Introduction
Glaucoma is a progressive degenerative optic neuropathy that results in visual field defects and eventually blindness. More than 60.5 million people in the world are affected by glaucoma and it is the leading cause of irreversible blindness in the world.1 It is characterized by death of retinal ganglion cells (RGCs) in the inner retina and loss of their axons in the optic nerve. This leads to structural thinning of the retinal nerve fiber layer (RNFL) and excavation of the optic cup that can be detected clinically by ophthalmoscopy and imaging devices such as optical coherence tomography (OCT) and laser scanning polarimetry.2 Functionally, RGC loss leads to visual field defects that often start in the periphery and gradually encroach on central vision.3 However, atrophy of the RNFL precedes visual field loss.4 Up to 40% of the RNFL can be lost before visual field defects can be detected by conventional perimetry.5 Hence, detection of RGC damage is critical for early identification and monitoring of disease and prevention of vision loss. In recent years, promising approaches of imaging RGC death and dysfunction in vivo have been developed. The detection of apoptotic retinal cells (DARC) technology can be used for in vivo quantification of RGC apoptosis and has the potential of identifying patients with glaucoma before irreversible vision loss.6 Another technique is TcapQ, a cell-penetrating probe that has been validated in animal models in vivo to detect RGC apoptosis. In addition, flavoprotein fluorescence (FPF) has been used to detect mitochondrial dysfunction in glaucoma patients, while adaptive optics can be used to visualize individual RGCs in vivo in humans. In this review, we discuss recent developments in these techniques and discuss the implications for the diagnosis and management of glaucoma.
Mechanisms of RGC Death
RGC death in most cases of glaucoma happens in the context of elevated intraocular pressure (IOP), although it can also occur with normal IOP. Thus far, IOP is the only modifiable risk factor in glaucoma, and reduction of IOP is associated with delayed progression of the disease.1,7,8 The mechanisms of RGC death are not fully understood and are likely multifactorial. These include deprivation of neurotrophic factors, mitochondrial dysfunction, glial activation, excitotoxicity, failure of axonal transport and oxidative stress.9,10 Regardless of the mechanism of injury, RGCs are thought to ultimately die via apoptosis.1,11
Apoptosis is programmed cell death that leads to self-destruction of a cell. It is characterized by cell shrinkage, nuclear fragmentation, exposure of phosphatidylserine (PS) on the cell surface, and loss of mitochondrial transmembrane potential. These biochemical features are activated by caspases, a family of proteases responsible for killing the cell.12 Apoptotic cells are subsequently engulfed by macrophages and microglia. DNA fragmentation pattern characteristic of apoptosis has been found to be at increased levels in glaucoma patients using the TUNEL [terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling] assay.11
Mitochondrial metabolic dysfunction has been proposed as a marker that precedes apoptosis. Mitochondrial dysfunction increases production of reactive oxygen species, causing oxidative stress.13 Oxidative stress is thought to contribute to the pathogenesis of many neurodegenerative diseases, including glaucoma. Oxidative stress can induce RGC apoptosis from direct neurotoxicity, or indirectly by causing damage to glial cells.14 There is evidence that mitochondrial dysfunction is present in some glaucoma patients. Studies have shown that primary open-angle glaucoma (POAG) patients have more mitochondrial DNA mutations and impaired complex-I-mediated ATP synthesis and respiration.15,16
Detecting RGC Apoptosis With DARC Technology
One of the early events in apoptosis is the translocation of PS from the inner leaflet to the outer leaflet of the cell membrane. Exposed PS serves as a signal for macrophages to engulf the apoptotic cell. Annexin V is a phospholipid-binding protein with a high affinity for PS in the presence of calcium.17 This property has been exploited for many years in for detecting apoptotic cells using fluorescently labelled annexin in vitro.18,19 More recently, Technetium-99m labelled annexin has been used in vivo to detect apoptosis in many conditions such as myocardial infarction, breast cancer, lymphoma and ischemic brain injury.20–23
DARC technology has been developed to noninvasively image apoptotic RGCs. It utilizes fluorescently labelled annexin V, which binds to translocated PS in the outer leaflet of the membrane of apoptotic RGCs. Individual RGCs undergoing apoptosis (annexin V-positive cells) are then visualized using a wide-angle confocal laser scanning ophthalmoscope.24 DARC has been used in experimental models to study the pathogenesis of neurodegeneration in glaucoma. Prior work has shown that RGC apoptosis is correlated with elevated IOP and changes in the extracellular matrix in the retina and optic nerve head.25 Another study showed that amyloid-beta colocalizes with apoptotic RGCs and induces RGC apoptosis in a dose-dependent and time-dependent manner, implicating that targeting the amyloid-beta formation and aggregation pathway may reduce glaucomatous RGC apoptosis.26 DARC has also been used to gage the neuroprotective effect of existing and novel candidate drugs.27 For example, the alpha-2 adrenergic receptor agonists brimonidine and clonidine have been shown to have neuroprotective effects via the amyloid-beta and secreted amyloid precursor protein pathways that are independent of IOP.28 DARC has been used to evaluate candidate drugs that target glutamate excitotoxicity, and the broad-spectrum NMDA receptor antagonist MK801 combined with group II metabotropic glutamate receptor (mGluR) agonist LY354740 was the most effective in a study of the role of glutamate modulation in glaucoma-related RGC apoptosis.29
A recent proof-of-concept phase I clinical trial demonstrated that DARC can be used in vivo in the human retina to visualize individual RGCs undergoing apoptosis.30 Eight progressing glaucoma patients and 8 healthy subjects were randomized to receive varying doses of intravenous fluorescently conjugated annexin V. DARC enabled individual apoptotic RGCs to be visualized in the human retina (Fig. 1). The level of apoptosis (DARC count) was significantly higher in glaucoma patients compared with controls, and was also significantly greater in patients who later had increasing rates of progression. Fluorescently conjugated annexin V was well tolerated and there were no serious adverse events in the study. The dye was rapidly absorbed and had a short half-life (10 to 36 min). This study suggests that DARC can be used to identify neurodegeneration in the retina. Moreover, DARC count may be a surrogate marker of disease activity, predictive of increased rates of progression, thus allowing for earlier treatment and prevention of vision loss. Compared with visual field testing, it may provide more repeatable data as it does not depend on the patient’s ability to comply with the test, and can detect early RGC loss that precedes visual field changes. Compared with OCT imaging, it may enable an earlier diagnosis in patients with mild glaucoma before detectable structural changes in the RNFL. It may also be helpful in patients with advanced glaucoma with very thin RNFLs when it is increasingly difficult to detect nerve fiber loss.
