Abstract
Wolffian duct (WD) maintenance and differentiation is predominantly driven by the androgen action, which is mediated by the androgen receptor (AR). It is well established that the mesenchyme indicates the fate and differentiation of epithelial cells. However, in vivo developmental requirement of mesenchymal AR in WD development is still undefined. By designing a mesenchyme-specific Ar knockout (ARcKO), we discovered that the loss of mesenchymal Ar led to the bilateral or unilateral degeneration of caudal WDs and cystic formation at the cranial WDs. Ex vivo culture of ARcKO WDs invariably resulted in bilateral defects, suggesting that some factor(s) originating from surrounding tissues in vivo might promote WD survival and growth even in the absence of mesenchymal Ar. Mechanistically, we found cell proliferation was significantly reduced in both epithelial and mesenchymal compartments; but cell apoptosis was not affected. Transcriptomic analysis by RNA sequencing of E14.5 mesonephroi revealed 131 differentially expressed genes. Multiple downregulated genes (Top2a, Wnt9b, Lama2, and Lamc2) were associated with morphological and cellular changes in ARcKO male embryos (ie, reduced cell proliferation and decreased number of epithelial cells). Mesenchymal differentiation into smooth muscle cells that are critical for morphogenesis was also impaired in ARcKO male embryos. Taken together, our results demonstrate the crucial roles of the mesenchymal AR in WD maintenance and morphogenesis in mice.
Keywords: Wolffian duct, androgens, androgen receptor, mesenchyme, epididymis
Androgen signaling is essential for Wolffian duct (WD) maintenance and its differentiation into male reproductive tract organs including the epididymis, vas deferens, and seminal vesicle (1-3). In the 1940s, Jost demonstrated that castrated rabbit embryos were born without WD derivatives and androgen replacement restored the WD structure (4, 5). The predominant action of androgens in WD development is mediated through the androgen receptor (AR), which is mainly a ligand-activated transcription factor (6). Global AR knockout or loss-of-function AR mutations result in WD regression in the male mice (7-10). AR mutations in XY human individuals can cause androgen insensitivity syndrome characterized by feminization of the external genitalia and underdevelopment or loss of the internal male reproductive tract organs (11). These observations demonstrate that the androgen-AR signaling is required for WD development.
Androgen-AR signaling regulates WD development through epithelial-mesenchymal interactions (1, 3). In male mouse embryos, AR expression first appears in the mesenchyme surrounding WDs at embryonic day (E) 12.5 but is absent in WD epithelium until E15.5 to E16.5 (7, 12). Epithelium-specific deletion of Ar does not impair WD survival, elongation, or morphogenesis (7), indicating that functional AR action occurs primarily in the paracrine mesenchyme. Classic tissue-recombinant studies have also demonstrated that mesenchymal androgen actions govern androgen-induced epithelial morphogenesis and differentiation. For example, when the epithelium from the upper WD (future epididymis) was combined with the lower WD mesenchyme (future seminal vesicle), the epithelium lost its epididymal identity and became seminal vesicle-like structures (13). The inductive capability of the mesenchyme was also found in another androgen target organ, the prostate. AR-deficient prostate epithelium recombined with wild-type prostate mesenchyme still exhibited ductal morphogenesis; by contrast, wild-type prostate epithelium recombined with AR-deficient prostate mesenchyme failed to undergo prostatic morphogenesis (14).
Those results from epithelium-specific Ar knockout and tissue recombination experiments provide evidence that the morphogenesis and differentiation of the WD requires the AR action in the mesenchyme. However, in vivo developmental requirement of mesenchymal androgen signaling in fetal WD maintenance and morphogenesis has not yet been elucidated. To address this knowledge gap, we set out to use the mouse Cre-flox system to achieve the efficient ablation of Ar in the WD mesenchyme at early development. We then studied the morphological, cellular, and molecular consequences of deleting mesenchymal Ar on WD development.
Methods and Materials
Mice
An Osr2-Cre (knockin Cre) line was provided by Rulang Jiang (Cincinnati Children's Hospital Medical Center) and maintained in its original genetic background (129/Sv X C57BL/6J) (15). Ar-flox (strain No. 018450) and Rosa26-tdTomato (strain No. 007909) were purchased from Jackson Laboratory. Timed mating was set up by housing 1 stud Osr2Cre+ male with 2 or 3 sexually mature XAr-floxXAr-flox females in the late afternoon. Vaginal plugs were checked early the next morning, and the day of plug detection was designated as E0.5. The male embryos harboring XAr-floxfY and Osr2Cre+;XAr-floxY were designated as control and Ar conditional knockout (ARcKO), respectively. All procedures involving animals were approved by the University of Wisconsin–Madison (UW-Madison) Animal Care and Use Committees and follow UW-Madison–approved animal study proposals and public laws.
Genotyping
Polymerase chain reaction was performed with primers (forward 5'-AAAATGCCTCCTTTTGAC CA-3', reverse 5'-AAGATGACAGTCCCCACGAG-3') for Ar-flox allele and primers (Cre83: 5'-GTCCAATTTACTGACCGTACACC-3', Cre85: 5'-GTTATTCGGATCATCAGCTACACC-3') for Osr2-Cre allele (15). Platinum II Taq Hot-Start DNA Polymerase (Invitrogen, catalog No. 14966001) was used to run the thermal cycle. The conditions for the thermal cycle were 94 °C for 2 minutes, 34 cycles of (94 °C for 15 seconds, 60 °C for 5 seconds, and 68 °C for 15 seconds) followed by 68 °C for 5 min.
Tissue Processing, Embedding, and Sectioning
Tissues were fixed in 10% neutral buffered formalin (Leica, catalog No. 3800598) overnight at room temperature, underwent 3 10-minute 1 × phosphate-buffered saline (PBS) washes, and dehydrated by a series of ethanol (70%, 80%, and 95% for 30 minutes each; 100% ethanol I and II for 50 minutes each). Tissues were then cleared and embedded in paraffin as described previously (16). These embedded tissues were then sectioned at 5 μm using a microtome (TN6000, Tanner Scientific) from the cranial to caudal epididymis with 50 μm between sections.
