Abstract
Patient: Female, 35-year-old
Final Diagnosis: Breast cancer
Symptoms: Breast mass
Clinical Procedure: —
Specialty: Oncology
Objective:
Rare coexistence of disease or pathology
Background:
Down syndrome (DS) is the most common genetic disorder, and individuals with DS are known to have a low risk for solid tumors, including breast cancer. In contrast, Breast Cancer Susceptibility Gene (BRCA) pathogenic variant can cause breast cancer. We report a case of primary breast cancer harboring a BRCA2 pathogenic variant in a 35-year-old woman with DS.
Case Report:
A 35-year-old woman with DS presented with a palpable 2-cm mass in the upper-inner quadrant of the left breast. A biopsy confirmed an invasive ductal carcinoma of the breast. Her clinical diagnosis was cT2, N0, M0, cStageIIA. A left modified radical mastectomy with axillary node dissection was performed. Her final pathological diagnosis was invasive ductal carcinoma (T2, pN1, M0, stageIIB), positive estrogen receptors, negative progesterone receptors, negative human epidermal receptor-2 status. She was started on adjuvant hormonal therapy. Unfortunately, 23 months after the operation, multiple metastases were detected. Testing for a BRCA pathogenic variant was performed, and a BRCA2 pathogenic variant was detected. Olaparib was orally administered, and the levels of tumor markers rapidly declined; however, the levels of the tumor markers started to increase again 5 months after the initiation of olaparib. Subsequently, she developed bilateral carcinomatous lymphangiomatosis and died 59 months after the operation.
Conclusions:
This report highlights a rare case of primary breast cancer harboring a germline BRCA2 pathogenic variant in an individual with DS. Our study highlights the importance of genetic testing as part of breast cancer management in these patients.
Keywords: Breast Neoplasms; Down Syndrome; Genes, BRCA2
Background
Down syndrome results from a trisomy of all or part of human chromosome 21. This syndrome is the most common genetic disorder, causing various developmental and functional defects. Individuals with Down syndrome display a unique pattern of malignant disorders, which is characterized by a high frequency of acute leukemia and testicular cancer and a low risk of other types of solid tumors, including breast cancer. On the contrary, BRCA pathogenic variant can cause various types of cancer, including breast, ovarian, pancreatic, and prostate cancer.
We report a case of primary breast cancer harboring a BRCA2 pathogenic variant in a 35-year-old woman with Down syndrome.
Case Report
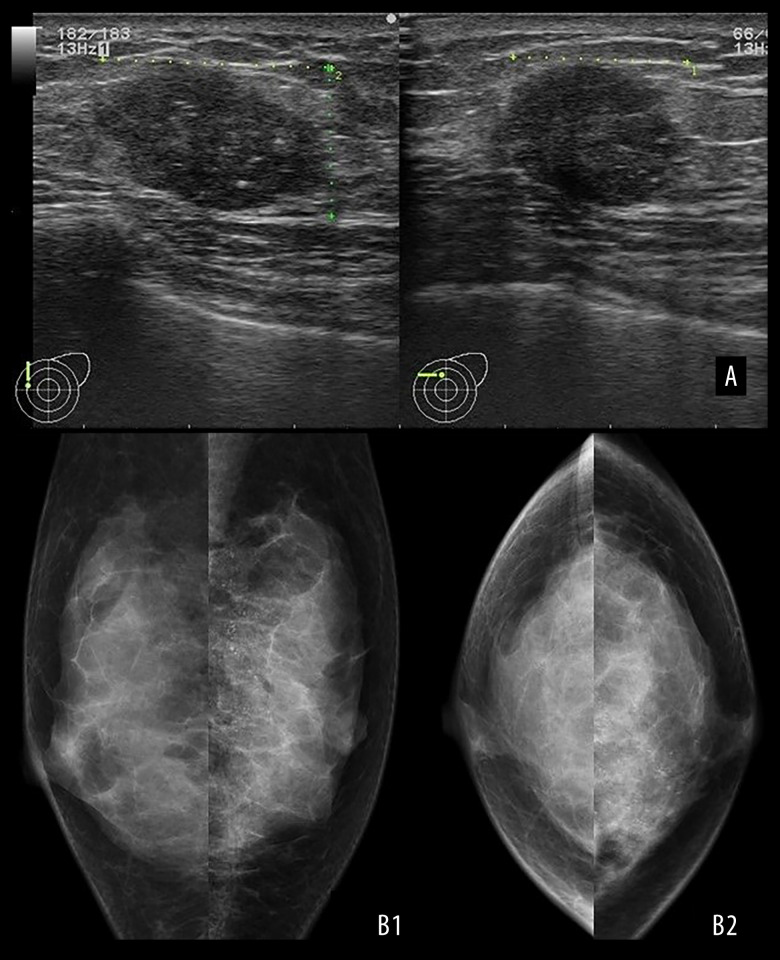
A 35-year-old premenopausal woman with Down syndrome presented to the Hokushin General Hospital Department of Surgery with a palpable 2-cm mass in the upper-inner quadrant of the left breast. She had histories of surgical repair of an atrioventricular septal defect at the age of 13 years and a duodenal stenosis at the age of 15 years. She had no family history of breast or ovarian cancer; however, her paternal grandfather had a history of prostatic cancer. Ultrasound sonography revealed a 2.2×1.7-cm irregularly shaped heterogeneous hypoechoic mass with a partially unclear boundary and multiple dotted calcifications (Figure 1A). Mammography revealed segmentally spread pleomorphic calcifications in the upper-inner quadrant of the left breast (Figure 1B).