Figure 1.
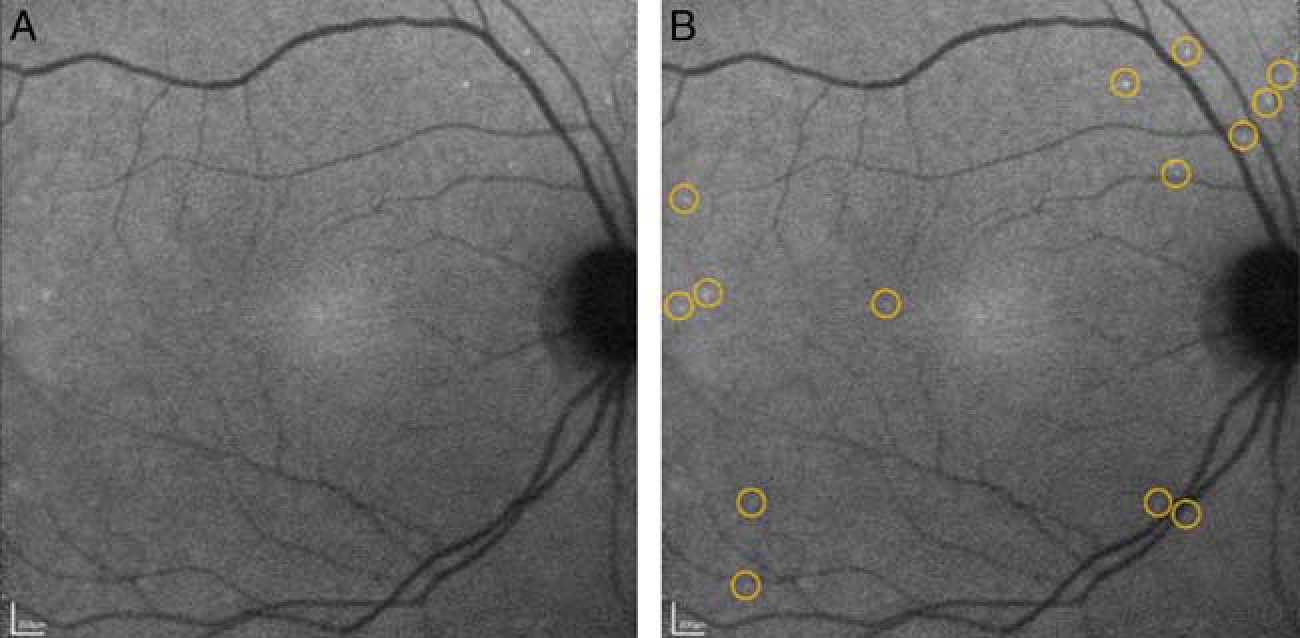
Detection of apoptotic retinal cells counts in a glaucoma patient showing single cell apoptosis in the retina. 0.4 mg of fluorescently conjugated annexin V was injected intravenously and retinal images were obtained at 240 minutes. Unmarked (A) and marked (B) annexin-positive spots are shown with yellow rings highlighting individual spots. Adapted from Cordeiro et al.30 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
For this technology to be successful in clinical applications, it will be crucial to distinguish between DARC counts of healthy and glaucomatous individuals, and within those with glaucoma to distinguish between the different stages of disease. A phase II clinical trial is currently underway to study DARC in up to 120 patients, including those with glaucoma, optic neuritis, age-related macular degeneration, Down syndrome (as a model for Alzheimer disease), and healthy subjects. It seeks to define the range of DARC counts seen in healthy subjects and those with different diseases.24 Future studies would also have to determine the specificity of annexin binding to PS in human eyes. The phase I trial did not include a scrambled annexin control. Hence, it is difficult to determine if the detected fluorescence represents annexin binding to PS or nonspecific binding, especially as increased dosage of annexin did not always correlate with increased DARC count.30
One fundamental criticism of annexin V imaging is that annexin binds to PS regardless of whether the phospholipid is exposed on the external or internal cell leaflet. Cells that undergo necrosis have defects in their cell membranes and can allow passage of large molecules such as annexin, enabling annexin to bind to PS in the inner leaflet. Hence, labelled annexin may not be able to distinguish between apoptosis and necrosis.31,32 In addition, exposure of PS to the cell surface can also be seen in infected and injured cells,33 and may be reversible in viable neurons under conditions of inflammation,34 and therefore may not be unique to cells committed to the apoptotic cell death pathway. This may confound the ability to track glaucomatous changes in eyes with other pathologies besides glaucoma.
