Abstract
BACKGROUND
Angiomatoid fibrous histiocytoma (AFH) is an exceptionally rare soft tissue neoplasm. This tumor primarily presents as a benign soft tissue lesion in children with an average age of 14 years. The standard treatment regimen is wide local excision with interval follow-up. However, newer reports have demonstrated malignant potential with the possibility of intracranial metastasis.
OBSERVATIONS
A 45-year-old male with no soft tissue primary tumor presented with a primary intracranial lesion and thoracic spine metastasis refractory to chemotherapy and radiation treatment.
LESSONS
This report illustrates the potential for a highly malignant nature of metastatic AFH. In addition, the authors demonstrate an incidence of AFH in a middle-aged male without a primary soft tissue or skin lesion. This report highlights the importance of prompt treatment and excision for AFH, as there is still little understanding of successful options for systemic therapy.
Keywords: angiomatoid fibrous histiocytoma, cerebral metastasis, spine metastasis, spine oncology
ABBREVIATIONS: AFH = angiomatoid fibrous histiocytoma, CT = computed tomography, EMA = epithelial membrane antigen, EWSR1-CREB1 = Ewing sarcoma breakpoint region 1/EWS RNA binding protein 1 to cAMP responsive binding protein element 1 gene translocation, EWSR1-ATF1 = Ewing sarcoma breakpoint region 1/EWS RNA binding protein 1 to activating transcription factor 1 gene translocation, FUS-ATF1 = Fused in Sarcoma to activating transcription factor 1 gene translocation, IR = interventional radiology, LCA = leukocyte common antigen, MFH = malignant fibrous histiocytoma, MRI = magnetic resonance imaging, PET = positron emission tomography, PR = progesterone receptor, SALL4 = sal-like protein 4, SOX10 = SRY-related HMG-box, TTF-1 = thyroid transcription factor-1
Angiomatoid fibrous histiocytoma (AFH) is a poorly understood rare soft tissue neoplasm. To our understanding, this is the first reported case of a confirmed primary intracranial lesion with spinal metastasis. AFH is diagnostically challenging as it lacks a specific immunoprofile, making immunohistochemical studies supportive rather than diagnostic. The differential is wide and can include hemangioma, malignant fibrous histiocytoma (MFH), myxoid chondrosarcoma, malignant ossifying fibromyxoid tumor, and various other sarcomas.1–3 Its most common presentation is a slow-growing, superficial nodular mass, with a median patient age of 14 years.2 There is no known sex predilection.4 Metastases are rare, occurring in less than 5% of cases.5,6 AFH was previously considered angiomatoid MFH. However, in 2020, the World Health Organization reclassified MFH into an entity of undifferentiated pleomorphic sarcomas, accounting for 5% of adult soft tissue sarcomas. AFH was reallocated to tumors of uncertain definition.3 The management of AFH is traditionally limited to wide local excision with close follow-up. Adjuvant radiation or chemotherapy is indicated if the case is metastatic or unresectable. We report the case of a 45-year-old male with biopsy-proven brain and spine metastasis of AFH.
Illustrative Case
A 45-year-old healthy male with no past medical history presented with headache in April 2019 and word-finding difficulty for 4 days. Initial head computed tomography (CT) (Fig. 1) demonstrated a left frontal lobe hematoma with moderate vasogenic edema, midline shift, and an adjacent peripherally centered, 2.9-cm, hyperdense mass-like lesion. Brain magnetic resonance imaging (MRI) demonstrated an extra-axial contrast-enhancing mass with hemorrhage and mass effect (Fig. 1).
FIG. 1.