Figure 1.
Image findings. (A) Breast ultrasonography revealed an irregularly shaped mass measuring 2.2×1.7×1.4 cm with an inhomogeneous inside and punctate high-echo contents. (B) Mammography showing pleomorphic calcifications spreading segmentary in the upper-inner quadrant of the left breast. (B1: MLO, B2: CC)
Core needle biopsy results confirmed invasive ductal carcinoma in the left breast. No distant metastasis was detected on body computed tomography (CT) or bone scintigraphy. She underwent a left modified radical mastectomy with axial lymph node dissection.
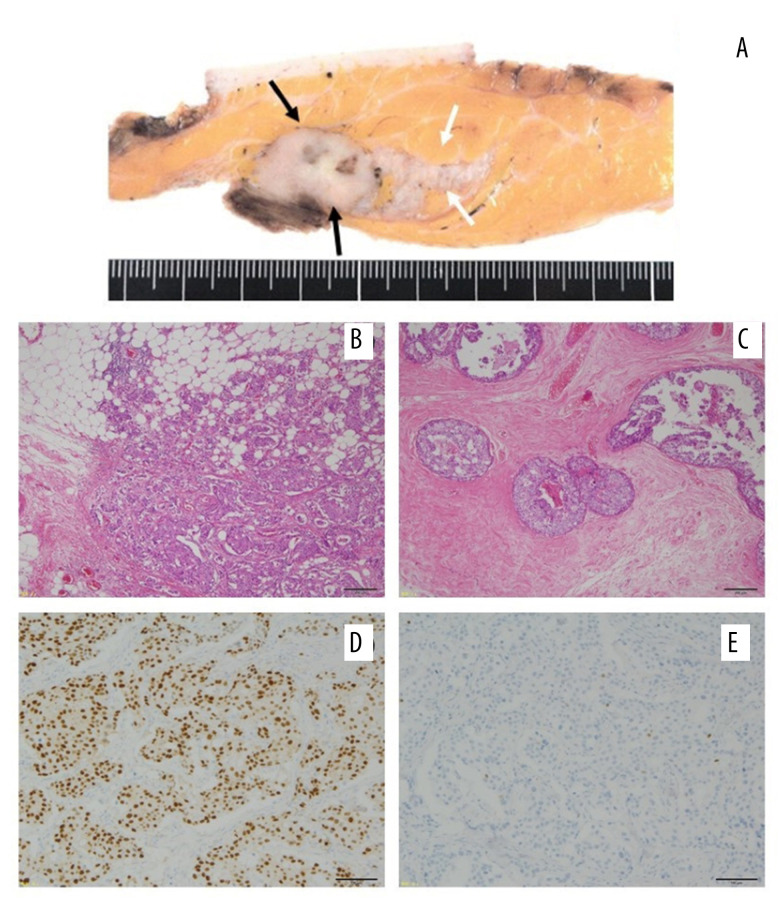
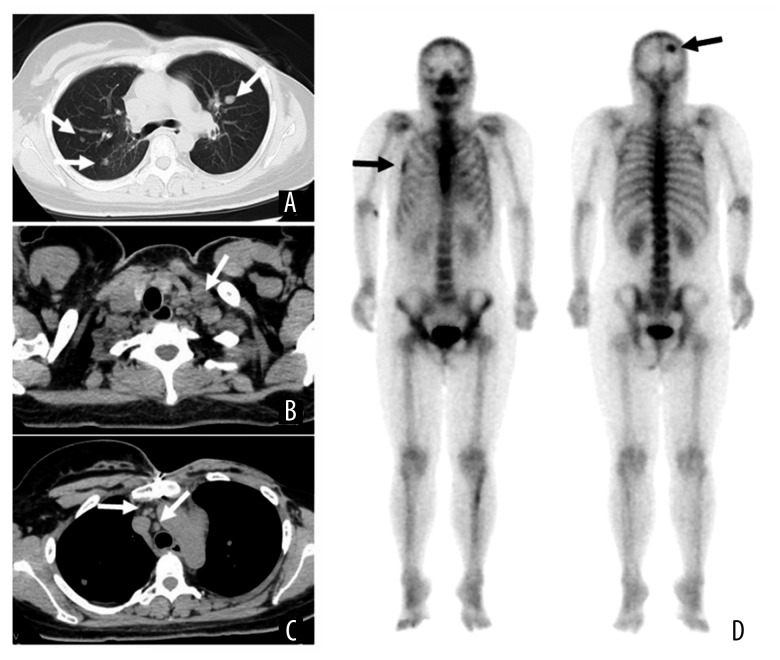
Postoperative histopathological examination revealed a 2.5×2.2-cm invasive ductal carcinoma, associated with a wide intraductal spread measuring 6.5 cm in diameter (Figure 2A–2C). The surgical margin was negative for cancer cells. Dissection of the axillary lymph nodes showed metastatic deposits in 1 of the 14 lymph nodes. Immunohistochemical analysis showed strong estrogen receptor positivity (90%) and negative progesterone receptor positivity (less than 1%; Figure 2E, 2E). The Ki-67 marker was positive in 62.5% of the tumor cells. Immunohistochemical analysis showed equivocal results for the human epidermal receptor (HER-2), and fluorescence in situ hybridization confirmed the negative results for HER-2. After surgery, the patient received adjuvant endocrine therapy with an LH-RH agonist and tamoxifen. Twenty-three months after the operation, while still under adjuvant hormonal therapy, a palpable left supraclavicular lymph node was detected. CT and bone scintigraphy revealed multiple pulmonary metastases, lymph node metastases, and multiple bone metastases, including in the skull and rib bones (Figure 3). Chemotherapy, consisting of weekly paclitaxel plus biweekly bevacizumab, was administered. The left supraclavicular metastatic lymph node rapidly reduced in size and became impalpable 2 months after the initiation of chemotherapy; however, the levels of the tumor markers (CEA and CA15-3) started to increase 9 months after the administration of chemotherapy and gradually increased.
