Abstract
Background
Lower testosterone levels in men have been consistently associated with metabolic abnormalities, particularly obesity. This study aims to investigate the relationship between testosterone and obesity by analyzing the correlation between testosterone levels and body fat percentage using data from the NHANES (National Health and Nutrition Examination Survey) database.
Methods
The study included a total of 5959 participants from the NHANES 2011–2016. Multivariable linear regression models were used to assess the association between testosterone levels and body composition parameters, including total percent fat (TPF), android percent fat (APF), gynoid percent fat (GPF), android to gynoid ratio (A/G), and lean mass percent (LMP). Subgroup analyses stratified by sex were conducted using multivariable linear regression. To account for potential non-linear relationships, fitted smoothing curves and generalized additive models were utilized. A separate analysis of participants with a BMI ≥ 30 kg/m2 was conducted to validate the conclusions.
Result
Among males, testosterone levels showed a significant negative correlation with TPF (β = -11.97, P <0.0001), APF (β = -9.36, P<0.0001), GPF (β = -10.29, P <0.0001), and A/G (β = -320.93, P<0.0001), while a positive correlation was observed between LMP and testosterone levels (β = 12.62, P<0.0001). In females, a contrasting pattern emerged in the relationship between testosterone and body fat, but no significant correlation was found between testosterone and body composition in obese women.
Conclusions
The findings of this study support a negative association between body fat and testosterone levels in males.
Introduction
Obesity is a metabolic disease characterized by an excess of adipose tissue, with a BMI (Body Mass Index) of ≥30kg/m2. The prevalence of obesity has significantly increased worldwide, accompanied by a rise in many health problems such as cardiovascular disease, type 2 diabetes, hypertension, musculoskeletal disorders, respiratory diseases, and certain cancers [1]. These diseases can cause disability and premature death, severely affecting quality of life and life expectancy. In recent years, the relation between obesity and hypogonadism was becoming a hot topic. Extensive research has reported the differing contributions of testosterone to obesity in males and females。
Testosterone is one of the most important representatives of the androgen hormone family. It is mainly synthesized in the testes and to a lesser extent in the ovaries and adrenal glands of females. Testosterone can regulate the development and function of male and female reproductive organs by combining with estrogen, and play an important role in other organs of the body. Testosterone can also affect muscle quality, fat distribution, and metabolic status by regulating protein and fat metabolism [2]. In males, testosterone deficiency can lead to increased fat deposition, fat synthesis, and fat cell proliferation, as well as decreased skeletal muscle mass and basal metabolic rate, thereby exacerbating weight gain, increased body fat percentage, and metabolic syndrome, among other issues [3–5].
However, these studies have been limited by small sample sizes, which may limit their generalizability and statistical power. Therefore, in this study, we aim to investigate the relationship between testosterone levels and obesity using a large dataset from the NHANES (National Health and Nutrition Examination Survey) database. Specifically, we aim to examine the effects of testosterone on body composition, as measured by DXA (Dual-energy X-ray absorptiometry), in both sexes. The results of this study may help to improve our understanding of the role of testosterone in obesity and its associated health risks, and may have important implications for the development of effective interventions for obesity management.
Materials and methods
Study population
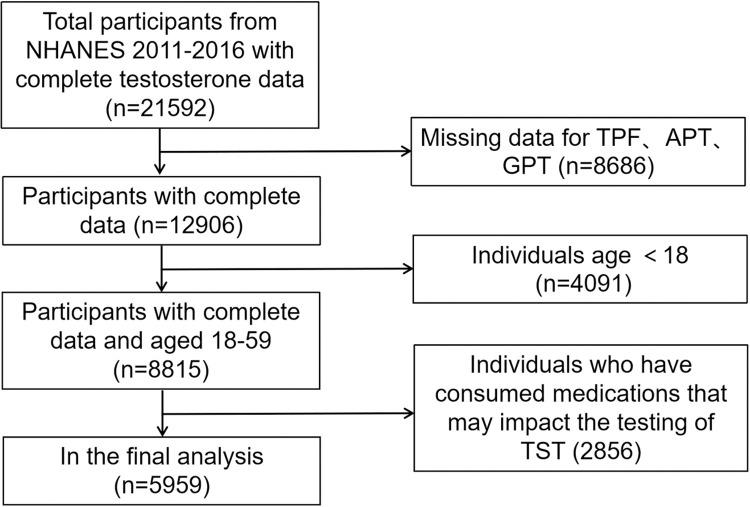
The data for this study were obtained from the National Health and Nutrition Examination Survey (NHANES) conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS) between 2011 and 2016. Participant inclusion and exclusion criteria are illustrated in Fig 1. A total of 21,592 participants with complete testosterone data were initially identified. We excluded 8,686 participants with incomplete body composition data, 4,091 participants under the age of 18 and 2,856 participants who had taken medications known to potentially affect testosterone levels (e.g., birth control pills, estrogen, aldactone). Consequently, our analysis focused on 5959 aged 18–59 years old, who had complete data and underwent DXA scans. The National Center for Health Statistics Research Ethics Review Board has thoroughly examined and endorsed the NHANES study and its protocols. Prior to participating, all individuals willingly gave their informed consent by signing the consent form. The specific NHANES cycle for which we obtained approval is a continuation of Protocol #2011–17. For further details, please visit the CDC’s website at: [insert URL: https://www.cdc.gov/nchs/nhanes/irba98.htm].
