Abstract
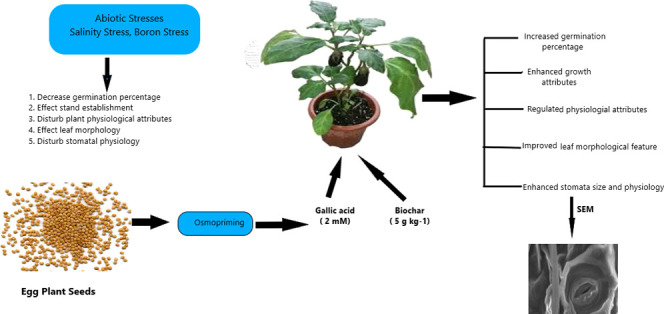
Dynamic shifts in climatic patterns increase soil salinity and boron levels, which are the major abiotic factors that affect plant growth and secondary metabolism. The present study assessed the role of growth regulators, including biochar (5 g kg–1) and gallic acid (GA, 2 mM), in altering leaf morpho-anatomical and physiological responses of Solanum melongena L. exposed to boron (25 mg kg–1) and salinity stresses (150 mM NaCl). These growth regulators enhanced leaf fresh weight (LFW) (70%), leaf dry weight (LDW) (20%), leaf area (LA), leaf area index (LAI) (85%), leaf moisture content (LMC) (98%), and relative water content (RWC) (115%) under salinity and boron stresses. Physiological attributes were analyzed to determine the stress levels and antioxidant protection. Photosynthetic pigments were negatively affected by salinity and boron stresses along with a nonsignificant reduction in trehalose, GA, osmoprotectant, and catalase (CAT) and ascorbate peroxidase (APX) activity. These parameters were improved by biochar application to soil and presoaking seeds in GA (p < 0.05) in both varieties of S. melongena L. Scanning electron microscopy (SEM) and light microscopy revealed that application of biochar and GA improved the stomatal regulation, trichome density, epidermal vigor, stomata size (SS) (13 381 μm), stomata index (SI) (354 mm2), upper epidermis thickness (UET) (123 μm), lower epidermis thickness (LET) (153 μm), cuticle thickness (CT) (11.4 μm), trichome density (TD) (23 per mm2), vein islet number (VIN) (14 per mm2), vein termination number (VTN) (19 per mm2), midrib thickness (MT) (5546 μm), and TD (27.4 mm2) under salinity and boron stresses. These results indicate that the use of inexpensive and easily available biochar and seed priming with GA can improve morpho-anatomical and physiological responses of S. melongena L. under oxidative stress conditions.
1. Introduction
Climate change is a current global issue and is estimated to increase the mean global temperature by 1.0–5.7 °C at the end of the 21st century.1−3 Changing climate patterns negatively affect crop growth and yield,4,5 with adverse effects on global food production.6−11 A variety of biotic12−14 and abiotic stresses15−17 created by environmental conditions include salinity,18,19 drought,20−22 temperature,23 floods, and heavy-metal stress.24−28 Salinity stress affects approximately 20% of irrigated land globally.29,30 It negatively affects the photosynthesis rate by inhibiting photochemical routes via reduction in stomata size (SS), stomatal closing, inhibiting nutrient uptake,31−33 and disrupting water balance.34,35 High levels of salts in the soil decrease stomatal conductivity, which further restricts the inward movement of CO2 and thus disrupts gaseous exchange.36 This subsequently interrupts electron transport chain reactions and decreases the photosynthesis rate.37−40 Salinity stress increases the trichome density (TD) and leaf size and adversely affects the plant height, total leaf area, and stomatal density.41 Salinity disrupts different physiological and biochemical functions of plants.29,42,43 For example, NaCl toxicity stimulates oxidative stress via formation of reactive oxygen species (ROS) that result in lipid peroxidation and damage to biomolecules.44−46
In addition to salinity stress, arid and semi-arid areas are subject to boron stress.47,48 Ferguson et al.49 characterized the toxic effect of boron stress that causes leaf injuries in pistachio plants. Boron stress decreases total dry mass and flower bud formation,49 reduces shoot growth, and damages the cell wall.50 Sang et al.51 reported that the toxic effect of high boron levels is due to alterations of protein biosynthesis and carbohydrate metabolism. Boron reacts with some basic metabolites and creates stable complexes that cause physiological impairment inside cells and disrupts plant growth and development.52 Boron also damages cell membranes via formation of ROS, which cause cell and tissue damage.53 In response to osmotic stress, plants synthesize compatible solutes such as soluble protein, soluble sugars, trehalose sugar (TS), carotenoids, proline, and antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT), that detoxify ROS.54−56 Boron stress potentiated salt toxicity in tomato and cucumber plants. Excess of boron in the soil imposed extracellular pressure on photosynthetic activity and limits the growth and yield of tomato and spinach under salt stress.57
Biochar is a biomass of carbon, a low-cost porous pyrogenous matter formed by heating organic waste58,59 (such as crop deposits or animal manure) under zero or inadequate oxygen environments in a closed furnace at elevated temperatures ≤700 °C through pyrolysis.60 Biochar is extensively used due to its appropriate carbon content in nutrient-poor and degraded soils. Biochar provides minerals and nutrients (such as Mg, S, Ca, K, and P) and improves soil physical status (such as bulk density, aggregate stability, high cation exchange capacity [CEC], porosity, and saturated hydraulic conductivity).61 In detail, it increased microbial biomass carbon in the saline soil and the activities of urease, invertase, and phosphatase in bulk soils and rhizosphere soils under maize cultivation. When applied in saline soils, composted biochar increased the soil organic matter content and CEC and decreased the exchangeable Na and soil pH.62 Thus, enhancing soil chemical and biological characteristics subsequently increases crop antioxidant defense mechanisms, yield, and microbial activity and reduces leaching of nutrients.63,64 Similarly, among different allelochemicals, phenolic compounds not only act as efficient free-radical scavengers but also inhibit the lipid peroxidation process, stabilize cell membranes, and act as cell defense system against ROS.19,65,66 Gallic acid (GA; 3,4,5-trihydroxybenzoic acid) is extracted from many plants and has antioxidant potential.67 GA was used in maintaining crop capacity to improve the growth rate and photosynthetic ability under induced abiotic stress in Glycine max L.68 GA induced tolerance in rice seedlings against NaCl stress by enhancing the activities of H2O2-scavenging enzymes, such as POD, SOD, CAT, and APX, and thus protected cell membranes from oxidative damage caused by ROS and lipo-peroxide accumulation.69
Eggplant (Solanum melongena L.) is the second most valuable crop of the family Solanaceae. S. melongena L. is widely grown and consumed in Southeast Asia and in the southern parts of Pakistan.70 Globally, the cultivation area of S. melongena L. is approximately 1.86 million hectares.71 In Pakistan, eggplant occupies an area of 9044 ha with an average yield of 88 148 tons ha–1.72 In Pakistan, most agricultural practices take place in arid and semi-arid areas in the warm season, with low precipitation and in soils with high salt content. Scanning electron microscopy (SEM) and UV–vis spectrophotometry are extensively applied tools for investigating the surface features of plant leaves, including the morphology of stomata, trichomes, stomatal density, epidermal characteristics, cuticular layer, quantification of nanoparticles, infection, and physicochemical components.73
The objectives of this study were to utilize UV–vis spectrophotometry, SEM, and light microscopy tools and energy-dispersive X-ray (EDX) to characterize leaf physicochemical components, leaf surface, cross-sectional anatomy, and elemental composition and to examine the responses of growth stimulators by activating natural defense systems both enzymatically and nonenzymatically under stress conditions. Currently, there is no published scientific report on the possible preventive roles of gallic acid and biochar on individual and combined effect of salinity and boron stresses in eggplant. In addition, there is no scientific report present on anatomical features and agronomic and physio-biochemical attributes of eggplant under such induced abiotic stressors and its amelioration through biochar and gallic acid treatment.
2. Materials and Methods
2.1. Biochar Preparation and Physicochemical Analysis by SEM and EDX
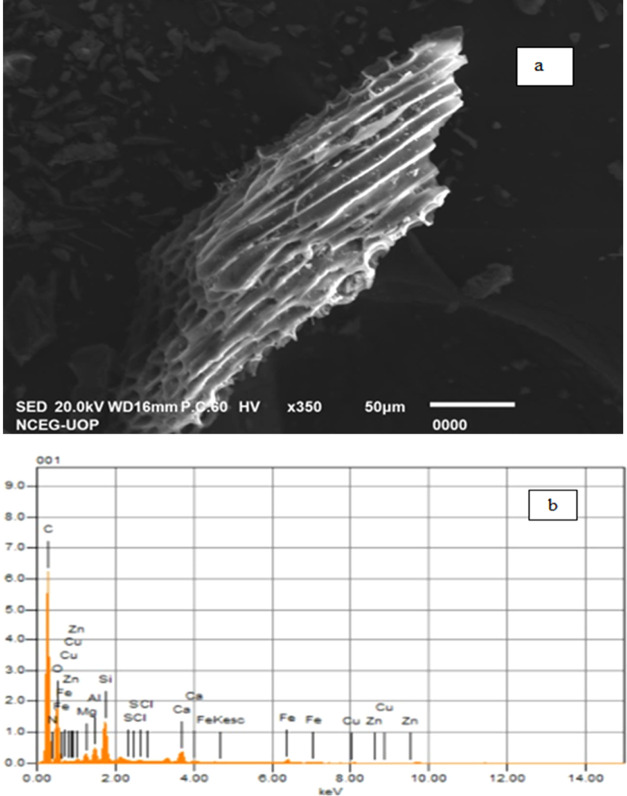
Biochar used in the experiment was produced from hardwood of Vachellia nilotica L. in traditional kilns with domestic charcoal. The kiln internal temperature was maintained at 500–550 °C observed with a thermocouple for 24–48 h of pyrolyzing. Prior to application, the produced biochar was air-dried, crushed, and sieved through a 2 mm sieve. Well-powdered biochar was analyzed with exposure to gold glaze placed on Spi coating segments for morphological characteristics using a scanning electron microscope (JSMIT100-JEOL-JAPAN) according to the methodology of Lalay et al.74 The biochar had large pores and rod-shaped morphology with sharp cracks; this unique structure has the capacity of water holding, araciality of carbon content in soil environments, and invites more microbes, including fungi, ascomycetes, and algae that improve soil fertility. The electrical conductivity (EC) of saturated soils (6.7 dS m–1) was measured at 25 °C by a calibrated EC meter (BANTE, DDS-12DW, China). Energy-dispersive X-ray spectroscopy (INCA200/Oxford instruments, U.K.) was used to determine EC (6.7 dS m–1), bulk density (0.48 g cm–3), cation exchange capacity (5.1 cmol kg–1), and perform elemental analysis, including maximum carbon (56.33%), oxygen (35.34%), silicon (3.12%), calcium (1.36%), iron (1.34%), aluminum (1.06%), nitrogen and magnesium (0.63%), chlorine (0.10%), copper (0.06%), zinc (0.04%), and sulfur (0.01%) (Figure 1).
Figure 1.
Morphological structure and elemental analysis of biochar obtained through (a) scanning electron microscopy and (b) energy-dispersive X-ray analysis.
2.2. Site Description and Seed Sterilization
Pot experiments were performed at the University of Peshawar (34°1′33.3012″N and 71°33′36.4860″E), KPK Pakistan, during the 2021 growing seasons. The locality of Peshawar lies in the Iranian plateau area with 513 mm of mean annual rainfall. Soil physicochemical studies revealed a silt clay soil texture class as determined by a hydrometer method.75 A soil–water suspension (w/v, 1:2.5) was prepared and shaken for 1 h to determine pH with a calibrated pH meter (WTW 7110, Weilheim, Germany). Exchangeable sodium percentage (ESP) of soil (7.5–8.1%) was measured by the method of Page et al.76 A soil–water suspension (w/v, 1:2.5) was prepared to determine pH (6.2) with a calibrated pH meter (WTW 7110, Weilheim, Germany). Energy-dispersive X-ray spectroscopy was used to perform soil elemental analysis, including calcium (7.86%), aluminum (7.46%), potassium (2.47%), silicon (20.2%), oxygen (54.86%), iron (6.91%), and zinc (0.20%). The seeds of two varieties (Neelam & BSS 513) of S. melongena L. were collected from the National Institute of Food and Agriculture (NIFA), Pakistan. Seeds were surface sterilized with 0.1% mercuric chloride solution, followed by 70% ethanol (5 min) and then washed with deionized water.77
2.3. Experimental Design and Growth Conditions
Pots were carefully maintained at a nursery in a complete randomized block design (CRBD) at a space of 5 cm away from each other with a net plot size of 3.0 × 2.0 m2 for proper air passage. The experiment was performed in a greenhouse in a 2 × 2 × 2 design (two varieties, two levels of abiotic stress-treated and nontreated soil with biochar and seed with GA) between 24/13 °C day/night temperature with an average humidity of 41 and 57% light intensity. Both varieties of eggplant were assessed under 12 treatments in triplicates and were divided into three groups (Table 1). One group served as a control (no stress treatment); in the second group, the seeds were primed with 2 mM GA solution (3,4,5-triphydroxyl-benzoic acid) in 100 mL of water at 4 °C for 1 h68 before sowing; in the third group, biochar was mixed at a ratio of 5:1 g kg–1 with soil dried for 24 h after sowing. Treatments were designed as the following.
Table 1. Experimental Design for Biochar and Seed Priming Technique.
| treatments | description |
|---|---|
| T1 | control (untreated) |
| T2 | 25 mg kg–1 boric acid |
| T3 | 120 mM NaCl |
| T4 | 25 mg kg–1 boric acid + 120 mM NaCl |
| T5 | 5 g kg–1 biochar |
| T6 | 5 g kg–1 biochar + 25 mg kg–1 boric acid |
| T7 | 5 g kg–1 biochar + 120 mM NaCl |
| T8 | 5 g kg–1 biochar + 25 mg kg–1 boric acid + 120 mM NaCl |
| T9 | 2 mM gallic acid |
| T10 | 2 mM gallic acid + 25 mg kg–1 boric acid |
| T11 | 2 mM gallic acid + 120 mM NaCl |
| T12 | 2 mM gallic acid + 25 mg kg–1 boric acid + 120 mM NaCl |
Before seed sowing, 72 pots were well plowed and filled with 2 kg of silt clay (1:2) soil along with farmyard manure. Ten seeds of each variety were sown in earthen pots of 18 cm top and bottom diameter with 20 cm height and placed 10 cm apart in triplicates. Pots were thinned after a week of seed germination, and five healthy seedlings were maintained. Data counted for germination parameters was taken from day 1 to day 10 before applying stress. After 15 days of germination, plants were subjected to salinity stress (120 mM NaCl solution)69 or boron stress (boric acid powder of 25 mg kg–1 soil).78 After induction of stresses on day 25, plants were uprooted and washed with distilled water to remove adhered dust particles for measurements of agronomic properties. After absorbing moisture from the root surface, the fresh weights of root and shoot were measured. The shoot and root lengths and leaf area via dry weights were determined after drying in an oven at 30 °C for 72 h until the weight became constant. Undamaged fresh leaves of plants per treatment in replicates were collected and stored at 4 °C for quantification of photosynthetic pigments, osmoprotectants, and plant antioxidant enzymes via spectroscopy (Spectronic UV-1700, Shimadzu, Japan). Some leaves per treatment were shade-dried for a week and used for morphological studies via SEM. Fresh leaf disks were used for anatomical evaluations by light microscopy.
2.4. Determination of Agronomic Characteristics
2.4.1. Relative Water Content (RWC) of Leaves
A fully expanded leaf of three plants per replicate was used to determine the relative water content (RWC) of the leaves by following the standard method of Ogbaga et al.79 Three leaf disks of 10 mm were cut by a cork borer through the interveinal area. The fresh weight (Wf) of mean disks was calculated immediately. Preweighed leaf disks were kept in distilled water for 4 h at 20 °C with dim illumination until complete hydration. The fully saturated weight (Ws) of leaf disks was estimated by measuring the dry weight (Wd) for 4 h at 70 °C.
| 1 |
2.4.2. Root–Shoot Ratio (RSR)
The dry weight of the shoot and root in all treatments per replicate was measured by the proposed formula of Chuyong and Acidri.80
| 2 |
2.4.3. Net Assimilation Rate of Leaves
The net assimilation rate (NAR) is the increase in dry mass of a plant per unit time per unit increase in the assimilatory surface. NAR was estimated by the formula of Ghule et al.81
| 3 |
where A1 and A2 are the leaf areas (cm2) by the length and width, respectively, while W1 and W2 are dry mass in grams at initial time t1 and final time t2, respectively.