Detecting RGC Apoptosis With TcapQ Technology
TcapQ uses a non–annexin-based technology to detect RGC apoptosis. It comprises of a cell-penetrating Tat peptide and a fluorophore-quencher pair flanking an effector caspase consensus sequence. Fluorescence is activated by caspase 3 and 7 in apoptotic cells.35 Conjugation to a cell-penetrating peptide enables rapid intracellular translocation of the probe via endocytosis, and enables selective uptake by RGCs following intravitreal injection.36 Subsequently, TcapQ was validated in an ex vivo model of NMDA-induced RGC degeneration with confirmation by TUNEL staining.37 More recently, using real-time imaging of a live rat NMDA-induced RGC degeneration model with a confocal scanning laser ophthalmoscope, the kinetics of probe activation, signal-to-noise ratios, and probe safety profiles were characterized in vivo. Fluorescence fundus imaging showed activation of TcapQ in single cells undergoing RGC apoptosis (Fig. 2), and the density of fluorescent RGCs increased with rising NMDA concentrations. The peak activation of probe was 12 hours after intravitreal injection. Electroretinography (ERG) performed following intravitreal injections of TcapQ showed no significant probe-related toxicity.38
Figure 2.
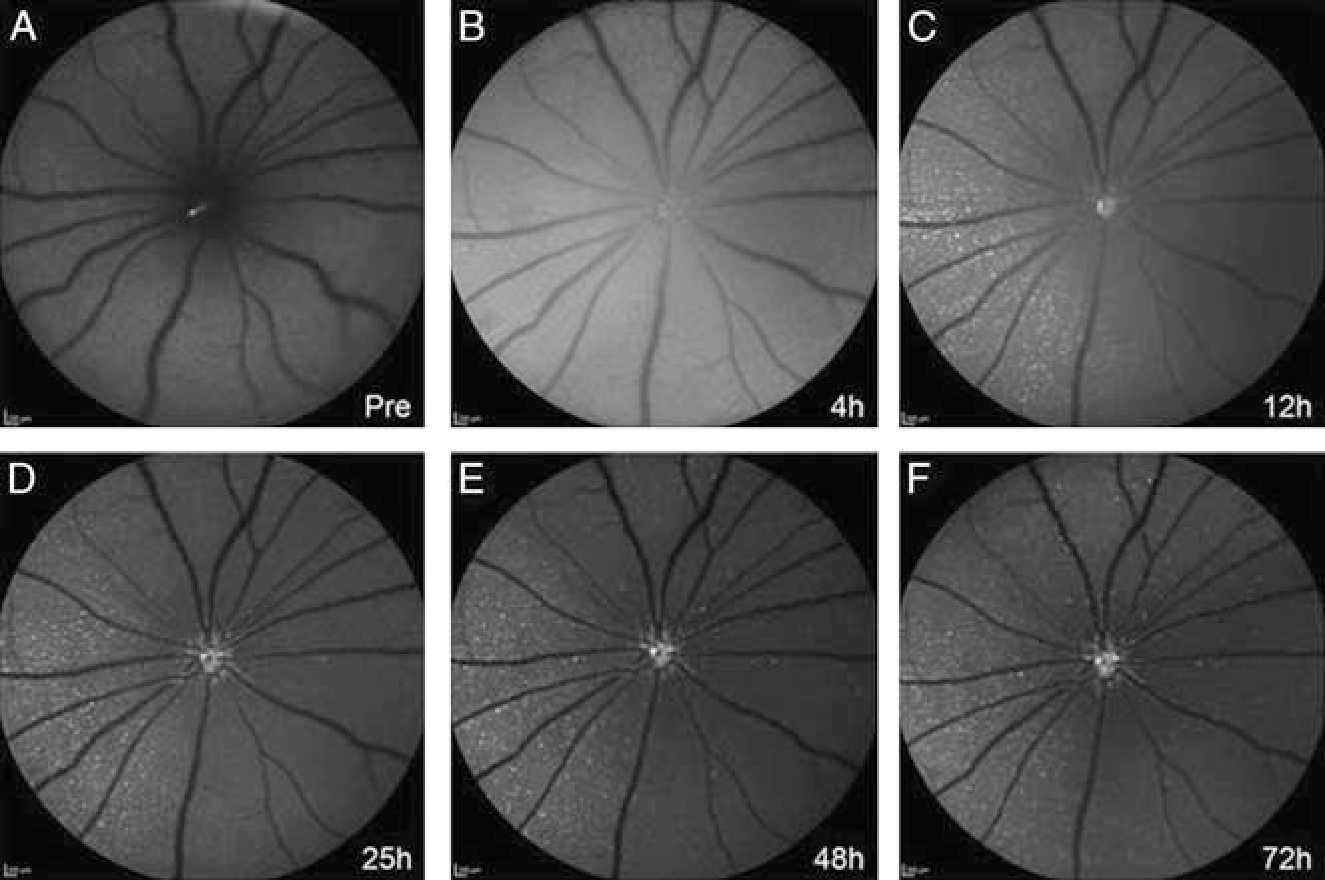
TcapQ activation in vivo. In vivo images were taken in a rat eye pretreated with 12.5 mM NMDA immediately before (A) and at 4 hours (B), 12 hours (C), 25 hours (D), 48 hours (E), and 72 hours (F) postintravitreal injection of 0.313 nmol TcapQ488. Evidence of initial probe activation was noted at 4 hours after TcapQ488 injection (B). Scale bar, 200 μm in all images. Reproduced from Qiu et al.38
An advantage of an activatable peptide probe is that fluorescence signal should only occur after uptake and cleavage by the specific target enzyme. However, when probe dose was high, there was some activation of the probe in the absence of NMDA. The cause is not entirely clear, and was hypothesized to be due to nonspecific dequenching of fluorescence not related to probe activation, or probe toxicity causing RGC apoptosis.38 Nonetheless, it was reassuring that no probe toxicity was detected via ERG. Another advantage of cell-penetrating probes is that they may be used to deliver other molecular imaging probes to RGCs, and may be modified to target specific retinal cell types, or be activated by other proteinases and kinases. As TcapQ moves towards further preclinical validation and use in glaucoma, it will be helpful to utilize a model of RGC degeneration resulting from elevated IOP that more closely resembles glaucoma.
