Summary
The genes encoding the killer immunoglobulin-like receptors (KIR) are situated within a segment of DNA that has undergone expansion and contraction over time due in large part to unequal crossing over. Consequently, individuals exhibit considerable haplotypic variation in terms of gene content. The highly polymorphic human leukocyte antigen (HLA) class I loci encode ligands for the KIR; thus, it is not surprising that KIR genes also show significant allelic polymorphism. As a result of the receptor-ligand relationship between KIR and HLA, functionally relevant KIR–HLA combinations need to be considered in the analysis of these genes as they relate to disease outcomes. This chapter will describe a genotyping method for identifying the presence/absence of the KIR genes and general approaches to data analysis in disease association studies.
Keywords: KIR genotyping, sequence-specific priming (SSP), natural killer (NK) cells, HLA class I ligands, KIR haplotypes
1. Introduction
Natural killer (NK) cells comprise about 15% of peripheral blood lymphocytes and are an important component of the innate immune system. They also play a critical role in bridging the innate and adaptive immune response to infection by the production of cytokines and chemokines that mediate activation of effector cells of the adaptive immune system (1, 2). NK cell activity is controlled by the balance of inhibitory and stimulatory signals generated upon ligand binding to a plethora of receptors located on their cell surfaces. The killer immunoglobulin-like receptor (KIR) locus comprises a family of related genes that encode a group of these activating and inhibitory receptors expressed on NK cells. Recognition of self-human leukocyte antigen (HLA) class I ligands by inhibitory KIR allows NK cells to identify and inhibit the response to normal cells (3, 4, 5). On the contrary, activation of NK cells through stimulatory receptors, including some KIR molecules, is directed toward cells with little or no expression of major histocompatibility complex (MHC) class I, consistent with the so-called “missing self” hypothesis, which was originally proposed by Ljunggren and Karre (6). A contemporary version of the “missing self” hypothesis proposes that, rather than completely terminating NK cell effector function, inhibitory receptors might instead dampen it depending on the amount of MHC class I expression. Thus, when multiple activating receptors are engaged, the signal might be sufficiently potent to overcome inhibition. In other words, NK cell cytotoxicity is essentially controlled by a balance between activating and inhibitory signals mediated by corresponding NK cell receptors (7).
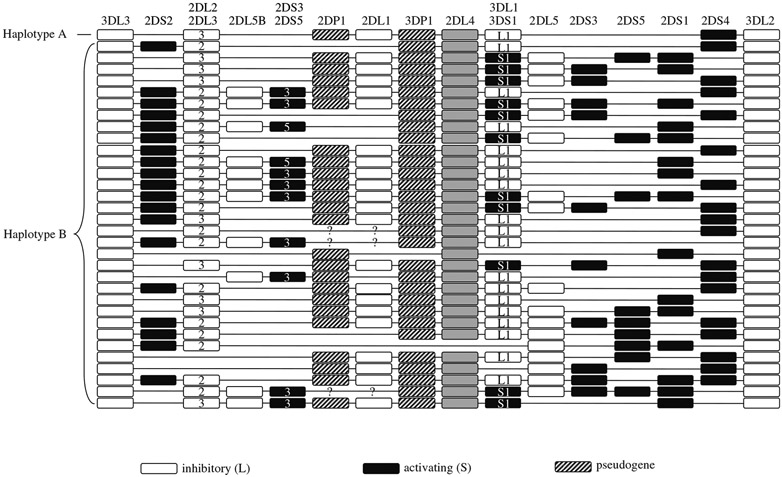
The KIR locus maps to chromosome 19q13.4 within the leukocyte receptor complex (LRC) and comprises a family of polymorphic and highly homologous genes that are tandemly arrayed over about 150 kb. On the basis of gene content, the haplotypes have been divided into two types termed A and B (8) (see Fig. 1). Haplotype A is invariant in terms of gene content, whereas haplotype B is quite variable, due in large part to the presence or absence of activating KIR, because most of the inhibitory KIR are present on nearly all haplotypes (8).
Fig. 1.
KIR haplotypes identified by segregation analysis. Haplotypes are derived from refs. 26-30 and unpublished observations (M.C.). Haplotypes A and B are labeled as such. White boxes represent inhibitory receptors, black boxes represent activating receptors, and hatched boxes represent pseudogenes. KIR2DL4 is shown as grey boxes. This receptor has features of both inhibitory and activating receptors, although functional data suggest that it is primarily activating (31, 32).