2.4.4. Leaf Moisture Content (LMC)
Fresh leaves from approximately three per plant in each treatment was taken and weighed. After measuring fresh weight in grams, the leaf was dried in an oven for 72 h at 30 °C and the dry mass was determined. The percent leaf moisture content (LMC) was measured by the proposed formula of Ullah et al.82
| 4 |
2.4.5. Leaf Area Index (LAI)
The leaf width and length were taken randomly from three leaf disks of a single plant per treatment, and the mean leaf area index (LAI) was measured by the formula of Shah et al.83
| 5 |
2.4.6. Leaf Area Ratio
The leaf area was measured by taking the width and length of three leaf disks per treatment from each plant with a portable leaf area meter (Panomex Inc.) while drying the leaf in an oven for 72 h. The leaf area ratio (LAR) was evaluated following the formula of Shah et al.83
| 6 |
2.4.7. Stomata Index (SI)
The stomata index (SI) was calculated by the method of Dubberstein et al.84 A cross section of a leaf about 1 mm was cut, its upper epidermis was peeled, and then the section was rinsed with distilled water and 70% ethanol three times. The cell structure of the samples was mounted in Canada balsam, and the number of stomata and epidermal cells was imaged and counted under a Nikon Eclipse E600 microscope (DS-U3, Nikon, Japan). In eq 7, S stands for stomata and E for epidermal cells.
| 7 |
2.5. Determination of Physiological Components
2.5.1. Quantification of Photosynthetic Pigments
The chlorophyll content in leaves was evaluated by the standard methodology of Zou et al.85 Fresh leaves (0.2 g) were ground with a mortar and pestle in 80% acetone and incubated for 24 h in the dark and then centrifuged. The chlorophyll a content was determined by measuring absorbance values at 649 nm, chlorophyll b content at 663 nm, and carotenoid content at 430 nm. Absorbance was measured with a spectrophotometer against 80% acetone blank.
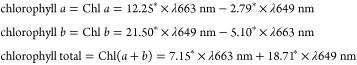 |
2.5.2. Total Proline Content (TPC)
The total proline content (TPC) of fresh foliar materials was determined following the standard method of Khanam and Mohammad.86 A total of 0.5 g of leaves were ground in 10 mL of 3% sulfosalicylic acid with a mortar and pestle. The solution was passed through a Whatman No. 2 filter paper and collected into a test tube. 3% aqueous sulfosalicylic acid was added to make a final volume of 10 mL. 2 mL of glacial acetic acid and acid ninhydrin were mixed in the filtrate. The mixture was boiled for 1 h at 100 °C and then cooled in an ice bath. The reaction was treated with 4 mL of toluene for extraction with vigorous shaking for 15–20 min. The absorbance was measured with a spectrophotometer at 520 nm with red color toluene against a blank toluene. TPC in the sample was calculated using the standard curve for proline in the sample, ranging from 0.1 to 36 μmol on the basis of the fresh mass of the sample. The proline content was expressed in μmol g–1 FM.
2.5.3. Determination of Trehalose Sugar
Determination of trehalose sugar (TS) was performed on fresh foliar material according to the standard methodology of Liu et al.87 0.2 g of leaves was crushed with 1 mL of 0.5 M trichloroacetic acid (TCA) in an ice bath, followed by vigorous shaking for 2 h at 0 °C. The mixture was then centrifuged for 10 min at 10 000 rpm, followed by the addition of 0.2 mL of 0.2 N H2SO4. The solution was then boiled for 10 min at 100 °C and then cooled. Approximately 4 mL of anthrone reagent (0.2 g of anthrone + 100 mL of cold 95% sulfuric acid) was then mixed in the reaction mixture, followed by boiling at 100 °C for 10 min and then cooled. Absorbance was measured with a spectrophotometer at 630 nm.
2.5.4. Determination of Gallic Acid
The GA content was analyzed in fresh leaves by grinding approximately 0.25 g of leaves in 95% methanol. The homogenate was centrifuged at 13 000 rpm for 5 min at 25 °C. The supernatant of 200 mL was then treated with 200 mL of 10% Folin–Ciocalteu (F–C) reagent along with 200 mL of 95% methanol solution. The reaction mixture was treated with 800 mL of 700 mM Na2CO3 and incubated for 2 h. Optical density (OD) was recorded at 760 nm according to the standard methodology of Yetişsin and Kurt.67
2.5.5. Estimation of Catalase Activity (CA)
Catalase activity (CA) was assessed by determining the disappearance of H2O2 at the initial rate following the method of Khanam and Mohammad.86 0.5 g of fresh leaf was homogenized in 5 mL of buffer solution. 0.1 mL of enzyme extract was added to 3 mL of 30 mM H2O2 by diluting 0.34 mL of 30% H2O2 to 100 mL phosphate buffer (pH 7). The decomposition of H2O2 was followed by decline in optical density (OD) at 240 nm as measured by a spectrophotometer. The interval was 30 s using a blank buffer solution along with the enzyme extract. Enzyme activity was measured in μM H2O2 kg–1 FM s–1.
2.5.6. Estimation of Ascorbate Peroxidase Activity
The ascorbate peroxidase assay (APX) was performed according to the method of Salimi et al.88 0.5 g of fresh foliar material was crushed with a mortar and pestle and homogenized in 5 mL of buffer solution. The reaction mixture was then centrifuged at 3000 rpm for 10 min at room temperature. 0.1 mL of enzyme extract was then added to 1.8 mL of 50 mM potassium phosphate (KPO4) buffer (pH 7.0), followed by the mixing 0.1 mL of 0.5 mM ascorbic acid solution and 1 mL of 30% H2O2 in a test tube. Absorbance was measured as the decrease rate of H2O2 for 3 min at 290 nm optical density against the blank of 30% H2O2.
2.6. Determination of Leaf Anatomy
For leaf analysis, the second oldest fully expanded leaf of a randomly selected plant within each pot was carefully removed using a pair of forceps. One leaf per replicate was harvested and immediately submerged into liquid nitrogen for 1 min, followed by 30 s submersion in a methanol gradient (50, 75, 90, and 100% v/v) and 1 min submersion in hexamethyl disilazane. For SEM analysis, the samples were dried and mounted on SEM cylinder specimen mounts (JSMIT100-JEOL-JAPAN, aluminum, grooved edge, ⌀32 mm). Leaves were oriented such that the adaxial surface could be examined. Mounted leaves were coated with carbon using a JEOL-EC-32010CC coating system for 30 nm. A JEOL scanning electron microscope was used to examine leaf surface anatomy, including epidermis, stomata, and trichomes of each variety at high 20 kV, working distance range 15–16 mm, magnification range 2.20–5.00k×, with a specimen stage T = −10 to +90° and R = 360°.
The standard methodology of Liu et al.87 was used for leaf anatomy studies with light microscopy. On day 15 after salinity and boron stress treatment, mature leaves were randomly obtained from each treatment and cut into small pieces (1 cm × 3 mm) from the region between the main vein to the margin of the leaf and a cross section of midrib with a double-sided blade. Samples were cut carefully to approximately 10 μm thickness and preserved in formalin acetic alcohol solution (90% ethanol, 5% formalin, 5% acetic acid) at 4 °C until dehydration. The cell structure of the samples was mounted in Canada balsam and imaged under a Nikon Eclipse E600 microscope (DS-U3, Nikon, Japan). Leaf pieces from three separate plants per treatment were obtained, and the values were expressed in micrometers. The stomata size, epicuticular thickness, and lamina and midrib thickness (MT) were measured by microscope graticules. Values were the mean of three measurements. The stomata index, vein islet, and vein termination number (VTN) were also recorded in a 1 mm leaf area.
2.7. Statistical Analysis
Statistical analysis was a factorial design with induced salinity and boron stress. The analyses were performed in triplicate (n = 3) for various parameters, including agronomic, anatomical, physiological, and biochemical attributes and were analyzed by Statistix 10 and IBM SPSS Statistics 22 (SPSS Inc., Chicago IL). Three-way analysis of variance (ANOVA) at significance difference (p ≤ 0.05) for all measurements, mean separation, and standard deviations (SD) were compared by Tukey’s multiple range test at p ≤ 0.05 for each variety separately. Pearson correlation (R) was measured by the same software.
3. Results
3.1. Determination of Growth Attributes
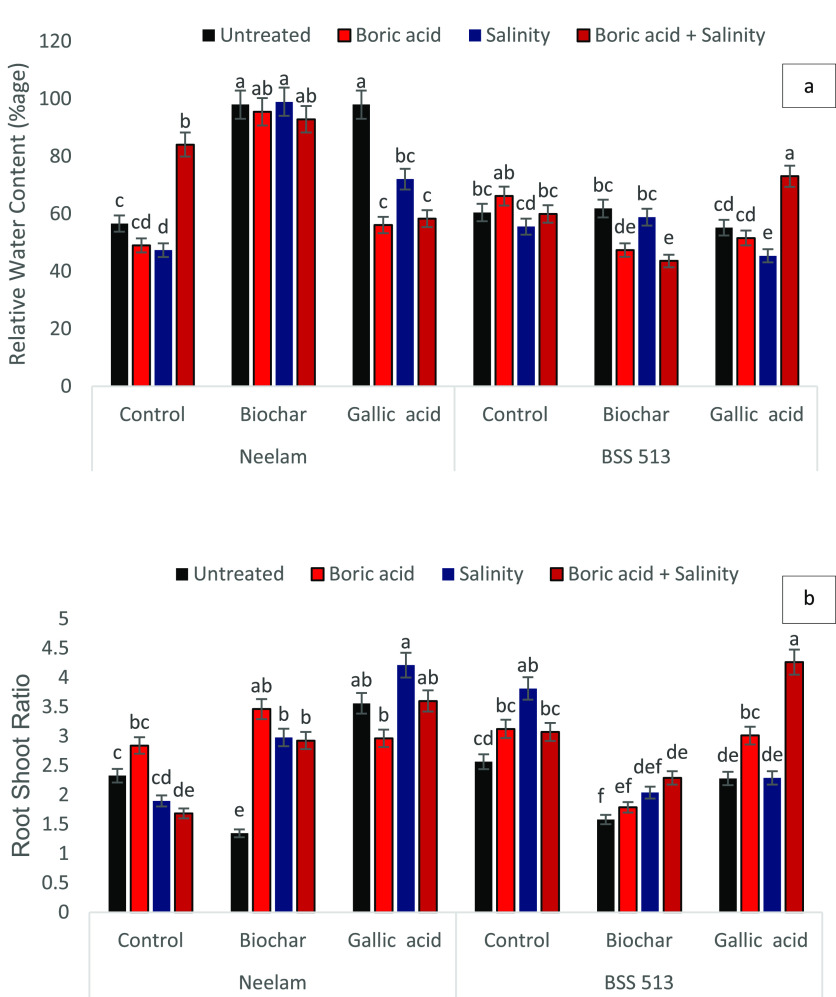
Statistical analysis revealed that leaf fresh weight (LFW), leaf dry weight (LDW), and LMC differed with and without the addition of biochar to soil and seed presoaking under abiotic stresses (Table 2). Boron stress reduced LFW (42%), LDW (3.6%), and LMC (18%) nonsignificantly in both varieties. In BSS 513, boron (T10) and salinity stress (T9) reduced LFW and LMC up to 54 and 18%, respectively. A significant increase in these attributes was observed in GA-pre-soaked seeds and after biochar application to soil. With biochar treatment, LFW was increased by 70 and 91%, while LMC was increased by 98 and 86% in both varieties. Likewise, LDW was increased by 12% in Neelam and 31% in BSS 513 with GA-pre-treated seeds under boron stress (T9) and combined abiotic stress (T12). In Neelam, salinity stress with GA-primed seeds (T11) resulted in a significantly reduced leaf area (LA) and LAI by 30 and 16%, respectively, while LAR decreased by 36% under T5. However, a significant (p < 0.05) increase in these parameters was observed in GA-pre-soaked seeds under combined abiotic stress. Nonetheless, LA, LAR, and LAI in BSS 513 were decreased by boron stress and combined abiotic stress by 51 and 62%, respectively, without growth regulators and 20% in GA-treated plants. LA and LAI were significantly increased by 85 and 29%, respectively, when soil was treated with biochar (T5) and GA-pre-soaked seeds under salinity stress (T10). LAR increased up to 99%. Furthermore, applied biochar to soil increased leaf RWC significantly (p < 0.05) by 115 and 66% in both varieties. GA priming under abiotic stress enhances the ability of biochar to conserve water in soil and thus improve the vigor and viability of plants under stress (Figure 2). Similarly, the root–shoot ratio of plants with respect to abiotic stress was observed after exposure to individual and combined abiotic stress in GA-treated plants in both varieties (Figure 2). However, biochar treatment of soil decreased RSR in Neelam and control untreated (T1) plants of BSS 513. Likewise, no significant difference was measured in leaf NAR of both varieties. ANOVA results through the F-ratio (Table 5) represented that all interactions between genotype, treatment, growth regulators, G × T, G × GR, T × GR, and G × T × GR were significant at p < 0.05.
Table 2. Effect of Biochar (5 g kg–1) and Gallic Acid (2 mM) on Growth Indices of S. melongena L. under Induced Abiotic Stressesa,b,c.
| variety | treatments | LFW | LDW | LMC | LA | LAR | LAI | NAR |
|---|---|---|---|---|---|---|---|---|
| Neelam | T1 | 47 ± 15bcd | 12 ± 1.5cde | 48.23 ± 9.4bcd | 41 ± 5.3de | 50 ± 24def | 2.32 ± 0.2de | 0.0883 ± 0.0a |
| T2 | 42 ± 7.02cd | 3.67 ± 3.06f | 18.29 ± 20cd | 40 ± 2.4de | 73 ± 10cde | 2.23 ± 0.1de | 0.0806 ± 0.0bc | |
| T3 | 46 ± 9.29cd | 13 ± 6.2cde | 67.82 ± 6.7abc | 45 ± 2.5de | 90 ± 53cde | 2.53 ± 0.1de | 0.0883 ± 0.0a | |
| T4 | 49 ± 10bcd | 10 ± 5.1def | 32.95 ± 11cd | 39 ± 6.0de | 43 ± 17efg | 2.18 ± 0.3de | 0.0827 ± 0.0bc | |
| T5 | 70 ± 7.5abc | 11 ± 2.0cde | 91.84 ± 3.64a | 49 ± 3.3cd | 36 ± 10.0fg | 2.77 ± 0.1cd | 0.0839 ± 0.0abc | |
| T6 | 44 ± 14.7cd | 5.33 ± 5.1ef | 65.61 ± 8.7ab | 43 ± 4.9de | 62 ± 4.2cde | 2.40 ± 0.2de | 0.0844 ± 0.0ab | |
| T7 | 51 ± 25bcd | 12 ± 7.9cde | 67.72 ± 6.8ab | 51 ± 6.0bc | 84 ± 52cde | 2.84 ± 0.3bc | 0.0825 ± 0.0bc | |
| T8 | 59 ± 6abcd | 10 ± 1.7def | 70.09 ± 4.7ab | 42 ± 6.8de | 53 ± 3.1def | 2.34 ± 0.3de | 0.085 ± 0.0ab | |
| T9 | 52 ± 15bcd | 12 ± 6.5cde | 55.63 ± 15bc | 52 ± 5.9ab | 48 ± 11efg | 2.92 ± 0.3ab | 0.0831 ± 0.0abc | |
| T10 | 43.1 ± 20cd | 7.6 ± 1.5def | 44.54 ± 27bc | 42 ± 5.3de | 51 ± 13def | 2.37 ± 0.2de | 0.0812 ± 0.0bc | |
| T11 | 38 ± 7.94cd | 4.6 ± 1.53ef | 74.53 ± 8.4ab | 30 ± 26.0e | 63 ± 58cde | 1.66 ± 1.40e | 0.0832 ± 0.0abc | |
| T12 | 54 ± 17bcd | 10 ± 3.6def | 75.07 ± 5.7ab | 54 ± 5.2ab | 92 ± 72abc | 3.02 ± 0.2ab | 0.0831 ± 0.0abc | |
| BSS 513 | T1 | 93 ± 10.9ab | 36 ± 12.86a | 60.24 ± 20bc | 81 ± 11.8a | 53 ± 14def | 4.53 ± 0.60a | 0.0835 ± 0.0abc |
| T2 | 80 ± 48abc | 11 ± 5.5cde | 54.07 ± 22bc | 51 ± 7.9bc | 96.0 ± 89a | 2.88 ± 0.4bc | 0.0846 ± 0.0abc | |
| T3 | 78±34abc | 20 ± 1.5abc | 60.56 ± 27bc | 81 ± 7.74a | 96 ± 22bcd | 4.54 ± 0.40a | 0.0835 ± 0.0abc | |
| T4 | 80 ± 29abc | 17 ± 3.6bcd | 71.40 ± 1.4ab | 61 ± 116ab | 98 ± 21abc | 3.42 ± 0.6ab | 0.0787 ± 0.0c | |
| T5 | 87 ± 33abc | 18 ± 5.0bcd | 86.98 ± 12ab | 85 ± 13.7a | 79 ± 29cde | 4.76 ± 0.70a | 0.0837 ± 0.0abc | |
| T6 | 78 ± 40abc | 23 ± 13.2cd | 80.45 ± 7.6ab | 55 ± 7.2ab | 62 ± 13cde | 3.08 ± 0.4ab | 0.0827 ± 0.0bc | |
| T7 | 79 ± 24abc | 21 ± 3.06cd | 85.14 ± 5.9ab | 58 ± 1.1ab | 70 ± 14cde | 3.24 ± 0.0ab | 0.0831 ± 0.0abc | |
| T8 | 65 ± 39bcd | 19 ± 2.0cde | 52.08 ± 58bc | 72 ± 26abc | 99 ± 46abc | 4.03 ± 14ab | 0.0848 ± 0.0ab | |
| T9 | 68 ± 46bcd | 15 ± 2.0def | 18.05 ± 65cd | 84 ± 11.8a | 64 ± 2.5cd | 4.68 ± 0.6ab | 0.0819 ± 0.0bc | |
| T10 | 54 ± 47bcd | 12 ± 1.7def | 84.95 ± 6.6ab | 53 ± 14ab | 99 ± 16abc | 2.92 ± 0.7ab | 0.0831 ± 0.0abc | |
| T11 | 74 ± 48abc | 28 ± 7.02ab | 10.48 ± 123d | 52 ± 6.2ab | 49 ± 21efg | 2.89 ± 0.3ab | 0.0846 ± 0.0ab | |
| T12 | 98 ± 28ab | 31 ± 4.7ab | 70.02 ± 9.1ab | 37 ± 9.4e | 98 ± 41abc | 2.08 ± 0.5e | 0.084 ± 0.0ab |
LFW = leaf fresh weight, LDW = leaf dry weight, LMC = leaf moisture content, LA = leaf area, LAR = leaf area ratio, LAI = leaf area index, and NAR = net assimilation rate.