One drawback to these cellular labeling techniques, including DARC and TcapQ, is that the specificity of these probes for RGCs is not well characterized. TcapQ has been shown to have preferential uptake in RGCs after intravitreal injection in rats. However, a subset of inner nuclear layer cells was also labeled.38 If other retinal cells are labeled as well, these techniques can overestimate the amount of RGC apoptosis. These approaches also capture the number of cells undergoing apoptosis at a certain timepoint but do not track the RGCs that have already been lost or those that remain healthy. Hence, it may be difficult to track disease progression. The duration of RGC apoptosis measured by annexin labeling has been estimated to last around 1 to 2 hours, and the short duration of fluorescence signal may lead to variability in imaging results.39 As clinical trials move forward with these techniques, it would be important to show that pattern of RGC apoptosis follows the same pattern of axonal loss as previously demonstrated in glaucoma, as optic disc cupping with corresponding arcuate visual field loss is a hallmark of glaucoma.
Detecting Mitochondrial Dysfunction via FPF
Reactive oxygen species production as a result of mitochondrial dysfunction leads to oxidation of mitochondrial flavoproteins, which increases their blue light-stimulated fluorescence.40,41 This property renders FPF a potential biomarker for mitochondrial dysfunction. Patients with ophthalmic diseases such as idiopathic intracranial hypertension, diabetic retinopathy, and age-related macular degeneration have increased FPF over controls.42–44 In cellular and animal models of glaucoma, increased IOP has also been shown to cause mitochondrial dysfunction.45,46
A recent study sought to investigate if retinal FPF could be a biomarker for early RGC injury in glaucoma by measuring retinal FPF in ocular hypertension (OHT) and primary POAG subjects.47 Eyes were imaged on a custom fundus camera modified to measure full retinal thickness fluorescence at a wavelength optimized to detect FPF. Macular FPF and the ratio of macular FPF to ganglion cell layer and inner plexiform layer (RGC+) thickness on OCT were measured. The RGC+ thickness was used as a surrogate to for the total number of mitochondria, and the ratio of macular FPF to RGC+ thickness adjusted FPF to account for ganglion cell layer thinning that occurred with progression of glaucoma. The study found increased macular FPF and macular FPF to RGC+ thickness ratio in OHT compared with control eyes. This suggests that mitochondrial dysfunction at the macula precedes structural or functional damage in glaucoma. However, in POAG eyes, FPF was not significantly increased over control eyes, possibly because of RGC atrophy and decrease in the total pool of contributing mitochondria. Nonetheless, the FPF to RGC+ thickness ratio was increased, again implicating mitochondrial dysfunction and oxidative stress in POAG. There was also a significant correlation between and age and FPF in POAG, suggesting that metabolic dysfunction may contribute to increased risk of developing POAG with age.47
This study was performed with a small cohort of patients (36 control eyes, 16 OHT eyes and 40 POAG eyes), and these results will have to be validated in larger studies. Nonetheless, FPF shows promise in early detection of glaucomatous injury before structural and functional changes. Future developments of the technology may allow correlation between areas of increased FPF and regions of RNFL loss and visual field deficits. Further analysis of POAG FPF patterns may also reveal distinct patterns of FPF that may predict risk of progression, and may clarify if FPF can be a useful predictive tool the management of glaucoma.
