Abstract
Background/aim
The clinical presentation of pediatric coronavirus disease 2019 (COVID-19) is associated with a milder disease course than the adult COVID-19 syndrome. The disease course of COVID-19 has three clinicobiological phases: initiation, propagation, and complication. This study aimed to assess the pathobiological alterations affecting the distinct clinical courses of COVID-19 in pediatric age groups versus the adult population. We hypothesized that critical biogenomic marker expressions drive the mild clinical presentations of pediatric COVID-19.
Materials and methods
Blood samples were obtained from 72 patients with COVID-19 hospitalized at Ankara City Hospital between March and July 2021. Peripheral blood mononuclear cells were isolated using Ficoll-Paque and density-gradient sedimentation. The groups were compared using a t-test and limma analyses. Mean standardized gene expression levels were used to hierarchically cluster genes employing Euclidean Gene Cluster 3.0. The expression levels of identified genes were determined using reverse transcription-polymerase chain reaction.
Results
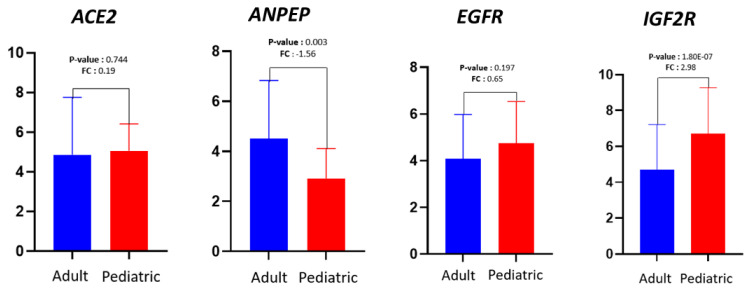
This study found that ANPEP gene expression was significantly downregulated in the pediatric group (p < 0.05, FC: 1.57) and IGF2R gene expression was significantly upregulated in the adult group (p < 0.05, FC: 2.98). The study results indicated that the expression of critical biogenomic markers, such as the first-phase (ACE2 and ANPEP) and second-phase (EGFR and IGF2R) receptor genes, was crucial in the genesis of mild clinical presentations of pediatric COVID-19. ANPEP gene expression was lower in pediatric COVID-19.
Conclusion
The interrelationship between the ANPEP and ACE2 genes may prevent the progression of COVID-19 from initiation to the propagating phase in pediatric patients. High IGF2R gene expression could potentially contribute to a protective effect and may be a contributing factor for the mild clinical course observed in pediatric patients.
Keywords: Pediatric COVID-19, adult COVID-19, ACE2, ANPEP, EGFR, IGF2R
1. Introduction
The clinicobiological course of coronavirus disease 2019 (COVID-19) due to the SARS-CoV-2 virus is significantly different in the pediatric population compared to adults [1]. Clinical presentations in adults can be heterogeneous and progressive, ranging from asymptomatic infection or minor upper respiratory tract illness to severe viral pneumonia with respiratory failure [2]. Regarding the severity of the illness, COVID-19 infections can affect multiple organs in adults, such as the respiratory, immunological, hematopoietic, cardiovascular, gastrointestinal, neurological, or musculoskeletal systems [3]. Clinical observations to date point to milder infections and better prognosis in the pediatric population compared to adults [1]. The immune system develops as we grow [4], and it is postulated that children with COVID-19 may have increased levels of inflammatory markers [5]. During the initiation and propagation stages of infection, several renin-angiotensin system (RAS) family genes (e.g., ACE2 and ANPEP) are upregulated, whereas others (e.g., epidermal growth factor receptor - EGFR and insulin-like growth factor 2 receptor - IGF2R) are downregulated [6,7]. Clinically, the first phase of COVID-19 is the asymptomatic/presymptomatic phase. The ACE2 and ANPEP genes play important roles in this phase. The second clinical phase of COVID-19 is the respiratory phase with mild/moderate/severe symptoms. These are related to the EGFR and IGF2R genes. The final clinical phase entails multisystemic clinical syndrome with impaired/disproportionate and/or defective immunity, which is associated with immune system genes [6,7]. We reported in the past that lung epithelial cells treated with SARS-CoV-2 revealed high expressions of the ACE2 and aminopeptidase N (ANPEP) genes in the initial phase at 12–24 h. ACE2 has been found to be an important receptor for the SARS-CoV-2 virus in the initial phase. In addition to ACE2, ANPEP has also been shown to act as a co-receptor in the first phase and has been suggested to be very important for the virus to develop the infection [6,7]. It seems that ACE and ANPEP not only affect the attachment of the virus and its entry into the cell but also save time for the virus to perform replication [8]. In addition to changes in the expression of the ACE2 and ANPEP genes, which function as key receptors and co-receptors in the early phase of COVID-19 infection, a decrease in RNA levels is found associated with the EGFR and IGF2R genes in the propagating phase. This phase is strongly tied to the initial phase and the complicating phase, both of which play key roles in determining the infection’s severity. Toll-like receptors together with EGFR are essential for innate immune response generation [9,10]. Therefore, EGFR downregulation may inhibit an early immunological response to an infection. In addition to the EGFR transcript, IGF2R is also a low-expressed gene during the propagation phase of the viral infection. IGF2R encodes insulin-like growth factor 2 and the mannose 6-phosphate receptor. Low IGF2R expression is likely a crucial element in the endocytosis-mediated entrance of the virus into the cell [11].