Values are the mean ± SD of plants from each treatment.
The treatments exhibit dissimilar letters within rows that represent significance (p ≤ 0.05) level.
Figure 2.
Effects of biochar (5 g kg–1) and gallic acid (2 mM) on the (a) relative water content and (b) root–shoot ratio in foliar (mean ± standard) under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stress. Vertical bars indicate standard errors with least significance difference among mean values at p < 0.05.
Table 5. F-Ratio and Significant Level of Morpho-Anatomical and Physiological Attributes in S. melongena L.a.
| source
of variation |
|||||||
|---|---|---|---|---|---|---|---|
| trait | genotype (G) | treatment (T) | growth regulator (GR) | G × T | G × GR | T × GR | G × T × GR |
| SS | 110*** | 25.3*** | 26.5*** | 33*** | 6*** | 12.9*** | 12*** |
| SI | 4*** | 193*** | 459*** | 60*** | 105*** | 111*** | 54*** |
| UET | 98*** | 21*** | 9*** | 6*** | 14*** | 18*** | 8*** |
| LET | 270*** | 53*** | 22*** | 18*** | 41*** | 28*** | 8*** |
| CT | 87*** | 13.9*** | 37*** | 13*** | 101*** | 16*** | 45*** |
| LT | 3.1 | 50*** | 27*** | 5.5*** | 27*** | 10*** | 8.5*** |
| VIN | 3*** | 50*** | 27*** | 5*** | 27*** | 10*** | 8*** |
| VTN | 40*** | 30*** | 3** | 11*** | 26*** | 17*** | 18*** |
| MT | 1889*** | 354*** | 667*** | 258*** | 13*** | 266*** | 105*** |
| TD | 140*** | 47*** | 72*** | 44*** | 153*** | 69*** | 71*** |
| LFW | 22*** | 0.80 | 0.26 | 0.03 | 0.46 | 0.48 | 044 |
| LDW | 47*** | 6.1*** | 0.05 | 0.34 | 0.23 | 4.1*** | 5.1*** |
| LMC | 0.04 | 2.31 | 1.33 | 1.32 | 1.61 | 0.80 | 1.49 |
| LA | 33*** | 9.7*** | 2.2 | 4.9*** | 3.1* | 2.3* | 2.6* |
| LAR | 5.1* | 4.4*** | 2.4 | 4.1** | 1.3 | 2.1 | 3.8*** |
| LAI | 63*** | 9.7*** | 2.2 | 4.9*** | 3.1* | 2.3* | 2.6* |
| NAR | 0.4 | 0.6 | 1.3 | 0.9 | 1.6 | 1 | 1.1 |
| RWC | 15*** | 1 | 4** | 0.6 | 8*** | 1.2 | 1 |
| RSR | 7.8*** | 8.3*** | 6.6*** | 8.6*** | 0.1 | 6.1*** | 3.1** |
| TCC | 7.1** | 0.9 | 3.5* | 0.5 | 0.5 | 4.8*** | 2.1 |
| CABR | 1.6 | 0.4 | 3.6* | 0.4 | 0.2 | 1.5 | 2.7* |
| CC | 0.07 | 2.2 | 5.1*** | 3.1* | 3.6* | 3.3*** | 0.8 |
| TPC | 2.7 | 0.4 | 3.6*** | 1.2 | 1.9 | 1.2 | 2.1* |
| TS | 92*** | 0.1 | 0.2 | 0.7 | 4.1* | 1.3 | 1.7 |
| GA | 20*** | 1.9 | 6.6*** | 1.3 | 1.7 | 1.8 | 8.8*** |
| PPO | 9*** | 3.1** | 0.1 | 1.2 | 11*** | 4.2*** | 3.4*** |
| CAT | 0.35 | 2.6 | 2.2 | 1.1 | 0.3 | 1.9 | 0.5 |
| APOX | 45*** | 7.6*** | 35*** | 21*** | 44*** | 9.5*** | 11*** |
LFW = leaf fresh weight, LDW = leaf dry weight, LMC = leaf moisture content, LA = leaf area, LAR = leaf area ratio, LAI = leaf area index, NAR = net assimilation rate, SS = stomata size (1 mm), SI = stomata index, UET = upper epidermis thickness (μm), LET = lower epidermis thickness (μm), CT = cuticle thickness (μm), TD = trichome density, VIN = vein islet number, VTN = vein termination number, MT = midrib thickness (μm), LT = lamina thickness (μm), TCC = total chlorophyll content, CABR = chlorophyll a/b ratio, CC = carotenoid content, TPC = total proline content, TS = trehalose sugar, GA = gallic acid, CAT = catalase, APOX = ascorbate peroxidase. *, **, and *** significant at p = 0.05. p = 0.01, and p = 0.001 probability levels, respectively.
3.2. Determination of Physiological and Biochemical Attributes
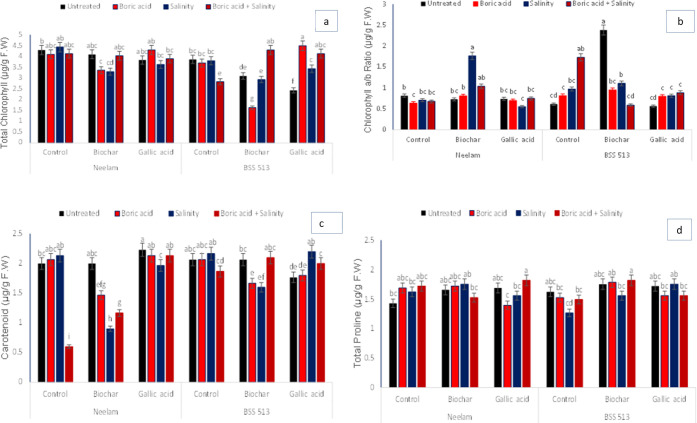
The concentration of photosynthetic components, including the total chlorophyll content (TCC) and chlorophyll a/b ratio (CABR), revealed that salinity stress decreased TCC and CABR with biochar (T7) and GA (T11) treatment significantly (p ≤ 0.05) in Neelam, whereas in variety BSS 513, boron stress (T6) and combined salinity and boron stresses (T8) reduced TCC and CABR with biochar. A greater increase in TCC and CABR in Neelam was observed with gallic acid treatment under boron stress (T10) and biochar amendment to soil under salinity stress (T7). In addition, GA-treated seeds after exposure to boron stress (T10) and in biochar (T5)-amended soil exhibited enhanced TCC and CABR in BSS 513. ANOVA results revealed a positive interaction between GR, T × GR and G × T × GR at p < 0.05 (Figure 3 and Table 5).
Figure 3.
Effects of biochar (5 g kg–1) and gallic acid (2 mM) on the (a) total chlorophyll a, (b) chlorophyll a/b ratio, (c) carotenoid, and (d) total proline in foliar (mean ± standard) under induced salinily (120 mM NaCl) and boron (25 mg kg–1) stress. Vertical bars indicate for standard errors with least significance difference among mean values at p < 0.05.
Under salt and boron stress, the carotenoid content (CC) and TPC increased; the increase was greater with GA treatment and biochar application in both varieties. Thus, GA treatment (T9) and biochar-amended soil under applied salinity stress (T7) regulated CC and TPC in the variety Neelam. In contrast to variety BSS 513, GA treatment under salinity stress (T11) and biochar application under combined abiotic stress (T8) enhanced CC and TPC significantly. Likewise, a large reduction in CC under combined stress (T4) in both varieties and TPC was observed in boron stress (T10) in Neelam and salinity (T3) in the variety BSS 513. The interactions were significant (p < 0.05) between growth regulators, T × GR, and G × T × GR (Figure 3 and Table 5).
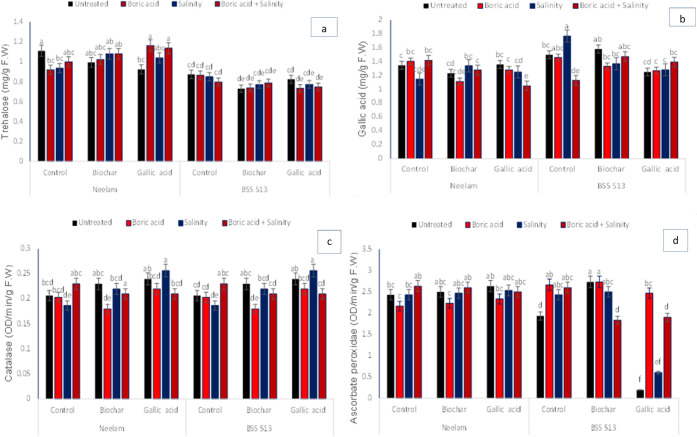
The highest values of TS and GA of foliar material treated with gallic acid (T12) and without gallic acid (T4) under combined stress were observed in Neelam. In addition, GA non-stress-treated (T9) and combined abiotic stress-treated plants with gallic acid (T12) reduced TS and GA contents in the variety Neelam. However, an increase in the same parameters in the variety BSS 513 was observed with boron (T2) and salinity stress (T3), while a decrease in TS and GA was observed under biochar applied to soil (T5) and combined stress treatments (T4). The F-ratio revealed significant results in terms of interactions between genotype, growth regulators, G × GR, and G × T × GR (Figure 4 and Table 5).
Figure 4.
Effects of biochar (5 g kg–1) and gallic acid (2 mM) on (a) trehalose, (b) gallic acid, (c) catalase, and (d) ascorbate peroxidase in foliar (mean ± standard) under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stress. Vertical bars indicate standard errors with least significance difference among mean values at p < 0.05.
To assess the effect of biochar and GA on resistance to boron and salinity stress, the activity of antioxidant enzymes in foliar plant materials was analyzed (Figure 4). GA pretreatment of seeds enhanced CAT in both varieties under salinity stress (T11), whereas low-level activity was observed under boron stress with biochar treatment (T6). In contrast, the APX content of Neelam increased with GA treatment (T9) and biochar amendment to soil under boron stress (T6) but decreased under boron stress (T2) in the Neelam variety and in plants treated with GA (T9) in BSS 513. ANOVA results revealed significant results for all interactions for APX (Table 5).