Fig 1. Flow chart of sample selection from the NHANES 2011–2016.
Variables
The exposure variables in this study included total percent fat (TPF), android percent fat (APF), gynoid percent fat (GPF), android to gynoid ratio (A/G) and lean mass percent (LMP). These variables were quantified using the Hologic Discovery model A densitometers (Hologic, Inc., Bedford, Massachusetts) with software version Apex 3.2. The measurements were conducted by trained and certified radiology technologists. TPF was determined by calculating the ratio of total fat mass to total fat and lean mass. The android area was defined as the lower trunk region bounded by two lines: the lower pelvic horizontal cut line and a line automatically placed above it. The upper gynoid line was positioned 1.5 times of the height of android region below the pelvic line, while the lower gynoid line was positioned to have a distance between the two gynoid lines twice the height of the android region [6]. The A/G ratio was derived from the ratio of APF to GPF,and LMP was calculated by dividing the total lean mass by the sum of total fat and lean mass. The placement of all these lines was automatically determined by the Hologic software.
The testosterone levels, which served as the outcome variable, were measured using the isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) method. This method was routinely developed by the CDC. The specific details regarding the testosterone measurements can be found in the Laboratory Data section, specifically the Sex Steroid Hormone chapter for the 2013–2014 and 2015–2016 cycles, as well as the Total Testosterone chapter for the 2011–2012 cycle.
Additional factors considered in this study encompassed demographic characteristics such as age, gender, ethnicity, and BMI (Body Mass Index), as well as socioeconomic factors including the ratio of family income to poverty. Physical measurements such as arm circumference and waist circumference were also taken into account. Moreover, the presence of hypertension, hypercholesteremia, and diabetes, as well as smoking status (defined as having smoked at least 100 cigarettes in one’s lifetime), vigorous work activity, and various laboratory markers (including cholesterol levels, triglycerides, HDL cholesterol, LDL cholesterol, creatinine, blood urea nitrogen, and uric acid) were included as covariates. Detailed information pertaining to these variables can be accessed on the official NHANES website.
Statistical analyses
The demographic characteristics of the individuals participating in the study were reported as mean ± standard deviation (SD) for continuous variables with a normal distribution, and as Median (Q1, Q3) for variables without a normal distribution. Categorical variables were expressed as percentages. Multivariable linear regression models were utilized to determine the association between body composition and testosterone levels. Model 1 did not include any covariates, while Model 2 included adjustments for age, sex, and race. Model 3 built upon Model 2 by additionally adjusting for hypertension, diabetes, hypercholesteremia, smoking status, and vigorous work activity. Additionally, a subgroup analysis stratified by sex was conducted using multivariable regression. To account for any potential nonlinear relationship between body composition and testosterone levels, smoothing techniques and generalized additive models were employed. All statistical analyses were carried out using R (http://www.Rproject.org) and EmpowerStats (http://www.empowerstats.com). A significance level below P < 0.05 was deemed to indicate statistical significance.
Results
A total of 5959 participants with complete data were included in this study. Table 1 presents the weighted characteristics of the study samples based on sex. There are noticeable disparities between males and females in terms of baseline characteristics. Firstly, males exhibit significantly higher levels of testosterone when compared to females (p<0.001). Secondly, despite males having higher overall energy intake and consuming more protein, carbohydrates, and fat than females, they possess lower measures of TPF, APF, and GPF in comparison to females. Conversely, males exhibit higher A/G ratio, LMP, arm circumference, and waist circumference, all with p-values <0.001. Furthermore, male BMI is lower than that of females (p<0.01). Moreover, females display lower proportions of high blood pressure, hyperlipidemia, smoking, and engagement in physical activity. There is no significant difference between the two genders in terms of diabetes prevalence.
Table 1. Weighted characteristics of study samples based on sex.