The aim of this study is to assess pathobiological alterations affecting the distinct clinical courses of COVID-19 syndrome in pediatric populations versus adults. Therefore, the hypothesis of our study is that critical biogenomic marker expressions such as the expressions of the first-phase (ACE2 and ANPEP) and second-phase (EGFR and IGF2R) receptor genes cause the mild clinical presentations of pediatric COVID-19 infections. Elucidation of the physiopathological basis of mild pediatric COVID-19 infection will be helpful for better clinical management of the syndrome in this age group.
2. Materials and methods
2.1. Blood samples
This research included 72 patients with COVID-19 pneumonia hospitalized at Ankara City Hospital in Ankara, Türkiye, between March and July 2021. The inclusion criteria were as follows: 1) diagnosed with COVID-19 infection; 2) hospitalized at Ankara City Hospital. Patients from whom blood samples could not be taken were excluded from the study. To determine if the expression of the first- and second-phase receptor genes was age-dependent, patients were categorized into pediatric (n = 23) and adult (n = 49) groups. Based on the US Department of Health and Food and Drug Administration’s estimated age ranges for stages of life [12], nine members of our cohort were children (range: 4–11 years, median: 7), 14 were adolescents (range: 14–19 years, median: 16), and 49 were adults (range: 27–91 years, median: 63.5). Information on the codes, ages, groups, and genders of the patients included in the study are given in Supplementary Data File 1. The local ethics committee approved the research protocol with approval number E1-20-1396. Each patient or the patient’s legal guardian signed a written permission form, and tests were conducted in accordance with the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report.
2.2. Blood collection and PBMC isolation
Up to 10 mL of blood was collected from patients in VACUETTE tubes containing ethylenediamine-tetra acetic acid (EDTA) or in acid citrate dextrose (ACD) tubes. Blood samples were processed immediately. Isolation of peripheral blood mononuclear cells (PBMCs) was performed by density-gradient sedimentation using Ficoll-Paque according to the standard procedures [13].
2.3. Expression profiling of genes by q-RT-PCR
In order to determine the expression of the first-phase (ACE2 and ANPEP) and second-phase (EGFR and IGF2R) receptor genes, RNA extraction, DNA fragmentation, cDNA production, and qPCR experiments were respectively performed. Gene expression was profiled quantitatively using a 7500 Real-Time PCR System and SYBR® Green PCR Master Mix for quantitative reverse transcription-polymerase chain reaction (q-RT-PCR). The PCR procedures were carried out in a cycling environment as recommended by the manufacturer. Every response was checked against GAPDH as an endogenous standard. The Primer3 tool (https://primer3.ut.ee/) was used to design the PCR primers (Table 1). The delta-delta Ct method was used to determine gene expression levels [14].
Table 1.
Primer sequences for first- and second-phases genes.
| Symbol | Forward | Reverse |
|---|---|---|
| ACE2 | TGGGTCTTCAGTGCTCTCAG | ACCCCACATATCACCAAGCA |
| ANPEP | GGCCCCATGAAGAACTACCT | TCAGCCTCATTGACCAGTGT |
| GAPDH | CCAGAACATCATCCCTGCCT | CCTGCTTCACCACCTTCTTG |
| EGFR | TCATGCTCTACAACCCCACC | GCACTTCTTACACTTGCGGA |
| IGF2R | CTTTGACAGCGAGAATCCCG | TCACTGTTTCCCTCCTCTCC |
2.4. Hierarchical clustering
Genes determined in linear regression analysis were hierarchically clustered with mean standardized gene expression values with the Euclidean Gene Cluster 3.0 program (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm) [15]. Data were standardized after cluster analysis, and the standardized data were viewed using TreeView (http://jtreeview.sourceforge.net/) [16]. Hierarchical clustering was performed by clustering both genes and arrays using Euclidian distance as a similarity metric and complete linkage as a clustering method.
2.5. Statistical analyses
To determine if there was a statistically significant difference between the expression of the relevant genes in the pediatric population versus adults, we performed a t-test and limma analysis [17]. The limma package is a core component of Bioconductor, an R-based open-source software development project for statistical genomics. It has proven a popular choice for the analysis of data from experiments involving microarrays, PCR, protein arrays, and other platforms. It contains rich features for handling complex experimental designs and for information-borrowing to overcome the problem of small sample sizes [17]. We also performed the Pearson product-moment correlation coefficient test to describe the strength and direction of the linear relationships between the relevant gene expressions and age. Additionally, we performed post hoc power analysis using G*Power software version 3.1.9.7 to determine the statistical power of the study based on the observed effect size and sample size.