Given the role of KIR in the immune response and their extensive genomic diversity, it is conceivable that KIR gene variation affects resistance and susceptibility to the pathogenesis of a number of diseases, and as such, they have become an attractive target for disease association studies. This chapter will describe a method for KIR genotyping, which detects the presence or absence of each gene in a given individual, thus providing a KIR profile. The method involves gene-specific polymerase chain reaction (PCR) amplification using sequence-specific primers (PCR-SSP). A general approach to the analysis of KIR data in disease association studies is also provided.
2. Materials
2.1. PCR
High-quality DNA isolated from tissue or cells using standard protocols, at a concentration of 50–100 ng/μl (see Notes 1-2).
Oligonucleotide primer mixes containing forward and reverse primers (5 μM) specific for each KIR gene and internal control (see Table 1 for primer sequences) (see Note 3).
50 mM MgCl2.
25 mM dNTP mix (mix equal quantities of 100 mM each dATP, dTTP, dGTP, and dCTP).
10× PCR buffer: 200 mM Tris–HCl, pH 8.4, 500 mM KCl.
Taq DNA polymerase (see Note 4).
384-well PCR plates. Each sample will use 30 wells, thus allowing typing of 12 samples per plate.
Multichannel pipettors ( 8 and 16 channel).
Table 1.
KIR Genotyping Primers
| Gene | Primers | Sequences | Exon | Position* | Size (bp) | Comments |
|---|---|---|---|---|---|---|
| 2DL1 | F1 | GTTGGTCAGATGTCATGTTTGAA | 4 | 437–459 | 146 | |
| R1 | GGTCCCTGCCAGGTCTTGCG | 4 | 563–582 | |||
| F2 | TGGACCAAGAGTCTGCAGGA | 8 | 1125–1144 | ~330 | Misses *005, Sees 2DL2*004 | |
| R2 | TGTTGTCTCCCTAGAAGACG | 3’UTR | 1369–1388 | |||
| 2DL2 | F1 | CTGGCCCACCCAGGTCG | 4 | 385–401 | 173 | Misses *004 |
| R1 | GGACCGATGGAGAAGTTGGCT | 4 | 537–557 | |||
| F2 | GAGGGGGAGGCCCATGAAT | 5 | 778–796 | 151 | ||
| R2 | TCGAGTTTGACCACTCGTAT | 5 | 909–928 | |||
| 2DL3 | F1 | CTTCATCGCTGGTGCTG | 7 | 1084–1100 | ~550 | Misses *007 |
| R1 | AGGCTCTTGGTCCATTACAA | 8 | 1119–1138 | |||
| F2 | TCCTTCATCGCTGGTGCTG | 7 | 1082–1100 | ~800 | ||
| R2 | GGCAGGAGACAACTTTGGATCA | 9 | 1316–1337 | |||
| 2DL4 | F1 | CAGGACAAGCCCTTCTGC | 3 | 82–99 | 254 | |
| R1 | CTGGGTGCCGACCACT | 3 | 320–335 | |||
| F2 | ACCTTCGCTTACAGCCCG | 5 | 675–692 | 288 | ||
| R2 | CCTCACCTGTGACAGAAACAG | 5 | 941-intron | |||
| 2DS2 | F1 | TTCTGCACAGAGAGGGGAAGTA | 4 | 467–488 | 175 | |
| R1 | GGGTCACTGGGAGCTGACAA | 4 | 622–641 | |||
| F2 | CGGGCCCCACGGTTT | 5 | 695–709 | 240 | ||
| R2 | GGTCACTCGAGTTTGACCACTCA | 5 | 912–934 | |||
| 2DS3 | F1 | TGGCCCACCCAGGTCG | 4 | 386–401 | 242 | |
| R1 | TGAAAACTGATAGGGGGAGTGAGG | 4 | 604–627 | |||
| F2 | CTATGACATGTACCATCTATCCAC | 5 | 753–776 | 190 | ||
| R2 | AAGCAGTGGGTCACTTGAC | 5 | 924–942 | |||
