Abstract
Background.
The ability to manage emotions is an important social-cognitive domain impaired in schizophrenia and linked to functional outcome. The goal of our study was to examine the impact of cognitive enhancement therapy (CET) on the ability to manage emotions and brain functional connectivity in early-course schizophrenia.
Methods.
Participants were randomly assigned to CET (n = 55) or an enriched supportive therapy (EST) control group (n = 45). The resting-state functional magnetic resonance imaging scans and measures of emotion management performances were collected at baseline, 9, and 18 months follow-up. The final sample consisted of 37 CET and 25 EST participants, including 19 CET and 12 EST participants with imaging data. Linear mixed-effects models investigated the impact of treatment on emotion management and functional connectivity from the amygdala to ventrolateral and dorsolateral prefrontal cortex (dlPFC).
Results.
The CET group showed significant improvement over time in emotion management compared to EST. Neither functional connectivity changes nor main group differences were observed following treatment. However, a significant between-group interaction showed that improved emotion management ability was associated with increased functional connectivity between the left amygdala and the left dlPFC in the CET group exclusively.
Conclusion.
Our results replicate the previous work demonstrating that CET is effective at improving some aspects of social cognition in schizophrenia. We found evidence that improvement in emotion management may be associated with a change in amygdala-dlPFC connectivity. This fronto-limbic circuit may provide a mechanistic link between the biology of emotion management processes that can be enhanced in individuals with schizophrenia.
1. Introduction
Social cognitive impairments in schizophrenia manifest as difficulties with emotion recognition, the theory of mind, and emotion management (Green & Horan, 2010). Emotion management can be defined as the ability to be open to feelings and to modulate them in oneself as well as in others (Mayer, Salovey, & Caruso, 2002). Emotion management is an important aspect of social cognition for independent living, work ability and social functioning (Eack et al., 2010; Kee et al., 2009). Emotion management is also suggested to mediate the effects of cognitive remediation therapy on functional outcomes in schizophrenia (Eack, Pogue-Geile, Greenwald, Hogarty, & Keshavan, 2011). Therefore, improving the ability to manage emotions is a critical treatment target in the schizophrenia population.
An early meta-analysis showed moderate benefits of cognitive remediation therapy at improving social cognition in schizophrenia (Wykes, Huddy, Cellard, McGurk, & Czobor, 2011), while more modest benefits were observed in a more recent meta-analysis (Kambeitz-Ilankovic et al., 2019). Cognitive remediation therapies that focus on social cognitive skills demonstrated significant improvement in various social cognitive domains, such as facial affect identification and theory-of-mind (Grynszpan et al., 2011; Kurtz & Richardson, 2012; Kurtz, Gagen, Rocha, Machado, & Penn, 2016), as well as emotion management (Gohar, Hamdi, El Ray, Horan, & Green, 2013; Horan et al., 2011). A better understanding of the neural underpinnings of such behavioral changes may help improve and optimize the effect of cognitive remediation treatments on specific social cognitive domains in schizophrenia.
Cognitive enhancement therapy (CET; Hogarty et al., 2004) is a form of cognitive remediation that specifically treats both neurocognition and social cognition (Eack, Hogarty, Greenwald, Hogarty, & Keshavan, 2007; Hogarty et al., 2004; Wojtalik et al., 2016). It is a comprehensive, step-wise approach to remediation that seeks to facilitate the development of both neurocognitive and social cognitive milestones in schizophrenia. Youth within the early course of schizophrenia who received CET have been noted to show improved neurocognition and social cognition, as well as functional gains in employment, social functioning, and daily activities (Eack et al., 2009). In a recent study, individuals with schizophrenia who received CET showed increased right dorsolateral prefrontal cortex (dlPFC) activity during an executive function task that was associated with improved neurocognitive performance, but not with social cognitive performance (Keshavan, Eack, Prasad, Haller, & Cho, 2017). This finding is in line with observations from several studies showing an association between neurocognitive improvements after cognitive remediation and increased activity in the dlPFC in schizophrenia (Guimond et al., 2018a, 2018b; Mothersill & Donohoe, 2019; Penadés et al., 2017; Ramsay, Nienow, Marggraf, & MacDonald, 2017). While several studies implicate the dlPFC in cognitive remediation-related improvements in executive function in schizophrenia (Keshavan et al., 2017; Ramsay & MacDonald, 2015; Wei et al., 2016), the neural basis of cognitive remediation-related improvements in social cognitive domains, including emotion management, remains unclear (Campos et al., 2016).
The relationship between activity in brain regions subserving executive function and limbic regions could play an important role in emotion management. Functional connectivity between the amygdala and both the ventrolateral prefrontal cortex (vlPFC) and the dlPFC is associated with the ability to manage emotions, which is an important component of social cognition in neurotypical individuals (Banks, Eddy, Angstadt, Nathan, & Luan Phan, 2007; Ochsner, Silvers, & Buhle, 2012; Morawetz, Bode, Baudewig, & Heekeren, 2017). Individuals with schizophrenia also show abnormal functional connectivity among these regions (Cole, Anticevic, Repovs, & Barch, 2011; Liu et al., 2017; Yoon et al., 2008). Evidence has shown that abnormalities of the amygdala-prefrontal cortex pathway underlie emotion management dysfunctions in schizophrenia (Anticevic, Repovs, & Barch, 2012; Morris, Sparks, Mitchell, Weickert, & Green, 2012). More specifically, reduced functional connectivity between the amygdala, vlPFC, and dlPFC is related to impaired emotion management abilities in schizophrenia (Park, Chun, Park, Kim, & Kim, 2018; Szabó et al., 2017). It is, therefore, possible that treatments that target and modulate this network could enhance the ability to manage emotions.
In this study, we sought to characterize changes in amygdala-vlPFC and amygdala-dlPFC connectivity in a sample of people within the early course of schizophrenia who were randomized to 18 months of either CET or active control intervention. The active control intervention was an enriched supportive therapy (EST) that fosters illness management by teaching how to apply coping strategies and providing psychoeducation (Hogarty et al., 2004). We hypothesized that CET would enhance emotion management capacity, as well as amygdala-vlPFC and amygdala-dlPFC resting-state functional connectivity, while this would not be the case for EST. We also hypothesized that increased connectivity in these neural circuits would be related to improved emotion management ability observed after CET but not EST.
2. Methods
2.1. Participants
A total of 106 participants with schizophrenia or schizoaffective disorder within the early course of the illness were enrolled as part of the longitudinal, randomized-controlled clinical trial ‘Brain Imaging, Cognitive Enhancement and Early Schizophrenia’ (BICEPS), at Beth Israel Deaconess Medical Center, Boston, MA and the University of Pittsburgh, Pittsburgh, PA (Wojtalik, et al., submitted). The Institutional Review Boards of both sites approved the study, and all the participants gave written informed consent before participating.