| Males (n = 4434) | Females (n = 1525) | P-value | |
|---|---|---|---|
| Testosterone (ng/dL) | 403.32 (304.00, 525.34) | 22.30 (15.50, 30.80) | <0.001 |
| Total percent fat (%) | 27.09 ± 6.07 | 38.67 ± 6.33 | <0.001 |
| Android percent fat (%) | 31.36 ± 8.32 | 38.68 ± 8.31 | <0.001 |
| Gynoid percent fat (%) | 28.63 ± 5.64 | 42.29 ± 5.28 | <0.001 |
| Android to gynoid ratio | 1.09 ± 0.18 | 0.91 ± 0.15 | <0.001 |
| Lean mass percent (%) | 69.87 ± 5.64 | 58.48 ± 5.85 | <0.001 |
| Age (years) | 38.26 ± 12.28 | 35.73 ± 12.26 | <0.001 |
| Ratio of family income to poverty | 2.11 (1.06–4.12) | 1.49 (0.82–3.17) | <0.001 |
| BMI (kg/m2) | 28.41 ± 5.84 | 28.99 ± 7.68 | <0.01 |
| Arm circumference (cm) | 34.39 ± 4.31 | 31.98 ± 5.64 | <0.001 |
| Waist circumference (cm) | 99.01 ± 15.41 | 94.86 ± 17.59 | <0.001 |
| Cholesterol (mmol/L) | 4.92 ± 1.08 | 4.76 ± 0.98 | <0.001 |
| Triglycerides (mmol/L) | 1.42 (0.91–2.33) | 1.12 (0.74–1.71) | <0.001 |
| HDL Cholesterol (mmol/L) | 1.23 ± 0.34 | 1.44 ± 0.39 | <0.001 |
| LDL Cholesterol (mmol/L) | 2.97 ± 0.90 | 2.81 ± 0.88 | <0.001 |
| Creatinine (μmol/L) | 83.10 (74.26–92.82) | 60.11 (52.16–68.07) | <0.001 |
| Blood urea nitrogen (mmol/L) | 4.64 (3.57–5.36) | 3.93 (3.21–4.64) | <0.001 |
| Serum uric acid (μmol/L) | 358.48 ± 71.73 | 273.42 ± 62.90 | <0.001 |
| Energy intake (kcal) | 2510.07 ± 903.12 | 1812.64 ± 703.12 | <0.001 |
| Protein intake (g) | 99.50 ± 41.28 | 70.31 ± 29.61 | <0.001 |
| Carbohydrate intake (g) | 294.03 ± 118.90 | 226.34 ± 92.84 | <0.001 |
| Fat intake (g) | 95.44 ± 42.03 | 69.20 ± 33.64 | <0.001 |
| Race (%) | <0.001 | ||
| Hispanic | 18.25 | 27.34 | |
| Non-Hispanic White | 62.18 | 44.53 | |
| Non-Hispanic Black | 10.55 | 13.10 | |
| Others | 9.02 | 15.03 | |
| Hypertension (%) | <0.001 | ||
| Yes | 22.81 | 17.33 | |
| No | 77.19 | 82.67 | |
| Hypercholesteremia(%) | <0.001 | ||
| Yes | 27.47 | 17.35 | |
| No | 72.53 | 82.65 | |
| Diabetes (%) | 0.34 | ||
| Yes | 8.90 | 8.05 | |
| No | 91.10 | 91.95 | |
| Smoking status (%) | <0.001 | ||
| Yes | 45.76 | 27.00 | |
| No | 54.24 | 73.00 | |
| Vigorous work activity (%) | <0.001 | ||
| Yes | 33.27 | 14.78 | |
| No | 66.73 | 85.22 |
Notes: Mean ± SD and median (Q1, Q3) for continuous variables, p-values were obtained using the weighted linear regression model and Kruskal-Wallis rank sum test. % for categorical variables and P value was calculated by weighted chi-square test.
Correlation between body composition and testosterone
After adjusting for age, sex, race, hypertension, diabetes, hypercholesteremia, smoking status, and vigorous work activity in model 3, significant negative correlations were observed between TPF, APF, GPF, A/G ratio, and testosterone levels. Conversely, a significantly positive association was found between LMP and testosterone levels. Subgroup analyses stratified by sex revealed a similar trend in males, while in females, a reverse correlation pattern was observed, with testosterone levels positively associated with body fat percentage and negatively associated with muscle mass, as presented in Table 2.
Table 2. The association between body composition and testosterone (ng/dL).