In Vivo Imaging of Individual RGCs With Adaptive Optics
The development of OCT 2 decades ago revolutionized how glaucoma is diagnosed by enabling the measurement of RNFL thickness. The recent incorporation of adaptive optics to OCT creates the possibility of performing noninvasive in vivo high-resolution cellular imaging of RGCs in humans. Adaptive optics, first invented for astronomical imaging48 and recently used for high-resolution microscopic imaging through deep, turbid biological tissues,49 is an optical technique that corrects for wavefront aberrations introduced by the scattering of the imaging medium. It could be performed at the hardware level using the Shack-Hartmann wavefront sensor and a digital deformable micromirror array in real-time,50 or at the computational level where the wavefront aberration sensing and correction are conducted offline.51 Cone photoreceptor cells have been imaged via their intrinsic reflectance using adaptive optics confocal scanning laser ophthalmoscope (AO-SLO), but RGCs have proven more challenging to image due to their near transparency.52
Recently, adaptive optics have been combined with ophthalmic OCT imaging, allowing for in vivo imaging of human photoreceptors51,53,54 and RGCs55,56 with increased resolution. Advances in real-time eye-motion tracking enable correction of motion artifacts (eg, heartbeat, breathing) and involuntary eye movements (eg, saccades, micro-tremors). Using coherent averaging techniques to suppress the common speckle noise that plague OCT images, high-resolution static images of individual RGC somas (field of view: 1.5×1.5 degrees; transverse resolution: 2.4 μm) were obtained in healthy human subjects (Fig. 3). Individual nerve fiber bundles of human subjects can also be observed in vivo.57 Adaptive optics combined with OCT imaging technique may enable longitudinal monitoring of the shrinkage, dropouts and remodeling of diseased RGCs at the cellular level as glaucoma progresses. It may also lead to improved visualization of the RNFL, allowing for detection of subtle changes. A recent study showed that AO-SLO can reveal structural defects in the RNFL that cannot be detected with OCT alone.58
Figure 3.
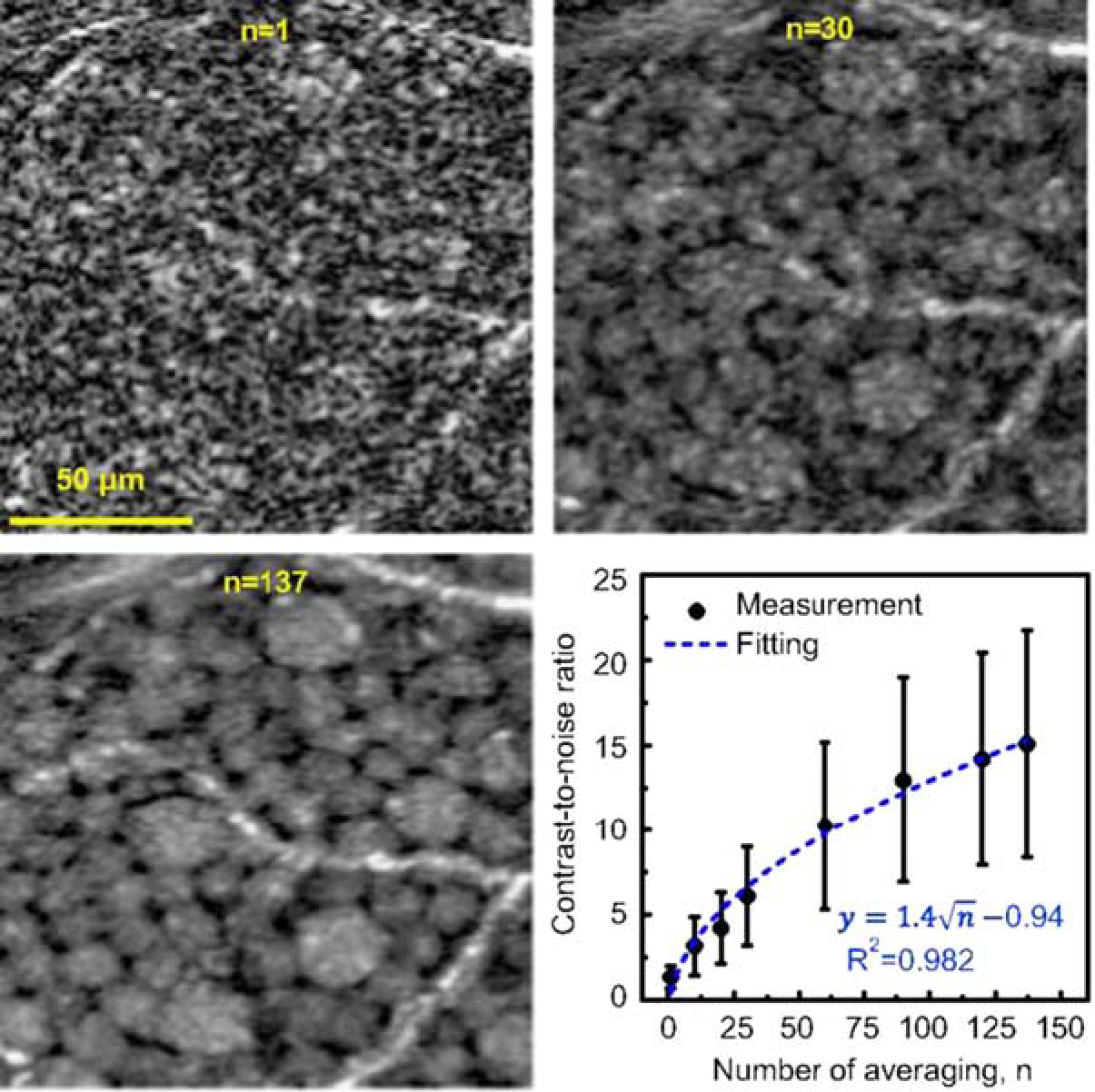
Ganglion cell layer (GCL) somas viewed with averaging registered adaptive optics optical coherence tomography images. Magnified view of the same small patch of retina is shown with different amounts of averaging (n = 1, 30, and 137 images). Images are from 12 to 13.5 degrees temporal to the fovea in subject S3. Plot shows the contrast-to-noise ratio (CNR) of 120 individual GCL somas computed as a function of images averaged. Error bars denote ± 1 SD. CNR increase follows the square root of the number of images (dashed curve). Reproduced from Liu et al.55
Currently, these novel approaches of RGC imaging with cellular resolution using adaptive optics have a limited field of view as a trade-off. The hardware is also complex, requiring expensive cameras with high frame rates and well-aligned adaptive optics modules. Laborious segmentation and registration with heavy computational reconstruction are required to restore the optical phase stability compromised by the drifts and fluctuations during imaging. Other technical challenges include long-term phase stability, speckle noises, multiple scattering artifacts, trade-offs between high-speed imaging and signal-to-noise ratio, and management of large data volumes. These need to be addressed before this technique can be adapted for clinical use.