The structured clinical interview for the DSM-IV (SCID) was used to confirm the diagnosis by trained raters. Inclusion criteria were: (1) 18–55 years of age; (2) IQ ⩾ 80 as assessed using the two-subtest form of the Wechsler Abbreviated Scale Intelligence 2nd Edition (WASI-II); (3) a sixth-grade reading level as assessed by the reading subtest of the Wide Range Achievement Test 4th Edition and fluent spoken English; (4) diagnosis of schizophrenia, schizophreniform, or schizoaffective disorder by the SCID; (5) illness duration ⩽10 years; (6) on antipsychotic medication or otherwise clinically stabilized for at least 2 months prior to study enrollment; and (7) displaying significant social and cognitive disability as assessed using the cognitive style and social cognition eligibility interview as used in previous CET studies (Eack et al., 2009, 2015; Hogarty et al., 2004). Participants were excluded if they had: (1) other medical conditions associated with cognitive impairment; (2) persistent suicidal or homicidal behavior; (3) substance abuse or dependence within 3 months; or (4) contrain-dications for magnetic resonance imaging (MRI; e.g. claustrophobia; MRI-incompatible implants or medical devices or history of injury involving metal fragments).
2.2. Treatments
The participants were randomly assigned to either CET or EST (control treatment) and treated for 18 months. The CET protocol is an integrated neurocognitive and social cognitive approach that begins with 60 h in participant-pairs of computer-based neurocognitive training in attention, memory, and problem solving, followed by integration into 45 weekly in-person group sessions of 6–8 participants. These structured weekly sessions focused on a wide range of social-cognitive abilities, such as perspective-taking, emotion management, reading non-verbal cues, and social context appraisal (see Hogarty et al., 2006). The EST protocol consisted of weekly 30–60-min personal therapy (Hogarty, 2002) focused on psychoeducation, stress, and illness management. The sessions were reduced to biweekly as the treatment progressed. The same clinicians provided both treatments, thereby avoiding potential clinician effects. The possibility of treatment contamination was minimized by the fact that both interventions were manual-driven, and were supervised by experts in both therapeutic conditions (Susan S. Hogarty, Deborah P. Greenwald, Shaun M. Eack). Both treatments have been described in detail in previous publications (Hogarty et al., 2004; Keshavan et al., 2017).
Neuroimaging and emotion management measurements were acquired at baseline and 9 and 18 months follow-up. Figure 1 shows the treatment group and data collection flowchart. Demographic, clinical, and cognitive information comparing the two groups included in our analysis at baseline is shown in Table 1. Age significantly differed between the groups, with the CET group being younger (p = 0.04). No significant differences were observed between the groups in terms of race, diagnosis, chlorpromazine equivalent medication dosage, or duration of illness. Table 2 shows that no significant demographic differences were found between treatment groups in the imaging sample. No significant demographic differences were found between the analysis sample and the randomization sample (all p > 0.18; Supplementary Table 2).
Fig. 1.
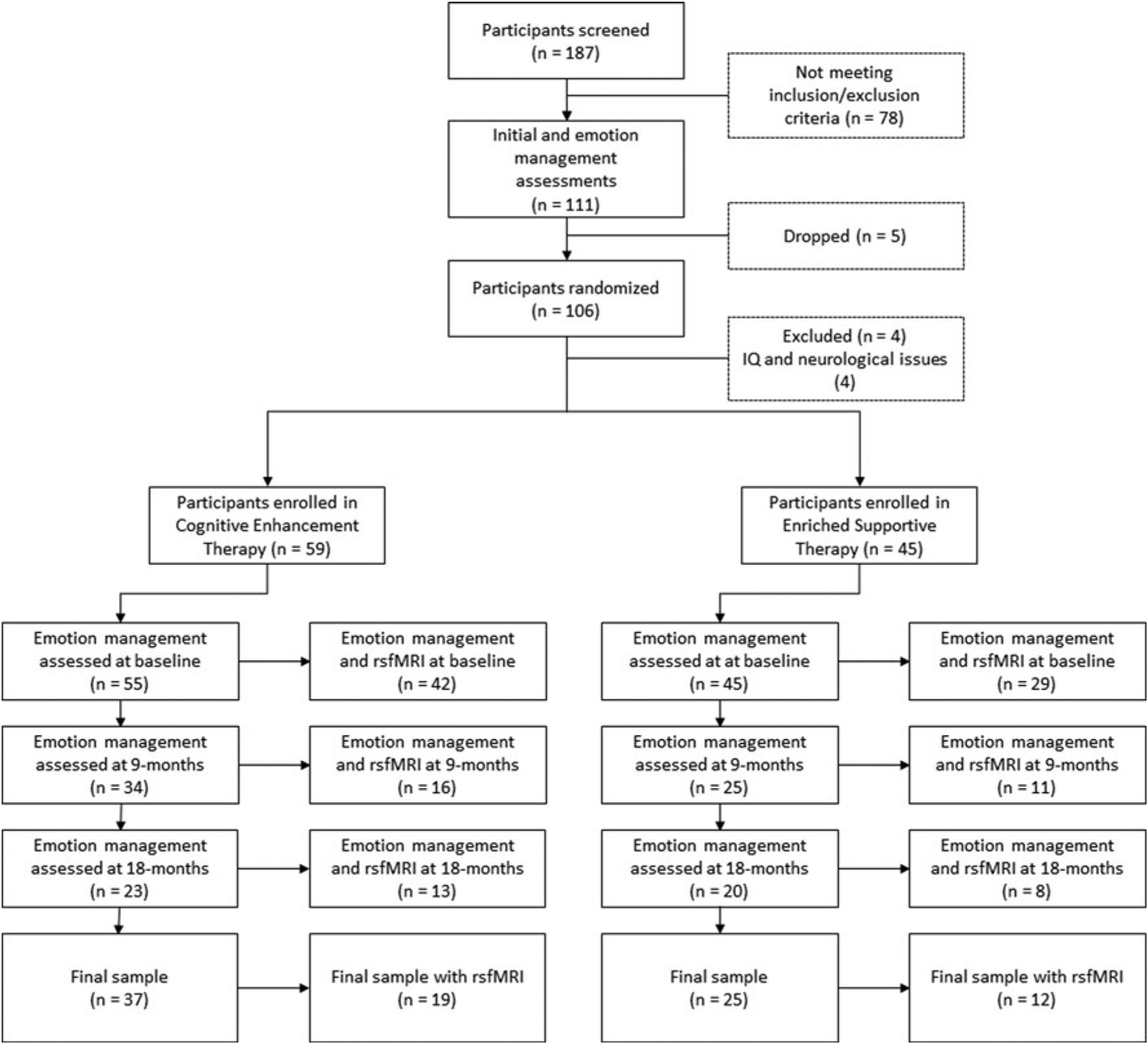
Randomization and data collection flowchart. Note: The flowchart represents how many participants were screened, have completed initial assessments, were randomized, and then were enrolled in both treatment conditions. It also delineates the number of participants with good quality data that were included in our analyses at baseline, 9, and 18 months. The final sample consists of participants who have been assessed at least twice during the study period. rsfMRI, resting-state functional magnetic resonance imaging.