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| β (95% CI) P value | β (95% CI) P value | β (95% CI) P value | |
| Total | |||
| TPF (%) | -18.84 (-19.41, -18.27) <0.001 | -9.25 (-9.90, -8.61) <0.001 | -9.39 (-10.01, -8.77) <0.001 |
| APF (%) | -12.84 (-13.40, -12.27) <0.001 | -7.33 (-7.81, -6.85) <0.001 | -7.44 (-7.90, -6.98) <0.001 |
| GPF (%) | -18.91 (-19.46, -18.36) <0.001 | -8.36 (-9.06, -7.66) <0.001 | -8.42 (-9.10, -7.73) <0.001 |
| A/G | 76.68 (46.61, 106.75) <0.001 | -270.4 (-294.44, -246.37) <0.001 | -283.28 (-306.52, -260.03) <0.001 |
| LMP (%) | 20.11 (19.51, 20.70) <0.001 | 9.78 (9.09, 10.47) <0.001 | 9.94 (9.26, 10.61) <0.001 |
| Sex | |||
| Males | |||
| TPF (%) | -12.44 (-13.21, -11.66) <0.001 | -11.97 (-12.80, -11.15) <0.001 | -12.09 (-12.89, -11.29) <0.001 |
| APF (%) | -9.52 (-10.07, -8.96) <0.001 | -9.36 (-9.97, -8.75) <0.001 | -9.46 (-10.05, -8.87) <0.001 |
| GPF (%) | -10.72 (-11.58, -9.85) <0.001 | -10.29 (-11.18, -9.40) <0.001 | -10.35 (-11.22, -9.48) <0.001 |
| A/G | -337.36 (-364.02, -310.71)<0.01 | -320.93 (-351.37, -290.5) <0.01 | -337.24 (-366.67, -307.82) <0.001 |
| LMP (%) | 13.17 (12.33, 14.00) <0.001 | 12.62 (11.73, 13.51) <0.001 | 12.76 (11.90, 13.63) <0.001 |
| Females | |||
| TPF (%) | 0.08 (-0.05, 0.21) 0.24 | 0.19 (0.06, 0.33) <0.01 | 0.20 (0.07, 0.33) <0.01 |
| APF (%) | 0.08 (-0.01, 0.18) 0.09 | 0.15 (0.05, 0.25) 0.0043 | 0.17 (0.07, 0.26) <0.01 |
| GPF (%) | 0.08 (-0.08, 0.24) 0.31 | 0.15 (-0.01, 0.31) 0.06 | 0.15 (-0.00, 0.30) 0.06 |
| A/G | 4.65 (-0.94, 10.24) 0.10 | 7.83 (2.00, 13.67) <0.01 | 8.93 (3.37, 14.49) <0.01 |
| LMP (%) | -0.09 (-0.23, 0.05) 0.22 | -0.21 (-0.35, -0.06) <0.01 | -0.22 (-0.36, -0.08) <0.01 |
Notes: No covariate was adjusted in Model 1. Model 2 indicates that analysis was adjusted for age, sex, and race. Model 3 indicates model 2 adjustment plus the adjustment for hypertension, diabetes, hypercholesteremia, smoking status, vigorous work activity.
Sex was not adjusted in the sex-stratified subgroup analyses.
In detail, in the fully adjusted model 3, negative correlations were found between TPF, APF, GPF, A/G ratio, and testosterone levels in males. Conversely, a positive relationship was observed between LMP and testosterone level. Specifically, testosterone decreased by 11.97 ng/dL with every 1% increase in TPF, by 9.36 ng/dL with every 1% increase in APF, and by 10.29 ng/dL with every 1% increase in GPF. Furthermore, for every unit increase in A/G ratio, testosterone levels decreased by 320.93 ng/dL, while testosterone levels increased by 12.62 ng/dL for every 1% increase in LMP.
For females, there were a positive correlations between TPF, APF, GPF, A/G ratio with testosterone level. A negative correlation was found between LMP and testosterone levels. Specifically, when TPF increased by 1%, testosterone levels increased by 0.19 ng/dL. For every 1% increase in APF, testosterone increased by 0.15ng/dL, and for every 1% increase in GPF, testosterone levels decreased by 0.15 ng/dL. When A/G increased by 1, testosterone increased by 7.83 ng/dL. Additionally, testosterone decreased by 0.21 ng/dL for every 1% increase in LMP.
Furthermore, we conducted an analysis on the population with a BMI ≥ 30, which is classified as obese, and observed consistent findings with those reported earlier in males. However, in females, no significant correlations were observed between testosterone levels and TPF,APF, GPF, or LMP. These results are presented in Table 3.
Table 3. The association between body composition and testosterone (ng/dL) of participants with BMI ≥ 30.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| β (95% CI) P value | β (95% CI) P value | β (95% CI) P value | |
| Total | |||
| TPF (%) | -16.88 (-17.79, -15.97) <0.001 | -5.57 (-6.70, -4.44) <0.001 | -5.32 (-6.47, -4.16) <0.001 |
| APF (%) | -14.51 (-15.65, -13.37) <0.001 | -5.04 (-6.03, -4.04) <0.001 | -4.82 (-5.83, -3.80) <0.001 |
| GPF (%) | -14.80 (-15.68, -13.92) <0.001 | -2.88 (-3.99, -1.77) <0.001 | -2.86 (-3.98, -1.73) <0.001 |
| A/G | 355.57 (305.74, 405.40) <0.001 | -123.59 (-165.31, -81.87) <0.001 | -111.92 (-154.41, -69.43) <0.001 |
| LMP (%) | 17.56 (16.62, 18.50) <0.001 | 5.63 (4.44, 6.82) <0.001 | 5.37 (4.15, 6.58) <0.001 |
| Sex | |||
| Males | |||