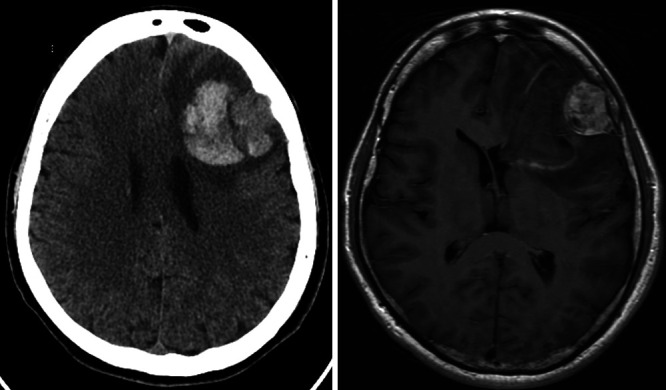
Left: Axial noncontrast computed tomography (CT) demonstrating a left frontal lobe hematoma with surrounding vasogenic edema and mild midline shift, as well as an adjacent peripherally centered, 2.9-cm, hyperdense mass-like lesion with erosion of the inner table of the skull. Right: Axial T1-weighted fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) with contrast demonstrating a left frontal, extra-axial, heterogeneously enhancing mass with hemorrhagic changes and mass effect.
The patient was taken to the operating room for left frontal craniotomy for resection of the hemorrhage and tumor. Postoperative MRI demonstrated complete resection of the mass. The patient was stable and oriented to person, place, and time; had an equal pupillary response; and was following commands antigravity without drift. He made mild paraphasic errors. He was discharged home on postoperative day 5.
Final pathology was consistent with a poorly differentiated, malignant, blue cell neoplasm with neuroendocrine expression positive for synaptophysin, Ki-67, and epithelial membrane antigen (EMA) (Fig. 2). Cells demonstrated a plasmacytoid appearance with a densely cellular blue cell neoplasm and abundant hemosiderin. The sample demonstrated relevant negative staining for SRY-related HMG-box (SOX10), HMB45, sal-like protein 4 (SALL4), progesterone receptor (PR), chromogranin, pancytokeratin, and thyroid transcription factor-1 (TTF-1), and the tumor was negative for leukocyte common antigen (LCA), CD138, Epstein-Barr encoding region in situ hybridization (EBER ISH), signal transducer and activator of transcription 6 (STAT6), and calcitonin. However, the final tumor pathology was not defined. Systemic workup with CT of the chest, abdomen, and pelvis was negative. A positron emission tomography (PET) scan demonstrated no definite evidence of residual malignancy in the brain and no suspicious uptake in the neck, chest, abdomen, or pelvis to suggest metastatic etiology for the brain lesion. The patient was treated with stereotactic fractionated radiotherapy in 5 fractions of 6 Gy in May 2019. An interval CT of the chest, abdomen, and pelvis in September demonstrated new scattered lytic osseous lesions, greatest at the T8 and L2 vertebral bodies. PET scanning confirmed an abnormal update at these lesions in addition to left anterior acetabulum and left posterior triangle neck lymph node. In October, an interventional radiology (IR)–guided needle biopsy of the T8 vertebral body yielded intermediate-sized cells with ovoid to histiocytoid nuclei arranged in small clusters and sheets (Fig. 3). This sample also demonstrated EMA and focal synaptophysin positivity.
FIG. 2.
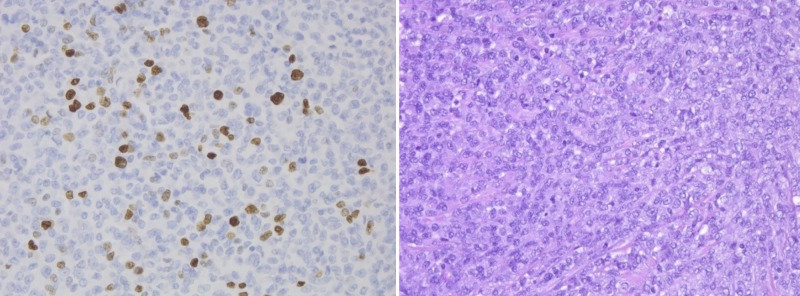
Left: Immunohistochemical staining from the frontal mass demonstrating Ki-67 positivity. Ki-67, original magnification ×20. Right: Cytological preparations demonstrate a blue cell tumor with moderate nuclear pleomorphism and high nuclear to cytoplasmic ratio. Some cells demonstrate a plasmacytoid appearance. There are frequent mitoses, up to 12 per high-power field. Hematoxylin and eosin (H&E), original magnification ×20.