3. Results
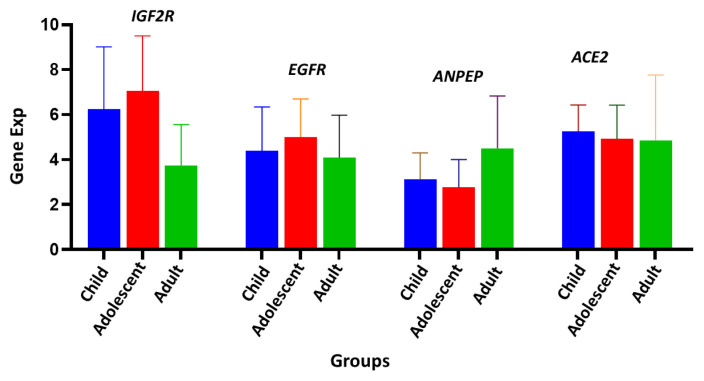
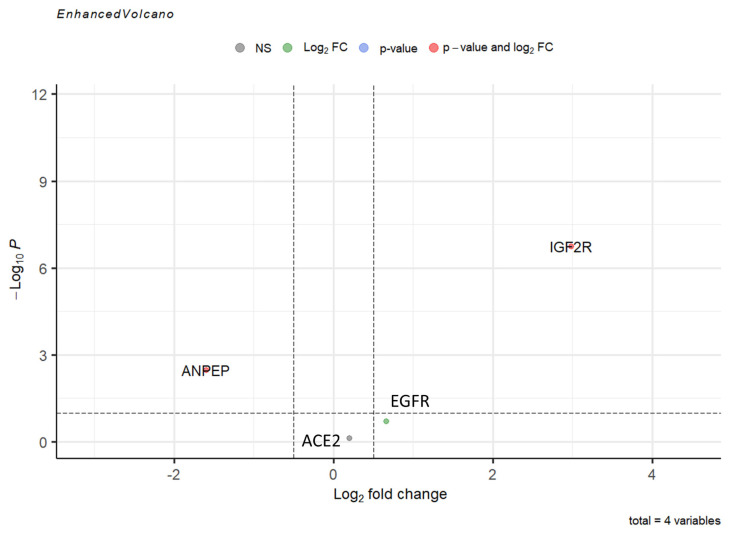
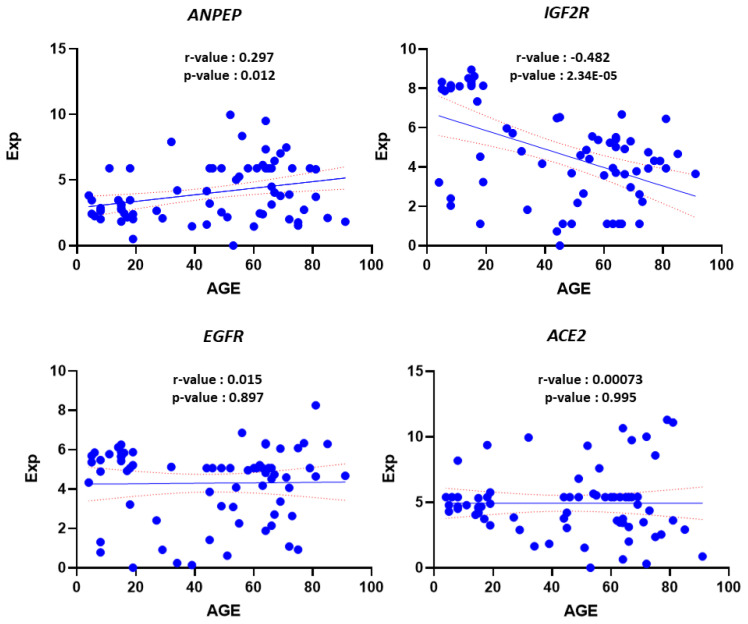
Polymorphonuclear leukocyte (PMNL) cells were isolated from 70 out of the total collected 72 blood samples from COVID-19 patients (Table 2). Our sample population was divided into two groups of pediatric and adult patients. Infancy, childhood, and adolescence were the subgroups of the pediatric age group. The frequency and percentage of each gene within these groups are presented in Table 3. In terms of gender distribution, there were 15 male and 8 female patients in the pediatric group, while there were 32 male and 16 female patients in the adult group (Table 3). Approximately 1–2 × 107 PMNL cells were isolated from each patient’s blood sample. Approximately 50–100 ng of RNA was extracted from each sample and used for RT-PCR to determine the level of gene expression. Six cases with active bacterial infection were excluded from analysis since active bacterial infection can alter immunogenomic features. Figure 1 depicts the expression levels of each gene in the different groups. To determine whether there was a statistically significant difference in the expression levels of genes (ACE2, ANPEP, EGFR, and IGF2R) between the groups, the average expression values of the genes were compared using the t-test and limma statistical results (Figure 2). According to the results of the Student two-tailed t-test, while the ANPEP gene was significantly downregulated in the pediatric group (p < 0.05, FC: 1.57), the IGF2R gene was significantly upregulated in the adult group (p < 0.05, FC: 2.98). EGFR and IGF2R expression levels were not significantly different between the adult and pediatric groups (p > 0.05). In addition, limma analysis results demonstrated that two genes (ANPEP and IGF2R) were expressed differently in the pediatric and adult groups (Figure 3). Post hoc power analysis showed that the study achieved statistical power of 0.87 with an effect size of 0.8 and sample sizes of 23 for the pediatric group and 49 for the adult group, with a significance level of p < 0.05. Comparing the male and female samples from different groups revealed no statistically significant differences in the expressions of the genes (data not shown). The results of Pearson correlation analysis revealed that the ANPEP gene had a significant direct relationship with age, whereas the IGF2R gene had a significant inverse relationship with age. Neither ACE2 nor EGFR demonstrated a significant correlation with age (Figure 4). Hierarchical clustering analysis showed that the ANPEP and IGF2R genes could distinguish between pediatric and adult patients very well after the exclusion of samples with expression values beyond the mean expression values of the groups (Figure 5).