Future Directions in Monitoring RGC Function and Viability
An ideal method for imaging and labelling RGCs would be minimally invasive, have single cell resolution, be able to detect changes in cell function, and be stable enough to allow for longitudinal monitoring. Functional imaging of RGCs using genetically encoded labels has been trialed in animals, and has the potential for monitoring of RGC function over time. Genetically coded calcium indicators implemented with adaptive optics have been used for in vivo imaging of foveal RGCs in macaques. The light responses of RGCs can be imaged repeatedly, allowing for long-term monitoring of RGC function.59 Recently, adeno-associated viral (AAV) vectors have been used to deliver fluorescent probes into RGCs via intravitreal injection in mice.60 Fluorescence was detected in vivo via confocal scanning laser ophthalmoscope imaging. Reassuringly, there were no structural or functional changes to the retina detected by OCT imaging and ERG after AAV injection. One advantage of this technique is the high specificity of the AAV vectors to RGCs. Future work would have to elucidate if AAVs can transduce human RGCs with specificity and reliability, and whether there are adverse effects of viral transduction. Such gene-based labelling strategies allow for longitudinal monitoring of both quantity and function of RGCs in vivo, offering the potential of tracking disease progression in glaucoma.
There have been attempts to harness the changes in the physical properties of neurons as an endogenous contrast agent for monitoring neuronal activity.61–63 Although ophthalmic OCT has micron-scale axial resolution, motion-compensated phase-sensitive OCT enables the time-resolved detection of an increase of the optical path length (in the order of tens of nanometers) in the RGC layers during patterned photostimulation.64,65 The origin of the size increase is still under debate, and is potentially due to osmotic swelling or increased cell membrane tension during neuronal depolarization.65,66 Future studies will have to determine how RGC injury in glaucoma and other pathologies alters its electrical activity and physical response to photostimulation.
Finally, intracellular motility is a promising endogenous contrast agent that may reflect the cellular viability of RGCs. On the microscopic scale, intracellular organelles including the mitochondria are not quiet, but are constantly undergoing Brownian motion.67 The amplitude of Brownian motion could be related to their metabolic activity and hence cellular viability. Using techniques including dynamic full-field OCT68 and dynamic light scattering OCT,69 intracellular organelle motility can be detected as information-carrying fluctuations imprinted in the backscattering optical signals. If the fluctuations are correlated with mitochondrial activity, one may expect a reduction in the dynamic OCT signal during mitochondrial dysfunction. Indeed, in 2 recent ex vivo studies of retinal explants of mice and macaques, healthy and stressed RGCs may be distinguished based on the diffusion constants or signal decorrelation time of intracellular organelles extracted by dynamic light scattering OCT.70,71 Currently, it is unclear whether the current state-of-the-art OCT engine has sufficient phase stability to utilize the cellular motility contrast for detecting RGC dysfunction in vivo.
Summary
The current commonly used strategies for diagnosing and monitoring glaucoma include visual field testing, examination of the optic disc, and imaging of the optic nerve and RNFL by OCT. These techniques assess RGC loss and cannot identify early RGC injury before irreversible death occurs. Hence, there is a need for novel techniques to detect and monitor the course of RGC injury over time in glaucoma and to assess the response to therapy. Herein we have discussed the use of molecular probes that detect apoptosis, FAF, adaptive optics and functional imaging to image individual RGCs in glaucoma. Although these innovative techniques still require extensive testing and validation before clinical use in humans, they represent an exciting opportunity to identify RGC dysfunction and death early on in the course of the disease, and to initiate appropriate treatment before irreversible damage takes place. Furthermore, we expect that techniques shown to be useful for imaging RGC injury may have applications for the diagnosis and management of other ophthalmic diseases such as age-related macular degeneration and other degenerative conditions of the retina, as well as neurodegenerative diseases such as Alzheimer disease.
Acknowledgments
The authors thank Pui Chuen Hui for helpful discussions and contributions to the section on OCT and adaptive optics. M.A.M. received funding from NIH/NEI K12 EY016335.
Footnotes
The authors declare that they have no conflicts of interest to disclose.