3.3. Determination of Stomata and Trichomes
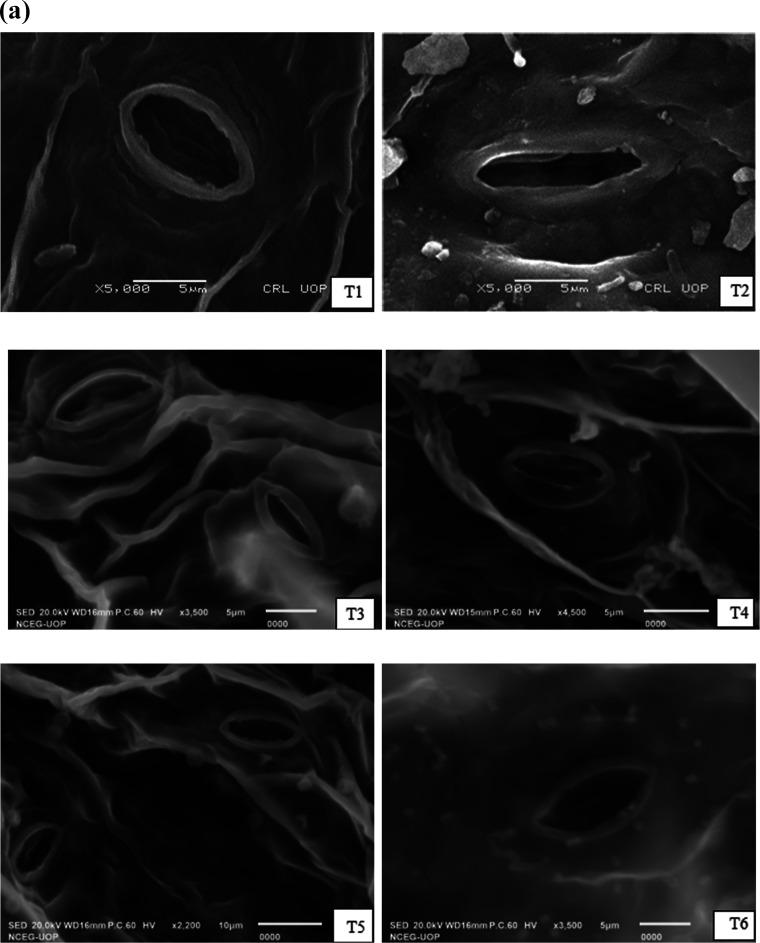
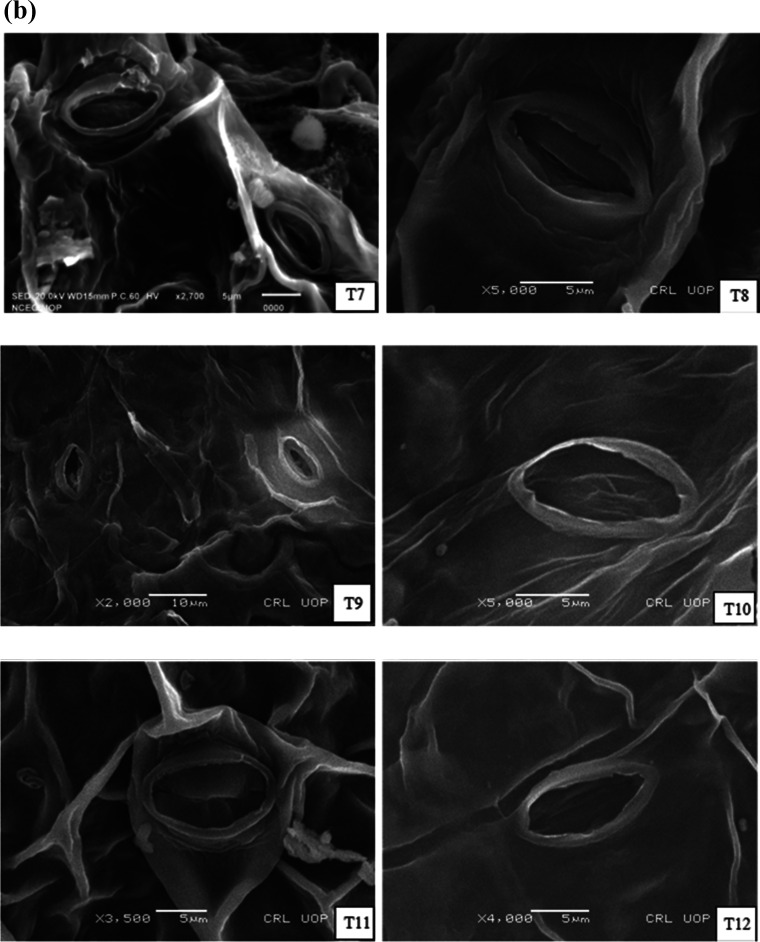
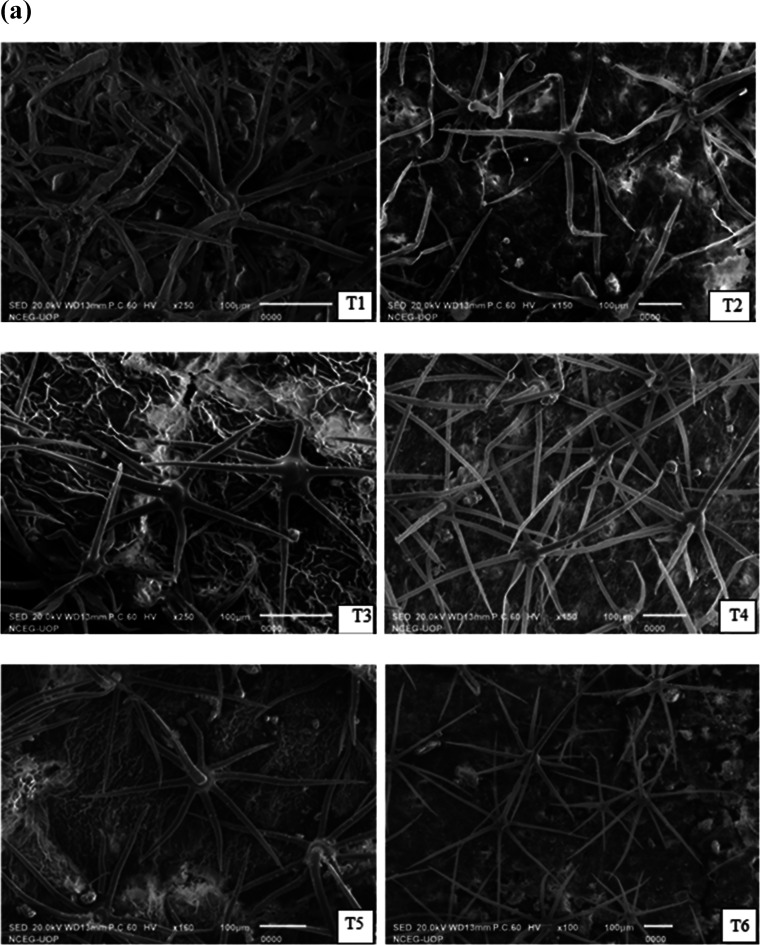
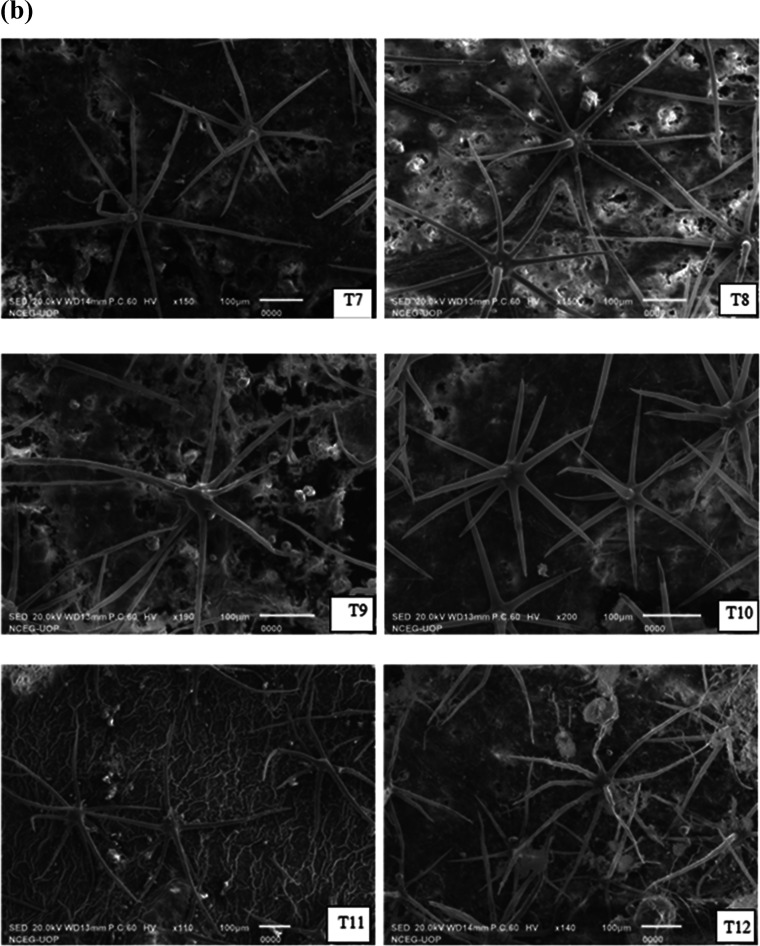
Stomata modify their physiology to regulate water absorption by opening and closing along with the size, density, and turgidity of guard cells. The stomatal morphology and epidermal vigor of the susceptible variety Neelam was significantly (p = 0.05) affected by combined salinity and boron stress (Figure 5a,b). Sunken and partially and completely closed stomatal apertures with flaccid guard cells were observed under T8 and T12. The subsidiary cells appeared as clusters and somewhat dehydrated due to water insufficiency under osmotic stress. However, biochar and GA treatments significantly increased resistance to the toxic effect of individual abiotic stress and nonsignificantly under combined abiotic stresses. The stomata aperture and epidermal vigor of BSS 513 were also significantly affected by combined abiotic stresses under T4 and T8 (Figure 5c,d). Stomatal alterations in response to combined salinity and boron stress were observed as indicated by quenched and reduced stomatal aperture size and epidermal vigor. However, biochar and GA treatments significantly improved the physiology of stomata and subsidiary cells by opening stomatal apertures and increasing the stomata size, water potential of guard cells, and epidermal vigor, which increases resistance to abiotic stresses. SEM results indicate that biochar GA treatments improved the stomata size and physiology, guard cell turgidity, and epidermal vigor under individual and combined abiotic stresses.
Figure 5.
(a, b) SEM micrographs indicating changes in the leaf stomata size and physiology of the S. melongena L. variety Neelam under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stresses. (c, d) SEM micrographs indicating changes in the leaf stomata size and physiology of the S. melongena L. variety BSS 513 under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stresses.
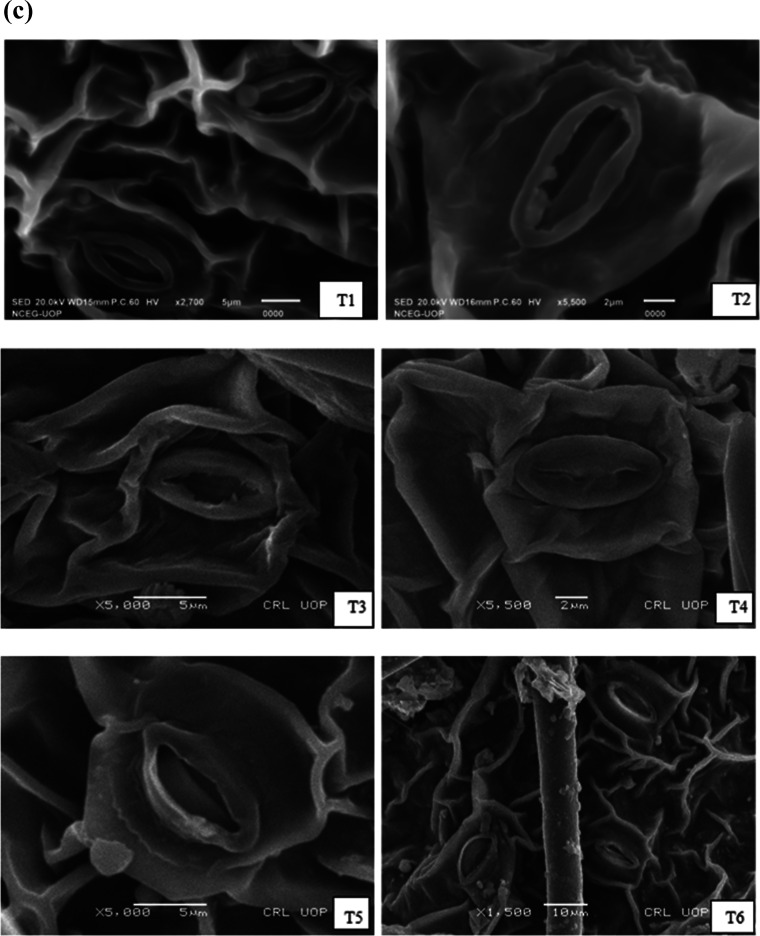
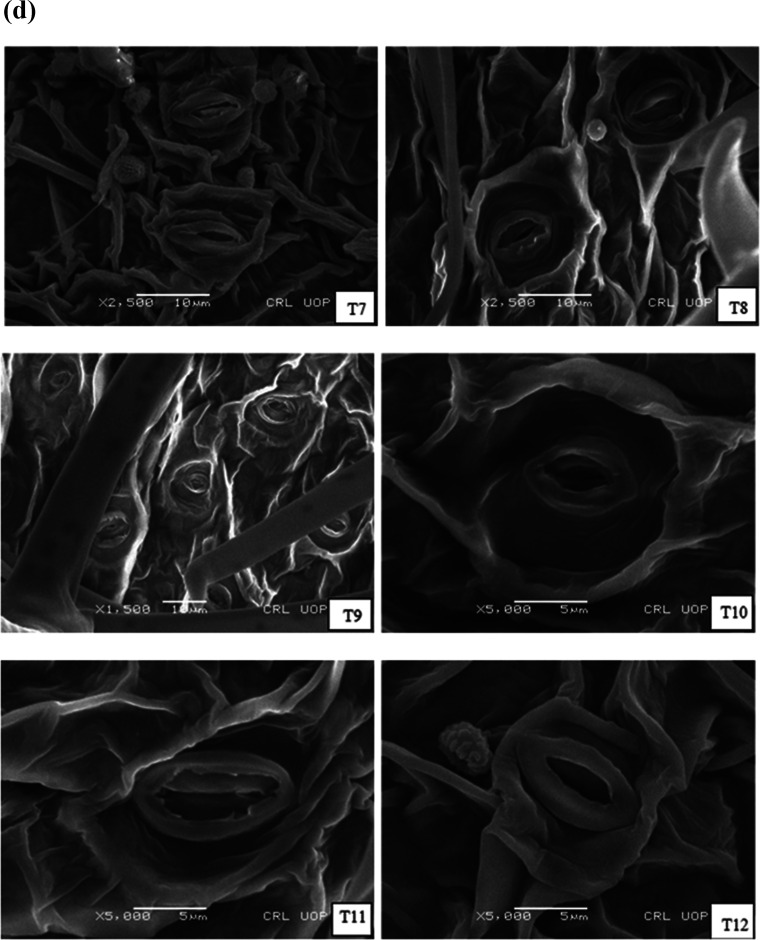
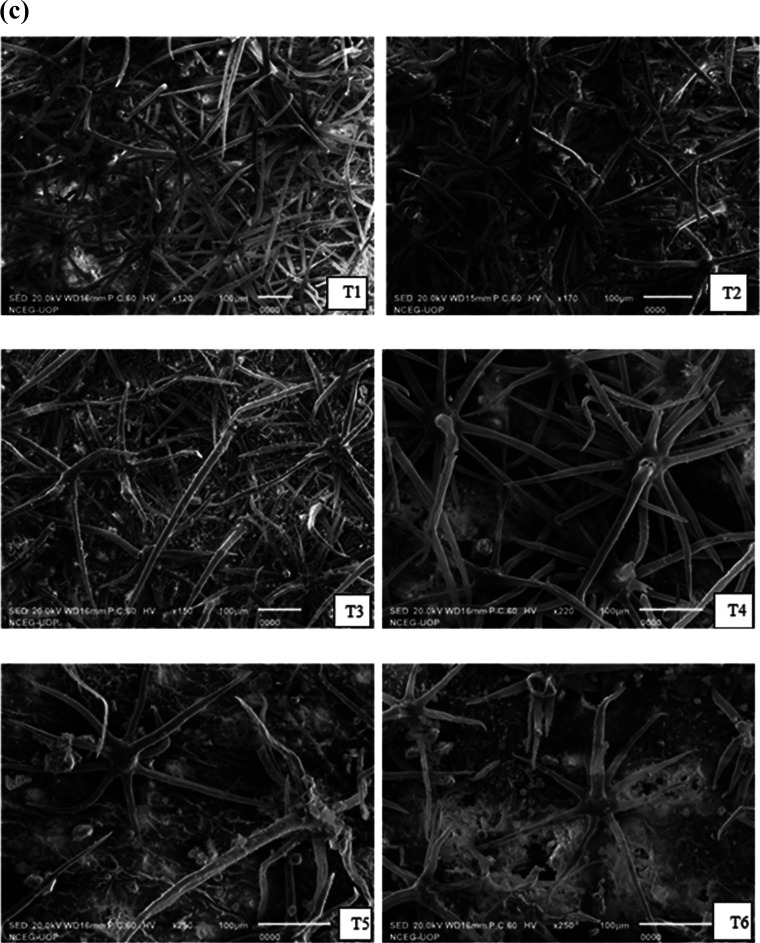
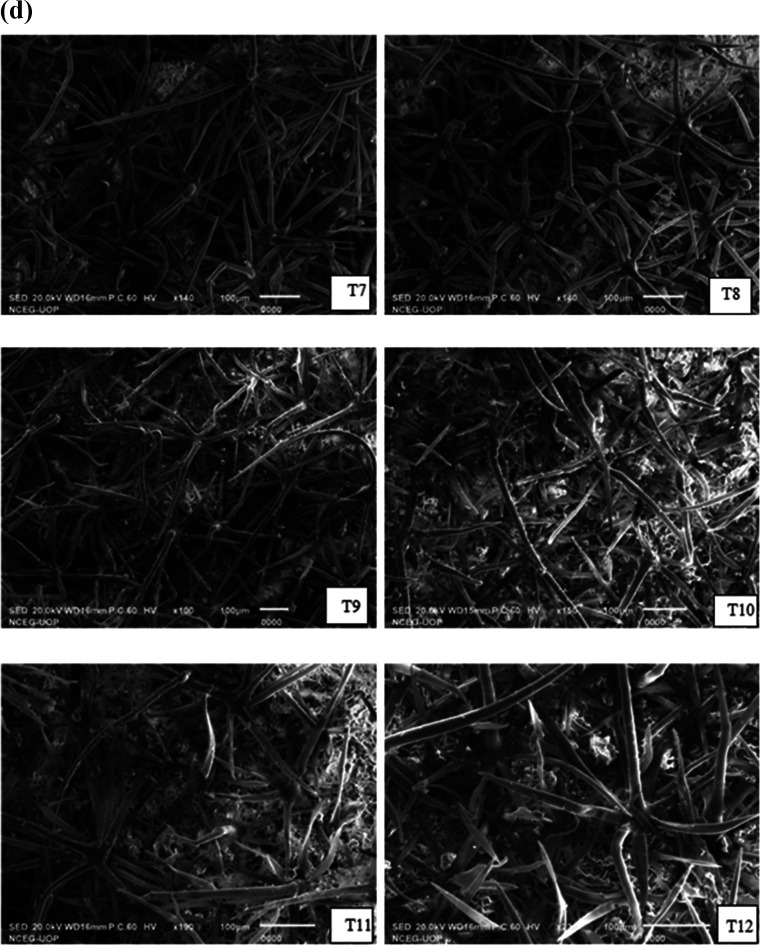
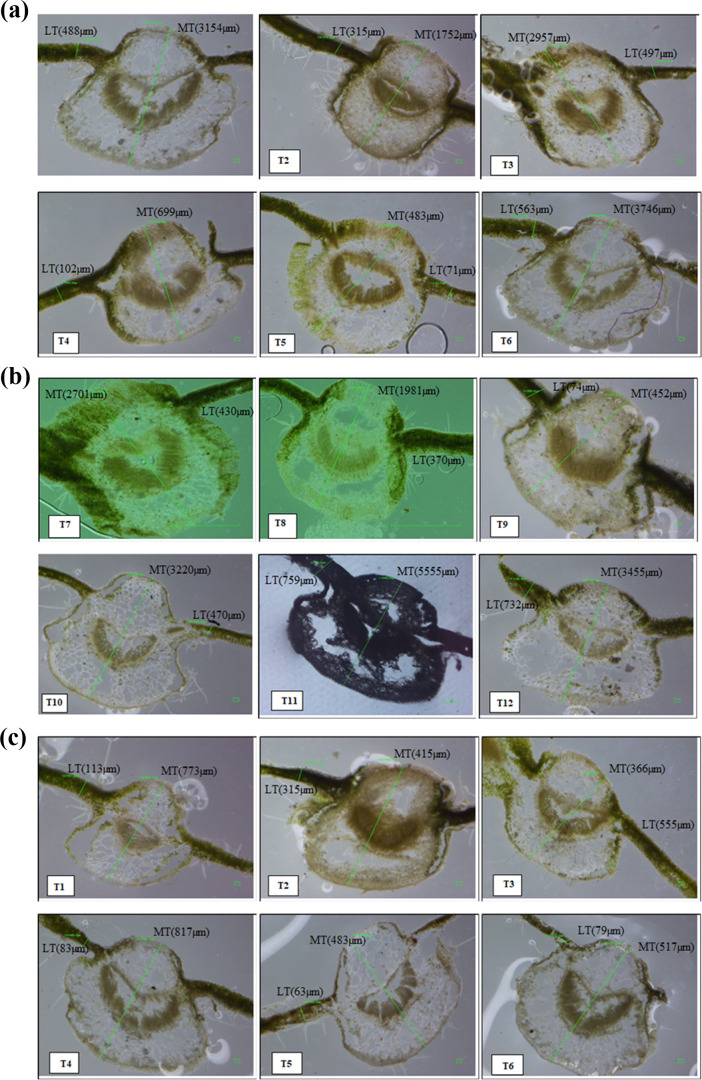
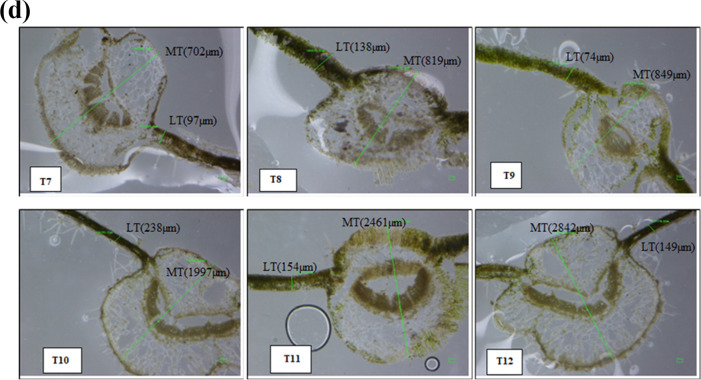
SEM revealed nonglandular stellate-shaped trichomes with pointed ends with a size range from 270 to 3125 μm in length (Figure 6a,b and Table 3). Salinity stress decreased the average density of trichomes by 5.3 mm2. Treatment with GA significantly (p < 0.05) increased trichome density by 11 mm2 in Neelam under combined abiotic stress. Biochar alone (T5) reduced the trichome density by 5.0 mm2 in BSS 513, which was significantly improved by 23 mm2 with GA treatment when compared with the control (Figure 6c,d and Table 3). These results suggest that GA treatment under abiotic stress mediates plant molecular mechanisms that enhance the size and density of trichomes to conserve water and reduce osmotic stress inside the cell.