Table 2.
Descriptive statistics: number of valid and missing samples for each gene, respectively.
| ACE2 | ANPEP | EGFR | IGF2R | ||
|---|---|---|---|---|---|
| N | Valid | 70 | 70 | 69 | 69 |
| Missing | 2 | 2 | 3 | 3 | |
Table 3.
Frequency and percent analysis of valid and missing samples belonging to different age groups.
| Frequency | Percent | Valid percent | Cumulative percent | ||
|---|---|---|---|---|---|
| ACE2 Valid | Infancy | 0 | 0% | 0% | 0% |
| Childhood | 9 | 12.85% | 12.85% | 12.85% | |
| Adolescence | 13 | 18.57% | 18.57% | 18.57% | |
| Adult | 48 | 68.57% | 68.57% | 68.57% | |
| Total | 70 | 100% | 100% | 100% | |
| ANPEP Valid | Infancy | 0 | 0% | 0% | 0% |
| Childhood | 9 | 12.85% | 12.85% | 12.85% | |
| Adolescence | 13 | 18.57% | 18.57% | 18.57% | |
| Adult | 48 | 68.57% | 68.57% | 68.57% | |
| Total | 70 | 100% | 100% | 100% | |
| EGFR Valid | Infancy | 0 | 0% | 0% | 0% |
| Childhood | 9 | 12.85% | 12.85% | 12.85% | |
| Adolescence | 12 | 17.39% | 17.39% | 17.39% | |
| Adult | 48 | 68.57% | 68.57% | 68.57% | |
| Total | 69 | 99% | 99% | 99% | |
| IGF2R Valid | Infancy | 0 | 0% | 0% | 0% |
| Childhood | 9 | 12.85% | 12.85% | 12.85% | |
| Adolescence | 13 | 18.84% | 18.84% | 18.84% | |
| Adult | 47 | 68.11% | 68.11% | 68.11% | |
| Total | 69 | 99% | 99% | 99% | |
Figure 1.
Gene expression analysis of first- and second-phase biomarker genes. IGF2R, EGFR, ANPEP, and ACE2 were respectively quantified by real-time qPCR in child, adolescent, and adult groups. GAPDH was used as an endogenous control.
Figure 2.
Comparison of first- and second-phase biomarker genes between adult and pediatric groups. The p-values indicate t-test comparisons of expression levels between adult and pediatric groups. FC values describe the change in the expression level of each gene.
Figure 3.
Comparison of average expression levels of first- and second-phase biomarker genes. The limma analysis showed that, among the four genes, two (ANPEP and IGF2R) were expressed statistically significantly differently between the groups.
Figure 4.
Linear correlation analysis of gene expression values with age. Analysis showed that ANPEP and IGF2R correlated significantly with age.
Figure 5.