FIG. 3.
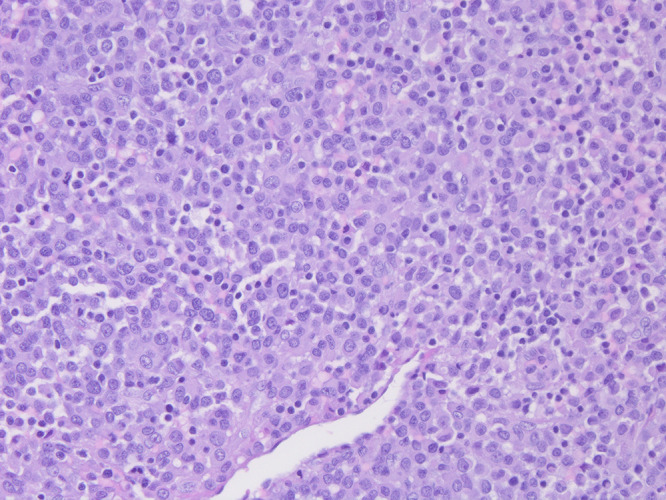
Pathology from the T7 mass. The tumor is composed of solid nodules to sheets of small to intermediate-sized, uniform, round to polygonal histiocytoid cells. Cells have eosinophilic cytoplasm with focal clearing. Abundant plasma cells and lymphocytes are adjacent to the tumor. H&E, original magnification ×20.
One week later, the patient presented with 5 days of bilateral lower-extremity weakness, gait instability, and bowel and bladder incontinence. Strength was diffusely 4/5 in bilateral lower-extremity muscle groups with bilateral clonus and 4+ patellar reflexes. MRI of the lumbar and thoracic spine with and without contrast demonstrated a T7 and T8 expansile metastatic lesion with Bilsky grade 3 compression of the spinal cord. There were two enhancing lesions of the L2 and L3 vertebral bodies with L2 Bilsky grade 1a epidural extension (Fig. 4).
FIG. 4.
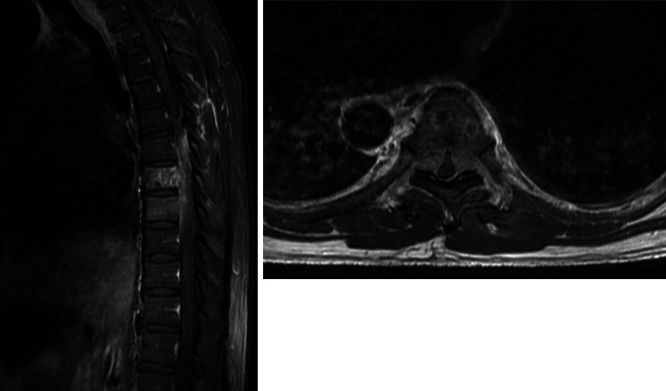
Left: Sagittal postcontrast T1-weighted MRI of the thoracic spine demonstrating T7 and T8 enhancing lesions of the vertebral body with epidural extension. Right: Axial postcontrast T1-weighted MRI of the thoracic spine through the T7 level demonstrating bilateral epidural extension with complete effacement of the cerebrospinal fluid (CSF) spaces and spinal cord compression.
Because of his rapidly progressive myelopathy and significant ventral compression, he was taken to the operating room for a posterior thoracic laminectomy at T5–10 with pedicle screw instrumentation at T4–11 and T7 and T8 corpectomies (Fig. 5). Final pathology demonstrated spindled to ovoid cells with prominent nucleoli with positive EMA, synaptophysin, and desmin staining. Additional immunohistochemical testing was negative for pancytokeratin, chromogranin, S100, SOX10, SALL4, PR, calcitonin, TTF-1, and LCA. Chromosomal testing demonstrated Ewing sarcoma breakpoint region 1/EWS RNA binding protein 1 to cAMP responsive binding protein element 1 gene translocation (EWSR1-CREB1) fusion and Ewing sarcoma breakpoint region 1/EWS RNA binding protein 1 to activating transcription factor 1 gene translocation (EWSR1-ATF1) which are both pathognomonic for AFH, confirming the diagnosis. This additional chromosomal testing was not performed on the intracranial lesion, leading to a nonconfirmatory diagnosis after his initial surgery. Postoperatively, his strength was 5/5 in his lower extremities. He progressed well and was discharged to rehabilitation 8 days postoperatively.
