Abstract
BACKGROUND
Developmental venous anomaly (DVA) is a rare cause of hemifacial spasm (HFS). The treatment of HFS caused by a DVA varies in the literature and includes medication management, botulinum toxin injections, and microvascular decompression (MVD).
OBSERVATIONS
A 64-year-old woman presented with right-sided HFS. Preoperative magnetic resonance imaging showed a DVA in the right inferior pons, with an enlarged segment compressing the facial nerve at its root detachment point prior to drainage into the superior petrosal sinus. MVD was performed, and the facial nerve was decompressed without sacrifice of the vein. Immediately following the procedure, the patient had significantly reduced spasms. The patient became spasm-free 3 months after MVD and maintained spasm freedom for 3 months. Six months after MVD, the patient had a partial return of spasms. At 8 months, the patient continued to have reduced and intermittent spasms in the right orbicularis oculi muscle.
LESSONS
MVD for HFS caused by a DVA is a safe procedure and can be effective at reducing spasm frequency and severity.
Keywords: developmental venous anomaly, case report, hemifacial spasm, microvascular decompression, pontine
ABBREVIATIONS: DVA = developmental venous anomaly, HFS = hemifacial spasm, MRI = magnetic resonance imaging, MVD = microvascular decompression
Hemifacial spasm (HFS) is a condition of hyperactivity in the facial nerve causing involuntary and irregular contraction of the ipsilateral facial muscles.1 Spasms begin as intermittent twitching of a single facial muscle and become more intense and frequent as the condition progresses. HFS is predominantly caused by arterial contact or compression of the centrally myelinated portion of the facial nerve.2–4 Less common causes of HFS include cerebellopontine angle tumors, vascular malformations, and veins.5,6 First-line nonoperative management of HFS is various preparations of botulinum toxin, and standard surgical treatment is microvascular decompression (MVD).7,8 Developmental venous anomaly (DVA) is a rare cause of HFS. DVAs are the most common vascular malformation, with one study reporting an incidence of 2.6% in 4069 brain autopsies.9 DVAs are typically asymptomatic but can present as hemorrhage, hydrocephalus, or cranial nerve dysfunction.10,11
There are three reported cases of MVD for DVA-associated HFS. One case was a DVA in the internal auditory canal, and two cases were cerebellar DVAs.12–14 We present a unique case of HFS caused by facial nerve compression by a pontine DVA treated with MVD.
Illustrative Case
A 64-year-old woman with a past medical history of Hashimoto’s thyroiditis presented with right-sided HFS. Spasms began in the right orbicularis oculi muscle when she was 56 years old and spread to ipsilateral muscles innervated by the facial nerve. Spasms were involuntary, intermittent, and debilitating. The patient had previously undergone 11 botulinum toxin treatments for HFS.
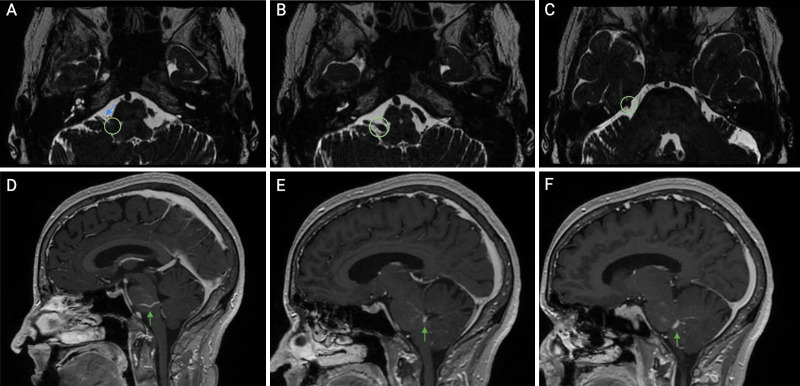
Preoperative magnetic resonance imaging (MRI) showed compression of the central myelinized portion of the right facial nerve by a DVA in the right inferior pons with drainage into the superior petrosal sinus (Fig. 1).
FIG. 1.
Preoperative axial (A–C) and sagittal (D–F) magnetic resonance imaging (MRI) sequences with contrast illustrating a developmental venous anomaly (DVA) (green arrow), the DVA (green circle, A) compressing the facial nerve (blue arrow), and the DVA (green circle, B and C) draining into the superior petrosal sinus.