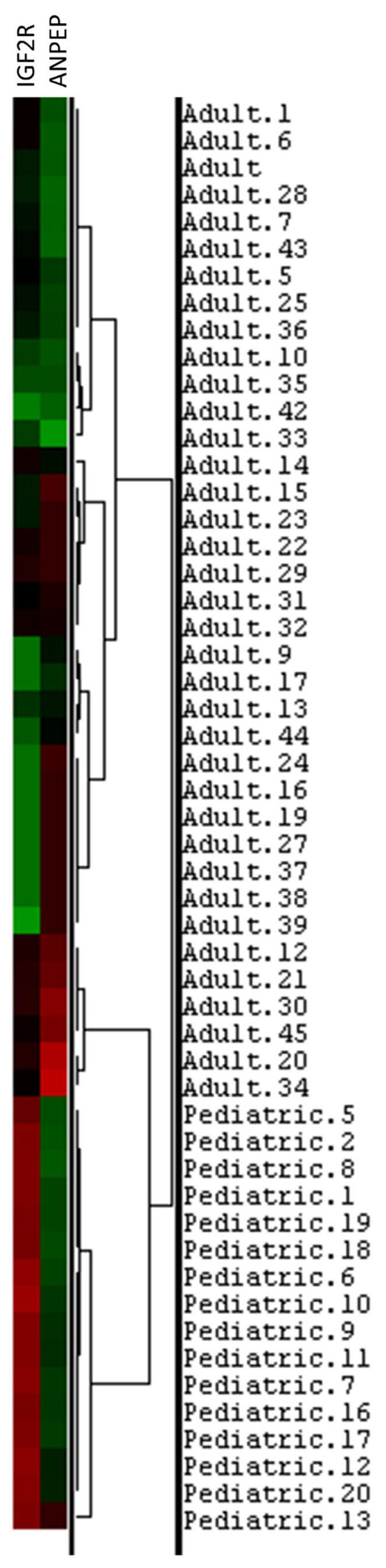
Hierarchical analysis confirmed that IGF2R and ANPEP can distinguish between adult and pediatric groups well.
4. Discussion
The results of the current study support the hypothesis that the mild clinical presentations of pediatric COVID-19 are driven by the expression of critical biogenomic markers, specifically the first-phase receptor genes (ACE2 and ANPEP) and second-phase receptor genes (EGFR and IGF2R). In a previous study, our group described the three clinicobiological phases (initiation, propagation, and complication phases) of human SARS-associated coronavirus infections [6]. The interferon (IFN) family plays a crucial role in the impact of SARS-CoV-2 [18]. We previously demonstrated that important IFN gene expressions are altered upon exposure to the SARS-CoV-2 virus [18]. One of the primary mechanisms for early infection control is the detection of continuously exposed pathogens, which stimulates the immediate replenishment of innate immune cells from the early stages of their development through emergency hematopoiesis [19]. However, the functional success of this mechanism can become dangerous when excessive stimulating signals accelerate immunological catastrophes. Metabolic or hyperinflammatory conditions in aging exacerbate this mechanism and lead to uncontrolled emergency myelopoiesis, resulting in severe COVID-19 disease [19]. This mechanism may explain the different clinical courses observed in pediatric and adult COVID-19 cases. Furthermore, adult COVID-19 patients with hypertension may more frequently and easily progress from the propagation phase to the complication phase due to alterations in the EGFR and IGF2R genes [7]. The higher incidence of hypertension in adult patients may also contribute to the differences in clinical course observed in COVID-19 between pediatric and adult populations. Gastrointestinal manifestations of COVID-19 can present as acute abdomen, which may be caused by conditions such as acute pancreatitis, acute appendicitis, intestinal obstruction, bowel ischemia, hemoperitoneum, or abdominal compartment syndrome [20]. The involvement of the gastrointestinal system in COVID-19 can be attributed to direct viral injury and/or an inflammatory immune response. This involvement can lead to malabsorption, an imbalance in intestinal secretions and gut mucosal integrity, and stimulation of the enteric nervous system [20].
Additionally, the ANPEP gene (also known as CD13) has been associated with gastrointestinal system involvement in COVID-19 [6]. ANPEP and ACE are two genes that have been implicated in COVID-19 due to their involvement in the renin-angiotensin-aldosterone system (RAS). The RAS is a complex hormonal pathway that regulates blood pressure, fluid balance, and electrolyte homeostasis. ACE is a key enzyme in this system, converting angiotensin I to angiotensin II, a potent vasoconstrictor that promotes inflammation [21]. The ANPEP and ACE genes are also known to participate in other important pathways. ACE, for instance, is involved in the ACE inhibitor pathway, early SARS-CoV-2 infection events, and peptide hormone metabolism [22–24]. On the other hand, ANPEP is involved in the innate immune system, glutathione conjugation, and protein metabolism pathways [25–27]. The SARS-CoV-2 virus, responsible for causing COVID-19, utilizes the ACE2 receptor to enter human cells. ACE2 is expressed in different tissues of the body, including the lungs. Recent studies have indicated that ANPEP, which codes for CD13, may also play a role in the viral entry process. In certain cell types, ANPEP can function as an alternative or co-receptor for the virus [28].