Figure 2.
Histopathological findings. (A) Macroscopic image; (B) hematoxylin and eosin staining ×40; (C) hematoxylin and eosin staining ×40; (D) estrogen receptor staining; (E) progesterone receptor staining. (A) Macroscopically, the tumor was a well-defined, solid mass measuring 2.5×2.2 cm in size (black arrows) with wide extension in the direction of the left papillary side (white arrow). Microscopic findings revealing (B) invasive ductal carcinoma with (C) wide intraductal spreading. The tumor cells are strongly positive for (D) estrogen receptors and (E) almost negative for progesterone receptors.
Figure 3.
Image findings. (A–C) Cervicothoracic computed tomography (CT) examination; (D) bone scintigraphy; (A) CT revealing multiple lung metastases (white arrow); (B) left supraclavicular lymph node metastases multiple lymph node metastases left supraclavicular lymph node (white arrow); (C) mediastinal lymph node (white arrow); (D) bone scintigraphy showed skull and right 5th ribs (black arrow).
Testing for the BRCA mutation was thought to be necessary for determining the need for second-line chemotherapy. BRCA analysis (Myriad Genetics) detected a BRCA2 pathogenic variant (c.2097 del); hence, olaparib was orally administered 15 months after the initiation of the first-line chemotherapy. The patient was started on olaparib at a dose of 300 mg twice a day. Six weeks after the initiation of olaparib, grade 3 anemia (hemoglobin 7.1 g/dL) occurred. Olaparib was suspended for 3 weeks and resumed at a reduced dosage (250 mg twice a day).
The levels of the tumor markers (CEA and CA15-3) rapidly declined after the administration of olaparib, and both levels fell within normal ranges 13 weeks after the initiation of olaparib; however, the levels of the tumor markers started to increase again 5 months after the initiation of olaparib (Figure 4). Eribulin in exchange for olaparib was started 12 months after the initiation of olaparib, owing to the progression of her disease. Subsequently, she developed bilateral carcinomatous lymphangiomatosis and died 59 months after the operation.