| 2DS4 | F1 | CTGGCCCTCCCAGGTCA | 4 | 385–401 | 204 | |
| R1 | TCTGTAGGTTCCTGCAAGGACAG | 4 | 566–588 | |||
| F2 | GTTCAGGCAGGAGAGAAT | 5 | 706–723 | 197/219 | ||
| R2 | GTTTGACCACTCGTAGGGAGC | 5 | 904–924 | |||
| 2DS5 | F1 | TGATGGGGTCTCCAAGGG | 4 | 522–539 | 126 | Misses *003 |
| R1 | TCCAGAGGGTCACTGGGC | 4 | 630–647 | |||
| F2 | ACAGAGAGGGGACGTTTAACC | 4 | 473–493 | 178 | ||
| R2 | ATGTCCAGAGGGTCACTGGG | 4 | 631–650 | |||
| 3DL1 | F1 | CGCTGTGGTGCCTCGA | 3 | 114–129 | 191 | Misses *009 |
| R1 | GGTGTGAACCCCGACATG | 3 | 287–304 | |||
| F2 | CCCTGGTGAAATCAGGAGAGAG | 4 | 401–422 | 186 | ||
| R2 | TGTAGGTCCCTGCAAGGGCAA | 4 | 566–586 | |||
| 3DL2 | F1 | CAAACCCTTCCTGTCTGCCC | 3 | 121–140 | 211 | Misses *013, *014 |
| R1 | GTGCCGACCACCCAGTGA | 3 | 314–331 | |||
| F2 | CCCATGAACGTAGGCTCCG | 5 | 788–806 | 130 | ||
| R2 | CACACGCAGGGCAGGG | 5 | 902–917 | |||
| 3DS1 | F1 | AGCCTGCAGGGAACAGAAG | 8 | 1134–1154 | ~300 | |
| R1 | GCCTGACTGTGGTGCTCG | 3’UTR | 1340–1357 | |||
| F2 | CCTGGTGAAATCAGGAGAGAG | 4 | 402–422 | 180 | ||
| R2 | GTCCCTGCAAGGGCAC | 4 | 566–581 | |||
| 3DL3 | F1 | GTCAGGACAAGCCCTTCCTC | 3 | 441–461 | 232 | |
| R1 | GAGTGTGGGTGTGAACTGCA | 3 | 530–552 | |||
| F2 | TTCTGCACAGAGAGGGGATCA | 4 | 737–753 | 165 | ||
| R2 | GAGCCGACAACTCATAGGGTA | 4 | 910–926 | |||
| 2DL5 | F1 | GCGCTGTGGTGCCTCG | 3 | 113–128 | 214 | |
| R1 | GACCACTCAATGGGGGAGC | 3 | 308–326 | |||
| F2 | TGCAGCTCCAGGAGCTCA | 5 | 736–753 | 191 | ||
| R2 | GGGTCTGACCACTCATAGGGT | 5 | 906–926 | |||
| 2DP1 | F1 | GTCTGCCTGGCCCAGCT | 3 | 99–115 | 205 | |
| R1 | GTGTGAACCCCGACATCTGTAC | 3 | 282–303 | |||
| F2 | CCATCGGTCCCATGATGG | 4 | 548–565 | 89 | ||
| R2 | CACTGGGAGCTGACAACTGATG | 4 | 615–637 | |||
| 2DS1 | F1 | CTTCTCCATCAGTCGCATGAA | 4 | 543–563 | 102 | |
| F2 | CTTCTCCATCAGTCGCATGAG | 4 | 543–563 | |||
| R1 | AGAGGGTCACTGGGAGCTGAC | 4 | 624–644 | |||
| DRB1 (control)(21) | F1 | TGCCAAGTGGAGCACCCAA | Intron 3 | 796 | ||
| R1 | GCATCTTGCTCTGTGCAGAT | Intron 3 |
Numbering based on KIR alignment from http://www.ebi.ac.uk/ipd/kir/
2.2. Gel Electrophoresis
Orange G loading buffer: 0.5% Orange G, 20% Ficoll, 100 mM ethylenediaminetetraacetic acid (EDTA).
10× TAE electrophoresis buffer: 400 mM Tris, 200 mM acetic acid, 10 mM EDTA.
100 bp DNA ladder.
Electrophoresis grade agarose.
Horizontal electrophoresis chamber.
Gel-casting tray and 50-well combs with teeth appropriately separated for use with multichannel pipettors. Note that the Owl Centipede™ horizontal model D3-14 wide gel system (Woburn, MA) will accommodate four combs per gel, allowing electrophoresis of 200 wells per gel.
Ethidium bromide solution (10 mg/ml).
High-voltage power supply.
Photographic means of gel documentation [ultraviolet (UV) light source, digital or conventional camera].