Immunofluorescence
Paraffin sections were deparaffinized and rehydrated. Sections were then exposed to antigen unmasking solution (VECTOR, catalog No. H-3300) for antigen retrieval using a microwave oven. After cooling, the slides were transferred to the Sequenza Immunostaining Center System (Thermo Fisher Scientific, catalog No. 73310017; cover plates: catalog No. 72110017) and washed with PBS and then PBST (1 × PBS with 0.1% Triton X-100). Sections were then incubated in a blocking buffer (5% normal donkey serum in PBST) for 1 hour followed by overnight incubation with primary antibodies: rabbit anti-Ki67 (Abcam, ab15580, RRID:AB_443209); rabbit anti-cleaved PARP1 (Abcam, ab32064, RRID:AB_777102); rabbit anti-alpha-smooth muscle actin (αSMA) (Abcam, ab5694, RRID:AB_2223021); rabbit anti-laminin (Novus Biologicals, NB-300, RRID:AB_10001146); rabbit anti-DsRed Polyclonal Antibody (Takara, 632496, RRID:AB_10013483) in blocking buffer at 4 °C using a 1:200 ratio. The next day, sections were washed 3 times with PBST and incubated for 1 hour at room temperature with the secondary antibodies (donkey anti-rabbit Alexa Fluor 488 conjugate; Invitrogen, A-21206, RRID:AB_2535792) (1:200). Two more PBST washes were then completed, and the slides were counterstained with DAPI (4′,6-diamidino-2-phenylindole; 1:1000, Thermo Fisher Scientific, 62248). Lastly, slides were mounted in EverBrite mounting medium (Biotium, 23003) and prepared for imaging under a Leica TCS SP8 confocal microscope.
Quantification of Proliferation, Apoptosis, and Smooth Muscles
ImageJ was used to analyze these end points in 3 to 5 sections per mouse. Mesenchymal compartment within 0.6 μm away from the WD basement membrane was used for quantifying the numbers of cleaved-PARP1+ and Ki67+ cells in the mesenchyme. The distance (0.6 μm) was determined to exclude the neighbor mesenchyme surrounding müllerian ducts in our data analysis. The total numbers of cells (DAPI staining for individual nucleus), cleaved-PARP1+ and Ki67+ cells in epithelial and mesenchymal compartments in each section were counted twice and averaged. The percentage of apoptotic or proliferating cells was calculated as the fraction of cleaved-PARP1+ or Ki67+ cells over the total number of cells for subsequent statistical analysis. For smooth muscle quantifications, the outer circumference of αSMA+ cell and WD lumens were outlined and the areas between the two outlines were calculated in ImageJ.
RNAScope
We followed the manufacturer's protocol as previously described (17). Briefly, after the tissues were prepared, deparaffinized, and rehydrated, they were treated with hydrogen peroxide, antigen retrieval buffer, and proteinase. Sections were then exposed to a Wnt9b probe and subjected to a series of rinsing and signaling detection at designated temperatures. All sections were then imaged under a compound microscope.
Immunohistochemistry
Paraffin sections were subjected to deparaffinization, rehydration, and antigen retrieval as described for the immunofluorescence procedure. Sections were treated with 3% H2O2 to eliminate endogenous peroxidase activities, washed with PBST (1 × PBS with 0.1% Triton X-100), and then incubated in the blocking buffer for 1 hour followed by incubation with primary antibody rabbit anti-androgen receptor (Abcam,133273, RRID:AB_11156085) (1:200) in the blocking buffer at 4 °C overnight. The following day, sections were washed with PBST 3 times and then incubated with biotinylated donkey anti-rabbit immunoglobulin G H&L (Abcam, ab97062, RRID:AB_10679678) (1:200) at room temperature for 30 minutes. Sections were then incubated with ABComplex/HRP (VECTOR, PK-6100) and DAB substrate (VECTOR, SK-4100), counterstained with hematoxylin, and mounted for imaging.
Reverse-Transcription Polymerase Chain Reaction
Gonads were snap-frozen on dry ice. RNA extraction and cDNA synthesis were performed using PicoPure RNA Isolation kit (Life technologies catalog No. KIT0204 Life Technologies) and the Superscript cDNA synthesis kit (Qiagen, catalog No. 330411, Qiagen), according to manufacturer's protocols. A total of 200 ng RNA in each sample was used for cDNA synthesis. SYBR Green PCR kit (Qiagen, catalog No. 208052) and 3 other primers (Harvard Primer Bank, Hsd3b1: 6680289a1; Cyp17a1: 6681097a1; Gapdh, 6679937a1) were used to run thermal cycles. All samples were analyzed in duplicates and normalized to the housekeeping gene Gapdh. The relative expression in knockout tissues was reported as a ratio of the expression relative to those of control mice.
RNA Sequencing and Analysis
RNA was sent to Novogene for an RNA quality check, library preparation, and sequencing (paired-end 150 nt reads). The RNA integrity number (RIN) of RNAs were all above 7.5. The raw sequencing data were uploaded and processed using Partek Flow. The Partek Flow pipeline includes trimming of low-quality bases using a Phred quality score of Q35, mm10 genome alignment using the STAR aligner (18), quantification of counts by gene based on the Refseq transcripts release 93. gene count normalization (transcripts per million), and DEseq2 differential gene expression analysis with a default setting. Differentially expressed genes (DEGs) were defined using an adjusted P-value threshold of less than .1. Gene ontology (GO) and pathway analyses were performed using Enrichr (19). Sequencing files were deposited to GEO under accession number GSE246141.