Figure 6.
(a, b) SEM micrographs indicating changes in the trichome density of the S. melongena L. variety Neelam under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stresses. (c, d) SEM micrographs indicating changes in the trichome density of the S. melongena L. variety BSS 513 through SEM under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stresses.
Table 3. Effect of Biochar (5 g kg–1) and Gallic Acid (2 mM) on Leaf Anatomical Characteristics of S. melongena L. under Induced Abiotic Stressesa,b,c.
| SS (μm) |
SI (1 mm) |
UET
(μm) |
LET
(μm) |
CT
(μm) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| treatment | Neelam | BSS 513 | Neelam | BSS 513 | Neelam | BSS 513 | Neelam | BSS 513 | Neelam | BSS 513 |
| T1 | 17 589 ± 578bcd | 475.0 ± 101.0b | 21.10 ± 0.29a | 25.4 ± 0.20bcd | 64 ± 0.9abcd | 21.0 ± 0.20g | 57 ± 1.6bcde | 14.0 ± 0.40g | 4.80 ± 1.30f | 5.20 ± 1.5c |
| T2 | 9989.0 ± 907cde | 400.0 ± 76.00b | 31.7 ± 0.96de | 24.00 ± 0.50de | 82.0 ± 3.2abc | 32.0 ± 0.80d | 93.0 ± 4.50b | 26.0 ± 1.2de | 5.6 ± 1.70ef | 5.40 ± 1.5c |
| T3 | 1589.0 ± 16.00e | 485.0 ± 174.0b | 20.6 ± 0.410f | 23.200 ± 0.67e | 117.0 ± 2.4a | 28.0 ± 0.80e | 72.0 ± 3.6bc | 30.0 ± 0.8cd | 6.2 ± 1.90de | 5.20 ± 1.5c |
| T4 | 299.00 ± 64.00e | 334.0 ± 90.0b | 18.0 ± 0.49b | 19.80 ± 0.600f | 69 ± 56abcd | 08.0 ± 0.20i | 65.0 ± 37bcd | 12.00 ± 0.2g | 10 ± 3.800a | 6.1 ± 1.9bc |
| T5 | 442.0 ± 125.00e | 741.0 ± 70.0b | 28.9 ± 0.23de | 35.400 ± 0.13a | 21.0 ± 1.10d | 23 ± 0.40fg | 25.0 ± 3.2de | 23 ± 2.50ef | 8.3 ± 2.9bc | 6.5 ± 1.4bc |
| T6 | 32 857 ± 1283ab | 424.0 ± 72.0b | 21.40 ± 1.2de | 27.30 ± 0.560b | 102 ± 9.006a | 27 ± 0.20ef | 153.0 ± 8.0a | 33.0 ± 0.0bc | 8.8 ± 3.20b | 4.90 ± 1.3c |
| T7 | 3666.0 ± 623de | 1460.0 ± 421b | 22.10 ± 0.82b | 26.30 ± 0.78bc | 40 ± 8.10bcd | 29 ± 0..8de | 99.0 ± 7.70b | 34.00 ± 2.0b | 9.0 ± 3.00b | 6.20 ± 2bc |
| T8 | 2833 ± 1312.0de | 3836.0 ± 340b | 28.80 ± 0.31c | 25.40 ± 0.36cd | 22.0 ± 1.80d | 32 ± 0.20d | 100 ± 8.10b | 33.0 ± 0.3bc | 11.0 ± 4.7a | 5.10 ± 1.4c |
| T9 | 938.00 ± 242.00e | 5146 ± 2483b | 25.2 ± 0.60de | 23.90 ± 0.58de | 26.0 ± 2.0cd | 15.0 ± 0.30h | 31.0 ± 13cde | 13.0 ± 0.20g | 5.7 ± 1.8ef | 7.5 ± 2.5ab |
| T10 | 39 246.0 ± 1425a | 1050.0 ± 170b | 20.90 ± 0.32e | 19.40 ± 0.320f | 96.0 ± 2.1ab | 42.0 ± 0.8b | 57 ± 1.6bcde | 26.0 ± 0.8de | 7.3 ± 2.5cd | 6.6 ± 2.2bc |
| T11 | 13 381 ± 3478cde | 796.00 ± 294b | 20.20 ± 0.33d | 18.6 ± 0.410fg | 13.00 ± 0.2d | 37.0 ± 1.2c | 15.0 ± 0.40e | 21.0 ± 1.60f | 8.2 ± 2.8bc | 5.40 ± 1.5c |
| T12 | 24 470 ± 6981abc | 11346 ± 5044a | 22.50 ± 0.36a | 16.940 ± 0.69g | 123.0 ± 0.8a | 75.0 ± 2.8a | 90.0 ± 0.80b | 95.0 ± 0.80a | 5.4 ± 1.0ef | 9.0 ± 3.30a |
SS = stomata size (1 mm), SI = stomata index, UET = upper epidermis thickness (μm), LET = lower epidermis thickness (μm), and CT = cuticle thickness (μm).
Values are the mean ± SD of plants from each treatment.
The treatments exhibit dissimilar letters within rows that represent significance (p ≤ 0.05) level.
3.4. Determination of Leaf Anatomy
Leaf structure analysis (Table 3) of images from an optical microscope (DS-U3, Nikon, Japan) revealed that GA treatment (T10) significantly increased the stomata size by 13 381 μm under salinity stress in Neelam and by 11 346 μm under combined salt and boron stress (T12) in BSS 513. However, both stresses in combined form decreased the trait more negatively in treatment T4 by 299 μm. In contrast, the biochar treatment of soil significantly (p < 0.05) enhanced leaf SI in both varieties (range 28.9–35.4 mm2). Leaf SI decreased up to 18 mm2 with combined salt and boron stress (T4) without growth regulators in the Neelam variety and with growth regulators in BSS 513. Our results confirm the adverse effects of combined stress conditions on leaf photosynthetic aperture by decreasing the number of stomata per epidermal cell compared to gallic acid and biochar-treated plants.
Plants treated with GA under combined abiotic stress (T12) exhibited increased thickness of upper epidermis (UET) by 123 μm in Neelam and 75 μm in BSS 513. The thickness of lower epidermis (LET) was significantly improved by biochar treatment of soil by 153 μm in Neelam and 95 μm in BSS 513 for boron stress (T6) and combined abiotic stress (T12). However, salinity stress reduced UET and LET by 13 and 15 μm in the variety Neelam under T11, while in BSS 513, a decrease was observed by 0.8 and 12 μm in UET and LET under combined abiotic stress, respectively. Both biochar- and GA-treated plants exhibited markedly improved leaf morphology and resisted these abiotic stresses when compared with control (untreated) plants. Similarly, cuticle thickness (CT) was enhanced by 11.4 μm in Neelam and 9.0 μm in variety BSS 513 for T12 in both varieties after salinity and boron stress (T8) when compared with the control. However, boron stresses without and with biochar reduced cuticle thickness by a mean of 5.6–4.9 μm for T2 and T6 in the variety Neelam and BSS 513 (Table 4).
Table 4. Effect of Biochar (5 g kg–1) and Gallic Acid (2 mM) on Leaf Anatomical Characteristics of S. melongena L. under Induced Abiotic Stressesa,b,c.
| TD (μm) |
VIN (1 mm) |
VTN (1 mm) |
MT (μm) |
LT
(μm) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| treatments | Neelam | BSS 513 | Neelam | BSS 513 | Neelam | BSS 513 | Neelam | BSS 513 | Neelam | BSS 513 |
| T1 | 5.6 ± 2.1de | 9 ± 7.8ghi | 13.0 ± 0.810a | 10 ± 0.810bcd | 17.00 ± 0.810a | 14 ± 0.81bcd | 3014 ± 107b | 773 ± 33d | 111 ± 13c | 471 ± 1.60a |
| T2 | 7.0 ± 2bcd | 12 ± 2.5ef | 9.00 ± 0.810c | 8 ± 0.810cdef | 11.0 ± 0.81cde | 10 ± 0.810ef | 1399 ± 255d | 471 ± 41fg | 427 ± 16d | 293.0 ± 79a |
| T3 | 6.6 ± 1.1cd | 10.0 ± 2gh | 8.3 ± 0.940cd | 5.00 ± 0.810f | 7.00 ± 2.100e | 8.0 ± 0.810f | 467.0 ± 10e | 387 ± 20g | 230 ± 53b | 565.0 ± 14a |
| T4 | 10 ± 1.50ab | 8.0 ± 3.6hi | 7.3 ± 0.470cd | 10 ± 0.81bcd | 10.0 ± 1.60cde | 17 ± 0.810ab | 758.0 ± 92e | 819 ± 1.6d | 85.00 ± 3e | 107.00 ± 2a |
| T5 | 6.6 ± 0.5cd | 5.0 ± 1.7ij | 10.0 ± 0.81bc | 11.0 ± 0.81bc | 13.6 ± 1.20abc | 14 ± 0.81bcd | 3286 ± 320b | 482 ± 0.8f | 62.00 ± 3e | 67.0 ± 0.8a |
| T6 | 10 ± 1.1ab | 9 ± 3.1ghi | 6.0 ± 0.810d | 5.00 ± 0.810f | 7.00 ± 0.8100e | 13 ± 0.81cde | 3511 ± 193b | 514.0 ± 2f | 81.00 ± 6b | 556 ± 4.30a |
| T7 | 4.00 ± 1.0e | 16 ±± 1cd | 12.0 ± 0.81ab | 7.0 ± 0.81def | 16.0 ± 0.810ab | 11 ± 0.81def | 2473 ± 157c | 681 ± 15.0e | 91.0 ± 16c | 410 ± 4.40a |
| T8 | 5.3 ± 0.5de | 17 ± 3.6bc | 100 ± 0.810bc | 6.0 ± 0.81ef | 12 ± 0.810bcd | 10.0 ± 0.81ef | 1868 ± 10d | 818 ± 0.8d | 140 ± 24d | 336 ± 5.00a |
| T9 | 6.3 ± 1.5cd | 22 ± 2.1ab | 13.0 ± 0.810a | 14.00 ± 1.60a | 10.3 ± 0.81cde | 19.30 ± 1.60a | 446.0 ± 5.3e | 847 ± 1.6d | 73.0 ± 2.0e | 71.0 ± 1.20a |
| T10 | 9.6 ± 1.1bc | 23 ± 7.50a | 8.20 ± 0.81cd | 12.0 ± 0.81ab | 10.0 ± 0.81cde | 16.0 ± 0.81bc | 3218 ± 2.4b | 1916 ± 54c | 236.0 ± 2c | 468 ± 1.20a |
| T11 | 9.6 ± 3.7bc | 12 ± 2.50ef | 9.00 ± 0.810c | 11.0 ± 0.81bc | 12.0 ± 1.60bcd | 13. ± 0.81cde | 5546 ± 7.9a | 2399 ± 43b | 153 ± 17a | 736 ± 0.80a |
| T12 | 11.0 ± 6.1a | 19 ± 1.1abc | 8.0 ± 0.810cd | 90 ± 0.81bcde | 9.00 ± 0.810de | 12.6 ± 0.47de | 3322 ± 88b | 2841 ± 0.4a | 141 ± 1.2a | 690 ± 2.40a |
TD = trichome density, VIN = vein islet number, VTN = vein termination number, MT = midrib thickness (μm), and LT = lamina thickness (μm).
Values are the mean ± SD of plants from each treatment.
The treatments exhibit dissimilar letters within rows that represent significance (p ≤ 0.05) level.
The variation in the vein islet number (VIN) ranged from 13 per mm2 in Neelam to 14 per mm2 in BSS 513 with GA treatment (T9) and was reduced under salinity stress (Table 4). Our results indicate that the interactions of boron stress and biochar (T7) enhanced vein termination numbers (VTN) from 16 per mm2, which was the apparent minimum under salinity stress without applied growth regulators (T3), irrespective of the variety. In BSS 513, VTN was 19 per mm2 in GA-treated plants (T9). Moreover, GA treatment under salinity stress (T11) in Neelam and combined abiotic stress (T12) in BSS 513 significantly increased midrib thickness (MT) by 5546 and 2841 μm, respectively. Lamina thickness (LT) increased by 236 and 736 μm for boron stress (T10) and salinity stress (T11), respectively (Figure 7a–d and Table 5). Furthermore, LT decreased in both varieties with biochar amendment (T5) by 62–67 μm. Applied salinity stress lowered MT by 387–467 μm in both varieties. Micrographs revealed significant differences in leaf anatomical features between the two varieties.
Figure 7.
(a, b) Light micrographs indicate changes in the midrib thickness (MT) and lamina thickness (LT) of the S. melongena L. variety Neelam under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stresses. (c, d) Light micrographs indicate changes in the midrib thickness (MT) and lamina thickness (LT) of the S. melongena L. variety BSS 513 under induced salinity (120 mM NaCl) and boron (25 mg kg–1) stresses.