3. Methods
3.1. PCR
Using an 8-multichannel pipettor, dispense 1 μl of each primer mix into separate wells in 384-well plates. Each sample will require two vertical rows of wells, thus allowing genotyping of 12 samples per plate.
Prepare PCR cocktails for each sample in a total volume of 132 μl (enough for 33 reactions) as follows: 200 ng DNA, 16.5 μl 10× PCR buffer (final concentration 1×), 4.95 μl MgCl2 (final concentration 1.5 mM), 1.32 μl dNTP (final concentration 200 mM), 0.825 μl Taq polymerase.
Add 4 μl PCR cocktail to each primer mix (total PCR volume = 5 μl).
Cover plates with acetate film and centrifuge briefly.
Amplify in a programmable thermal cycler with a heated lid using the following parameters: 3 min at 94°C, 5 cycles of 15 s at 94°C, 15 s at 65°C, 30 s at 72°C; 21 cycles of 15 s at 94°C, 15 s at 60°C, 30 s at 72°C; 4 cycles of 15 s at 94°C, 1 min at 55°C, 2 min at 72°C, with a final 7-min extension step at 72°C (see Notes 5 and 6).
3.2. Gel Electrophoresis
Prepare 150 ml of 3% agarose in 1× TAE per gel and heat until the agarose has completely gone into solution.
Cool gel mixture to 65°C, add ~5 μl ethidium bromide, and gently mix to avoid bubble formation.
Pour gel mixture into the gel-casting tray, insert four 50-well combs, and allow the gel to solidify for 20 min.
Fill the electrophoresis chamber with 800 ml of 1× TAE buffer and submerge the gel into the chamber. Remove the combs.
Add 5 μl Orange G loading buffer to each PCR and centrifuge briefly.
Load 2 μl of the 100 bp DNA ladder to the first and last well of each row of wells.
Using a 16-channel pipettor, load 5 μl of each PCR product into the gel. Save the remaining PCR product from the wells containing primers for 2DS4 if subtyping of this gene is planned (see Subheading 3.3).
Electrophorese for 30 min at 100 V or until the Orange G has migrated 3 cm.
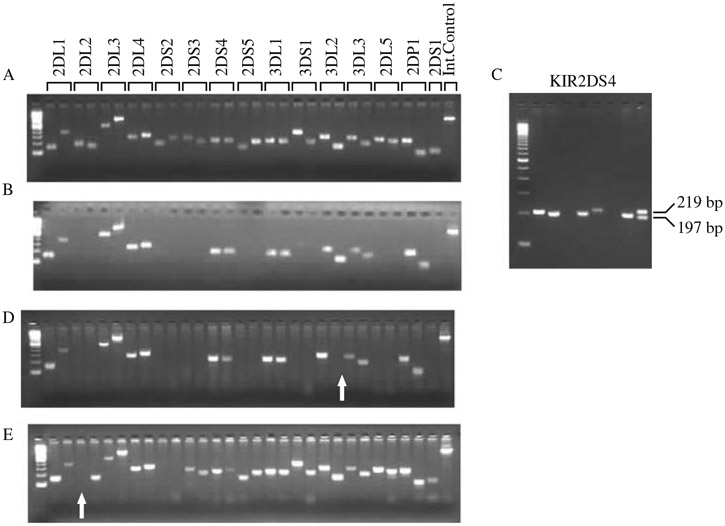
Visualize the gel using a UV light source and photograph the gel for a permanent record (see Fig. 2A and B).
Fig. 2.
KIR genotyping results by PCR-SSP. (A) Gel electrophoresis showing the presence of all genes tested. (B) Gel electrophoresis results from an individual homozygous for haplotype A. (C) Gel electrophoresis of KIR2DS4 showing the 197 and 219 bp alleles. (D) A gel showing the absence of amplicon (arrow) for one of the two primer pairs for KIR3DL2. The PCR for KIR3DL2 was repeated, indicating the presence of amplicon for both pairs of primers (i.e., one pair of primers did not amplify in the initial PCR due to technical error). (E) This gel shows the absence of amplicon for one of the primer pairs for KIR2DL2 (arrow), which was confirmed after repeating the PCR. The sample was sequenced for KIR2DL2 and was found to be allele *004, which is not amplified by this primer pair (see Table 1). The order of the genes for B, D, and E is as shown in A.