Microvascular decompression was performed through a right retromastoid craniectomy with the patient in the left lateral decubitus position, as previously described.15 The facial nerve was decompressed with multiple interpositional felt pledgets (Bard PTFE felt pledget; Becton Dickinson). Before and after dissecting the DVA from the nerve, the nerve was stimulated at 0.1 mA, and the nerve responded robustly. The vein was not sacrificed due to concerns of stroke.
Immediately following the procedure, the patient had significantly reduced spasms, with less frequency and intensity. She spent 1 night in the hospital. She became spasm-free 3 months after MVD and maintained spasm freedom for 3 months. Six months following MVD, the patient had a partial return of spasms in the setting of several significant stressors. At 8 months, the patient continued to have intermittent spasms in the right orbicularis oculi muscle that were reduced in frequency and intensity compared with before surgery. The patient’s spasm status will continue to be monitored.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Developmental venous anomaly–associated HFS management remains controversial. The majority of DVAs are asymptomatic and therefore are typically not treated. The largest risk factor for hemorrhage is concurrent cavernous angioma. Hemorrhage in the setting of a DVA is likely attributable to concurrent cavernous angiomas.16,17 However, symptomatic DVAs can cause severe and life-threatening symptoms and can require treatment. Symptomatic DVAs are divided into three categories: flow related, mechanical, and idiopathic. Flow-related causes are further divided into an increase of inflow to the DVA or a decrease in outflow from the DVA. Symptoms from flow-related causes include headache, neurological deficit, seizures, and coma secondary to hemorrhage or infarction. Mechanical causes include obstructive hydrocephalus and nerve compression, causing trigeminal neuralgia and HFS. Cases with symptoms attributed to DVA, such as headache, but with no identifiable patho-mechanism, are classified as idiopathic.5 Here, we present an example of mechanical compression of the facial nerve by a pontine DVA ostensibly causing HFS. Although the patient ultimately had a partial recurrence of spasms, she did not experience any surgical or postoperative complications, providing evidence that MVD for HFS due to compression of the facial nerve by a pontine DVA can be a safe treatment.
Observations
There are few cases of brainstem DVAs associated with HFS in the literature, and only a portion of these cases were treated with MVD. As outlined in Table 1, Rizek et al.18 presented a case of left-sided HFS associated with a pontine DVA that was managed with botulinum toxin treatment. No long-term follow-up data are provided for this case. Chiaramonte et al.19 described a man with left-sided HFS with a left cerebellar DVA that was treated with carbamazepine with no long-term follow-up. Arita et al.20 reported a case of left HFS associated with a left pontine DVA. In this case, a left retrosigmoid craniotomy showed no evidence of compression, so MVD was not performed. The patient continued to have spasms postoperatively. Mahran et al.12 presented a case of right-sided HFS with an internal auditory canal DVA treated with MVD and removal of the DVA. The patient was spasm-free immediately following surgery and 3 months later. Chen et al.13 reported left-sided HFS associated with a left cerebellar DVA treated with MVD. The patient was spasm-free at 1 week, but no long-term follow-up was provided. Finally, Grigoryan et al.14 presented a case of left-sided HFS secondary to a left cerebellar DVA with two previous MVDs. The patient had a third MVD with evidence of DVA thrombosis on postoperative MRI and was spasm-free immediately and at the 3-month follow-up. Of the six reported cases with HFS associated with DVA, the longest patient follow-up was 3 months. In our case, the patient was spasm-free from postoperative months 3 to 6 and subsequently had a recurrence of spasms at the 6-month mark. In our and others’ experience, it can take 2 years for spasms to resolve following MVD.21 Two of the three cases of DVA treated with MVD resulted in DVA removal or thrombosis, and both cases remained spasm-free at 3 months after MVD. However, it is difficult to determine if removal or thrombosis of a DVA is associated with long-term spasm relief, as both of these cases only had 3 months of follow-up. Chen et al. did not sacrifice the DVA during MVD, and the patient was spasm-free immediately following the operation, but only 1 week of follow-up was reported. All three previous cases of MVD for DVA-associated HFS resulted in immediate spasm relief; however, none had long-term follow-up. In our case, the DVA was not sacrificed, and the patient ultimately had a return of spasms.
TABLE 1.