FIG. 5.
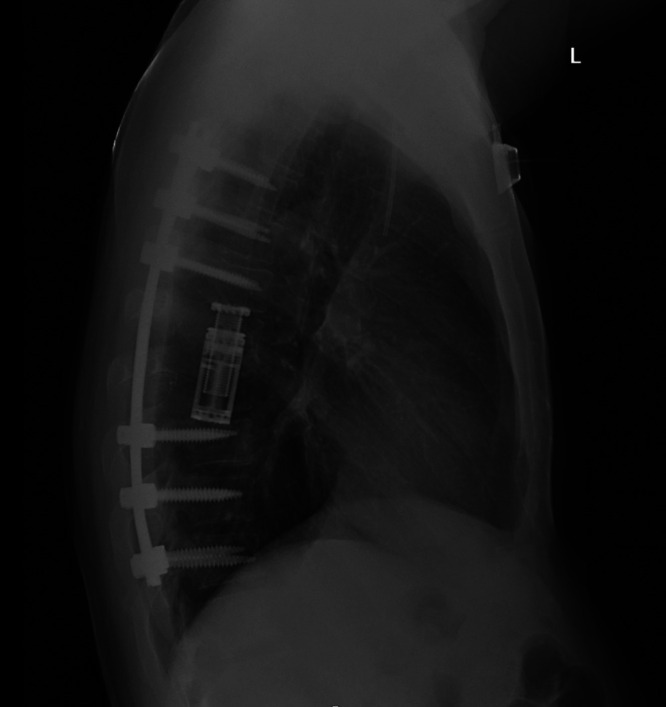
Lateral chest radiograph demonstrating the T7–8 corpectomy with T4–11 instrumentation and fusion.
He was seen for follow-up 1 month after surgery and was ambulating well with a walker. His instability had improved, and he was participating in outpatient physical therapy. He received palliative radiation therapy to the postoperative bed in the thoracic spine and lumbar spine radiation therapy to the existing metastasis. This was followed by chemotherapy with Adriamycin (Pfizer Inc.) and ifosfamide. Two months later, bevacizumab and temozolomide chemotherapy was delivered without benefit. He developed new, diffuse metastasis to the iliopsoas muscle, ilium, sphenoid wing, and frontal bone. He was ultimately discharged to hospice care 20 months after initial diagnosis.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
This case represents an exceptionally rare presentation of AFH with initial presentation of a frontal hemorrhagic brain mass with indeterminate pathology followed by rapid metastasis. Multiple biopsies of thoracic lesions confirmed AFH and, compared with brain pathology, are most indicative of a primary brain lesion of AFH with metastasis to the vertebral bodies with epidural compression.