In the current study, we observed that the expression of the ANPEP gene was lower in pediatric patients with COVID-19. This lower expression may have a protective effect in the pediatric age group, as the ANPEP gene is involved in the intestinal penetration of SARS-CoV-2 [7]. Moreover, the dysregulation of the sodium-dependent glucose transporter (SGLT1 or SLC5A1) in the intestinal epithelium, controlled by ACE2, has been linked to the pathogenesis of diabetes mellitus, which may contribute to the higher mortality observed in COVID-19 patients with diabetes [29].
The high expression of ACE2 in mucosal cells of the intestine suggests that they could serve as potential entry sites for the virus and support its replication [29].
The interrelationship between the ANPEP and ACE genes might impede the progression of COVID-19 from the initiation phase to the propagating phase in pediatric patients. Notably, both low ANPEP gene expression levels and mild clinical disease courses were common features in pediatric COVID-19 patients and in adult patients without gastrointestinal involvement. Thus, the ACE and ANPEP genes may contribute to the severity and progression of COVID-19 by influencing the virus’s ability to enter human cells and modulating the response of the RAS to the infection.
Understanding the genetic factors that influence the susceptibility and severity of COVID-19 could help identify individuals at higher risk and guide the development of new therapeutic approaches. The present study has revealed that the expression of the IGF2R gene is higher in pediatric patients with COVID-19. Previous research demonstrated that with increasing exposure time to SARS-CoV-2, two receptors, EGFR and IGF2R, which play crucial roles in the RAS signaling pathway, are significantly downregulated in infected human bronchial epithelial cells during the propagating phase of COVID-19 [7].
EGFR is a gene involved in cell growth and proliferation. It has been found to be upregulated in lung cells infected with SARS-CoV-2. This upregulation may contribute to the development of lung inflammation and the lung damage observed in severe cases of COVID-19. Furthermore, certain studies suggest that drugs targeting EGFR may hold potential as treatments for COVID-19, although further research is required to explore this possibility [30]. It is important to note that while the roles of EGFR and IGF2R in the context of COVID-19 have been investigated, their precise significance in the disease is still being explored. Ongoing research aims to provide a better understanding of the involvement of these genes in COVID-19 and their potential as therapeutic targets. IGF2R is a gene that regulates the activity of insulin-like growth factors, which are involved in cell growth and division. In lung cells infected with SARS-CoV-2, IGF2R has been found to be downregulated, and this downregulation may contribute to the development of lung inflammation and damage observed in severe cases of COVID-19 [7]. Additionally, some studies have suggested that IGF2R may play a role in the immune response to viral infections, although further research is necessary to fully understand this role [31].
Furthermore, insulin-like growth factor 2 (IGF2) has been found to be decreased in the serum of individuals with diabetes [32]. Diabetic patients generally exhibit a poorer prognosis in COVID-19, as diabetes is considered a risk factor for adverse outcomes [33]. In light of these data, the high expression of the IGF2R gene in pediatric patients may have a protective role and could contribute to the milder clinical course of COVID-19 observed in this population. The interrelationship between the EGFR and IGF2R genes might impede the progression of COVID-19 from the propagating phase to the complicating phase in pediatric patients.
Overall, although the EGFR and IGF2R genes have been implicated in COVID-19, further research is required to fully comprehend their significance in the disease and to identify potential targets for treatments.
According to our previous study, COVID-19 exhibits three distinct phases: the initiating, propagating, and complicating phases. Each phase is associated with specific genes: ACE2 and ANPEP in the initiating phase, EGFR and IGF2R in the propagating phase, and immune system-related genes in the complicating phase [6].
Generally, the clinical course of COVID-19 tends to be more severe in adults compared to pediatric patients. In the initiating phase and in some cases in the propagating phase, COVID-19 treatment can be managed in outpatient clinic settings. However, if patients progress to the complicating phase, their clinical condition deteriorates, necessitating hospitalization and, in some cases, admission to intensive care units. One possible explanation for the divergent clinical courses observed between adult and pediatric COVID-19 patients is alterations in the ANPEP and IGF2R genes. Particularly in adult patients, screening for ANPEP and IGF2R gene expressions could be valuable for predicting the severity of the clinical course of COVID-19. This predictive capability may have implications for future treatment algorithms in COVID-19.
It is important to note that further research and investigation are required to fully understand the roles of these genes and their potential implications for COVID-19 treatment and management. Our research group has proposed a model suggesting that the incorporation of the SARS-CoV-2 virus into the human virobiota occurs through three distinct pathobiological phases: induction, consolidation, and maintenance [34].
This chimerism process involves the integration of the SARS-CoV-2 virus into the human genome, particularly within the intestinal virobiota [34]. Additionally, it has been observed that the Omicron variant of the virus possesses unique characteristics that enhance chimerism-mediated immunotherapy without causing high mortality rates by evading interferon gene expression [35].