3.3. KIR2DS4 Analysis
A variant of the KIR2DS4 gene that has a 22-bp deletion in exon 5 can be detected by the following protocol. The primer pair in exon 5 amplifies a 219-bp product if the full-length gene is present and a 197-bp product if the deletion variant is present (see Fig. 2C and Note 7).
Prepare the gel as described in Subheading 3.2., but use a single 50-well comb per gel.
Load 2 μl of the 100 bp DNA ladder to the first and last well in the row.
Load the remainder of the KIR2DS4 PCR products (~5 μl) into the gel.
Electrophorese for 1 h at 100 V or until the Orange G has migrated ~7 cm. It is necessary to run the gel for a sufficient period of time to allow separation of the 197-bp and 219-bp products.
Visualize the gel using a UV light source and photograph the gel for a permanent record.
3.4. Interpretation of Results
Interpretation of the PCR-SSP results is relatively straightforward. Positive gene-specific amplifications are identified by PCR products of the correct sizes, whereas absence of a product implies absence of the gene specific for the corresponding primer mix (see Fig. 2 and Notes 8-10). There are two specific pairs of primers for each gene (except for KIR2DS1, see Table 1), and both primers should have a visible product if the gene is present or no product if the gene is absent (see Notes 11 and 12). Samples exhibiting discrepant results for primer pairs that recognize the same gene are repeated, and if discrepant results are confirmed, the gene is sequenced (see Fig. 2D and E). The internal control amplicon should be visible in all samples. The results are recorded in an appropriate spreadsheet, such as Microsoft Excel, indicating which genes are present and which genes are absent for each sample.
3.5. Data Analysis
Some general guidelines regarding analysis of KIR data in disease association studies are given in this section (see ref. 9 for a recent review). There are no specific rules; rather, the type of analysis performed will depend on the questions being asked. HLA class I ligands for several of the inhibitory KIR molecules have been identified; so, an account of KIR genotypes in combination with HLA class I data is often necessary. One problem with studying polymorphic loci such as KIR and HLA is the inherent lack of statistical power, particularly when it is necessary to consider functionally relevant combinations of variants at the two unlinked gene complexes. In some cases, it is possible to take a specific hypothesis-driven approach in which only specific KIR genes are considered, as exemplified by our previous study of KIR3DS1 effects on HIV disease progression (10). This approach is reasonable when specific KIR/HLA associations can be addressed based on previously defined functional or genetic data, but it is often necessary to test all genes for any potential associations. Thus, it is recommended that testing for KIR/HLA effects be performed in study groups that are large enough to provide sufficient statistical power. An approach to disease association analysis is given below.
3.5.1. Disease Association Analysis
An obvious starting point is to test for disease associations with presence/absence of individual KIR genes. For example, this approach was used in the analysis of KIR in scleroderma (11), where the combination of the presence of 2DS2 and the absence of 2DL2 was associated with susceptibility.
The effect of combinations of KIR genes with HLA alleles based on known receptor–ligand relationships should be tested. KIR/HLA associations have been described for a number of diseases such as psoriasis (12), type 1 diabetes (13), HIV-1 disease progression (10), and rheumatoid vasculitis (14).
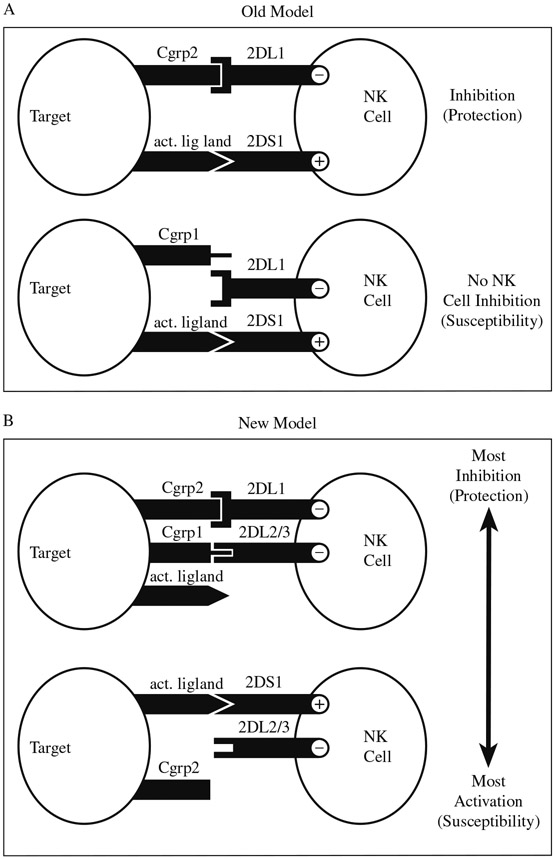
Grouping of KIR genes based on functional characteristics, while also accounting for HLA, may provide additional information regarding the nature of the disease association. This process is exemplified in our study of psoriatic arthritis (PsA) (15). This study also illustrates the challenges involved in the analysis of multiple linked and unlinked loci. Initially, we concluded that individuals with certain activating KIR were susceptible to developing PsA when HLA ligands for the corresponding homologous inhibitory receptors were missing. With our increased knowledge of KIR function in the regulation of NK cell activity, however, we were able to propose a more logical model to explain our results (see Fig. 3). In the old model, the assumption was made that only the corresponding inhibitory receptor could counteract the activity of the homologous activating receptor (15). The new model on the contrary proposes that genotypes conferring the most inhibition are protective against PsA, whereas those conferring the most activation are susceptible (16) (see Note 13 and Fig. 3).