4. Discussion
Changes in climate conditions are the main source of abiotic stresses,89,90 which have adverse effects on agricultural lands.91 Such changes in climatic conditions are interlinked with each other in various aspects that negatively affect plant physiology.92,93 The studies presented here were focused on the mitigating effects of woody biochar of V. nilotica L. and GA on morpho-anatomical, physiological, and biochemical aspects of S. melongena L. under induced salinity and boron stress. Biochar is generally a carbon-rich compound that resists decomposition when applied to soil and increases EC, pH, and crop yield. Biochar also reduces nutrient leaching from soil.94 However, Biochar–Na action mechanisms extend from basically no explanation to broad advantageous action or exchange Na+ ions with biochar-borne Mg++ and Ca++ ions. This electronegativity effect sorbs base cations such as Na+ to a higher level than other base cations (Ca and Mg) because of large radii and weak hydration soft base cations (Na); other base cations which are hard (Ca and Mg) may generate weak cation−π electron action due to their greater hydrated energies and thus have less efficaciously sorbed via biochar-delocalized π electrons.94 SEM and EDX analysis in the present study revealed more cracks and the porous nature of biochar with high EC and pH, improved cation exchange capacity, and high carbon/oxygen ratio. Hao et al.95 and Méndez et al.96 analyzed large pore size with cracks generated due to high-temperature pyrolysis that enhanced carbon stability in soil. Ahmad et al.97 and Kim et al.98 observed high bulk density and cation exchange capacity with an increased carbon/oxygen ratio. These studies are also in agreement with the findings of Lalay et al.,74 as the same keV of elemental peaks exposed the nutritional availability of various elements to crops that may be a possible approach to improve growth and productivity.
Disruption in plant growth and physiological activities of are primarily due to abiotic stress.99,100 These disturbances may occur when the plant is exposed to physical dehydration and the subsequent osmotic effects that block the passage of water in xylem vessels via ion toxicity.101 We observed a significant reduction in LDW, LAI, and LAR and a nonsignificant decrease in LFW and LMC under boron and salinity stresses. Growth attributes were significantly improved with biochar and GA treatments. Ibrahim et al.102 and Kanwal et al.103 revealed that salinity stress (150 mM) with 2 and 5% biochar application improved the leaf moisture content, leaf area, leaf fresh mass, and dry mass by 16, 16.5, 26.2, and 27.4%, respectively. In addition, salinity and salinity combined with boron stress led to a significant increase in the root–shoot ratio, which was then reduced by biochar amendment to soil. These results are consistent with the findings of Khan et al.104 and Shi et al.105 Chlorophyll is the main photosynthetic component of plants and is affected by ROS, including H2O2 and O2–, which cause lipid peroxidation and thus degeneration of chlorophyll.106 We observed a decline in chlorophyll content under stress regimes, which was improved by GA treatment. Taffouo et al.107 and Manuchehri and Salehi108 observed greater osmotic potential in stressed plants due to the elevated salt content, which significantly degraded and inhibited photosynthetic pigments. Parallel to our results, García-Sánchez et al.109 observed a pronounced reduction in total chlorophyll content due to salinity stress, which was ameliorated by GA and β-carotene application. The total content of chlorophyll in leaves was increased by 10% biochar, and the lowest was recorded in 12 dS m–1 salinity without biochar.110 Similar findings were described in seeds of rice primed with GA and rutin66 and through biochar in Syngonium podophyllum.111
Osmoprotectants, such as carotenoids, proline, and trehalose, are synthesized and accumulated in plant cells as a defense against hyperosmotic stress.112 As carotenoids, proline and trehalose play a defensive role against abiotic stresses in plants. Bhamburdekar and Chavan113 observed a reduction in carotenoid, proline, and trehalose contents under salinity and combined abiotic stresses. According to their findings, carotenoid and proline contents decreased under salinity and boron stresses in chickpea and wheat leaves. We observed that biochar and GA treatment enhanced the carotenoid and proline contents in S. melongena L. under abiotic stresses. Akladious and Mohamed114 and Linić et al.115 reported the highest level of carotenoids and proline in Capsicum annuum and Chinese cabbage with phenolic acid application under salinity stress. As an osmoprotectant, trehalose plays an important physiological role in maintaining the cellular osmotic balance and protects biological molecules under abiotic stress conditions.116 On exposure to salinity stress, we observed a reduction in trehalose content, which was improved by biochar and GA treatment under combined salinity and boron stresses. Our results are consistent with the previous work of Mehdizadeh et al.,117 who revealed that use of biochar enhances sugar levels by increasing NaCl. Similar results were also reported by Khodary,118 who suggested that the use of phenolic acid may trigger sugar metabolism by disrupting the enzymatic system for hydrolysis of polysaccharides under high osmotic pressure.
In natural environments, phenolic compounds are associated with scavenging systems for quenching ROS and therefore protect plants against tissue oxidation from free radicals.83,111 In the present study, the lowest GA levels were observed under saline conditions without growth regulators. Nevertheless, the opposite trend was observed under high boron concentration and combined abiotic stress through GA seed priming. The results are consistent with those of Tavallali et al.119 Lim et al.120 observed that accumulation of phenolic compounds was primarily due to rutin, isoorientin, and orientin. Babaei et al.121 observed that β-carotene and GA treatment of seeds enhanced levels of phenolic compounds upon exposure to salinity stress, consistent with our results. Consequently, higher antioxidant enzymatic activities likely provide greater resistance under abiotic stress conditions.122 The current study revealed that catalase and ascorbate activities increased after GA treatment under salt stress and decreased under boron stress. These outcomes are consistent with Babaei et al.,121 who observed that GA can participate in H2O2 detoxification by enhancing the activity of various antioxidant enzymes, including CAT, APX, SOD, and GPX in Lepidium sativum L. Similarly, Singh et al.66 also observed low H2O2 levels in plants treated with GA when compared with the control. Similar studies on the beneficial role of GA in improving antioxidant mechanisms in plants under salinity and boron stress have been described by Gunes et al.57 in Vitis vinifera and Ferro et al.123 in Solanum esculentum.
Leaf anatomical characteristics and internal leaf elemental compartmentalization were examined to elucidate relative salinity tolerance mechanisms in the varieties Neelam and BSS 513, which have superior salinity tolerance relative to other genotypes of the same species in prior screenings. SEM investigations of stomatal physiology revealed that leaves of S. melongena L. were sensitive to induced salt stress compared with boron stress and led to a considerable reduction in leaf size and density. Abnormal subsidiary cells may be due to osmotic stress, as salinity stress led to water shortage in these cells. Investigations with SEM by Torabi et al.124 and Orsini et al.125 revealed lower stomatal density, transpiration rate, and conductance as a means to resist salt stress in strawberry and borage. Our results indicate that modification in stomatal status had a significant effect on its movement and density and particularly turgidity of guard cells in plants after GA treatment. Consistent with this study, Ma et al.126 observed that treatment with phenolic acid reduced the negative impact of salinity stress under 0.3 and 0.6% NaCl treatment on stomatal and nonstomatal factors by enhancing stomatal density, thus improving photosynthetic ability in Dianthus superbus L.
Trichomes are epidermal cell developments in aerial parts of plants that facilitate responses to abiotic stress.127 SEM analysis on the density of covering trichomes on the abaxial epidermis in plants after GA treatment revealed an increased level of trichome density in both varieties but were decreased under abiotic stresses. Similar findings were obtained by Torabi et al.,124 where SEM micrographs revealed a reduced density of covering trichomes on the abaxial epidermis in plants exposed to salinity stress when compared with control plants. These outcomes were further corroborated by the results of Passinho-Soares et al.,128 who reported that growth regulators influenced quantitative and qualitative profiles of trichome distribution on the leaf surface. Bose et al.129 observed that growth regulators have a major role in trichome development. Likewise, Chakraborty130 observed that priming of tomato seeds with phenolic acid led to modified physiological attributes, including the seed vigor index, trichome density, and lengths of the shoot and root.
Leaf plasticity and changes in the morphological structure modulate both physiological and biochemical attributes in plants.87 In the present study, optical microscopy revealed an increase in SS and SI after biochar application in soil; a pronounced decrease was observed under salinity and boron stresses. Our results are consistent with previous work on tomato plants,131 which revealed that stomatal aperture and density were reduced under salinity stress. Similar findings were obtained by Akhtar et al.,132 who observed that biochar application improved both the stomatal aperture and density. A significant reduction in UET and LET were observed in stressed plants; this reduction was not observed with biochar application and GA treatment. These results are consistent with the observations of Hafez et al.133 in barley under stress and Leite et al.134 in Astronium fraxinifolium. Cutin is a polyester formed by hydroxy and hydroxy–epoxy (C16 and C18) fatty acid monomers135 that play a key role in reinforcing cuticle layers in leaves and roots to prevent water loss and thus maintain cellular turgidity. We observed improved cuticle thickness under combined stresses with applied biochar and GA compared with control groups. These results are consistent with those of Ndiate et al.,136 who observed formation of fatty acids in cuticle production in plants under stress conditions after biochar application. Moreover, the greater abundance of functional enzymes associated with production of medium chain fatty acids confirmed that biochar enhanced production for biosynthesis of the β-oxidation pathway and the fatty acid pathway for cutin production.137
Vein islet is a small photosynthetic tissue encircled by an area of conducting channel where the end terminal of the vein is veinlet termination points per millimeter on the surface of the leaf. Light microscopy revealed decreased VIN, VTN, MT, and LT under abiotic stresses, which were improved after biochar application or GA treatment in both varieties. Tak and Kakde138 observed a significant decrease in vein islet and termination number when tree foliage was exposed to pollutant stress. Our results confirmed the findings of Darwish et al.,139 who observed that riboflavin minimized the toxic effects of salt stress in tecoma plants, which reduced midrib and lamina thickness.
5. Conclusions
Biochar treatment of soil enhanced soil fertility and pH and maintained the moisture content. In addition, biochar application and GA treatment improved leaf parameters, chlorophyll content, osmoprotectants, phenolic compounds, and concentrations of antioxidant enzymes in S. melongena L. under salinity and boron stresses either individually or combined. SEM and light microscopy indicated that both biochar application and GA treatment alleviated the adverse effects of NaCl and boron toxicity by improving leaf morphology, epidermal vigor, and stomatal regulation. Moreover, a thick epicuticular layer and trichome density facilitated resistance to these stresses. The present study also revealed the susceptible nature of Neelam and the greater resistance of BSS 513 to these stresses. Given the conditions created by climate change, fulfilling the food demands of the rapidly increasing global population is a challenge for the scientific community. The results of this study suggest that application of growth regulators and employing priming techniques may open a new avenue to a sustainable agriculture by enhancing crop production and improving their abiotic stress resistance.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project Number (RSP2023R483), King Saud University, Riyadh, Saudi Arabia.
Glossary
Abbreviations
- LDW
leaf dry weight
- LFW
leaf fresh weight
- LA
leaf area
- LAI
leaf area index
- LMC
leaf moisture content
- RWC
relative water content
- SEM
scanning electron microscopy
- SS
stomata size
- SI
stomata index
- UET
upper epidermis
- LET
lower epidermis
- CT
cuticle thickness
- TL
trichome length
- VIN
vein islet number
- VTN
vein termination number
- CAT
catalase
- APX
ascorbate peroxidase
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
S.: conceptualization; S. and W.S.: data curation; S.U., B.A.: formal analysis; S., B.A., A.H.: investigation; S., S.U., W.S.: resources; M.S., S.E., and A.A.A.-G.: software; A.H., B.A., S.U.: supervision; S.U.: validation; S.U., A.A.A.-G., and M.S.E.: visualization; S., S.U., W.S.: funding acquisition; M.S.E., S.E., A.A.A.G.; writing—original draft; S.U., W.S., B.A., S.: writing—review and editing; S., W.S., B.A., S.K., S.U., S.E., M.S.E., A.H., A.A.A.-G.
Researchers supported through Project Number (RSP2023R483).
The authors declare no competing financial interest.
Notes
The seeds of two varieties (Neelam & BSS 513) of S. melongena L. were collected from the National Institute of Food and Agriculture (NIFA), Pakistan. All of the experiments were performed in accordance with relevant guidelines and regulations.