Organ Culture
E14.5 mesonephroi with testes attached were cultured at 37 °C with 5% CO2/95% air on MilliCELL-CM culture insert 0.4-µm filters (MilliporeSigma, PICM03050) in Dulbecco’s modified Eagle’s medium/F12 (Gibco, 21041025) supplemented with 100 U/mL penicillin–streptomycin (Gibco, 15070063) (20). Media was changed every other day. Bright-field images were taken daily with a Leica S9 scope.
Statistical Analysis
Quantitative data are presented as mean ±SD and the sample sizes are indicated in figure legends. Two-tail t test was used for evaluating the significant difference between control and ARcKO groups. The statistical significance level was set at P less than .05.
Results
Ablation of Mesenchymal Ar Caused Defective Wolffian Duct Morphogenesis.
To achieve genetic ablation of Ar in the mesenchyme surrounding WDs, we set out to use the previously characterized constitutive knockin Cre line Osr2-Cre (15). When we crossed Osr2-Cre with Rosa26-tdTomato to generate Osr2Cre+;RosatdTomato+ male embryos, the tdTomato expression was detected in the surrounding mesenchyme at E11.5 (before the initiation of androgen production) and on 2 later time points, E12.5 (the initiation of androgen production (21) and E16.5 (during WD morphogenesis) (Fig. 1A). Of note, the Cre line used in our studies did not have the problem of ectopic Cre activity found in another line Osr2-CreIRES, in which Cre was inserted into the 3’ untranslated regions of the Osr2 gene (22). These results confirm that the Cre activity is restricted to the mesenchyme surrounding the WD, and the onset of the Cre activity is prior to androgen production.
Figure 1.
Mesenchyme-specific ablation of Ar leads to defective Wolffian duct morphogenesis. A, Immunofluorescent staining of DsRed for tdTomato expression in Osr2Cre+; RosatdTomato+ male embryos at 3 time points (E11.5, E12.5, and E14.5). Dashed white lines demarcate Wolffian duct epithelium, which is negative for tdTomato expression. Scale bar: 25 μm. B, Immunohistochemical staining of androgen receptor (AR) at E14.5. Blue arrows: Wolffian duct epithelium. Scale bar: 50 μm. C, Brightfield image of PND0 control and ARcKO mice. Scale bar: 4.0 mm.
Next, we set up crossings of Osr2Cre+ males with Arflox/flox females to generate control (Arflox/Y) and mesenchyme-specific AR knockout (ARcKO, Osr2Cre+;ArfloxY) littermates (7). In the control group, AR was detected in the nuclei of mesenchymal cells surrounding the WD epithelium but was not detected in the WD epithelium at E14.5 (Fig. 1B). Our observation of the AR expression pattern at E14.5 was consistent with the previous report (12). In the ARcKO group, AR expression was absent in the peritubular mesenchyme, demonstrating the efficient ablation of Ar in the mesenchyme. To our surprise, WD epithelium in the ARcKO group expressed nuclear AR (see Fig. 1B), which was an unexpected gene expression change in the epithelium.
To discover the consequence of the loss of the mesenchymal Ar on WD development, we examined control and ARcKO mice at birth (postnatal day 0, PND0). In the control, the anterior region of WDs developed into the coiled epididymis (N = 23); however, in their knockout littermates, the WD partially degenerated, and the maintained WD at the anterior region lost its characteristic coiling and became cystic (N = 17). The epididymal cystic formation occurred bilaterally in N = 7 of ARcKO male embryos and unilaterally in N = 10 (left horns were affected in 9 embryos while right horns were affected in 1 embryo) (Fig. 1C). In those ARcKO male embryos with the unilateral cystic epididymis, the contralateral epididymis still underwent coiling, although the extent of coiling (number of turns per mm) was reduced (P = .053) (Supplementary Fig. S1A and S1B) (23). We confirmed that mesenchymal Ar on both sides was successfully ablated (Supplementary Fig. S2) (23).
The Osr2-CRE activity is expected in the fetal Leydig cell where Osr2 expression is enriched (24). We also observed its activity in the interstitial cells of the testis (Supplementary Fig. S3A and S3B) (23). In global Ar knockout males, androgen production is unaffected and the testis appears normal until PND5 (25). In addition, the specific loss of Ar in the somatic cells of the fetal testis has no effect on normal androgen production and androgen-dependent urogenital organ development (26-28). In our study, the testis of ARcKO male embryos looked normal on PND0 (Fig. 1C) and the expression of 2 key steroidogenic enzymes, Cyp17a1 and Hsd3b1 (29), were comparable between control and ARcKO testes at E14.5 (Supplementary Fig. S4) (23). We thus conclude that androgen production is not compromised in our ARcKO mice, and the defective WD development results from the local loss of the mesenchymal Ar. Taken together, these results demonstrate that the mesenchymal AR is essential for WD maintenance and morphogenesis.
Ex Vivo Culture of ARcKO Wolffian Ducts (WDs) Revealed Progression of Abnormal WD Development.
To understand how the morphological defect in ARcKO WDs arises over time in the same embryo, we decided to culture the mesonephroi with testes starting from E14.5. We used our established ex vivo explant culture method that enables recapitulation of in vivo morphological changes of WDs (20). After 2 days of culture, WDs in the control group became elongated and developed 2 to 3 turns in the cranial regions (Fig. 2). After 2 additional days, extensive coiling was observed in both the cranial and caudal regions of the presumed epididymis (see Fig. 2). By contrast, WDs in the ARcKO group were underdeveloped and did not undergo coiling after 2 days of culture (see Fig. 2). In addition, the cranial region of WDs degenerated (see Fig. 2). After 4 days of culture, cysts had formed in the cranial region, comparable to the phenotype observed in vivo (see Fig. 2). These results demonstrated the progression of abnormal WD development in ARcKO embryos: decreased tubular growth, partial WD degeneration, and then cyst formation. All ARcKO tissues showed bilateral degeneration and cyst formation in the cranial region in ex vivo culture condition (100%, N = 6); however, during the in vivo condition, cystic formation occurred bilaterally in 41% (7/17) of ARcKO male embryos and unilaterally in 59% (10/17) ARcKO male embryos. These observations suggest signaling originating from surrounding tissues might provide a compensation mechanism for promoting WD survival and growth in vivo.