Table 1.
Baseline demographic, clinical and cognitive data for the final sample
| CET (n = 37) | EST (n = 25) | t-statistic | p value | |||
|---|---|---|---|---|---|---|
| Mean (S.D.) | Range | Mean (S.D.) | Range | |||
| Age (years) | 24.8 (4.5) | 19–43 | 28.1 (7.7) | 18–50 | 2.11 | 0.04 |
| Emotion management performance (t-score) | 41.9 (13.2) | 13–73 | 45.9 (11.7) | 20–68 | 1.23 | 0.23 |
| CPZ eq (mg) | 493.3 (388.4) | 50–1560 | 486.3 (313.1) | 100–1433 | −0.07 | 0.94 |
| Duration of illness (years) | 4.5 (2.5) | 0.3–9.5 | 4.6 (2.7) | 1.3–10.4 | 0.23 | 0.82 |
| IQ | 110.9 (8.0) | 96–125 | 109.5 (13.1) | 89–139 | −0.48 | 0.63 |
| CET | EST | χ2-statistic | p value | |||
| Site | 0.005 | 0.94 | ||||
| Boston | 16 | 11 | ||||
| Pittsburgh | 21 | 14 | ||||
| Sex | 0.77 | 0.38 | ||||
| Female | 7 | 8 | ||||
| Male | 30 | 17 | ||||
| Race | 0.71 | 0.70 | ||||
| African-American | 7 | 7 | ||||
| Caucasian | 23 | 14 | ||||
| Others | 7 | 4 | ||||
| Diagnosis | 1.54 | 0.21 | ||||
| Schizoaffective disorder | 6 | 8 | ||||
| Schizophrenia | 31 | 17 | ||||
Note: CPZ eq, chlorpromazine equivalence; IQ, Intelligence Quotient estimate based on two-subtest form of the Wechsler Abbreviated Scale Intelligence, 2nd Edition (WASI-II).
Table 2.
Baseline demographic, clinical and cognitive data for the final sample with imaging data
| CET (n = 19) | EST (n = 12) | t-statistic | p value | |||
|---|---|---|---|---|---|---|
| Mean (S.D.) | Range | Mean (S.D.) | Range | |||
| Age (years) | 25.5 (5.7) | 19.3–43.2 | 27.9 (5.5) | 20.6–36.3 | 1.17 | 0.25 |
| Emotion management performance (t-score) | 44.8 (8.7) | 28–63 | 47.8 (11.4) | 32–63 | 0.83 | 0.41 |
| CPZ eq (mg) | 287.8 (235.2) | 25.0–750.0 | 468.8 (364.4) | 100.0–1433.5 | 1.66 | 0.11 |
| Duration of illness (years) | 4.5 (2.7) | 0.3–9.2 | 4.1 (2.2) | 1.3–8.3 | −0.32 | 0.75 |
| IQ | 111.0 (7.7) | 97–125 | 115 (16.7) | 89–139 | 0.92 | 0.36 |
| CET | EST | χ2-statistic | p value | |||
| Site | 0.12 | 0.73 | ||||
| Boston | 4 | 4 | ||||
| Pittsburgh | 15 | 8 | ||||
| Sex | 0.77 | 0.38 | ||||
| Female | 4 | 3 | ||||
| Male | 15 | 9 | ||||
| Race | 0.71 | 0.70 | ||||
| African-American | 3 | 3 | ||||
| Caucasian | 14 | 7 | ||||
| Others | 2 | 2 | ||||
| Diagnosis | 1.31 | 0.25 | ||||
| Schizoaffective disorder | 3 | 4 | ||||
| Schizophrenia | 16 | 8 | ||||
Note: CPZ eq, chlorpromazine equivalence; IQ, Intelligence quotient estimate based on two-subtest form of the Wechsler Abbreviated Scale Intelligence 2nd Edition (WASI-II).
2.3. Measures
2.3.1. Emotion management
We chose to use the managing emotions branch of the Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT; Mayer, Salovey, Caruso, and Sitarenios, 2003) as it is strongly correlated with functional outcome (Eack et al., 2010; Kee et al., 2009), and hence it is used as part of the measurement and treatment research to improve cognition in schizophrenia consensus cognitive battery (Nuechterlein et al., 2008). This measure also performed well on test–retest reliability, repeated measure utility, response to pharmacological intervention, as well as practicality and tolerability (Mayer, Salovey & Caruso, 2012; Nuechterlein et al., 2008). Furthermore, this measure assesses how people manage their emotions, which is of particular interest when investigating brain functional networks that are known to be important for regulating and managing emotions (Banks et al., 2007; Morawetz et al., 2017; Ochsner et al., 2012). Uncorrected t-scores were used in all analyses.
2.3.2. Resting-state functional MRI
A 3T Siemens Trio (TIM upgrade) scanner was used at the Boston site to acquire resting-state functional magnetic resonance imaging (fMRI) data, while a 3T Siemens Verio scanner was used at the Pittsburgh site. The imaging parameters at both sites consisted of 3000-ms repetition time, 30-ms echo time, 85° flip angle, and 3 × 3 × 3-mm3 voxels. Forty-seven axial sections were collected at Boston while 45 were collected at Pittsburgh, both with interleaved acquisition and no gap. All participants were instructed to ‘remain still, stay awake, and keep their eyes open’ during the resting-state fMRI run of 6.2 min (124 time points). A high-resolution T1 image was also taken for pre-processing purposes.
Resting-state fMRI data were preprocessed using data processing assistant for resting-state (DPABI) image processing software (Version 4.0 190305; Yan, Wang, Zuo, and Zang, 2016) for a total of 186 scans including all time points (CET, n = 105; EST, n = 81). The first four volumes were removed from every scan to minimize the effects of the scanner signal stabilization. The scans with head motion exceeding 4-mm maximum translation or 4° maximum rotation were discarded (CET, n = 19; EST, n = 20). Functional and structural images were co-registered. Structural images were normalized and segmented into gray matter, white matter, and cerebral spinal fluid (CSF) partitions using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) method (Ashburner, 2007). The Friston 24-parameter model was used to regress out head motion effects from the data (Friston, Williams, Howard, Frackowiak, & Turner, 1996). CSF, WM and global signals were regressed out (Yan et al., 2013). The images were realigned and corrected for slice timing. Framewise displacement was calculated and all volumes exceeding the threshold of 0.5-mm were censored. Scans with more than 50% of volumes requiring censoring were discarded (CET, n = 9; EST, n = 8). There was no significant treatment group difference in terms of volumes that were censored ( p = 0.55). The data had nuisance covariates regressed out and were band-pass filtered for 0.01–0.08-Hz signals. Normalization was then performed via DARTEL in Montreal Neurological Institute space and smoothing was performed using an 8-mm Gaussian kernel full-width-at-maximum-height. A total of 96 participants had at least one scan collected during the study. From these 96 participants, a total of 87 participants (91%) remained after quality control and filtering as described above. Of these 87 participants, 71 (82%) had a baseline scan, but only 31 of those 71 participants (44%) also had a scan obtained at a second-time point. Therefore, a total of 31 participants (19 CET and 12 EST) were included in the longitudinal resting-state fMRI analysis.