Figure 4.
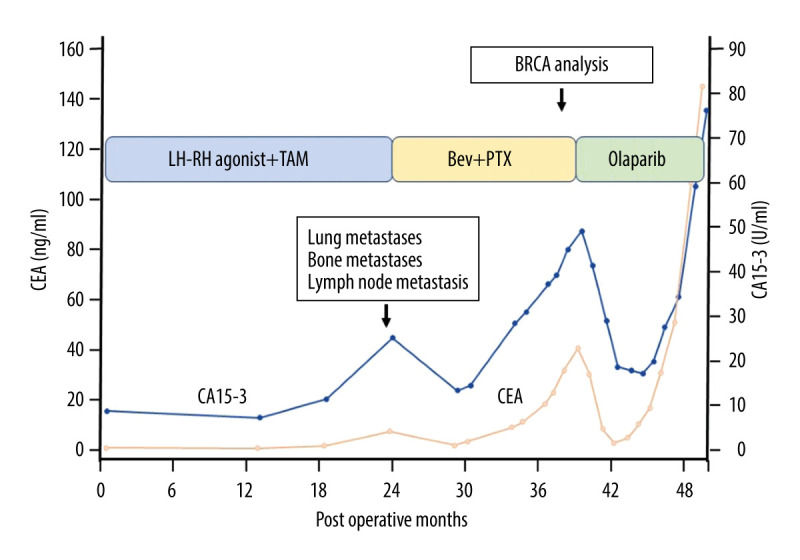
Postoperative patient’s clinical course. The process of treatment and the levels of tumor markers (CEA and CA15-3) are shown. Thirty-three months after the operation, BRCA analysis was performed under the first-line chemotherapy for multiple metastases of the breast cancer. The mutation of BRCA 2 was detected, and olaparib was administered for the second line chemotherapy. TAM – tamoxifen citrate; Bev – bevacizumab; PTX – paclitaxel.
Discussion
Down syndrome is the most common chromosomal aberration, occurring in 1 in 450 to 500 live births in Japan [1]. It is characterized by the presence of an extra copy of chromosome 21, and there are 3 forms: nondisjunction, translocation, and mosaic. Down syndrome is associated with various health issues, including intellectual disabilities, congenital heart diseases, gastrointestinal defects, celiac disease, and hypothyroidism [2,3].
However, individuals with Down syndrome have a decreased risk of solid tumors throughout life [4]. Some studies showed that the standardized incidence ratio of breast cancer in patients with Down syndrome was less than 0.5 [4–6]. Even though women with Down syndrome show a higher prevalence of risk factors for breast cancer, such as nulliparity, a lack of breast feeding, a high body mass index, and physical inactivity, the incidence of breast cancer is lower than that in the general population.
Women with Down syndrome generally have a short life compared to the general population, and they could die before attaining the susceptible age for breast cancer. The reduced life expectancy of women with Down syndrome compared with the general population could be linked to the small number of breast cancer patients; moreover, hormonal or environmental protective factors, such as reduced estrogen exposure, early menopause, or no alcohol/no tobacco use, can contribute to the lower incidence of breast cancer. However, these epidemiological, hormonal, or environmental factors alone are not sufficient to explain the low frequency of breast cancer.
The basis for the decreased incidence of solid tumors, including breast cancer, in Down syndrome remains unclear. One of the reasons for the low incidence of solid tumors in Down syndrome may be the “gene dosage effects” [7] or “increased dosage effects” [8], which means an extra copy of chromo-some 21 leads to higher levels of the gene product that protects solid tumor growth. Some protein products encoded by the genes on chromosome 21 are thought to be candidates for antitumor agents. The protein product encoded by DSCR1 (Down syndrome candidate resion-1/Down syndrome critical region-1) located on chromosome 21 inhibits angiogenesis mediated by vascular endothelial growth factor [9,10]. Another candidate protein implicated as a protective agent against the development of solid tumors in Down syndrome is endostatin, which is a product of collagen XVIII, encoded by the COL18A1 gene on chromosome 21 and a potent angio-genesis inhibitor. Serum endostatin levels are elevated in patients with Down syndrome [8]. Other genes located on chromosome 21 are thought to be tumor repressors, such as Ets2, ADAMTS1, and JAM-B [11]. Apart from the proteins related to angiogenesis inhibitor, the extracellular matrix produced by trisomy 21 fibroblasts is indicated as the other tumor-developing protection factor [12].