References
- 1.Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. [DOI] [PubMed] [Google Scholar]
- 2.Werkmeister RM, Cherecheanu AP, Garhofer G, et al. Imaging of retinal ganglion cells in glaucoma: pitfalls and challenges. Cell Tissue Res. 2013;353:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JM, Kyung H, Shim SH, et al. Location of initial visual field defects in glaucoma and their modes of deterioration. Invest Ophthalmol Vis Sci. 2015;56:7956–7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–146. [DOI] [PubMed] [Google Scholar]
- 6.Yang E, Al-Mugheiry TS, Normando EM, et al. Real-time imaging of retinal cell apoptosis by confocal scanning laser ophthalmoscopy and its role in glaucoma. Front Neurol. 2018;9:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720; discussion 829–830. [DOI] [PubMed] [Google Scholar]
- 8.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. [DOI] [PubMed] [Google Scholar]
- 9.Almasieh M, Wilson AM, Morquette B, et al. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. [DOI] [PubMed] [Google Scholar]
- 10.Qu J, Wang D, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010;91:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerrigan LA, Zack DJ, Quigley HA, et al. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. [DOI] [PubMed] [Google Scholar]
- 12.Nagata S Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36:489–517. [DOI] [PubMed] [Google Scholar]
- 13.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. [DOI] [PubMed] [Google Scholar]
- 14.Chrysostomou V, Rezania F, Trounce IA, et al. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol. 2013;13:12–15. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–2541. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Sheck L, Crowston JG, et al. Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Invest Ophthalmol Vis Sci. 2012;53:2431–2437. [DOI] [PubMed] [Google Scholar]
- 17.Meers P, Mealy T. Calcium-dependent annexin V binding to phospholipids: stoichiometry, specificity, and the role of negative charge. Biochemistry. 1993;32:11711–11721. [DOI] [PubMed] [Google Scholar]
- 18.Munoz LE, Frey B, Pausch F, et al. The role of annexin A5 in the modulation of the immune response against dying and dead cells. Curr Med Chem. 2007;14:271–277. [DOI] [PubMed] [Google Scholar]
- 19.Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. [DOI] [PubMed] [Google Scholar]
- 20.Barr PM, Lazarus HM, Cooper BW, et al. Phase II study of bryostatin 1 and vincristine for aggressive non-Hodgkin lymphoma relapsing after an autologous stem cell transplant. Am J Hematol. 2009;84:484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blankenberg FG, Strauss HW. Will imaging of apoptosis play a role in clinical care? A tale of mice and men. Apoptosis. 2001;6:117–123. [DOI] [PubMed] [Google Scholar]
- 22.Campian ME, Tan HL, van Moerkerken AF, et al. Imaging of programmed cell death in arrhythmogenic right ventricle cardiomyopathy/dysplasia. Eur J Nucl Med Mol Imaging. 2011;38:1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurihara H, Yang DJ, Cristofanilli M, et al. Imaging and dosimetry of 99mTc EC annexin V: preliminary clinical study targeting apoptosis in breast tumors. Appl Radiat Isot. 2008;66:1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yap TE, Donna P, Almonte MT, et al. Real-time imaging of retinal ganglion cell apoptosis. Cells. 2018;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo L, Moss SE, Alexander RA, et al. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L, Salt TE, Luong V, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007;104:13444–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordeiro MF, Guo L, Coxon KM, et al. Imaging multiple phases of neurodegeneration: a novel approach to assessing cell death in vivo. Cell Death Dis. 2010;1:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nizari S, Guo L, Davis BM, et al. Non-amyloidogenic effects of alpha2 adrenergic agonists: implications for brimonidine-mediated neuroprotection. Cell Death Dis. 2016;7:e2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo L, Salt TE, Maass A, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordeiro MF, Normando EM, Cardoso MJ, et al. Real-time imaging of single neuronal cell apoptosis in patients with glaucoma. Brain. 2017;140:1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouckaert G, Kalai M, Krysko DV, et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell. 2004;15:1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirt UA, Leist M. Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ. 2003;10:1156–1164. [DOI] [PubMed] [Google Scholar]
- 33.Goth SR, Stephens RS. Rapid, transient phosphatidylserine externalization induced in host cells by infection with Chlamydia spp. Infect Immun. 2001;69:1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neher JJ, Neniskyte U, Brown GC. Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front Pharmacol. 2012;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullok K, Piwnica-Worms D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J Med Chem. 2005;48:5404–5407. [DOI] [PubMed] [Google Scholar]
- 36.Barnett EM, Elangovan B, Bullok KE, et al. Selective cell uptake of modified Tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest Ophthalmol Vis Sci. 2006;47:2589–2595. [DOI] [PubMed] [Google Scholar]
- 37.Barnett EM, Zhang X, Maxwell D, et al. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc Natl Acad Sci U S A. 2009;106:9391–9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu X, Johnson JR, Wilson BS, et al. Single-cell resolution imaging of retinal ganglion cell apoptosis in vivo using a cell-penetrating caspase-activatable peptide probe. PLoS One. 2014;9:e88855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzke F, Tsatourian V, Guo L, et al. Time–course of single cell apoptosis in vivo using video and image analysis of retinal ganglion cell disease model. Invest Ophthalmol Vis Sci. 2005;46:4823. [Google Scholar]
- 40.Kindzelskii A, Petty HR. Fluorescence spectroscopic detection of mitochondrial flavoprotein redox oscillations and transient reduction of the NADPH oxidase-associated flavoprotein in leukocytes. Eur Biophys J. 2004;33:291–299. [DOI] [PubMed] [Google Scholar]