Our a priori hypothesis was to determine the strength of correlation of the pathway between the amygdala and the lateral prefrontal cortex using anatomical selected seeds that relate to emotion management (Anticevic et al., 2012; Hoptman et al., 2010; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008) and that have previously been used in the literature (Morawetz et al., 2017). Therefore, bilateral amygdala, dlPFC, and vlPFC were selected as a priori regions-of-interest (ROIs) for our analyses using the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002; see details in Supplementary Table 1).
2.4. Statistical analyses
1All statistical analyses were performed in R. linear mixed-effects models were performed with the lmerTest package (Kuznetsova, Brockhoff, & Christensen, 2017) and adjusted for random intercept for each participant. All analyses were corrected for sites to account for possible differences between assessors and scanners. Age and sex were entered as covariates in the models as these variables are known to affect the MSCEIT scores as well as the brain functional connectivity measures (Cabello, Sorrel, Fernández-Pinto, Extremera, & Fernández-Berrocal, 2016; Zhang et al., 2016). Post-hoc within-group effects were also examined. Multiple comparison correction for Type 1 error was applied using the false discovery rate Benjamin–Hochberg procedure and q values are reported.
First, a linear mixed-effect model was used to analyze the effect of treatment groups on emotion management performance over time.
Next, functional connectivity was assessed using a seed-based analysis. ROI-to-ROI functional connectivity values were extracted and z-transformed values were calculated using the DPABI toolbox (Yan et al., 2016). A series of linear mixed-effects models were then used to analyze the effect of the treatment group on these z-transformed connectivity values over time between our ROIs.
Finally, linear mixed-effects models were used to explore the association between change over time in functional connectivity and change over time in emotion management performance, and the interaction of treatment groups on this longitudinal relationship. When the between-group interactions were significant, post-hoc linear mixed-effects models were then performed in each group separately.
3. Results
3.1. Effect of treatment on emotion management performance
We observed a significant time by group interaction on emotion management performance (B = −0.215, t = −2.34, p = 0.021; see Fig. 2). Post-hoc within-group analysis revealed that only participants in the CET group displayed a significant improvement in emotion management over time (CET: B = 0.213, t = 2.95, p = 0.0043; EST: B = 0.0147, t = 0.85, p = 0.183). The interaction term was also significant in the subsample of participants included in the rsfMRI analysis (B = −0.269, t = −2.47, p = 0.018; see Supplementary Figure 1).
Fig. 2.
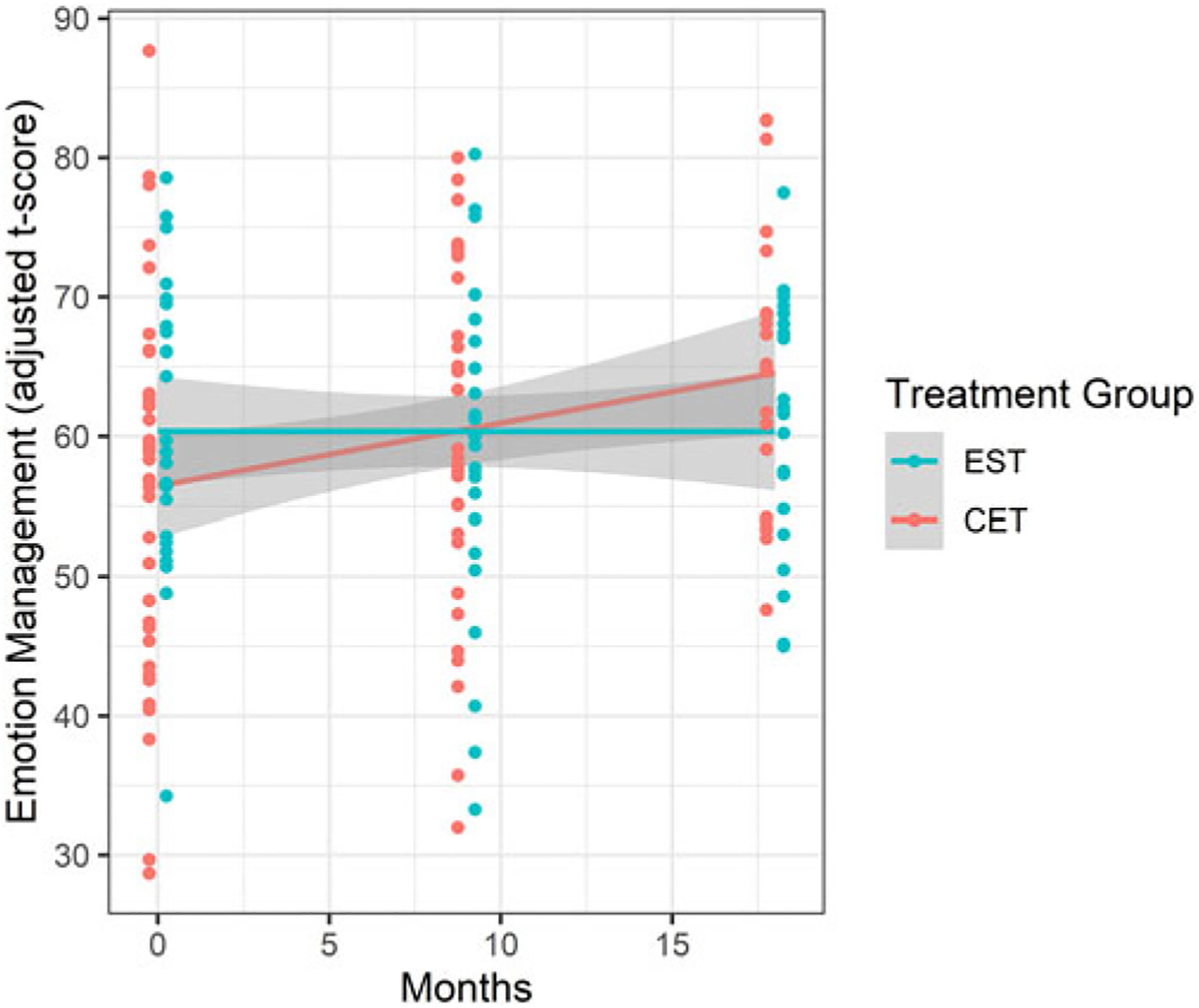
Patients enrolled in CET significantly improved their emotion management performance over time compared to patients enrolled in EST. Note: emotion management t-scores are adjusted for age, sex and site. EST, enriched supportive therapy; CET, cognitive enhancement therapy.