| TPF (%) | -6.74 (-8.19, -5.29) <0.001 | -6.80 (-8.26, -5.33) <0.001 | -6.54 (-8.04, -5.04) <0.001 |
| APF (%) | -6.27 (-7.56, -4.98) <0.001 | -6.29 (-7.59, -4.99) <0.001 | -6.07 (-7.39, -4.74) <0.001 |
| GPF (%) | -3.40 (-4.84, -1.96) <0.001 | -3.61 (-5.09, -2.14) <0.001 | -3.61 (-5.10, -2.12) <0.001 |
| A/G | -143.20 (-194.59, -91.81) <0.001 | -147.85 (-201.21, -94.48) <0.001 | -131.90 (-186.31, -77.48) <0.001 |
| LMP (%) | 6.81 (5.28, 8.34) <0.001 | 6.87 (5.32, 8.41) <0.001 | 6.59 (5.01, 8.17) <0.001 |
| Females | |||
| TPF (%) | 0.07 (-0.29, 0.43) 0.72 | 0.14 (-0.22, 0.49) 0.46 | 0.19 (-0.18, 0.56) 0.31 |
| APF (%) | 0.19 (-0.11, 0.49) 0.220 | 0.18 (-0.12, 0.48) 0.24 | 0.21 (-0.10, 0.51) 0.18 |
| GPF (%) | 0.03 (-0.29, 0.36) 0.85 | -0.02 (-0.34, 0.31) 0.92 | 0.05 (-0.28, 0.38) 0.7736 |
| A/G | 10.39 (-4.31, 25.08) 0.17 | 13.01 (-1.47, 27.48) 0.08 | 12.00 (-3.02, 27.01) 0.12 |
| LMP (%) | -0.06 (-0.44, 0.32) 0.77 | -0.12 (-0.50, 0.26) 0.53 | -0.18 (-0.57, 0.21) 0.36 |
Notes: No covariate was adjusted in Model 1. Model 2 indicates that analysis was adjusted for age, sex, and race. Model 3 indicates model 2 adjustment plus the adjustment for hypertension, diabetes, hypercholesteremia, smoking status, vigorous work activity.
Sex was not adjusted in the sex-stratified subgroup analyses.
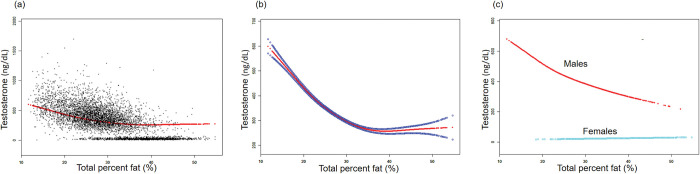
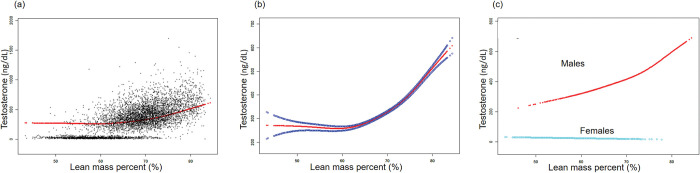
The non-linear relationships between TPF, LMP, and testosterone level were further analyzed using smooth curve fittings and generalized additive models (Figs 2 and 3). Additionally, the results of subgroup analysis stratified by sex are presented in Table 2. The smooth curve fittings between APF, GPF, and testosterone levels were similar to those of TPF with testosterone levels, which are presented in the S1 and S2 Figs.
Fig 2. The association between total percent fat (%) and testosterone (ng/dL).
(a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence bands derived from the fit. (c) Stratified by sex. Age, sex, race, hypertension, diabetes, hypercholesteremia, smoking status, vigorous work activity were adjusted (c was not sex-adjusted).
Fig 3. The association between lean mass percent (%) and testosterone (ng/dL).
(a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence bands derived from the fit. (c) Stratified by sex. Age, sex, race, hypertension, diabetes, hypercholesteremia, smoking status, vigorous work activity were adjusted (c was not sex-adjusted).
We also performed subgroup analyses based on the presence of diabetes, which showed a negative correlation between testosterone levels and body fat percentage in both non-diabetic and diabetic population. These results are presented in the S1 Table.
Discussion
This study revealed notable gender differences in energy intake and body composition. Specifically, it was observed that male participants exhibited higher overall energy intake, while simultaneously presenting lower levels of total protein intake (TPF), amino acid intake (APF), glucose intake (GPF), and body mass index (BMI) in comparison to their female counterparts. Conversely, males displayed higher A/G ratio, lean mass percentage (LMP), arm circumference, and waist circumference as opposed to females. Further investigations revealed, in males, testosterone levels were negatively correlated with TPF, APF, and GPF, and positively correlated with LBM. However in females, there is no significant correlation between testosterone and body composition. These results suggest that testosterone may exert different physiological effects in men and women.
In males, testosterone is known to play a critical role in the regulation of metabolism, and studies have shown that low testosterone levels may lead to obesity. Studies have shown that testosterone deprivation treatment can lead to an increase in visceral, abdominal subcutaneous, and total fat in patients with prostate cancer [7]. Conversely, testosterone supplementation has been shown to decrease abdominal and thigh subcutaneous fat in men with low testosterone levels and high waist circumference [8]. Additionally, weight loss and bariatric surgery in young men have been associated with an increase in total and free testosterone concentrations [9]. These findings suggest bidirectional regulatory effects between serum testosterone and obesity in males.