Epidemiology
In the landmark paper on AFH by Enzinger1 in 1979, ages ranged from 6 to 43 years with a median age of 13 years. Moreover, 88% of patients were younger than 30, 68% younger than 27, and 35% younger than 14 years. There was a mild predilection for males. However, more recent reviews have reported no sex predilection.4 Its most frequent presentation is a superficial, nontender, slow-growing skin lesion that can be mistaken for other soft tissue tumors like hemangioma, sarcoma, and hematoma.5
Occurrences outside of somatic soft tissues are exceptionally rare but have been documented in the brain,7,8 lungs,7,8 mediastinum,8–10 omentum,9 vulva,9 ovary,9 and bone.9,10 Extrasomatic AFH tends to have a higher mean age of presentation, 35 years, with a male predilection.9 Patients with extrasomatic occurrences can present with paraneoplastic inflammatory syndrome with systemic symptoms including pyrexia, malaise, and anemia. It is theorized that these metastases produce inflammatory cytokines through interleukin-6 (IL-6) expression, which is upregulated by the EWSR1-CREB1 translocation.9
In 2020, Bin Abdulqader et al.11 conducted a review of the existing 22 cases of intracranial AFH in the literature since the initial case documented by Dunham et al.7 in 2008. Only 7 of the 22 cases were older than 20 years (31.8%). The most common location was frontal (36%), with only three cases (13.6%) demonstrating a dural origin, as in our case. The most common presenting symptom was headaches followed by seizure.11
While AFH has been documented in the bones, there is no previous documentation of spine metastasis. Tumors have been located in the pelvis, femur, mandible, humerus, ulna, radius, and other peripheral long bones, but there has been no documentation of prior thoracic spinal metastasis.9 In addition, no other case reports have demonstrated a primary cranial lesion with metastasis to the spine with progression of disease and trials of adjuvant therapy.
Radiology
Radiologically, these lesions characteristically have multiple internal cystic cavities with fluid levels when observed in peripheral bones. This is not surprising considering the frequency of intralesional hemorrhage and hemosiderin deposition.12 In the case report and literature review by Bin Abdulqader et al.,11 there were three lesions that demonstrated an extra-axial mass with a dural tail similar in presentation to our case. In addition, the intracranial lesions demonstrated a peripheral rim of enhancement with intralesional heterogeneous enhancement. These findings were congruent with our patient’s intracranial lesion. However, some of the other examples of intra-axial lesions had more of a multilobulated and cystic appearance with fluid levels, as observed in pelvic and long bone lesions. There is no direct evidence to consider for AFH spinal lesions, but a retrospective review of our patient’s lesion demonstrated a peripherally enhancing mass with heterogeneous enhancement of the T7 vertebral body. There were no cystic changes of fluid levels.
Histology
Histologically, AFH is a benign-appearing tumor consisting of ovoid, epithelioid, or spindle cells with minimal cytological atypia.1,13 A small percentage of lesions may demonstrate pleomorphism, but cellular atypia and mitotic figures were not correlated with worse outcomes.4 Intralesional hemorrhage is noted in most cases regardless of whether the lesion presents with gross hemorrhage. This is believed to be due to the formation of blood-filled pseudo-angiomatous spaces lined by flattened neoplastic cells.4 The immunohistochemistry is variable, but AFH often shows positivity for desmin, CD68, and EMA.3,9,14 Moreover, 75% of cases in a short case series demonstrated positivity for synpatophysin.14 AFHs are consistently negative for skeletal muscle markers such as myogenin or MyoD1.4 Vascular endothelial markers are negative, including CD31, CD34, CD35, S100, cytokeratin, and lyzosome.15 In a case series (n = 8), the Ki-67 proliferation index was found to be low, from 2% to 4%.9
Genetics
There is a consensus of three associated gene fusions: EWSR1-CREB1, EWSR1-ATF1, and rarely Fused in Sarcoma to activating transcription factor 1 gene translocation (FUS-ATF1).1,14,16,17 EWSR-CREB1 and EWSR1-ATF1 fusions are not unique to AFH and are present in a variety of soft tissue neoplasms, including clear cell sarcoma-like tumor of the gastrointestinal tract and primary pulmonary myxoid sarcoma.4,16,18 EWSR1-ATF1 t(22q12)(q13;q12) and FUS-ATF1 (t21q22)(q13;q12) are seen in 7%–10% of cases. The FUS-ATF1 gene translocation is the only one unique to AFH. The EWSR1-CREB1 gene fusion is present in a group of aggressive mesenchymal neoplasms, including primary pulmonary myxoid sarcoma, clear cell sarcoma-like tumor of the gastrointestinal tract, AFH, and clear cell tumor. The mechanism by which these gene fusions affect oncogenes, downstream messaging, and tumorigenesis is not yet understood.15,17
Treatment Options and Outcomes
The mainstay of treatment for AFH is wide excision.1 With complete excision, local recurrence is limited to 2%–10%. In soft tissue primary lesions, there was an 11% chance of local recurrence and a 1% chance of distal metastasis.1 The case report and literature review of intracranial lesions by Dunham et al.7 demonstrated recurrence in 7 (53.8%) of 13 cases with follow-up. Of the eight cases listed as having complete resection, only 37.5% (3/8) had a recurrence. This series suggests that, like the local skin lesion form of AFH, complete wide resection is linked to better outcomes.8 However, more research is needed, especially in regard to spine metastasis and the benefits of radiation therapy.