Figure 2.

Visualization of Wolffian duct development over time in control and ARcKO groups. Ex vivo organ culture of paired male reproductive tracts with testes attached in control and ARcKO groups starting at E14.5 (day 0) for 4 days. Blue and purple arrows indicate cranial and caudal Wolffian duct, respectively. Scale bar: 2.0 mm.
Attenuated Ductal Growth and Cellular Proliferation in ARcKO Wolffian Ducts
As a way to elucidate cellular mechanisms underlying WD stunted elongation and degeneration in ARcKO male embryos, we sectioned E14.5 mesonephros to investigate cell proliferation and apoptosis, two major events involved in WD elongation (30) and regression (7), respectively. When we examined the cross-sections, we immediately noticed a reduced diameter of WDs in the ARcKO groups: The circumference outlining the WD basement border and the average number of epithelial cells per section were significantly reduced (Fig. 3A and 3B). The percentages of proliferating cells (Ki67+) in the epithelial and mesenchymal compartment were significantly increased in the ARcKO groups (Fig. 3C and 3D). In contrast, the percentage of apoptotic cells in either epithelial or mesenchymal compartment was not significantly different between the control and ARcKO groups (Fig. 3C and 3E). These results indicate that mesenchymal AR regulates the proliferation of both mesenchymal and epithelial cells but not cell survival during WD development at E14.5.
Figure 3.
Attenuated ductal growth and cellular proliferation in ARcKO Wolffian ducts. A and B, Quantification of the Wolffian duct circumference (μm) A, and the average number of epithelial cells per section, B, in the control and ARcKO groups. C, Immunofluorescent staining of cell proliferation marker Ki67 and cell apoptotic marker cleaved-PARP1 in the control group and ARcKO groups at E14.5. Scale bar: 25 μm. Dashed blue lines outline Wolffian duct epithelial layers. D and E, Quantifications of the percentage of proliferating and apoptotic cells in Wolffian duct epithelium and mesenchyme in control ARcKO groups. Results were shown as mean ± SD and analyzed by 2-tailed t test. NS, no significance. P less than .05; *P less than .05 compared to the control group. N = 5 for each group.
RNA-Sequencing Profiling Identified Differentially Expressed Genes and Pathways in ARcKO Mesonephroi
To gain insight into the molecular action of mesenchymal AR, we performed RNA sequencing (RNA-seq) on control and ARcKO mesonephroi at E14.5, which identified 131 DEGs (Fig. 4A). Among 131 DEGs, 52 were upregulated and 79 genes were downregulated in ARcKO mesonephroi. Ar was one of the top downregulated genes, confirming the efficient ablation of Ar in ARcKO mesonephroi. The top biological processes enriched in these DEGs included regulation of cell growth (Fig. 4B). For example, Top2a, a proliferation marker (31), was decreased (Fig. 4C). Those results were consistent with the observation that the percentage of proliferating cells was reduced in ARcKO mesonephroi (Fig. 3D). It is also worth noting the decreased expression of Wnt9b, which is a WD epithelium–derived WNT ligand and plays a central role in WD maintenance (32). The decreased Wnt9b expression could be either the cause or outcome of epithelial degeneration in ARcKO mesonephroi. To address this question, we performed RNAScope on sections and discovered that Wnt9b was expressed robustly in WD epithelium in both control and ARcKO groups (Fig. 5). These observations suggest that decreased Wnt9b was the outcome of epithelial degeneration in ARcKO mesonephroi.
Figure 4.
Identification of differentially expressed genes and pathways by RNA sequencing in ARcKO mesonephroi. A, Volcano plot displaying gene expression at E14.5. Differentially expressed genes (DEGs; adjusted P values <.1) in the ARcKO group are highlighted in blue (downregulated genes) and red (upregulated genes), respectively. B, Gene ontology (GO) analysis showing enriched cellular components and biological processes associated with DEGs. C, Dot plots illustrating the expression levels of 4 selected genes (Ar, Top2a, Wnt9b, and Lama2). All 4 genes were significantly downregulated in the ARcKO group. TMP, transcripts per million.
Figure 5.
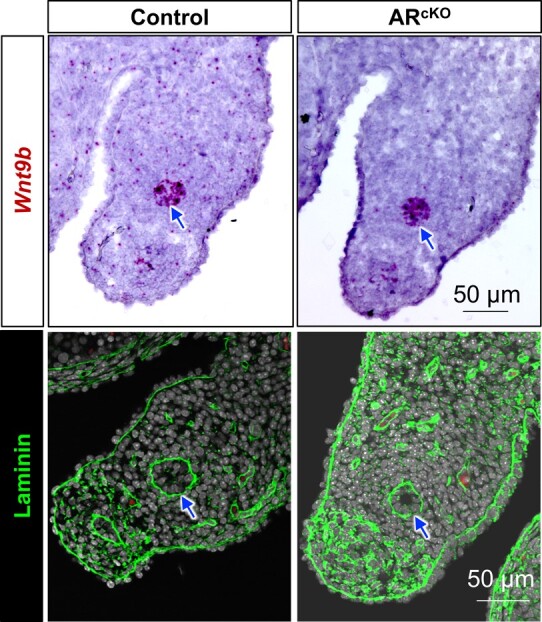
Expression of Wnt9b and laminin in control and ARcKO mesonephroi. Wnt9b and Laminin expression were localized by RNAscope and immunofluorescent staining, respectively. Blue arrows indicate Wolffian ducts. Scale bar: 50 μm.