- 41.Reinert KC, Dunbar RL, Gao W, et al. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo. J Neurophysiol. 2004;92:199–211. [DOI] [PubMed] [Google Scholar]
- 42.Elner VM, Park S, Cornblath W, et al. Flavoprotein autofluorescence detection of early ocular dysfunction. Arch Ophthalmol. 2008;126:259–260. [DOI] [PubMed] [Google Scholar]
- 43.Field MG, Comer GM, Kawaji T, et al. Noninvasive imaging of mitochondrial dysfunction in dry age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2012;43:358–365. [DOI] [PubMed] [Google Scholar]
- 44.Field MG, Elner VM, Puro DG, et al. Rapid, noninvasive detection of diabetes-induced retinal metabolic stress. Arch Ophthalmol. 2008;126:934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ju WK, Liu Q, Kim KY, et al. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest Ophthalmol Vis Sci. 2007;48:2145–2151. [DOI] [PubMed] [Google Scholar]
- 46.Moreno MC, Campanelli J, Sande P, et al. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. [DOI] [PubMed] [Google Scholar]
- 47.Geyman LS, Suwan Y, Garg R, et al. Noninvasive detection of mitochondrial dysfunction in ocular hypertension and primary open-angle glaucoma. J Glaucoma. 2018;27:592–599. [DOI] [PubMed] [Google Scholar]
- 48.Wizinowich PL, Le Mignant D, Bouchez AH, et al. The WM Keck Observatory laser guide star adaptive optics system: overview. Publ Astron Soc Pac. 2006;118:297. [Google Scholar]
- 49.Ji N, Sato TR, Betzig E. Characterization and adaptive optical correction of aberrations during in vivo imaging in the mouse cortex. Proc Natl Acad Sci U S A. 2012;109:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roorda A, Romero-Borja F, Donnelly Iii W, et al. Adaptive optics scanning laser ophthalmoscopy. Opt Express. 2002;10:405–412. [DOI] [PubMed] [Google Scholar]
- 51.Ginner L, Kumar A, Fechtig D, et al. Noniterative digital aberration correction for cellular resolution retinal optical coherence tomography in vivo. Optica. 2017;4:924–931. [Google Scholar]
- 52.Roorda A, Williams DR. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–522. [DOI] [PubMed] [Google Scholar]
- 53.Sudkamp H, Koch P, Spahr H, et al. In-vivo retinal imaging with off-axis full-field time-domain optical coherence tomography. Opt Lett. 2016;41:4987–4990. [DOI] [PubMed] [Google Scholar]
- 54.Hillmann D, Spahr H, Hain C, et al. Aberration-free volumetric high-speed imaging of in vivo retina. Sci Rep. 2016;6:35209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, Kurokawa K, Zhang F, et al. Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proc Natl Acad Sci U S A. 2017;114:12803–12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossi EA, Granger CE, Sharma R, et al. Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proc Natl Acad Sci U S A. 2017;114:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takayama K, Ooto S, Hangai M, et al. High-resolution imaging of the retinal nerve fiber layer in normal eyes using adaptive optics scanning laser ophthalmoscopy. PLoS One. 2012;7:e33158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen MF, Chui TY, Alhadeff P, et al. Adaptive optics imaging of healthy and abnormal regions of retinal nerve fiber bundles of patients with glaucoma. Invest Ophthalmol Vis Sci. 2015;56:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin L, Masella B, Dalkara D, et al. Imaging light responses of foveal ganglion cells in the living macaque eye. J Neurosci. 2014;34:6596–6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith CA, Chauhan BC. In vivo imaging of adeno-associated viral vector labelled retinal ganglion cells. Sci Rep. 2018;8:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stepnoski RA, LaPorta A, Raccuia-Behling F, et al. Noninvasive detection of changes in membrane potential in cultured neurons by light scattering. Proc Natl Acad Sci U S A. 1991;88:9382–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batabyal S, Satpathy S, Bui L, et al. Label-free optical detection of action potential in mammalian neurons. Biomed Opt Express. 2017;8:3700–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen LB, Keynes RD, Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968;218:438–441. [DOI] [PubMed] [Google Scholar]
- 64.Hillmann D, Spahr H, Pfaffle C, et al. In vivo optical imaging of physiological responses to photostimulation in human photoreceptors. Proc Natl Acad Sci U S A. 2016;113:13138–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfäffle C, Hillmann D, Spahr H, et al. Functional imaging of ganglion and receptor cells in living human retina by osmotic contrast. ArXiv e-prints. 2018. Available at: https://ui.adsabs.harvard.edu/#abs/2018arXiv180902812P. Accessed September 1, 2018. [Google Scholar]
- 66.Ling T, Boyle KC, Goetz G, et al. Full-field interferometric imaging of propagating action potentials. ArXiv e-prints. 2018. Available at: https://ui.adsabs.harvard.edu/#abs/2018arXiv180703269L. Accessed July 1, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovacs R, Kardos J, Heinemann U, et al. Mitochondrial calcium ion and membrane potential transients follow the pattern of epileptiform discharges in hippocampal slice cultures. J Neurosci. 2005;25:4260–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Apelian C, Harms F, Thouvenin O, et al. Dynamic full field optical coherence tomography: subcellular metabolic contrast revealed in tissues by interferometric signals temporal analysis. Biomed Opt Express. 2016;7:1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J, Wu W, Jiang JY, et al. Dynamic light scattering optical coherence tomography. Opt Express. 2012;20:22262–22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thouvenin O, Boccara C, Fink M, et al. Cell motility as contrast agent in retinal explant imaging with full-field optical coherence tomography. Invest Ophthalmol Vis Sci. 2017;58:4605–4615. [DOI] [PubMed] [Google Scholar]
- 71.Lee JS, Eom K, Polucha C, et al. Standard-unit measurement of cellular viability using dynamic light scattering optical coherence microscopy. Biomed Opt Express. 2018;9:5227–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]