In the setting of metastatic disease, adjuvant radiotherapy and chemotherapy have been considered. The support for chemotherapy is minimal and is limited to a few case reports, mostly in children.5 It is generally considered to be fatal if the lesion is in a nonresectable location or the disease has metastasized. Our patient had two trials of chemotherapy in addition to spinal Gamma Knife radiation to the residual lesions. He ultimately developed further metastasis and was transitioned to hospice 20 months after initial diagnosis.
Observations
AFH is a rare soft tissue tumor that is classically considered to be a nonmalignant disease of children. However, we illustrated the case of a 45-year-old male with rapid, malignant transformation of AFH without a somatic lesion. Diagnosis was first suggested after the left frontal mass was discovered and was confirmed 4 months later with multiple metastatic bone lesions and a large thoracic epidural lesion requiring urgent decompression, corpectomy, and fusion. This patient was positive for classic markers of AFH, including desmin, EMA, synaptophysin, and the EWSR1-CREB1 translocation.
Lessons
AFH should be within the differential of hemorrhagic lesions, especially in the younger population. More data are needed to determine the best treatment algorithm for spine metastasis and expected clinical course. Current research efforts demonstrate few trials of chemotherapy or radiation therapy. While our patient received multiple different chemotherapy regimens and spinal Gamma Knife radiation, none were effective. This leaves an opportunity for further studies into this unique tumor, its markers, and possible treatment options.
Author Contributions
Conception and design: Demand, Barber, Britz. Acquisition of data: Demand, Barber, Britz. Analysis and interpretation of data: Demand, Barber. Drafting of the article: Demand. Critically revising the article: Demand, Barber, Britz. Reviewed submitted version of the manuscript: Demand, Barber. Approved the final version of the manuscript on behalf of all authors: Demand. Administrative/technical/material support: Demand. Study supervision: Barber. Pathology: Powell.
References
- 1. Enzinger FM. Angiomatoid malignant fibrous histiocytoma: a distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm. Cancer. 1979;44(6):2147–2157. doi: 10.1002/1097-0142(197912)44:6<2147::aid-cncr2820440627>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2. Chow LT, Allen PW, Kumta SM, Griffith J, Li CK, Leung PC. Angiomatoid malignant fibrous histiocytoma: report of an unusual case with highly aggressive clinical course. J Foot Ankle Surg. 1998;37(3):235–238. doi: 10.1016/s1067-2516(98)80117-8. [DOI] [PubMed] [Google Scholar]
- 3. Fletcher CD. The evolving classification of soft tissue tumours—an update based on the new 2013 WHO classification. Histopathology. 2014;64(1):2–11. doi: 10.1111/his.12267. [DOI] [PubMed] [Google Scholar]
- 4. Thway K, Fisher C. Angiomatoid fibrous histiocytoma: the current status of pathology and genetics. Arch Pathol Lab Med. 2015;139(5):674–682. doi: 10.5858/arpa.2014-0234-RA. [DOI] [PubMed] [Google Scholar]