KIR profile or haplotypic analyses might also be appropriate in some cases. One example is the analysis of KIR profiles with increasing numbers of activating KIR. We recently showed that there was a trend toward increasing susceptibility to nasopharyngeal carcinoma with ≥5 activating KIR (17). Analysis of the effect of haplotypes A and B on disease outcomes might also be useful. Hiby et al. (18) reported that maternal homozygosity for haplotype A (which lacks most or all activating KIR) combined with the presence of HLA-C group 2 in the fetus was associated with increased risk of pre-eclampsia, a disorder attributed to poor placental perfusion. The authors suggested that the combination of fetal HLA-C group 2 and maternal Haplotype A results in strong NK cell inhibition, which impairs the remodeling of maternal blood vessels, a process which is in part due to NK cell activation.
Allelic analysis of specific genes can also be performed if allelic subtyping is available. This is particularly useful for loci that are highly polymorphic, such as the KIR3DL1 and KIR3DL2 loci. An allele of KIR3DL2 was recently shown to be associated with an increased interaction of NK cells with red blood cells infected with the malaria parasite in vitro, and this interaction resulted in induction of interferon-γ synthesis (19).
Fig. 3.
Susceptibility to psoriatic arthritis. (A) Old model. NK cell activation (by KIR2DS1 in this case) is quenched when HLA ligand (HLA-C group 2) for the corresponding homologous inhibitory receptor (KIR2DL1) is present (protection), but not when ligand is absent (susceptibility). (B) New model. There is a trend in susceptibility to PsA such that genotypes conferring the most inhibition are protective, whereas those conferring the most activation are susceptible (adapted from ref. 16).
4. Notes
Good quality DNA is essential for reliable PCR-SSP results. Heparinized blood should be avoided because heparin is a PCR inhibitor (20).
The protocol requires approximately 5 ng of DNA per specific primer reaction, hence, the requirement for a total of 150–200 ng DNA. If this quantity of DNA is not available, it is possible (although not desirable) to use less DNA. In such a case, the total number of cycles should be increased to 35 by increasing the second-cycle sequence from 21 to 25 cycles. One drawback to this protocol alteration is that increasing the number of cycles is also likely to increase the likelihood of false-positive reactions.
All primer mixes should be tested with positive and negative controls when new working dilution aliquots are made. In addition, careful attention should be given to the concentration of primers to ensure maximum amplification efficiency.
The use of Platinum® Taq DNA polymerase (Invitrogen, Carlsbad, CA) is recommended. This enzyme is complexed with a proprietary antibody that inhibits polymerase activity at ambient temperatures. Polymerase activity is regained after the initial denaturation step, thus providing an automatic “hot start” for the PCR and resulting in improved PCR efficiency and specificity.
A modified “stepdown” program is utilized to increase the specificity of the PCR (21). It might be necessary to alter the PCR conditions slightly depending on the PCR machine being used. A fast ramping thermocycler with a heated cover is recommended for best results.
PCR machines should be checked regularly to ensure that all wells are amplifying uniformly. It is also important to ensure that the PCR plates fit snugly into the machine.