Reported cases of HFS associated with DVA
| Authors & Year | Age (yrs)/Sex | Age at HFS Onset (yrs) | Lesion | Treatment |
|---|---|---|---|---|
| Mahran et al., 199112
|
58/F |
60*
|
Venous angioma located in IAC; compression of rt CN VII & VIII |
MVD–rt retrosigmoid craniectomy |
| Chen et al., 199613
|
53/F |
51 |
Lt cerebellar hemisphere venous angioma; compression of lt CN VII |
MVD–lt retromastoid craniectomy |
| Arita et al., 201220
|
38/F |
21 |
Lt pontine cavernous angioma w/ venous angioma exiting ventrally to CN VII in prepontine cistern (noncompressive) |
Botulinum toxin (unsatisfactory); lt retromastoid craniotomy, but no evidence of compression |
| Chiaramonte et al., 201319
|
59/M |
59 |
Lt cerebellar DVA; compression of lt CN VII |
Carbamazepine 600 mg/day |
| Rizek et al., 201618
|
32/F |
5 |
Lt pontine DVA; compression of lt CN VII at REZ |
Botulinum toxin preferred by patient over surgery |
| Grigoryan et al., 202014
|
30/F |
18 |
Lt cerebellar DVA; compression of lt CN VII at REZ |
MVD–lt retromastoid craniotomy, 2 previous at MVDs age 26 & 27 |
| Present case | 64/F | 56 | Rt pontine DVA; deformation of rt CN VII at REZ | MVD–rt retromastoid craniectomy |
CN = cranial nerve; IAC = internal auditory canal; REZ = root exit zone.
DVA was discovered at age 58, and the patient subsequently developed HFS at age 60.
To our knowledge, this is the first report in the literature of a patient with HFS caused by facial nerve compression by a pontine DVA treated with MVD. This case report presents the longest follow-up for any DVA causing HFS, including those managed medically and surgically. It provides evidence that MVD is a safe treatment for HFS caused by a pontine DVA. Although MVD did reduce spasm intensity and frequency, it did not eliminate the spasms completely. It is difficult to assess the outcomes of MVD for DVA-associated HFS with the limited number of cases in the literature.12–14 Higher complication rates have been noted in MVD of veins for HFS.6 Therefore, caution should be taken when considering the manipulation of brainstem veins.
This study is limited by a lack of long-term follow-up. A follow-up of at least 2 years would be beneficial to assess longer-term outcomes of MVD to treat HFS secondary to a pontine DVA. A prospective cohort study to assess the efficacy of MVD to treat HFS associated with a pontine DVA would provide stronger evidence to guide future management recommendations.
Lessons
This report describes a unique case of right HFS caused by a right pontine DVA compressing the facial nerve and treated with MVD. The patient had no complications, suggesting that MVD is a safe procedure for HFS caused by a DVA. However, the patient had a partial return of spasms 6 months after MVD. Spasms were reduced in frequency and intensity, suggesting that MVD can be effective in treating HFS due to facial nerve compression by a DVA.
Author Contributions
Conception and design: both authors. Acquisition of data: both authors. Analysis and interpretation of data: both authors. Drafting of the article: both authors. Critically revising the article: both authors. Reviewed submitted version of the manuscript: both authors. Approved the final version of the manuscript on behalf of both authors: Sekula.