The nitric oxide biosynthesis and metabolism processes were also enriched. Four genes (endothelin 1, Edn1; dimethylarginine dimethylaminohydrolase, Ddah1; angiotensin II receptor type 2, Agtr2; and clusterin, Clu) corresponding to these processes were all downregulated in ARcKO, suggesting that AR might be a positive regulator of nitric oxide synthesis in the mesenchyme. Nitric oxide signaling has been implicated in testicular and penis functions (33); however, its potential role in WD development and functions remains undefined, which could be an exciting direction for future investigations.
Our pathway analysis identified only one significant pathway enriched in the DEGs, the hedgehog signaling pathway (Shh, Ptch1, Ccnd2, and Hhip). It is known that WD epithelium synthesizes and secretes SHH, which activates its receptor PTCH1 in the mesenchyme to induce Ccnd2 and Hhip expression (34-36). All 4 of these genes were downregulated in the ARcKO group, suggesting that SHH signaling activity was reduced in the absence of mesenchymal Ar.
GO analysis of these DEGs revealed gene enrichment in the basement membrane and collagen-containing extracellular matrix (see Fig. 4B). For example, subunits of the basement membrane protein laminin, Lama2 (see Fig. 4C) and Lamc2, were both downregulated in the ARcKO group. By performing immunofluorescence, we observed laminin expression at the basement membrane of WDs both in the control and ARcKO groups (see Fig. 5). However, the circumference of laminin-positive basement was reduced in the ARcKO groups, consistent with our histological observation in Fig. 3. Of note, 2 genes (Cldn1 and Cldn8) that are known to form tight junctions in the epididymis (37) were downregulated in the ARcKO group, suggesting a potential loss of epithelial impermeability.
Taken together, our transcriptome profiling not only validates our observations that the loss of mesenchymal AR reduced the cell proliferation and the number of epithelial cells at E14.5, but also provides a few pathways potentially interacting with mesenchymal AR.
Impaired Differentiation of Wolffian Ducts Mesenchyme in the Absence of Mesenchymal Ar
Under the androgen action, the mesenchyme surrounding the WD differentiates into smooth muscle cells (38), which provides an important mechanical force for proper WD morphogenesis (39). We therefore examined the smooth muscle marker αSMA at the initiation of smooth muscle differentiation (E16.5) (Fig. 6A) (17). We observed multiple layers of αSMA+ cells in the inner stroma in the control group; however, in the absence of mesenchymal Ar, the αSMA+ cellular area was reduced, which was confirmed quantitatively (Fig. 6B). These observations indicate that mesenchymal cell differentiation was impaired in the absence of mesenchymal Ar.
Figure 6.
Reduced smooth muscle differentiation in Wolffian ducts of ARcKO male embryos. A, Immunofluorescent staining of the smooth muscle marker alpha-smooth muscle actin (αSMA) in the control group and ARcKO group at E16.5. Blue dashed lines outline Wolffian duct epithelium. Scale bar: 50 μm. B, Quantifications of the average area (μm2) of αSMA+ smooth muscle cells in the control and ARcKO groups. Results were shown as mean ± SD. and analyzed by 2-tailed unpaired t test. *P less than .05 compared to the control group. N = 5 for each group.
Discussion
Mesenchymal Androgen Receptor Plays an Essential Role in Wolffian Duct Maintenance
During WD development, AR expression first appeared in the mesenchyme at E12.5 and then in the epithelium at E16.5 (7). When we removed Ar in the mesenchyme, the WDs degenerated predominantly in the caudal region at birth. This phenotype is less severe than that of global AR-deficient mice (testicular feminization Tfm mice), in which both cranial and caudal regions degenerate (40). These observations suggest that other factors may exist to promote survival of cranial WDs in males in the absence of mesenchymal AR signaling.
In male embryos, the degeneration of WDs in the absence of androgen signaling is believed to result from 2 cellular processes, increased apoptosis and decreased epithelial proliferation (7, 40). We observed decreased epithelial proliferation but did not observe an increased number of apoptotic cells in the absence of mesenchymal Ar at E14.5. Our results are consistent with the observation of no evident epithelial apoptosis in Tfm male embryos at E15.5 and E16.5 (40). Nevertheless, another study identified epithelial apoptosis as a major cellular process in WD degeneration in Tfm male embryos at E14.5 (7). This discrepancy may result from different AR ablation models (mesenchyme-specific Ar ablation in our model vs global AR deficiency) and/or distinct examination time points (E14.5 (7) vs E15.5 and E16.5 (40)).
Intriguingly, in the ARcKO group, epithelial cells at E14.5 expressed AR, which is normally not present in the epithelium until E16.5 (7). The consequence of premature expression of epithelial AR is not clear. Classic tissue recombinant studies and an epithelium-specific AR deletion genetic study have demonstrated that epithelial AR is not essential for WD maintenance and morphogenesis (7, 41); however, epithelial AR was essential for epithelial secretory function (42). We speculate that epithelial AR may contribute to the cystic formation observed in our ARcKO model by promoting epithelial secretion. This increased epithelial secretion might result in fluid accumulation, especially since the drainage was blocked due to the caudal ductal regression. Mesenchyme-derived morphogen Inhba was proposed to be responsible for the upregulation of AR in the WD epithelium (43); however, Inhba was not differentially expressed in our RNA-seq. Therefore the signal for promoting epithelial AR expression remains to be determined.
Mesenchymal Androgen Receptor Regulates Smooth Muscle Differentiation in Male Reproductive Tract Development
It is known that androgen-AR signaling is critical for smooth muscle differentiation in the WD. When male rat embryos were exposed to the AR antagonist flutamide during the masculinization window, the αSMA+ cellular layer surrounding WDs was significantly reduced without causing apparent epithelial damage (44). AR is expressed both in epithelial and mesenchymal cells at the initiation of αSMA expression in the mesenchyme. A previous study showed that deletion of the epithelial AR did not affect smooth muscle differentiation, suggesting that mesenchymal AR might be the key player. Here, by ablating Ar specifically in the mesenchyme, we provided the direct evidence that mesenchymal AR is required for proper smooth muscle differentiation. In addition, we observed a reduced number of proliferating cells in the mesenchymal compartment in the absence of mesenchymal Ar. Therefore, mesenchymal AR might regulate smooth muscle proliferation as well.