3.2. Effect of treatment on functional connectivity
Neither significant longitudinal change over time nor group differences in functional connectivity were observed between any of our ROIs (all p > 0.300; see Supplementary Table 3).
3.3. Relationship between change in emotion management performance and functional connectivity
Significant between-group interactions were observed in terms of the association between change in emotion management abilities and change in functional connectivity between the left amygdala and bilateral dlPFC (left amygdala-to-left dlPFC, B = −0.703, t = −3.44, p = 0.002, q = 0.009; left amygdala-to-right dlPFC, B = −0.760, t = −3.51, p = 0.002, q = 0.009), as well as between right amygdala and right dlPFC (right amygdala-to-right, B = −0.638, t = −2.75, p = 0.01, q = 0.03).
As demonstrated in Fig. 3, post-hoc within-group analysis revealed that the more the participants in the CET group improved their emotion management performance over time, the more they tend to show an increase in functional connectivity between the left amygdala and the left dlPFC (B = 0.466, t = 2.47, p = 0.027), while this relationship was not observed in the EST group (B = −0.498, t = −1.60, p = 0.154).
Fig. 3.
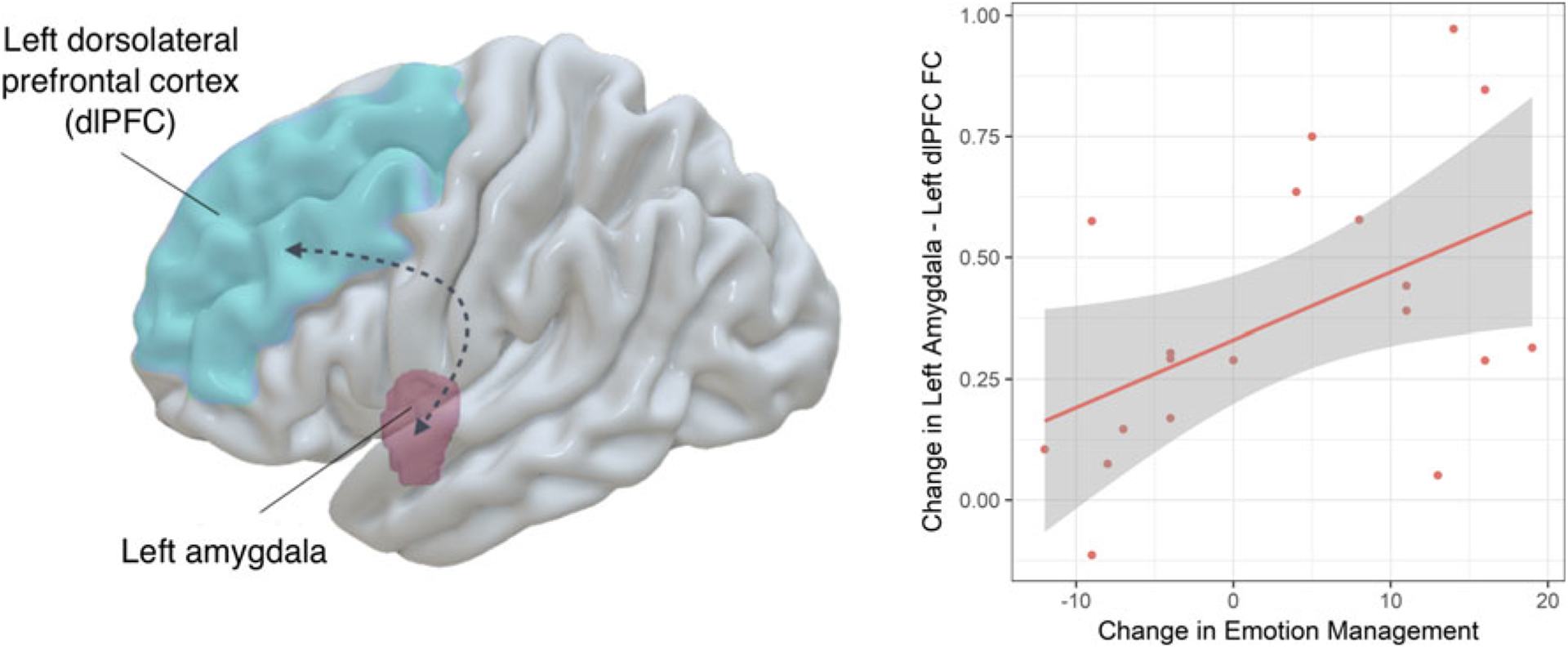
Emotion management improvement over time in people enrolled in CET was associated with increased functional connectivity between the left amygdala and the left dlPFC. Note: Graphical representation of the two regions of interest (left panel); scatter plot showing association between change in emotion management performance and change in functional connectivity following cognitive enhancement therapy (CET) (right panel). Change in functional connectivity z-scores are adjusted for age, sex and site. dlPFC, dorsolateral prefrontal cortex; FC, functional connectivity; EST, enriched supportive therapy; CET, cognitive enhancement therapy.
Post-hoc within-group analysis showed that significant group interactions related to functional connectivity between the bilateral amygdala and the right dlPFC were instead driven by reduced connectivity between these brain regions being related to better emotion management performances in patients enrolled in EST (left amygdala-to-right dlPFC, B = −0.955, t = −3.62, p = 0.008; right amygdala-to-right dlPFC, B = −1.23, t = −12, p < 0.001), while such a negative relationship was not observed in patients enrolled in CET (left amygdala-to-right dlPFC, B = 0.300, t = 1.19, p = 0.254; right amygdala-to-right dlPFC, B = 0.292, t = 1.29, p = 0.217).
There was neither significant overall relationship between change in emotion management abilities and change in functional connectivity observed across treatment groups (see Supplementary Tables 4) nor group differences for any of the measures of functional connectivity at baseline (see Supplementary Tables 5).
4. Discussion
In this study, we investigated changes in emotion management performance and amygdala-vlPFC/dlPFC connectivity in individuals within the early course of schizophrenia receiving CET. Our results show that emotion management ability in patients with schizophrenia improves significantly after undergoing CET as compared to EST. Our findings also denote that individuals who showed greater improvements in emotion management performance over the course of CET were more likely to show increased connectivity between the left amygdala and the left dlPFC. These findings suggest that increased functional connectivity between these two brain regions could be an underlying mechanism involved in the amelioration of emotion management impairments in individuals with schizophrenia receiving CET.