References
- 1. Schultze F. Linksseitiger facialiskrampf in folge eines aneurysma der arteria vertebralis sinistra. Arch. Pathol. Anat. 1875;65:385–391. [Google Scholar]
- 2. Campos-Benitez M, Kaufmann AM. Neurovascular compression findings in hemifacial spasm. J Neurosurg. 2008;109(3):416–420. doi: 10.3171/JNS/2008/109/9/0416. [DOI] [PubMed] [Google Scholar]
- 3. Maroon JC. Hemifacial spasm. A vascular cause. Arch Neurol. 1978;35(8):481–483. doi: 10.1001/archneur.1978.00500320001001. [DOI] [PubMed] [Google Scholar]
- 4. Sekula RF, Jr, Frederickson AM, Branstetter BF, 4th, et al. Thin-slice T2 MRI imaging predicts vascular pathology in hemifacial spasm: a case-control study. Mov Disord. 2014;29(10):1299–1303. doi: 10.1002/mds.25947. [DOI] [PubMed] [Google Scholar]
- 5. Arseni C, Petrovici I. Persistent tonic facial spasm in brain stem tumours. J Neurol Sci. 1968;7(1):107–114. doi: 10.1016/0022-510x(68)90007-5. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Thirumala PD, Shah A, et al. The role of vein in microvascular decompression for hemifacial spasm: a clinical analysis of 15 cases. Neurol Res. 2013;35(4):389–394. doi: 10.1179/1743132812Y.0000000153. [DOI] [PubMed] [Google Scholar]
- 7. Park YC, Lim JK, Lee DK, Yi SD. Botulinum a toxin treatment of hemifacial spasm and blepharospasm. J Korean Med Sci. 1993;8(5):334–340. doi: 10.3346/jkms.1993.8.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995;82(2):201–210. doi: 10.3171/jns.1995.82.2.0201. [DOI] [PubMed] [Google Scholar]
- 9. Sarwar M, McCormick WF. Intracerebral venous angioma. Case report and review. Arch Neurol. 1978;35(5):323–325. doi: 10.1001/archneur.1978.00500290069012. [DOI] [PubMed] [Google Scholar]
- 10. Pereira VM, Geibprasert S, Krings T, et al. Pathomechanisms of symptomatic developmental venous anomalies. Stroke. 2008;39(12):3201–3215. doi: 10.1161/STROKEAHA.108.521799. [DOI] [PubMed] [Google Scholar]
- 11. Acioly MA, Simões EL, Parise M, Telles C, Nigri F. Developmental venous anomaly causing trigeminal neuralgia. Arq Neuropsiquiatr. 2010;68(5):822–825. doi: 10.1590/s0004-282x2010000500031. [DOI] [PubMed] [Google Scholar]
- 12. Mahran A, Samii M, Penkert G, Ostertag H. Vascular lesions of the internal auditory canal. Skull Base Surg. 1991;1(2):78–84. doi: 10.1055/s-2008-1056985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen HJ, Lee TC, Lui CC. Hemifacial spasm caused by a venous angioma. Case report. J Neurosurg. 1996;85(4):716–717. doi: 10.3171/jns.1996.85.4.0716. [DOI] [PubMed] [Google Scholar]
- 14. Grigoryan G, Sitnikov A, Grigoryan Y. Hemifacial spasm caused by the brainstem developmental venous anomaly: a case report and review of the literature. Surg Neurol Int. 2020;11:141. doi: 10.25259/SNI_56_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thirumala P, Frederickson AM, Balzer J, et al. Reduction in high-frequency hearing loss following technical modifications to microvascular decompression for hemifacial spasm. J Neurosurg. 2015;123(4):1059–1064. doi: 10.3171/2014.12.JNS141699. [DOI] [PubMed] [Google Scholar]
- 16. Huber G, Henkes H, Hermes M, Felber S, Terstegge K, Piepgras U. Regional association of developmental venous anomalies with angiographically occult vascular malformations. Eur Radiol. 1996;6(1):30–37. doi: 10.1007/BF00619949. [DOI] [PubMed] [Google Scholar]
- 17. McLaughlin MR, Kondziolka D, Flickinger JC, Lunsford S, Lunsford LD. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998;43(2):195–201. doi: 10.1097/00006123-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 18. Rizek P, Kumar N, Sharma M, et al. Brainstem developmental venous anomaly causing hemifacial spasm—case report and review of the literature. Can J Neurol Sci. 2016;43(4):606–608. doi: 10.1017/cjn.2016.33. [DOI] [PubMed] [Google Scholar]
- 19. Chiaramonte R, Bonfiglio M, D’Amore A, Chiaramonte I. Developmental venous anomaly responsible for hemifacial spasm. Neuroradiol J. 2013;26(2):201–207. doi: 10.1177/197140091302600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arita H, Kishima H, Hosomi K, et al. Hemifacial spasm caused by intra-axial brainstem cavernous angioma with venous angiomas. Br J Neurosurg. 2012;26(2):281–283. doi: 10.3109/02688697.2011.609605. [DOI] [PubMed] [Google Scholar]
- 21. Payner TD, Tew JMJ., Jr Recurrence of hemifacial spasm after microvascular decompression. Neurosurgery. 1996;38(4):686–690, discussion 690–691. [PubMed] [Google Scholar]