Obesity may induce male hypogonadism. Adipocytes in obese individuals exhibit elevated levels of aromatase, which catalyzes the conversion of testosterone to estradiol [10]. Estradiol, along with other adipocytokines such as TNF, IL-6, and leptin, can negatively impact testosterone synthesis through a variety of mechanisms, including inhibition of the hypothalamic-pituitary-testicular axis [11], suppression of kisspeptin neuron function [12], and direct inhibition of gonadotropin release by leptin [13]. Conversely, testosterone deficiency can promote the differentiation of pluripotent stem cells into adipocytes, potentially exacerbating obesity [14].
On the other hand, in males, testosterone supplementation can promote lipolysis by increasing expression of norepinephrine and β-adrenergic receptors in adipocytes [15], and inhibit lipid storage by reducing LPL activity on adipocyte surfaces [16]. Additionally, testosterone can reduce expression of PPARγ in preadipocytes, inhibiting their differentiation into adipocytes [14]. Studies have also shown that testosterone supplementation can increase lipid catabolism and mitochondrial biomarkers in adipose tissue, indicating a potential acceleration of energy consumption in adipocytes [17].
Additionally sufficient testosterone concentration is essential for maintaining muscle mass in men, and testosterone deficiency may result in muscle mass loss. In patients with prostate cancer receiving GnRHa therapy for 48 weeks, the muscle mass percentage decreased by 2.7%. Arik Davidyan et al. explored the effect of testosterone on maintaining muscle mass and found that muscle mass in castrated mice significantly decreased [18].
Testosterone supplementation increases muscle mass and strength. Several studies have reported that testosterone therapy increases lean body mass in men, with gains ranging from 1.9 kg to 3.6 kg [19–21], depending on the duration and dose of treatment. Additionally, testosterone therapy has been shown to improve muscle function and gait performance [21]. The mechanism of action for testosterone-induced muscle growth involves hypertrophy of type I and type II fibers and an increase in the number of muscle bundles and satellite cells [22].
The relationship between testosterone levels and body composition in women remains ambiguous. Some studies suggest a positive correlation between testosterone levels and body fat [23, 24], while others indicate no clear correlation between the two [25, 26]. This suggests that the relationship between testosterone levels and body composition in women is complex and may be influenced by factors such as BMI, age, and hormone balance.
Women with pathologically elevated androgen levels, such as those with polycystic ovary syndrome (PCOS), have a high incidence of overweight and obesity, which is associated with insulin resistance and metabolic disorders [27]. The increased activity of steroid-converting enzymes in PCOS patients indicates that adipose tissue function is influenced by these sex steroid hormones at the tissue level. However, the relationship between obesity and high androgen status in the PCOS population is not yet fully established. In most studies of the PCOS population, total testosterone levels were positively correlated with the presence and amount of abdominal obesity [28, 29], but some studies also reported no association between fat accumulation and total testosterone levels [30].
The complex relationship between testosterone levels and female fat distribution remains unclear, mainly because existing mechanism studies have not shown a clear sex difference in testosterone’s regulation of fat metabolism, such as inhibiting adipocyte differentiation, promoting adipocyte lipolysis, and promoting lipid metabolism [31]. In addition, some studies have shown that appropriate testosterone supplementation plays an important role in maintaining female metabolism. Davis et al. found that testosterone replacement therapy improved body composition, including reducing body fat percentage and increasing muscle mass, in women with low testosterone levels [32]. Liang et al. found that higher TT levels were associated with a gradually lower risk of metabolic syndrome in adult women [33].
Therefore, the results of this study contribute to a deeper understanding of the relationship between testosterone and fat distribution, and further exploration of the impact of testosterone levels and female body composition in high androgen-related diseases such as PCOS and hyperandrogenism.
Strength and limitation
This study has several strengths, including the use of a large and nationally representative sample of US adults with complete data on TPF, LMP, and testosterone levels, and the use of DXA to measure body composition accurately. The large sample size also allowed for sex-specific subgroup analysis, revealing a previously unreported pattern between testosterone and TPF.
However, this study is limited by its cross-sectional design, which cannot establish causality. Additionally, there may be other unmeasured confounding factors that could affect the observed associations, such as natural differences in TPF and LMP between men and women.
Conclusion
In conclusion, our results showed that lower testosterone levels in males are linked to more total fat percent and less lean mass percent. Instead, there is no significant correlation between testosterone and body composition in females. The underlying mechanisms of the different patterns of this relationship in males and females still need further investigation.
Supporting information
(a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence bands derived from the fit. (c) Stratified by sex. Age, sex, race, hypertension, diabetes, hyperlipidemia, smoking status, vigorous work activity were adjusted (c was not sex-adjusted).
(TIF)
(a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence bands derived from the fit. (c) Stratified by sex. Age, sex, race, hypertension, diabetes, hyperlipidemia, smoking status, vigorous work activity were adjusted (c was not sex-adjusted).
(TIF)
Notes: No covariate was adjusted in Model 1. Model 2 indicates that analysis was adjusted for age, sex, and race. Model 3 indicates model 2 adjustment plus the adjustment for hypertension, hyperlipidemia, smoking status, vigorous work activity.