The focus of this paper has been exploring the reasons behind the divergent clinical courses observed in pediatric and adult COVID-19 patients. We have clinically validated our previous hypothesis regarding the three critical clinicobiological phases of human SARS-associated coronavirus infections [6], along with their corresponding genes for each phase. Furthermore, we have shown that COVID-19 induces differential gene expression patterns in adult and pediatric patients, which may contribute to the observed differences in clinical course between these two age groups. Specifically, we have emphasized the significance of the ANPEP and IGF2R genes, which play unique roles in the progression of COVID-19 from the initiating phase to the propagating phase.
The findings presented in this study can serve as a foundation for future investigations focusing on the clinical implications of distinct gene expression patterns in COVID-19. One important limitation of this study is the relatively small number of study participants, which was due to corruption of the samples. The limited sample size might have had an impact on the generalizability and statistical power of the findings. It is crucial to replicate and validate the results of this study using larger sample sizes in future studies. With the inclusion of a larger number of participants, the findings would be more robust and reliable, providing a stronger basis for drawing conclusions and identifying clinical implications.
It is also worth noting that additional factors, such as demographic characteristics, comorbidities, and environmental factors, could potentially influence the clinical course of COVID-19. Controlling for these factors and conducting further investigations with larger cohorts would provide a more comprehensive understanding of the relationship between gene expression patterns and the clinical outcomes of COVID-19.
In conclusion, the expression of critical biogenomic markers, including the ACE2, ANPEP, EGFR, and IGF2R genes, plays a significant role in the development of mild clinical presentations in pediatric COVID-19 cases. Pediatric patients exhibit lower ANPEP gene expressions, which may contribute to their milder clinical courses. The interplay between the ANPEP and ACE genes could potentially prevent the progression of COVID-19 from the initiation phase to the propagating phase in pediatric patients. Furthermore, the higher expression of the IGF2R gene in pediatric patients may confer a protective effect and contribute to the mild clinical course observed in this age group. The interrelationship between EGFR and IGF2R might also play a role in impeding the progression of COVID-19 from the propagating phase to the complicating phase in pediatric patients.
However, further research with larger sample sizes is needed to validate these findings and better understand the complex genetic mechanisms underlying the clinical course of COVID-19 in pediatric patients. Exploring the interplay of these genes could have implications for predicting disease severity and guiding future treatment strategies for pediatric COVID-19 cases.
Supplementary Information
Acknowledgment/disclaimers/conflict of interest
This study was supported by the Ankara Yıldırım Beyazıt University Scientific Research Projects Coordination Unit (TSA-2021-2250).
The authors declare that there is no conflict of interest regarding the publication of this article.
The data supporting this study are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.
Funding Statement
This study was supported by the Ankara Yıldırım Beyazıt University Scientific Research Projects Coordination Unit (TSA-2021-2250).
Footnotes
Informed consent: The local ethics committee approved the research protocol with approval number E1-20-1396. Each patient or the patient’s legal guardian signed a written permission form, and tests were conducted in accordance with the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report.
References
- 1.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temgoua MN, Endomba FT, Nkeck JR, Kenfack GU, Tochie JN, et al. Coronavirus disease 2019 (COVID-19) as a multi-systemic disease and its impact in low- and middle-income countries (LMICs) SN Comprehensive Clinical Medicine. 2020;2(9):1377–1387. doi: 10.1007/s42399-020-00417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1821):20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clinical Chemistry and Laboratory Medicine. 2020;58(7):1135–1138. doi: 10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 6.Turk C, Turk S, Malkan UY, Haznedaroglu IC. Three critical clinicobiological phases of the human SARS-associated coronavirus infections. European Review for Medical and Pharmacological Sciences. 2020;24(16):8606–8620. doi: 10.26355/eurrev_202008_22660. [DOI] [PubMed] [Google Scholar]
- 7.Turk C, Turk S, Temirci ES, Malkan UY, Haznedaroglu IC. In vitro analysis of the renin-angiotensin system and inflammatory gene transcripts in human bronchial epithelial cells after infection with severe acute respiratory syndrome coronavirus. Journal of the Renin-Angiotensin-Aldosterone System. 2020;21(2):1470320320928872. doi: 10.1177/1470320320928872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Çiftçiler R, Haznedaroğlu İC. COVID-19, renin-angiotensin system, and hematopoiesis. Turkish Journal of Hematology. 2020;37(3):207–208. doi: 10.4274/tjh.galenos.2020.2020.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2008;294(6):L1068–1075. doi: 10.1152/ajplung.00025.2008. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell HD, Eisfeld AJ, Stratton KG, Heller NC, Bramer LM, et al. The role of EGFR in influenza pathogenicity: multiple network-based approaches to identify a key regulator of non-lethal infections. Frontiers in Cell and Developmental Biology. 2019;7:200. doi: 10.3389/fcell.2019.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin DH, Ruley HE. Cellular genetics of host susceptibility and resistance to virus infection. Critical Reviews in Eukaryotic Gene Expression. 2006;16(2):155–170. doi: 10.1615/critreveukargeneexpr.v16.i2.40. [DOI] [PubMed] [Google Scholar]