References
- Afridi M. S.; Javed M. A.; Ali S.; De Medeiros F. H. V.; Ali B.; Salam A.; et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 1–22. 10.3389/fpls.2022.899464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amna; Ali B.; Azeem M. A.; Qayyum A.; Mustafa G.; Ahmad M. A.. et al. Bio-Fabricated Silver Nanoparticles: A Sustainable Approach for Augmentation of Plant Growth and Pathogen Control. Sustainable Agriculture Reviews 53; Springer, 2021; pp 345–371. [Google Scholar]
- Afridi M. S.; Javed M. A.; Ali S.; De Medeiros F. H. V.; Ali B.; Salam A.; et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464 10.3389/fpls.2022.899464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A.; Adnan M.; Basir A.; Fhad S.; Hafeez A.; Subhan F.. et al. Impact of tillage, potassium levels and sources on growth, yield and yield attributes of wheat. Pak. J. Bot. 2022, 55( (1), ). 10.30848/PJB2023-1(30). [DOI]
- Saini A.; Manuja S.; Kumar S.; Hafeez A.; Ali B.; Poczai P. Impact of Cultivation Practices and Varieties on Productivity, Profitability, and Nutrient Uptake of Rice (Oryza sativa L.) and Wheat (Triticum aestivum L.) Cropping System in India. Agriculture 2022, 12, 1678 10.3390/agriculture12101678. [DOI] [Google Scholar]
- Ma J.; Ali S.; Saleem M. H.; Mumtaz S.; Yasin G.; Ali B.; et al. Short-term responses of Spinach (Spinacia oleracea L.) to the individual and combinatorial effects of Nitrogen, Phosphorus and Potassium and silicon in the soil contaminated by boron. Front. Plant Sci. 2022, 13, 983156 10.3389/fpls.2022.983156. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ma J.; Saleem M. H.; Ali B.; Rasheed R.; Ashraf M. A.; Aziz H.; et al. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 2022, 13, 950120 10.3389/fpls.2022.950120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Saleem M. H.; Yasin G.; Mumtaz S.; Qureshi F. F.; Ali B.; et al. Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front. Plant Sci. 2022, 13, 973740 10.3389/fpls.2022.973740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood S.; Khatoon Z.; Amna; Ahmad I.; Muneer M. A.; Kamran M. A.; et al. Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2021, 197–211. 10.1080/03650340.2021.1971654. [DOI] [Google Scholar]
- Zainab N.; Amna; Khan A. A.; Azeem M. A.; Ali B.; Wang T.; et al. Pgpr-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. In industrially contaminated soils. Agronomy 2021, 11, 1820 10.3390/agronomy11091820. [DOI] [Google Scholar]
- Fahad S.; Chavan S. B.; Chichaghare A. R.; Uthappa A. R.; Kumar M.; Kakade V.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877 10.3390/su142214877. [DOI] [Google Scholar]
- Al-Zaban M. I.; Alhag S. K.; Dablool A. S.; Ahmed A. E.; Alghamdi S.; Ali B.; et al. Manufactured nano-objects confer viral protection against cucurbit chlorotic yellows virus (CCYV) infecting nicotiana benthamiana. Microorganisms 2022, 10, 1837 10.3390/microorganisms10091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanki M. K.; Solanki A. C.; Rai S.; Srivastava S.; Kashyap B. K.; Divvela P. K.; et al. Functional interplay between antagonistic bacteria and Rhizoctonia solani in the tomato plant rhizosphere. Front. Microbiol. 2022, 13, 990850 10.3389/fmicb.2022.990850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervat M. H. A.; El-Naby A.; Shimaa S. I.; Noha A. M.; Heba H.; Abdou S.. et al. Omani Frankincense Nanoemulsion Formulation efficacy and its latent effects on biological aspects of the Spiny bollworm Earias insulana (Boisd.) Front Physiol., 21291001136 10.3389/fphys.2022.1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeem M.; Pirjan K.; Qasim M.; Mahmood A.; Javed T.; Muhammad H.; et al. Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci. Rep. 2023, 13, 2895 10.1038/s41598-023-29954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali J.; Jan I.; Ullah H.; Fahad S.; Saud S.; Adnan M.; et al. Biochemical Response of Okra (Abelmoschus esculentus L.) to Selenium (Se) under Drought Stress. Sustainability 2023, 15, 5694 10.3390/su15075694. [DOI] [Google Scholar]
- Shahzadi E.; Nawaz M.; Iqbal N.; Ali B.; Adnan M.; Saleem M. H.; et al. Silicic and Ascorbic Acid Induced Modulations in Photosynthetic, Mineral Uptake, and Yield Attributes of Mung Bean (Vigna radiata L. Wilczek) under Ozone Stress. ACS Omega 2023, 8, 13971–13981. 10.1021/acsomega.3c00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeed A. H. A.; Saleem M. H.; Asghar M. A.; Mumtaz S.; Ameer A.; Ali B.; et al. Ameliorative Effects of Exogenous Potassium Nitrate on Antioxidant Defense System and Mineral Nutrient Uptake in Radish (Raphanus sativus L.) under Salinity Stress. ACS Omega 2023, 8, 22575–22588. 10.1021/acsomega.3c01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B.; Hafeez A.; Afridi M. S.; Javed M. A.; Sumaira; Suleman F.; et al. Bacterial-Mediated Salinity Stress Tolerance in Maize (Zea mays L.): A Fortunate Way toward Sustainable Agriculture. ACS Omega 2023, 8, 20471–20487. 10.1021/acsomega.3c00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab A.; Abdi G.; Saleem M. H.; Ali B.; Ullah S.; Shah W.; et al. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620 10.3390/plants11131620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dola D. B.; Mannan M. A.; Sarker U.; Mamun M. A. A.; Islam T.; Ercisli S.; et al. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535 10.3389/fpls.2022.992535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq T. H.; Rafy M.; Basit H.; Shakoor A.; Shabbir R.; Riaz M. U.. et al. Morpho-Physiological Growth Performance and Phytoremediation Capabilities of Selected Xerophyte Grass Species Towards Cr and Pb Stress 2022997120 10.3389/fpls.2022.997120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S.; Ullah A.; Ullah S.; Noor J.; Ali B.; Khan M. N.; et al. Validating the Impact of Water Potential and Temperature on Seed Germination of Wheat (Triticum aestivum L.) via Hydrothermal Time Model. Life 2022, 12, 983 10.3390/life12070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi S.; Ullah S.; Hafeez A.; Khan M. N.; Javed M. A.; Ali B.; et al. Exogenous Ca/Mg quotient reduces the inhibitory effects of PEG induced osmotic stress on Avena sativa L. Braz. J. Biol. 2022, 84, e264642 10.1590/1519-6984.264642. [DOI] [PubMed] [Google Scholar]
- Naz R.; Khan M. S.; Hafeez A.; Fazil M.; Khan M. N.; Ali B.; et al. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted industrial soils. Braz. J. Biol. 2022, 84, e264473 10.1590/1519-6984.264473. [DOI] [PubMed] [Google Scholar]
- Nawaz H.; Ali A.; Saleem M. H.; Ameer A.; Hafeez A.; Alharbi K.; et al. Comparative effectiveness of EDTA and citric acid assisted phytoremediation of Ni contaminated soil by using canola (Brassica napus). Braz. J. Biol. 2022, 82, e261785 10.1590/1519-6984.261785. [DOI] [PubMed] [Google Scholar]
- Saleem K.; Asghar M. A.; Saleem M. H.; Raza A.; Kocsy G.; Iqbal N.; et al. Chrysotile-Asbestos-Induced Damage in Panicum virgatum and Phleum pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824 10.3390/su141710824. [DOI] [Google Scholar]
- Long X.-X.; Ju H.; Wang J.-D.; Gong S.-H.; Li G.-Y. Impact of climate change on wheat yield and quality in the Yellow River Basin under RCP8. 5 during 2020–2050. Adv. Clim. Changes Res. 2022, 13, 397. 10.1016/j.accre.2022.02.006. [DOI] [Google Scholar]
- Ali B.; Hafeez A.; Ahmad S.; Javed M. A.; Cavalu S.; Afridi M. S.; et al. Bacillus thuringiensis PM25 Ameliorates Oxidative Damage of Salinity Stress in Maize via Regulating Growth, Leaf Pigments, Antioxidant Defense System, and Stress Responsive Gene Expression. Front. Plant Sci. 2022, 13, 921668 10.3389/fpls.2022.921668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S. S.; Rasheed M.; Hamzah Saleem M.; Ahmed Z. I.; Hafeez A.; Jilani G., et al. Salt tolerance in maize with melatonin priming to achieve sustainability in yield on salt affected soils. Pak. J. Bot. 2023;55 10.30848/PJB2023-1(27). [DOI] [Google Scholar]
- Adnan M.; Fahad S.; Saleem M. H.; Ali B.; Mussart M.; Ullah R.; et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997 10.1038/s41598-022-16035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M.; Ishaq M.; Shah W. A.; Adnan M.; Fahad S.; Saleem M. H.; et al. Managing Phosphorus Availability from Organic and Inorganic Sources for Optimum Wheat Production in Calcareous Soils. Sustainability 2022, 14, 7669 10.3390/su14137669. [DOI] [Google Scholar]
- Umar U. d.; Ahmed N.; Zafar M. Z.; Rehman A.; Naqvi S. A. H.; Zulfiqar M. A.; et al. Micronutrients Foliar and Drench Application Mitigate Mango Sudden Decline Disorder and Impact Fruit Yield. Agronomy 2022, 12, 2449 10.3390/agronomy12102449. [DOI] [Google Scholar]
- Karlidag H.; Yildirim E.; Turan M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci. Agric. 2009, 66, 180–187. 10.1590/S0103-90162009000200006. [DOI] [Google Scholar]
- Hafsi C.; Romero-Puertas M. C.; Gupta D. K.; Luis A.; Sandalio L. M.; Abdelly C. Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environ. Exp. Bot. 2010, 69, 129–136. 10.1016/j.envexpbot.2010.04.008. [DOI] [Google Scholar]
- Remorini D.; Melgar J. C.; Guidi L.; Degl’Innocenti E.; Castelli S.; Traversi M. L.; et al. Interaction effects of root-zone salinity and solar irradiance on the physiology and biochemistry of Olea europaea. Environ. Exp. Bot. 2009, 65, 210–219. 10.1016/j.envexpbot.2008.12.004. [DOI] [Google Scholar]
- Saleem A.; Zulfiqar A.; Ali B.; Naseeb M. A.; Almasaudi A. S.; Harakeh S. Iron Sulfate (FeSO4) Improved Physiological Attributes and Antioxidant Capacity by Reducing Oxidative Stress of Oryza sativa L. Cultivars in Alkaline Soil. Sustainability 2022, 14, 16845 10.3390/su142416845. [DOI] [Google Scholar]
- Faryal S.; Ullah R.; Khan M. N.; Ali B.; Hafeez A.; Jaremko M.; Qureshi K. A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744 10.3390/molecules27185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram N. A.; Saleem M. H.; Shafiq S.; Naz H.; Farid-ul-Haq M.; Ali B.; et al. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498 10.3390/su142114498. [DOI] [Google Scholar]
- Deinlein U.; Stephan A. B.; Horie T.; Luo W.; Xu G.; Schroeder J. I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P.; Yang Y.; Fang M.; Wang F.; Chen H. Physiological response of young seedlings from five accessions of Diospyros L. under salinity stress. Hortic. Sci. Technol. 2016, 34, 564–577. 10.12972/kjhst.20160058. [DOI] [Google Scholar]
- Ali B.; Hafeez A.; Javed M. A.; Afridi M. S.; Abbasi H. A.; Qayyum A.; et al. Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense: A comprehensive review. S. Afr. J. Bot. 2022, 151, 33–46. 10.1016/j.sajb.2022.09.036. [DOI] [Google Scholar]
- Ali B.; Wang X.; Saleem M. H.; Azeem M. A.; Afridi M. S.; Nadeem M.; et al. Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219 10.3390/life12020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M.; Anwar-Ul-Haq M.; Javed T.; Hussain S.; Ahmad I.; Ashraf Sumrah M.; et al. Response of Contrasting Rice Genotypes to Zinc Sources under Saline Conditions. Phyton 2023, 92, 1361–1375. 10.32604/phyton.2023.026620. [DOI] [Google Scholar]
- Ali S.; Ullah S.; Khan M. N.; Khan W. M.; Razak S. A.; Wahab S.; et al. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability 2022, 14, 12924 10.3390/su141912924. [DOI] [Google Scholar]
- Salam A.; Afridi M. S.; Javed M. A.; Saleem A.; Hafeez A.; Khan A. R.; et al. Nano-Priming against Abiotic Stress: A Way Forward towards Sustainable Agriculture. Sustainability 2022, 14, 14880 10.3390/su142214880. [DOI] [Google Scholar]
- Anwar T.; Qureshi H.; Perveen N.; Mahmood S.; Zulqurnain Haider M.; Mumtaz S.. et al. Herbicidal effectiveness of wild poisonous plant Rhazya stricta using different media by the sandwich method. Pak. J. Bot. 2023, 55( (2), ). 10.30848/PJB2023-2(10). [DOI]
- Camacho-Cristóbal J. J.; Rexach J.; González-Fontes A. Boron in plants: deficiency and toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. 10.1111/j.1744-7909.2008.00742.x. [DOI] [PubMed] [Google Scholar]
- Ferguson I. B.; Boyd L. M.. Inorganic Nutrients and Fruit Quality. Fruit Quality and Its Biological Basis; CRC Press, 2002; pp 15–45. [Google Scholar]
- Aftab T.; Khan M. M. A.; Idrees M.; Naeem M.; Ram M. Boron induced oxidative stress, antioxidant defence response and changes in artemisinin content in Artemisia annua L. J. Agron. Crop Sci. 2010, 196, 423–430. 10.1111/j.1439-037X.2010.00427.x. [DOI] [Google Scholar]
- Sang W.; Huang Z.-R.; Yang L.-T.; Guo P.; Ye X.; Chen L.-S. Effects of high toxic boron concentration on protein profiles in roots of two citrus species differing in boron-tolerance revealed by a 2-DE based MS approach. Front. Plant Sci. 2017, 8, 180 10.3389/fpls.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgun Y.; Çöl B.; Bürün B. 24-Epibrassinolide ameliorates the effects of boron toxicity on Arabidopsis thaliana (L.) Heynh by activating an antioxidant system and decreasing boron accumulation. Acta Physiol. Plant. 2016, 38, 71 10.1007/s11738-016-2088-8. [DOI] [Google Scholar]
- Masood S.; Wimmer M. A.; Witzel K.; Zörb C.; Mühling K. H. Interactive effects of high boron and NaCl stresses on subcellular localization of chloride and boron in wheat leaves. J. Agron. Crop Sci. 2012, 198, 227–235. 10.1111/j.1439-037X.2011.00501.x. [DOI] [Google Scholar]
- Asma; Hussain I.; Ashraf M. Y.; Saleem M. H.; Ashraf M. A.; Ali B.; et al. Alleviating effects of salicylic acid spray on stage-based growth and antioxidative defense system in two drought-stressed rice (Oryza sativa L.) cultivars. Turk. J. Agric. For. 2023, 47, 79–99. 10.55730/1300-011X.3066. [DOI] [Google Scholar]
- Al-Huqail A. A.; Saleem M. H.; Ali B.; Azeem M.; Mumtaz S.; Yasin G.; et al. Efficacy of priming wheat (Triticum aestivum) seeds with a benzothiazine derivative to improve drought stress tolerance. Funct. Plant Biol. 2023, 10.1071/FP22140. [DOI] [PubMed] [Google Scholar]
- Talbi S.; Romero-Puertas M. C.; Hernández A.; Terrón L.; Ferchichi A.; Sandalio L. M. Drought tolerance in a Saharian plant Oudneya africana: role of antioxidant defences. Environ. Exp. Bot. 2015, 111, 114–126. 10.1016/j.envexpbot.2014.11.004. [DOI] [Google Scholar]
- Gunes A.; Inal A.; Bagci E. G.; Pilbeam D. J. Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil 2007, 290, 103–114. 10.1007/s11104-006-9137-9. [DOI] [PubMed] [Google Scholar]
- Elkhlifi Z.; Iftikhar J.; Sarraf M.; Ali B.; Saleem M. H.; Ibranshahib I.; et al. Potential Role of Biochar on Capturing Soil Nutrients, Carbon Sequestration and Managing Environmental Challenges: A Review. Sustainability 2023, 15, 2527 10.3390/su15032527. [DOI] [Google Scholar]
- Javed M. A.; Khan M. N.; Ali B.; Wahab S.; Din I. U.; Razak S. A.. Positive and Negative Impacts of Biochar on Microbial Diversity. In Sustainable Agriculture Reviews 61. Sustainable Agriculture Reviews; Fahad S.; Danish S.; Datta R.; Saud S.; Lichtfouse E., Eds.; Springer International Publishing: Cham, 2023; pp 311–330. [Google Scholar]
- Lutfunnahar S. J.; Piash M. I.; Rahman M. H. Impact of MgCl2 Modified Biochar on Phosphorus and Nitrogen Fractions in Coastal Saline Soil. Open J. Soil Sci. 2021, 11, 331–351. 10.4236/ojss.2021.116017. [DOI] [Google Scholar]
- Chaganti V. N.; Crohn D. M. Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 2015, 259–260, 45–55. 10.1016/j.geoderma.2015.05.005. [DOI] [Google Scholar]
- Peng H.; Gao P.; Chu G.; Pan B.; Peng J.; Xing B. Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. 10.1016/j.envpol.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Muhammad H.; Fahad S.; Saud S.; Hassan S.; Nasim W.; Ali B.; et al. A Paradigm Shift towards Beneficial Microbes Enhancing the Efficiency of Organic and Inorganic Nitrogen Sources for a Sustainable Environment. Land 2023, 12, 680 10.3390/land12030680. [DOI] [Google Scholar]
- Haider M. W.; Nafees M.; Ahmad I.; Ali B.; Iqbal R.; Vodnar D. C.; et al. Postharvest dormancy-related changes of endogenous hormones in relation to different dormancy-breaking methods of potato (Solanum tuberosum L.) tubers. Front. Plant Sci. 2022, 13, 945256 10.3389/fpls.2022.945256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B.; Wang X.; Saleem M. H.; Hafeez A.; Afridi M. S.; Khan S.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345 10.3390/plants11030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Gupta R.; Pandey R. Exogenous application of rutin and gallic acid regulate antioxidants and alleviate reactive oxygen generation in Oryza sativa L. Physiol. Mol. Biol. Plants 2017, 23, 301–309. 10.1007/s12298-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetişsin F.; Kurt F. Gallic acid (GA) alleviating copper (Cu) toxicity in maize (Zea mays L.) seedlings. Int. J. Phytorem. 2020, 22, 420–426. 10.1080/15226514.2019.1667953. [DOI] [PubMed] [Google Scholar]
