surg Oncol 2013;22:1–13 [DOI] [PubMed] [Google Scholar]
- 28. Feroci F, Lenzi E, Garzi A, Vannucchi A, Cantafio S, Scatizzi M. Intracorporeal versus extracorporeal anastomosis after laparoscopic right hemicolectomy for cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2013;28:1177–1186 [DOI] [PubMed] [Google Scholar]
- 29. Ricci C, Casadei R, Alagna V, Zani E, Taffurelli G, Pacilio CAet al. A critical and comprehensive systematic review and meta-analysis of studies comparing intracorporeal and extracorporeal anastomosis in laparoscopic right hemicolectomy. Langenbecks Arch Surg 2017;402:417–427 [DOI] [PubMed] [Google Scholar]
- 30. Wu Q, Jin C, Hu T, Wei M, Wang Z. Intracorporeal versus extracorporeal anastomosis in laparoscopic right colectomy: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 2017;27:348–357 [DOI] [PubMed] [Google Scholar]
- 31. den Hartog FPJ, van Egmond S, Poelman MM, Menon AG, Kleinrensink G-J, Lange JFet al. The incidence of extraction site incisional hernia after minimally invasive colorectal surgery: a systematic review and meta-analysis. Colorectal Dis 2023;25:586–599 [DOI] [PubMed] [Google Scholar]
- 32. Greemland I, Raveh G, Gavrielli S, Sadot E, Kashtan H, Wasserberg N. High rates of incisional hernia after laparoscopic right colectomy with midline extraction site. Surg Laparosc Endosc Percutan Tech 2021;31:722–728 [DOI] [PubMed] [Google Scholar]
- 33. Lee L, Mata J, Droeser RA, Kaneva P, Liberman S, Charlebois Pet al. Incisional hernia after midline versus transverse specimen extraction incision: a randomized trial in patients undergoing laparoscopic colectomy. Ann Surg 2018;268:41–47 [DOI] [PubMed] [Google Scholar]
- 34. Bosker R, Groen H, Hoff C, Totte E, Ploeg R, Pierie JP. Effect of proctoring on implementation and results of elective laparoscopic colon surgery. Int J Colorectal Dis 2011;26:941–947 [DOI] [PubMed] [Google Scholar]
- 35. Varban OA, Thumma JR, Carlin AM, Finks JF, Ghaferi AA, Dimick JB. Peer assessment of operative videos with sleeve gastrectomy to determine optimal operative technique. J Am Coll Surg 2020;231:470–477 [DOI] [PubMed] [Google Scholar]
- 36. Miskovic D, Foster J, Agha A, Delaney CP, Francis N, Hasegawa Het al. Standardization of laparoscopic total mesorectal excision for rectal cancer: a structured international expert consensus. Ann Surg 2015;261:716–722 [DOI] [PubMed] [Google Scholar]
- 37. Markar SR, Wiggins T, Ni M, Steyerberg EW, Van Lanschot JJB, Sasako Met al. Assessment of the quality of surgery within randomised controlled trials for the treatment of gastro-oesophageal cancer: a systematic review. Lancet Oncol 2015;16:e23–e31 [DOI] [PubMed] [Google Scholar]
- 38. Foster JD, Mackenzie H, Nelson H, Hanna GB, Francis NK. Methods of quality assurance in multicenter trials in laparoscopic colorectal surgery: a systematic review. Ann Surg 2014;260:220–229 [DOI] [PubMed] [Google Scholar]
- 39. Kurashima Y, Kitagami H, Teramura K, Poudel S, Ebihara Y, Inaki Net al. Validation study of a skill assessment tool for education and outcome prediction of laparoscopic distal gastrectomy. Surg Endosc 2022;36:8807–8816 [DOI] [PubMed] [Google Scholar]
- 40. Mackenzie H, Ni M, Miskovic D, Motson RW, Gudgeon M, Khan Zet al. Clinical validity of consultant technical skills assessment in the English National Training Programme for Laparoscopic Colorectal Surgery. Br J Surg 2015;102:991–997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study data are not openly available. The authors are willing to share the data upon reasonable request.