Our current results support previous findings that indicate that CET is an effective approach for improving various aspects of social cognition in individuals with schizophrenia (Hogarty et al., 2004; 2006; Lee et al., 2013; Wojtalik et al., 2016). More specifically, our findings suggest that improvements in emotion management performance in individuals who receive CET are related to increased functional connectivity between the left amygdala and left dlPFC, two important regions for emotion management in neurotypical individuals (Banks et al., 2007; Lee, Heller, van Reekum, Nelson, & Davidson, 2012) and in schizophrenia (Ursu et al., 2011). In line with these results, Wojtalik et al., in an independent sample, found CET treatment to increase activity in prefrontal and limbic regions while participants performed an emotion regulation task (Wojtalik et al., 2016). Taken together, these findings show that activity changes in fronto-limbic networks are associated with performance improvements in managing emotions following CET in patients with schizophrenia.
While we observed an association between increased emotion management ability and increased functional connectivity over time between the left amygdala and left dlPFC in the CET group and not in the EST group, we found no main effect of groups on functional connectivity. Furthermore, no significant changes were detected between the amygdala and vlPFC, which was surprising given that both regions are implicated in emotion processing in neurotypical individuals (Banks et al., 2007; Lee et al., 2012; Morawetz et al., 2017). However, it is possible that the vlPFC is less involved in individuals in whom this ability is impaired, and more research is needed to investigate this further. Moreover, as we performed a longitudinal fMRI study and follow-up scans were sparse, our study was limited in sample size, which may have reduced our power to detect group differences in these measures.
Our findings also revealed an unexpected negative relationship between change in the bilateral amygdala and right dlPFC connectivity and emotion management performance in our active control group receiving EST. More specifically, individuals who received EST showed lower emotion management performance at follow-up, but increased functional connectivity between the bilateral amygdala and right dlPFC. This surprising finding may be a result of our control condition or relate to individual differences in declining emotion management abilities throughout the current study. This also raises the possibility that increased functional connectivity between the bilateral amygdala and right dlPFC may occur in the natural course of schizophrenia. This could be an inefficient neural response, since it is associated with reduced emotional management abilities, suggesting a maladaptive compensatory effect. Nonetheless, it is important to note that our final EST control group sample was small. Subsequently, additional research in a larger sample is needed to elucidate this intriguing result.
Previous reports suggest that both right and left dlPFC play a differential role in the top–down regulation of emotions (Ligeza, Wyczesany, Tymorek, & Kamiński, 2016; MacDonald, Cohen, Stenger, & Carter, 2000; Sanchez-Lopez, Vanderhasselt, Allaert, Baeken, & De Raedt, 2018). Brain stimulation studies showed that increased activity of the left dlPFC may help reduce attention toward negative emotions, while stimulation of the right dlPFC would result in impaired disengagement toward these negative emotions in neurotypical individuals (De Raedt et al., 2010; Sanchez-Lopez et al., 2018). While increased activity in the left dlPFC would be critical for improving emotion regulation, this may not be the case for the right dlPFC in neurotypical individuals (Sanchez-Lopez et al., 2018) as well as in people with depression (De Raedt & Koster, 2010; Disner, Beevers, Haigh, & Beck, 2011). This could explain the differential lateralized effect in functional connectivity we observed between CET and EST across both hemispheres of the dlPFC.
This study is the first to investigate resting-state functional connectivity underlying changes in emotion management performances after CET in individuals within the early course of schizophrenia. Our results provide evidence that the functional corticolimbic connectivity between the left amygdala and left dlPFC may be a potential mechanism underlying emotion management outcomes following CET in schizophrenia. Nonetheless, the findings from our study should be interpreted considering its limitations. First, the current sample consists of individuals with unusually high baseline IQ and may not be a representative of most people within the early course of schizophrenia. Hence, future studies are needed to confirm our current findings in people within the early course of schizophrenia with lower IQ as well as in individuals in more advanced stages of the illness. It is important to note that the current findings related to emotion management are limited by the use of the MSCEIT as our measure of social cognitive outcome. This aspect of social cognition depends on cognitive processes including verbal memory, comprehension, and more general cognitive processing. Further research is required to investigate the cognitive components of CET that may contribute to emotion management task improvement. Many other domains of social cognition are impaired in schizophrenia and should be investigated in future studies (e.g. emotion recognition, attention bias, theory of mind) (Mike et al., 2019). These domains of social cognition are likely to be supported by different brain networks (Laillier et al., 2019; Lee & Siegle, 2012; Sanchez-Lopez et al., 2018). Hence, more research is needed to investigate the brain correlates of improvement in other aspects of social cognition following CET.
Furthermore, since we excluded brain imaging data containing excessive motion and many participants did not have a brain scan post-baseline, the final sample size of participants included in our functional connectivity analysis was relatively small. This may reduce the generalizability of our results. Nonetheless, the demographics from the initial sample (n = 100) were similar to those of our final sample (see Supplementary Table 2). The small sample size for the fMRI analysis also reduced our power and as such, may have increased the risk of false-negative results. This lack of power also limits our ability to further investigate whole-brain functional changes over time. Future studies should explore the link between improvements in emotion management and other resting-state networks, such as the default mode network (Mars et al., 2012). In addition, CET is an extended cognitive remediation program and we observed high levels of attrition over time (55% from baseline to 18 months). While the attrition was similar in both CET and EST groups (see Fig. 1), the current results may be biased by the type of individuals who remain in the study over the 18-month period, as it is likely that those who dropped out experienced fewer benefits from treatment. In the future, a larger longitudinal sample should be used to account for these limitations and replicate our current findings. This also highlights the importance of closer monitoring of quality control during imaging data collection for further similar clinical trials to avoid such attrition. This study was conducted at two different sites with two different scanners. While we harmonized data acquisition procedures between both sites and included site as a covariate in all our analyses, this is an additional limitation to our study. Finally, more efforts are needed to increase the retention of participants in CET trials and to further develop shorter-duration cognitive interventions that may similarly improve emotion management performance and social cognition in schizophrenia.
In summary, this study suggests that functional connectivity between the left amygdala and left dlPFC is a possible underlying brain mechanism of emotion management improvement following CET in early course schizophrenia. A better understanding of the role of functional connectivity between the amygdala and the dlPFC in these social cognitive outcomes may guide the development of more targeted treatment approaches. In the future, studies may try to target this specific brain circuit with neuromodulation techniques to enhance plasticity and thereby maximize the efficacy of CET in improving emotion management ability. Furthermore, additional neuroimaging research is required to unravel the complex influence of CET on resting-state functional connectivity. The association of neurocognitive and social-cognitive aspects of CET treatment, and their interaction, on improved functional connectivity, will be an important concept to subsequent investigations.