(DOC)
Acknowledgments
The authors would like to express their gratitude to the dedicated staff and participants of the NHANES study for their valuable contributions.
Data Availability
The survey data from the NHANES study can be accessed by data users and researchers worldwide through the following website: http://www.cdc.gov/nchs/nhanes/. All included variables can be found in the demographics, examination, laboratory, and questionnaire data parts of NHANES cycles 2011-2012, 2013-2014 and 2015-2016.
Funding Statement
National High Level Hospital Clinical Research Funding (2022-PUMCH-D-002), CAMS Innovation Fund for Medical Sciences (CIFMS 2021-I2M-1-003).Peking Union Medical College Hospital Youth Research Funding(pumch201911009).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. National Natural Science Foundation of China (Grant No. 81971375), and Natural Science Foundation of Beijing (Grant No. 7212080).The funder is Jiangfeng Mao who designed the experiments and supervised the study.
References
- 1.Chen Z, Iona A, Parish S, Chen Y, Guo Y, Bragg F, et al. Adiposity and risk of ischaemic and haemorrhagic stroke in 0·5 million Chinese men and women: a prospective cohort study. Lancet Glob Health. 2018;6(6):e630–e40. doi: 10.1016/s2214-109x(18)30216-x ; PubMed Central PMCID: PMC5960068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45. Epub 20130429. doi: 10.1530/JOE-12-0455 . [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Jackson G, Jones TH, Matsumoto AM, Nehra A, Perelman MA, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34(7):1669–75. doi: 10.2337/dc10-2339 ; PubMed Central PMCID: PMC3120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):224–32. doi: 10.1097/MED.0b013e3283398ee2 . [DOI] [PubMed] [Google Scholar]
- 5.Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40(1):189–207. Epub 20100924. doi: 10.1093/ije/dyq158 . [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Earp JE, LoBuono DL, Greene GW. The Relationship of Physical Activity and Dietary Quality with Android Fat Composition and Distribution in US Adults. Nutrients. 2022;14(14). Epub 20220708. doi: 10.3390/nu14142804 ; PubMed Central PMCID: PMC9318818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, Bolton D, et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf). 2011;74(3):377–83. doi: 10.1111/j.1365-2265.2010.03942.x . [DOI] [PubMed] [Google Scholar]
- 8.Frederiksen L, Højlund K, Hougaard DM, Mosbech TH, Larsen R, Flyvbjerg A, et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol. 2012;166(3):469–76. Epub 20111221. doi: 10.1530/EJE-11-0565 . [DOI] [PubMed] [Google Scholar]
- 9.Dhindsa S, Ghanim H, Jenkins T, Inge TH, Harmon CM, Ghoshal A, et al. High prevalence of subnormal testosterone in obese adolescent males: reversal with bariatric surgery. Eur J Endocrinol. 2022;186(3):319–27. Epub 20220201. doi: 10.1530/EJE-21-0545 . [DOI] [PubMed] [Google Scholar]
- 10.Cohen PG. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt—a major factor in the genesis of morbid obesity. Med Hypotheses. 1999;52(1):49–51. doi: 10.1054/mehy.1997.0624 . [DOI] [PubMed] [Google Scholar]
- 11.Jones TH. Testosterone Associations with Erectile Dysfunction, Diabetes, and the Metabolic Syndrome. European Urology Supplements. 2007;6(16):847–57. doi: 10.1016/j.eursup.2007.07.002 [DOI] [Google Scholar]
- 12.Jones TH. Effects of testosterone on Type 2 diabetes and components of the metabolic syndrome. J Diabetes. 2010;2(3):146–56. doi: 10.1111/j.1753-0407.2010.00085.x . [DOI] [PubMed] [Google Scholar]
- 13.George JT, Millar RP, Anderson RA. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology. 2010;91(4):302–7. Epub 20100610. doi: 10.1159/000299767 . [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144(11):5081–8. Epub 20030724. doi: 10.1210/en.2003-0741 . [DOI] [PubMed] [Google Scholar]
- 15.De Pergola G. The adipose tissue metabolism: role of testosterone and dehydroepiandrosterone. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S59–63. doi: 10.1038/sj.ijo.0801280 . [DOI] [PubMed] [Google Scholar]
- 16.Mårin P, Lönn L, Andersson B, Odén B, Olbe L, Bengtsson BA, et al. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab. 1996;81(3):1018–22. doi: 10.1210/jcem.81.3.8772568 . [DOI] [PubMed] [Google Scholar]