KIR2DS4 is the only short-tailed receptor gene present on haplotype A, which is also the most common KIR haplotype (in terms of gene content) across most populations. The deletion allele is also quite common with a frequency of ~80% in Caucasians. Detection of the KIR2DS4 deletion allele might be useful when performing data analysis, because individuals who are homozygous for haplotype A and the deletion allele have no expressed activating KIR, apart from KIR2DL4, a gene that may have tissue-specific function (22). If activating KIR genes are beneficial against certain types of diseases, then individuals homozygous for the null KIR2DS4 allele on an AA genotype background may be at increased risk for the development of those diseases.
To ensure the highest degree of accuracy for PCR-SSP typing, which in many cases involves using locus-specific primers that recognize a single base difference relative to the other loci, it is important to routinely check the KIR database (http://www.ebi.ac.uk/ipd/kir/) for new alleles. This helps to ensure that the primers being used for a gene will amplify all known alleles of that gene and will not amplify alleles of another gene. As new alleles are discovered, it may be necessary to repeat genotyping performed before the knowledge of the new alleles.
The KIR genes are highly (80–90%) similar and appear to have arisen by gene duplication from a common ancestor. Furthermore, the gene content and sequence of some KIR alleles strongly suggest that unequal crossing over in the region may account for a substantial amount of the diversity at this locus (23, 24, 25). Therefore, the presence of a chimeric gene for example could result in an unusual PCR profile.
As a result of linkage disequilibrium, some genes are found together at much higher frequencies than expected, and this information can be used to validate the results obtained. Figure 1 shows a list of haplotypes based on segregation analysis, and it is clear that there are certain combinations of genes that are almost always found together across different haplotypes, for example, KIR2DL2 and KIR2DS2, KIR2DP1 and KIR2DL1, and KIR3DL1 and KIR2DS4.
As previously noted above, the KIR genes are highly similar, and this poses a challenge in designing primers that will amplify all alleles of a single locus specifically. As shown in Table 1, some primers miss one or two alleles of a gene and others cross-react with a non-target gene.
Most of the KIR gene sequences available have been obtained largely from Caucasians, and it is likely that as more populations are studied, the number of known alleles for each gene will increase, some of which may be population-specific. Consequently, it is also likely that some of the primers currently being used for genotyping might not be appropriate for all populations.
It is essential to continually consider appropriate models when analyzing data pertaining to complex genetic loci such as KIR in human diseases. As our knowledge and understanding of KIR biology advances, modification of models proposed previously may be necessary.
Acknowledgments
The authors thank Arman Bashirova for helpful comments. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Cooper MA, Fehniger TA, and Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22, 633–40. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, and Biron CA (2002) Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol 169, 4279–87. [DOI] [PubMed] [Google Scholar]
- 3.Storkus WJ, Alexander J, Payne JA, Dawson JR, and Cresswell P (1989) Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci USA 86, 2361–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karre K, Ljunggren HG, Piontek G, and Kiessling R (1986) Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–8. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu Y, and DeMars R (1989) Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur J Immunol 19, 447–51. [DOI] [PubMed] [Google Scholar]
- 6.Ljunggren HG, and Karre K (1990) In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 11, 237–44. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23, 225–74. [DOI] [PubMed] [Google Scholar]
- 8.Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, Tyan D, Lanier LL, and Parham P (1997) Human diversity in killer cell inhibitory receptor genes. Immunity 7, 753–63. [DOI] [PubMed] [Google Scholar]
- 9.Carrington M, and Martin MP (2006) The impact of variation at the KIR gene cluster on human disease. Curr Top Microbiol Immunol 298, 225–57. [DOI] [PubMed] [Google Scholar]
- 10.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O’Brien SJ, and Carrington M (2002) Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31, 429–34. [DOI] [PubMed] [Google Scholar]