- 5. Bernini JC, Fort DW, Pritchard M, Rogers BB, Winick NJ. Adjuvant chemotherapy for treatment of unresectable and metastatic angiomatoid malignant fibrous histiocytoma. Cancer. 1994;74(3):962–964. doi: 10.1002/1097-0142(19940801)74:3<962::aid-cncr2820740327>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6. Saito K, Kobayashi E, Yoshida A, et al. Angiomatoid fibrous histiocytoma: a series of seven cases including genetically confirmed aggressive cases and a literature review. BMC Musculoskelet Disord. 2017;18(1):31. doi: 10.1186/s12891-017-1390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunham C, Hussong J, Seiff M, Pfeifer J, Perry A. Primary intracerebral angiomatoid fibrous histiocytoma: report of a case with a t(12;22)(q13;q12) causing type 1 fusion of the EWS and ATF-1 genes. Am J Surg Pathol. 2008;32(3):478–484. doi: 10.1097/PAS.0b013e3181453451. [DOI] [PubMed] [Google Scholar]
- 8. Ochalski PG, Edinger JT, Horowitz MB, et al. Intracranial angiomatoid fibrous histiocytoma presenting as recurrent multifocal intraparenchymal hemorrhage. J Neurosurg. 2010;112(5):978–982. doi: 10.3171/2009.8.JNS081518. [DOI] [PubMed] [Google Scholar]
- 9. Chen G, Folpe AL, Colby TV, et al. Angiomatoid fibrous histiocytoma: unusual sites and unusual morphology. Mod Pathol. 2011;24(12):1560–1570. doi: 10.1038/modpathol.2011.126. [DOI] [PubMed] [Google Scholar]
- 10. Mangham DC, Williams A, Lalam RK, Brundler MA, Leahy MG, Cool WP. Angiomatoid fibrous histiocytoma of bone: a calcifying sclerosing variant mimicking osteosarcoma. Am J Surg Pathol. 2010;34(2):279–285. doi: 10.1097/PAS.0b013e3181cb4017. [DOI] [PubMed] [Google Scholar]
- 11. Bin Abdulqader S, Altuhaini K, Tallab R, et al. Primary intracranial angiomatoid fibrous histiocytoma: two case reports and literature review. World Neurosurg. 2020;143:398–404. doi: 10.1016/j.wneu.2020.07.225. [DOI] [PubMed] [Google Scholar]
- 12. Ajlan AM, Sayegh K, Powell T, et al. Angiomatoid fibrous histiocytoma: magnetic resonance imaging appearance in 2 cases. J Comput Assist Tomogr. 2010;34(5):791–794. doi: 10.1097/RCT.0b013e3181e39755. [DOI] [PubMed] [Google Scholar]
- 13. Fanburg-Smith JC, Miettinen M. Angiomatoid “malignant” fibrous histiocytoma: a clinicopathologic study of 158 cases and further exploration of the myoid phenotype. Hum Pathol. 1999;30(11):1336–1343. doi: 10.1016/s0046-8177(99)90065-5. [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa T, Seki K, Ono K, Hirohashi S. Angiomatoid (malignant) fibrous histiocytoma: a peculiar low-grade tumor showing immunophenotypic heterogeneity and ultrastructural variations. Pathol Int. 2000;50(9):731–738. doi: 10.1046/j.1440-1827.2000.01112.x. [DOI] [PubMed] [Google Scholar]
- 15. Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36(7):e1–e11. doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 16. Rossi S, Szuhai K, Ijszenga M, et al. EWSR1-CREB1 and EWSR1-ATF1 fusion genes in angiomatoid fibrous histiocytoma. Clin Cancer Res. 2007;13(24):7322–7328. doi: 10.1158/1078-0432.CCR-07-1744. [DOI] [PubMed] [Google Scholar]
- 17. Thway K, Stefanaki K, Papadakis V, Fisher C. Metastatic angiomatoid fibrous histiocytoma of the scalp, with EWSR1-CREB1 gene fusions in primary tumor and nodal metastasis. Hum Pathol. 2013;44(2):289–293. doi: 10.1016/j.humpath.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 18. Hallor KH, Mertens F, Jin Y, et al. Fusion of the EWSR1 and ATF1 genes without expression of the MITF-M transcript in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer. 2005;44(1):97–102. doi: 10.1002/gcc.20201. [DOI] [PubMed] [Google Scholar]