Unfortunately, our patient was also found to have an abnormal BRCA gene. BRCA are tumor-suppressor genes that encode proteins for the high-fidelity repair of DNA double-strand breaks by the homologous recombination repair pathway [13]. Mutation of the gene leads to defective DNA double-strand repair. Patients harboring pathogenic variants of the BRCA genes are at increased risk of developing solid tumors, especially breast and/or ovarian cancers. The cumulative risk for breast cancer by age 70 is 60% to 65% in those with BRCA1 pathogenic variants and 45% to 55% in those with BRCA2 pathogenic variants [14,15].
In our case, there were 2 opposite genetic conditions present simultaneously in the individual: the chromosome 21 trisomy, which may be a protective factor against cancer development, and the BRCA2 pathogenic variant, which can cause breast cancer with a high frequency. To the best of our knowledge, this is the first reported case of breast cancer harboring a germline BRCA2 pathogenic variant in a patient with Down syndrome.
Conclusions
Herein, we report an extremely rare case of primary breast cancer harboring a germline BRCA2 pathogenic variant in an individual with Down syndrome. Given that breast cancer is rare in patients with Down Syndrome, our case illustrates the importance for genetic testing upfront when a patient with Down Syndrome presents with breast cancer, as it may have significant impact on cancer therapy and, subsequently, the prognosis of the patient.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
Department of Surgery, Hokushin General Hospital, Nakano, Nagano, Japan.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Sasaki A, Sago H. Equipoise of recent estimated Down syndrome live births in Japan. Am J Med Genet. 2019;179A:1815–19. doi: 10.1002/ajmg.a.61298. [DOI] [PubMed] [Google Scholar]
- 2.Weijerman ME, de Winter JP. Clinical practice. The care of children with Down syndrome. Eur J Pediatr. 2010;169:1445–52. doi: 10.1007/s00431-010-1253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roizen NJ, Patterson D. Down’s syndrome. Lancet. 2003;361:1281–89. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- 4.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355:165–69. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 5.Patja K, Pukkala E, Sund R, et al. Cancer incidence of persons with Down syndrome in Finland: A population-based study. Int J Cancer. 2006;118:1769–72. doi: 10.1002/ijc.21518. [DOI] [PubMed] [Google Scholar]
- 6.Hill DA, Gridley G, Cnattingius S, et al. Mortality and cancer incidence among individuals with Down syndrome. Arch Intern Med. 2003;163:705–11. doi: 10.1001/archinte.163.6.705. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds LE, Watson AR, Baker M, et al. Tumour angiogenesis is reduced in the Tc1 mouse model of Down Syndrome. Nature. 2010;465(7299):813–17. doi: 10.1038/nature09106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorick TS, Mustacchi Z, Bando SY, et al. High serum endostatin levels in Down syndrome: Implications for improved treatment and prevention of solid tumours. Eur J Hum Genet. 2001;9:811–14. doi: 10.1038/sj.ejhg.5200721. [DOI] [PubMed] [Google Scholar]
- 9.Baek KH, Zaslavsky A, Lynch RC, et al. Down syndrome suppression of tumor growth and the role of the calcineurin inhibitor DSCR1. Nature. 2009;459(7250):1126–30. doi: 10.1038/nature08062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minami T, Horiuchi K, Miura M, et al. Vascular endothelial growth factor-and thrombin-induced termination factor, Down syndrome critical resion-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem. 2004;279:50537–54. doi: 10.1074/jbc.M406454200. [DOI] [PubMed] [Google Scholar]
- 11.Sussan TE, Yang A, Li F, Ostrowski MC, Reeves RH. Trisomy represses ApcMin-mediated tumours in mouse models of Down’s syndrome. Nature. 2008;451:73–75. doi: 10.1038/nature06446. [DOI] [PubMed] [Google Scholar]
- 12.Bénard J, Béron-Gaillard N, Satgé D. Down’s syndrome protects against breast cancer: Is a constitutional cell microenvironment the key? Int J Cancer. 2005;113:168–70. doi: 10.1002/ijc.20532. [DOI] [PubMed] [Google Scholar]
- 13.Walsh CS. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137:343–50. doi: 10.1016/j.ygyno.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Antoniou A, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–30. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mavaddat N, Peock S, Frost D, et al. EMBRACE Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105(11):812–22. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]