Supplementary Material
Acknowledgements.
The authors thank all the participants who took part in the study, as well as Annaliese Lausberg, Josh Golt, Summer McKnight, Scott Barb, and Taylor Nichols for their help with data collection, as well as Rachel Templeton for her help with the preliminary analyses.
Financial support.
This work was funded by an operating grant from NIMH MH 92440; MSK, PI, Clinicatrials.gov #NCT01561859). SG was supported by a postdoctoral training fellowship from the Fonds de recherche du Québec - Santé (FRQS) and by an Emerging Research Innovators in Mental Health (eRIMh) award. GC was supported by the European Union’s research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 749201. RB was supported by NIH grant R01 MH116170.
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720004110
Conflicts of interest. None.
Ethical standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Anticevic A, Repovs G, & Barch DM (2012). Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophrenia Bulletin, 38(5), 967–980. 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. Neuroimage, 38(1), 95–113. 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Luan Phan K (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello R, Sorrel MA, Fernández-Pinto I, Extremera N, & Fernández-Berrocal P (2016). Age and gender differences in ability emotional intelligence in adults: a cross-sectional study. Developmental Psychology, 52(9), 1486–1492. 10.1037/dev0000191. [DOI] [PubMed] [Google Scholar]
- Campos C, Santos S, Gagen E, Machado S, Rocha S, Kurtz MM, … Rocha NB (2016). Neuroplastic changes following social cognition training in schizophrenia: a systematic review. Neuropsychology Review, 26(3), 310–328. 10.1007/s11065-016-9326-0. [DOI] [PubMed] [Google Scholar]
- Cole M, Anticevic A, Repovs G, & Barch D (2011). Variable global dysconnectivity and individual differences in schizophrenia. Biological Psychiatry, 70(1), 43–50. 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raedt R, & Koster EHW (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective and Behavioral Neuroscience, 10(1), 50–70. 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- De Raedt R, Leyman L, Baeken C, Van Schuerbeek P, Luypaert R, Vanderhasselt MA, & Dannlowski U (2010). Neurocognitive effects of HF-rTMS over the dorsolateral prefrontal cortex on the attentional processing of emotional information in healthy women: an event-related fMRI study. Biological Psychology, 85(3), 487–495. 10.1016/j.biop-sycho.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12(8), 467–477. 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, & Keshavan MS (2010). Assessing social-cognitive deficits in schizophrenia with the Mayer–Salovey–Caruso emotional intelligence test. Schizophrenia Bulletin, 36, 370–380. 10.1093/schbul/sbn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, & Keshavan MS (2009). Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatric Services, 60(11), 1468–1476. 10.1176/ps.2009.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Greenwald DP, Hogarty SS, & Keshavan MS (2007). Cognitive enhancement therapy improves emotional intelligence in early course schizophrenia: preliminary effects. Schizophrenia Research, 89 (1–3), 308–311. 10.1016/j.schres.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack S, Hogarty S, Greenwald D, Litschge M, Mcknight S, Bangalore S, … Cornelius J (2015). Cognitive enhancement therapy in substance misusing schizophrenia: results of an 18-month feasibility trial. Schizophrenia Research, 161(2–3), 478–483. 10.1016/j.schres.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Pogue-Geile MF, Greenwald DP, Hogarty SS, & Keshavan MS (2011). Mechanisms of functional improvement in a 2-year trial of cognitive enhancement therapy for early schizophrenia. Psychological Medicine, 41, 1253–1261. 10.1017/S0033291710001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, & Turner R. (1996). Movement-Related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355. 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gohar S, Hamdi E, El Ray L, Horan W, & Green M (2013). Adapting and evaluating a social cognitive remediation program for schizophrenia in arabic. Schizophrenia Research, 148(1), 12–17. 10.1016/j.schres.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Green M, & Horan W (2010). Social cognition in schizophrenia. Current Directions in Psychological Science, 19(4), 243–248. 10.1177/0963721410377600. [DOI] [Google Scholar]
- Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S, & Perez-Diaz F (2011). Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychological Medicine, 41(1), 163–173. 10.1017/S0033291710000607. [DOI] [PubMed] [Google Scholar]
- Guimond S, Béland S, & Lepage M (2018a). Strategy for semantic association memory (SESAME) training: effects on brain functioning in schizophrenia. Psychiatry Research - Neuroimaging, 271, 50–58. 10.1016/j.pscychresns.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Guimond S, Padani S, Lutz O, Eack S, Thermenos H, & Keshavan M (2018b). Impaired regulation of emotional distractors during working memory load in schizophrenia. Journal of Psychiatric Research, 101, 14–20. 10.1016/j.jpsychires.2018.02.028. [DOI] [PubMed] [Google Scholar]
- Hogarty GE (2002). Personal therapy for schizophrenia and related disorders: A guide to individualized treatment. New York, US: Guilford Press. [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, … Zoretich R (2004). Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry, 61(9), 866–876. 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Hogarty GE, Greenwald DP, & Eack SM (2006). Special section: a memorial tribute: durability and mechanism of effects of cognitive enhancement therapy. Psychiatric Services, 57(12), 1751–1757. 10.1176/ps.2006.57.12.1751. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, D’Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AMC, … Milham MP (2010). Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophrenia Bulletin, 36(5), 1020–1028. 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Tripp C, Hellemann G, Wynn JK, Bell M, … Green MF (2011). Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. Journal of Psychiatric Research, 45 (8), 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz-Ilankovic L, Betz LT, Dominke C, Haas SS, Subramaniam K, Fisher M, … Kambeitz J (2019). Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neuroscience and Biobehavioral Reviews, 107, 828–845. 10.1016/j.neubiorev.2019.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee KS, Horan WP, Salovey P, Kern RS, Sergi MJ, Fiske AP, … Green MF (2009). Emotional intelligence in schizophrenia. Schizophrenia Research, 107, 61–68. 10.1016/j.schres.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Eack SM, Prasad KM, Haller CS, & Cho RY (2017). Longitudinal functional brain imaging study in early course schizophrenia before and after cognitive enhancement therapy. NeuroImage, 151, 55–64. 10.1016/j.neuroimage.2016.11.060. [DOI] [PubMed] [Google Scholar]