- 12.Hardin AP, Hackell JM Committee on Practice and Ambulatory Medicine. Age limit of pediatrics. Pediatrics. 2017;140(3):e20172151. doi: 10.1542/peds.2017-2151. [DOI] [PubMed] [Google Scholar]
- 13.Weiskopf N, Suckling J, Williams G, Correia MM, Inkster B, et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: a multi-center validation. Frontiers in Neuroscience. 2013;7:95. doi: 10.3389/fnins.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.de Hoon MJL, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20(9):1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 16.Saldanha AJ. Java TreeView--extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malkan UY, Turk S, Turk C, Temirci ES, Koker I, et al. Interferon-gene family alterations following the SARS-Cov infection in association with iron metabolism and lymphoid biology. UHOD-International Journal of Hematology and Oncology. 2021;32(2):67–78. doi: 10.4999/uhod.214646. [DOI] [Google Scholar]
- 19.Balandrán JC, Zamora-Herrera G, Romo-Rodríguez R, Pelayo R. Emergency hematopoiesis in the pathobiology of COVID-19: the dark side of an early innate protective mechanism. Journal of Interferon & Cytokine Research. 2022;42(8):393–405. doi: 10.1089/jir.2022.0028. [DOI] [PubMed] [Google Scholar]
- 20.Kariyawasam JC, Jayarajah U, Riza R, Abeysuriya V, Seneviratne SL. Gastrointestinal manifestations in COVID-19. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2021;115(12):1362–1388. doi: 10.1093/trstmh/trab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Molecular Medicine. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ni W, Yang X, Yang D, Bao J, Li R, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Critical Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. European Journal of Clinical Microbiology & Infectious Diseases. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polak Y, Speth RC. Metabolism of angiotensin peptides by angiotensin converting enzyme 2 (ACE2) and analysis of the effect of excess zinc on ACE2 enzymatic activity. Peptides. 2021;137:170477. doi: 10.1016/j.peptides.2020.170477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH, Afridi R, Cho E, Yoon JH, Lim YH, et al. Soluble ANPEP released from human astrocytes as a positive regulator of microglial activation and neuroinflammation: brain renin-angiotensin system in astrocyte-microglia crosstalk. Molecular & Cellular Proteomics. 2022;21(11):100424. doi: 10.1016/j.mcpro.2022.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pompella A, Visvikis A, Paolicchi A, Tata VD, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochemical Pharmacology. 2003;66(8):1499–1503. doi: 10.1016/S0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 27.Seli E, Senturk LM, Bahtiyar OM, Kayisli UA, Arici A. Expression of aminopeptidase N in human endometrium and regulation of its activity by estrogen. Fertility and Sterility. 2001;75(6):1172–1176. doi: 10.1016/s0015-0282(01)01779-4. [DOI] [PubMed] [Google Scholar]
- 28.López-Cortés GI, Palacios-Pérez M, Hernández-Aguilar MM, Veledíaz HF, José MV. Human coronavirus cell receptors provide challenging therapeutic targets. Vaccines (Basel) 2023;11(1):174. doi: 10.3390/vaccines11010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Faiq MA, Pareek V, Raza K, Narayan RK, et al. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Medical Hypotheses. 2020;144:110271. doi: 10.1016/j.mehy.2020.110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Londres HD, Armada JJ, Martínez AH, Cuza AAA, Sánchez YH, et al. Blocking EGFR with nimotuzumab: a novel strategy for COVID-19 treatment. Immunotherapy. 2022;14(7):521–530. doi: 10.2217/imt-2022-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G, Zhou L, Zhang C, Shi Y, Dong D, et al. Insulin-like growth factor 1 regulates acute inflammatory lung injury mediated by influenza virus infection. Frontiers in Microbiology. 2019;10:2541. doi: 10.3389/fmicb.2019.02541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zolov SN, Imai H, Losiewicz MK, Singh RSJ, Fort PE, et al. Insulin-like growth factor-2 regulates basal retinal insulin receptor activity. Journal of Biological Chemistry. 2021;296:100712. doi: 10.1016/j.jbc.2021.100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19) Diabetes/Metabolism Research and Reviews. 2021;37(2):e3377. doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Türk S, Türk C, Malkan ÜY, Temirci ES, Peker MÇ, et al. Current community transmission and future perspectives on the COVID-19 process. Turkish Journal of Medical Sciences. 2021;51(3):1001–1011. doi: 10.3906/sag-2012-310. [DOI] [PubMed] [Google Scholar]
- 35.Malkan UY, Haznedaroglu IC. Hematological aspects of the COVID-19 syndrome. European Review for Medical and Pharmacological Sciences. 2022;26(12):4463–4476. doi: 10.26355/eurrev_202206_29086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.