- 17.Maseroli E, Comeglio P, Corno C, Cellai I, Filippi S, Mello T, et al. Testosterone treatment is associated with reduced adipose tissue dysfunction and nonalcoholic fatty liver disease in obese hypogonadal men. J Endocrinol Invest. 2021;44(4):819–42. Epub 20200808. doi: 10.1007/s40618-020-01381-8 ; PubMed Central PMCID: PMC7946690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidyan A, Pathak S, Baar K, Bodine SC. Maintenance of muscle mass in adult male mice is independent of testosterone. PLoS One. 2021;16(3):e0240278. Epub 20210325. doi: 10.1371/journal.pone.0240278 ; PubMed Central PMCID: PMC7993603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frederiksen L, Højlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dordr). 2012;34(1):145–56. Epub 20110224. doi: 10.1007/s11357-011-9213-9 ; PubMed Central PMCID: PMC3260358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14(1):153. Epub 20161007. doi: 10.1186/s12916-016-0700-9 ; PubMed Central PMCID: PMC5054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolind MI, Christensen LL, Caserotti P, Andersen MS, Glintborg D. Muscle function following testosterone replacement in men on opioid therapy for chronic non-cancer pain: A randomized controlled trial. Andrology. 2022;10(3):551–9. Epub 20211230. doi: 10.1111/andr.13147 . [DOI] [PubMed] [Google Scholar]
- 22.Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, et al. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2(3):146–59. doi: 10.1038/ncpendmet0120 ; PubMed Central PMCID: PMC2072878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carranza-Lira S, Velasco Díaz G, Olivares A, Chán Verdugo R, Herrera J. Correlation of Kupperman’s index with estrogen and androgen levels, according to weight and body fat distribution in postmenopausal women from Mexico City. Int J Fertil Womens Med. 2006;51(2):83–8. . [PubMed] [Google Scholar]
- 24.Garaulet M, Pérex-Llamas F, Fuente T, Zamora S, Tebar FJ. Anthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur J Endocrinol. 2000;143(5):657–66. doi: 10.1530/eje.0.1430657 . [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Zhang S, Zou S, Xia X. The relationship between endogenous androgens and body fat distribution in early and late postmenopausal women. PLoS One. 2013;8(3):e58448. Epub 20130304. doi: 10.1371/journal.pone.0058448 ; PubMed Central PMCID: PMC3587576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casson PR, Toth MJ, Johnson JV, Stanczyk FZ, Casey CL, Dixon ME. Correlation of serum androgens with anthropometric and metabolic indices in healthy, nonobese postmenopausal women. J Clin Endocrinol Metab. 2010;95(9):4276–82. Epub 20100621. doi: 10.1210/jc.2009-2390 ; PubMed Central PMCID: PMC2936067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. Epub 20111206. doi: 10.1016/j.fertnstert.2011.09.024 . [DOI] [PubMed] [Google Scholar]
- 28.Douchi T, Yoshimitsu N, Nagata Y. Relationships among serum testosterone levels, body fat and muscle mass distribution in women with polycystic ovary syndrome. Endocr J. 2001;48(6):685–9. doi: 10.1507/endocrj.48.685 . [DOI] [PubMed] [Google Scholar]
- 29.Evans DJ, Barth JH, Burke CW. Body fat topography in women with androgen excess. Int J Obes. 1988;12(2):157–62. . [PubMed] [Google Scholar]
- 30.Dong Z, Chen X, Li L, Huang J, Yin Q, Yang D. Free testosterone level correlated with the metabolic abnormalities dependent on central obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2012;120(6):355–60. Epub 20120510. doi: 10.1055/s-0032-1311643 . [DOI] [PubMed] [Google Scholar]
- 31.Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf). 2010;72(2):176–88. Epub 20090602. doi: 10.1111/j.1365-2265.2009.03645.x . [DOI] [PubMed] [Google Scholar]
- 32.Davis SR, Wahlin-Jacobsen S. Testosterone in women—the clinical significance. Lancet Diabetes Endocrinol. 2015;3(12):980–92. Epub 20150907. doi: 10.1016/S2213-8587(15)00284-3 . [DOI] [PubMed] [Google Scholar]
- 33.Liang J, Peng Q, Yang X, Yang C. The association between serum testosterone levels and metabolic syndrome among women. Diabetol Metab Syndr. 2021;13(1):26. Epub 20210306. doi: 10.1186/s13098-021-00643-6 ; PubMed Central PMCID: PMC7937283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence bands derived from the fit. (c) Stratified by sex. Age, sex, race, hypertension, diabetes, hyperlipidemia, smoking status, vigorous work activity were adjusted (c was not sex-adjusted).
(TIF)
(a) Each black point represents a sample. (b) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence bands derived from the fit. (c) Stratified by sex. Age, sex, race, hypertension, diabetes, hyperlipidemia, smoking status, vigorous work activity were adjusted (c was not sex-adjusted).
(TIF)
Notes: No covariate was adjusted in Model 1. Model 2 indicates that analysis was adjusted for age, sex, and race. Model 3 indicates model 2 adjustment plus the adjustment for hypertension, hyperlipidemia, smoking status, vigorous work activity.
(DOC)
Data Availability Statement
The survey data from the NHANES study can be accessed by data users and researchers worldwide through the following website: http://www.cdc.gov/nchs/nhanes/. All included variables can be found in the demographics, examination, laboratory, and questionnaire data parts of NHANES cycles 2011-2012, 2013-2014 and 2015-2016.