- 11.Momot T, Koch S, Hunzelmann N, Krieg T, Ulbricht K, Schmidt RE, and Witte T (2004) Association of killer cell immunoglobulin-like receptors with scleroderma. Arthritis Rheum 50, 1561–5. [DOI] [PubMed] [Google Scholar]
- 12.Luszczek W, Manczak M, Cislo M, Nockowski P, Wisniewski A, Jasek M, and Kusnierczyk P (2004) Gene for the activating natural killer cell receptor, KIR2DS1, is associated with susceptibility to psoriasis vulgaris. Hum Immunol 65, 758–66. [DOI] [PubMed] [Google Scholar]
- 13.van der Slik AR, Koeleman BP, Verduijn W, Bruining GJ, Roep BO, and Giphart MJ (2003) KIR in type 1 diabetes: disparate distribution of activating and inhibitory natural killer cell receptors in patients versus HLA-matched control subjects. Diabetes 52, 2639–42. [DOI] [PubMed] [Google Scholar]
- 14.Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, Weyand CM, and Goronzy JJ (2001) Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med 193, 1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin MP, Nelson G, Lee JH, Pellett F, Gao X, Wade J, Wilson MJ, Trowsdale J, Gladman D, and Carrington M (2002) Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol 169, 2818–22. [DOI] [PubMed] [Google Scholar]
- 16.Nelson GW, Martin MP, Gladman D, Wade J, Trowsdale J, and Carrington M (2004) Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig-like receptor combinations in psoriatic arthritis. J Immunol 173, 4273–6. [DOI] [PubMed] [Google Scholar]
- 17.Butsch Kovacic M, Martin M, Gao X, Fuksenko T, Chen CJ, Cheng YJ, Chen JY, Apple R, Hildesheim A, and Carrington M (2005) Variation of the killer cell immunoglobulin-like receptors and HLA-C genes in nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 14, 2673–7. [DOI] [PubMed] [Google Scholar]
- 18.Hiby SE, Walker JJ, O’Shaughnessy K M, Redman CW, Carrington M, Trowsdale J, and Moffett A (2004) Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med 200, 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artavanis-Tsakonas K, Eleme K, McQueen KL, Cheng NW, Parham P, Davis DM, and Riley EM (2003) Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol 171, 5396–405. [DOI] [PubMed] [Google Scholar]
- 20.Satsangi J, Jewell DP, Welsh K, Bunce M, and Bell JI (1994) Effect of heparin on polymerase chain reaction. Lancet 343, 1509–10. [DOI] [PubMed] [Google Scholar]
- 21.Bunce M, O’Neill CM, Barnardo MC, Krausa P, Browning MJ, Morris PJ, and Welsh KI (1995) Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens 46, 355–67. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, and Long EO (2006) Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol 4, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Lozano N, Estefania E, Williams F, Halfpenny I, Middleton D, Solis R, and Vilches C (2005) The silent KIR3DP1 gene (CD158c) is transcribed and might encode a secreted receptor in a minority of humans, in whom the KIR3DP1, KIR2DL4 and KIR3DL1/KIR3DS1 genes are duplicated. Eur J Immunol 35, 16–24. [DOI] [PubMed] [Google Scholar]
- 24.Martin MP, Bashirova A, Traherne J, Trowsdale J, and Carrington M (2003) Cutting edge: expansion of the KIR locus by unequal crossing over. J Immunol 171, 2192–5. [DOI] [PubMed] [Google Scholar]
- 25.Shilling HG, Lienert-Weidenbach K, Valiante NM, Uhrberg M, and Parham P (1998) Evidence for recombination as a mechanism for KIR diversification. Immunogenetics 48, 413–6. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Lozano N, Gardiner CM, Parham P, and Vilches C (2002) Some human KIR haplotypes contain two KIR2DL5 genes: KIR2DL5A and KIR2DL5B. Immunogenetics 54, 314–9. [DOI] [PubMed] [Google Scholar]
- 27.Hsu KC, Chida S, Dupont B, and Geraghty DE (2002) The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunol Rev 190, 40–52. [DOI] [PubMed] [Google Scholar]
- 28.Hsu KC, Liu XR, Selvakumar A, Mickelson E, O’Reilly RJ, and Dupont B (2002) Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol 169, 5118–29. [DOI] [PubMed] [Google Scholar]
- 29.Shilling HG, Guethlein LA, Cheng NW, Gardiner CM, Rodriguez R, Tyan D, and Parham P (2002) Allelic polymorphism synergizes with variable gene content to individualize human KIR genotype. J Immunol 168, 2307–15. [DOI] [PubMed] [Google Scholar]
- 30.Uhrberg M, Parham P, and Wernet P (2002) Definition of gene content for nine common group B haplotypes of the Caucasoid population: KIR haplotypes contain between seven and eleven KIR genes. Immunogenetics 54, 221–9. [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi-Maki A, Yusa S, Catina TL, and Campbell KS (2003) KIR2DL4 is an IL-2-regulated NK cell receptor that exhibits limited expression in humans but triggers strong IFN-gamma production. J Immunol 171, 3415–25. [DOI] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Fu J, and Long EO (2001) Cutting edge: induction of IFN-gamma production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol 167, 1877–81. [DOI] [PubMed] [Google Scholar]