Of note, αSMA+ cell formation was not completely abolished in the absence of the mesenchymal Ar, suggesting that other inductive signals exist in promoting smooth muscle differentiation. In another androgen-AR signaling target organ, the prostate, both androgens and epithelium-derived signals direct smooth muscle differentiation (45). When the mesenchyme of the urogenital sinus (primordium of the prostate) was grafted alone to an intact male, few αSMA+ cells were induced and no organization of smooth muscle tissue were established. SHH is expressed in the WD and is known to play a critical role in smooth muscle development in the bladder (46) and small intestine (47). However, the deletion of Shh in the WD did not affect smooth muscle formation or overall WD development (48). Therefore, epithelium-derived factor(s) for regulating smooth muscle differentiation remain to be discovered.
Smooth muscle cells provide both mechanical forces and growth factors for proper WD morphogenesis. Inhibition of αSMA expression (presumably smooth muscle differentiation) decreased mechanical resistance of the tissues surrounding the tubule, which led to increased tubule diameter (39). In the ureter development, delayed onset of smooth muscle cell differentiation leads to hydroureter formation in mice (49). When Ar was deleted in smooth muscles of seminal vesicles, seminal vesicles in adulthood became smaller with reduced epithelial cell height (50). Therefore, impairment of smooth muscle differentiation in our study may reduce mechanical force and growth factor signaling to the epithelium, contributing to cystic formation in the cranial region.
Loss of Mesenchymal Androgen Receptor Does not Always Result in Bilateral Wolffian Duct Degeneration
Congenital bilateral/unilateral absence of the vas deferens (the caudal WD) in humans is found in 1% to 2% of men with infertility (51). This birth defect can be caused by mutations in CFTR (cystic fibrosis transmembrane conductance regulator) (52) and ADGRG2 (adhesion G protein–coupled receptor G2) (53). CFTR and ADGRG2 are both expressed in the epithelium and control the luminal environment by regulating anion transport and fluid reabsorption, respectively. In rats, testosterone regulates the CFTR level in seminal vesicles and the vas deferens (54, 55). In human caput epididymal epithelium, AR binding sites were found in the CFTR and ADGRG2 promoters (56). These results suggest that CFTR and ADGRG2 might be two potential AR targets in the epithelium. It remains to be determined whether AR signaling disruption might contribute to bilateral/unilateral absence of the vas deferens in humans.
One unique finding of our mesenchymal ARcKO model was that male embryos manifested unilateral WD defects (10/17), which predominantly affected the left side (9/10, 90%). It has been long noticed that congenital defects of male reproductive tracts can occur unilaterally in mammalian species, including humans (57-60). It seems that unilateral abnormalities of the urogenital system tend to occur with a left bias. For example, unilateral anomalies of the kidney and urinary tract occur more often on the left side (53%-63.2% depending on anomalies) than the anticipated 50% (61). The left-sided abnormalities in androgen-dependent testicular descent were also observed in 2 mouse models that deleted Wt1 or Wnt4 in the gubernacular (62, 63). It was speculated that the asymmetrical position of the internal organs might create mechanistic restrictions on the movements of the gonads, or driver genes for left-right patterning might contribute to left-sided defects (62, 63). In our mesenchymal ARcKO model, when paired mesonephroi grew in an ex vivo culture condition, the unilateralism of WD degeneration observed in vivo was abolished: WDs on both sides underwent regression in the caudal region. These observations suggest there might be some growth factor(s) deriving from the right side of the embryo that promote WD maintenance even in the absence of mesenchymal Ar. A potential growth factor candidate is Fgf8, which is detected in the adjacent somite but not in the mesonephros after E11.5 (64). Ablation of Fgf8 in the mesodermal lineages including somite caused the loss of WDs in mouse male embryos (64). Right asymmetric expression of Fgf8 has been noticed in chicken embryos; it would be intriguing to investigate whether Fgf8 expression during sexual differentiation is asymmetric in mouse embryos (65).
Acknowledgments
We would like to thank Dr Rulang Jiang from Cincinnati Children's Hospital Medical Center, Division of Developmental Biology, for providing the Osr2-Cre knockin mice. We are also thankful to the animal care staff at the University of Wisconsin–Madison School of Veterinary Medicine for taking care of the mouse colonies.
Abbreviations
- αSMA
alpha-smooth muscle actin
- AR
androgen receptor
- ARcKO
mesenchyme-specific Ar knockout
- cDNA
complementary DNA
- DEG
differentially expressed gene
- E
embryonic day
- PBS
phosphate-buffered saline
- PND
postnatal day
- RNA-seq
RNA sequencing
- WD
Wolffian duct
Contributor Information
Jillian Wilbourne, Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA.
Shuai Jia, Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA.
Allyssa Fogarty, Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA; Comparative Biomedical Sciences Graduate Program, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA.
Motoki Takaku, Department of Biomedical Sciences, School of Medicine, University of North Dakota, Grand Forks, ND 58202, USA.
Fei Zhao, Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA; Comparative Biomedical Sciences Graduate Program, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (grant Nos. R00HD096051 and R01HD111425 to F.Z.).
Disclosures
The authors declare that they have no competing interests.
Data Availability
All data are available in the main text or the supplementary materials on reasonable request. Bulk RNA-seq data have been deposited in the GEO database under the accession code number GSE246141.