- Yildiztugay E.; Ozfidan-Konakci C.; Kucukoduk M. Improvement of cold stress resistance via free radical scavenging ability and promoted water status and photosynthetic capacity of gallic acid in soybean leaves. J. Soil Sci. Plant Nutr. 2017, 17, 366–384. 10.4067/S0718-95162017005000027. [DOI] [Google Scholar]
- Ozfidan-Konakci C.; Yildiztugay E.; Kucukoduk M. Protective roles of exogenously applied gallic acid in Oryza sativa subjected to salt and osmotic stresses: effects on the total antioxidant capacity. Plant Growth Regul. 2015, 75, 219–234. 10.1007/s10725-014-9946-4. [DOI] [Google Scholar]
- Díaz-Pérez J. C.; Eaton T. E. Eggplant (Solanum melongena L.) plant growth and fruit yield as affected by drip irrigation rate. HortScience 2015, 50, 1709–1714. 10.21273/HORTSCI.50.11.1709. [DOI] [Google Scholar]
- FAO F . Food and Agriculture Organization of the United Nations; FAO: Rome, 2018. http//faostatfaoorg. [Google Scholar]
- Habib K.; Khan I. A.; Rasheed Akbar M. S.; Farid A.; Ali I.; Alam M. Response of Brinjal, Solanum melongena L. (Solanales: Solanaceae), Genotypes against Insect Pests in Peshawar, Pakistan. J. Entomol. Zool. Stud. 2015, 3, 423–427. [Google Scholar]
- Matthaeus W. J.; Schmidt J.; White J. D.; Zechmann B. Novel perspectives on stomatal impressions: Rapid and non-invasive surface characterization of plant leaves by scanning electron microscopy. PLoS One 2020, 15, e0238589 10.1371/journal.pone.0238589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalay G.; Ullah S.; Ahmed I. Physiological and biochemical responses of Brassica napus L. to drought-induced stress by the application of biochar and Plant Growth Promoting Rhizobacteria. Microsc. Res. Tech. 2022, 85, 1267–1281. 10.1002/jemt.23993. [DOI] [PubMed] [Google Scholar]
- Gee G. W.; Or D.. 2.4 Particle-Size Analysis, Methods of Soil Analysis: Part 4 Physical Methods, 5.4; John Wiley & Sons, Inc., 2002; Vol. 5, pp 255–293. [Google Scholar]
- Page A. L.; Miller R. H.; Keeney D. R.. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley & Sons, Inc., 1982; Vol. 2, pp 643–698. [Google Scholar]
- Kandoliya S. I.; Kandoliya U. K.; Bhadja N. V.; Golakiya B. A. The effect of seed priming with plant growth promoting rhizobacteria (PGPR) on growth of Coriander (Coriandrum sativum L.) seedling. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1926–1934. 10.20546/ijcmas.2017.603.219. [DOI] [Google Scholar]
- Metwally A. M.; Radi A. A.; El-Shazoly R. M.; Hamada A. M. The role of calcium, silicon and salicylic acid treatment in protection of canola plants against boron toxicity stress. J. Plant Res. 2018, 131, 1015–1028. 10.1007/s10265-018-1008-y. [DOI] [PubMed] [Google Scholar]
- Ogbaga C. C.; Stepien P.; Johnson G. N. Sorghum (Sorghum bicolor) varieties adopt strongly contrasting strategies in response to drought. Physiol. Plant. 2014, 152, 389–401. 10.1111/ppl.12196. [DOI] [PubMed] [Google Scholar]
- Chuyong G. B.; Acidri T. Light and moisture levels affect growth and physiological parameters differently in Faidherbia albida (Delile) A. Chev. seedlings. Acta Physiol. Plant. 2017, 39, 117 10.1007/s11738-017-2410-0. [DOI] [Google Scholar]
- Ghule P. L.; Dahiphale V. V.; Jadhav J. D.; Palve D. K. Absolute growth rate, relative growth rate, net assimilation rate as influenced on dry matter weight of Bt cotton. Int. Res. J. Agric. Econ. Stat. 2013, 4, 42–46. [Google Scholar]
- Ullah S.; Zada J.; Ali S. Effect of nephthyl acetic acid foliar spray on amelioration of drought stress tolerance in maize (Zea mays L.). Commun. Soil Sci. Plant Anal. 2016, 47, 1542–1558. 10.1080/00103624.2016.1194994. [DOI] [Google Scholar]
- Shah W.; Ullah S.; Ali S.; Idrees M.; Khan M. N.; Ali K.; et al. Effect of exogenous alpha-tocopherol on physio-biochemical attributes and agronomic performance of lentil (Lens culinaris Medik.) under drought stress. PLoS One 2021, 16, e0248200 10.1371/journal.pone.0248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubberstein D.; Oliveira M. G.; Aoyama E. M.; Guilhen J. H.; Ferreira A.; Marques I.; et al. Diversity of Leaf Stomatal Traits among Coffea canephora Pierre ex A. Froehner Genotypes. Agronomy 2021, 11, 1126 10.3390/agronomy11061126. [DOI] [Google Scholar]
- Zou M.; Yuan L.; Zhu S.; Liu S.; Ge J.; Wang C. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol. Plant. 2017, 39, 30 10.1007/s11738-016-2319-z. [DOI] [Google Scholar]
- Khanam D.; Mohammad F. Plant growth regulators ameliorate the ill effect of salt stress through improved growth, photosynthesis, antioxidant system, yield and quality attributes in Mentha piperita L. Acta Physiol. Plant. 2018, 40, 188 10.1007/s11738-018-2769-6. [DOI] [Google Scholar]
- Liu Y.; He Z.; Xie Y.; Su L.; Zhang R.; Wang H.; et al. Drought resistance mechanisms of Phedimus aizoon L. Sci. Rep. 2021, 11, 13600 10.1038/s41598-021-93118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi F.; Shekari F.; Hamzei J. Methyl jasmonate improves salinity resistance in German chamomile (Matricaria chamomilla L.) by increasing activity of antioxidant enzymes. Acta Physiol. Plant. 2016, 38, 1 10.1007/s11738-015-2023-4. [DOI] [Google Scholar]
- Alshegaihi R. M.; Mfarrej M. F. B.; Saleem M. H.; Parveen A.; Ahmad K. S.; Ali B.; et al. Effective citric acid and EDTA treatments in cadmium stress tolerance in pepper (Capsicum annuum L.) seedlings by regulating specific gene expression. S. Afr. J. Bot. 2023, 159, 367–380. 10.1016/j.sajb.2023.06.024. [DOI] [Google Scholar]
- Ameen F.; Mumtaz S.; Ali B.; Hussain I.; Hafeez A.; Gul A.; et al. The impact of Cu-polluted and organic soil on the fibrous plant; insights into plant growth promotion, antioxidant defences system, and oxidative stress. Funct. Plant Biol. 2023, 10.1071/FP23027. [DOI] [PubMed] [Google Scholar]
- Kakar H. A.; Ullah S.; Shah W.; Ali B.; Satti S. Z.; Ullah R.; et al. Seed Priming Modulates Physiological and Agronomic Attributes of Maize (Zea mays L.) under Induced Polyethylene Glycol Osmotic Stress. ACS Omega 2023, 8, 22788–22808. 10.1021/acsomega.3c01715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool A.; Ghani A.; Nawaz R.; Ahmad S.; Shahzad K.; Rebi A.; et al. Effects of Poultry Manure on the Growth, Physiology, Yield, and Yield-Related Traits of Maize Varieties. ACS Omega 2023, 10.1021/acsomega.3c00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A.; Razzaq A.; Mehmood S. S.; Zou X.; Zhang X.; Lv Y.; Xu J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34 10.3390/plants8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman L. A.; Harpole W. S. Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 2013, 5, 202–214. 10.1111/gcbb.12037. [DOI] [Google Scholar]
- Hao F.; Zhao X.; Ouyang W.; Lin C.; Chen S.; Shan Y.; Lai X. Molecular Structure of Corncob-Derived Biochars and the Mechanism of Atrazine Sorption. Agron. J. 2013, 105, 773–782. 10.2134/agronj2012.0311. [DOI] [Google Scholar]
- Méndez A.; Terradillos M.; Gascó G. Physicochemical and agronomic properties of biochar from sewage sludge pyrolysed at different temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 124–130. 10.1016/j.jaap.2013.03.006. [DOI] [Google Scholar]
- Ahmad M.; Lee S. S.; Yang J. E.; Ro H.-M.; Lee Y. H.; Ok Y. S. Effects of soil dilution and amendments (mussel shell, cow bone, and biochar) on Pb availability and phytotoxicity in military shooting range soil. Ecotoxicol. Environ. Saf. 2012, 79, 225–231. 10.1016/j.ecoenv.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Kim K. H.; Kim J.-Y.; Cho T.-S.; Choi J. W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. 10.1016/j.biortech.2012.04.094. [DOI] [PubMed] [Google Scholar]
- Ullah S.; Khan M.; Khan N.; Ali U.; Ali B.; Iqbal R.; et al. Efficacy of Naphthyl Acetic Acid Foliar Spray in Moderating Drought Effects on the Morphological and Physiological Traits of Maize Plants (Zea mays L.). ACS Omega 2023, 8, 20488–20504. 10.1021/acsomega.3c00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood N.; Saeed M.; Zafarullah S.; Hyder S.; Rizvi Z. F.; Gondal A. S.; et al. Multifaceted Impacts of Plant-Beneficial Pseudomonas spp. in Managing Various Plant Diseases and Crop Yield Improvement. ACS Omega 2023, 8, 22296. 10.1021/acsomega.3c00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R.; Tester M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Hussien Ibrahim M. E.; Adam Ali A. Y.; Zhou G.; Ibrahim Elsiddig A. M.; Zhu G.; Ahmed Nimir N. E.; Ahmad I. Biochar application affects forage sorghum under salinity stress. Chil. J. Agric. Res. 2020, 80, 317–325. 10.4067/S0718-58392020000300317. [DOI] [Google Scholar]
- Kanwal S.; Ilyas N.; Shabir S.; Saeed M.; Gul R.; Zahoor M.; et al. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. 2018, 41, 526–538. 10.1080/01904167.2017.1392568. [DOI] [Google Scholar]
- Khan M. A.; Islam E.; Shirazi M. U.; Mumtaz S.; Mujtaba S. M.; Khan M. A.; et al. Physiological responses of various wheat genotypes to salinity. Pak. J. Bot. 2010, 42, 3497–3505. [Google Scholar]
- Shi L.; Yang J.; Liu J.; Li R.; Long Y.; Xu F.; Meng J. Identification of quantitative trait loci associated with low boron stress that regulate root and shoot growth in Brassica napus seedlings. Mol. Breed. 2012, 30, 393–406. 10.1007/s11032-011-9629-z. [DOI] [Google Scholar]
- Nxele X.; Klein A.; Ndimba B. K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. 10.1016/j.sajb.2016.11.003. [DOI] [Google Scholar]
- Taffouo V. D.; Nouck A. H.; Dibong S. D.; Amougou A. Effects of salinity stress on seedlings growth, mineral nutrients and total chlorophyll of some tomato (Lycopersicum esculentum L.) cultivars. Afr. J. Biotechnol. 2010, 9, 5366–5372. [Google Scholar]
- Manuchehri R.; Salehi H. Physiological and biochemical changes of common bermudagrass (Cynodon dactylon [L.] Pers.) under combined salinity and deficit irrigation stresses. S. Afr. J. Bot. 2014, 92, 83–88. 10.1016/j.sajb.2014.02.006. [DOI] [Google Scholar]
- García-Sánchez F.; Jifon J. L.; Carvajal M.; Syvertsen J. P. Gas exchange, chlorophyll and nutrient contents in relation to Na+ and Cl– accumulation in ‘Sunburst’ mandarin grafted on different rootstocks. Plant Sci. 2002, 162, 705–712. 10.1016/S0168-9452(02)00010-9. [DOI] [Google Scholar]
- Farhangi-Abriz S.; Torabian S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. 10.1016/j.ecoenv.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Zulfiqar F.; Younis A.; Chen J. Biochar or biochar-compost amendment to a peat-based substrate improves growth of Syngonium podophyllum. Agronomy 2019, 9, 460 10.3390/agronomy9080460. [DOI] [Google Scholar]
- Chen H.; Jiang J.-G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. 10.1139/A10-014. [DOI] [Google Scholar]
- Bhamburdekar S. B.; Chavan P. D. Effect of some stresses on free proline content during pigeonpea (Cajanas cajan) seed germination. J. Stress Physiol. Biochem. 2011, 7, 235–241. [Google Scholar]
- Akladious S. A.; Mohamed H. I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. 10.1016/j.scienta.2018.03.047. [DOI] [Google Scholar]
- Linić I.; Mlinarić S.; Brkljačić L.; Pavlović I.; Smolko A.; Salopek-Sondi B. Ferulic acid and Salicylic acid foliar treatments reduce short-term salt stress in Chinese cabbage by increasing phenolic compounds accumulation and photosynthetic performance. Plants 2021, 10, 2346 10.3390/plants10112346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadak M. S. Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull. Natl. Res. Cent. 2019, 43, 53 10.1186/s42269-019-0098-6. [DOI] [Google Scholar]
- Mehdizadeh L.; Moghaddam M.; Lakzian A. Alleviating negative effects of salinity stress in summer savory (Satureja hortensis L.) by biochar application. Acta Physiol. Plant. 2019, 41, 1–13. 10.1007/s11738-019-2900-3. [DOI] [Google Scholar]
- Khodary S. E. A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Tavallali V.; Karimi S.; Espargham O. Boron enhances antioxidative defense in the leaves of salt-affected Pistacia vera seedlings. Hortic. J. 2018, 87, 55–62. 10.2503/hortj.OKD-062. [DOI] [Google Scholar]
- Lim J.-H.; Park K.-J.; Kim B.-K.; Jeong J.-W.; Kim H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. 10.1016/j.foodchem.2012.05.068. [DOI] [PubMed] [Google Scholar]
- Babaei M.; Shabani L. Improving the Effects of Salt Stress by β-carotene and Gallic Acid Using Increasing Antioxidant Activity and Regulating Ion Uptake in Lepidium sativum L. Bot. Stud. 2021, 22 10.1186/s40529-022-00352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam R. K.; Srivastava G. C. Induction of oxidative stress and antioxidant activity by hydrogen peroxide treatment in tolerant and susceptible wheat genotypes. Biol. Plant. 2000, 43, 381–386. 10.1023/A:1026730008917. [DOI] [Google Scholar]
- Ferro M.; Sgherri C.; Romani M.; Carvalho M. d. F.; Izzo R. Tomato irrigated with boron enriched fresh water: effects on leaves and berries. Agrochimica 2010, 54, 370–380. [Google Scholar]
- Torabi F.; Majd A.; Enteshari S. The effect of silicon on alleviation of salt stress in borage (Borago officinalis L.). Soil Sci. Plant Nutr. 2015, 61, 788–798. 10.1080/00380768.2015.1005540. [DOI] [Google Scholar]
- Orsini F.; Alnayef M.; Bona S.; Maggio A.; Gianquinto G. Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ. Exp. Bot. 2012, 81, 1–10. 10.1016/j.envexpbot.2012.02.005. [DOI] [Google Scholar]
- Ma X.; Zheng J.; Zhang X.; Hu Q.; Qian R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600 10.3389/fpls.2017.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.; Chen X.-Y. Development: a new function of plant trichomes. Nat. Plants 2016, 2, 16096 10.1038/nplants.2016.96. [DOI] [PubMed] [Google Scholar]
- Passinho-Soares H. C.; David J. P.; de Santana J. R. F.; David J. M.; Rodrigues F. d. M.; Mesquita P. R. R.; et al. Influence of growth regulators on distribution of trichomes and the production of volatiles in micropropagated plants of Plectranthus ornatus. Rev. Bras. Farmacogn. 2017, 27, 679–690. 10.1016/j.bjp.2017.10.001. [DOI] [Google Scholar]
- Bose S. K.; Yadav R. K.; Mishra S.; Sangwan R. S.; Singh A. K.; Mishra B.; et al. Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol. Biochem. 2013, 66, 150–158. 10.1016/j.plaphy.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Chakraborty N. Salicylic acid and nitric oxide cross-talks to improve innate immunity and plant vigor in tomato against Fusarium oxysporum stress. Plant Cell Rep. 2021, 40, 1415–1427. 10.1007/s00299-021-02729-x. [DOI] [PubMed] [Google Scholar]
- Akhtar N. Effect of physical and chemical mutagens on morphological behavior of tomato (Solanum lycopersicum) CV. Plant Breed. Seed Sci. 2014, 70, 69–79. 10.1515/plass-2015-0014. [DOI] [Google Scholar]
- Akhtar S. S.; Andersen M. N.; Liu F. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. 10.1111/jac.12132. [DOI] [Google Scholar]
- Hafez Y.; Attia K.; Alamery S.; Ghazy A.; Al-Doss A.; Ibrahim E.; et al. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 2020, 10, 630 10.3390/agronomy10050630. [DOI] [Google Scholar]
- Leite M. C. M.; Araujo M. A.; Souza L. A.; Martins A. R.; Camargos L. S. Ecophysiological response of Astronium fraxinifolium (Anacardiaceae) in degraded and non-degraded brazilian Cerrado. Rodriguésia 2021, 72, 1–10. 10.1590/2175-7860202172066. [DOI] [Google Scholar]
- Fernández V.; Guzmán-Delgado P.; Graça J.; Santos S.; Gil L. Cuticle structure in relation to chemical composition: re-assessing the prevailing model. Front. Plant Sci. 2016, 7, 427 10.3389/fpls.2016.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiate N. I.; Zaman Q. u.; Francis I. N.; Dada O. A.; Rehman A.; Asif M.; et al. Soil Amendment with Arbuscular Mycorrhizal Fungi and Biochar Improves Salinity Tolerance, Growth, and Lipid Metabolism of Common Wheat (Triticum aestivum L.). Sustainability 2022, 14, 3210 10.3390/su14063210. [DOI] [Google Scholar]
- Wu S.-L.; Wei W.; Xu Q.; Huang X.; Sun J.; Dai X.; Ni B. J. Revealing the mechanism of biochar enhancing the production of medium chain fatty acids from waste activated sludge alkaline fermentation liquor. ACS ES&T Water 2021, 1, 1014–1024. 10.1021/acsestwater.0c00278. [DOI] [Google Scholar]
- Tak A. A.; Kakde U. B. Biochemical, morphological and anatomical changes in tree foliage exposed to vehicular-pollution. Int. J. Environ. Agric. Biotechnol. 2020, 5, 699–708. 10.22161/ijeab.53.23. [DOI] [Google Scholar]
- Darwish M. A.; Nassar R. M. A.; Abdel-Aziz N. G.; Abdel-Aal A. S. Riboflavin minimizes the deleterious effects of salinity stress on growth, chloroplast pigments, free proline, activity of antioxidant enzyme catalase and leaf anatomy of Tecoma capensis (Thumb.) Lindl. Middle East J Agric. Res. 2017, 6, 757–765. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.