- Kurtz M, Gagen E, Rocha N, Machado S, & Penn D (2016). Comprehensive treatments for social cognitive deficits in schizophrenia: a critical review and effect-size analysis of controlled studies. Clinical Psychology Review, 43, 80–89. 10.1016/j.cpr.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, & Richardson CL (2012). Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophrenia Bulletin, 38(5), 1092–1104. 10.1093/schbul/sbr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). Lmertest package: tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Laillier R, Viard A, Caillaud M, Duclos H, Bejanin A, de La Sayette V, … Laisney M (2019). Neurocognitive determinants of theory of mind across the adult lifespan. Brain and Cognition, 136, 103588. 10.1016/j.bandc.2019.103588. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, & Davidson RJ (2012). Amygdala-prefrontal coupling underlies individual differences in emotion regulation. NeuroImage, 62(3), 1575–1581. 10.1016/j.neuro-image.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RSC, Redoblado-Hodge MA, Naismith SL, Hermens DF, Porter MA, & Hickie IB (2013). Cognitive remediation improves memory and psychosocial functioning in first-episode psychiatric out-patients. Psychological Medicine, 43(6), 1161–1173. 10.1017/S0033291712002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, & Siegle GJ (2012). Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Social Cognitive and Affective Neuroscience, 7(5), 521–534. 10.1093/scan/nsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeza TS, Wyczesany M, Tymorek AD, & Kamiński M (2016). Interactions between the prefrontal Cortex and attentional systems during volitional affective regulation: an effective connectivity reappraisal study. Brain Topography, 29(2), 253–261. 10.1007/s10548-015-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhang W, Chen G, Tian H, Li J, Qu H, … Zhuo C (2017). Aberrant patterns of local and long-range functional connectivity densities in schizophrenia. Oncotarget, 8(29), 48196–48203. 10.18632/oncotarget.18441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A, Cohen J, Stenger V, & Carter C (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MAP, Sallet J, Toni I, & Rushworth MFS (2012). On the relationship between the “default mode network” and the “social brain.” Frontiers in Human Neuroscience. 2012(6), 189. 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, & Caruso DR (2002) Mayer–Salovey–Caruso emotional intelligence test (MSCEIT). Toronto, Ontario: Multi-Health Systems, Inc. http://www.eiconsortium.org/measures/msceit.html. [Google Scholar]
- Mayer JD, Salovey P, & Caruso DR (2012). The validity of the MSCEIT: additional analyses and evidence. Emotion Review, 4(4), 403–408. 10.1177/1754073912445815. [DOI] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, & Sitarenios G (2003). Measuring emotional intelligence with the MSCEIT V2.0. Emotion, 3(1), 97–105. 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- Mike L, Guimond S, Kelly S, Thermenos H, Mesholam-Gately R, Eack S, & Keshavan M (2019). Social cognition in early course of schizophrenia: exploratory factor analysis. Psychiatry research, 272, 737–743. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, & Heekeren HR (2017). Effective amygdala-prefrontal connectivity predicts individual differences in successful emotion regulation. Social Cognitive and Affective Neuroscience, 12(4), 569–585. 10.1093/scan/nsw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Sparks A, Mitchell PB, Weickert CS, & Green MJ (2012). Lack of cortico-limbic coupling in bipolar disorder and schizophrenia during emotion regulation. Translational Psychiatry, 2(3), e90. 10.1038/tp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill D, & Donohoe G (2019). Neural effects of cognitive training in schizophrenia: a systematic review and activation likelihood estimation meta-analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(8), 688–696. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, … Marder SR (2008). The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry, 165(2), 203–213. 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251 (1), E1–E24. 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Chun JW, Park HJ, Kim E, & Kim JJ (2018). Involvement of amygdala–prefrontal dysfunction in the influence of negative emotion on the resolution of cognitive conflict in patients with schizophrenia. Brain and Behavior, 8(8). 10.1002/brb3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penadés R, González-Rodríguez A, Catalán R, Segura B, Bernardo M, & Junqué C (2017). Neuroimaging studies of cognitive remediation in schizophrenia: a systematic and critical review. World Journal of Psychiatry, 7(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay I, & MacDonald A (2015). Brain correlates of cognitive remediation in schizophrenia: activation likelihood analysis shows preliminary evidence of neural target engagement. Schizophrenia Bulletin, 41(6), 1276–1284. 10.1093/schbul/sbv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay IS, Nienow TM, Marggraf MP, & MacDonald AW (2017). Neuroplastic changes in patients with schizophrenia undergoing cognitive remediation: triple-blind trial. The British Journal of Psychiatry, 210(3), 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lopez A, Vanderhasselt MA, Allaert J, Baeken C, & De Raedt R (2018). Neurocognitive mechanisms behind emotional attention: inverse effects of anodal tDCS over the left and right DLPFC on gaze disengagement from emotional faces. Cognitive, Affective and Behavioral Neuroscience, 18(3), 485–494. 10.3758/s13415-018-0582-8. [DOI] [PubMed] [Google Scholar]
- Szabó ÁG, Farkas K, Marosi C, Kozák LR, Rudas G, Réthelyi J, & Csukly G (2017). Impaired mixed emotion processing in the right ventrolateral prefrontal cortex in schizophrenia: an fMRI study. BMC Psychiatry, 17(1), 391. 10.1186/s12888-017-1558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Gard MG, Minzenberg MJ, Yoon JH, Ragland JD, … Carter CS (2011). Prefrontal cortical deficits and impaired cognition–emotion interactions in schizophrenia. American Journal of Psychiatry, 168(3), 276–285. 10.1176/appi.ajp.2010.09081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YY, Wang JJ, Yan C, Li ZQ, Pan X, Cui Y, … Tang YX (2016). Correlation between brain activation changes and cognitive improvement following cognitive remediation therapy in schizophrenia: an activation likelihood estimation meta-analysis. Chinese Medical Journal, 129(5), 578–585. 10.4103/0366-6999.176983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtalik JA, Hogarty SS, Cornelius JR, Phillips ML, Keshavan MS, Newhill CE, & Eack SM (2016). Cognitive enhancement therapy improves frontolimbic regulation of emotion in alcohol and/or cannabis misusing schizophrenia: a preliminary study. Frontiers in Psychiatry, 6 (JAN), 186. 10.3389/fpsyt.2015.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtalik JA, Mesholam-Gately R, Hogarty SS, Greenwald DP, Litschge MY, Sandoval LR, … Eack SM (submitted). Confirmatory efficacy of cognitive enhancement therapy for early course schizophrenia: results from a multi-site randomized controlled trial. Psychiatric Services. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, & Czobor P (2011). A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry, 168(5), 472–485. 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, … Milham MP (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage, 76, 183–201. doi: doi: 10.1016/j.neuroimage.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Wang XD, Zuo XN, & Zang YF (2016). DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics, 14(3), 339–351. 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yoon J, Minzenberg M, Ursu S, Ryan Walter B, Walters R, Wendelken C, … Yoon J (2008). Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. The American Journal of Psychiatry, 165(8), 1006–1014. 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Cahill ND, Arbabshirani MR, White T, Baum SA, & Michael AM (2016). Sex and age effects of functional connectivity in early adulthood. Brain connectivity, 6(9), 700–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