References
- 1. Murashima A, Kishigami S, Thomson A, Yamada G. Androgens and mammalian male reproductive tract development. Biochim Biophys Acta. 2015;1849(2):163‐170. [DOI] [PubMed] [Google Scholar]
- 2. Sharpe RM. Androgens and the masculinization programming window: human-rodent differences. Biochem Soc Trans. 2020;48(4):1725‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amato CM, Yao HH, Zhao F. One tool for many jobs: divergent and conserved actions of androgen signaling in male internal reproductive tract and external genitalia. Front Endocrinol (Lausanne). 2022;13:910964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jost A. Hormonal factors in the sex differentiation of the mammalian foetus. Philos Trans R Soc Lond B Biol Sci. 1970;259(828):119‐130. [DOI] [PubMed] [Google Scholar]
- 5. Jost A. Problems of fetal endocrinology—the gonadal and hypophyseal hormones. Recent Prog Horm Res. 1953;8:379‐418. [Google Scholar]
- 6. Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37(1):3‐15. [PMC free article] [PubMed] [Google Scholar]
- 7. Murashima A, Miyagawa S, Ogino Y, et al. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology. 2011;152(4):1640‐1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaspar ML, Meo T, Bourgarel P, Guenet JL, Tosi M. A single base deletion in the Tfm androgen receptor gene creates a short-lived messenger RNA that directs internal translation initiation. Proc Natl Acad Sci U S A. 1991;88(19):8606‐8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeh S, Tsai MY, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99(21):13498‐13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooke PS, Walker WH. Male fertility in mice requires classical and nonclassical androgen signaling. Cell Rep. 2021;36(7):109557. [DOI] [PubMed] [Google Scholar]
- 11. Batista RL, Costa EMF, Rodrigues AS, et al. Androgen insensitivity syndrome: a review. Arch Endocrinol Metab. 2018;62(2):227‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991;128(6):2867‐2873. [DOI] [PubMed] [Google Scholar]
- 13. Higgins SJ, Young P, Cunha GR. Induction of functional cytodifferentiation in the epithelium of tissue recombinants. II. Instructive induction of Wolffian duct epithelia by neonatal seminal vesicle mesenchyme. Development. 1989;106(2):235‐250. [DOI] [PubMed] [Google Scholar]
- 14. Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differentiation. 2008;76(6):578‐586. [DOI] [PubMed] [Google Scholar]
- 15. Chen J, Lan Y, Baek JA, Gao Y, Jiang R. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol. 2009;334(1):174‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jia S, Wilbourne J, Crossen MJ, Zhao F. Morphogenesis of the female reproductive tract along antero-posterior and dorso-ventral axes is dependent on Amhr2+ mesenchyme in mice. Biol Reprod. 2022;107(6):1477‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao F, Grimm SA, Jia S, Yao HH. Contribution of the Wolffian duct mesenchyme to the formation of the female reproductive tract. PNAS Nexus. 2022;1(4):pgac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29(1):15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuleshov MV, Jones MR, Rouillard AD, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44(W1):W90‐W97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia S, Zhao F. Ex vivo development of the entire mouse fetal reproductive tract by using microdissection and membrane-based organ culture techniques. Differentiation. 2022;123:42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Shaughnessy PJ, Baker P. et al. et al. Fetal development of Leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology. 1998;139(3):1141‐1146. [DOI] [PubMed] [Google Scholar]
- 22. Lan Y, Wang Q, Ovitt CE, Jiang R. A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis. 2007;45(10):618‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilbourne J, Jia S, Fogarty A, Takaku M, Zhao F. Crucial roles of the mesenchymal androgen receptor in Wolffian duct development. Endocrinology. 2023:bqad193. doi: 10.1210/endocr/bqad193 [DOI] [PMC free article] [PubMed]
- 24. McClelland KS, Bell K, Larney C, et al. Purification and transcriptomic analysis of mouse fetal Leydig cells reveals candidate genes for specification of gonadal steroidogenic cells. Biol Reprod. 2015;92(6):145. [DOI] [PubMed] [Google Scholar]
- 25. O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci. 2002;115(17):3491‐3496. [DOI] [PubMed] [Google Scholar]
- 26. De Gendt K, Swinnen JV, Saunders PT, et al. A sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101(5):1327‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holdcraft RW, Braun RE. Androgen receptor function is required in sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131(2):459‐467. [DOI] [PubMed] [Google Scholar]
- 28. Shima Y. Development of fetal and adult Leydig cells. Reprod Med Biol. 2019;18(4):323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao F, Franco HL, Rodriguez KF, et al. Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science. 2017;357(6352):717‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hinton BT, Galdamez MM, Sutherland A, et al. How do you get six meters of epididymis inside a human scrotum? J Androl. 2011;32(6):558‐564. [DOI] [PubMed] [Google Scholar]
- 31. Neubauer E, Wirtz RM, Kaemmerer D, et al. Comparative evaluation of three proliferation markers, Ki-67, TOP2A, and RacGAP1, in bronchopulmonary neuroendocrine neoplasms: issues and prospects. Oncotarget. 2016;7(27):41959‐41973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9(2):283‐292. [DOI] [PubMed] [Google Scholar]
- 33. Luo Y, Zhu Y, Basang W, Wang X, Li C, Zhou X. Roles of nitric oxide in the regulation of reproduction: a review. Front Endocrinol (Lausanne). 2021;12:752410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franco HL, Yao HH. Sex and hedgehog: roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res. 2012;20(1):247‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barsoum I, Yao HH. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal Leydig cells. Biol Reprod. 2011;84(5):894‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raleigh DR, Reiter JF. Misactivation of hedgehog signaling causes inherited and sporadic cancers. J Clin Invest. 2019;129(2):465‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cyr DG, Dufresne J, Gregory M. Cellular junctions in the epididymis, a critical parameter for understanding male reproductive toxicology. Reprod Toxicol. 2018;81:207‐219. [DOI] [PubMed] [Google Scholar]
- 38. Welsh M, Saunders PT, Sharpe RM. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology. 2007;148(7):3185‐3195. [DOI] [PubMed] [Google Scholar]
- 39. Hirashima T. Pattern formation of an epithelial tubule by mechanical instability during epididymal development. Cell Rep. 2014;9(3):866‐873. [DOI] [PubMed] [Google Scholar]
- 40. Welsh M, Sharpe RM, Walker M, Smith LB, Saunders PT. New insights into the role of androgens in wolffian duct stabilization in male and female rodents. Endocrinology. 2009;150(5):2472‐2480. [DOI] [PubMed] [Google Scholar]
- 41. Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205(2):181‐193. [DOI] [PubMed] [Google Scholar]
- 42. Donjacour AA, Cunha GR. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;132(6):2342‐2350. [DOI] [PubMed] [Google Scholar]
- 43. Tomaszewski J, Joseph A, Archambeault D, Yao HH. Essential roles of inhibin beta A in mouse epididymal coiling. Proc Natl Acad Sci U S A. 2007;104(27):11322‐11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Welsh M, Saunders PT, Marchetti NI, Sharpe RM. Androgen-dependent mechanisms of Wolffian duct development and their perturbation by flutamide. Endocrinology. 2006;147(10):4820‐4830. [DOI] [PubMed] [Google Scholar]
- 45. Cunha GR, Hayward SW, Dahiya R, Foster BA. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat (Basel). 1996;155(1):63‐72. [DOI] [PubMed] [Google Scholar]
- 46. Shiroyanagi Y, Liu B, Cao M, et al. Urothelial sonic hedgehog signaling plays an important role in bladder smooth muscle formation. Differentiation. 2007;75(10):968‐977. [DOI] [PubMed] [Google Scholar]
- 47. Huycke TR, Miller BM, Gill HK, et al. Genetic and mechanical regulation of intestinal smooth muscle development. Cell. 2019;179(1):90‐105.e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murashima A, Akita H, Okazawa M, et al. Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm. Dev Biol. 2014;386(1):216‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weiss AC, Bohnenpoll T, Kurz J, et al. Delayed onset of smooth muscle cell differentiation leads to hydroureter formation in mice with conditional loss of the zinc finger transcription factor gene Gata2 in the ureteric mesenchyme. J Pathol. 2019;248(4):452‐463. [DOI] [PubMed] [Google Scholar]
- 50. Welsh M, Moffat L, Jack L, et al. Deletion of androgen receptor in the smooth muscle of the seminal vesicles impairs secretory function and alters its responsiveness to exogenous testosterone and estradiol. Endocrinology. 2010;151(7):3374‐3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Souza DAS, Faucz FR, Pereira-Ferrari L, Sotomaior VS, Raskin S. Congenital bilateral absence of the vas deferens as an atypical form of cystic fibrosis: reproductive implications and genetic counseling. Andrology. 2018;6(1):127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bieth E, Hamdi SM, Mieusset R. Genetics of the congenital absence of the vas deferens. Hum Genet. 2021;140(1):59‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patat O, Pagin A, Siegfried A, et al. Truncating mutations in the adhesion G protein-coupled receptor G2 gene ADGRG2 cause an X-linked congenital bilateral absence of vas Deferens. Am J Hum Genet. 2016;99(2):437‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramli NS, Giribabu N, Muniandy S, Salleh N. Testosterone regulates levels of cystic fibrosis transmembrane regulator, adenylate cyclase, and cAMP in the seminal vesicles of orchidectomized rats. Theriogenology. 2016;85(2):238‐246. [DOI] [PubMed] [Google Scholar]
- 55. Khadijah Ramli NS, Giribabu N, Salleh N. Testosterone enhances expression and functional activity of epithelial sodium channel (ENaC), cystic fibrosis transmembrane regulator (CFTR) and sodium hydrogen exchanger (NHE) in vas deferens of sex-steroid deficient male rats. Steroids. 2018;138:117‐133. [DOI] [PubMed] [Google Scholar]
- 56. Yang R, Browne JA, Eggener SE, Leir SH, Harris A. A novel transcriptional network for the androgen receptor in human epididymis epithelial cells. Mol Hum Reprod. 2018;24(9):433‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mieusset R, Bieth E, Daudin M, et al. Male partners of infertile couples with congenital unilateral absence of the vas deferens are mainly non-azoospermic. Andrology. 2020;8(3):645‐653. [DOI] [PubMed] [Google Scholar]
- 58. Donohue RE, Fauver HE. Unilateral absence of the vas deferens. A useful clinical sign. JAMA. 1989;261(8):1180‐1182. [PubMed] [Google Scholar]
- 59. Moon JH, Yoo DY, Jo YK, et al. Unilateral cryptorchidism induces morphological changes of testes and hyperplasia of sertoli cells in a dog. Lab Anim Res. 2014;30(4):185‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hahn KL, Beres B, Rowton MJ, et al. A deficiency of lunatic fringe is associated with cystic dilation of the rete testis. Reproduction. 2009;137(1):79‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schreuder MF. Unilateral anomalies of kidney development: why is left not right? Kidney Int. 2011;80(7):740‐745. [DOI] [PubMed] [Google Scholar]
- 62. Kaftanovskaya EM, Neukirchner G, Huff V, Agoulnik AI. Left-sided cryptorchidism in mice with Wilms’ tumour 1 gene deletion in gubernaculum testis. J Pathol. 2013;230(1):39‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seth A, Bournat JC, Medina-Martinez O, et al. Loss of WNT4 in the gubernaculum causes unilateral cryptorchidism and fertility defects. Development. 2022;149(23):dev201093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kitagaki J, Ueda Y, Chi X, et al. FGF8 is essential for formation of the ductal system in the male reproductive tract. Development. 2011;138(24):5369‐5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boettger T, Wittler L, Kessel M. FGF8 functions in the specification of the right body side of the chick. Curr Biol. 1999;9(5):277‐280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the main text or the supplementary materials on reasonable request. Bulk RNA-seq data have been deposited in the GEO database under the accession code number GSE246141.






